Abstract
Ageing is a leading risk factor predisposing cartilage to osteoarthritis. However, little research has been conducted on the effect of ageing on the expression of small non-coding RNAs (sncRNAs). RNA from young and old chondrocytes from macroscopically normal equine metacarpophalangeal joints was extracted and subjected to small RNA sequencing (RNA-seq). Differential expression analysis was performed in R using package DESeq2. For transfer RNA (tRNA) fragment analysis, tRNA reads were aligned to horse tRNA sequences using Bowtie2 version 2.2.5. Selected microRNA (miRNAs or miRs) and small nucleolar RNA (snoRNA) findings were validated using real-time quantitative Polymerase Chain Reaction (qRT-PCR) in an extended cohort of equine chondrocytes. tRNA fragments were further investigated in low- and high-grade OA human cartilage tissue. In total, 83 sncRNAs were differentially expressed between young and old equine chondrocytes, including miRNAs, snoRNAs, small nuclear RNAs (snRNAs), and tRNAs. qRT-PCR analysis confirmed findings. tRNA fragment analysis revealed that tRNA halves (tiRNAs), tiRNA-5035-GluCTC and tiRNA-5031-GluCTC-1 were reduced in both high grade OA human cartilage and old equine chondrocytes. For the first time, we have measured the effect of ageing on the expression of sncRNAs in equine chondrocytes. Changes were detected in a number of different sncRNA species. This study supports a role for sncRNAs in ageing cartilage and their potential involvement in age-related cartilage diseases.
Keywords: chondrocyte, ageing, equine, small non-coding RNA
1. Introduction
Articular cartilage is a specialised connective tissue of diarthrodial joints. Its smooth lubricated surface assists joint movement and its mechanical properties facilitate load bearing in the joint. The tissue harbours one cell type; the chondrocyte, and is devoid of blood vessels and nerves, receiving nutrients from synovial fluid and subchondral bone [1]. Articular cartilage is characterised by an extracellular matrix (ECM) consisting of mainly collagen type 2 and proteoglycans, which give the tissue many of its properties. After reaching maturity, cartilage displays a limited repairing capacity as indicated by low chondrocyte proliferation and low collagen turnover [2].
There are a number of factors affecting the homeostatic properties of cartilage such as genetics and obesity [3]. However, ageing is a leading risk factor that predisposes cartilage to pathological changes and disease, such as osteoarthritis (OA), the most common joint disease [3]. These age-related changes affect both chondrocyte physiology and ECM properties. Aged chondrocytes display increased senescence and higher expression of catabolic markers; features also evident in OA chondrocytes [3,4]. Moreover, in humans, aged knee cartilage is thinner compared to younger cartilage and is characterised by increased collagen crosslinking and altered proteoglycan content. These changes affect matrix stiffness, make cartilage susceptible to fractures and lower its ability to sense mechanical stimuli [3,5,6].
The exact mechanisms through which age can affect cartilage health remain elusive, though it is believed to be a cumulative combination of many molecular pathways rather than a single aetiology. Recent advances in the field have recognised epigenetics in ageing and diseased articular cartilage as an area of growing interest [6,7]. A class of epigenetic modifications that have attracted increasing attention are small non-coding RNAs (sncRNAs). They are short, typically <200 bp, RNA species, which are not translated into protein but have other structural or regulatory biological roles. These include microRNAs (miRNAs or miRs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), piwi-interacting RNAs and transfer RNAs (tRNAs). SncRNAs are promising candidates for targeted therapeutics due to their small size and diverse cellular functions. By using specific synthetic oligonucleotides, aberrant expression of sncRNAs in disease could be modified, resulting in delay or reversal of pathological changes [8]. Furthermore, sncRNAs could be used as biomarkers to monitor disease initiation and progression or response to treatment [9,10]. This is of high importance in OA, as treatment is currently symptomatic and most patients with end-stage OA require joint replacement; a procedure with a high social and economic burden for patients and the healthcare system respectively [11].
MiRNAs, the most studied sncRNAs, regulate gene expression by binding complementary sequences in the 3’ untranslated region of their messenger RNA (mRNA) targets, thus inhibiting mRNA translation [8]. MiRNAs have been linked to ageing and diseased cartilage; miR-140 is important for cartilage development and deletion of miR-140 in mice causes skeletal defects [12]. Moreover, OA chondrocytes show decreased expression of miR-24, resulting in increased expression of the senescent marker p16INK4a, highlighting the link between OA and senescence; a hallmark of ageing [4].
In addition to miRNAs, snoRNAs are increasingly studied in ageing and OA. Snora73 expression increases in the joint and serum of old mice compared to young mice [9] and we have also previously identified a catalogue of age-related snoRNAs in human knee cartilage [13]. SnoRNAs have canonical roles in the post transcriptional modification of RNA substrates including ribosomal RNAs, and mRNAs, but can also exhibit non-canonical functions such as miRNA-like activity [14]. Their aberrant expression has also been associated with the development of some diseases [15]. Our previously conducted mouse study demonstrated alterations in the snoRNA profile of young, old and OA joints in mice when compared to healthy controls, highlighting the potential of snoRNAs to be used as novel markers for this disease [9]. We have also identified changing snoRNA profiles in ageing and OA human cartilage [16], synovial fluid from horses with early OA and diseased anterior cruciate ligaments from OA joints [17].
tRNAs are adaptor molecules of ~73–90 nucleotides long consisting of a T-loop, D-loop, variable loop, and the anticodon loop. Protein translation requires amino acids to be linked together into polypeptides and tRNAs recruit these amino acids to the translating ribosome. Recent studies have shown that tRNAs are a major source of sncRNAs with an active role in gene regulation [18]. tRNA fragments result from specific processing of tRNAs. These include tRNA halves (tiRNAs), which are 28–36 nucleotide long fragments formed by Angiogenin (ANG). ANG divides the tRNA into two halves at the anticodon loop [19] giving rise to the 3′ tiRNA and 5′ tiRNA halves. tRNAs are also processed into smaller fragments; tRNA-derived small RNA fragments (tRFs); tRF-1, tRF-2, tRF-3 and tRF-5, however the naming conventions of these classes is still not consistent [20]. RNase Z, or its cytoplasmic homologue ELAC2, [21] cleaves the 3′ trailer fragment of pre-tRNAs resulting in tRF-1 formation. The enzyme responsible for the cleavage of tRF-2 fragments is still unclear. The tRF-2 fragment consists of the anticodon loop of the tRNA and has been detected in breast cancer MDA-231 cells [22]. Dicer and ANG cleave tRNAs into ~15–30 nucleotide tRF-3 and tRF-5 fragments. TRF-3 fragments are cleaved at the T loop by Dicer [23] and ANG [24] and tRF-5 fragments are derived from the cleavage of the D-loop by Dicer [23]. The final category of tRFs are i-tRFs, which are internal to the respective tRNA and can straddle the anticodon loop [25]. Limited knowledge of the expression and role of tRNA and tRFs is available in health and disease [26] with even less in musculoskeletal biology [27,28].
In this study, we investigated the expression changes of sncRNAs in chondrocytes isolated from healthy metacarpophalangeal joints of young and old horses. Findings for tRNAs are compared to a human OA data set. Age-related changes may predispose cartilage to disease by altering the complex sncRNA expression profile. This provides a sncRNA-wide insight into age-related targets for future therapeutic approaches.
2. Materials and Methods
All reagents were from Thermo-Fisher-Scientific, Loughborough, UK unless stated.
2.1. Sample Collection and Preparation
Samples were collected from an abattoir as a by-product of the agricultural industry. Specifically, the Animal (Scientific procedures) Act 1986, Schedule 2, does not define collection from these sources as scientific procedures. Ethical approval was therefore not required. Full thickness equine cartilage was removed from the entire surface of macroscopically normal metacarpophalangeal joints of young n = 5 (age mean ± standard deviation; 4 ± 1 years) and old n = 5 (17.4 ± 1.9 years) non-Thoroughbred horses. Scoring of the metacarpophalangeal joint was undertaken using a macroscopic grading system, as previously described [29] and samples with no macroscopic perturbations were selected (combined score of zero). Freshly isolated chondrocytes were removed from all 10 samples of harvested cartilage, as previously described [30], plated to confluence and RNA extracted from two million cells per donor.
In addition to the above samples, RNA from chondrocytes from young n = 2 (age mean ± standard deviation; 0.75 ± 0.3 years) and old n = 6 (age mean ± standard deviation; 19.3 ± 3.6 years) non-Thoroughbred horses were used for validation.
2.2. RNA Isolation, cDNA Library Preparation, and Small RNA Sequencing (RNA-seq)
Total RNA including small RNAs was extracted, as previously described [31], and purified using the miRNAeasy kit (Qiagen, Crawley, UK) according to manufacturer’s instructions and including an on-column DNAse step to remove residual genomic DNA. The integrity of the RNA was assessed on the Agilent 2100 Bioanalyser system using an RNA Pico chip (Agilent, Stockport, UK). The NEBNext® Small RNA Library Prep Set for Illumina® was used for library preparation (New England Biolabs, Ipswich, MA, USA) but with the addition of a Cap-Clip™ Acid Pyrophosphatase (Cell script, Madison, WI, USA) step to remove potential 5′ caps found on some snoRNAs. Samples were amplified for 15 cycles and size was selected. The libraries were sequenced on an Illumina MiSEq platform (Illumina, San Diego, CA, USA) with version 2 chemistry using sequencing by synthesis technology to generate 2 × 150 bp paired-end reads with >12 million clusters per run.
2.3. Data Processing
Sequence data were processed through a number of steps to obtain sncRNA expression values including basecalling and de-multiplexing of indexed reads using CASAVA version 1.8.2 [32]; adapter and quality trimming using Cutadapt version 1.2.1 [33] and Sickle version 1.200 to obtain fastq files of trimmed reads; aligning reads to horse genome reference sequences (GCF_002863925.1) using Tophat version 2.0.10 [34] with option “–g 1”; counting aligned reads using HTSeq-count against the annotated features which are combined annotation information from the sources: NCBI Equus caballus 3.0 genome annotation, miRBase horse micro RNA annotation, Rfam snoRNA annotation.
Differential expression analysis was performed in R environment using package DESeq2 [35]. The processes and technical details of the analysis included: assessing data variation and detecting outlier samples through comparing variations of within and between sample groups using principle component analysis (PCA) and correlation analysis; handling library size variation using the DESeq2 default method; formulating data variation using negative binomial distributions; modelling data using a generalised linear model; computing log2 Fold Change (logFC) values for required contrasts based on model fitting results through contrast fitting approach, evaluating the significance of estimated logFC values by Wald test; adjusting the effects of multiple tests using False Discovery Rate (FDR) approach to obtain FDR [36] adjusted P-values.
2.4. Pathway Analysis
In order to identify miRNA targets, bioinformatic analysis was performed by uploading differentially expressed miRNA data into the MicroRNA Target Filter module within Ingenuity Pathway Analysis software (IPA) (Qiagen Redwood City, CA, USA) along with previously identified differentially expressed mRNAs from our ageing equine cartilage study following RNA-seq [31]. In IPA we selected miRNA-target genes based on the direction of differential expression (for example, if a miRNA was reduced in expression it was only matched to mRNAs that demonstrated increased expression). We then identified the networks, functions, and canonical pathways of these miRNA-target genes.
2.5. qRT-PCR Validation
Validation of the small RNA-seq results was undertaken using real-time quantitative PCR (qRT-PCR) analysis in the samples used for sequencing as well as additional samples. SncRNAs were chosen based on level of differential expression. Total RNA was extracted and quantified as above. cDNA was synthesized using 200 ng RNA and the miScript II RT Kit according to the manufacturer’s protocol (Qiagen, Crawley, UK). qRT-PCR mastermix was prepared using the miScript SYBR Green PCR Kit (Qiagen, Crawley, UK) and the appropriate miScript Primer Assay (Qiagen, Crawley, UK) (File S1) using 1 ng/μL cDNA according to manufacturer’s guidelines. qRT-PCR was undertaken using a LightCycler® 96 system (Roche, Welwyn Garden City, UK). Relative expression levels were normalised to U6 (as this was stable in the small RNA-seq data set) and calculated using the 2-ΔCt method [37].
2.6. tRNA Fragment Analysis
Following the alignment of trimmed reads to NCBI horse genome reference sequences (version 3.0) using Tophat version 2.1.0 [38], the candidate tRNA reads were extracted from the BAM files according to whether they overlapped the ranges covered by tRNA features. The read pairs were stitched into RNA fragments using PEAR (version 0.9.10) [39]. The output reads were aligned to horse tRNA sequences (defined in NCBI GCF_002863925.1_EquCab3.0_genomic.gff) using Bowtie2 version 2.2.5. Only the perfectly mapped fragments were extracted and taken as tRNA fragments for further explorations. Finally, statistical analyses were mainly focused on the fragment length and the mapping start location, which generated the length distribution and the mapping start position distribution of observed tRNA fragments, as well as the summary table for observed tRNA fragments and their target tRNAs.
2.7. Novel snoRNA Analysis
Putative snoRNAs were detected from the raw pair-end reads using ShortStack 3.8.4 [40] with the setting “--mincov 5”, which specifies that the clusters of small RNAs must have at least five alignments. The ShortStack results were subsequently fed into SnoReport 2.0 [41], which uses RNA secondary structure prediction combined with machine learning as the basis to identify the two main classes of snoRNAs; the box H/ACA and the box C/D. Putative snoRNAs were annotated from our experimental small RNA-seq data using ShortStack and SnoReport.
2.8. Statistical Analysis
3D PCA score plots and heat maps were carried out using MetaboAnalyst 3.5 [42] (http://www.metaboanalyst.ca) which uses the R package of statistical computing software [43].
For statistical evaluation of qRT-PCR results, following normality testing, Mann-Whitney tests were performed using GraphPad Prism version 7.03 for Windows, (GraphPad Software, La Jolla, CA, USA); p values are indicated.
2.9. Human Sample Collection and Preparation
De-identified human OA cartilage approved by Northeast Ohio Medical University (NEOMED) Institutional Review Board and Summa Health Systems, Barberton, Ohio as ‘non-human subject study under 45 CFR’ was used. Total RNA was extracted from smooth, macroscopically intact human OA cartilage with a Mankin score of 2 or less (n = 1, female, 60 years old) and damaged OA cartilage with a Mankin score of 4 or higher (n = 1, female, 80 years old) using MiRNeasy Kit (Qiagen, Germantown, MD, USA). RNA was quantified using the Nanodrop 1000 Spectrophotometer (Thermo Fisher, Waltham, MA, USA). TapeStation 4200 (Agilent Technologies, Santa Clara, CA, USA) was used to determine RNA integrity using High Sensitivity RNA Screentape analysis kit (Agilent, Santa Clara, CA, USA).
2.10. Removal of tRF Modifications from Human Cartilage RNA
The rtStar™ tRF&tiRNA Pretreatment Kit (ArrayStar, Rockville, MD, USA) was used according to the manufacture’s description. For cDNA qRT-PCR library construction, tRF modifications were removed from RNA. The kit removes 3′-aminoacyl and 3′-cP for 3′ adaptor ligation, phosphorylates 5′-OH for 5′-adaptor ligation, and demethylates m1A, m1G, and m3C for efficient cDNA reverse transcription.
2.11. tRF 3′ and 5′ Adaptor Ligation for Human Cartilage cDNA Synthesis
The rtStar™ First-Strand cDNA Synthesis Kit (ArrayStar, Rockville, MD USA) was used according to the manufacturer’s description. The kit sequentially ligates 3′-Adaptor with its 5′-end to the 3′-end of the RNAs, and 5′-Adaptor with its 3′-end to the 5′-end of the RNAs. The non-ligation ends of 3′ and 5′ Adaptors were blocked by modifications. A universal priming site for reverse transcription was contained within the 3′ adaptor. Spike-in RNA was used for monitoring the cDNA synthesis efficiency and as a quantitative reference.
2.12. tRF and tRNA Human qPCR Arrays
The nrStar™ Human tRF and tiRNA PCR Array (ArrayStar, Rockville, MD, USA) was used according to the manufacturers description to profile 185 tRF and tiRNA fragments, of which 101 are derived from tRF and tiRNA database [44,45] and the other 84 from recently published papers [46,47,48]. RNA Spike in control and a positive PCR control were used to evaluate PCR efficiency and a genomic DNA control was used to monitor genomic DNA contamination. To profile parent tRNAs, the nrStat Human tRNA PCR Array (ArrayStar, Rockville, MD, USA) was used according to the manufacturer’s protocol. This array consisted of 163 PCR-distinguishable nuclear tRNA isodecoders and 22 PCR distinguishable mitochondrial tRNA species covering all anti-codons compiled in GtTNAdb [49,50] and tRNAdb [51] databases. Genomic DNA and positive PCR controls were included to monitor the quality of RNA sample.
qRT-PCR reactions were conducted using Power SYBR Green master mix (Life technologies, Carlsband, CA, USA) on a Step One Plus (Applied Biosystems, Waltham, MA, USA) machine. U6, SNORD43 and SNORD45 were used as endogenous controls for normalisation of tRF, tiRNA and tRNA detection. Target tRNA, tiRNA and tRF levels were determined as fold change differences utilising the ΔΔCt method [37].
3. Results
3.1. Preliminary Analysis of Small RNA-seq Data
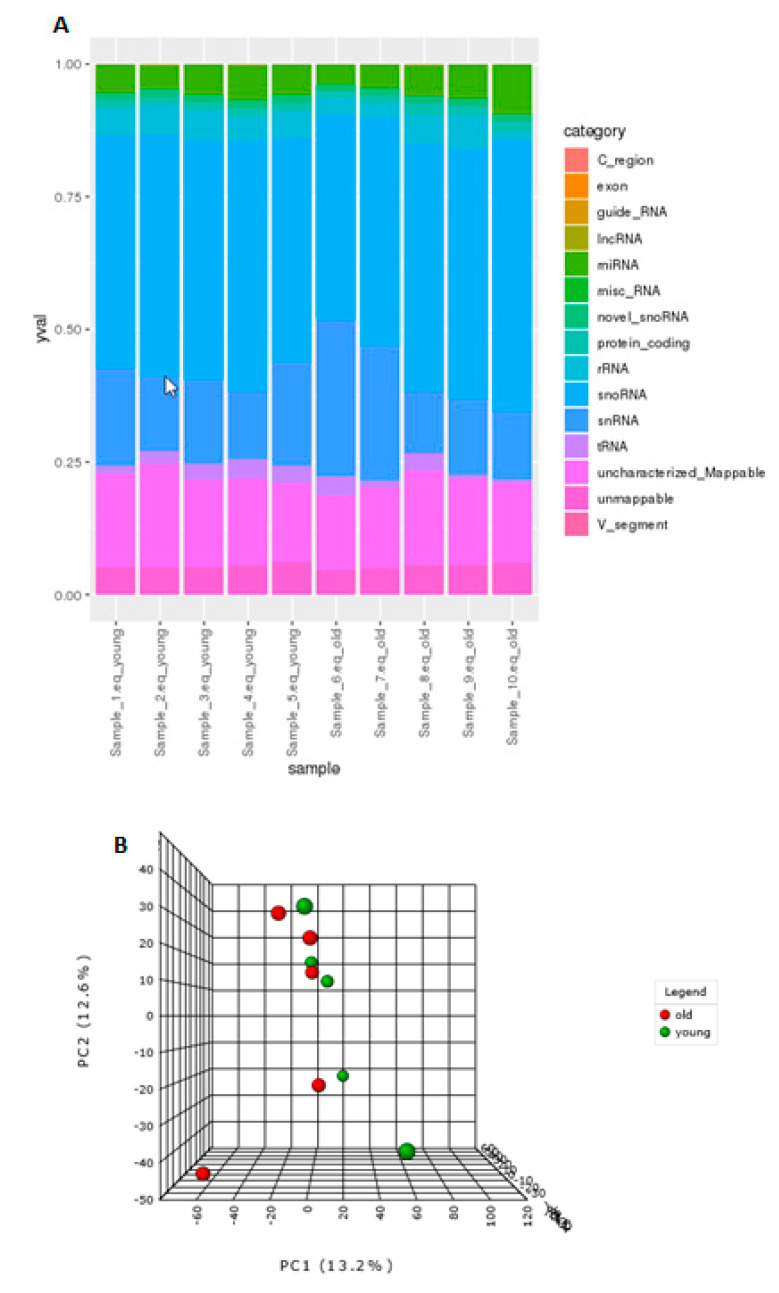
To identify differential expression of sncRNAs in ageing, Illumina MiSeq was utilised. Summaries of raw, trimmed reads, and mapped reads to Equus caballus database can be found in File S2. Reads mapping percentages were between 92 to 94.3%. There were 2128 sncRNAs identified. The categories of non-coding RNAs identified are in Figure 1A and File S3 and included miRNAs, snoRNAs, novel snoRNAs, tRNAs, snRNAs, and long non-coding RNAs (lncRNAs).
Figure 1.
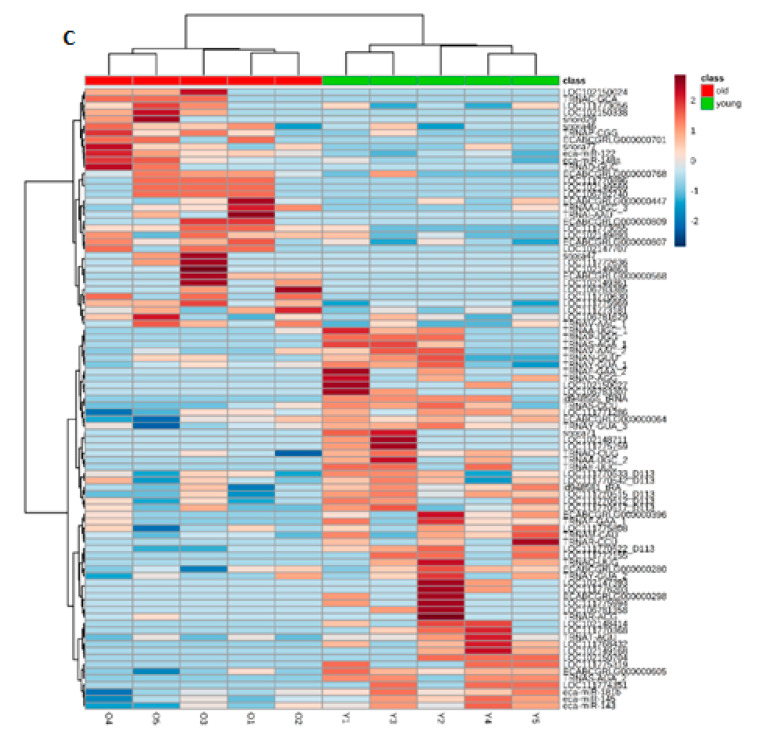
Age-related differential small non-coding RNA (sncRNA) gene expression. (A) The categories of non-coding RNAs identified in young and old equine chondrocytes; long non-coding RNAs (lncRNAs), microRNAs (miRNAs or miRs), small nucleolar RNAs (snoRNAs), small nuclear RNAs (snRNAs), transfer RNAs (tRNAs). (B) 3D principle component analysis (PCA) plot between the selected principle components (PCs). The explained variances are shown in brackets. (C). Clustering results shown as a heatmap (distance measure using Euclidean, and clustering algorithm using Ward) for the top 90 molecules.
3.2. Age-Related Differential Small Non-Coding RNA Gene Expression
There were no overall differences in the distribution of classes of sncRNAs in ageing. The effect of age on the expression of sncRNAs was weak and the separation of young and old samples was not clear. The 3D PCA plot (Figure 1B) indicated that few, but very specific changes in the expression of sncRNAs were found, and samples from old donors were more variable than those from young. A heat map of hierarchical clusters of correlations among samples (Figure 1C) depicts that the sncRNA expression of young and old groups are not very different.
There were 83 sncRNAs differentially expressed with age; six snoRNAs, 11 novel snoRNAs, three snRNAs, 31 lncRNAs, 27 tRNAs (p < 0.05) and five miRNAs (FDR-adjusted p < 0.05) (Table 1). Data is deposited on NCBI GEO, accession; E-MTAB-8112.
Table 1.
Differentially expressed sncRNAs in ageing chondrocytes. Log2 fold change values were derived with young as the reference group. A positive log2 fold change equates to higher expression in old, whereas a negative log2 fold change equates to lower expression in old. All are significant at p < 0.05, except for miRNAs that are significant with a false-discovery rate (FDR-)adjusted p value of p < 0.05.
| Gene/Transcript Name | Gene Biotype | Log2 Fold Change |
|---|---|---|
| eca-miR-143 | miRNA | −1.3 |
| eca-miR-145 | miRNA | −1.8 |
| eca-miR-181b | miRNA | −1.8 |
| eca-miR-122 | miRNA | 2.3 |
| eca-miR-148a | miRNA | 1.3 |
| snora71 | snoRNA | −3.2 |
| snord113 | snoRNA | −2.4 |
| snora46 | snoRNA | 1.0 |
| snora77 | snoRNA | 2.0 |
| snora47 | snoRNA | 2.5 |
| snord29 | snoRNA | 2.5 |
| ECABCGRLG0000003960 | novel snoRNA | −2.6 |
| ECABCGRLG0000002980 | novel snoRNA | −2.5 |
| ECABCGRLG0000006050 | novel snoRNA | −1.6 |
| ECABCGRLG0000000640 | novel snoRNA | −1.1 |
| ECABCGRLG0000002800 | novel snoRNA | −1.0 |
| ECABCGRLG0000007680 | novel snoRNA | 1.8 |
| ECABCGRLG0000008070 | novel snoRNA | 2.0 |
| ECABCGRLG0000007010 | novel snoRNA | 2.5 |
| ECABCGRLG0000008090 | novel snoRNA | 2.9 |
| ECABCGRLG0000004470 | novel snoRNA | 3.0 |
| ECABCGRLG0000005680 | novel snoRNA | 3.1 |
| LOC111775808 | snRNA | −1.3 |
| LOC111773055 | snRNA | 2.2 |
| LOC111772636 | snRNA | 2.5 |
| LOC111770368 | lncRNA | −3.4 |
| LOC111768432 | lncRNA | −3.2 |
| LOC102148414 | lncRNA | −3.2 |
| LOC102149168 | lncRNA | −2.9 |
| LOC111772155 | lncRNA | −2.5 |
| LOC106783307 | lncRNA | −2.5 |
| LOC111775319 | lncRNA | −2.5 |
| LOC102148711 | lncRNA | −2.5 |
| LOC111775759 | lncRNA | −2.5 |
| LOC111774351 | lncRNA | −2.5 |
| LOC102150704 | lncRNA | −2.5 |
| LOC111775994 | lncRNA | −2.5 |
| LOC102150027 | lncRNA | −2.5 |
| LOC106781358 | lncRNA | −2.5 |
| LOC102147393 | lncRNA | −2.5 |
| LOC111776203 | lncRNA | −2.5 |
| LOC111771286 | lncRNA | −1.3 |
| LOC102149893 | lncRNA | 1.7 |
| LOC111775969 | lncRNA | 1.7 |
| LOC106781629 | lncRNA | 1.9 |
| LOC111773181 | lncRNA | 2.0 |
| LOC102149863 | lncRNA | 2.5 |
| LOC102149361 | lncRNA | 2.5 |
| LOC111770630 | lncRNA | 2.5 |
| LOC102147707 | lncRNA | 2.5 |
| LOC106783385 | lncRNA | 2.5 |
| LOC102149569 | lncRNA | 2.5 |
| LOC106782740 | lncRNA | 2.5 |
| LOC111770896 | lncRNA | 2.5 |
| LOC102150024 | lncRNA | 2.9 |
| LOC102150338 | lncRNA | 2.9 |
| TRNAR-ACG | tRNA | −3.4 |
| TRNAR-CCU | tRNA | −3.2 |
| TRNAS-AGA | tRNA | −3.2 |
| TRNAS-GCU | tRNA | −3.1 |
| TRNAA-UGC | tRNA | −3.0 |
| TRNAP-AGG | tRNA | −2.9 |
| TRNAQ-UUG | tRNA | −2.9 |
| TRNAS-AGA | tRNA | −2.9 |
| TRNAA-UGC | tRNA | −2.9 |
| TRNAF-GAA | tRNA | −2.5 |
| TRNAP-UGG | tRNA | −2.5 |
| TRNAE-UUC | tRNA | −2.5 |
| TRNAF-GAA | tRNA | −2.2 |
| TRNAV-AAC | tRNA | −2.2 |
| TRNAM-CAU | tRNA | −2.1 |
| TRNAT-AGU | tRNA | −1.9 |
| TRNAY-GUA | tRNA | −1.9 |
| TRNAQ-CUG | tRNA | −1.4 |
| TRNAN-GUU | tRNA | −1.4 |
| TRNAY-GUA | tRNA | −1.4 |
| TRNAY-GUA | tRNA | −1.2 |
| TRNAV-AAC | tRNA | 1.9 |
| TRNAP-CGG | tRNA | 2.2 |
| TRNAA-UGC | tRNA | 2.4 |
| TRNAC-GCA | tRNA | 2.5 |
| TRNAI-AAU | tRNA | 2.9 |
| TRNAD-GUC | tRNA | 3.4 |
3.3. Age Specific miRNA Interactome
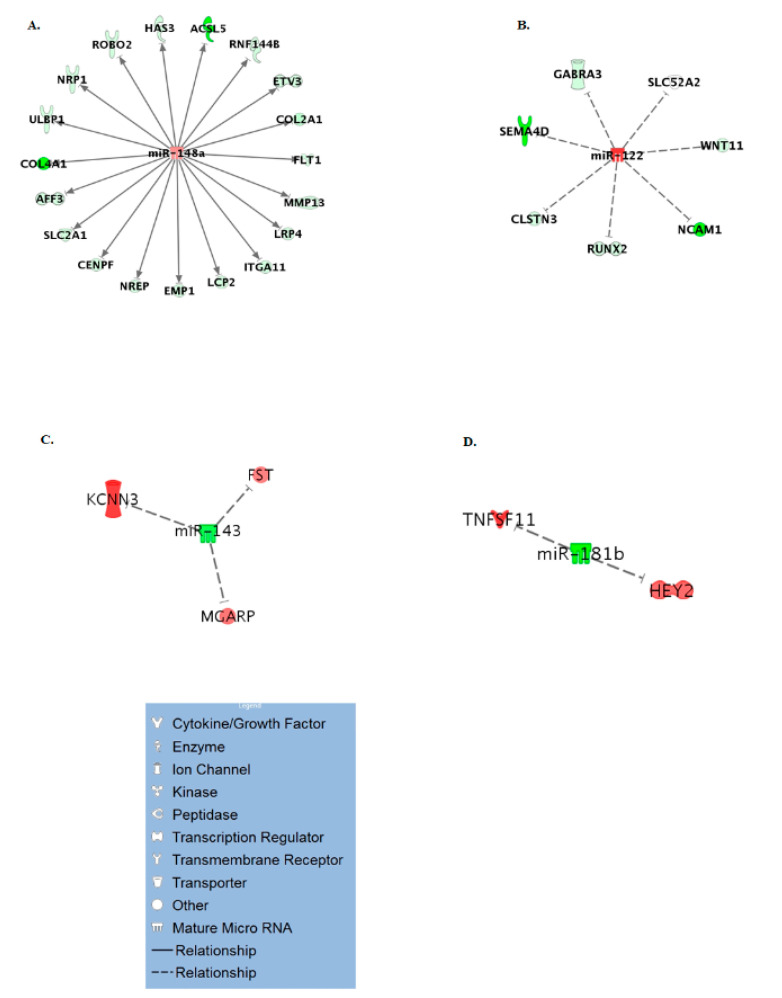
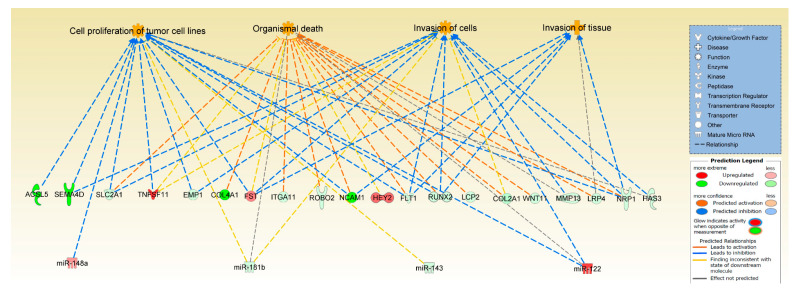
To generate an age-specific miRNA interactome of the most likely miRNA-mRNA target pairs, analysis was performed to identify miRNA targets of the five differentially expressed miRNAs from this study. In IPA, the five miRNAs (miR-143, miR-145, miR-181b, miR-122, miR-148a) were paired with 351 protein coding genes differentially expressed in our previous RNA-seq study on ageing equine cartilage [31]. In File S4, the miRNA-mRNA pairings in the correct direction (miR increase and mRNA decrease or vice versa) are shown, including the target predictions and /or experimental validation in the respective database. Four of the five miRNAs interacted in IPA with 31 different differentially expressed target genes reflecting that miRNAs target many mRNAs. These mRNAs targeted by the miRNAs were used in IPA as network-eligible molecules and overlaid onto molecular networks based on information from the IPA Database. Networks for the four miRNAs (miR-148a, miR-122, miR-143, and miR-181b) were generated based on connectivity (Figure 2A–D). Interesting age-related features were determined from the gene networks inferred. Among the top canonical pathways were hepatic fibrosis (p = 1.51 × 10−3), glycoprotein 6 (GP6) signalling (p = 5.67 × 10−4), and osteoarthritis pathway (p = 2.93 × 10−3). The top diseases and cellular functions associated with this network are shown in Table 2 and Figure 3. All IPA results are in File S5.
Figure 2.
miR-mRNA interactome for differentially expressed miRs in ageing. Significantly differentially expressed miRs were paired with differentially expressed mRNAs from our original cartilage study. (A) miR-148a, (B) miR-122, (C) miR-143, (D) miR-181b. The legend for individual molecules is shown. Genes in red are upregulated and green downregulated in old chondrocytes/cartilage compared to young, and depth of colour correlates to fold expression change.
Table 2.
IPA mRNA target diseases and functions.
| a. Top Molecular and Cellular Functions | p-Value Range | Number of Molecules |
|---|---|---|
| Cellular Movement | 1.41 × 10−3–4.23 × 10−10 | 21 |
| Cell Morphology | 1.37 × 10−3–3.52 × 10−8 | 16 |
| Cellular Development | 1.42 × 10−3–4.94 × 10−7 | 24 |
| Cell Death and Survival | 1.37 × 10−3–1.08 × 10−5 | 18 |
| Cell-To-Cell Signalling and Interaction | 1.37 × 10−3–1.88 × 10−5 | 20 |
| b. Top Diseases and Disorders | ||
| Skeletal and Muscular Disorders | 1.50 × 10−3–1.22 × 10-9 | 16 |
| Connective Tissue Disorders | 1.50 × 10−3–3.52 × 10-8 | 16 |
| Organismal Injury and Abnormalities | 1.50 × 10−3–3.52 × 10-8 | 31 |
| Cancer | 1.49 × 10−3–5.30 × 10-7 | 31 |
| Developmental Disorder | 1.37 × 10−3–5.30 × 10-7 | 17 |
Figure 3.
Significant pathways and networks affected in cartilage ageing. IPA was used to pair differentially expressed mRNA and miRNA data from ageing equine cartilage and chondrocytes. Figure is a graphical representation between molecules identified in our data in their respective networks. Red nodes; upregulated gene expression in old group; green nodes; downregulated gene expression in old group. Intensity of colour is related to higher fold-change. The key to the main features in the networks is shown.
3.4. Confirmation of Differential Gene Expression Using qRT-PCR
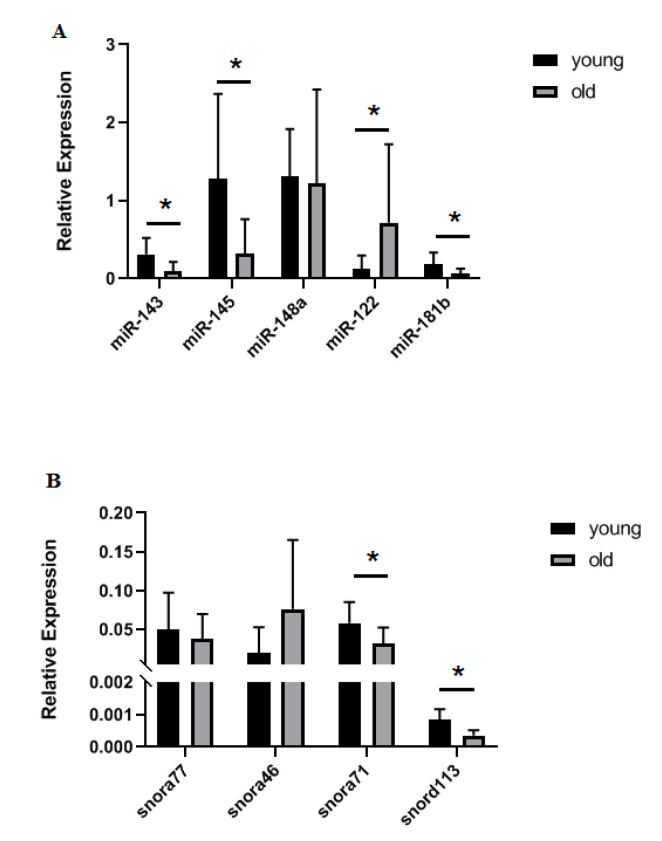
For selected snoRNAs (snora46, snora71, snora77, and snord113) and miRNAs (miR-143, miR-145, miR-148a, miR-122, and miR-181b) we used qRT-PCR to validate the small RNA-Seq findings in an extended cohort of chondrocytes. Findings for snora71, snord113, miR-143, miR-145, miR-122, and miR-181b were validated (Figure 4).
Figure 4.
Validation of selected sncRNAs in an extended cohort of equine young and old chondrocytes. Real-time quantitative Polymerase Chain Reaction (qRT-PCR) was used to validate findings from the small RNA sequencing (A) microRNAs and (B) snoRNAs, n = 5–12 per group. Statistical analysis was undertaken using a Mann Whitney test in GraphPad Prism. Mean and standard errors are shown with * denoting p < 0.05.
3.5. tRNA Fragment Changes in Equine Ageing
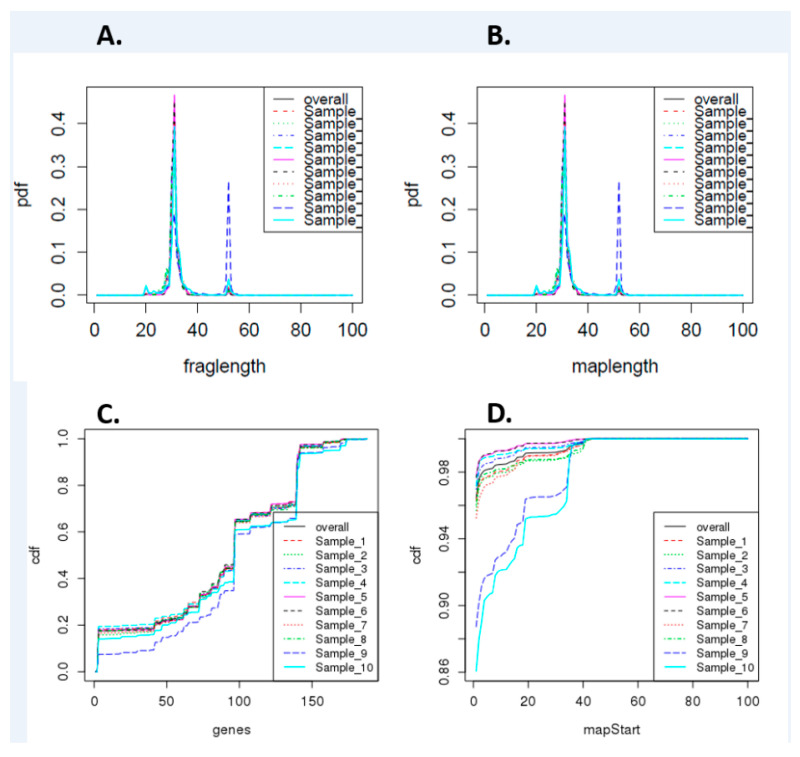
As we identified differentially expressed tRNAs in ageing equine chondrocytes, we undertook additional analysis to identify tiRNAs and tRFs, as increasing experimental evidence suggests their functional roles in OA [27]. Figure 5 shows cumulative density of tRNA fragment length, alignment length, gene counts, and map start position. On the assessment of data variation using PCA, samples in group “old” did not scatter very closely. Differential expression analysis of all young versus all old did not identify any differentially expressed tRFs (FDR < 0.05). Therefore, we reanalysed the data with the old group divided into two subgroups based on PCA findings: ‘old 1’ (samples 6, 7, 8) and ‘old 2’ (samples 9 and 10) for further differential expression analysis. There were 81 differentially expressed tiRNAs/tRFs; 44 higher in ‘old 2’ and 37 lower in ‘old 2’ compared to young (File S6).
Figure 5.
Summary of differentially expressed tRNA fragment data. (A) Cumulative density of tRNA fragment length, (B) alignment length, (C) gene counts, and (D) map start position. Samples 1–5 are derived from young donors and 6–10 are old donors.
3.6. Human tRNA and tRF Profiles Compared to Equine tRNA and tRF Profiles
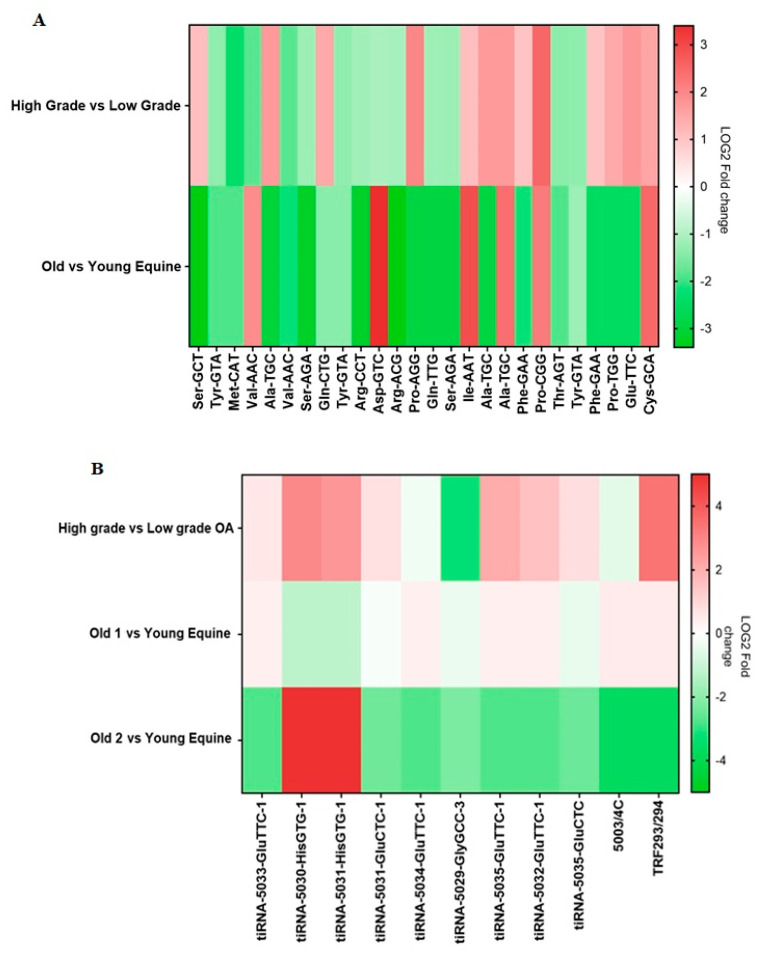
In both human and equine samples, 26 parent tRNAs were detected. Of these 26 tRNAs, 13 tRNAs were induced and 13 were reduced in cartilage from high grade OA compared to cartilage from low grade OA. In equine chondrocytes, 6 parent tRNAs were expressed higher and 20 parent tRNAs were expressed lower in old samples compared to young. Four parent tRNAs were induced in both high grade OA cartilage compared to low grade OA cartilage and in old versus young equine samples. Eleven parent tRNAs were reduced in high grade OA cartilage compared to low grade OA cartilage and in the old versus young equine samples (Figure 6A).
Figure 6.
tRNA analysis of human osteoarthritic cartilage. (A) High grade versus low grade OA human cartilage and old versus young equine chondrocyte tRNA profiles. Heatmap of Log2 fold change expression of 26 tRNAs detected in both human and equine samples. Human cartilage tRNAs detected using a Human tRNA PCR Array. Red highlights induced tRNA expression in high grade OA human cartilage compared to low grade and in old equine chondrocytes compared to young. Green highlights reduced tRNA expression in high grade OA human cartilage compared to low grade and in old equine chondrocytes compared to young. (B) High grade OA versus low grade OA human cartilage and equine tiRNA/tRF profiles. Heatmap of Log2 foldchange expression of 11 tRF/tiRNA fragments detected in both human and equine samples. Human cartilage tRF/tiRNA detected using a Human tRF&tiRNA PCR Array. Red highlights induced tRF/tiRNA expression in human high grade versus low grade OA samples and in old versus young equine samples. Green highlights reduced tRF/tiRNA expression in human high grade versus low grade OA samples and in old versus young equine samples.
In both human and equine samples, the tRF-5 fragment known as tRF293/294 and 10 tiRNAs were detected (Figure 6B). In high grade OA compared to low grade OA cartilage, seven tiRNAs and tRF293/294 were induced and three tiRNAs were reduced. In old 1 versus young equine, five tiRNAs and tRF293/294 were induced and five tiRNAs were reduced. In old 2 versus young equine, two tiRNAs were induced and eight tiRNAs and tRF293/294 were reduced. Of these tRNA fragments, three tiRNAs and tRF293/294 were induced and tiRNA-5029-GlyGCC-3 was reduced in high versus low grade OA cartilage and in old 1 versus young equine. Two tiRNAs were induced and three tiRNAs were reduced in high versus low grade OA cartilage and in old 2 versus young equine. In high versus low grade OA cartilage and old 1 versus young equine tiRNA-5029-GlyGCC was reduced.
4. Discussion
Our study investigated the changing sncRNA landscape in ageing chondrocytes. Several risk factors exist that influence cartilage health and chondrocyte homeostasis. Among them, ageing is one of the leading risk factors contributing to cartilage-related diseases, such as OA [52]. Many studies have shown that ageing can affect cartilage in different ways, both at cellular and molecular level. Increased chondrocyte death, apoptosis, and a shift towards a catabolic profile have been observed in aged chondrocytes [53]. Additional age-related changes in articular cartilage include increased chondrocyte senescence [54], oxidative stress [55], and changes in the composition and structure of ECM [56]. Although the underlying molecular causes of these changes are not completely understood, it is hypothesised that aged chondrocytes respond differently to various stimuli, such as growth factors, [53,57] and demonstrate altered molecular signatures [6].
SncRNAs are a subset of epigenetic modifiers and their role in cartilage ageing has been studied increasingly in the last decade [6,31,58]. In the current study, we have used small RNA-seq to identify alterations in sncRNAs between young and old equine chondrocytes. The horse is a good model to study musculoskeletal ageing and disease as we could assess the whole joint for pathological perturbations during tissue collection. It is very challenging to source aged human cartilage that has no OA changes, whereas this is easily undertaken in equine samples. Moreover, the horse has been used as a model of OA and there has been significant research on equine joint anatomy and pathophysiology [59,60].
Within our set of differentially expressed sncRNAs, miRNAs are the best studied in musculoskeletal ageing and cartilage. In old chondrocytes, we identified two miRNAs with higher expression; miR-122 and miR-148a, and three miRNAs with lower expression; miR-143, miR-145, and miR-181b. Of these five miRNAs, all except miR-148a were validated in an extended cohort of young and old equine chondrocytes with qRT-PCR. MiR-122 has been researched extensively in liver [61,62], but its role in musculoskeletal ageing is less clear. MiR-122 was decreased in the serum and plasma of patients with osteoporosis, the most common age-related bone disease [63], but was significantly upregulated in senescent human fibroblasts [64] and was shown to upregulate p53 which is induced in senescence [65]. MiR-143 was downregulated in muscle satellite cells from old mice and primary myoblasts from old humans and mice [66]. In addition, circulating miR-143 was upregulated in young individuals following resistant exercise, but was downregulated in older individuals after resistant exercise [67]. MiR-145 was downregulated in old OA patients [68] as well as in experimental OA rat chondrocytes treated with tumour necrosis factor (TNF) [69] and, finally, miR-181b was downregulated in skeletal muscle of old rhesus monkeys [70].
To further investigate the potential role of the differentially expressed miRNAs identified in this study, we used IPA to combine them with the differentially expressed mRNAs from our previous equine cartilage study [31]. IPA miRNA ‘Target Filter and Expression Pairing’ identified 31 potential target genes. IPA core analysis of these genes revealed canonical pathways associated with cartilage physiology, such as role of chondrocytes in rheumatoid arthritis, OA-related pathways and bone morphogenic protein (BMP) signalling, all of which have been reported to change with ageing [56,71,72]. Moreover, top diseases and disorders linked to these genes, as identified by IPA, included skeletal and muscular disorders and connective tissue disorders. Of note, follistatin (FST) which was upregulated in old equine cartilage and was predicted by IPA as a target of the downregulated miR-143, was overexpressed in human OA chondrocytes [73] and canine OA cartilage [74] and was induced by telomere shortening [75], a process associated with ageing. Moreover, TNF ligand superfamily member 11 (TNFSF11), also known as receptor activator of nuclear factor kappa-Β ligand (RANKL) was upregulated in old equine cartilage and was identified as a predicted target of the downregulated miR-181b. Higher expression of TNFSF11/RANKL, which correlated with bone loss, was reported in old C57BL/6 mice [76], rabbits with chronic antigen-induced arthritis [77], and in human high grade OA cartilage [78]. These results demonstrate the adverse effect of ageing on miRNA levels and their potential use as biomarkers or therapeutic targets for age-related musculoskeletal diseases.
Six snoRNAs were identified as differentially expressed due to ageing in chondrocytes. This conserved class of non-coding RNAs are principally characterised as guiding site-specific post-transcriptional modifications in ribosomal RNA [79] (canonical snoRNAs), but can also modify additional classes of RNAs including other snoRNAs, tRNAs and mRNAs; so called non-canonical snoRNAs [80,81]. Examples of age-related snoRNAs in equine ageing chondrocytes with canonical functions include snora71 and snord29. Novel non-canonical functions reported for snoRNAs including the modulation of alternative splicing [82], an essential involvement in stress response pathways [83] and the modulation of mRNA 3′ end processing [84]. Similar to miRNAs, snoRNAs are emerging as important regulators of cellular function and disease development [15], related to their ability to fine-tune ribosomes accommodating changing requirements for protein production during development, normal function, and disease [85]. We have previously identified a role for snoRNAs in cartilage ageing and OA [16] and their potential use as biomarkers for OA [9]. Interestingly there was a reduction in snord113/114 in ageing chondrocytes which agrees with our previous findings in equine cartilage ageing [31]. We have also previously demonstrated that SNORD113 was reduced in ageing human knee cartilage but increased in OA [16] and increased in human anterior cruciate ligament [17]. SNORD113/114 are located in imprinted loci and may play a role in the evolution and/or mechanism of epigenetic imprinting. Although belonging to the C/D box class of snoRNAs which direct site-specific 2′-O-ribose methylation of substrate RNAs, they differ from other C/D box snoRNAs in their tissue specific expression profiles (including fibroblast, chondrocytes and osteoblasts) and the lack of known substrate RNA complementarity. This currently classifies them as orphan snoRNAs as they are not predicted to guide the 2′-O-ribose methylation but have novel, unknown roles [86]. Additionally, SNORD113 functions as a tumour suppressor in hepatic cell carcinoma by reducing cell growth, and it inactivates the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 and mothers against decapentaplegic homolog (SMAD) 2/3 in mitogen-activated protein kinase (MAPK)/ERK and transforming growth factor beta (TGF-β) pathways [87]. Together, our snoRNA findings indicate that age-related changes in chondrocyte snoRNAs could have important implications through both canonical and non-canonical snoRNA routes.
This is the first study to detect tRNA and tRNA fragments in equine chondrocytes and to compare these findings with tRNA and tRNA fragments detected in human OA cartilage. For our tRNA data, old donors clustered into two groups and further analysis was undertaken with these subgroups. There were no apparent differences in these subgroups with regards to age or sex and scores were all zero. The parent tRNA Cys-GCA was found to be increased in both aged equine chondrocytes and high grade OA human cartilage samples. tRNA Cys-GCA levels have previously been reported to be increased in human chondrocytes induced with the cytokine interleukin (IL) 1 beta resulting in the production of the tRNA fragment tRF-3003a, a type 3-tRF produced by the cleavage of Cys-GCA. tRF-3003a has been shown to post-transcriptionally regulate the Janus Kinase 3 (JAK3) expression through sequence complementarity via the Argonaute (AGO) / RNA-induced silencing complex (RISC) in human chondrocytes [27].
The Janus Kinase and Signal Transducer and Activator of Transcription (JAK-STAT) pathway is the target of several cytokines such as interferon-γ, IL-2, IL-4, IL-6, IL-7, IL-10, IL-12, and IL-15. Many of these cytokines are known to play important roles in synovial inflammation during OA pathogenesis [88,89].
The tRNA fragments detected in equine samples that matched with human samples consisted of 5′ tiRNA and tRF-5 fragments. Many of the equine fragments detected did not fall into the classical tRF-3, tRF-5, or tiRNA size ranges and instead may likely be i-tRFs, which are internal to the respective tRNA and can straddle the anticodon loop. 5′ tiRNA, 3′ tiRNA, tRF-3, and tRF-5 fragments were detected in the low-grade OA cartilage and in the high grade OA cartilage. In our human studies, base modifications found on tRNAs and tRF/tiRNA fragments that would normally block reverse transcription were removed and this may account for some of the differences found between the equine and human tRNA/tRF profiles.
The importance of modulation of tRNA levels and tRNA fragments in articular cartilage homeostasis remains an unexplored area. This is the first evidence that aged equine samples have changes in the expression of specific tRNAs and tRFs when compared to young equine samples. We report for the first time several 5′ tiRNAs, such as tiRNA Glu-TTC and tiRNA His-GTG, were induced in old compared to young equine chondrocytes and in high grade compared to low grade human OA cartilage. Previous reports have shown that 5′ tiRNAs can be produced by cell stress in mammalian cells and these 5′ tiRNA half fragments may have a role in inhibiting cell translation and could be involved in stress granule formation [90]. Further studies are required to find the mechanism by which these fragments are produced and whether the changes in the profile of fragments found in old compared to young equine chondrocytes or high compared to low grade OA cartilage potentially contribute to the development of OA.
We are aware our study has a number of limitations. The effect of ageing between young and old equine chondrocytes was small on the differential expression of sncRNAs. However, it is likely that we are therefore interrogating highly specific changes that are age dependent. Furthermore, we cannot rule out changes related to the use of chondrocytes instead of cartilage tissue. Even though chondrocytes of low passage were used, collagenase digestion and plating of cells could have affected their phenotype and gene expression. The choice of chondrocytes over cartilage tissue was made based on RNA from cartilage tissue is of low quality (in our hands) and heavily contaminated with proteoglycans [91], usually making it challenging for sequencing. In our previous study interrogating snoRNAs in human knee cartilage ageing and OA [16] we utilized cartilage tissue as opposed to chondrocytes. The old non-OA cartilage was derived from the grossly normal condyle of OA joints. In the study we found a number of age and OA specific snoRNAs, but we could not define the cartilage as truly normal as it was derived from an OA joint. Nevertheless, we identified two age related, no species specific snoRNAs that were DE in both our studies, snord113 and snord29. The use of the Illumina MiSeq platform would have contributed to the low number of differentially expressed sncRNAs as it offers less depth coverage compared to other platforms, such as the HiSeq platform. Finally, we have used a relatively small number of samples per group. Given the use of primary cells and the degree of variability observed, especially for the old group, the inclusion of five samples pre group may have contributed to the small number of differentially expressed sncRNAs in ageing equine chondrocytes on our study.
5. Conclusions
For the first time we have described, using unbiased methods, the effect of ageing on the expression of sncRNAs in equine chondrocytes. We detected variable classes of sncRNAs (the small non-coding RNAome) in young and old chondrocytes, which are differentially abundant, indicating that there are multiple levels of epigenetic control in cartilage and chondrocyte ageing. Among them, there were miRNAs, which are predicted to play a role in the development of the musculoskeletal system and in skeletal disorders. In addition, the current study is one of the few studies that have investigated tRNAs and tRNA fragments, in an attempt to uncover novel molecular signatures in aged and diseased chondrocytes/cartilage that could be useful in the future as therapeutic targets. Further research is needed to elucidate the role and function of these molecules and their potential link to disease.
Acknowledgments
We would like to thank Catarina Castanheira for her assistance with the laboratory work.
Abbreviations
| ECM | extracellular matrix |
| OA | osteoarthritis |
| sncRNAs | small non-coding RNAs |
| miRNAs or miRs | microRNAs |
| snRNAs | small nuclear RNAs |
| snoRNAs | small nucleolar RNAs |
| tRNAs | transfer RNAs |
| mRNA | messenger RNA |
| tiRNAs | tRNA halves |
| ANG | angiogenin |
| tRFs | tRNA-derived small RNA fragments |
| PCA | principle component analysis |
| logFC | log2 fold change |
| FDR | False Discovery Rate |
| IPA | Ingenuity Pathway Analysis |
| RNA-seq | RNA sequencing |
| qRT-PCR | real-time quantitative Polymerase Chain Reaction |
| lncRNAs | long non-coding RNAs |
| PCs | Principle components |
| GP6 | glycoprotein 6 |
| BMP | bone morphogenic protein |
| FST | follistatin |
| TNF | tumour necrosis factor |
| TNFSF11 | TNF ligand superfamily member 11 |
| RANKL | receptor activator of nuclear factor kappa-Β ligand |
| ERK | extracellular signal-regulated kinase |
| SMAD | mothers against decapentaplegic homolog |
| MAPK | mitogen-activated protein kinase |
| TGF-β | transforming growth factor beta |
| IL | interleukin |
| JAK3 | Janus Kinase 3 |
| AGO | Argonaute |
| RISC | RNA-induced silencing complex |
| JAK-STAT | Janus Kinase and Signal Transducer and Activator of Transcription |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/16/5675/s1. File S1. .xls, Small RNA assay details; File S2. .xls, MiSeq read mapping details; File S3. .xls, Types of small non-coding RNAs identified in MiSeq data; File S4. .xls, miRNA-mRNA pairings in the correct direction; File S5. .xls, IPA analysis summary; File S6. .xls, differentially expressed tRNA halves/tRFs
Author Contributions
M.J.P., P.B., T.J.M.W., J.A.G. and T.M.H. conceived the study and designed the work; P.B., P.D., J.A.G., M.J.P., Y.A.K. acquired and analysed the data, Y.F., X.L., interpreted the data; all authors have drafted the work. All authors have approved the submitted version. All authors have agreed both to be personally accountable for the author’s own contributions and to ensure that questions related to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and the resolution documented in the literature. All authors have read and agreed to the published version of the manuscript.
Funding
Mandy Peffers is funded through a Wellcome Trust Intermediate Clinical Fellowship (107471/Z/15/Z). Panagiotis Balaskas is funded through an MRC-DTP studentship supported by the Medical Research Council (MRC). Both are supported through Versus Arthritis as part of the MRC Versus Arthritis Centre for Integrated research into Musculoskeletal Ageing (CIMA). This work was also supported in part by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) and the National Centre for Complementary and Integrative Health (NCCIH) of the National Institutes of Health (NIH) under award numbers R01-AR067056, and R01-AT007373 respectively and funds from NEOMED to TMH.
Conflicts of Interest
T.J.M Welting is listed as inventors on patents WO2017178251 and WO2017178253. T.J.M Welting has shares in Chondropeptix. The remaining authors declare that they have no competing interests.
References
- 1.Messina O.D., Wilman M.V., Neira L.F.V. Nutrition, osteoarthritis and cartilage metabolism. Aging Clin. Exp. Res. 2019;31:807–813. doi: 10.1007/s40520-019-01191-w. [DOI] [PubMed] [Google Scholar]
- 2.Decker R.S. Articular cartilage and joint development from embryogenesis to adulthood. Semin. Cell Dev. Biol. 2016;62:50–56. doi: 10.1016/j.semcdb.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sacitharan P.K., Vincent T.L. Cellular ageing mechanisms in osteoarthritis. Mamm. Genome. 2016;27:421–429. doi: 10.1007/s00335-016-9641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philipot D., Guérit D., Platano D., Chuchana P., Olivotto E., Espinoza F., Dorandeu A., Pers Y.M., Piette J., Borzì R., et al. p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis. Arthritis Res. Ther. 2014;16:R58. doi: 10.1186/ar4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verzijl N., DeGroot J., Ben Zaken C., Braun-Benjamin O., Maroudas A., Bank R.A., Mizrahi J., Schalkwijk C.G., Thorpe S.R., Baynes J.W., et al. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002;46:114–123. doi: 10.1002/1529-0131(200201)46:1<114::AID-ART10025>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 6.Zhang M., Theleman J.L., Lygrisse K.A., Wang J. Epigenetic Mechanisms Underlying the Aging of Articular Cartilage and Osteoarthritis. Gerontology. 2019;65:387–396. doi: 10.1159/000496688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greene M.A., Loeser R.F. Aging-related inflammation in osteoarthritis. Osteoarthr. Cartil. 2015;23:1966–1971. doi: 10.1016/j.joca.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sondag G.R., Haqqi T.M. The Role of MicroRNAs and Their Targets in Osteoarthritis. Curr. Rheumatol. Rep. 2016;18:56. doi: 10.1007/s11926-016-0604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steinbusch M.M.F., Fang Y., Milner P.I., Clegg P.D., Young D.A., Welting T.J.M., Peffers M.J. Serum snoRNAs as biomarkers for joint ageing and post traumatic osteoarthritis. Sci. Rep. 2017;7:43558. doi: 10.1038/srep43558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L., Yang M., Marks P., White L., Hurtig M., Mi Q.-S., Divine G., Gibson G. Serum non-coding RNAs as biomarkers for osteoarthritis progression after ACL injury. Osteoarthr. Cartil. 2012;20:1631–1637. doi: 10.1016/j.joca.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kamaruzaman H., Kinghorn P., Oppong R. Cost-effectiveness of surgical interventions for the management of osteoarthritis: A systematic review of the literature. BMC Musculoskelet. Disord. 2017;18:183. doi: 10.1186/s12891-017-1540-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyaki S., Sato T., Inoue A., Otsuki S., Ito Y., Yokoyama S., Kato Y., Takemoto F., Nakasa T., Yamashita S., et al. MicroRNA-140 plays dual roles in both cartilage development and homeostasis. Genes Dev. 2010;24:1173–1185. doi: 10.1101/gad.1915510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peffers M., Caron M., Cremers A., Surtel D., Fang Y., Dyer P., Balaskas P., Welting T. snoRNA signatures in cartilage ageing and osteoarthritis. Osteoarthr. Cartil. 2018;26:S164. doi: 10.1016/j.joca.2018.02.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brameier M., Herwig A., Reinhardt R., Walter L., Gruber J. Human box C/D snoRNAs with miRNA like functions: Expanding the range of regulatory RNAs. Nucleic Acids Res. 2011;39:675–686. doi: 10.1093/nar/gkq776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stepanov G.A., Filippova J.A., Komissarov A.B., Kuligina E.V., Richter V.A., Semenov D.V. Regulatory Role of Small Nucleolar RNAs in Human Diseases. BioMed Res. Int. 2015;2015:1–10. doi: 10.1155/2015/206849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peffers M.J., Chabronova A., Balaskas P., Fang Y., Dyer P., Cremers A., Emans P.J., Feczko P.Z., Caron M.M., Welting T.J.M. SnoRNA signatures in cartilage ageing and osteoarthritis. Sci. Rep. 2020;10:1–10. doi: 10.1038/s41598-020-67446-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharaz Y.A., Fang Y., Welting T., Peffers M., Comerford E. Small RNA signatures of the anterior cruciate ligament from patients with knee 1 joint osteoarthritis. medRxiv. 2020 doi: 10.1101/2020.05.14.20101048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raina M., Ibba M. tRNAs as regulators of biological processes. Front. Genet. 2014;5:171. doi: 10.3389/fgene.2014.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamasaki S., Ivanov P., Hu G.-F., Anderson P. Angiogenin cleaves tRNA and promotes stress-induced translational repression. J. Cell Biol. 2009;185:35–42. doi: 10.1083/jcb.200811106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee Y.S., Shibata Y., Malhotra A., Dutta A. A novel class of small RNAs: tRNA-derived RNA fragments (tRFs) Genes Dev. 2009;23:2639–2649. doi: 10.1101/gad.1837609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takaku H., Minagawa A., Takagi M., Nashimoto M. A candidate prostate cancer susceptibility gene encodes tRNA 3′ processing endoribonuclease. Nucleic Acids Res. 2003;31:2272–2278. doi: 10.1093/nar/gkg337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodarzi H., Liu X., Nguyen H.C., Zhang S., Fish L., Tavazoie S.F. Endogenous tRNA-Derived Fragments Suppress Breast Cancer Progression via YBX1 Displacement. Cell. 2015;161:790–802. doi: 10.1016/j.cell.2015.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cole C., Sobala A., Lu C., Thatcher S.R., Bowman A., Brown J.W., Green P.J., Barton G.J., Hutvagner G. Filtering of deep sequencing data reveals the existence of abundant Dicer-dependent small RNAs derived from tRNAs. RNA. 2009;15:2147–2160. doi: 10.1261/rna.1738409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Z., Ender C., Meister G., Moore P.S., Chang Y., John B. Extensive terminal and asymmetric processing of small RNAs from rRNAs, snoRNAs, snRNAs, and tRNAs. Nucleic Acids Res. 2012;40:6787–6799. doi: 10.1093/nar/gks307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telonis A.G., Loher P., Honda S., Jing Y., Palazzo J., Kirino Y., Rigoutsos I. Dissecting tRNA-derived fragment complexities using personalized transcriptomes reveals novel fragment classes and unexpected dependencies. Oncotarget. 2015;6:24797–24822. doi: 10.18632/oncotarget.4695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang P., Yan F. tiRNAs & tRFs Biogenesis and Regulation of Diseases: A Review. Curr. Med. Chem. 2019;26:5849–5861. doi: 10.2174/0929867326666190124123831. [DOI] [PubMed] [Google Scholar]
- 27.Green J., Ansari M., Ball H., Haqqi T.M. tRNA-derived fragments (tRFs) regulate post-transcriptional gene expression via AGO-dependent mechanism in IL-1β stimulated chondrocytes. Osteoarthr. Cartil. 2020;28:1102–1110. doi: 10.1016/j.joca.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ormseth M.J., Solus J.F., Sheng Q., Ye F., Song H., Wu Q., Guo Y., Oeser A.M., Allen R.M., Vickers K.C., et al. The Endogenous Plasma Small RNAome of Rheumatoid Arthritis. ACR Open Rheumatol. 2020;2:97–105. doi: 10.1002/acr2.11098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawcak C., Frisbie D., Werpy N., Park R., McIlwraith C. Effects of exercise vs experimental osteoarthritis on imaging outcomes. Osteoarthr. Cartil. 2008;16:1519–1525. doi: 10.1016/j.joca.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Peffers M.J., Milner P., Tew S., Clegg P.D. Regulation of SOX9 in normal and osteoarthritic equine articular chondrocytes by hyperosmotic loading. Osteoarthr. Cartil. 2010;18:1502–1508. doi: 10.1016/j.joca.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peffers M., Liu X., Clegg P. Transcriptomic signatures in cartilage ageing. Arthritis Res. Ther. 2013;15:R98. doi: 10.1186/ar4278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.User Guide. [(accessed on 5 August 2020)];2011 Available online: https://emea.support.illumina.com/sequencing/sequencing_software/casava2/questions.html.
- 33.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 2011;17:10. doi: 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 34.Kim D., Pertea G., Trapnell C., Pimentel H., Kelley R., Salzberg S. TopHat2: Accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 2013;14:R36. doi: 10.1186/gb-2013-14-4-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:002832. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benjamini Y., Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. doi: 10.1111/j.2517-6161.1995.tb02031.x. [DOI] [Google Scholar]
- 37.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J., Kobert K., Flouri T., Stamatakis A. PEAR: A fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2014;30:614–620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson N., Yeoh J.M., Coruh C., Axtell M.J. Improved Placement of Multi-mapping Small RNAs. Genes Genomes Genetics. 2016;6:2103–2111. doi: 10.1534/g3.116.030452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oliveira J.V.D.A., Costa F., Backofen R., Stadler P.F., Walter M.E.M.T., Hertel J. SnoReport 2.0: New features and a refined Support Vector Machine to improve snoRNA identification. BMC Bioinform. 2016;17:464–486. doi: 10.1186/s12859-016-1345-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pang Z., Chong J., Li S., Xia J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites. 2020;10:186. doi: 10.3390/metabo10050186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xia J., Wishart D.S. Using MetaboAnalyst 3.0 for Comprehensive Metabolomics Data Analysis. Curr. Protoc. Bioinform. 2016;55:14.10.1–14.10.91. doi: 10.1002/cpbi.11. [DOI] [PubMed] [Google Scholar]
- 44.Kumar P., Anaya J., Mudunuri S.B., Dutta A. Meta-analysis of tRNA derived RNA fragments reveals that they are evolutionarily conserved and associate with AGO proteins to recognize specific RNA targets. BMC Biol. 2014;12:78.:78. doi: 10.1186/s12915-014-0078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kumar P., Mudunuri S.B., Anaya J., Dutta A. tRFdb: A database for transfer RNA fragments. Nucleic Acids Res. 2014;43:D141–D145. doi: 10.1093/nar/gku1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olvedy M., Scaravilli M., Hoogstrate Y., Visakorpi T., Jenster G., Martens-Uzunova E. A comprehensive repertoire of tRNA-derived fragments in prostate cancer. Oncotarget. 2016;7:24766–24777. doi: 10.18632/oncotarget.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selitsky S.R., Baran-Gale J., Honda M., Yamane D., Masaki T., Fannin E.E., Guerra B., Shirasaki T., Shimakami T., Kaneko S., et al. Small tRNA-derived RNAs are increased and more abundant than microRNAs in chronic hepatitis B and C. Sci. Rep. 2015;5:7675. doi: 10.1038/srep07675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang Q., Lee I., Ren J., Ajay S.S., Lee Y.S., Bao X. Identification and Functional Characterization of tRNA-derived RNA Fragments (tRFs) in Respiratory Syncytial Virus Infection. Mol. Ther. 2013;21:368–379. doi: 10.1038/mt.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chan P.P., Lowe T.M. GtRNAdb: A database of transfer RNA genes detected in genomic sequence. Nucleic Acids Res. 2009;37:D93–D97. doi: 10.1093/nar/gkn787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chan P.P., Lowe T.M. GtRNAdb 2.0: An expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 2016;44:D184–D189. doi: 10.1093/nar/gkv1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jühling F., Mörl M., Hartmann R.K., Sprinzl M., Stadler P.F., Pütz J. tRNAdb 2009: Compilation of tRNA sequences and tRNA genes. Nucleic Acids Res. 2009;37:D159–D162. doi: 10.1093/nar/gkn772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lotz M.K., Loeser R.F. Effects of aging on articular cartilage homeostasis. Bone. 2012;51:241–248. doi: 10.1016/j.bone.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y., Wei X., Zhou J., Wei L. The Age-Related Changes in Cartilage and Osteoarthritis. BioMed Res. Int. 2013;2013:1–12. doi: 10.1155/2013/916530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Martin J.A., Buckwalter J.A. Telomere erosion and senescence in human articular cartilage chondrocytes. J. Gerontol. Ser. A Biol. Sci. Med Sci. 2001;56:B172–B179. doi: 10.1093/gerona/56.4.B172. [DOI] [PubMed] [Google Scholar]
- 55.Jallali N., Ridha H., Thrasivoulou C., Underwood C., Butler P.E., Cowen T. Vulnerability to ROS-induced cell death in ageing articular cartilage: The role of antioxidant enzyme activity. Osteoarthr. Cartil. 2005;13:614–622. doi: 10.1016/j.joca.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 56.Rahmati M., Nalesso G., Mobasheri A., Mozafari M. Aging and osteoarthritis: Central role of the extracellular matrix. Ageing Res. Rev. 2017;40:20–30. doi: 10.1016/j.arr.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 57.Hodgson D., Rowan A.D., Falciani F., Proctor C.J. Systems biology reveals how altered TGFbeta signalling with age reduces protection against pro-inflammatory stimuli. PLoS Comput. Biol. 2019;15:e1006685. doi: 10.1371/journal.pcbi.1006685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Weilner S., Grillari-Voglauer R., Redl H., Grillari J., Nau T. The role of microRNAs in cellular senescence and age-related conditions of cartilage and bone. Acta Orthop. 2015;86:92–99. doi: 10.3109/17453674.2014.957079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goodrich L.R., Nixon A.J. Medical treatment of osteoarthritis in the horse—A review. Veter. J. 2006;171:51–69. doi: 10.1016/j.tvjl.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 60.McIlwraith C., Frisbie D.D., Kawcak C.E. The horse as a model of naturally occurring osteoarthritis. Bone Jt. Res. 2012;1:297–309. doi: 10.1302/2046-3758.111.2000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu Y., Li P., Liu L., Zhang Y. The diagnostic role of miR-122 in drug-induced liver injury. Medicine. 2018;97:e13478. doi: 10.1097/MD.0000000000013478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thakral S., Ghoshal K. miR-122 is a unique molecule with great potential in diagnosis, prognosis of liver disease, and therapy both as miRNA mimic and antimir. Curr. Gene Ther. 2015;15:142–150. doi: 10.2174/1566523214666141224095610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mandourah A.Y., Ranganath L., Barraclough R., Vinjamuri S., Hof R.V., Hamill S., Czanner G., Dera A.A., Wang D., Barraclough D.L. Circulating microRNAs as potential diagnostic biomarkers for osteoporosis. Sci. Rep. 2018;8:8421. doi: 10.1038/s41598-018-26525-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Markopoulos G.S., Roupakia E., Tokamani M., Vartholomatos G., Tzavaras T., Hatziapostolou M., Fackelmayer F.O., Sandaltzopoulos R., Polytarchou C., Kolettas E. Senescence-associated microRNAs target cell cycle regulatory genes in normal human lung fibroblasts. Exp. Gerontol. 2017;96:110–122. doi: 10.1016/j.exger.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 65.Burns D.M., D’Ambrogio A., Nottrott S., Richter J.D. CPEB and two poly(A) polymerases control miR-122 stability and p53 mRNA translation. Nature. 2011;473:105–108. doi: 10.1038/nature09908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Soriano-Arroquia A., McCormick R., Molloy A.P., McArdle A., Goljanek-Whysall K. Age-related changes in miR-143-3p:Igfbp5 interactions affect muscle regeneration. Aging Cell. 2016;15:361–369. doi: 10.1111/acel.12442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Margolis L., Lessard S.J., Ezzyat Y., Fielding R.A., Rivas D. Circulating MicroRNA Are Predictive of Aging and Acute Adaptive Response to Resistance Exercise in Men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017;72:1319–1326. doi: 10.1093/gerona/glw243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu C., Ren S., Zhao S. Wang. LncRNA MALAT1/MiR-145 Adjusts IL-1beta-Induced Chondrocytes Viability and Cartilage Matrix Degradation by Regulating ADAMTS5 in Human Osteoarthritis. Yonsei Med. J. 2019;60:1081–1092. doi: 10.3349/ymj.2019.60.11.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hu G., Zhao X., Wang C., Geng Y., Zhao J., Xu J., Zuo B., Zhao C., Wang C., Zhang X.-L. MicroRNA-145 attenuates TNF-α-driven cartilage matrix degradation in osteoarthritis via direct suppression of MKK4. Cell Death Dis. 2017;8:e3140. doi: 10.1038/cddis.2017.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mercken E.M., Majounie E., Ding J., Guo R., Kim J., Bernier M., Mattison J., Cookson M.R., Gorospe M., Decabo R., et al. Age-associated miRNA Alterations in Skeletal Muscle from Rhesus Monkeys reversed by caloric restriction. Aging. 2013;5:692–703. doi: 10.18632/aging.100598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Collins J.A., Diekman B.O., Loeser R. Targeting aging for disease modification in osteoarthritis. Curr. Opin. Rheumatol. 2018;30:101–107. doi: 10.1097/BOR.0000000000000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thielen N.G.M., van der Kraan P.M., van Caam A.P.M. TGFbeta/BMP Signaling Pathway in Cartilage Homeostasis. Cells. 2019;8:969. doi: 10.3390/cells8090969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tardif G., Hum D., Pelletier J.-P., Boileau C., Ranger P., Martel-Pelletier J. Differential gene expression and regulation of the bone morphogenetic protein antagonists follistatin and gremlin in normal and osteoarthritic human chondrocytes and synovial fibroblasts. Arthritis Rheum. 2004;50:2521–2530. doi: 10.1002/art.20441. [DOI] [PubMed] [Google Scholar]
- 74.Tardif G., Pelletier J.-P., Boileau C., Martel-Pelletier J. The BMP antagonists follistatin and gremlin in normal and early osteoarthritic cartilage: An immunohistochemical study. Osteoarthr. Cartil. 2009;17:263–270. doi: 10.1016/j.joca.2008.06.022. [DOI] [PubMed] [Google Scholar]
- 75.Liu N., Yin Y., Wang H., Zhou Z., Sheng X., Fu H., Guo R., Wang H., Yang J., Gong P., et al. Telomere dysfunction impairs epidermal stem cell specification and differentiation by disrupting BMP/pSmad/P63 signaling. PLoS Genet. 2019;15:e1008368. doi: 10.1371/journal.pgen.1008368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cao J., Venton L., Sakata T., Halloran B.P. Expression of RANKL and OPG Correlates With Age-Related Bone Loss in Male C57BL/6 Mice. J. Bone Miner. Res. 2003;18:270–277. doi: 10.1359/jbmr.2003.18.2.270. [DOI] [PubMed] [Google Scholar]
- 77.Martínez-Calatrava M.J., Prieto-Potín I., Roman-Blas J.A., Tardío L., Largo R., Herrero-Beaumont G. RANKL synthesized by articular chondrocytes contributes to juxta-articular bone loss in chronic arthritis. Arthritis Res. Ther. 2012;14:R149. doi: 10.1186/ar3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Upton A.R., Holding C.A., Dharmapatni A.A.S.S.K., Haynes D. The expression of RANKL and OPG in the various grades of osteoarthritic cartilage. Rheumatol. Int. 2012;32:535–540. doi: 10.1007/s00296-010-1733-6. [DOI] [PubMed] [Google Scholar]
- 79.Dieci G., Preti M., Montanini B. Eukaryotic snoRNAs: A paradigm for gene expression flexibility. Genomics. 2009;94:83–88. doi: 10.1016/j.ygeno.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 80.Dupuis-Sandoval F., Poirier M., Scott M.S. The emerging landscape of small nucleolar RNAs in cell biology. Wiley Interdiscip. Rev. RNA. 2015;6:381–397. doi: 10.1002/wrna.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kishore S., Gruber A.R., Jedlinski D.J., Syed A.P., Jorjani H., Zavolan M. Insights into snoRNA biogenesis and processing from PAR-CLIP of snoRNA core proteins and small RNA sequencing. Genome Biol. 2013;14:R45. doi: 10.1186/gb-2013-14-5-r45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khanna A., Stamm S. Regulation of alternative splicing by short non-coding nuclear RNAs. RNA Biol. 2010;7:480–485. doi: 10.4161/rna.7.4.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Michel C.I., Holley C.L., Scruggs B.S., Sidhu R., Brookheart R.T., Listenberger L.L., A Behlke M., Ory D.S., Schaffer J.E. Small Nucleolar RNAs U32a, U33, and U35a Are Critical Mediators of Metabolic Stress. Cell Metab. 2011;14:33–44. doi: 10.1016/j.cmet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Huang C., Shi J., Guo Y., Huang W., Huang S., Ming S., Wu X., Zhang R., Ding J., Zhao W., et al. A snoRNA modulates mRNA 3′ end processing and regulates the expression of a subset of mRNAs. Nucleic Acids Res. 2017;45:8647–8660. doi: 10.1093/nar/gkx651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Montanaro L., Treré D., Derenzini M. Nucleolus, Ribosomes, and Cancer. Am. J. Pathol. 2008;173:301–310. doi: 10.2353/ajpath.2008.070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jorjani H., Kehr S., Jedlinski D.J., Gumienny R., Hertel J., Stadler P.F., Zavolan M., Gruber A.R. An updated human snoRNAome. Nucleic Acids Res. 2016;44:5068–5082. doi: 10.1093/nar/gkw386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xu G., Yang F., Ding C.-L., Zhao L.-J., Ren H., Zhao P., Wang W., Qi Z. Small nucleolar RNA 113–1 suppresses tumorigenesis in hepatocellular carcinoma. Mol. Cancer. 2014;13:216. doi: 10.1186/1476-4598-13-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldring M.B. The role of cytokines as inflammatory mediators in osteoarthritis: Lessons from animal models. Connect. Tissue Res. 1999;40:1–11. doi: 10.3109/03008209909005273. [DOI] [PubMed] [Google Scholar]
- 89.Kapoor M., Martel-Pelletier J., Lajeunesse D., Pelletier J.-P., Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 90.Emara M.M., Ivanov P., Hickman T., Dawra N., Tisdale S., Kedersha N., Hu G.-F., Anderson P. Angiogenin-induced tRNA-derived Stress-induced RNAs Promote Stress-induced Stress Granule Assembly. J. Biol. Chem. 2010;285:10959–10968. doi: 10.1074/jbc.M109.077560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ali S.A., Alman B. RNA extraction from human articular cartilage by chondrocyte isolation. Anal. Biochem. 2012;429:39–41. doi: 10.1016/j.ab.2012.06.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.