Abstract
Aim
The aim of this paper is to describe the clinical features of COVID‐19‐related encephalopathy and their metabolic correlates using brain 2‐desoxy‐2‐fluoro‐D‐glucose (FDG)‐positron‐emission tomography (PET)/computed tomography (CT) imaging.
Background and purpose
A variety of neurological manifestations have been reported in association with COVID‐19. COVID‐19‐related encephalopathy has seldom been reported and studied.
Methods
We report four cases of COVID‐19‐related encephalopathy. The diagnosis was made in patients with confirmed COVID‐19 who presented with new‐onset cognitive disturbances, central focal neurological signs, or seizures. All patients underwent cognitive screening, brain magnetic resonance imaging (MRI), lumbar puncture, and brain 2‐desoxy‐2‐fluoro‐D‐glucose (FDG)‐positron‐emission tomography (PET)/computed tomography (CT) (FDG‐PET/CT).
Results
The four patients were aged 60 years or older, and presented with various degrees of cognitive impairment, with predominant frontal lobe impairment. Two patients presented with cerebellar syndrome, one patient had myoclonus, one had psychiatric manifestations, and one had status epilepticus. The delay between first COVID‐19 symptoms and onset of neurological symptoms was between 0 and 12 days. None of the patients had MRI features of encephalitis nor significant cerebrospinal fluid (CSF) abnormalities. SARS‐CoV‐2 RT‐PCR in the CSF was negative for all patients. All patients presented with a consistent brain FDG‐PET/CT pattern of abnormalities, namely frontal hypometabolism and cerebellar hypermetabolism. All patients improved after immunotherapy.
Conclusions
Despite varied clinical presentations, all patients presented with a consistent FDG‐PET pattern, which may reflect an immune mechanism.
Keywords: COVID‐19, encephalitis, encephalopathy, FDG‐PET/CT, SARS‐CoV‐2
Introduction
Since December 2019, an outbreak of infection caused by the SARS‐CoV‐2 virus has rapidly spread worldwide. A variety of neurological symptoms have been reported in association with COVID‐19, including anosmia and ageusia, cerebrovascular events, and encephalopathy. In a retrospective study of 214 patients, more than a third presented with neurological manifestations [1]. In a report of patients hospitalized in the intensive care unit (ICU), more than a third had dysexecutive symptoms on discharge, and all brain magnetic resonance imaging (MRI) showed bilateral fronto‐parietal hypoperfusion [2]. While the neuro‐invasive potential of SARS‐CoV‐2, like that of other closely related coronaviruses, is suspected, there are surprisingly few reports of COVID‐19 encephalitis [3, 4, 5, 6]. We report four cases of patients with COVID‐19‐related encephalopathy of suspected immune mechanism, presenting with acute cognitive impairment and brain metabolic abnormalities on 2‐desoxy‐2‐fluoro‐D‐glucose (FDG) positron emission tomography‐computed tomography (PET/CT) imaging.
Materials and methods
We report consecutive cases of COVID‐19‐related encephalopathy, managed by our multidisciplinary team of infectious diseases physicians, neurologists and psychiatrists, from 20 March to 16 May 2020 in the Pitié‐Salpêtrière Hospital in Paris.
The diagnosis of COVID‐19‐related encephalopathy was made in patients with new‐onset cognitive disturbances, with central focal neurological signs or seizures in the context of COVID‐19, in the absence of another cause of encephalopathy. The diagnosis of COVID‐19 was confirmed for all patients by a positive RT‐PCR assay from a nasopharyngeal swab sample. Cognitive performances were assessed using the following validated screening tests, depending on attentional abilities: the Mini‐Mental State Evaluation (MMSE), the Frontal Assessment Battery (FAB) and praxis abilities. All patients underwent cerebrospinal fluid (CSF) analysis, electroencephalogram (EEG), brain MRI, and brain FDG‐PET/CT imaging. A comprehensive evaluation was carried out in all patients to exclude alternative diagnoses of encephalopathy and encephalitis, including PCR‐detected herpesvirus in the CSF, and anti‐neuronal antibodies in the blood and CSF.
Patients received information and agreed to the use of their medical data in accordance with French regulations. The study received approval from the Sorbonne University Ethics Committee (CER‐202028 on 24 April 2020).
Results
The main clinical, biological and imaging features of the patients are summarized in Table 1.
Table 1.
Clinical and laboratory features of four patients with COVID‐19 and acute cognitive impairment
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | |
|---|---|---|---|---|
| Age, years | 72 | 66 | 60 | 69 |
| Sex | Male | Female | Female | Male |
| Medical history/comorbidities | None | None | Temporal lobe epilepsy (hippocampal sclerosis) | Type 2 diabetes mellitus, hypertension |
| Clinical features at admission | ||||
| COVID‐19 symptoms | Fever, cough, anosmia | Fever, fatigue, shortness of breath | Fever, cough, diarrhea | Fever, fatigue, anosmia, ageusia |
| Supplemental oxygen | Yes | Yes | No | Yes |
| Mechanical ventilation | No | No | No | Yes |
| Delay between COVID‐19 onset and neurological symptoms, days | 12 | 7 | 0 | 7 |
| Neurological symptoms | Psychomotor agitation, frontal lobe syndrome (MMSE 25/28, FAB 13/18), cerebellar syndrome (static), myoclonus | Psychomotor slowing, frontal lobe syndrome, apraxia (MMSE 9/30, FAB 2/18) | Psychomotor agitation, anxiety, depressed mood, dysexecutive syndrome (FAB 11/18) cerebellar syndrome (hypotonia, gait ataxia, dysmetria, dysarthria, nystagmus) | Generalized convulsive status epilepticus, frontal lobe syndrome (FAB 3/18) |
| CSF testing | ||||
|
Cellularity, cells/mm3 (reference <5) |
6 | 1 | 0 | 1 |
|
Protein levels, g/l (reference 0.15–0.45) |
0.23 | 0.3 | 0.25 | 0.66 |
| Oligoclonal bands | Absent | Absent | Absent | Absent |
| IL‐6 levels in CSF, pg/ml (reference value <6.5 pg/ml) | 13 | NP | NP | 16 |
| SARS‐CoV‐2 RT‐PCR assay | Negative | Negative | Negative | Negative |
| EEG results | Normal | Generalized periodic discharges, slowed background activity, irregular anterior rhythms | Normal | Lateralized periodic discharges in the right frontal lobe |
| Brain MRI results | Unremarkable | Non‐specific white‐matter hyperintensities | Right mesial sclerosis (already known) | Right T2 orbitofrontal hyperintensity |
| Brain FDG‐PET/CT results |
Hypometabolism within the bilateral prefrontal cortex and left‐sided parieto‐temporal cortex. Slight hypermetabolism within the cerebellar vermis |
Hypometabolism within the bilateral prefrontal and associative posterior cortices. Hypermetabolism within the bilateral striatum and the cerebellar vermis |
Hypometabolism within the bilateral orbito‐frontal cortices. Slight hypermetabolism within the bilateral striatum and cerebellar vermis. |
Hypometabolism within the bilateral prefrontal and associative posterior cortices. Hypermetabolism within the cerebellar vermis |
CSF, cerebrospinal fluid; EEG, electroencephalogram; FAB, Frontal Assessment Battery; FDG‐PET/CT, 2‐desoxy‐2‐fluoro‐D‐glucose positron‐emission tomography/computed tomography; IL‐6, interleukin 6; MMSE, Mini‐Mental State Evaluation; MRI, magnetic resonance imaging; NP, not performed; RT‐PCR, real‐time polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus.
Patient 1
Patient 1 was a 72‐year‐old man, whose initial COVID‐19 symptoms were fever, cough and anosmia. Fifteen days after COVID‐19 onset, he presented with acute psychomotor agitation, cognitive and behavioural frontal lobe syndrome, upper limbs myoclonus, and cerebellar ataxia. CSF examination revealed 6 cells/mm3. Brain FDG‐PET/CT imaging on day 23 after COVID‐19 onset showed bilateral prefrontal and left‐sided parieto‐temporal hypometabolism and a slight hypermetabolism within the cerebellar vermis (Fig. 1). COVID‐19‐related encephalitis was suspected. Intravenous polyvalent immunoglobulins (IVIg) 2 g/kg were administered and preferred to corticosteroids because of the psychomotor agitation. Neurological symptoms improved gradually after IVIg, with resolution of myoclonus, cerebellar syndrome, and improvement of frontal signs. Six weeks after COVID‐19 onset, cognitive examination was normal.
Figure 1.
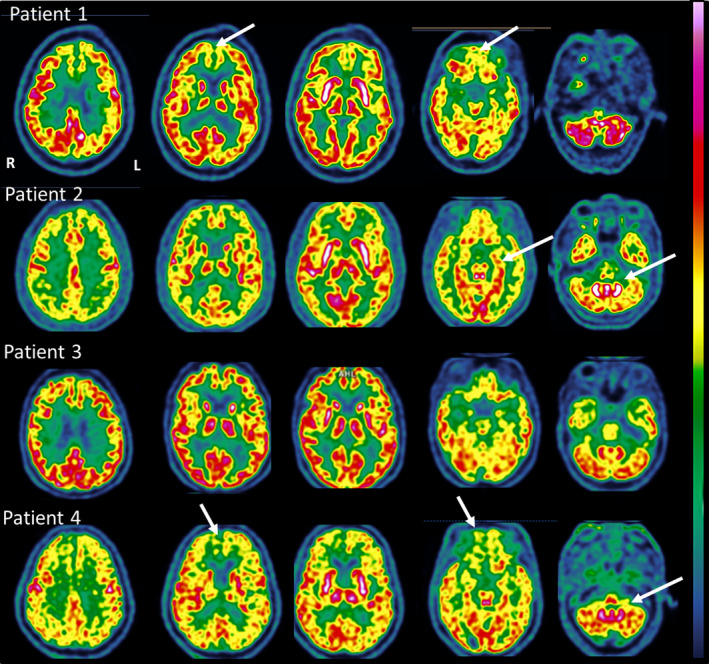
Brain FDG‐postitron‐emission tomography/computed tomography imaging in four patients with COVID‐19 and acute cognitive impairment.
Patient 2
Patient 2 was a 66‐year‐old woman, who initially presented with fatigue, fever and shortness of breath, and who experienced acute cognitive impairment 7 days after COVID‐19 onset. Neurological examination was notable for psychomotor slowing, a cognitive and behavioural frontal lobe syndrome with perseveration, and severe apraxia. Laboratory examinations revealed hypercalcemia (3.2 mmol/l corrected; reference range 2.20–2.55 mmol/l). EEG showed irregular anterior rhythms. Due to persisting cognitive impairment despite correction of hypercalcemia, we administered IVIg from day 14 to day 18 after COVID‐19 onset, without notable improvement. Brain FDG‐PET/CT imaging on day 21 showed marked hypometabolism within the bilateral prefrontal and associative posterior cortices and hypermetabolism within the bilateral striatum and the cerebellar vermis (Fig. 1). Because of the persisting severe cognitive impairment and the FDG‐PET results, we administered intravenous pulse corticosteroids (2 mg/kg/day for 3 days then 1 g/day for 3 days). The neurological examination findings were markedly improved in the following days and normalized within 2 weeks, including cognitive examination (MMSE 28/30, FAB 16/18). Repeat CSF examination showed low beta‐amyloid 1–42 (385 pg/ml, normal range 650–2000 pg/ml).
Patient 3
Patient 3 was a 60‐year‐old woman, who complained of acute anxiety, depressed mood, akathisia and gait imbalance on the same day as COVID‐19 symptom onset (fever, cough and diarrhea). On day 10, neurological examination revealed psychomotor agitation, dysexecutive syndrome, and cerebellar ataxia. Brain FDG‐PET/CT on day 14 showed hypometabolism within the bilateral orbito‐frontal cortices, and a slight hypermetabolism in the bilateral striatum and cerebellar vermis. Pulse corticosteroids (2 mg/kg/day for 3 days) were administered. Resolution of the cerebellar syndrome was noted over a few days. Akathisia and anxiety improved within 10 days with antidepressant treatment (paroxetine 20 mg and mirtazapine 30 mg). Six weeks after COVID‐19 onset, cognitive examination was normal.
Patient 4
Patient 4 was a 69‐year‐old man who was hospitalized in the ICU and intubated due to generalized convulsive status epilepticus 7 days after initial COVID‐19 symptom onset (fever, fatigue, anosmia and ageusia). EEG showed lateralized periodic discharges in the right frontal lobe, and brain MRI imaging was notable for right orbitofrontal hyperintensities on T2‐weighted images. A detailed report of the clinical history, EEG and MRI imaging of patient 4 is under consideration for publication elsewhere. He was treated with antiepileptics (levetiracetam, lacosamide) and IVIg from day 10 to day 14 after COVID‐19 onset. After extubation on day 39, neurological examination showed marked psychomotor slowing, frontal cognitive and behavioural syndrome and dyspraxia. Brain FDG‐PET/CT on day 41 showed hypometabolism within the bilateral prefrontal and associative posterior cortices, and hypermetabolism within the cerebellar vermis. Pulse corticosteroids (1 g/day for 5 days) were introduced from day 51 to day 55, with slow improvement ever since. Clinical examination 10 weeks after COVID‐19 onset showed improvement of psychomotor slowing, persisting dysexecutive syndrome, improvement of behavioural frontal disturbances and praxis. Repeat CSF examination revealed high total tau protein (>2000 pg/ml, normal range 150–450 pg/ml) and low beta‐amyloid 1–42 (570 pg/ml, normal range 650–2000 pg/ml).
Discussion
We report four patients presenting with varying degrees of acute cognitive impairment and focal neurological signs in the context of COVID‐19, with a consistent pattern of metabolic abnormalities on brain FDG‐PET. Patients were older than 60 years, and had predominant executive and frontal behavioural disorders, sometimes associated with anosmia (Patients 1 and 4), cerebellar syndrome (Patients 1 and 3), myoclonus (Patient 1), or seizures (Patient 4). None of the patients presented with significant CSF abnormalities. SARS‐CoV‐2 RT‐PCR in the CSF was negative for all patients. MRI showed no specific abnormalities. All patients fulfilled the diagnosis criteria for possible immune encephalitis according to Graus criteria [7].
Our case series is the first to illustrate FDG‐PET/CT findings in patients with COVID‐19‐related encephalopathy. One case of COVID‐19‐related anosmia was investigated by brain FDG‐PET/CT, which showed asymmetrical frontal hypometabolism [8]. Despite varied neurological presentations, our patients presented with a common pattern of hypometabolism in the prefrontal or orbito‐frontal cortices and hypermetabolism in the cerebellar vermis. This pattern is distinct from that typically seen in patients with delirium who exhibit global cortical hypometabolism [9]. An association of cortical hypometabolism and striatal, mesiotemporal, and/or cerebellar hypermetabolism has rarely been described in infectious encephalitis, such as in varicella zoster virus encephalitis [10]. Cerebellar hypermetabolism has seldom been reported in paraneoplastic cerebellar degeneration [11]. The local glucose consumption, and thus 18F‐FDG cerebral uptake, correlates with local neuronal and synaptic activity [12]. Neurotransmission and signal transduction are the processes with the highest energetic requirements. Connections between neurons are carried out mainly by excitatory glutamatergic synapses, which account for the great majority of all cortical synapses, yielding an energetic consumption of approximately 80% of total cortical consumption. A large body of literature demonstrated that 18F‐FDG‐PET/CT adds value to MRI and diagnostic evaluation of several neurological diseases [13, 14]. In encephalitis, cortical hypometabolism may be the consequence of varied neuropathological mechanisms: direct blockade of receptors or ion channels by antibodies, direct toxicity/cellular damage due to pathogenic agents or post‐infectious healing mechanism. An increased FDG uptake may reflect compensatory mechanisms [15], electro‐convulsive phenomena or inflammatory processes (infectious or immune origin) that increase the energy turnover of affected cells.
To our knowledge, there have only been two published cases of COVID‐19 with a positive SARS‐CoV‐2 RT‐PCR assay in the CSF [4, 6], and most cases of acute cognitive impairment reported in patients with COVID‐19 had no pleocytosis [2], or only mildly elevated CSF cell counts [5]. In our cases, the new‐onset central focal neurological signs or seizures, occurring within the second week after COVID‐19 onset in three of the four patients, the absence of SARS‐CoV‐2 in the CSF and meningitis, and the FDG‐PET/CT findings point towards a parainfectious cytokine storm, post‐infectious antibody‐ or cell‐mediated immune mechanism, rather than direct viral neuro‐invasion. The presence of increased interleukin‐6 in the CSF in 2/2 patients further supports this mechanism. Clinical improvement after immunotherapy is also in keeping with an immune process, although we cannot exclude spontaneous amelioration. Interestingly, similar descriptions of acute cognitive impairment with diffuse cortical hypometabolism on FDG‐PET/CT were reported after therapy with chimeric antigen receptor T cells, which are known to induce a cytokine storm [16].
In the instance of severe COVID‐19, cognitive impairment may result from other factors such as hypoxic encephalopathy, metabolic disturbances and side effects of sedation in the case of patients treated in the ICU. The diagnosis of COVID‐19‐related encephalitis or encephalopathy may be further confounded by acute delirium revealing underlying cognitive deficits, or epilepsy. Notably, cognitive disturbances in patient 2 could be partly induced by hypercalcemia, and patient 4’s initial psychomotor slowing could have been secondary to prolonged sedation and/or postictal confusion. Importantly, patient 2 and patient 4 both presented with anterior EEG abnormalities, consistent with what has been previously shown in COVID‐19 patients with encephalopathy [17]. It is noteworthy that these two patients had abnormal neurodegenerative markers in the CSF, namely, isolated low beta‐amyloid for patient 2 and high tau with low beta amyloid for patient 4. It is possible that an underlying neurodegenerative disease may have been a predisposing factor for neurological manifestations in the context of COVID‐19. Elevated tau protein in patient 4 could also reflect neuronal damage due to encephalitis and/or status epilepticus.
Brain FDG‐PET/CT imaging should be considered in patients with COVID‐19 presenting with acute central nervous system impairment. Further studies with longitudinal FDG‐PET/CT imaging will be important to determine the pathophysiological bases of the pattern of cortical hypometabolism and cerebellar hypermetabolism, and whether these abnormalities are attributable to functional inactivation rather than irreversible brain damage.
Disclosure of conflicts of interest
Vincent Navarro has served as a board member for UCB Pharma, LivaNova, GW Pharma and EISAI.
Acknowledgements
The authors thank the Cohort COVID‐19 Neurosciences (CoCo Neurosciences) study, sponsored by APHP– ICM and consequently the CoCo‐Neurosciences study group and the COVID SMIT PSL study group. Steering Committee (Pitié‐Salpêtrière Hospital, Paris): Cecile Delorme, Jean‐Christophe Corvol, Jean‐Yves Delattre, Stephanie Carvalho, Sandrine Sagnes. Scientific Committee (Pitié‐Salpêtrière Hospital, Paris): Bruno Dubois, Vincent Navarro, Celine Louapre, Tanya Stojkovic, Ahmed Idbaih, Charlotte Rosso, David Grabli, Ana Zenovia Gales, Bruno Millet, Benjamin Rohaut, Eleonore Bayen, Sophie Dupont, Gaelle Bruneteau, Stephane Lehericy, Danielle Seilhean, Alexandra Durr, Foudil Lamari, Marion Houot, Vanessa Batista Brochard. Principal investigators: Pitié‐Salpêtrière Hospital (Paris): Sophie Dupont, Catherine Lubetzki, Danielle Seilhean, Pascale Pradat‐Diehl, Charlotte Rosso, Khe Hoang‐Xuan, Bertrand Fontaine, Lionel Naccache, Philippe Fossati, Isabelle Arnulf, Alexandra Durr, Alexandre Carpentier, Stephane Lehericy, Yves Edel; Rothschild Hospital (Paris): Gilberte Robain, Philippe Thoumie; Avicenne Hospital (Bobigny): Bertrand Degos; Sainte‐Anne Hospital (Paris): Tarek Sharshar; Saint‐Antoine Hospital (Paris): Sonia Alamowitch, Emmanuelle Apartis‐Bourdieu, Charles‐Siegried Peretti; Saint‐Louis Hospital (Paris): Renata Ursu; Tenon Hospital (Paris): Nathalie Dzierzynski; Charles Foix Hospital (Ivry): Kiyoka Kinugawa Bourron, Joel Belmin, Bruno Oquendo, Eric Pautas, Marc Verny. Co‐investigators: Pitié‐Salpêtrière Hospital (Paris): Cecile Delorme, Jean‐Christophe Corvol, Jean‐Yves Delattre, Yves Samson, Sara Leder, Anne Leger, Sandrine Deltour, Flore Baronnet, Ana Zenovia Gales,Stephanie Bombois, Mehdi Touat, Ahmed Idbaih, Marc Sanson, Caroline Dehais, Caroline Houillier, Florence Laigle‐Donadey, Dimitri Psimaras, Agusti Alenton, Nadia Younan, Nicolas Villain, David Grabli, Maria del Mar Amador, Gaelle Bruneteau, Celine Louapre, Louise‐Laure Mariani, Nicolas Mezouar, Graziella Mangone, Aurelie Meneret, Andreas Hartmann, Clement Tarrano, David Bendetowicz, Pierre‐François Pradat, Michel Baulac, Sara Sambin, Phintip Pichit, Florence Chochon, Adele Hesters, Bastien HerlinAn Hung Nguyen, Valerie Procher, Alexandre Demoule, Elise Morawiec, Julien Mayaux, Morgan Faure, Claire Ewenczyk, Giulia Coarelli, Anna Heinzmann, Tanya Stojkovic, Marion Masingue, Guillaume Bassez, Vincent Navarro, Isabelle An, Yulia Worbe, Virginie Lambrecq, Rabab Debs, Esteban Munoz Musat, Timothee Lenglet, Virginie Lambrecq, Aurelie Hanin, Lydia Chougar, Nathalia Shor, Nadya Pyatigorskaya, Damien Galanaud, Delphine Leclercq, Sophie Demeret, Benjamin Rohaut, Albert Cao, Clemence Marois, Nicolas Weiss, Salimata Gassama, Loic Le Guennec, Vincent Degos, Alice Jacquens, Thomas Similowski, Capucine MorelotPanzini, Jean‐Yves Rotge, Bertrand Saudreau, Bruno Millet, Victor Pitron, Nassim Sarni, Nathalie Girault, Redwan Maatoug, Ana Zenovia Gales, Smaranda Leu, Eleonore Bayen, Lionel Thivard, Karima Mokhtari, Isabelle Plu; Sainte‐Anne Hospital (Paris): Bruno Gonçalves; Saint‐Antoine Hospital (Paris): Laure Bottin, Marion Yger; Rothschild Hospital (Paris): Gaelle Ouvrard, Rebecca Haddad; Charles Foix Hospital (Ivry): Flora Ketz, Carmelo Lafuente, Christel Oasi. Other Contributors: Associated centers (Lariboisière Hospital, Paris): Bruno Megabarne, Dominique Herve; Clinical Research Associates (ICM, Pitié‐Salpêtrière Hospital, Paris): Haysam Salman, Armelle Rametti‐Lacroux, Alize Chalançon, Anais Herve, Hugo Royer, Florence Beauzor, Valentine Maheo, Christelle Laganot, Camille Minelli, Aurelie Fekete, Abel Grine, Marie Biet, Rania Hilab, Aurore Besnard, Meriem Bouguerra, Gwen Goudard, Saida Houairi, Saba Al‐Youssef, Christine Pires, Anissa Oukhedouma, Katarzyna Siuda‐Krzywicka, Tal Seidel Malkinson; (Saint‐Louis Hospital, Paris): Hanane Agguini; Data Manager (ICM, Paris): Safia Said; Statistician (ICM, Paris): Marion Houot.
Funding information: This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors.
Data availability statement
Detailed data are available from the corresponding author upon request.
References
- 1. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol 2020; 77: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med 2020; 382: 2268–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Poyiadji N, Shahin G, Noujaim D, Stone M, Patel S, Griffith B. COVID‐19‐associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 2020; 296: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis 2020; 94: 55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernard‐Valnet R, Pizzarotti B, Anichini A, et al. Two patients with acute meningo‐encephalitis concomitant to SARS‐CoV‐2 infection. Eur J Neurol 2020; 7: e43–e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hanna Huang Y, Jiang D, Huang JT. A case of COVID‐19 encephalitis. Brain Behav Immun 2020; 87: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15(4): 391–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Karimi‐Galougahi M, Yousefi‐Koma A, Bakhshayeshkaram M, Raad N, Haseli S. 18FDG PET/CT scan reveals hypoactive orbitofrontal cortex in anosmia of COVID‐19. Acad Radiol 2020; 27: 1042–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Haggstrom LR, Nelson JA, Wegner EA, Caplan GA. 2–18 F‐fluoro‐2‐deoxyglucose positron emission tomography in delirium. J Cereb Blood Flow Metab 2017; 37: 3556–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coiffard B, Guedj E, Daumas A, Leveque P, Villani P. Brain PET metabolic abnormalities in a case of varicella‐zoster virus encephalitis. Clin Nucl Med 2014; 39: e389–e391. [DOI] [PubMed] [Google Scholar]
- 11. Choi K‐D. Cerebellar hypermetabolism in paraneoplastic cerebellar degeneration. J Neurol Neurosurg Psychiatry 2006; 77: 525–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Berti V, Mosconi L, Pupi A. Brain: normal variations and benign findings in fluorodeoxyglucose‐PET/computed tomography imaging. PET Clin 2014; 9: 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morbelli S, Djekidel M, Hesse S, et al. Role of (18)F‐FDG‐PET imaging in the diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15: 1009–1010. [DOI] [PubMed] [Google Scholar]
- 14. Deuschl C, Rüber T, Ernst L, et al. 18F‐FDG‐PET/MRI in the diagnostic work‐up of limbic encephalitis. PLoS One 2020; 15(1): e0227906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ritz L, Segobin S, Lannuzel C, et al. Cerebellar hypermetabolism in alcohol use disorder: compensatory mechanism or maladaptive plasticity? Alcohol Clin Exp Res 2019; 43: 2212–2221. [DOI] [PubMed] [Google Scholar]
- 16. Rubin DB, Danish HH, Ali AB, et al. Neurological toxicities associated with chimeric antigen receptor T‐cell therapy. Brain 2019; 142(5): 1334–1348. [DOI] [PubMed] [Google Scholar]
- 17. Galanopoulou AS, Ferastraoaru V, Correa DJ, et al. EEG findings in acutely ill patients investigated for SARS‐CoV‐2/COVID‐19: a small case series preliminary report. Epilepsia Open 2020; 5: 314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Detailed data are available from the corresponding author upon request.


