A 35‐year‐old previously healthy female presented with fever, cough, sneezing, rhinorrhea, diarrhea, myalgia, anosmia, and hypogeusia. She was diagnosed with mild coronavirus disease 2019 (COVID‐19) infection (positive quantitative reverse transcription polymerase chain reaction test for severe acute respiratory syndrome coronavirus 2 [SARS‐COV‐2] performed by nasopharyngeal swab) and did not require hospitalization. After 10 days, she recovered from respiratory symptoms but developed paresthesia and difficulty in moving her right arm. After 1 day, she became unable to move the right arm and developed a lower voice tone, generalized rigidity, and slowness of movement with gait unsteadiness.
Two weeks after the onset of neurological symptoms, physical examination revealed decreased facial expression, eyelid retraction, and slow and hypometric saccades as well as hypophonia. She also had generalized and asymmetric (right worse than the left) bradykinesia and cogwheel rigidity, stooped posture, gait with reduced arm swing, en bloc turning, and decreased stride length. The Movement Disorder Society Unified Parkinson's Disease Rating Scale Part III score on the first evaluation was 49. After 4 days of therapy with 200/50 mg of levodopa/benserazide three times a day, there was significant improvement of facial expression, dysarthria, bradykinesia, and arm swinging. The Movement Disorder Society Unified Parkinson's Disease Rating Scale Part III score decreased to 32 (see the Supplementary Information for Movement Disorder Society Unified Parkinson's Disease Rating Scale Part III detailed description; physical examination features are shown in Video S1). Cognition was normal, and Sniffin' Sticks confirmed moderate hyposmia (9/16 correct answers). The proband had no family history of parkinsonism.
Laboratory workup included a normal cerebrospinal fluid analysis (cells, 0; protein, 36 mg/dL; glucose, 60 mg/dL). The 3‐Tesla magnetic resonance imaging was unremarkable, including an evaluation of nigrosome‐1 and neuromelanin imaging. Brain fluorodeoxyglucose–positron emission tomography showed normal glucose metabolism. There was decreased dopamine transporter density on the left putamen (more evident in the mid‐putamen, different from the posterior involvement usual of idiopathic Parkinson's disease). Such finding contrasted with the patient's bilateral symptoms, what could be attributed to microstructural changes in other brain pathways (Fig. 1).
FIG 1.
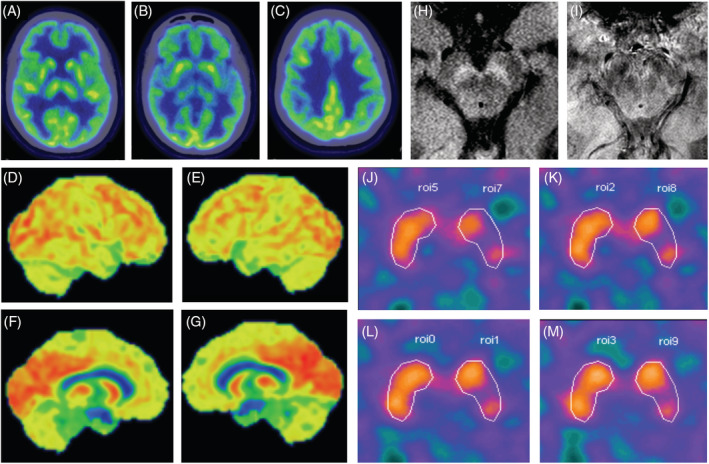
Comprehensive neuroimaging ancillary evaluation: fluorodeoxyglucose–positron emission tomography, neuromelanin and nigrosome‐1 magnetic resonance imaging, and 99mTc‐TRODAT‐1 (Technetium‐99m labeled tropane derivative) single‐photon emission computed tomography). (A–C) Fluorodeoxyglucose–positron emission tomography scan axial slices (through the thalamus, striatum, and corona radiata) show cerebral glucose metabolism within normal limits in the basal ganglia and cortical regions. (D–G) Three‐dimensional stereotactic surface projection (3d‐SSP) projection of the cortical radiotracer uptake to a lateral and medial surface perspective of both hemispheres shows normal glucose metabolism. (H) Normal neuromelanin content is seen in an axial magnetic resonance imaging slice of the midbrain through the substantia nigra. (I) Nigrossome‐1 and its related “swallow‐tail” sign are readily identifiable in both cerebral peduncles. (J–M) Nigrostriatal denervation at the left mid‐putamen (semiquantification of dopamine transporter binding—0.60), contralateral to the most significant clinically affected side. The specific dopamine transporter binding potential in the basal ganglia is calculated as the difference between dorsal putamen activity (PUT) and the reference region, that is, the occipital activity (OA), divided by the OA: (PUT−OA)/OA. [Color figure can be viewed at wileyonlinelibrary.com]
Our patient clearly has akinetic‐rigid parkinsonism. The abnormal dopamine transporter scan and levodopa improvement confirm the presynaptic nature of the parkinsonian syndrome. As the patient was neurologically normal prior to the COVID‐19 infection, we conclude that SARS‐CoV‐2 infection is responsible for the parkinsonism. Méndez‐Guerrero and colleagues 1 recently described another case of parkinsonism associated with COVID‐19 affecting a critically ill patient with pleomorphic neurologic symptomatology.
Neurologic manifestations in COVID‐19 most commonly described include headache, seizures, stroke, hyposmia, and altered mental status. 2 , 3 As they may occur regardless of respiratory symptoms, most likely there is direct viral neurotoxicity. 4 Since the outbreak of encephalitis lethargica in the 1920s, 5 an ever‐growing number of viruses have been implicated in postencephalitic parkinsonism, including influenza, Epstein‐Barr, West Nile, and Japanese encephalitis. 6 In contrast, the COVID‐19 clinical and laboratory pictures of our patient do not suggest she had encephalitis. In parkinsonism associated with viral infections, neuropathology varies from direct acute infection to postinfectious neuroinflammation, both culminating with dopaminergic cell loss. SARS‐CoV‐2 might infect neurons in the central nervous system through either retrograde axonal transport or hematogenic routes. 4 Our patient probably had a direct SARS‐CoV‐2 lesion of the nigro‐striatal system. 7
In conclusion, we report a case of levodopa‐responsive parkinsonism probably caused by direct SARS‐CoV‐2 infection, broadening the disease clinical spectrum.
Author Roles
(1) Research Project: A. Conception, B. Organization; (2) Manuscript: A. Writing of the First Draft, B. Review and Critique.
I.F.: 1A, 1B, 1C, 2A, 2B
P.P.B.: 1A, 1B, 1C, 2A, 2B
F..M.: 1B, 1C, 2B
D..D.CB.: 1B, 1C, 2B
F.B.M.: 1B, 1C, 2B
F.C.: 1A 2B
Financial Disclosures of all authors (for the preceeding 12 months)
Ingrid Faber: research support from Sabin Laboratory. Pedro Renato P. Brandão: research support from Sabin Laboratory. Fiorella Menegatti: none. Diógenes Diego de Carvalho Bispo: none. Fernando Bisinoto Maluf: none Francisco Cardoso: none
Supporting information
Table S1.Unified Parkinson's Disease Rating Scale Part III before and after levodopa
Video S1. Supporting information
Acknowledgments
We thank Dr. Alaor Barra Sobrinho, Director of Imagens Médicas de Brasilia, for kindly giving support for nuclear medicine complementary exams.
Ingrid Faber and Pedro R.P. Brandão contributed equally to this work.
Relevant conflicts of interest/financial disclosures: Nothing to report.
References
- 1. Méndez‐Guerrero A, Laespada‐García MI, Gómez‐Grande A, et al. Acute hypokinetic‐rigid syndrome following SARS‐CoV‐2 infection. Neurology [published online ahead of print July 8, 2020]. 10.1212/WNL.0000000000010282. [DOI] [PubMed] [Google Scholar]
- 2. Munhoz RP, Pedroso JL, Nascimento FA, et al. Neurological complications in patients with SARS‐CoV‐2 infection: a systematic review. Arq Neuropsiquiatr 2020;78(5):290–300. 10.1590/0004-282x20200051. [DOI] [PubMed] [Google Scholar]
- 3. Ellul MA, Benjamin L, Singh B, et al. Neurological associations of COVID‐19. Lancet Neurol 2020;19(9):767–783. 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol 2020;92(7):699–702. 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hoffman LA, Vilensky JA. Encephalitis lethargica: 100 years after the epidemic. Brain 2017;140(8):2246–2251. 10.1093/brain/awx177. [DOI] [PubMed] [Google Scholar]
- 6. Limphaibool N, Iwanowski P, Holstad MJV, Kobylarek D, Kozubski W. Infectious etiologies of Parkinsonism: pathomechanisms and clinical implications. Front Neurol 2019;10:652. 10.3389/fneur.2019.00652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zubair AS, McAlpine LS, Gardin T, Farhadian S, Kuruvilla DE, Spudich S. Neuropathogenesis and neurologic manifestations of the coronaviruses in the age of coronavirus disease 2019: a review. JAMA Neurol 2020;77(8):1018–1027. 10.1001/jamaneurol.2020.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.Unified Parkinson's Disease Rating Scale Part III before and after levodopa
Video S1. Supporting information


