Abstract
COVID‐19 is a highly contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). It has rapidly spread to 216 countries and territories since first outbreak in December of 2019, posing a substantial economic losses and extraordinary threats to the public health worldwide. Although bats have been suggested as the natural host of SARS‐CoV‐2, transmission chains of this virus, role of animals during cross‐species transmission, and future concerns remain unclear. Diverse animal coronaviruses have extensively been studied since the discovery of avian coronavirus in 1930s. The current article comprehensively reviews and discusses the current understanding about animal coronaviruses and SARS‐CoV‐2 for their emergence, transmission, zoonotic potential, alteration of tissue/host tropism, evolution, status of vaccines and surveillance. This study aims at providing guidance for control of COVID‐19 and preventative strategies for possible future outbreaks of zoonotic coronavirus via cross‐species transmission.
Keywords: animal coronaviruses, SARS‐CoV‐2, tissue host tropism, viral evolution, zoonotic coronavirus
1. INTRODUCTION
The coronavirus disease 2019 (COVID‐19) broke out in Wuhan, China, in December 2019 and spread rapidly across the world. On March 11th of 2020, World Health Organization (WHO) announced COVID‐19 a pandemic. As of April 7, just four months since its first outbreak, more than 3.4 million confirmed cases and 238,000 deaths have been recorded in 215 countries, areas and territories, and moreover, it seems that severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) that causes COVID‐19 will probably continue to circulate around the globe (https://www.who.int/emergencies/diseases/novel‐coronavirus‐2019/situation‐reports/). The health authorities and governments of affected countries have paid attention to current pandemic and have taken immediate measures to block COVID‐19 transmission, including utilization of personal protective equipment, quarantine, epidemiological investigation, isolation, clinical data analysis and sharing, public health education, maintaining social distance, the creation of diagnostics, therapeutics and vaccines (Xiao & Torok, 2020). The new information is rapidly accumulating and uncovers the nature of SARS‐CoV‐2, but many questions remain to be answered.
2. CORONAVIRUSES WITH ZOONOTIC POTENTIALS
Coronaviruses (CoVs) are large, enveloped viruses that contain a single‐strand, positive‐sense RNA as the genome and are classified into four genera: Alphacoronavirus, Betacoronavirus, Gammacoronavirus and Deltacoronavirus. CoVs are able to infect a wide range of hosts including humans, pigs, poultry, mice, cats, dogs, camels, bats, pangolins and other species of animals (rabbits, minks, beluga whales, ducks, Canada gooses and guinea fowls). Such a wide host range may be owing a large part to the high mutation rates and recombination events of the viruses in nature (Erles, Toomey, Brooks, & Brownlie, 2003; Li et al., 2018; Tang et al., 2020; Wang, Vlasova, Kenney, & Saif, 2019).
Specific engagement of viral spike (S) proteins with cell surface receptors is the key step for CoVs to initiate and establish infection. The S protein is composed of three functional domains; ectodomain, transmembrane anchor and a short cytoplasmic tail. The ectodomain is responsible for the entry of viruses to host cells and consisted of the S1 and S2 subunits. S1 harbours the receptor‐binding domain (RBD) and mediates virus binding to the cell surface via interaction with diverse cell surface receptors, whereas S2 fuses viral membrane with host cell membrane (Li, 2016; Tortorici et al., 2019). Importantly, RBD also contains viral‐neutralizing epitopes (Shahwan, Hesse, Mork, Herrler, & Winter, 2013; Tortorici et al., 2019; Tusell, Schittone, & Holmes, 2007; Wang, Chen, et al., 2020; Wang et al., 2018). Numerous studies demonstrated that alteration in genes for coding S protein or associated factors can alter CoV virulence, tissue tropism, host range or host immune response (Hulswit, de Haan, & Bosch, 2016; Li et al., 2018). Two amino acid alterations in the S protein of feline CoVs could contribute to the shift of cell tropism from epithelial cells to macrophages (Chang, Egberink, Halpin, Spiro, & Rottier, 2012; Rottier, Nakamura, Schellen, Volders, & Haijema, 2005), and two amino acid changes in the S1 subunit of porcine transmissible gastroenteritis coronavirus (TGEV) could result in the loss of its enteric tropism (Ballesteros, Sanchez, & Enjuanes, 1997). Furthermore, changes in the S protein probably foster the expansion of SARS‐CoV host tropism from bats to civets and then to humans, continually achieving interspecies transmission (Chinese, 2004; Guan et al., 2003; Sheahan, Rockx, Donaldson, Corti, & Baric, 2008). The most striking examples of cross‐species transmission of CoVs are severe acute respiratory syndrome coronavirus (SARS‐CoV), Middle East respiratory syndrome coronavirus (MERS‐CoV) and SARS‐CoV‐2. Moreover, genetic recombination in S gene of CoVs is frequent and complex, significantly contributing to alteration of CoV virulence, tissue tropism and even emergence of new CoVs (Graham & Baric, 2010; Lam et al., 2020; Li, Giorgi, et al., 2020; Xiao, Zhai, et al., 2020; Yi, 2020). It was proven that SARS‐CoV, MERS‐CoV and SARS‐CoV‐2 were the consequences of genetic recombination with the involvement of S gene, and the RBD of SARS‐CoV‐2 was introduced via recombination with pangolin CoVs (Cui, Li, & Shi, 2019; Li, Giorgi, et al., 2020). Genetic recombination between various CoVs within a common host can be a key step to the emergence of recombinant CoVs, and the recombinant CoVs may further undergo cross‐species transmissions under certain conditions. Notably, researchers have shown that a SARS‐CoV‐2 variant with the D614G mutation in S protein became a predominant form around the world with an increased infectivity, although some researchers hold different opinions (Zhang, Jackson et al., 2020; Korber et al., 2020; Yurkovetskiy et al., 2020). However, the particular effects of this mutation in S protein on viral virulence, viral transmission, development of disease and vaccine development await further investigation.
Human CoVs (OC43, 229E, HKU1 and NL63) generally cause mild clinical symptoms, and thus, CoVs had not drawn much attention until the outbreak of SARS in Guangdong province of China in 2002 and MERS in Saudi Arabia in 2012 (Lu et al., 2020). Interestingly, SARS‐CoV, MERS‐CoV and SARS‐CoV‐2 all belong to Betacoronaviruses. Today worldwide scientists have reached a consensus that bats are the natural reservoirs for a number of CoVs (human CoV NL63, human CoV 229E, SARS‐CoV, MERS‐CoV, swine acute diarrhoea syndrome virus (SADS‐CoV), SARS‐CoV‐2 and possibly porcine epidemic diarrhoea virus (PEDV)), but intermediate hosts vary largely. It is generally accepted that palm civets and raccoon dogs are the intermediate hosts for SARS‐CoV, and dromedary camels are the intermediate hosts for MERS‐CoV (Azhar et al., 2014; Chinese, 2004). Camelids and cattle are most likely the intermediates of human CoV 229E and human CoV OC43, respectively (Cui et al., 2019). CoVs infection often causes diseases in the respiratory tract and gastrointestinal tract, and may also cause damages to the liver, kidney, reproductive system or nervous system in different animal species (Niederwerder & Hesse, 2018; Paules, Marston, & Fauci, 2020).
3. CoVs INFECTION IN PIGS
Currently, six different CoVs have been reported from swine. Among them, TGEV, PEDV, porcine respiratory coronavirus (PRCV) and SADS‐CoV belong to Alphacoronaviruses, while porcine hemagglutinating encephalomyelitis virus (PHEV) and porcine deltacoronavirus (PDCoV) belong to Betacoronavirus and Deltacoronavirus, respectively (Wang et al., 2019). PEDV, TGEV, SADS‐CoV and PDCoV can cause gastroenteritis characterized by anorexia, dehydration, vomiting and diarrhoea, especially in sucking or nursing piglets, though some of these viruses may replicate in the lung (Niederwerder & Hesse, 2018). In general, based on clinical signs, porcine CoVs can be grouped into two types: enteric type and respiratory type, and accordingly faecal‐oral transmission and aerogenic transmission are the main routes for viral spread, respectively.
PRCV is a derivative of TGEV with a large deletion of 207–227 aa in the S protein. This deletion changes the tissue tropism of TGEV from enteric tract to the respiratory tract, albeit exhibiting mild respiratory symptoms (Wang et al., 2019). Notably, phylogenetic analysis suggested that PDCoV might have originated from birds, indicating a potential cross‐species transmission from birds to mammals (Jung, Hu, & Saif, 2016; Woo et al., 2012). Both TGEV and PRCV utilize aminopeptidase N (APN) as a cellular receptor for binding, while sialic acid is the binding receptor for PHEV (Nam & Lee, 2010; Reguera et al., 2012; Zhu et al., 2018) (Figure 1). Several groups demonstrated that SADS‐CoV does not utilize the functional CoV receptors reported so far angiotensin‐converting enzyme 2 (ACE2), aminopeptidase N (APN), dipeptidyl peptidase 4 (DPP4) and carcinoembryonic antigen‐related cell adhesion molecule (CECAM 1a) (Yang et al., 2019; Zhou et al., 2018). Currently, no report is available on the functional receptor utilization by PHEV (Table 1). These six CoVs usually cause infections in pigs, but PDCoV seems to have the ability to infect other species such as badgers, calves and cats (Li et al., 2018). Moreover, it was reported that PEDV could engage with human APN and infect human cells (Liu et al., 2015). Thus, PDCoV and PEDV may pose a potential risk for other animals and humans, suggesting a necessity to strengthen coronavirus surveillance in those possible reservoirs.
Figure 1.
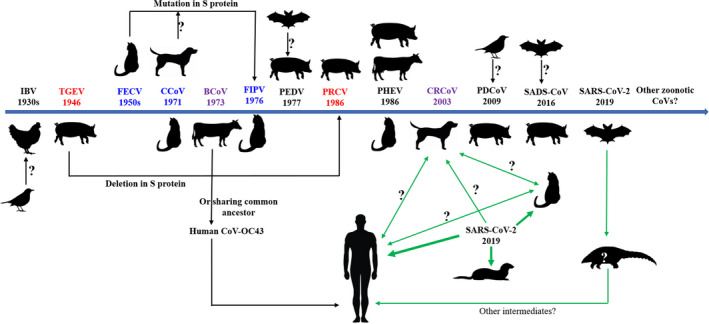
Timelines of the emergence of animal CoVs with significant impact on farm animals, pets, and humans. Arrow directions stand for the gene source or gene exchange direction. Animals locating vertically at individual CoV are viral hosts. CoVs in the same colour (red, green, purple) indicate that they have close relationship either gene exchange or sharing common ancestors. Blue line stands for the possible transmission route of SARS‐CoV‐2 between hosts. Thick lines strand for relative strength of infectivity [Colour figure can be viewed at wileyonlinelibrary.com]
Table 1.
Pathobiologic characteristics of some animal coronaviruses (CoVs)
| CoV | Representative species | Host | Zoonotic nature | Tissue tropism | Common clinical symptoms | Receptor for entry | References | |
|---|---|---|---|---|---|---|---|---|
| Porcine CoV | Alphacoronavirus | PEDV, TGEV, PRCV, PHEV | Mainly Pigs, occasionally Badgers, Calves, Cats | No | Lungs, Intestine, Brain | Pneumonia, Diarrhoea, Neurological symptoms | Sugar, APN, APN, ND | Wang et al. (2019), Li et al. (2018), Liu et al. (2015), Hulswit et al. (2019), Reguera et al. (2012) |
| Avian CoV | Gammacoronavirus | IBV | Poultry | No | Respiratory Tract, Kidney Oviduct, Testes, Alimentary Tract | Rales and sneezing, Renal swelling and brittle, Poor egg production, Alloplasia, Inflammation, etc | Sialic Acid | (Shahwan et al. (2013), Li, Chen, et al. (2020), Cavanagh, 2007) |
| Canine CoV | Alphacoronavirus | CCoV, CRCoV | Dogs | No | Intestine, Tonsils, Lungs, Liver | Vomiting, Diarrhoea, Drowsiness | APN (for both canine and feline); Sialic Acid, HLA1 | Szczepanski et al. (2019), Regan et al. (2012) |
| Feline CoV | Alphacoronavirus | FECV, FIPV | Cats | No | Intestine, Monocytes | Cough, Vomiting, Diarrhoea, Peritonitis | APN | Wang et al. (2013), Rottier et al. (2005) |
| Bovine CoV | Betacoronavirus | BCoV | Cattle | No | Respiratory Tract, intestines, Nasal turbinates, Trachea, Lungs | Diarrhoea, Winter dysentery, Respiratory illness | Sialic Acid | Szczepanski et al. (2019), Hulswit et al. (2019) |
| SARS‐CoV‐2 | Betacoronavirus | SARS‐COV‐2 | Bats | Yes | Respiratory Tract, Gastrointestinal Tract, Spleen, Lymph Nodes, Conjunctival Sac, Uterus, Blood Vessels, Liver, Heart, Kidney | Fever, Cough, Fatigue, Dyspnoea, Diarrhoea, Multiple organ dysfunction syndrome, Lymphocytopenia | ACE2, CD147 | Wang, Chen, Strych, et al. (2020), Zhou, Yang, et al. (2020), Zhou, Zeng, et al. (2020), Chen, Feng, et al. (2020), Szczepanski et al. (2019 |
Abbreviations: ACE2, Angiotensin‐converting enzyme 2; APN, aminopeptidase N; CoVs, coronaviruses; HLA1, Human leucocyte antigen; IBV, infectious bronchitis virus; ND, not determined.
Vaccination is considered the most effective approach to the prevention and control of porcine coronavirus infections in pig farms. However, currently vaccines are only available for TGEV and PEDV, and the majority of them are subunit vaccines and live attenuated vaccines (Gerdts & Zakhartchouk, 2017; Hou et al., 2019; Zuniga, Pascual‐Iglesias, Sanchez, Sola, & Enjuanes, 2016). Notably, the latest progress in developing a vaccine candidate for PEDV is the combination of 2′‐O‐MTase inactivation and removal of endocytic signal in the S protein (Hou et al., 2019), which seems to be a very promising strategy to design and develop vaccines for other porcine CoVs.
Our phylogenetic analysis with S protein shows that PEDV and TGEV share only 42.8%–43.5% of genome similarity with SARS‐CoV‐2, and PHEV and PDCoV share 49.2%–49.3% and 40.3%–40.4% genome similarity with SARS‐CoV‐2, respectively (Table 2). Obviously, porcine CoVs are unlikely the origin of SARS‐CoV‐2. However, the RBD of SARS‐CoV‐2 is likely to recognize porcine ACE2 based on the high similarity of critical virus‐binding residues between porcine ACE2 and human ACE2 (Wan, Shang, Graham, Shang, Graham, Baric, & Li, 2020), raising a reasonable speculation about SARS‐CoV‐2’s ability to infect pigs and even potential recombination with diverse porcine CoVs to generate a new strain with a pandemic potential. A recent study shows that pigs are not susceptible to SARS‐CoV‐2 (Shi et al., 2020), but a possibility remains that SARS‐CoV‐2 may jump and adapt to pigs either via mutations or recombination with circulating porcine CoVs. Therefore, even more strict hygiene and appropriate biosafety measures, such as ensuring a safe source of porcine feed, regular test of swineherds and relevant personnel in the farms, staggering workers by working unit, prohibiting the import of hogwash and leftovers from restaurants, limiting the frequencies of visitors and transport from outside, etc., should be implemented in advance to cut off the possible transmission of SARS‐CoV‐2 from infected humans (particularly the farm workers with asymptomatic infection) to pigs.
Table 2.
Comparison of S protein identity (genome, nucleobase, amino acid) between common animal coronavirus and SARS‐CoV‐2
| SARS‐CoV‐2 | Alpha‐CoV | Beta‐CoV | Gamma‐CoV | Delta‐CoV | |||
|---|---|---|---|---|---|---|---|
| Porcine (PEDV, TGEV) | Canine | Feline | Porcine (PHEV) | Bovine | Chicken | Porcine (PDCoV) | |
| Genome | 42.8−43.5% | 43.6−44.0% | 43.3−43.6% | 49.2−49.3% | 49.2−49.3% | 43.0−43.2% | 40.3−40.4% |
| S Protein | |||||||
| Nucleobase | 37.1−38.7% | 38.5−40% | 39.20% | 43.4−43.5% | 43.90% | 40.9−41.1% | 38.5−39.0% |
| Amino acid | 20.3−22.1% | 20.80% | 20.30% | 28.8−29.0% | 28.8−29.2% | 22.2−22.3% | 21.9−22.6% |
4. INFECTIOUS BRONCHITIS VIRUS
Infectious bronchitis virus (IBV) is a highly contagious avian coronavirus. It can cause severe respiratory, urogenital, renal and reproductive disorders, clinically characterized by rales, sneezing, diarrhoea, and drop in egg quality and production (Li, Chen, et al., 2020; Santos Fernando et al., 2017). IBV belongs to Gammacoronavirus and has dozens of serotypes due to rapid evolution driven by genetic diversity, natural selection and human intervention (Franzo et al., 2019; Zhao et al., 2016). Based on diverse tissue tropism, IBVs are classified to respiratory type, nephrotropic type and reproductive type (Cook, Jackwood, & Jones, 2012). Comparative genome analysis shows that birds can serve as a gene source of Gammacoronavirus and Deltacoronavirus, fuelling constant evolution and dissemination of CoVs. However, this report did not prove that co‐infection facilitates recombination (Woo et al., 2012). May chickens or other birds serve as a mixing vessel for Gammacoronavirus and Deltacoronavirus? It seems reasonable since chickens have a close relationship with human habitats and birds can move for a long distance in a short period of time. The investigation needs to be explored. IBV normally binds to cell receptors via sialic acid for its attachment and entry (Shahwan et al., 2013; Toro, van Santen, & Jackwood, 2012) (Table 1). Live attenuated vaccines for IBV are usually adopted by the farms, which are developed by serial passages of virulent strains in embryonated chicken eggs (Baron, Iqbal, & Nair, 2018; Laconi et al., 2020; Masoudi, Pishraft Sabet, & Shahsavadi, 2020). The constant emergence of new serotypes and variants of IBV brings a new challenge for the control of this disease in poultry. This also reminds us to be vigilant in the potential evolution into diverse branches of SARS‐CoV‐2 in the future. Approximately, 43.0%–43.2% of the genome similarity is observed between IBV and SARS‐CoV‐2 (Table 2).
5. CANINE CoVs
Canine coronavirus (CCoV) and canine respiratory coronavirus (CRCoV) represent canine CoVs, and they belong to Alphacoronavirus and Betacoronavirus, respectively. CCoV, which is an enteric virus and populates in the alimentary tract, and its infection usually causes mild gastroenteritis featured by loss of appetite, vomiting, fluid diarrhoea and dehydration in dogs. Accordingly, the faecal‐oral transmission is the main route for CCoV spread. Notably, four CCoV strains could experience S gene exchange with TGEV at the N‐terminal domain, unravelling a novel mechanism for CCoV evolution and a possible interspecies circulation between dogs and pigs (Decaro et al., 2009). Canine APN receptor mediates the binding and entry of CCoV, and it is worthy to note that CCoV genotype II can not only engage with canine APN but also interact with feline APN, which is in a sharp contrast with the general concept that each virus should utilize a species‐specific receptor (Buonavoglia et al., 2006; Regan et al., 2012) (Table 1). This does imply a potential of cross‐species transmission of CCoV.
CRCoV infection leads to mild respiratory disease with clinical signs of cough and potential bronchopneumonia. Actually, CRCoV shares many gene and amino acid similarities with bovine CoV, suggesting its possible origin of a bovine or common ancestor (Lorusso et al., 2009). Sialic acids and perhaps human leucocyte antigen class I (HLA1) serve as the receptors for CRCoV binding and entry (Erles, Shiu, & Brownlie, 2007; Lu et al., 2016; Szczepanski et al., 2019) (Table 1). So far, only a few recombinant canine CoVs have been reported, and perhaps more attention needs to be paid on the recombination event for canine CoV. For the prevention of canine CoV infection, both inactivated and attenuated vaccines are available. However, in most cases, canine coronavirus infection only causes mild or subclinical infection in dogs, and therefore, the use of vaccines are usually not recommended by veterinarians and the World Small Animal Veterinary Association (Day, Horzinek, Schultz, & Squires, 2016).
Canine CoVs shares only 43.6%–44.0% genome similarity with SARS‐CoV‐2 (Table 2), implying an unlikely canine source of SARS‐CoV‐2. Currently, two dogs were reported to test positive with SARS‐CoV‐2 in Hong Kong. An OIE report shows that the antibody in a pet dog living with a COVID‐19‐infected patient in Hong Kong was originally weak positive against SARS‐CoV‐2 but became negative later after analysing the nasal, oral and rectal samples over time. This dog did not present any clinical symptoms while SARS‐CoV‐2 was isolated from its clinical samples (COVID‐19 (SARS‐COV‐2), Hong Kong (SAR – PRC) 18/03/2020). Very recently, a family dog in America was tested positive for SARS‐CoV‐2, and three members of this family were confirmed to be infected by SARS‐CoV‐2 prior to the report. Thus far, no evidence supports that pet dogs would play a significant role in the spread of SARS‐CoV‐2 between dogs and households.
6. FELINE CoVs
Feline CoVs consist of two biotypes: feline enteric CoV (FECV) and feline infectious peritonitis virus (FIPV). FECV mainly replicates in the intestinal epithelium of cats and causes inapparent enteritis (Vogel et al., 2010). In contrast, FIPV is capable of replicating in monocytes and can lead to systemic diseases, often fatal peritonitis characterized by immune complex vasculitis accompanied by necrosis and pyogenic granulomatous inflammation (Wang, Su, Hsieh, & Chueh, 2013). FECV and FIPV are indistinguishable antigenically, morphologically and serologically (Felten & Hartmann, 2019; Hartmann, 2005). Genetic analyses have demonstrated that FIPV acquired the tropism in macrophage through mutation in the S gene of FECV (Chang et al., 2012; Rottier et al., 2005; Licitra et al., 2013; Vennema, Poland, Foley, & Pedersen, 1998), not via the receptor usage, likely with the help of accessory proteins 3c and 7b for its tropism shift (Oguma, Ohno, Yoshida, & Sentsui, 2018; Pedersen, 2009; Pedersen et al., 2012). It is suggested that some strains of FIPV might have arisen from a genetic recombination between FECV and canine CoV (Herrewegh, Smeenk, Horzinek, Rottier, & de Groot, 1998). The host and tissue tropism expansion of CoVs may likely be the result of either genetic mutations or recombination in the S or other genes that may have occurred in feline or canine CoVs. APN serves as a binding receptor for both FECV and FIPV (Hohdatsu, Izumiya, Yokoyama, Kida, & Koyama, 1998) (Table 1). As shown in Table 2, feline CoV shares only 43.3%–43.6% genome similarity with SARS‐CoV‐2, also seemingly irrelevant to the SARS‐CoV‐2 emergence. Currently with regard to the control of feline CoV infection, vaccination against FIP is not recommended due to the possible occurrence of antibody‐dependent enhancement (ADE) (Day et al., 2016). Future studies are needed for exploring the pathogenesis of FIPV infection and developing new safe and effective vaccines.
Media coverage in Belgium reported that a cat was tested positive for SARS‐CoV‐2 one week after its owner was confirmed positive for COVID‐19. Interestingly, the cat exhibited some typical signs of COVID‐19 as in humans, including diarrhoea, vomiting and breathing difficulty. Although the viral genome was detected from the vomit and faecal samples, no direct evidence in reality points to the viral transmission from cats to cats or to humans. A modelled structure analysis showed that the receptor‐binding motif of SARS‐CoV‐2 RBD can engage with ACE2 of humans and cats at similar efficiencies (Wan, Shang, Graham, et al., 2020). Furthermore, a recent study illustrated that SARS‐CoV‐2 could infect outbred cats through intranasal inoculation effectively, and what's more, the virus could be further transmitted to other cats via respiratory droplets (Shi et al., 2020), indicating the high infectivity and transmissibility of SARS‐CoV‐2 in cats. In addition, a field investigation has shown a significant number of cats (15/102) with SARS‐CoV‐2 infection through a ELISA‐based antibody detection test in Wuhan, the epicentre of first COVID‐19 outbreak in China (Zhang, Zhang, Wu, & Zhang, 2020). Thus, now it seems clear that SARS‐CoV‐2 is able to infect cats at a notable efficiency. This urges a comprehensive surveillance of SARS‐CoV‐2 in cats to better understand and be vigilant about cat's role in SARS‐CoV‐2 spread between (stray and/or owned) cats and even to humans. A study ever suggested SARS‐CoV could even infect domestic cats without showing clinical signs and then be transmitted to naïve cats who were living together (Li, 2020; Martina et al., 2003). In consideration of the high genomic similarities between SARS‐CoV and SARS‐CoV‐2 (82%) (Chan et al., 2020) and the high sequence homology between feline ACE2 and human ACE2 (85%) (Guo et al., 2008), it is not surprising that SARS‐CoV‐2 is able to infect cats and very likely to transmit between cats or even to other species. However, currently those infected cats are only regarded as the victims of human SARS‐CoV‐2 infection, and not evidence justify the notion that SARS‐CoV‐2 can transmit from cats to humans. Notably, report showed that SARS‐CoV‐2 were transmitted from infected minks to at least 2 farm workers in holland (Oreshkova et al., 2020), making it the first case so far from animals to humans.
It is of interest to note that the seroprevalence of feline CoVs is highly variable (especially for cats in shelters), ranging from 31.2% to 90% and depending on the strains, ages and reported regions (Kipar, Meli, Baptiste, Bowker, & Lutz, 2010; Oguzoglu, Sahna, Ataseven, & Muz, 2010; Taharaguchi, Soma, & Hara, 2012). Therefore, it is particularly vigilant on potential in vivo recombination of SARS‐CoV‐2 and feline CoVs which may result in generating novel coronavirus with accompanying alterations of virulence, tissue tropism and host range. This scenario has been reported in other animals and viruses. Pigs and birds can serve as the 'mixing vessel' for influenza virus (Zhang et al., 2018). The emergence of A(H1N1) pandemic influenza virus is a good example. This virus was likely originated by triple reassortment of 4 different parental viruses (avian, human, swine, avian‐like), and global trade of live swine indeed plays a role during the emergence of pandemic virus (Krog, Hjulsager, Larsen, & Larsen, 2017; Mena et al., 2016; Neumann, Noda, & Kawaoka, 2009). Perhaps besides the humanized mouse, rhesus macaque and ferret models, it is worth considering cats as an additional animal model to explore viral pathogenesis as well as therapeutic agents and vaccines against SARS‐CoV‐2.
7. BOVINE CoVs (BCoVs)
BCoVs are members of the Betacoronavirus genus and cause neonatal diarrhoea, winter dysentery and respiratory illness in cattle. BCoV did not draw public health concerns until the isolation of a BCoV‐like enteric virus CoV‐44/US/94 from a child (Zhang, Herbst, Kousoulas, & Storz, 1994). Furthermore, a relaxed molecular clock approach showed that human Betacoronavirus OC43 that causes common cold in humans was relevant to BCoV and that BCoV was assumed to be an ancestor of human CoV‐OC43, or alternatively, they might have originated from a common ancestor (Hasoksuz et al., 2007). BCoV also utilizes sialic acids as the binding receptor for viral attachment and entry, and it is usually transmitted through faecal‐oral route or respiratory route (Table 1). Besides cattle, BCoV can also infect dogs, poultry and possibly giraffes (Hasoksuz et al., 2007; Ismail, Cho, Ward, Saif, & Saif, 2001; Kaneshima et al., 2007), indicating its broad host range. Our phylogenetic analysis shows that BCoV shares only 49.2%–49.3% genome similarity with SARS‐CoV‐2 (Table 2). For bovine CoV infection control, vaccination of pregnant cows is a common practice, which can confer protection for neonates through colostrum (Kanno, Ishihara, Hatama, & Uchida, 2013; Nemoto et al., 2017).
Taken together, porcine, bovine, avian, canine and feline CoVs exhibit their wide tissue tropisms in the gastrointestinal, respiratory, and nerve systems, and continuous host range changes. The phylogenetic analyses revealed that the CoVs of porcine, bovine, avian, canine and feline origins all shared a low level (<50%) of genome similarity with SARS‐CoV‐2 (Table 2). The genetic recombination and mutations, especially within the S protein of CoVs, are implicated as a driving factor for host range expansion. Typically, deletion in the N‐terminal domain of S protein in some strains of TGEV leads to their conversion to PRCV, whereas PDCoV may have emerged from birds despite the lack of a clear genetic causality. Experimental results proved that several point mutations introduction in the S protein of SARS‐CoV isolated from civets could confer the full infectivity of the virus in human cells (Sheahan et al., 2008). Nevertheless, much remains unknown about the underlying mechanisms responsible for CoVs cross‐species transmission.
8. SARS‐COV‐2
SARS‐CoV‐2 is the aetiologic agent of COVID‐19. It belongs to the genus Betacoronavirus and has a close relationship with SARS‐CoV, but these two viruses are different in biological, epidemiological and clinical characteristics (Jiang et al., 2020; Lu et al., 2020).
During the initial stage of COVID‐19 epidemic, typical symptoms of SARS‐CoV‐2‐infected patients seemed to mainly centre on the respiratory illness including fever, cough, fatigue, dyspnoea, diarrhoea and invasive lesions in the lungs consistently observed by chest radiographic examination. But later many patients appeared to die of multiple organ dysfunctions. Furthermore, some patients show symptoms like nausea and diarrhoea or the clinical signs of influenza infections. More importantly, a body of growing evidence strongly suggests asymptomatic carriers of SARS‐CoV‐2, reflecting the difficulty in containing the pandemic of COVID‐19 (Chen, Hu, et al., 2020; Xiao, Tang, et al., 2020; Yang et al., 2020). Additionally, it was reported that some of the severely ill patients would exhibit neurologic manifestations (Mao et al., 2020).
It is widely accepted that respiratory transmission is the main route for SARS‐CoV‐2 spread between people, and beyond this, viral RNA or antigen has been detected in the conjunctival sac, kidney tubules and faeces of the patients (Diao et al., 2020; Xiao, Tang, et al., 2020; Zhou, Zeng, Zeng, Tong, & Chen, 2020), suggesting that the urine‐oral and faecal‐oral transmissions might also contribute to the virus spread. Additionally, the presence or isolation of SARS‐CoV‐2 have been increasingly confirmed from aerosols, saliva, faeces, blood, urine and nerve tissues (e.g. cerebrospinal fluid) (Cyranoski, 2020; Mao et al., 2020; Xiao, Tang, et al., 2020; Young et al., 2020; Yu et al., 2020) (Table 1). All these findings unequivocally demonstrate the broad range of tissue tropism of SARS‐CoV‐2 within human body.
Bats are considered the natural reservoir of SARS‐CoV‐2 based on genome similarity (~96%) and similarity in RBD (~85%), and pangolins are presumably believed to be an intermediate host based on genome similarity (90.5%–91.02%) and similarity in RBD (~89%) in RBD (Cyranoski, 2020; Wong, Javornik, Cregeen, & Petrosino, 2020; Zhang, Wu, et al., 2020; Zhang & Holmes, 2020). Other possible intermediate hosts of SARS‐CoV‐2 need to be identified and confirmed (Deng et al., 2020). In order to depict the evolutionary relationship of SARS‐CoV‐2 with representative CoVs circulating in animals, phylogenetic trees have been constructed based on S protein of nine human SARS‐CoV‐2 isolates and 37 common animal CoVs (Figure 2). Our analyses found that SARS‐CoV‐2 has a closest evolutionary relationship to SARS‐CoV, with more than 74.8% amino acid identity, 72%–76.42% similarity in S protein and ~ 73.4% in RBD (Coronaviridae Study Group of the International Committee on Taxonomy of, 2020; Sun et al., 2020; Xu et al., 2020; Zhang & Holmes, 2020). The TGEV and PEDV showed 37.1% and 38.7% nucleotide identities and 20.3% and 22.1% amino acid identities in S protein sequence to SARS‐CoV‐2, respectively. Approximately, 43.4%–43.5% nucleotide identity and 28.8%–29.0% amino acid identity are discovered between S proteins of PHEV and SARS‐CoV‐2, respectively. PDCoV has about 38.5%–39.0% nucleotide identity and 21.9%–22.6% amino acid identity to the S sequence of SARS‐CoV‐2. Avian CoVs, bovine CoVs, canine CoVs and feline CoVs exhibit 40.9%–41.1%, 43.9%, 38.5%–40% and 39.2% nucleotide identity as well as 22.2%–22.3%, 28.8%, 20.8% and 20.3% amino acid identity with the S sequence of SARS‐CoV‐2, respectively. Additionally, the homology analyses of SARS‐CoV‐2 and various animal CoVs isolated from mink (Neovison vison), mouse (Mus musculus), horse (Equus caballus), rabbit (Oryctolagus cuniculus), duck (Anas platyrhynchos), turkey (Meleagridis gallopavo), goose (Anser anser) and Beluga Whale (Delphinapterus leucas) also show the nucleotide identities ranging from 40.9%–46.2% and amino acid identities of 22.1%–30.8% (Figure 2). These data prove that SARA‐CoV‐2 has low genome/S protein similarity with common animal CoVs. A complete list of all CoV strains analysed in this study can be found in Table S1.
Figure 2.
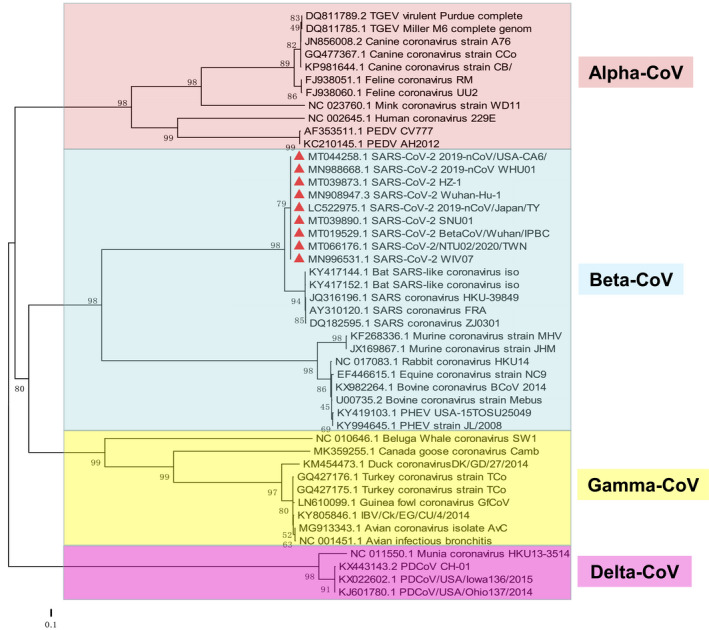
Phylogenetic analysis based on the genome of Coronavirus. The robustness of the neighbour‐joining tree was estimated by bootstrap analysis with 1,000 replicates [Colour figure can be viewed at wileyonlinelibrary.com]
9. RECEPTOR UTILIZATION BY SARS‐COV‐2
As for the receptors utilized by SARS‐CoV‐2, biophysical and structural data showed that the S protein engages with ACE2 with at least 10 times higher affinity compared to that of SARS‐CoV (Wrapp et al., 2020). In addition to ACE2, SARS‐CoV‐2 may also invade the cell via another receptor: CD147 (Ibrahim, Abdelmalek, Elshahat, & Elfiky, 2020; Wang, Chen, et al., 2020) (Table 1), somewhat resembling SARS‐CoV’s receptor binding which seemed to utilize more than one receptor (ACE2 and CD209L) for invasion as well. Furthermore, GRP78, Furin and heparan sulphate probably provide advantages during viral attachment and fusion with the cell membrane (Hao et al., 2020; Ibrahim et al., 2020; Qing & Gallagher, 2020). It was reported that Furin is involved in the cleavage of SARS‐CoV‐2 S protein and further facilitate viral entry (Shang et al., 2020). In principle, tissues with consistently high expression of ACE2 or CD147 are likely susceptible to SARS‐CoV‐2, including small intestine, kidney (renal tubular cells), testis (Leydig cells and cells in seminiferous ducts), gall bladder, heart, stomach and liver (Fagerberg et al., 2014). According to a recent report from the United States Department of Agriculture, a Malayan tiger (Panthera tigris jacksoni) and several lions (Panthera leo) in a zoo situated in New York was confirmed COVID‐19 positive (Agriculture, 2020). We blasted relevant sequences of the respective ACE2 of tigers and discovered a higher homology at the transcriptional level (83%) and the translational level (85%) between Amur tiger (Panthera tigris altaica) ACE2 and human ACE2, which was very similar to the level between cat ACE2 and human ACE2. Thus, it seems apparent that the Felidae family members are relatively susceptible to SARS‐CoV‐2.
10. SARS‐COV‐2 AND ANIMAL CoVs
SARS‐CoV‐2 may have risen either through natural selection in animals or humans before or after crossing the species barrier (Andersen, Rambaut, Lipkin, Holmes, & Garry, 2020; Cagliani, Forni, Clerici, & Sironi, 2020). In spite of no consensus on the mutations or evolution of SARS‐CoV‐2, it was proved by several laboratories that limited but uniformly distributed mutations were found in the SARS‐CoV‐2 genome according to the polymorphism data analyses (Shen et al., 2020; Tang et al., 2020; Wu et al., 2020). However, several groups identified some critical sites in RBD, which are closely associated with viral infectivity. Wan et al. revealed that a single mutation (N501T) in the RBD of SARS‐CoV‐2 S protein may have significantly enhanced the viral infectivity (Wan, Shang, Graham, et al., 2020), and some isolates in Europe and Hong Kong share identical mutations in RBD, leading to a higher binding affinity to human ACE2 (Ou et al., 2020). An elaborate survey for potential mutations at the critical sites in RBD should be further performed. Recently, phylogenetic network analysis show the existence of three main types of SARS‐CoV‐2 variants (type A, B or C), and amongst them, type A is the ancestral type prevalent in Americans, whereas type B and type C are derived from type A circulating in East Asians and Europeans, respectively (Forster, Forster, Renfrew, & Forster, 2020). Phylogenetic analysis by researchers from Institut Pasteur in Paris showed that SARS‐CoV‐2 might already circulated in France prior to first local case (Gámbaro et al., 2020). All of these data undoubtedly showed an intricate situation of this virus. Thus, more surveillance needs to be taken to explore the origin and diversity of this virus worldwide.
Importantly, the careful analyses demonstrate that some of the animal CoVs have evolved to tissue‐specific types, such as respiratory type (PRCV, CRCoV), gastrointestinal type (TGEV, CCoV, FECVs), reproductive type (IBV), renal type (IBV), neurotropic type (PHEV) and other types after a long‐term evolution. Currently, the SARS‐CoV‐2 pandemic is only in the initial stage in most regions, but this virus already exhibits very broad tissue tropism and strong affinity with host cells. Thus, a question arises, and what is future direction of viral evolution with regard to tissue tropism or host ranges. These aspects should be closely monitored clinically.
Moreover, since SARS‐CoV‐2 can affect a variety of organs and tissues, appropriate intervention of the affected organs or tissues should be fully managed for diagnosis and treatments. Some of the organs or tissues affected by SARS‐CoV‐2 as well as the clinical symptoms are remarkably similar to those of the animals infected by CoVs as described above, and thus, the experience from animal CoV prevention and control may represent the guidance for epidemiologic investigation, diagnostics, prevention and clinical treatments of SARS‐CoV‐2. Several points worthy of consideration are presented as follows: (a) Some animal CoVs, like FIPVs and PRCV, became capable of infecting new target tissues through the mutation‐based evolution. What would be the future evolutionary consequence of SARS‐CoV‐2 after a long‐term circulation in humans or other hosts? (b) Some animal CoVs potentially arose from genome recombination between CoVs, as discussed for the possible origin of CoV‐OC43 and FIPV in the previous paragraphs. An appropriate monitoring system should be deployed for different species of hosts, especially cats and pigs, to avoid emergence of recombination events in a potential 'mixing vessel' (c) Uncontrolled shifts from the natural and undetermined intermediate hosts of CoVs complicate the prevention and control of epidemics. Seroprevalence of feline CoVs is typically higher in cats in shelters than that in household cats. For control of SARS‐CoV‐2, movement of pets (especially cats) and wild animals (especially ferrets and non‐human primates) should be properly managed, and the safe distance and clear boundary should be maintained. (d) SARS‐CoV‐2 continues to spread and has shown the ability to target and damage multiple organs, leading to seemingly a systemic disease. This recalls clinical manifestation of certain coronavirus infections in animals, like FIPVs whose infection also causes a systemic and fatal disease in many cases. Investigating FIPV infection can aid to understand the pathogenic mechanisms of COVID‐19 in humans.
Regarding the prevention of CoV infection with vaccines, antibody‐dependent enhancement (ADE) and vaccine‐associated enhanced respiratory disease (VAERD) are two crucial considerations when evaluating the efficacy of a vaccine. ADE can benefit viral entry under certain conditions and further exacerbate the clinical diseases. That is why veterinary experts still hold different view on vaccination against FIPV infection in cats although some vaccines are available in the market (Satoh et al., 2011; Vennema et al., 1990). Moreover, ADE is often observed in member viruses of the Flaviviridae family, like dengue disease (Katzelnick et al., 2017; Libraty et al., 2009), and during infection with SARS‐CoV (Kam et al., 2007; Wan, Shang, Sun, et al., 2020). VAERD was originally reported in children when inactivated vaccine was given to combat respiratory syncytial virus infection, and inactivated vaccine led to enhanced respiratory disease compared with control children (Fulginiti et al., 1969). Generally, immune complex formation, complement deposition, and Th2‐biased immune response can arise in VAERD (Graham, 2020). Currently, more than 100 vaccine candidates for SARS‐CoV‐2 are under investigation, and a live attenuated vaccine attracts researchers’ attention due to its several advantages (Amanat & Krammer, 2020). Codagenix from America collaborated with Serum Institute of India to study the live attenuated vaccine, and most vaccine candidates have not started human clinical trials (Chen, Strych, Hotez, & Bottazzi, 2020). However, several aspects need to be considered when developing live attenuated vaccines, for example extensive tests for the sake of safety, potential of reversion to virulence and high risk for recombination in vivo (Amanat & Krammer, 2020). Currently, researchers from China, Europe and America are mainly focusing on the development of viral vector‐based vaccines (basically adenovirus) and mRNA vaccines, and some of the candidates are already in or completion of phase I/II clinical trials exhibiting promising clinical performance (Jackson et al., 2020; Zhu et al., 2020). Groups in China and Austria are also developing inactivated vaccines, and phase I clinical trials may be initiated later in 2020 (Gao et al., 2020; Wang, Zhang, et al., 2020). Scientists race to develop effective vaccines and therapeutics against COVID‐19, and both ADE and VAERD should be both taken into accounts during the assessment of the safety and efficacy of vaccine candidates. Therefore, it will still likely take some time before effective vaccines become available.
11. CLOSING REMARKS
A considerable amount of knowledge has been gained for many aspects of SARS‐CoV‐2 during past 4 months since the COVID‐19 outbreak. Still, the natural reservoir and intermediate hosts of the virus remain unclear, transmission chains of this pandemic, evolutionary history and future developments of the virus, clinical characteristics and treatment strategies, and intra‐species transmissions in animals and humans. Considering close relationship between cats and humans, potential cross‐species transmissions should be systemically monitored in residential communities, and public management practices should be implemented without delay. Genetic mutation of SARS‐CoV‐2 concurs with viral evolution, and this may lead to alteration and expansion of viral tropism, including changes of susceptible tissues and organs, expansion of host tropism and crossing of the species barrier. This novel invader virus may start targeting various organs in humans. Such possibilities have already been seen for other animal CoVs and are not unusual as described above. Viral evolution may proceed in unpredictable directions and SARS‐CoV‐2 may have originated through the recombination between bat CoV and pangolin CoV. Whether SARS‐CoV‐2 will further 'fuse' with other CoVs of wild animals, livestock or pets will be a challenge for the control of disease. A recent report shows that minks were infected by SARS‐CoV‐2 in two fur farms in the Netherlands and exhibited typical respiratory symptoms. An employee in the farms was considered the source of infection. Moreover, it has been reported that SARS‐CoV‐2 can be transmitted from minks back to human, and minks can be served as viral reservoir, posing a potential threat to public health. Thus, sustained surveys for zoonotic CoVs in animals, especially those in close relationship with humans, will allow us to prepare for effective countermeasures towards not only the current SARS‐CoV‐2 but also for other potential zoonotic CoVs in the future (Yoo & Yoo, 2020).
It is possible that SARS‐CoV‐2 can survive or preserved in vegetable, meats or other carriers especially during the cold chain transport; however, no direct evidence shows that SARS‐CoV‐2 can transmit from these food or food packages to humans. In addition, since SARS‐CoV‐2 can infect various tissues, collection of appropriate clinical samples from diverse tissues such as nasal swabs, faecal samples and secretions will minimize the rate of false‐negatives. The confirmed cases, transmission intensity and other parameters of COVID‐19 have recently decreased in some countries, whereas asymptomatic and sporadic cases rather increase, and thus, mass screening for COVID‐19 in individuals is necessary to avoid possible re‐emergence of SARS‐CoV‐2. The current mitigation strategies for prevention and control should be continued until an effective vaccine becomes available.
CONFLICT OF INTEREST
The authors declare that they have no competing financial interests.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to. No ethical approval was required as this is a review article with no original research data.
Supporting information
Table S1
Zhang G, Li B, Yoo D, et al. Animal coronaviruses and SARS‐CoV‐2. Transbound Emerg Dis.2021;68:1097–1110. 10.1111/tbed.13791
Zhang, Li and Yoo authors contributed equally to this work.
Funding information
This study was supported by Agricultural Science and Technology Innovation Program (ASTIP‐IAS15), Jiangsu province Natural Sciences Foundation (BK20190003), Beijing Innovation Consortium of Agriculture Research System (BAIC04–2020) and Agriculture and Food Research Initiative Competitive Grants no. 2018‐67015‐28287 from the US Department of Agriculture National Institute of Food and Agriculture awarded to DY.
Contributor Information
Xiaodong Zhang, Email: zhang_xd@jlu.edu.cn.
Yaxiong Jia, Email: jiayaxiong@caas.cn.
Shangjin Cui, Email: cuishangjin@caas.cn.
DATA AVAILABILITY STATEMENT
All data generated or analysed in this study are included in this manuscript and in the supplementary material.
REFERENCES
- Amanat, F. , & Krammer, F. (2020). SARS‐CoV‐2 Vaccines: Status Report. Immunity, 52, 583–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen, K. G. , Rambaut, A. , Lipkin, W. I. , Holmes, E. C. , & Garry, R. F. (2020). The proximal origin of SARS‐CoV‐2. Nature Medicine, 26(4), 450–452. 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azhar, E. I. , El‐Kafrawy, S. A. , Farraj, S. A. , Hassan, A. M. , Al‐Saeed, M. S. , Hashem, A. M. , & Madani, T. A. (2014). Evidence for camel‐to‐human transmission of MERS coronavirus. New England Journal of Medicine, 370, 2499–2505. [DOI] [PubMed] [Google Scholar]
- Ballesteros, M. L. , Sanchez, C. M. , & Enjuanes, L. (1997). Two amino acid changes at the N‐terminus of transmissible gastroenteritis coronavirus spike protein result in the loss of enteric tropism. Virology, 227, 378–388. 10.1006/viro.1996.8344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron, M. D. , Iqbal, M. , & Nair, V. (2018). Recent advances in viral vectors in veterinary vaccinology. Current Opinion in Virology, 29, 1–7. [DOI] [PubMed] [Google Scholar]
- Buonavoglia, C. , Decaro, N. , Martella, V. , Elia, G. , Campolo, M. , Desario, C. , … Tempesta, M. (2006). Canine coronavirus highly pathogenic for dogs. Emerging Infectious Diseases, 12, 492–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagliani, R. , Forni, D. , Clerici, M. , & Sironi, M. (2020). Computational inference of selection underlying the evolution of the novel coronavirus, SARS‐CoV‐2. Journal of Virology, 84, e00411‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh, D. (2007). Coronavirus avian infectious bronchitis virus. Veterinary Research, 38, 281–297. [DOI] [PubMed] [Google Scholar]
- Chan, J. F. , Kok, K. H. , Zhu, Z. , Chu, H. , To, K. K. , Yuan, S. , & Yuen, K. Y. (2020). Genomic characterization of the 2019 novel human‐pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerging Microbes Infection, 9, 221–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, H. W. , Egberink, H. F. , Halpin, R. , Spiro, D. J. , & Rottier, P. J. (2012). Spike protein fusion peptide and feline coronavirus virulence. Emerging Infectious Diseases, 18, 1089–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, W. H. , Strych, U. , Hotez, P. J. , & Bottazzi, M. E. (2020). The SARS‐CoV‐2 vaccine pipeline: An overview. Current Tropical Medicine Reports, 7(2), 61–64. 10.1007/s40475-020-00201-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, Y. , Feng, Z. , Diao, B. , Wang, R. , Wang, G. , Wang, C. , … Wu, Y. (2020). The Novel Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2) directly decimates human spleens and lymph nodes. medRxiv 2020.03.27.20045427. [Google Scholar]
- Chen, Z. , Hu, J. , Zhang, Z. , Jiang, S. , Wang, T. , Shi, Z. , … Zhang, Z. (2020). Caution: The clinical characteristics of COVID‐19 patients at admission are changing. medRxiv 2020.03.03.20030833. [Google Scholar]
- Chinese, SARS Molecular Epidemiology Consortium (2004). Molecular evolution of the SARS coronavirus during the course of the SARS epidemic in China. Science, 303, 1666–1669. [DOI] [PubMed] [Google Scholar]
- Cook, J. K. , Jackwood, M. , & Jones, R. C. (2012). The long view: 40 years of infectious bronchitis research. Avian Pathology, 41, 239–250. [DOI] [PubMed] [Google Scholar]
- Coronaviridae Study Group of the International Committee on Taxonomy of, Viruses (2020). The species severe acute respiratory syndrome‐related coronavirus: Classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiology, 5, 536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COVID‐19 (SARS‐COV‐2), Hong Kong (SAR ‐ PRC) (2020). https://www.oie.int/wahis_2/public/wahid.php/Reviewreport/Review?page_refer=MapFullEventReport&reportid=33892
- Cui, J. , Li, F. , & Shi, Z. L. (2019). Origin and evolution of pathogenic coronaviruses. Nature Reviews Microbiology, 17, 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyranoski, D. (2020). Mystery deepens over animal source of coronavirus. Nature, 579, 18–19. [DOI] [PubMed] [Google Scholar]
- Day, M. J. , Horzinek, M. C. , Schultz, R. D. , Squires, R. A. & Association Vaccination Guidelines Group of the World Small Animal Veterinary (2016). WSAVA Guidelines for the vaccination of dogs and cats. Journal of Small Animal Practice, 57, E1–E45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decaro, N. , Mari, V. , Campolo, M. , Lorusso, A. , Camero, M. , Elia, G. , … Buonavoglia, C. (2009). Recombinant canine coronaviruses related to transmissible gastroenteritis virus of Swine are circulating in dogs. Journal of Virology, 83, 1532–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, J. , Jin, Y. , Liu, Y. , Sun, J. , Hao, L. , Bai, J. , … Tian, K. (2020). Serological survey of SARS‐CoV‐2 for experimental, domestic, companion and wild animals excludes intermediate hosts of 35 different species of animals. Transboundary and Emerging Diseases, 67(4), 1745–1749. 10.1111/tbed.13577 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Diao, B. , Wang, C. , Wang, R. , Feng, Z. , Tan, Y. , Wang, H. , … Chen, Y. (2020). Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) Infection. medRxiv 2020.03.04.20031120. [Google Scholar]
- Erles, K. , Shiu, K. B. , & Brownlie, J. (2007). Isolation and sequence analysis of canine respiratory coronavirus. Virus Research, 124, 78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erles, K. , Toomey, C. , Brooks, H. W. , & Brownlie, J. (2003). Detection of a group 2 coronavirus in dogs with canine infectious respiratory disease. Virology, 310, 216–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerberg, L. , Hallstrom, B. M. , Oksvold, P. , Kampf, C. , Djureinovic, D. , Odeberg, J. , … Uhlen, M. (2014). Analysis of the human tissue‐specific expression by genome‐wide integration of transcriptomics and antibody‐based proteomics. Molecular & Cellular Proteomics, 13, 397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felten, S. , & Hartmann, K. (2019). Diagnosis of feline infectious peritonitis: a review of the current literature. Viruses, 11(11), 1068. 10.3390/v11111068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster, P. , Forster, L. , Renfrew, C. , & Forster, M. (2020). Phylogenetic network analysis of SARS‐CoV‐2 genomes. Proceedings of the National Academy of Sciences, USA, 117(17), 9241–9243. 10.1073/pnas.2004999117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzo, G. , Legnardi, M. , Tucciarone, C. M. , Drigo, M. , Martini, M. , & Cecchinato, M. (2019). Evolution of infectious bronchitis virus in the field after homologous vaccination introduction. Veterinary Research, 50, 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulginiti, V. A. , Eller, J. J. , Sieber, O. F. , Joyner, J. W. , Minamitani, M. , & Meiklejohn, G. (1969). Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum‐precipitated respiratory syncytial virus vaccine. American Journal of Epidemiology, 89, 435–448. [DOI] [PubMed] [Google Scholar]
- Gámbaro, F. , Behillil, S. , Baidaliuk, A. , Donati, F. , Albert, M. , Alexandru, A. , … Simon‐Loriere, E. (2020) Introductions and early spread of SARS‐CoV‐2 in France. bioRxiv 2020.04.24.059576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao, Q. , Bao, L. , Mao, H. , Wang, L. , Xu, K. , Yang, M. , & Qin, C. (2020) Rapid development of an inactivated vaccine for SARS‐CoV‐2. bioRxiv 2020.04.17.046375. [Google Scholar]
- Gerdts, V. , & Zakhartchouk, A. (2017). Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Veterinary Microbiology, 206, 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, B. S. (2020). Rapid COVID‐19 vaccine development. Science, 368, 945–946. [DOI] [PubMed] [Google Scholar]
- Graham, R. L. , & Baric, R. S. (2010). Recombination, reservoirs, and the modular spike: Mechanisms of coronavirus cross‐species transmission. Journal of Virology, 84, 3134–3146. 10.1128/JVI.01394-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, Y. , Zheng, B. J. , He, Y. Q. , Liu, X. L. , Zhuang, Z. X. , Cheung, C. L. , … Poon, L. L. (2003). Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science, 302, 276–278. [DOI] [PubMed] [Google Scholar]
- Guo, H. , Guo, A. , Wang, C. , Yan, B. , Lu, H. , & Chen, H. (2008). Expression of feline angiotensin converting enzyme 2 and its interaction with SARS‐CoV S1 protein. Research in Veterinary Science, 84, 494–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao, W. , Ma, B. , Li, Z. , Wang, X. , Gao, X. , Li, Y. , … Tan, Z. (2020) Binding of the SARS‐CoV‐2 spike protein to glycans. bioRxiv 2020.05.17.100537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, K. (2005). Feline infectious peritonitis. The Veterinary Clinics of North America. Small Animal Practice, 35(1), 39–79. 10.1016/j.cvsm.2004.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasoksuz, M. , Alekseev, K. , Vlasova, A. , Zhang, X. , Spiro, D. , Halpin, R. , … Saif, L. J. (2007). Biologic, antigenic, and full‐length genomic characterization of a bovine‐like coronavirus isolated from a giraffe. Journal of Virology, 81, 4981–4990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrewegh, A. A. P. M. , Smeenk, I. , Horzinek, M. C. , Rottier, P. J. M. , & de Groot, R. J. (1998). Feline coronavirus type II strains 79–1683 and 79–1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. Journal of Virology, 72, 4508–4514. 10.1128/JVI.72.5.4508-4514.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohdatsu, T. , Izumiya, Y. , Yokoyama, Y. , Kida, K. , & Koyama, H. (1998). Differences in virus receptor for type I and type II feline infectious peritonitis virus. Archives of Virology, 143, 839–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou, Y. , Ke, H. , Kim, J. , Yoo, D. , Su, Y. , Boley, P. , … Wang, Q. (2019). Engineering a live attenuated porcine epidemic diarrhea virus vaccine candidate via inactivation of the viral 2'‐O‐methyltransferase and the endocytosis signal of the spike protein. Journal of Virology, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulswit, R. J. G. , de Haan, C. A. M. , & Bosch, B. J. (2016). Coronavirus spike protein and tropism changes. Advances in Virus Research, Vol 96: Coronaviruses, 96: 29–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulswit, R. J. G. , Lang, Y. , Bakkers, M. J. G. , Li, W. , Li, Z. , Schouten, A. , … de Groot, R. J. (2019). Human coronaviruses OC43 and HKU1 bind to 9‐O‐acetylated sialic acids via a conserved receptor‐binding site in spike protein domain A. Proceedings of the National Academy of Sciences, USA, 116, 2681–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim, I. M. , Abdelmalek, D. H. , Elshahat, M. E. , & Elfiky, A. A. (2020). COVID‐19 spike‐host cell receptor GRP78 binding site prediction. Journal of Infection, 80, 554–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail, M. M. , Cho, K. O. , Ward, L. A. , Saif, L. J. , & Saif, Y. M. (2001). Experimental bovine coronavirus in turkey poults and young chickens. Avian Diseases, 45, 157–163. [PubMed] [Google Scholar]
- Jackson, L. A. , Anderson, E. J. , Rouphael, N. G. , Roberts, P. C. , Makhene, M. , Coler, R. N. , … Beigel, J. H. (2020). An mRNA vaccine against SARS‐CoV‐2 — Preliminary report. New England Journal of Medicine. 10.1056/NEJMoa2022483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, S. , Shi, Z. , Shu, Y. , Song, J. , Gao, G. F. , Tan, W. , & Guo, D. (2020). A distinct name is needed for the new coronavirus. Lancet, 395, 949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, K. , Hu, H. , & Saif, L. J. (2016). Porcine deltacoronavirus infection: Etiology, cell culture for virus isolation and propagation, molecular epidemiology and pathogenesis. Virus Research, 226, 50–59. 10.1016/j.virusres.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam, Y. W. , Kien, F. , Roberts, A. , Cheung, Y. C. , Lamirande, E. W. , Vogel, L. , … Altmeyer, R. (2007). Antibodies against trimeric S glycoprotein protect hamsters against SARS‐CoV challenge despite their capacity to mediate FcgammaRII‐dependent entry into B cells in vitro. Vaccine, 25, 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshima, T. , Hohdatsu, T. , Hagino, R. , Hosoya, S. , Nojiri, Y. , Murata, M. , … Koyama, H. (2007). The infectivity and pathogenicity of a group 2 bovine coronavirus in pups. Journal of Veterinary Medical Science, 69, 301–303. [DOI] [PubMed] [Google Scholar]
- Kanno, T. , Ishihara, R. , Hatama, S. , & Uchida, I. (2013). Antigenic variation among recent Japanese isolates of bovine coronaviruses belonging to phylogenetically distinct genetic groups. Archives of Virology, 158, 1047–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katzelnick, L. C. , Gresh, L. , Halloran, M. E. , Mercado, J. C. , Kuan, G. , Gordon, A. , … Harris, E. (2017). Antibody‐dependent enhancement of severe dengue disease in humans. Science, 358, 929–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kipar, A. , Meli, M. L. , Baptiste, K. E. , Bowker, L. J. , & Lutz, H. (2010). Sites of feline coronavirus persistence in healthy cats. Journal of General Virology, 91, 1698–1707. [DOI] [PubMed] [Google Scholar]
- Korber, B. , Fischer, W. M. , Gnanakaran, S. , Yoon, H. , Theiler, J. , Abfalterer, W. , … Wyles, M. D. (2020). Tracking changes in SARS‐CoV‐2 spike: Evidence that D614G increases infectivity of the COVID‐19 virus. Cell, 182, 812–827. 10.1016/j.cell.2020.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krog, J. S. , Hjulsager, C. K. , Larsen, M. A. , & Larsen, L. E. (2017). Triple‐reassortant influenza A virus with H3 of human seasonal origin, NA of swine origin, and internal A(H1N1) pandemic 2009 genes is established in Danish pigs. Influenza and Other Respiratory Viruses, 11, 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laconi, A. , Weerts, E. , Bloodgood, J. , Deniz Marrero, J. P. , Berends, A. J. , Cocciolo, G. , … Verheije, M. H. (2020). Attenuated live infectious bronchitis virus QX vaccine disseminates slowly to target organs distant from the site of inoculation. Vaccine, 38, 1486–1493. 10.1016/j.vaccine.2019.11.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, T. T. , Jia, N. , Zhang, Y. W. , Shum, M. H. , Jiang, J. F. , Zhu, H. C. , … Cao, W. C. (2020). Identifying SARS‐CoV‐2‐related coronaviruses in Malayan pangolins. Nature, 583, 282–285. [DOI] [PubMed] [Google Scholar]
- Li, F. (2016). Structure function, and evolution of coronavirus spike proteins. Annual Review of Virology, 3, 237–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. T. , Hulswit, R. J. G. , Kenney, S. P. , Widjaja, I. , Jung, K. , Alhamo, M. A. , … Bosch, B. J. (2018). Broad receptor engagement of an emerging global coronavirus may potentiate its diverse cross‐species transmissibility. Proceedings of the National Academy of Sciences of the United States of America, 115, E5135–E5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. (2020). Cats under the shadow of the SARS‐CoV‐2 pandemic. Transboundary and Emerging Diseases, 67(4), 1416–1417. 10.1111/tbed.13599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X. , Giorgi, E. E. , Honnayakanahalli Marichannegowda, M. , Foley, B. , Xiao, C. , Kong, X.‐P. , … Gao, F. (2020). Emergence of SARS‐CoV‐2 through recombination and strong purifying selection. Science Advances 6: eabb9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y. T. , Chen, T. C. , Lin, S. Y. , Mase, M. , Murakami, S. , Horimoto, T. , & Chen, H. W. (2020). Emerging lethal infectious bronchitis coronavirus variants with multiorgan tropism. Transboundary and Emerging Diseases, 67, 884–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libraty, D. H. , Acosta, L. P. , Tallo, V. , Segubre‐Mercado, E. , Bautista, A. , Potts, J. A. , … Capeding, R. Z. (2009). A prospective nested case‐control study of Dengue in infants: Rethinking and refining the antibody‐dependent enhancement dengue hemorrhagic fever model. PLoS Med, 6, e1000171. 10.1371/journal.pmed.1000171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licitra, B. N. , Millet, J. K. , Regan, A. D. , Hamilton, B. S. , Rinaldi, V. D. , Duhamel, G. E. , & Whittaker, G. R. (2013). Mutation in spike protein cleavage site and pathogenesis of feline coronavirus. Emerging Infectious Diseases, 19, 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, C. , Tang, J. , Ma, Y. , Liang, X. , Yang, Y. , Peng, G. , … Li, F. (2015). Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. Journal of Virology, 89, 6121–6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorusso, A. , Desario, C. , Mari, V. , Campolo, M. , Lorusso, E. , Elia, G. , … Decaro, N. (2009). Molecular characterization of a canine respiratory coronavirus strain detected in Italy. Virus Research, 141, 96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, R. , Zhao, X. , Li, J. , Niu, P. , Yang, B. , Wu, H. , … Tan, W. (2020). Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet, 395, 565–574. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, S. , Chen, Y. , Qin, K. , Zhou, J. , Lou, Y. , & Tan, W. (2016). Genetic and antigenic characterization of recombinant nucleocapsid proteins derived from canine coronavirus and canine respiratory coronavirus in China. Science China Life Science, 59, 615–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao, L. , Wang, M. , Chen, S. , He, Q. , Chang, J. , Hong, C. … Hu, B. (2020). Neurological manifestations of hospitalized patients with COVID‐19 in Wuhan, China: A retrospective case series study. SSRN Electronic Journal, 77, 683–690. 10.2139/ssrn.3544840 [DOI] [Google Scholar]
- Martina, B. E. , Haagmans, B. L. , Kuiken, T. , Fouchier, R. A. , Rimmelzwaan, G. F. , Van Amerongen, G. , … Osterhaus, A. D. (2003). Virology: SARS virus infection of cats and ferrets. Nature, 425, 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masoudi, S. , Pishraft Sabet, L. , & Shahsavadi, S. (2020). Immunogenicity and efficacy of live infectious bronchitis 793/B.08IR vaccine in SPF chickens. Archives of Razi Institute, 75, 23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mena, I. , Nelson, M. I. , Quezada‐Monroy, F. , Dutta, J. , Cortes‐Fernandez, R. , Lara‐Puente, J. H. , … Garcia‐Sastre, A. (2016). Origins of the 2009 H1N1 influenza pandemic in swine in Mexico. Elife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam, E. , & Lee, C. (2010). Contribution of the porcine aminopeptidase N (CD13) receptor density to porcine epidemic diarrhea virus infection. Veterinary Microbiology, 144, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto, M. , Kanno, T. , Bannai, H. , Tsujimura, K. , Yamanaka, T. , & Kokado, H. (2017). Antibody response to equine coronavirus in horses inoculated with a bovine coronavirus vaccine. Journal of Veterinary Medical Science, 79, 1889–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann, G. , Noda, T. , & Kawaoka, Y. (2009). Emergence and pandemic potential of swine‐origin H1N1 influenza virus. Nature, 459, 931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederwerder, M. C. , & Hesse, R. A. (2018). Swine enteric coronavirus disease: A review of 4 years with porcine epidemic diarrhoea virus and porcine deltacoronavirus in the United States and Canada. Transbound Emerging Disease, 65, 660–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguma, K. , Ohno, M. , Yoshida, M. , & Sentsui, H. (2018). Mutation of the S and 3c genes in genomes of feline coronaviruses. Journal of Veterinary Medical Science, 80, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oguzoglu, T. C. , Sahna, K. C. , Ataseven, V. S. , & Muz, D. (2010). Prevalence of feline coronavirus (FCoV) and feline leukemia virus (FeLV) in Turkish cats. Ankara Universitesi Veteriner Fakultesi Dergisi, 57, 271–274. [Google Scholar]
- Oreshkova, N. , Molenaar, R. J. , Vreman, S. , Harders, F. , Oude, B. B. , Munnink, R. W. , … Stegeman, A. (2020). SARS‐CoV‐2 infection in farmed minks, the Netherlands, April and May 2020. Eurosurveillance, 25, 2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou, J. , Zhou, Z. , Zhang, J. , Lan, W. , Zhao, S. , Wu, J. , … Seto, D. (2020). RBD mutations from circulating SARS‐CoV‐2 strains enhance the structure stability and infectivity of the spike protein. bioRxiv 2020.03.15.991844. [Google Scholar]
- Paules, C. I. , Marston, H. D. , & Fauci, A. S. (2020). Coronavirus infections‐more than just the common cold. JAMA, 323(8), 707. 10.1001/jama.2020.0757 [DOI] [PubMed] [Google Scholar]
- Pedersen, N. C. (2009). A review of feline infectious peritonitis virus infection: 1963–2008. Journal of Feline Medicine and Surgery, 11, 225–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen, N. C. , Liu, H. , Scarlett, J. , Leutenegger, C. M. , Golovko, L. , Kennedy, H. , & Kamal, F. M. (2012). Feline infectious peritonitis: Role of the feline coronavirus 3c gene in intestinal tropism and pathogenicity based upon isolates from resident and adopted shelter cats. Virus Research, 165, 17–28. 10.1016/j.virusres.2011.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing, E. , & Gallagher, T. (2020). SARS Coronavirus Redux. Trends in Immunology, 41, 271–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regan, A. D. , Millet, J. K. , Tse, L. P. , Chillag, Z. , Rinaldi, V. D. , Licitra, B. N. , … Whittaker, G. R. (2012). Characterization of a recombinant canine coronavirus with a distinct receptor‐binding (S1) domain. Virology, 430, 90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera, J. , Santiago, C. , Mudgal, G. , Ordono, D. , Enjuanes, L. , & Casasnovas, J. M. (2012). Structural bases of coronavirus attachment to host aminopeptidase N and its inhibition by neutralizing antibodies. PLoS Path, 8, e1002859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier, P. J. M. , Nakamura, K. , Schellen, P. , Volders, H. , & Haijema, B. J. (2005). Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. Journal of Virology, 79, 14122–14130. 10.1128/JVI.79.22.14122-14130.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos Fernando, F. , Coelho Kasmanas, T. , Diniz Lopes, P. , da Silva Montassier, M. F. , Zanella Mores, M. A. , Casagrande Mariguela, V. , … Montassier, H. J. (2017). Assessment of molecular and genetic evolution, antigenicity and virulence properties during the persistence of the infectious bronchitis virus in broiler breeders. Journal of General Virology, 98, 2470–2481. 10.1099/jgv.0.000893 [DOI] [PubMed] [Google Scholar]
- Satoh, R. , Furukawa, T. , Kotake, M. , Takano, T. , Motokawa, K. , Gemma, T. , … Hohdatsu, T. (2011). Screening and identification of T helper 1 and linear immunodominant antibody‐binding epitopes in the spike 2 domain and the nucleocapsid protein of feline infectious peritonitis virus. Vaccine, 29, 1791–1800. 10.1016/j.vaccine.2010.12.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahwan, K. , Hesse, M. , Mork, A. K. , Herrler, G. , & Winter, C. (2013). Sialic acid binding properties of soluble coronavirus spike (S1) proteins: Differences between infectious bronchitis virus and transmissible gastroenteritis virus. Viruses, 5, 1924–1933. 10.3390/v5081924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang, J. , Wan, Y. , Luo, C. , Ye, G. , Geng, Q. , Auerbach, A. , & Li, F. (2020). Cell entry mechanisms of SARS‐CoV‐2. Proceedings of the National Academy of Sciences, USA, 117(21), 11727–11734. 10.1073/pnas.2003138117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan, T. , Rockx, B. , Donaldson, E. , Corti, D. , & Baric, R. (2008). Pathways of cross‐species transmission of synthetically reconstructed zoonotic severe acute respiratory syndrome coronavirus. Journal of Virology, 82, 8721–8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Z. , Xiao, Y. , Kang, L. , Ma, W. , Shi, L. , Zhang, L. , … Li, M. (2020). Genomic diversity of SARS‐CoV‐2 in Coronavirus Disease 2019 patients. Clinical Infectious Diseases, 71, 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi, J. , Wen, Z. , Zhong, G. , Yang, H. , Wang, C. , Huang, B. , … Bu, Z. (2020). Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science, 368, 1016–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun, C. , Chen, L. , Yang, J. , Luo, C. , Zhang, Y. , Li, J. , & Yang, J. (2020). SARS‐CoV‐2 and SARS‐CoV spike‐RBD structure and receptor binding comparison and potential implications on neutralizing antibody and vaccine development. bioRxiv 2020.02.16.951723. [Google Scholar]
- Szczepanski, A. , Owczarek, K. , Bzowska, M. , Gula, K. , Drebot, I. , Ochman, M. , … Pyrc, K. (2019). Canine respiratory coronavirus, bovine coronavirus, and human coronavirus oc43: receptors and attachment factors. Viruses, 11(4), 328. 10.3390/v11040328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taharaguchi, S. , Soma, T. , & Hara, M. (2012). Prevalence of feline coronavirus antibodies in Japanese domestic cats during the past decade. Journal of Veterinary Medical Science, 74, 1355–1358. [DOI] [PubMed] [Google Scholar]
- Tang, X. , Wu, C. , Li, X. , Song, Y. , Yao, X. , Wu, X. , … Lu, J. (2020). On the origin and continuing evolution of SARS‐CoV‐2. National Science Review, 7(6), 1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toro, H. , van Santen, V. L. , & Jackwood, M. W. (2012). Genetic diversity and selection regulates evolution of infectious bronchitis virus. Avian Diseases, 56, 449–455. [DOI] [PubMed] [Google Scholar]
- Tortorici, M. A. , Walls, A. C. , Lang, Y. , Wang, C. , Li, Z. , Koerhuis, D. , … Veesler, D. (2019). Structural basis for human coronavirus attachment to sialic acid receptors. Nature Structural & Molecular Biology, 26, 481–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusell, S. M. , Schittone, S. A. , & Holmes, K. V. (2007). Mutational analysis of aminopeptidase N, a receptor for several group 1 coronaviruses, identifies key determinants of viral host range. Journal of Virology, 81, 1261–1273. 10.1128/JVI.01510-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- United States Department of Agriculture (2020). USDA Statement on the Confirmation of COVID‐19 in a Tiger in New York. https://www.aphis.usda.gov/aphis/newsroom/news/sa_by_date/sa‐2020/ny‐zoo‐covid‐19
- Vennema, H. , de Groot, R. J. , Harbour, D. A. , Dalderup, M. , Gruffydd‐Jones, T. , Horzinek, M. C. , & Spaan, W. J. (1990). Early death after feline infectious peritonitis virus challenge due to recombinant vaccinia virus immunization. Journal of Virology, 64, 1407–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennema, H. , Poland, A. , Foley, J. , & Pedersen, N. C. (1998). Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology, 243, 150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel, L. , Van der Lubben, M. , te Lintelo, E. G. , Bekker, C. P. , Geerts, T. , Schuijff, L. S. , … Rottier, P. J. (2010). Pathogenic characteristics of persistent feline enteric coronavirus infection in cats. Veterinary Research, 41, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. , Shang, J. , Graham, R. , Baric, R. S. , & Li, F. (2020). Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade‐long structural studies of SARS coronavirus. Journal of Virology, 94, e00127‐20. 10.1128/JVI.00127-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan, Y. , Shang, J. , Sun, S. , Tai, W. , Chen, J. , Geng, Q. , … Li, F. (2020). Molecular mechanism for antibody‐dependent enhancement of coronavirus entry. Journal of Virology, 94, e02015‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, H. , Zhang, Y. , Huang, B. , Deng, W. , Quan, Y. , Wang, W. , … Yang, X. (2020). Development of an inactivated vaccine candidate, BBIBP‐CorV, with potent protection against SARS‐CoV‐2. Cell, 182(3), 713–721.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Chen, W. , Zhou, Y.‐S. , Lian, J.‐Q. , Zhang, Z. , Du, P. , … Chen, Z.‐N. (2020). SARS‐CoV‐2 invades host cells via a novel route: CD147‐spike protein. bioRxiv 2020.03.14.988345. [Google Scholar]
- Wang, L. , Shi, W. , Chappell, J. D. , Joyce, M. G. , Zhang, Y. , Kanekiyo, M. , … Graham, B. S. (2018). Importance of neutralizing monoclonal antibodies targeting multiple antigenic sites on the middle east respiratory syndrome coronavirus spike glycoprotein to avoid neutralization escape. Journal of Virology, 92. 10.1128/JVI.02002-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Q. , Vlasova, A. N. , Kenney, S. P. , & Saif, L. J. (2019). Emerging and re‐emerging coronaviruses in pigs. Current Opinion in Virology, 34, 39–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. T. , Su, B. L. , Hsieh, L. E. , & Chueh, L. L. (2013). An outbreak of feline infectious peritonitis in a Taiwanese shelter: Epidemiologic and molecular evidence for horizontal transmission of a novel type II feline coronavirus. Veterinary Research, 44, 57. 10.1186/1297-9716-44-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, M. C. , Javornik, S. J. , Cregeen, N. J. , & Petrosino, J. F. (2020). Evidence of recombination in coronaviruses implicating pangolin origins of nCoV‐2019. bioRxiv 2020.02.07.939207. [Google Scholar]
- Woo, P. C. , Lau, S. K. , Lam, C. S. , Lau, C. C. , Tsang, A. K. , Lau, J. H. , … Yuen, K. Y. (2012). Discovery of seven novel Mammalian and avian coronaviruses in the genus deltacoronavirus supports bat coronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. Journal of Virology, 86, 3995–4008. 10.1128/JVI.06540-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp, D. , Wang, N. , Corbett, K. S. , Goldsmith, J. A. , Hsieh, C. L. , Abiona, O. , … McLellan, J. S. (2020). Cryo‐EM structure of the 2019‐nCoV spike in the prefusion conformation. Science, 367, 1260–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, A. , Niu, P. , Wang, L. , Zhou, H. , Zhao, X. , & Wang, W. … Cheng, G. (2020) Mutations, recombination and insertion in the evolution of 2019‐nCoV. bioRxiv 2020.02.29.971101. [Google Scholar]
- Xiao, F. , Tang, M. , Zheng, X. , Liu, Y. , Li, X. , & Shan, H. (2020). Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology, 158(6), 1831–1833.e3. 10.1053/j.gastro.2020.02.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, K. , Zhai, J. , Feng, Y. , Zhou, N. , Zhang, X. , Zou, J.‐J. , … Shen, Y. (2020). Isolation and characterization of 2019‐nCoV‐like coronavirus from Malayan Pangolins. bioRxiv 2020.02.17.951335. [Google Scholar]
- Xiao, Y. , & Torok, M. E. (2020). Taking the right measures to control COVID‐19. Lancet Infectious Diseases, 20, 523–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu, J. , Zhao, S. , Teng, T. , Abdalla, A. E. , Zhu, W. , Xie, L. , … Guo, X. (2020). Systematic comparison of two animal‐to‐human transmitted human coronaviruses: SARS‐CoV‐2 and SARS‐CoV. Viruses, 12, 244. 10.3390/v12020244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, X. , Yu, Y. , Xu, J. , Shu, H. , Xia, J. , Liu, H. , … Shang, Y. (2020). Clinical course and outcomes of critically ill patients with SARS‐CoV‐2 pneumonia in Wuhan, China: a single‐centered, retrospective, observational study. Lancet Respiratory Medicine, 8(5), 475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, Y. L. , Qin, P. , Wang, B. , Liu, Y. , Xu, G. H. , Peng, L. , … Huang, Y. W. (2019). Broad cross‐species infection of cultured cells by bat HKU2‐related swine acute diarrhea syndrome coronavirus and identification of its replication in murine dendritic cells in vivo highlight its potential for diverse interspecies transmission. Journal of Virology, 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi, H. (2020). 2019 novel coronavirus is undergoing active recombination. Clinical Infectious Diseases, 71(15), 884–887. 10.1093/cid/ciaa219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo, H. S. , & Yoo, D. (2020). 'COVID‐19 and veterinarians for one health, zoonotic‐ and reverse‐zoonotic transmissions. Journal of Veterinary Science, 21, e51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young, B. E. , Ong, S. W. X. , Kalimuddin, S. , Low, J. G. , Tan, S. Y. , Loh, J. , … Lye, D. C. (2020). Epidemiologic features and clinical course of patients infected with SARS‐CoV‐2 in Singapore. JAMA, 323(15), 1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu, Y. X. , Sun, L. , Yao, K. , Lou, X. T. , Liang, X. , Zhao, B. W. , … Zhang, H. (2020). Consideration and prevention for the aerosol transmission of 2019 novel coronavirus. Zhonghua Yan Ke Za Zhi, 56, E008. [DOI] [PubMed] [Google Scholar]
- Yurkovetskiy, L. , Pascal, K. E. , Tompkins‐Tinch, C. , Nyalile, T. , Wang, Y. , Baum, A. , … Luban, J. (2020). SARS‐CoV‐2 Spike protein variant D614G increases infectivity and retains sensitivity to antibodies that target the receptor binding domain. bioRxiv [Google Scholar]
- Zhang, L. , Jackson, C. B. , Mou, H. , Ojha, A. , Rangarajan, E. S. , Izard, T. , … Choe, H. (2020). The D614G mutation in the SARS‐CoV‐2 spike protein reduces S1 shedding and increases infectivity. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Q. , Zhang, H. , Huang, K. , Yang, Y. , Hui, X. , Gao, J. , … Jin, M. (2020). SARS‐CoV‐2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv 2020.04.01.021196. [Google Scholar]
- Zhang, T. , Wu, Q. , & Zhang, Z. (2020). Probable Pangolin Origin of SARS‐CoV‐2 associated with the COVID‐19 outbreak. Current Biology, 30, 1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. M. , Herbst, W. , Kousoulas, K. G. , & Storz, J. (1994). Biological and genetic characterization of a hemagglutinating coronavirus isolated from a diarrhoeic child. Journal of Medical Virology, 44, 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Sun, H. , Cunningham, F. L. , Li, L. , Hanson‐Dorr, K. , Hopken, M. W. , … Wan, X. F. (2018). Tissue tropisms opt for transmissible reassortants during avian and swine influenza A virus co‐infection in swine. PLoS Pathology, 14, e1007417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Y. Z. , & Holmes, E. C. (2020). A genomic perspective on the origin and emergence of SARS‐CoV‐2. Cell, 181, 223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, Y. , Zhang, H. , Zhao, J. , Zhong, Q. , Jin, J. H. , & Zhang, G. Z. (2016). Evolution of infectious bronchitis virus in China over the past two decades. Journal of General Virology, 97, 1566–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Fan, H. , Lan, T. , Yang, X. L. , Shi, W. F. , Zhang, W. , … Ma, J. Y. (2018). Fatal swine acute diarrhoea syndrome caused by an HKU2‐related coronavirus of bat origin. Nature, 556, 255–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, P. , Yang, X. L. , Wang, X. G. , Hu, B. , Zhang, L. , Zhang, W. , … Shi, Z. L. (2020). A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature, 579(7798), 270–273. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y. , Zeng, Y. , Tong, Y. , & Chen, C. Z. (2020). Ophthalmologic evidence against the interpersonal transmission of 2019 novel coronavirus through conjunctiva. medRxiv: 2020.02.11.20021956. [Google Scholar]
- Zhu, F. C. , Guan, X. H. , Li, Y. H. , Huang, J. Y. , Jiang, T. , Hou, L. H. … Chen, W. (2020) Immunogenicity and safety of a recombinant adenovirus type‐5‐vectored COVID‐19 vaccine in healthy adults aged 18 years or older: a randomised, double‐blind, placebo‐controlled, phase 2 trial. Lancet 396, 479–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu, X. , Liu, S. , Wang, X. , Luo, Z. , Shi, Y. , Wang, D. , … Xiao, S. (2018). Contribution of porcine aminopeptidase N to porcine deltacoronavirus infection. Emerging Microbes Infection, 7, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga, S. , Pascual‐Iglesias, A. , Sanchez, C. M. , Sola, I. , & Enjuanes, L. (2016). Virulence factors in porcine coronaviruses and vaccine design. Virus Research, 226, 142–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1
Data Availability Statement
All data generated or analysed in this study are included in this manuscript and in the supplementary material.


