Abstract
Coronavirus disease (COVID‐19) caused by the novel coronavirus (SARS‐CoV‐2) has rapidly spread across the globe affecting 213 countries or territories with greater than six million confirmed cases and about 0.37 million deaths, with World Health Organization categorizing it as a pandemic. Infected patients present with fever, cough, shortness of breath, and critical cases show acute respiratory infection and multiple organ failure. Likelihood of these severe indications is further enhanced by age as well as underlying comorbidities such as diabetes, cardiovascular, or thoracic problems, as well as due to an immunocompromised state. Currently, curative drugs or vaccines are lacking, and the standard of care is limited to symptom management. Natural products like ginger, turmeric, garlic, onion, cinnamon, lemon, neem, basil, and black pepper have been scientifically proven to have therapeutic benefits against acute respiratory tract infections including pulmonary fibrosis, diffuse alveolar damage, pneumonia, and acute respiratory distress syndrome, as well as associated septic shock, lung and kidney injury, all of which are symptoms associated with COVID‐19 infection. This review highlights the potential of these natural products to serve as home‐based, inexpensive, easily accessible, prophylactic agents against COVID‐19.
Keywords: acute respiratory distress syndrome, coronavirus disease, diffuse alveolar damage, natural products, pulmonary fibrosis, severe acute respiratory syndrome coronavirus 2
1. CORONAVIRUS AND ASSOCIATED CLINICAL SYMPTOMS
Coronaviruses (CoV; Cronaviridae) are a group of viruses discovered in the 1930s that mainly cause respiratory, gastrointestinal, liver, and neurological diseases in animals (Weiss & Navas‐Martin, 2005). These viruses are termed zoonotic and can be transmitted from animals to humans due to pathogen spillover (Salata, Calistri, Parolin, & Palù, 2019). The word “Corona” is derived from the Latin word which means “crown” signifying the crown‐like projections found on the surface of this virus. Coronaviruses are spherical, positive single‐stranded RNA viruses made of spike (S) protein required for attachment, membrane (M) protein that maintains virion shape, envelope (E) protein for assembly and release of viral particles, and nucleocapsid (N) proteins for RNA binding and viral packaging (Fehr & Perlman, 2015). Figure 1 shows the structure of the SARS‐CoV‐2 virus. Seven types of coronaviruses are known to cause infections in humans. Among these, four (NL63, 229E, OC43, and HUK1) cause common cold with associated mild respiratory tract infections (Singhal, 2020). The remaining three strains cause severe and acute respiratory tract infections, which are responsible for three major human pandemic outbreaks in the 21st century namely severe acute respiratory syndrome (SARS, caused by SARS‐CoV in 2002), Middle East Respiratory Syndrome (MERS, caused by MERS‐CoV in 2012), and Corona Virus Disease 2019 (COVID‐19, caused by SARS‐CoV‐2 in 2019) (Fan, Zhao, & Zhou, 2019; Guo et al., 2020).
FIGURE 1.
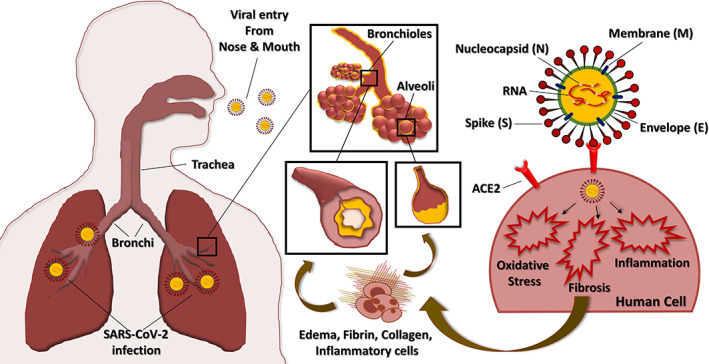
Structure of SARS‐CoV‐2 virus showing the single‐stranded RNA and nucleocapsid (N) along with spike (S), envelope (E), and membrane (M) proteins. Schematic representation shows viral entry through the respiratory tract causing lung infection by damaging bronchioles and alveoli. This is associated with edema or swelling and elevated levels of fibrin, collagen, and inflammatory cells leading to pulmonary fibrosis. The spike (S) protein of SARS‐CoV‐2 binds to ACE2 on the human bronchial and alveolar epithelial cells and activates fibrosis, oxidative stress, and inflammatory responses leading to acute lung infection. ACE2, angiotensin‐converting enzyme 2; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2 [Colour figure can be viewed at wileyonlinelibrary.com]
1.1. SARS and MERS
SARS emerged first in China in 2002 and spread across the world infecting around 8,096 individuals with 10% fatality (Gu & Korteweg, 2007). MERS emerged in Saudi Arabia in 2012 and spread mostly to the Middle East with a few cases in Europe, Asia and North America (Hemida, 2019). It accounted for around 2,494 cases with a higher mortality rate of 34.4% (WHO, 2019). Since 2012, there have been a few active sporadic outbreaks of MERS (Ramadan & Shaib, 2019). The symptoms of SARS and MERS are similar and include fever, cough, shortness of breath, myalgia or fatigue (muscle pain), and diarrhea. In severe cases, both these infections result in pneumonia, severe respiratory infection, acute respiratory distress syndrome (ARDS), sepsis, and multiple organ failure (Al Hajjar, Memish, & McIntosh, 2013; Peiris, Yuen, Osterhaus, & Stöhr, 2003). There is an increased risk of progression associated with age and comorbid conditions such as diabetes, hypertension, cardio, pulmonary, and renal diseases. Pathological analysis of SARS and MERS reveals diffused alveolar damage (DAD), edema, elevated levels of collagen, fibrin and inflammatory cells in alveoli leading to decline in lung function and acute lung injury (Alsaad et al., 2017; Franks et al., 2003). Bats were reported to be the natural hosts for SARS and MERS coronaviruses which were transmitted to intermediate hosts, and finally to Humans (Omrani, Al‐Tawfiq, & Memish, 2015; Salata et al., 2019). The transmission of SARS and MERS was by direct contact or through droplets of coughs and sneezes from the infected patients (Killerby, Biggs, Midgley, Gerber, & Watson, 2020; Peiris et al., 2003). Treatment for SARS and MERS involved administration of antiviral drugs in combination with corticosteroids and interferon‐α (IFN‐α), however, the treatment had minimal benefits to patients and severe adverse effects (Al Ghamdi et al., 2016; Tai, 2007).
1.2. COVID‐19
COVID‐19 first emerged in December 2019 in China causing acute respiratory tract infections (Guo et al., 2020). This new virus is highly contagious and has spread rapidly across the globe. WHO declared the outbreak as “Public Health Emergency of International Concern” (WHO, 2020b). By the end of May 2020, the virus had spread to 213 countries or territories with greater than six million confirmed cases and about 0.37 million deaths. Furthermore, during this period, there was a steep rise in the number of reported cases and associated mortality (WHO, 2020d). Although the overall fatality rate for COVID‐19‐infected patients is lower (5.9%) compared with SARS‐ and MERS‐infected subjects, the spread of the disease has been exceptionally rapid, causing a global pandemic. Figure 2 shows the COVID‐19 global pandemic curve with the total number of confirmed cases and deaths. The current statistics on the extent of COVID‐19 infections globally could be a significant underestimate given the lack of enough testing kits and a large number of asymptomatic carriers.
FIGURE 2.
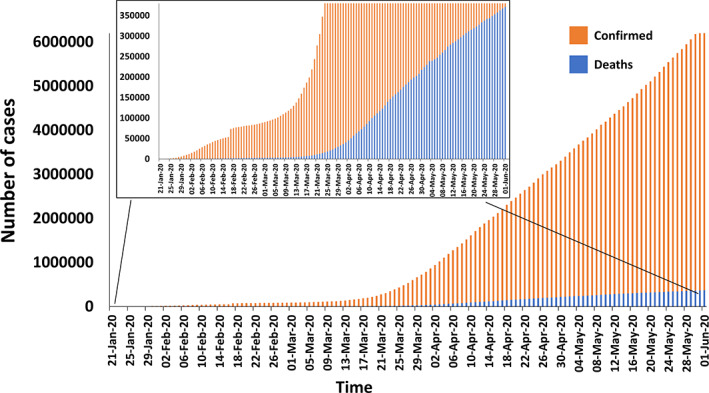
COVID‐19 pandemic curve showing the total number of confirmed cases (orange bars) and the total number of deaths globally (blue bars). In‐set highlights the increasing number of deaths (WHO, 2020d) [Colour figure can be viewed at wileyonlinelibrary.com]
COVID‐19 has very close similarities to the SARS virus and hence is named SARS‐CoV‐2 (Wu et al., 2020). Bats are believed to be the primary source of the virus and although the exact mechanism of transmission to humans is unclear, it is considered to have resulted either from direct contact, consumption of meat or through intermediate hosts (Guo et al., 2020).
1.2.1. Symptoms
Patients with mild infection show fever, fatigue, dry cough, slight nasal congestion, and muscle pain. In severe cases, shortness of breath (dyspnea) associated with dry cough or sputum/ phlegm production (expectoration), along with signs of pneumonia are observed (Guan et al., 2020). In critical cases, patients show ARDS associated with complete respiratory failure, sepsis, septic shock, and multiple organ dysfunction including heart, liver, and kidney. These patients need ventilators in the intensive care unit (Cascella, Rajnik, Cuomo, Dulebohn, & Di Napoli, 2020). Elderly patients with chronic comorbidities like diabetes, hypertension, cardiovascular, and cerebrovascular diseases are at greater risk of contracting COVID‐19 (Lai et al., 2020). Laboratory studies show low blood levels of lymphocytes and white blood cells, and high levels of C‐reactive protein and lactate dehydrogenase in COVID‐19‐infected patients (Tian et al., 2020; Wang, Hu, et al., 2020, p. 13).
Post‐mortem lung sections from COVID‐19‐infected patients revealed DAD with thickening of alveolar walls, edema, fibrin, and proteinaceous exudates in alveolar spaces, vascular congestion, and pneumocyte hyperplasia with viral inclusions and multinucleate giant cells (Tian et al., 2020; Zhang et al., 2020). The chest computed tomography (CT) of COVID‐19 patients shows bilateral multifocal ground‐glass opacity indicative of alveolar exudate and transudate, as well as pleural thickening (Guan et al., 2020; Wang, Hu, et al., 2020).
1.2.2. Transmission
The transmission of COVID‐19 virus occurs with close, prolonged, and unprotected contact with symptomatic or patients who test positive for the virus (person‐to‐person), (Ghinai et al., 2020), as well as via respiratory droplets (Cascella et al., 2020). Figure 1 shows the schematic representation of SARS‐CoV‐2 viral entry through the respiratory tract of a healthy individual leading to acute lung infection. It is thought that short distance aerosol transmission could also be a possible mode of transmission, although the evidence in support of this is not strong. Furthermore, the transmission of the infection from asymptomatic individuals has been reported leading to the fear of community transmission (Mizumoto, Kagaya, Zarebski, & Chowell, 2020; Rothe et al., 2020).
1.2.3. Chronological progression and severity
A retrospective study conducted in China examined a total of 225 patients including 116 individuals who recovered from COVID‐19 infection and 109 patients who succumbed to the infection. In this study, individuals who recovered had a median age of 40 years, whereas those who died were older with a median age of 69 years. Furthermore, the prevalence of comorbidities was significantly higher among the patients who failed to recover versus those who recovered. This included hypertension (36.7 vs. 15.5%), prior history of lung disease (20.2 vs. 2.6%), diabetes (15.6 vs. 7.8%), and heart disease (11.9 vs. 3.4%). Interestingly, the common symptoms associated with the infection like fever, muscle pain, fatigue, and cough were found to the same extent in both the patient groups with the exceptions of shortness of breath (70.6 vs. 19%) and expectoration (32.1 vs. 12.1%). Furthermore, patients who died had a higher incidence of ARDS (89.9 vs. 8.6%), acute cardiac injury (59.6 vs. 0.9%), acute kidney injury (18.3 vs. 0%), septic shock (11.9 vs. 0%) compared with individuals who showed complete recovery (Deng et al., 2020). Another study described 80% of infected individuals to exhibit generic symptoms, with only about 15% showing dyspnea with rapid and shallow breathing, pneumonia, and disturbed pulmonary gas exchange. Included within this group of individuals showing progressive symptoms was a smaller subset (5%) who developed acute symptoms of lung infection, sepsis, and organ failure requiring ICU admission. Importantly, the course of progression of this viral infection is relatively slow spanning an incubation period of 5–20 days from the time of exposure to onset of symptoms. In individuals who progress to a more severe state, this usually happens by around day 10 after appearance of the initial symptoms (Thomas‐Rüddel et al., 2020).
1.2.4. Prevention
Preventive measures to control the spread of COVID‐19 pandemic are crucial and should be followed strictly. Coronaviruses are inactivated by ethanol, chlorine‐containing disinfectants, and lipid solvents. Washing hands regularly with soap and sanitizers containing 70% ethanol/isopropanol to prevent the spread of viral infection is the recommended standard (Kampf & Kramer, 2004). Environmental surfaces have to be cleaned regularly with detergent or bleach such as sodium hypochlorite (NaOCl) solution. Close contact with symptomatic individuals has to be avoided, while also avoiding touching eyes, nose, and mouth. Individuals with a prior history of respiratory distress should wear masks and cover their coughs or sneezes. Health care workers (HCWs) should follow appropriate safety measures during the diagnosis, hospitalization, and isolation of COVID‐19 positive patients (WHO, 2020c). During sample collection, precautions should be taken to prevent direct contact or exposure to airborne droplets. It is very essential to quarantine symptomatic individuals prior to diagnostic testing with appropriate safety guidelines (Adhikari et al., 2020). HCWs caring for COVID‐19 patients should self‐monitor body temperature and respiratory symptoms. Strict hygiene protocols should be implemented in hospitals. Immunocompromised individuals should avoid public/private gatherings (Cascella et al., 2020).
1.2.5. Social distancing, personal hygiene, quarantine, and isolation
Social distancing or physical space between two individuals should be maintained to prevent the spread of the COVID‐19 pandemic. When symptomatic or asymptomatic COVID‐19‐infected person coughs or sneezes, the respiratory droplets (>5–10 μm size) (Atkinson et al., 2009) containing the SARS‐CoV‐2 virus are released into the air, which can infect a healthy individual within 1 m radius. The virus can enter into the subject through the mouth (oral), nose (mucosal), or eyes (conjunctiva). Given this, social distancing requires maintaining at least 2 m or 6 ft distance between individuals to avoid respiratory droplet transmission (Yu & Yang, 2020). It requires avoiding crowded places and public gatherings and implements staying at home and limiting the number of visitors. Respiratory hygiene should be followed by covering mouth and nose with a tissue or bent elbow while coughing and sneezing. In addition to the above, indirect transmission of the virus by contacting contaminated environmental surfaces has been described. Hence, touching eyes, nose or mouth should be avoided.
Quarantining refers to keeping an asymptomatic individual who has been exposed to COVID‐19, in isolation (Sharma et al., 2020). Quarantine can be implemented at home (self‐quarantine) or the individual can be secluded in a specially designed facility. Importantly, quarantined individuals should be monitored for sign of fever, cough, and any other respiratory symptoms for up to 14 days.
Isolation refers to segregating confirmed COVID‐19 patients away from healthy individuals to avoid the spread of the infection. This can be implemented either at home or in a hospital/isolation facility (Wilder‐Smith & Freedman, 2020; Tang et al., 2020). Infected patients are monitored and administered care to manage their symptoms.
1.2.6. Treatment and management
To date, treatment is symptomatic and supportive, with oxygen therapy and mechanical ventilation for ARDS and hypoxemia; fluid bolus therapy, vasopressors, and antibiotics for septic shock, as well as treatment to mitigate co‐infections (WHO, 2020a). There are no potentially effective drugs or vaccines available for the treatment of COVID‐19 and this has resulted in an explosion in research aimed at developing specific drugs. In parallel, existing drugs are being repurposed to test their efficacy against the SARS‐CoV‐2 virus. For example, broad‐spectrum antiviral drugs (Remdesivir, ribavirin, and IFN‐α) (Dong, Hu, & Gao, 2020), antimalarial drugs (chloroquine and hydroxychloroquine) (Gautret et al., 2020), and combination of retroviral drugs (Ritonavir/lopinavir) (Wang et al., 2020) are being evaluated in COVID‐19 patients. Early results suggest that patients treated with these repurposed drugs show no improvement in the mortality rate, while the viral RNA load seems to show a slight decrease. However, in most patients, increased adverse events have been observed (Cao et al., 2020), which in more pronounced in individuals with underlying comorbidities (Srinivasa, Tosounidou, & Gordon, 2017).
Given the lack of targeted drugs to treat COVID‐19, different approaches to mitigate COVID‐19‐associated complications are being evaluated. In this context, this review highlights the potential beneficial effects of natural products that are actively used in alternative/traditional medicines to treat many of the acute pulmonary infections, routinely seen in COVID‐19 patients. These natural products can also boost immunity which is key to resist COVID‐19 infection.
2. THERAPEUTIC PROPERTIES OF NATURAL PRODUCTS
Medicinal plants are the biggest age‐old source of therapeutically beneficial phytochemicals used for maintaining good health, and to prevent and treat many diseases. These include plants and herbs that are both used in Ayurveda, a traditional and alternative medicinal therapy based on holistic body healing, which originated in the Indian subcontinent. A huge body of research is currently focused on understanding the therapeutic efficacy and mechanism of action of these phytochemical agents. The following sections describe dietary supplements and home‐based remedies that have shown value as preventive agents for acute respiratory infections, pulmonary fibrosis, pneumonia, sepsis, and multiple organ failure; all of which are characteristic manifestations of severe COVID‐19 infection. In addition, many of these agents boost the immune system and imbues protection against infective agents. Figure 3 summarizes the beneficial properties of natural products against viral or chemically induced fibrosis, oxidative stress, inflammatory response, and associated acute lung injury in the setting of COVID‐19.
FIGURE 3.
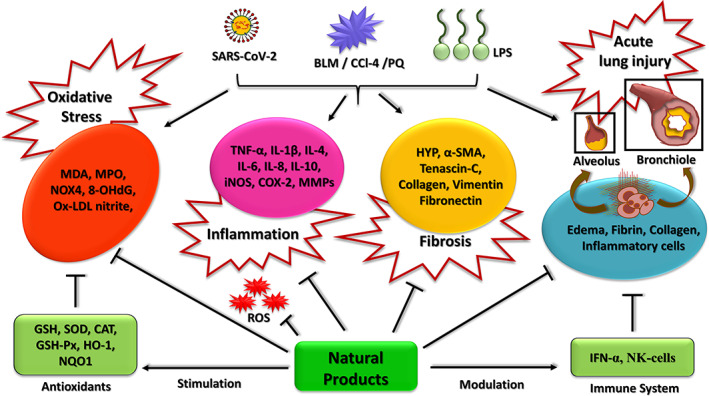
Schematic illustration summarizing the beneficial properties of natural products and their impact on oxidative stress, inflammatory response, pulmonary fibrosis, and acute lung injury [Colour figure can be viewed at wileyonlinelibrary.com]
Mechanistically, the available knowledge base shows that oxidative stress and dysfunctional immune system, in addition to existing comorbidities, contribute to many of the complications associated with COVID‐19 infection. For example, oxidative stress is an important factor resulting in pathogen‐induced pulmonary fibrosis (Cheresh, Kim, Tulasiram, & Kamp, 2013). Along the same lines, an effective immune system is essential for surveying pathogens and neutralizing them in an efficient and timely manner to protect the individual from the infection. The medicinal plants described here contain diverse phytochemicals that have antiviral, antifibrotic, antioxidant, antiinflammatory, and immunomodulatory properties. These, when used in combination, could have a synergistic effect as prophylactic or supportive agents to minimize certain clinical symptoms observed in COVID‐19‐infected patients. In addition, certain species of bacteria, algae, and fungi also exert therapeutic effects against pulmonary fibrosis and acute lung injury. Table 1 summarizes the evidence in the literature supporting the therapeutic value of specific species of bacteria, algae, and fungi, as well as plants. The detailed summary of the therapeutic properties for each of the key natural products discussed in this review is provided in Table S1. Among these, we have identified ginger, turmeric, garlic, onion, cinnamon, lemon, neem, basil, and pepper as well as mushrooms as readily available home‐based remedies that have shown efficacy against pulmonary symptoms associated with COVID‐19 infections in various pre‐clinical and clinical trials.
TABLE 1.
Literature‐based evidence supporting the therapeutic value of various species of bacteria, algae, fungi, and plants
| Source | Species | Bioactive compound | References |
|---|---|---|---|
| Bacteria | Cyanobacteria (blue‐green algae) | Phycocyanin | Leung, Lee, Kung, Tsai, & Chou, 2013; Li et al., 2017; Sun et al., 2011 |
| Algae | Laminaria japonica (sweet kelp) | Fucoidan | Wang et al., 2019 |
| Fungus |
Hirsutella Sinensis (Caterpillar fungus) Lentinula edodes (shiitake) Ganoderma Sinense (purple Reishi) |
Crude extracts, Lanostane‐type triterpenoids |
Chen et al., 2012; Di Pierro, Bertuccioli, & Cavecchia, 2020; Huang et al., 2015; Jung et al., 2019; Sato, Zhang, Ma, & Hattori, 2009; Shou et al., 2012; Sillapachaiyaporn, Nilkhet, Ung, & Chuchawankul, 2019 |
| Plants | Allium cepa (onion) | Quercetin, Apigenin, selenium | Shahabi, Anissian, Javadmoosavi, & Nasirinezhad, 2019; Zhang, Cai, Zhang, & Chen, 2018 |
| Allium sativum (garlic) |
s‐allyl cysteine (SAC), Alliin (SAC sulfoxide) SMFM (sucrose methyl 3‐formyl‐4‐methylpentanoate) |
Nie et al., 2019; Tsukioka et al., 2017 | |
| Andrographis panicula (green chiretta) | Andrographolide | Li et al., 2020; Zhu, Huang, Wang, Zhan, & Fan, 2011; Zhu, Zhang, Xiao, Chen, & Jin, 2013 | |
| Arenaria kansuensis | β‐carboline alkaloids | Cui et al., 2019 | |
|
Aucuba japonica (Japanese laurel) Eucommia ulmoides (Gutta‐percha) |
Aucubin | Qiu et al., 2018 | |
| Azadirachta indica (neem) | Nimbolide, leaf extracts | Lee et al., 2017; Prashanth Goud, Bale, Pulivendala, & Godugu, 2019 | |
| Carthamus tinctorius (safflower) | Hydroxysafflor yellow A | Jin, Wu, Wang, Zang, & Tan, 2016 | |
| Centella asiatica (Indian pennywort) | Asiatic acid | Dong, Liu, Wei, Tan, & Han, 2017 | |
| Cinnamomum verum or C. zeylanicum (cinnamon) | Cinnamaldehyde, Eugenol, linalool | Polansky & Lori, 2020; Zhuang et al., 2009 | |
| Citrus limon (lemon) | Vitamin‐C | Fisher et al., 2012; Rodrigues da Silva et al., 2018 | |
| Corydalis bungeana | Corynoline | Liu et al., 2017 | |
| Curcuma longa (turmeric) | Curcumin | Chen et al., 2017; Hosseini, Rasaie, Asl, Ahmadabadi, & Ranjbar, 2019 | |
| Dioscorea nipponica | Trillin | Jiang et al., 2016 | |
| Eclipta prostrata (Ecliptae Herba) |
Eclipta herb extracts, Ecliptasaponin A |
You et al., 2015 | |
| Gastrodiaelata blume (Tien Ma) | Gastrodin | Zhang et al., 2017 | |
| Humulus lupulus (hops) | Xanthohumol | Lv et al., 2017 | |
| Mangifera indica (mango) | Mangiferin | Impellizzeri et al., 2015; Jia et al., 2019 | |
| Nelumbo nucifera (lotus) | Neferine (bisbenzylisoquinline alkaloid) | Zhao et al., 2010 | |
| Nigella sativa (fennel flower) | Seed extracts | Gholamnezhad, Shakeri, Saadat, Ghorani, & Boskabady, 2019; Poursalehi et al., 2018 | |
| Ocimum sanctum (basil/tulsi) | Crude extracts | Mahajan, Rawal, Verma, Poddar, & Alok, 2013; Pattanayak, Behera, Das, & Panda, 2010 | |
| Oryza sativa (Asian rice) | Isovitexin (from rice hulls) | Lv et al., 2016 | |
| Paeonia lactiflora (common garden peony) | Paeoniflorin | Ji et al., 2016 | |
| Piper nigrum (black pepper) | Piperine | Butt et al., 2013; Vijayakumar, Surya, & Nalini, 2004 | |
| Pogostemon cablin (mint) | Pogostone, β‐Patchoulene | Chen et al., 2017; Sun et al., 2016 | |
| Rheum rhabarbarum (garden rhubarb) | Emodin | Guan et al., 2016; Tian, Yang, Liu, & Xu, 2018 | |
| Rhodiola rosea (Golden root) |
Salidroside, crude extracts |
Tang et al., 2016; Zhang et al., 2016 | |
| Rosmarinus officinalis (rosemary) | Leaf extract | Bahri et al., 2017 | |
| Salvia divinorum (magic mint) | Salvia + Ligustrazine | Huang, Li, Fan, Wang, & Zhan, 2013 | |
| Salvia miltiorrhiza (red sage) | Tanshinone IIA | Tang et al., 2015 | |
|
Schisandra chinensis (Magnolia‐vine) Glycyrrhiza glabra (Liquorice) |
Schizandrin B + Glycyrrhizic acid | Zhang et al., 2017 | |
| Sophora flavescens (Ku Shen) | Matrine + lycopene | Li et al., 2019 | |
| Tanacetum parthenium (feverfew) | Parthenolide | Li et al., 2018 | |
| Tripterygium wilfordii (thunder god vine) | Celastrol | Divya, Dineshbabu, Soumyakrishnan, Sureshkumar, & Sudhandiran, 2016, p. 2; Yang et al., 2020 | |
| Zingiber officinale (ginger) | Zingerone and crude extracts | Mansouri et al., 2019; Rodrigues et al., 2018 | |
| Other bioactive compounds | Foods rich in calcitriol:Dairy products, orange juice, soy milk, and cereals | Calcitriol (vitamin D3) | Tan et al., 2016 |
|
Foods rich in chicoric acid: Cichorium intybus (chicory) And also available in Echinacea purpurea, dandelion leaves, basil, and lemon balm |
Chicoric acid | Ding, Ci, Cheng, Yu, & Li, 2019 | |
| Foods rich in Epicatechin: Brewed green tea, Black tea, Black grapes, and blackberries | Epicatechin | Shariati, Kalantar, Pashmforoosh, Mansouri, & Khodayar, 2019 | |
| Foods rich in resveratrol: Peanuts, pistachios, grapes, red and white wine, blueberries, and cranberries | Resveratrol | Sener, Topaloğlu, Sehirli, Ercan, & Gedik, 2007 | |
| Foods rich in Sulforaphane:Broccoli sprouts, cauliflower, kale, and cabbage | Sulforaphane | Kyung et al., 2018 | |
| Crude venom of Naja naja atra | Cobrotoxin | Cui et al., 2014 |
2.1. Ginger
Ginger (Zingiber officinale) has therapeutic properties against pulmonary fibrosis, pneumonia, ARDS, sepsis, and acute kidney injury. In addition, ginger along with its phytochemicals has antiviral, antifibrotic, antioxidant, antiinflammatory, and hepatoprotective properties (Chang, Wang, Yeh, Shieh, & Chiang, 2013; Mao et al., 2019; Rahmani, shabrmi, & Aly, 2014).
Ginger has significantly reduced pulmonary fibrosis and mitigated oxidative stress and inflammatory response in chemically induced pulmonary fibrosis in animal models. For example, bleomycin, a cytotoxic antibiotic used in cancer treatment, has idiopathic pulmonary fibrosis (IPF) as a side effect. In bleomycin‐treated rats, zingerone, a bioactive compound in ginger has significantly reduced fibrosis score in histopathological sections of lungs, reduced levels of fibrosis marker, hydroxyproline and oxidative stress marker, and malondialdehyde (MDA). In addition, it increased levels of antioxidant markers like reduced glutathione (GSH), superoxide dismutase (SOD), and glutathione peroxidase (GSH‐Px) in the lungs (Mansouri et al., 2019). Similarly, in ethanol‐treated rats that exhibit symptoms of diffuse alveolar damage and acute lung injury leading to ARDS, extracts of ginger mitigated abnormalities in alveolar air space, wall thickening, infiltration of multinucleated cells and pneumocytes, lung cell proliferation, and fibrosis in the ethanol‐treated rats. In addition, ginger significantly reduced the oxidative stress markers namely 8‐hydroxy‐2′‐deoxyguanosine (8‐OHdG), oxidized low‐density lipoprotein (Ox‐LDL), and NADH oxidase levels. (Shirpoor, Gharalari, Rasmi, & Heshmati, 2017).
In a separate clinical study on 32 ARDS patients, 120 mg of ginger extract was shown to increase the tolerance of enteral feeding, significantly reduced nosocomial pneumonia and increased the ICU‐free and ventilator‐free days compared with the placebo group (Shariatpanahi, Taleban, Mokhtari, & Shahbazi, 2010). Ginger with its bioactive compounds has also ameliorated sepsis and acute kidney injury (AKI) induced by cecal ligation and puncture (CLP) in rats. Specifically, in this study, the authors demonstrated that 6‐gingerol and 10‐gingerol significantly reduced pathological levels of AKI markers, oliguria, blood urea nitrogen, urinary protein, serum creatinine levels, urinary sodium, and osmolarity in these rats. Both compounds have also reduced the levels of oxidative stress markers, MDA and nitrite, as well as increased the levels of antioxidants, GSH and SOD. In addition, they also reduced levels of inflammatory markers such as tumor necrosis factor‐α (TNF‐α), interleukin (IL)‐1β, and kidney injury marker, KIM‐1 (Rodrigues et al., 2018).
Pulmonary tuberculosis (TB) is another serious infectious lung disease leading to pulmonary fibrosis and acute lung injury (Ravimohan, Kornfeld, Weissman, & Bisson, 2018). In patients undergoing treatment for TB, ginger has significantly reduced levels of MDA and inflammatory markers such as TNF‐α and ferritin (Kulkarni & Deshpande, 2016). Overall, these clinical studies suggest that use of ginger extract could have beneficial effects in individuals having pulmonary problems such as ARDS, pulmonary fibrosis, and pneumonia, as well as those having inflammatory conditions such as sepsis, all of which are symptoms seen in patients infected with COVID‐19.
2.2. Turmeric
Turmeric (Curcuma longa) has potential therapeutic effects on pulmonary fibrosis, severe respiratory disorders, lung infections, liver abnormalities. Curcumin, the bioactive compound in turmeric has been shown to have antifibrotic, antioxidant, antiinflammatory, and immunomodulatory activities (Jurenka, 2009; Menon & Sudheer, 2007; Srivastava, Singh, Dubey, Misra, & Khar, 2011).
Curcumin has significantly enhanced pulmonary function and ameliorated pulmonary fibrosis, oxidative stress, and inflammation in pulmonary fibrosis‐animal models induced by virus, radiation, and toxic chemicals. Mice infected with Reovirus 1/L induce DAD with intra‐alveolar and interstitial exudate and fibrosis, as well as pneumonia resulting in acute lung injury and ARDS. In these mice, curcumin has significantly reduced infiltration of inflammatory cells into interstitial and intra‐alveolar space and decreased interstitial fibrosis in the lungs which are associated with the late phase of viral infection. Consistent with this, curcumin also reduced the fibrosis markers such as α‐smooth muscle actin (α‐SMA), tenascin‐C and E‐cadherin, as well as inflammatory markers like IL‐6, IL‐10, and IFN‐γ (Avasarala et al., 2013).
In bleomycin‐induced pulmonary fibrosis rats, curcumin increased the expression of cathepsins (CatK, CatL) which degrade collagen, and inhibited lung fibroblast proliferation by blocking transforming growth factor (TGF)‐β1 (Smith et al., 2010; Zhang et al., 2011). In these rats, curcumin also suppressed the inflammatory cytokine TNF‐α released by alveolar macrophages ameliorating pulmonary fibrosis (Punithavathi, Venkatesan, & Babu, 2000).
Paraquat is a toxic herbicide that leads to pulmonary fibrosis, edema, acute lung injury, and respiratory failure. In the paraquat‐induced pulmonary fibrosis rat model, curcumin reduced the deposition of collagen fiber and inhibited fibrosis. In parallel, it also improved the tidal volume (volume of air taken during normal breath) and arterial partial pressure of oxygen (PaO2) in the lungs (Chen, Yang, et al., 2017). In these rats, at the molecular level, curcumin decreased the levels of fibrosis marker hydroxyproline, as well as oxidative stress markers, and inhibited lung fibrosis (Hosseini et al., 2019). In another study, curcumin reduced levels of hydroxyproline and reactive oxygen species (ROS), and increased the levels of antioxidant marker heme oxygenase‐1(HO‐1), in mice irradiated with γ radiation showing symptoms of pneumonitis and acute pulmonary fibrosis (Lee et al., 2010).
In addition, it is well documented that impaired immune system with imbalances in inflammatory cells and cytokines can aggravate lung fibrosis (Hügle, 2011). In this context, it is important to note that curcumin is a potent immunomodulator and can regulate the function of dendritic cells, natural killer (NK) cells, neutrophils, macrophages, T cells, and B cells, as well as inflammatory cytokines (Gautam, Gao, & Dulchavsky, 2007). All the above pre‐clinical findings strongly implicate turmeric as an agent that can improve lung function, and protect against acute lung injury and associated DAD, pulmonary fibrosis, and ARDS, all of which are observed in COVID‐19 patients.
2.3. Garlic
Garlic (Allium sativum) has potential therapeutic effects against respiratory tract infections, intra‐alveolar edema and cell infiltration, pulmonary fibrosis, sepsis, and acute lung injury. Garlic along with its bioactive compounds, s‐allyl cysteine (SAC), alliin, and diallyl thiosulfonate (allicin), has been shown to have antiviral, antifibrotic, antioxidant, antiinflammatory, and immunomodulatory properties (Arreola et al., 2015; Bayan, Koulivand, & Gorji, 2014; Shang et al., 2019).
Garlic has significantly inhibited pulmonary fibrosis and acute lung injury, and subsequently mitigated oxidative stress and inflammatory response in chemically induced pulmonary fibrosis, sepsis, and acute lung injury rat models. For example, in multiple studies using either bleomycin‐ or carbon tetrachloride (CCl4)‐induced pulmonary fibrosis rat model, SAC significantly reduced the fibrotic lesions, alveolar wall thickening, and collagen deposition. In doing so, it reduced the expression of various collagens, fibronectin, as well as levels of various fibrotic markers including hydroxyproline, α‐SMA, and TGF‐β, possibly by inhibiting phosphoinositide 3‐kinases (PI3K)/protein kinase B (AKT) and nuclear factor‐κB (AKT/NF‐κB) signaling pathways (Nie et al., 2019). SAC also reduced inflammatory cells in the alveolar spaces and attenuated lung injury by reducing the expression of inflammatory genes namely iNOS, TNF‐α, and matrix metalloproteinase‐9 (MMP‐9), and reducing levels of oxidative stress markers like inducible NO synthase (iNOS) in lungs, total nitrites and nitrates (NOx), ROS, and lung lipid peroxides (Mizuguchi et al., 2006; Tsukioka et al., 2017).
Along the same lines, allilin (SAC sulfoxide) was shown to inhibit alveolar edema and damage, reduce neutrophils infiltration into alveoli and decrease inflammatory cytokines like TNF‐α and IL‐1β. In this process, allilin also inhibited the inflammatory response by inhibiting NF‐κB and peroxisome proliferator‐activated receptor γ (PPARγ) in lipopolysaccharides induced acute lung injury rat model (Wang, Guo, He, Chen, & Zhuang, 2017). Similarly, in CLP‐induced sepsis and acute lung inflammation models in rats, SMFM (sucrose methyl 3‐formyl‐4‐methylpentanoate) a natural compound isolated from garlic significantly inhibited alveolar damage, thrombotic lesions, and lung infection. In addition, SMFM also inhibited bacterial infection and proinflammatory cytokines TNF‐α, IL‐6, and IL‐1β in the peritoneal fluid which are known to cause vital organ damage (Lee et al., 2015).
Garlic is also a potent immunomodulator (Ishikawa et al., 2006). In a clinical study on humans, dietary consumption of 2 g of garlic every 2–3 days, boosted the basal plasma IFN‐α levels which are known to be protective against viral infections and prevent viral replication (Bhattacharyya, Girish, Karmohapatra, Samad, & Sinha, 2007). Importantly, these pre‐clinical studies highlight the efficacy of garlic in mitigating pulmonary fibrosis, lung injury, and sepsis‐associated organ failure, all of which are symptoms observed in patients with advanced COVID‐19 infection.
2.4. Onion
Onion (Allium cepa) has potential therapeutic benefits against acute respiratory tract infection and lung injury caused by collagen deposition, inflammatory cell infiltration, and pulmonary fibrosis. Onion along with its bioactive compounds, quercetin, apigenin, and selenium is known to exert antiviral, antifibrotic antioxidant, antiinflammatory, antiasthmatic and hepatoprotective properties (Kumar & Pandey, 2013; Marefati et al., 2018; Suleria, Butt, Anjum, Saeed, & Khalid, 2015).
Onion has been shown to significantly alleviate pulmonary fibrosis following attenuation of oxidative stress and inflammatory response in chemically induced pulmonary fibrosis rat models. For instance, in several bleomycin‐induced pulmonary fibrosis rat models, quercetin, apigenin, and selenium have significantly reduced lung fibrosis by reducing collagen deposition, decreasing inflammatory cell infiltration, reducing alveolar wall thickness, and increasing alveolar air space. Similarly, quercetin has been shown to reduce the levels of fibrosis markers such as hydroxyproline, fibronectin, α‐SMA, and several collagens in lung tissues of these mice. Quercetin has also ameliorated lung damage by enhancing levels of antioxidants (SOD and catalase), reducing the expression of oxidative stress markers (MDA), and decreasing inflammatory markers (TNF‐α and MMP‐7). At the molecular level, quercetin acts by inhibiting sphingosine kinase 1/sphingosine‐1‐phosphate (SphK1/S1P) signaling (Zhang et al., 2018).
Likewise, apigenin reduced expression levels of fibrotic marker hydroxyproline and leukocyte adhesion marker myeloperoxidase (MPO), besides enhancing antioxidant SOD activity. Apigenin and selenium independently ameliorated lung injury by reducing levels of inflammatory markers, TNF‐α, and TGF‐β1 (Chen & Zhao, 2016; Shahabi et al., 2019).
Along similar lines, in a rhinovirus‐induced chronic obstructive pulmonary disorder (COPD) mice model, quercetin ameliorated lung injury by reducing infiltration of neutrophils, macrophages, and T cells into alveoli, and lowering the expression of inflammatory cytokines, TNF‐α, IFN‐γ, and IL‐17A (Farazuddin et al., 2018).
In a separate clinical study, lower selenium levels were observed in patients with respiratory disorders admitted to the ICUs that correlated with decreased lymphocytes and increased C‐reactive protein levels (Lee et al., 2016). In other studies, conducted on hospitalized patients with pneumonia and bronchiolitis, 1 mg of sodium selenite was found to reduce signs of respiratory infection and improve the recovery time. At the molecular levels, it increased the levels of antioxidant glutathione peroxidase as well as the leukocyte count (Hu, Liu, Yin, & Xu, 1998; Liu, Yin, & Li, 1997). Together, these pre‐clinical and clinical studies highlight the potency of onion in ameliorating pulmonary fibrosis, acute respiratory tract infections, and lung injury which are the critical symptoms of COVID‐19 patients.
2.5. Cinnamon
Cinnamon (Cinnamomum verum or C. zeylanicum) along with its major bioactive compounds, cinnamaldehyde, eugenol, and linalool, has potent antiviral, antioxidant, antiinflammatory, and hepatoprotective properties (Jayaprakasha & Rao, 2011; Kawatra & Rajagopalan, 2015; Rao & Gan, 2014). Of the two varieties of cinnamon, on one hand, there is cassia that contains significant amounts of another bioactive compound coumarin, and on the other, there is ceylon (also known as true cinnamon) that has lesser amounts of coumarin. Coumarin if taken in larger quantities could be harmful. Care should be taken not to ingest cinnamon powder in large quantities as this could have potentially harmful effects (Grant‐Alfieri, Schaechter, & Lipshultz, 2013). In light of this, Ceylon is more widely used.
Cinnamoni cortex (CC) extract has shown in vitro dose‐dependent inhibition of wild‐type SARS‐CoV and a luciferase encoded HIV‐containing SARS‐CoV spike protein (HIV/SARS‐CoV S pseudovirus). The antiviral activity against SARS‐CoV could probably be due to the inhibition of viral replication and interference with viral endocytic pathways (Zhuang et al., 2009). Cinnamon extracts along with quercetin and selenium is also part of Gene‐Eden‐VIR/Novirin, a broad‐spectrum antiviral herbal treatment. Clinical trials have shown its efficacy against several viruses including herpes simplex virus (HSV), Epstein–Barr virus (EBV) and human cytomegalovirus (hCMV) (Polansky & Lori, 2020).
Proteolytic activation of SARS‐CoV‐2 spike protein by the host cell proteases is essential for infection. Studies have shown the role of trypsin and cathepsin L in cleavage and activation of spike protein and its binding to ACE2, during the SARS‐CoV infection (Belouzard, Chu, & Whittaker, 2009; Simmons et al., 2011). In this context, cinnamon as a trypsin inhibitor (Gopalakrishnan, Ediga, Reddy, Reddy, & Ismail, 2018; Shahwar, Raza, Shafiq‐Ur‐Rehman, Abbasi, & Atta‐Ur‐Rahman, 2012) could have a therapeutic role against the SARC‐CoV‐2 infection in COVID‐19.
In CCL4 and LPS‐stimulated rat and mice, cinnamon extracts reduced MDA and increased levels of antioxidant markers catalase and SOD. It also reduced antiinflammatory markers TNF‐ α/IL‐6, reduced phosphorylation of MAPKs (JNK, p38 and ERK1/2), and interfered with NF‐κB activation by inhibiting the degradation of IκBα. It has also reduced necrosis and infiltration of lymphocytes in the liver of rats with hepatic injury (Hong et al., 2012; Moselhy & Ali, 2009). Overall, these studies implicate promising therapeutic roles of cinnamon against the SARS‐CoV‐2 infection in COVID‐19.
2.6. Lemon
Lemon (Citrus limon) has potential therapeutic benefits against pulmonary fibrosis, pneumonia, ARDS, sepsis, acute lung, kidney, and liver injury. Lemons contain vitamin‐C (Vit‐C) or ascorbic acid (AA), which is an antifibrotic, antioxidant, antidiabetic, as well as an immunomodula tor. It is also documented to be protective against respiratory infections (Ashbel' & Arziaeva, 1965; Chambial, Dwivedi, Shukla, John, & Sharma, 2013; Hong, Lee, Lee, & Kim, 2018). Consistent with this, individuals with lower ascorbic acid levels are prone to severe infections and other acute diseases (Bakaev & Duntau, 2004).
In a case study involving a patient with dyspnoea, hypoxemia, and ARDS, placed on ventilator support, vitamin‐C (50 mg/kg body weight every 6 hr) administered intravenously improved bilateral lung opacities as seen by chest X‐ray, attenuated sepsis‐associated ARDS, and was extubated (Bharara et al., 2016).
In an independent clinical study containing 595 critically ill surgical patients from ICUs, patients receiving antioxidant therapy (AA and α‐tocopherol) had a relatively lower risk of pulmonary morbidity (a measure of ARDS and pneumonia), multiple organ failure and mortality compared with the standard of care patients. Importantly, patients on antioxidant therapy required mechanical ventilation and ICU admission for a shorter period of time (Nathens et al., 2002). In a separate clinical study, 57 elderly patients hospitalized for bronchitis and pneumonia were administered oral vitamin‐C (200 mg/day) and compared with the placebo arm. Vitamin‐C levels in the treated group were higher in the plasma and leukocytes, and these patients were showed pulmonary complications (Hunt, Chakravorty, Annan, Habibzadeh, & Schorah, 1994).
In a pre‐clinical study comparing the effect of sepsis in mice lacking the capacity to synthesize vitamin‐C (l‐gulono‐γ‐lactone oxidase deficient, −Gulo) versus wild‐type controls (+Gulo), lack of vitamin‐C resulted in multiple organ failure, pulmonary edema, and proinflammatory response, all of which were attenuated by intraperitoneal infusion of vitamin‐C (Fisher et al., 2014). Similar results were obtained in independent studies using sepsis and acute lung injury models treated with or without vitamin‐C (200 mg/kg) (Fisher et al., 2011, 2012). Along the same lines, in paraquat‐induced pulmonary fibrosis mice model, vitamin‐C has blocked infiltration of lymphocytes, neutrophils, macrophages, and attenuates pulmonary fibrosis. It also significantly decreased collagen deposition and reduced levels of pro‐inflammatory markers, TGF‐β, IL‐6, IL‐17, and enhanced antioxidant markers namely catalase and SOD (Rodrigues da Silva et al., 2018).
Vitamin‐C also has significant immunomodulatory properties. It gets accumulated in neutrophils and enhances phagocytosis, NK cell activity, and lymphocyte proliferation (Carr & Maggini, 2017; Wintergerst, Maggini, & Hornig, 2006). Taken together, preclinical and clinical studies suggest that vitamin‐C could have promising therapeutic benefits in individuals with pulmonary fibrosis, pneumonia, ARDS, sepsis, acute lung injury, and multiple organ dysfunction all of which are observed in advanced COVID‐19 patients.
2.7. Neem
Neem (Azadirachta indica) has potential therapeutic benefits against pulmonary fibrosis pulmonary inflammation, acute lung injury, and alveolar damage. The bioactive compounds in neem are azadirachtin, nimbolinin, nimbolide, quercetin, and β‐sitosterol. These are known to exhibit antiviral, antioxidant, and antiinflammatory (Alzohairy, 2016; Subapriya & Nagini, 2005; Tiwari, Darmani, Yue, & Shukla, 2010) properties.
In a bleomycin‐induced pulmonary fibrosis mice model, nimbolide has been shown to significantly reduce pulmonary fibrosis by decreasing infiltration of white blood cells (monocytes, lymphocytes, and neutrophils) into the bronchoalveolar lavage (BAL) fluid. At the molecular level, nimbolide was shown to reduce the expression of fibrosis and oxidative stress markers, while increasing the expression of the autophagy mediator Beclin‐1. Additional studies have implicated the inhibition of TGF‐β/Smad pathway as a possible mechanism for its antifibrotic properties (Prashanth Goud et al., 2019).
In an independent study using cigarette smoke (CS)‐lipopolysaccharide (LPS)‐induced pulmonary inflammation mice model, administration of neem leaf extract (NLE) significantly reduced infiltration of macrophages and neutrophils into BAL fluid and prevented acute lung injury (Lee et al., 2017).
2.8. Basil
Basil a.k.a. Tulsi (Ocimum sanctum) along with its bioactive compounds, quercetin, eugenol, and apigenin has been shown to exhibit antiviral, antioxidant, antiinflammatory, antiasthmatic, and immunomodulatory properties (Mahajan et al., 2013; Mediratta, Sharma, & Singh, 2002; Pattanayak et al., 2010; Saini, Sharma, & Chhibber, 2009). In healthy humans, 4 weeks oral administration of ethanol extracts of Tulsi significantly increased the levels of IFN‐γ, IL‐4, T‐helper cells, and NK‐cells (Mondal et al., 2011).
2.9. Black pepper
Black pepper (Piper nigrum), known as “king of spices”, has antiviral, antioxidant, and antiinflammatory properties (Butt et al., 2013; Vijayakumar et al., 2004). The bioactive compound piperine is known to enhance the bioavailability of many drugs and phytochemicals by increasing their absorption from the gastrointestinal tract (Pattanaik, Hota, Prabhakar, & Pandhi, 2009). For example, 20 mg black pepper when taken along with 2 g turmeric was shown to increase the bioavailability of the latter by 2000‐fold (Shoba et al., 1998). In light of this, one would envision that the bioavailability of the supplements discussed above could be enhanced by combining them with black pepper.
2.10. Medicinal mushrooms
Medicinal mushrooms are an untapped resource which show potential antiviral, antiinflammatory, and immunomodulatory properties against various viruses like HSV, EBV hepatitis C virus, (HCV), human immunodeficiency virus (HIV), H1N1 strain of flu, and influenza (Ellan et al., 2019; Linnakoski et al., 2018; Muszyńska, Grzywacz‐Kisielewska, Kała, & Gdula‐Argasińska, 2018). The secondary metabolites from these mushrooms such as alkaloids, non‐ribosomal peptides, polyketides, and terpenoids have shown protease inhibitory activities against HIV‐1 and hepatitis C virus (El‐Fakharany, Haroun, Ng, & Redwan, 2010; Sato et al., 2009; Sillapachaiyaporn et al., 2019). Some of the medicinal mushrooms like Ganoderma species [(G. lucidum, (Reishi) G. colosum,(Lingzhi) G. sinense (Purple Reishi)], Auricularia polytricha (wood ear/jelly ear), Chrysosporium merdarium, Cordyceps militaris (caterpillar fungus), Lignosus rhinoceros (tiger milk mushroom), Agaricus bisporus (button mushrooms) (Lu et al., 2020; Ying et al., 2016) might also have protease inhibitory activities against the SARS‐CoV‐2 papain‐like protease (Suwannarach et al., 2020). These mushroom extracts have bioactive polysaccharides (β‐glucans) and galactomannan that are known to have immunomodulatory properties and stimulate the production of antiviral cytokines such as TNF‐α, NK‐cells, and macrophages (Jung et al., 2019). Mushroom extracts from Lentinula edodes (Shiitake) mycelia (AHCC) are extensively studied for their immunostimulant effects against various viruses and could have therapeutic effects against COVID‐19 (Di Pierro et al., 2020).
2.11. Papaya leaf
Papaya (Carica papaya) along with its bioactive compounds show antiviral, antioxidant, and antiinflammatory properties (Joseph, Sankarganesh, Ichiyama, & Yamamoto, 2015; Panzarini, Dwikat, Mariano, Vergallo, & Dini, 2014). Thrombocytopenia or low blood platelet count could be another risk factor correlating with both, severity and higher mortality in COVID‐19 patients (Lippi, Plebani, & Henry, 2020). Similarly, low platelet count is also associated with IPF, multiple organ failure and acute kidney injury (Nguyen, Cruz, & Carcillo, 2015; Steiropoulos et al., 2014). Thrombocytopenia is a common clinical manifestation in dengue patients and studies suggest that activation of platelets leads to prothrombotic state in these patients (Jayashree, Manasa, Pallavi, & Manjunath, 2011; Ojha et al., 2017). Papaya leaf extract enhances thrombopoiesis and has significantly increased the platelet count in dengue patients and in mouse model (Dharmarathna, Wickramasinghe, Waduge, Rajapakse, & Kularatne, 2013; Subenthiran et al., 2013). Papaya leaf extracts might increase platelet count in COVID‐19 patients and may show possible beneficial effects. An invitro study suggests that papaya leaf extracts might stabilize erythrocyte membrane thereby preventing hemolysis (Ranasinghe et al., 2012).
2.12. Hot water
Inactivation of the virus is a critical step for containing the spread of COVID‐19 pandemic. Studies show that heat can render the virus inactive and non‐infectious. Consistent with this, SARS coronavirus was shown to survive at 4°C as well as at body temperature (37°C) but was inactivated at temperatures greater than 50°C (Rabenau et al., 2005). Similarly, UV radiation for 60‐min was also shown to inactivate the virus (Duan et al., 2003). In turn, these findings highlight the importance of boiling water before drinking. In addition, inhaling steam generated from water containing turmeric and tulsi is effective against respiratory tract infections (Saleem, Rani, & Daniel, 2019; Shuman, Raju, & Jogdeo, 2018; Singh, Singhi, & Walia, 1990). Furthermore, fumigating living rooms with medicated smoke from burnt neem leaves has also been shown to be effective in combating the virus (Khedekar, Goel, & Ojha, 2016). Taken together, the prophylactic measures to protect against coronaviruses could include avoiding cold beverages, boiling/or UV‐based sterilization of drinking water.
2.13. Mechanism of SARS‐CoV‐2 infection and natural products bioactivity
The spike protein (S‐protein) of SARS‐CoV‐2 virus recognizes and binds to the angiotensin‐converting enzyme 2 (ACE2, Figure 1) on bronchial and alveolar epithelial cells and vascular endothelial cells (Zhang, Penninger, Li, Zhong, & Slutsky, 2020). The viral membrane fuses with the host membrane, and the viral RNA along with nucleocapsid proteins is released and replicated further in the host cells. The viral infection triggers apoptosis of epithelial and endothelial cells leading to secretion of inflammatory cytokines, IL‐1β, IL‐4, IL‐10, TNF‐α, and IFN‐γ, that destroy the host cells (Fu, Cheng, & Wu, 2020). SARS CoV papain‐like protease (PLpro) upregulates the expression of TGF‐β1, a profibrotic cytokine (Li et al., 2012). The activation of TGF‐β1 by proteolytic cleavage of its latent complex form is carried out by MMP‐9 and MMP‐2 (Kobayashi et al., 2014; Wang et al., 2006). Interestingly, as shown in Figure 4, the bioactive compounds inhibit TGF‐β1 activation by suppressing these MMPs (Kim et al., 2013; Kumar, Kumar, Saravanan, & Singh, 2012).
FIGURE 4.
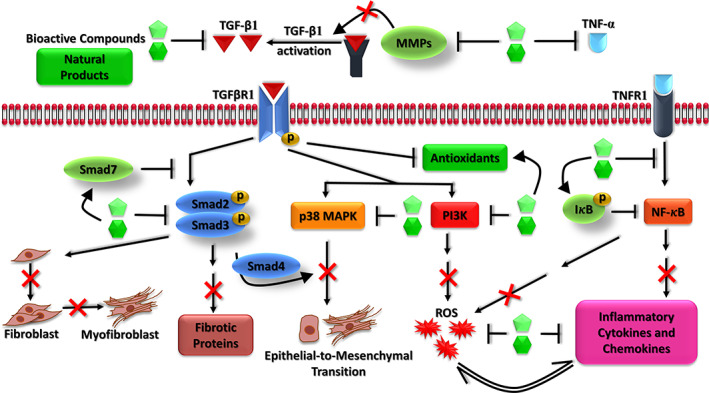
Inhibitory mechanism of bioactive compounds in TGF‐β1 and NF‐κB signaling pathways in pulmonary fibrosis. NF‐κB, nuclear factor‐κB; TGF, transforming growth factor [Colour figure can be viewed at wileyonlinelibrary.com]
TGF‐β1 induces proliferation of fibroblasts and their differentiation into myofibroblasts that secrete extracellular matrix (ECM) leading to fibrosis (Liu et al., 2016; Michalik et al., 2018). In the canonical pathway, TGF‐β1 phosphorylates the downstream effector proteins Smad2 and Smad3 that further activate the expression of pro‐fibrotic proteins namely fibronectin, collagen type I/III, α‐SMA, and vimentin (Malmström et al., 2004). The bioactive compounds inhibit the phosphorylated Smad2 and Smad3, and enhance the expression of Smad7 (a TGF‐beta antagonist), leading to downstream suppression of fibroblast proliferation and their differentiation into myofibroblasts, as well as inhibition of pro‐fibrotic gene expression (Nie et al., 2019; Smith et al., 2010).
In the non‐canonical pathway, TGF‐β1 activates p38 mitogen‐activated protein kinase (MAPK) that induces epithelial to mesenchymal transition (EMT) (Wang et al., 2017; Zhang, 2009). In addition, TGF‐β1 induces NOX4 expression and suppresses antioxidants, resulting in ROS generation and oxidative stress (Liu & Desai, 2015; Liu & Gaston Pravia, 2010). Bioactive compounds inhibit p38 MAPK, thereby ameliorating EMT (Avila‐Carrasco et al., 2019; Cai et al., 2018). They also inhibit PI3K leading to downstream suppression of ROS and oxidative stress (Huang, 2013; Park, Kim, & Lee, 2009). The non‐structural proteins (nsp1, nsp3a, and nsp7a) and spike proteins of SARS‐CoV activates NF‐κB, resulting in the inflammatory response and ROS, which further drives the pathogenesis (Liao et al., 2005). S protein also degrades IκB‐α, resulting in activation of NF‐κB, that further upregulates TNF‐α and IL‐6 (DeDiego et al., 2014; Wang et al., 2007). Bioactive compounds inhibit expression of TNF‐α and enhance IκB‐α expression, together leading to suppression of NF‐κB‐mediated expression of inflammatory cytokines and chemokines. These compounds also have direct inhibitory effect on ROS and inflammatory cytokines and enhance the expression of antioxidants. It has also been shown that the SARS‐CoV virus downregulates the ACE2 expression, and this could further trigger acute lung injury (Glowacka et al., 2010; Kuba et al., 2005).
3. DISCUSSION
COVID‐19 causes acute respiratory tract infections, pulmonary fibrosis, sepsis, and multiple organ failure, all of which could result in mortality. Study on the severity and progression of COVID‐19 suggests that, among the patients who died, 70% had shortness of breath and 32% had expectoration. This implies that underlying pathogenesis like the initiation of fibrosis and alveolar damage might begin early during the 14–20 days of incubation period after exposure to the virus. Natural products taken during these initial stages of viral infection could prevent further progression of the infection and stabilize the initial symptoms. Furthermore, among the patients who died, around 89% had ARDS, suggesting that the major cause of mortality was the acute lung infection, pulmonary fibrosis and pneumonia. Natural products that are effective against these pulmonary conditions could be beneficial supplements to promote the recovery of patients showing these advanced COVID‐19‐related symptoms.
Considering the widespread global outbreak of COVID‐19, controlled clinical trials might not be feasible. In some clinical settings, antimalarial, retroviral drugs, and corticosteroids are being repurposed and are showing adverse side effects. In this context, given the therapeutic efficacy of many of the natural products, these could be administered in combination with the clinical standard of care to mitigate treatment‐related side effects. Importantly, unlike chemotherapeutic supplements, natural products have no adverse effects.
In summary, natural products have shown therapeutic efficacy against multiple symptoms observed in advanced COVID‐19 patients. They are highly tolerated with no side effects and can be used in combination with existing clinical standard of care. In this setting, natural products have the potential to serve as prophylactic agents in populations that are at risk to develop COVID‐19 infection. These include elderly individuals as well as those who have underlying comorbid conditions. In addition, in symptomatic patients, natural product supplementation can halt the progression of the infection. In the case of patients who have progressed to an advanced stage, natural products can mitigate many of the complications and reduce mortality. Importantly, natural product supplementation constitutes home‐based remedies that are inexpensive and can be easily implemented on a community‐wide scale.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
Sai Manohar Thota conceptualized, designed, searched databases/articles, and wrote the manuscript. Venkatesh Balan and Venketesh Sivaramakrishnan contributed to manuscript writing and provided scientific guidance.
DEDICATION
Dedicated to India and her Traditional Medicine System—Ayurveda.
Supporting information
Table S1. Beneficial properties of various bacterial, algal, fungal and plant species against the COVID‐19 related symptoms. Overview shows the antifibrotic, antioxidant, anti‐inflammatory properties, histopathological and other lung properties of algal, fungal, plant and other phytochemicals (↑ up arrow represents the increase and ↓ down arrow represents decrease of a specific parameter) (Abbreviations used are given at the end of the table).
ACKNOWLEDGEMENTS
The authors thank Prof. Arun Sreekumar, Baylor College of Medicine, Houston, Texas, USA, for careful and insightful review of the manuscript. Mr. Thota and Dr. Sivaramakrishnan thank Central Research Instruments Facility, Sri Sathya Sai Institute of Higher Learning and also acknowledge the support of SERB‐DST (EMR/2017/005381), BSR‐UGC (F.25‐1/2013‐14(BSR)/7‐164/2007(BSR), BIF‐DBT (BT/BI/25/063/2012), FIST‐DST (SR/FST/LSI‐616/2014), SAP‐III‐UGC (F.3‐19/2018/DRS‐III(SAP‐ II); Dr. Balan thank the University of Houston for providing small equipment grant and state of Texas for startup funds.
Thota SM, Balan V, Sivaramakrishnan V. Natural products as home‐based prophylactic and symptom management agents in the setting of COVID‐19. Phytotherapy Research. 2020;34:3148–3167. 10.1002/ptr.6794
Funding information SERB, Department of Science and Technology, Govt. of India, Grant/Award Number: EMR/2017/005381; BSR Fellowship, University Grants Commission, Govt. of India, Grant/Award Number: F.25‐1/2013‐14(BSR)/7‐164/2007(BSR); BIF, Department of Biotechnology, Govt. of India, Grant/Award Number: BT/BI/25/063/2012; FIST, Department of Science and Technology, Govt. of India, Grant/Award Number: SR/FST/LSI‐616/2014; SAP‐III, University Grants Commission, Govt. of India, Grant/Award Number: F.3‐19 /2018/DRS‐III(SAP‐ II)
Contributor Information
Sai Manohar Thota, Email: saimanoharthota@gmail.com.
Venketesh Sivaramakrishnan, Email: svenketesh@sssihl.edu.in.
REFERENCES
- Adhikari, S. P. , Meng, S. , Wu, Y.‐J. , Mao, Y.‐P. , Ye, R.‐X. , Wang, Q.‐Z. , … Zhou, H. (2020). Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID‐19) during the early outbreak period: A scoping review. Infectious Diseases of Poverty, 9(1), 29. 10.1186/s40249-020-00646-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Ghamdi, M. , Alghamdi, K. M. , Ghandoora, Y. , Alzahrani, A. , Salah, F. , Alsulami, A. , … Sood, G. (2016). Treatment outcomes for patients with middle eastern respiratory syndrome coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infectious Diseases, 16, 174. 10.1186/s12879-016-1492-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Hajjar, S. , Memish, Z. A. , & McIntosh, K. (2013). Middle East respiratory syndrome coronavirus (MERS‐CoV): A perpetual challenge. Annals of Saudi Medicine, 33(5), 427–436. 10.5144/0256-4947.2013.427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsaad, K. , Hajeer, A. , Al Balwi, M. , Moaiqel, M. , Aloudah, N. , Ajlan, A. , … Arabi, Y. (2017). Histopathology of Middle East respiratory syndrome coronovirus (MERS‐CoV) infection—Clinicopathological and ultrastructural study. Histopathology, 72, 516–524. 10.1111/his.13379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzohairy, M. A. (2016). Therapeutics role of Azadirachta indica (Neem) and their active constituents in diseases prevention and treatment. Evidence‐Based Complementary and Alternative Medicine: ECAM, 2016, 2016–2011. 10.1155/2016/7382506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arreola, R. , Quintero‐Fabián, S. , López‐Roa, R. I. , Flores‐Gutiérrez, E. O. , Reyes‐Grajeda, J. P. , Carrera‐Quintanar, L. , & Ortuño‐Sahagún, D. (2015). Immunomodulation and anti‐inflammatory effects of garlic compounds. Journal of Immunology Research, 2015, 1–13. 10.1155/2015/401630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashbel', S. I. , & Arziaeva, E. I. (1965). Ascorbic acid aerosol therapy of patients with pneumosclerosis. Vrachebnoe Delo, 5, 141–142. [PubMed] [Google Scholar]
- Atkinson, J. , Chartier, Y. , Pessoa‐Silva, C. L. , Jensen, P. , Li, Y. , & Seto, W.‐H. (2009). Respiratory droplets. In Natural ventilation for infection control in health‐care settings, Geneva: World Health Organization. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK143281/ [PubMed] [Google Scholar]
- Avasarala, S. , Zhang, F. , Liu, G. , Wang, R. , London, S. D. , & London, L. (2013). Curcumin modulates the inflammatory response and inhibits subsequent fibrosis in a mouse model of viral‐induced acute respiratory distress syndrome. PLoS One, 8(2), e57285. 10.1371/journal.pone.0057285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila‐Carrasco, L. , Majano, P. , Sánchez‐Toméro, J. A. , Selgas, R. , López‐Cabrera, M. , Aguilera, A. , & González Mateo, G. (2019). Natural plants compounds as modulators of epithelial‐to‐Mesenchymal transition. Frontiers in Pharmacology, 10, Article 715, 1–24. 10.3389/fphar.2019.00715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahri, S. , Ben Ali, R. , Gasmi, K. , Mlika, M. , Fazaa, S. , Ksouri, R. , … Shlyonsky, V. (2017). Prophylactic and curative effect of rosemary leaves extract in a bleomycin model of pulmonary fibrosis. Pharmaceutical Biology, 55(1), 462–471. 10.1080/13880209.2016.1247881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaev, V. V. , & Duntau, A. P. (2004). Ascorbic acid in blood serum of patients with pulmonary tuberculosis and pneumonia. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union against Tuberculosis and Lung Disease, 8(2), 263–266. [PubMed] [Google Scholar]
- Bayan, L. , Koulivand, P. H. , & Gorji, A. (2014). Garlic: A review of potential therapeutic effects. Avicenna Journal of Phytomedicine, 4(1), 1–14. [PMC free article] [PubMed] [Google Scholar]
- Belouzard, S. , Chu, V. C. , & Whittaker, G. R. (2009). Activation of the SARS coronavirus spike protein via sequential proteolytic cleavage at two distinct sites. Proceedings of the National Academy of Sciences, 106(14), 5871–5876. 10.1073/pnas.0809524106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharara, A. , Grossman, C. , Grinnan, D. , Syed, A. , Fisher, B. , DeWilde, C. , … Fowler, A. A. (. B.). (2016). Intravenous vitamin C administered as adjunctive therapy for recurrent acute respiratory distress syndrome. Case Reports in Critical Care, 2016, 1–4. 10.1155/2016/8560871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya, M. , Girish, G. V. , Karmohapatra, S. K. , Samad, S. A. , & Sinha, A. K. (2007). Systemic production of IFN‐alpha by garlic (Allium sativum) in humans. Journal of Interferon & Cytokine Research: The Official Journal of the International Society for Interferon and Cytokine Research, 27(5), 377–382. 10.1089/jir.2006.0124 [DOI] [PubMed] [Google Scholar]
- Butt, M. S. , Pasha, I. , Sultan, M. T. , Randhawa, M. A. , Saeed, F. , & Ahmed, W. (2013). Black pepper and health claims: A comprehensive treatise. Critical Reviews in Food Science and Nutrition, 53(9), 875–886. 10.1080/10408398.2011.571799 [DOI] [PubMed] [Google Scholar]
- Cai, W. , Yu, D. , Fan, J. , Liang, X. , Jin, H. , Liu, C. , … Yu, J. (2018). Quercetin inhibits transforming growth factor β1‐induced epithelial–mesenchymal transition in human retinal pigment epithelial cells via the Smad pathway. Drug Design, Development and Therapy, 12, 4149–4161. 10.2147/DDDT.S185618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao, B. , Wang, Y. , Wen, D. , Liu, W. , Wang, J. , Fan, G. , … Wang, C. (2020). A trial of Lopinavir–Ritonavir in adults hospitalized with severe Covid‐19. New England Journal of Medicine, 382(19), 1787–1799. 10.1056/NEJMoa2001282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr, A. C. , & Maggini, S. (2017). Vitamin C and immune function. Nutrients, 9(11), Article 1211, 1–25. 10.3390/nu9111211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascella, M. , Rajnik, M. , Cuomo, A. , Dulebohn, S. C. , & Di Napoli, R. (2020). Features, evaluation and treatment coronavirus (COVID‐19). In StatPearls, Treasure Island (FL): StatPearls. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK554776/ [PubMed] [Google Scholar]
- Chambial, S. , Dwivedi, S. , Shukla, K. K. , John, P. J. , & Sharma, P. (2013). Vitamin C in disease prevention and cure: An overview. Indian Journal of Clinical Biochemistry, 28(4), 314–328. 10.1007/s12291-013-0375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang, J. S. , Wang, K. C. , Yeh, C. F. , Shieh, D. E. , & Chiang, L. C. (2013). Fresh ginger (Zingiber officinale) has anti‐viral activity against human respiratory syncytial virus in human respiratory tract cell lines. Journal of Ethnopharmacology, 145(1), 146–151. 10.1016/j.jep.2012.10.043 [DOI] [PubMed] [Google Scholar]
- Chen, H. , Yang, R. , Tang, Y. , Xu, J. , Feng, Y. , Liu, S. , … Hou, L. (2017). Effects of curcumin on pulmonary fibrosis and functions of paraquat‐challenged rats. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 29(11), 973–976. 10.3760/cma.j.issn.2095-4352.2017.11.003 [DOI] [PubMed] [Google Scholar]
- Chen, L. , & Zhao, W. (2016). Apigenin protects against bleomycin‐induced lung fibrosis in rats. Experimental and Therapeutic Medicine, 11(1), 230–234. 10.3892/etm.2015.2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , Cheung, F. W. K. , Chan, M. H. , Hui, P. K. , Ip, S.‐P. , Ling, Y. H. , … Liu, W. K. (2012). Protective roles of Cordyceps on lung fibrosis in cellular and rat models. Journal of Ethnopharmacology, 143(2), 448–454. 10.1016/j.jep.2012.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X.‐Y. , Dou, Y.‐X. , Luo, D.‐D. , Zhang, Z.‐B. , Li, C.‐L. , Zeng, H.‐F. , … Li, Y.‐C. (2017). β‐Patchoulene from patchouli oil protects against LPS‐induced acute lung injury via suppressing NF‐κB and activating Nrf2 pathways. International Immunopharmacology, 50, 270–278. 10.1016/j.intimp.2017.07.001 [DOI] [PubMed] [Google Scholar]
- Cheresh, P. , Kim, S.‐J. , Tulasiram, S. , & Kamp, D. W. (2013). Oxidative stress and pulmonary fibrosis. Biochimica et Biophysica Acta, 1832(7), 1028–1040. 10.1016/j.bbadis.2012.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, K. , Kou, J.‐Q. , Gu, J.‐H. , Han, R. , Wang, G. , Zhen, X. , & Qin, Z.‐H. (2014). Naja naja atra venom ameliorates pulmonary fibrosis by inhibiting inflammatory response and oxidative stress. BMC Complementary and Alternative Medicine, 14(1), Article 461, 1–11. 10.1186/1472-6882-14-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui, Y. , Jiang, L. , Yu, R. , Shao, Y. , Mei, L. , & Tao, Y. (2019). β‐carboline alkaloids attenuate bleomycin induced pulmonary fibrosis in mice through inhibiting NF‐kb/p65 phosphorylation and epithelial–mesenchymal transition. Journal of Ethnopharmacology, 243, 112096. 10.1016/j.jep.2019.112096 [DOI] [PubMed] [Google Scholar]
- DeDiego, M. L. , Nieto‐Torres, J. L. , Regla‐Nava, J. A. , Jimenez‐Guardeño, J. M. , Fernandez‐Delgado, R. , Fett, C. , … Enjuanes, L. (2014). Inhibition of NF‐κB‐mediated inflammation in severe acute respiratory syndrome coronavirus‐infected mice increases survival. Journal of Virology, 88(2), 913–924. 10.1128/JVI.02576-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng, Y. , Liu, W. , Liu, K. , Fang, Y.‐Y. , Shang, J. , Zhou, L. , … Liu, H.‐G. (2020). Clinical characteristics of fatal and recovered cases of coronavirus disease 2019 (COVID‐19) in Wuhan, China: A retrospective study. Chinese Medical Journal, 133, 1261–1267. 10.1097/CM9.0000000000000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmarathna, S. L. C. A. , Wickramasinghe, S. , Waduge, R. N. , Rajapakse, R. P. V. J. , & Kularatne, S. A. M. (2013). Does Carica papaya leaf‐extract increase the platelet count? An experimental study in a murine model. Asian Pacific Journal of Tropical Biomedicine, 3(9), 720–724. 10.1016/S2221-1691(13)60145-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pierro, F. , Bertuccioli, A. , & Cavecchia, I. (2020). Possible therapeutic role of a highly standardized mixture of active compounds derived from cultured Lentinula edodes mycelia (AHCC) in patients infected with 2019 novel coronavirus. Minerva Gastroenterologica E Dietologica, 66(2), 172–176. 10.23736/S1121-421X.20.02697-5 [DOI] [PubMed] [Google Scholar]
- Ding, H. , Ci, X. , Cheng, H. , Yu, Q. , & Li, D. (2019). Chicoric acid alleviates lipopolysaccharide‐induced acute lung injury in mice through anti‐inflammatory and anti‐oxidant activities. International Immunopharmacology, 66, 169–176. 10.1016/j.intimp.2018.10.042 [DOI] [PubMed] [Google Scholar]
- Divya, T. , Dineshbabu, V. , Soumyakrishnan, S. , Sureshkumar, A. , & Sudhandiran, G. (2016). Celastrol enhances Nrf2 mediated antioxidant enzymes and exhibits anti‐fibrotic effect through regulation of collagen production against bleomycin‐induced pulmonary fibrosis. Chemico‐Biological Interactions, 246, 52–62. 10.1016/j.cbi.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Dong, L. , Hu, S. , & Gao, J. (2020). Discovering drugs to treat coronavirus disease 2019 (COVID‐19). Drug Discoveries & Therapeutics, 14(1), 58–60. 10.5582/ddt.2020.01012 [DOI] [PubMed] [Google Scholar]
- Dong, S.‐H. , Liu, Y.‐W. , Wei, F. , Tan, H.‐Z. , & Han, Z.‐D. (2017). Asiatic acid ameliorates pulmonary fibrosis induced by bleomycin (BLM) via suppressing pro‐fibrotic and inflammatory signaling pathways. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 89, 1297–1309. 10.1016/j.biopha.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Duan, S.‐M. , Zhao, X.‐S. , Wen, R.‐F. , Huang, J.‐J. , Pi, G.‐H. , Zhang, S.‐X. , … SARS Research Team . (2003). Stability of SARS coronavirus in human specimens and environment and its sensitivity to heating and UV irradiation. Biomedical and Environmental Sciences: BES, 16(3), 246–255. [PubMed] [Google Scholar]
- El‐Fakharany, E. M. , Haroun, B. M. , Ng, T. B. , & Redwan, E. R. (2010). Oyster mushroom laccase inhibits hepatitis C virus entry into peripheral blood cells and hepatoma cells. Protein and Peptide Letters, 17(8), 1031–1039. 10.2174/092986610791498948 [DOI] [PubMed] [Google Scholar]
- Ellan, K. , Thayan, R. , Raman, J. , Hidari, K. I. P. J. , Ismail, N. , & Sabaratnam, V. (2019). Anti‐viral activity of culinary and medicinal mushroom extracts against dengue virus serotype 2: An in‐vitro study. BMC Complementary and Alternative Medicine, 19(1), 260. 10.1186/s12906-019-2629-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, Y. , Zhao, K. , & Zhou, P. (2019). Bat coronaviruses in China. Viruses, 11, 210. 10.3390/v11030210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farazuddin, M. , Mishra, R. , Jing, Y. , Srivastava, V. , Comstock, A. T. , & Sajjan, U. S. (2018). Quercetin prevents rhinovirus‐induced progression of lung disease in mice with COPD phenotype. PLoS One, 13(7), e0199612. 10.1371/journal.pone.0199612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr, A. R. , & Perlman, S. (2015). Coronaviruses: An overview of their replication and pathogenesis. Methods in Molecular Biology (Clifton, N.J.), 1282, 1–23. 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, B. J. , Kraskauskas, D. , Martin, E. J. , Farkas, D. , Puri, P. , Massey, H. D. , … Natarajan, R. (2014). Attenuation of sepsis‐induced organ injury in mice by vitamin C. JPEN: Journal of Parenteral and Enteral Nutrition, 38(7), 825–839. 10.1177/0148607113497760 [DOI] [PubMed] [Google Scholar]
- Fisher, B. J. , Kraskauskas, D. , Martin, E. J. , Farkas, D. , Wegelin, J. A. , Brophy, D. , … Natarajan, R. (2012). Mechanisms of attenuation of abdominal sepsis induced acute lung injury by ascorbic acid. American Journal of Physiology. Lung Cellular and Molecular Physiology, 303(1), L20–L32. 10.1152/ajplung.00300.2011 [DOI] [PubMed] [Google Scholar]
- Fisher, B. J. , Seropian, I. M. , Kraskauskas, D. , Thakkar, J. N. , Voelkel, N. F. , Fowler, A. A. , & Natarajan, R. (2011). Ascorbic acid attenuates lipopolysaccharide‐induced acute lung injury. Critical Care Medicine, 39(6), 1454–1460. 10.1097/CCM.0b013e3182120cb8 [DOI] [PubMed] [Google Scholar]
- Franks, T. J. , Chong, P. Y. , Chui, P. , Galvin, J. R. , Lourens, R. M. , Reid, A. H. , … Travis, W. D. (2003). Lung pathology of severe acute respiratory syndrome (SARS): A study of 8 autopsy cases from Singapore. Human Pathology, 34(8), 743–748. 10.1016/s0046-8177(03)00367-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, Y. , Cheng, Y. , & Wu, Y. (2020). Understanding SARS‐CoV‐2‐mediated inflammatory responses: From mechanisms to potential therapeutic tools. Virologica Sinica, 35(3), 266–271. 10.1007/s12250-020-00207-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam, S. C. , Gao, X. , & Dulchavsky, S. (2007). Immunomodulation by curcumin. Advances in Experimental Medicine and Biology, 595, 321–341. 10.1007/978-0-387-46401-5_14 [DOI] [PubMed] [Google Scholar]
- Gautret, P. , Lagier, J.‐C. , Parola, P. , Hoang, V. T. , Meddeb, L. , Mailhe, M. , … Raoult, D. (2020). Hydroxychloroquine and azithromycin as a treatment of COVID‐19: Results of an open‐label non‐randomized clinical trial. International Journal of Antimicrobial Agents, 105949. 10.1016/j.ijantimicag.2020.105949 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ghinai, I. , McPherson, T. D. , Hunter, J. C. , Kirking, H. L. , Christiansen, D. , Joshi, K. , … Layden, J. E. (2020). First known person‐to‐person transmission of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in the USA. The Lancet, 395(10230), 1137–1144. 10.1016/S0140-6736(20)30607-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholamnezhad, Z. , Shakeri, F. , Saadat, S. , Ghorani, V. , & Boskabady, M. H. (2019). Clinical and experimental effects of Nigella sativa and its constituents on respiratory and allergic disorders. Avicenna Journal of Phytomedicine, 9(3), 195–212. [PMC free article] [PubMed] [Google Scholar]
- Glowacka, I. , Bertram, S. , Herzog, P. , Pfefferle, S. , Steffen, I. , Muench, M. O. , … Pöhlmann, S. (2010). Differential downregulation of ACE2 by the spike proteins of severe acute respiratory syndrome coronavirus and human coronavirus NL63. Journal of Virology, 84(2), 1198–1205. 10.1128/JVI.01248-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan, S. , Ediga, H. H. , Reddy, S. S. , Reddy, G. B. , & Ismail, A. (2018). Procyanidin‐B2 enriched fraction of cinnamon acts as a proteasome inhibitor and anti‐proliferative agent in human prostate cancer cells. IUBMB Life, 70(5), 445–457. 10.1002/iub.1735 [DOI] [PubMed] [Google Scholar]
- Grant‐Alfieri, A. , Schaechter, J. , & Lipshultz, S. E. (2013). Ingesting and aspirating dry cinnamon by children and adolescents: The “cinnamon challenge”. Pediatrics, 131(5), 833–835. 10.1542/peds.2012-3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu, J. , & Korteweg, C. (2007). Pathology and pathogenesis of severe acute respiratory syndrome. The American Journal of Pathology, 170(4), 1136–1147. 10.2353/ajpath.2007.061088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, R. , Wang, X. , Zhao, X. , Song, N. , Zhu, J. , Wang, J. , … Shen, L. (2016). Emodin ameliorates bleomycin‐induced pulmonary fibrosis in rats by suppressing epithelial–mesenchymal transition and fibroblast activation. Scientific Reports, 6, 35696. 10.1038/srep35696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan, W. , Ni, Z. , Hu, Y. , Liang, W. , Ou, C. , He, J. , … Zhong, N. (2020). Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine, 382(18), 1708–1720. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, Y.‐R. , Cao, Q.‐D. , Hong, Z.‐S. , Tan, Y.‐Y. , Chen, S.‐D. , Jin, H.‐J. , … Yan, Y. (2020). The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak—An update on the status. Military Medical Research, 7(1), 11. 10.1186/s40779-020-00240-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida, M. G. (2019). Middle East respiratory syndrome coronavirus and the one health concept. PeerJ, 7, e7556. 10.7717/peerj.7556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, J.‐W. , Yang, G.‐E. , Kim, Y. B. , Eom, S. H. , Lew, J.‐H. , & Kang, H. (2012). Anti‐inflammatory activity of cinnamon water extract in vivo and in vitro LPS‐induced models. BMC Complementary and Alternative Medicine, 12, Article 237, 1–8. 10.1186/1472-6882-12-237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong, J. Y. , Lee, C. Y. , Lee, M. G. , & Kim, Y. S. (2018). Effects of dietary antioxidant vitamins on lung functions according to gender and smoking status in Korea: A population‐based cross‐sectional study. BMJ Open, 8(4), e020656. 10.1136/bmjopen-2017-020656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini, A. , Rasaie, D. , Asl, S. S. , Ahmadabadi, A. N. , & Ranjbar, A. (2019). Evaluation of the protective effects of curcumin and nanocurcumin against lung injury induced by sub‐acute exposure to paraquat in rats. Toxin Reviews, 1–9. 10.1080/15569543.2019.1675707 [DOI] [Google Scholar]
- Hu, S. , Liu, X. , Yin, S. A. , & Xu, Q. (1998). Effect of selenium on children suffered from Mycoplasma pneumonia. Wei Sheng Yan Jiu = Journal of Hygiene Research, 27(5), 344–347. [PubMed] [Google Scholar]
- Huang, C. , Li, Y. , Fan, X. , Wang, W. , & Zhan, X. (2013). Effects of combination of salvia and ligustrazine on TNF‐α and TGF‐β1 in serum and BALF of rats with pulmonary fibrosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chinese Journal of Cellular and Molecular Immunology, 29(7), 673–676. [PubMed] [Google Scholar]
- Huang, S. (2013). Inhibition of PI3K/Akt/mTOR signaling by natural products. Anti‐Cancer Agents in Medicinal Chemistry, 13(7), 967–970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang, T.‐T. , Lai, H.‐C. , Ko, Y.‐F. , Ojcius, D. M. , Lan, Y.‐W. , Martel, J. , … Chong, K.‐Y. (2015). Hirsutella sinensis mycelium attenuates bleomycin‐induced pulmonary inflammation and fibrosis in vivo. Scientific Reports, 5, 15282. 10.1038/srep15282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hügle, T. (2011). Immunology of fibrotic lung disease: Managing infections whilst preventing autoimmunity? Journal of Inflammation Research, 4, 21–27. 10.2147/JIR.S10602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt, C. , Chakravorty, N. K. , Annan, G. , Habibzadeh, N. , & Schorah, C. J. (1994). The clinical effects of vitamin C supplementation in elderly hospitalised patients with acute respiratory infections. International Journal for Vitamin and Nutrition Research. Internationale Zeitschrift Fur Vitamin‐ Und Ernahrungsforschung. Journal International De Vitaminologie et De Nutrition, 64(3), 212–219. [PubMed] [Google Scholar]
- Impellizzeri, D. , Talero, E. , Siracusa, R. , Alcaide, A. , Cordaro, M. , Maria Zubelia, J. , … Motilva, V. (2015). Protective effect of polyphenols in an inflammatory process associated with experimental pulmonary fibrosis in mice. The British Journal of Nutrition, 114(6), 853–865. 10.1017/S0007114515002597 [DOI] [PubMed] [Google Scholar]
- Ishikawa, H. , Saeki, T. , Otani, T. , Suzuki, T. , Shimozuma, K. , Nishino, H. , … Morimoto, K. (2006). Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. The Journal of Nutrition, 136(3 Suppl), 816S–820S. 10.1093/jn/136.3.816S [DOI] [PubMed] [Google Scholar]
- Jayaprakasha, G. K. , & Rao, L. J. M. (2011). Chemistry, biogenesis, and biological activities of Cinnamomum zeylanicum . Critical Reviews in Food Science and Nutrition, 51(6), 547–562. 10.1080/10408391003699550 [DOI] [PubMed] [Google Scholar]
- Jayashree, K. , Manasa, G. C. , Pallavi, P. , & Manjunath, G. V. (2011). Evaluation of platelets as predictive parameters in dengue fever. Indian Journal of Hematology & Blood Transfusion, 27(3), 127–130. 10.1007/s12288-011-0075-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji, Y. , Dou, Y.‐N. , Zhao, Q.‐W. , Zhang, J.‐Z. , Yang, Y. , Wang, T. , … Wei, Z.‐F. (2016). Paeoniflorin suppresses TGF‐β mediated epithelial–mesenchymal transition in pulmonary fibrosis through a Smad‐dependent pathway. Acta Pharmacologica Sinica, 37(6), 794–804. 10.1038/aps.2016.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia, L. , Sun, P. , Gao, H. , Shen, J. , Gao, Y. , Meng, C. , … Zhang, G. (2019). Mangiferin attenuates bleomycin‐induced pulmonary fibrosis in mice through inhibiting TLR4/p65 and TGF‐β1/Smad2/3 pathway. The Journal of Pharmacy and Pharmacology, 71(6), 1017–1028. 10.1111/jphp.13077 [DOI] [PubMed] [Google Scholar]
- Jiang, W. , Luo, F. , Lu, Q. , Liu, J. , Li, P. , Wang, X. , … Ding, X. (2016). The protective effect of Trillin LPS‐induced acute lung injury by the regulations of inflammation and oxidative state. Chemico‐Biological Interactions, 243, 127–134. 10.1016/j.cbi.2015.09.010 [DOI] [PubMed] [Google Scholar]
- Jin, M. , Wu, Y. , Wang, L. , Zang, B. , & Tan, L. (2016). Hydroxysafflor yellow a attenuates bleomycin‐induced pulmonary fibrosis in mice. Phytotherapy Research: PTR, 30(4), 577–587. 10.1002/ptr.5560 [DOI] [PubMed] [Google Scholar]
- Joseph, B. , Sankarganesh, P. , Ichiyama, K. , & Yamamoto, N. (2015). In vitro study on cytotoxic effect and anti‐DENV2 activity of Carica papaya L. leaf. Frontiers in Life Science, 8(1), 18–22. 10.1080/21553769.2014.924080 [DOI] [Google Scholar]
- Jung, S.‐J. , Jung, E.‐S. , Choi, E.‐K. , Sin, H.‐S. , Ha, K.‐C. , & Chae, S.‐W. (2019). Immunomodulatory effects of a mycelium extract of Cordyceps (Paecilomyces hepiali; CBG‐CS‐2): A randomized and double‐blind clinical trial. BMC Complementary and Alternative Medicine, 19(1), 77. 10.1186/s12906-019-2483-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurenka, J. S. (2009). Anti‐inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Alternative Medicine Review: A Journal of Clinical Therapeutic, 14(2), 141–153. [PubMed] [Google Scholar]
- Kampf, G. , & Kramer, A. (2004). Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clinical Microbiology Reviews, 17(4), 863–893. 10.1128/CMR.17.4.863-893.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawatra, P. , & Rajagopalan, R. (2015). Cinnamon: Mystic powers of a minute ingredient. Pharmacognosy Research, 7(Suppl 1), S1–S6. 10.4103/0974-8490.157990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedekar, S. , Goel, S. , & Ojha, N. (2016). Ayurveda Dhoopana (medicated smoke) chikitsa in present scenario: A review. International Journal of Research in Ayurveda & Pharmacy, 7, 98–102. 10.7897/2277-4343.07265 [DOI] [Google Scholar]
- Killerby, M. E. , Biggs, H. M. , Midgley, C. M. , Gerber, S. I. , & Watson, J. T. (2020). Middle East respiratory syndrome coronavirus transmission. Emerging Infectious Diseases, 26(2), 191–198. 10.3201/eid2602.190697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, S. , Jung, Y. , An, H. , Kim, D.‐H. , Jang, E. , Choi, Y. , … Chung, H.‐Y. (2013). Anti‐wrinkle and anti‐inflammatory effects of active garlic components and the inhibition of MMPs via NF‐κB signaling. PLoS One, 8, e73877. 10.1371/journal.pone.0073877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi, T. , Kim, H. , Liu, X. , Sugiura, H. , Kohyama, T. , Fang, Q. , … Rennard, S. I. (2014). Matrix metalloproteinase‐9 activates TGF‐β and stimulates fibroblast contraction of collagen gels. American Journal of Physiology. Lung Cellular and Molecular Physiology, 306(11), L1006–L1015. 10.1152/ajplung.00015.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuba, K. , Imai, Y. , Rao, S. , Gao, H. , Guo, F. , Guan, B. , … Penninger, J. M. (2005). A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus‐induced lung injury. Nature Medicine, 11(8), 875–879. 10.1038/nm1267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni, R. A. , & Deshpande, A. R. (2016). Anti‐inflammatory and antioxidant effect of ginger in tuberculosis. Journal of Complementary and Integrative Medicine, 13(2), 201–206. 10.1515/jcim-2015-0032 [DOI] [PubMed] [Google Scholar]
- Kumar, D. , Kumar, M. , Saravanan, C. , & Singh, S. K. (2012). Curcumin: A potential candidate for matrix metalloproteinase inhibitors. Expert Opinion on Therapeutic Targets, 16(10), 959–972. 10.1517/14728222.2012.710603 [DOI] [PubMed] [Google Scholar]
- Kumar, S. , & Pandey, A. K. (2013). Chemistry and biological activities of flavonoids: An overview. The Scientific World Journal, 2013, 1–16. 10.1155/2013/162750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyung, S. Y. , Kim, D. Y. , Yoon, J. Y. , Son, E. S. , Kim, Y. J. , Park, J. W. , & Jeong, S. H. (2018). Sulforaphane attenuates pulmonary fibrosis by inhibiting the epithelial–mesenchymal transition. BMC Pharmacology & Toxicology, 19(1), 13. 10.1186/s40360-018-0204-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai, C.‐C. , Liu, Y. H. , Wang, C.‐Y. , Wang, Y.‐H. , Hsueh, S.‐C. , Yen, M.‐Y. , … Hsueh, P.‐R. (2020). Asymptomatic carrier state, acute respiratory disease, and pneumonia due to severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2): Facts and myths. Journal of Microbiology, Immunology and Infection, 53, 404–412. 10.1016/j.jmii.2020.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. C. , Kinniry, P. A. , Arguiri, E. , Serota, M. , Kanterakis, S. , Chatterjee, S. , … Christofidou‐Solomidou, M. (2010). Dietary Curcumin increases antioxidant defenses in lung, ameliorates radiation‐induced pulmonary fibrosis, and improves survival in mice. Radiation Research, 173(5), 590–601. 10.1667/RR1522.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J.‐W. , Ryu, H. W. , Park, S.‐Y. , Park, H. A. , Kwon, O.‐K. , Yuk, H. J. , … Ahn, K.‐S. (2017). Protective effects of neem (Azadirachta indica A. Juss.) leaf extract against cigarette smoke‐ and lipopolysaccharide‐induced pulmonary inflammation. International Journal of Molecular Medicine, 40(6), 1932–1940. 10.3892/ijmm.2017.3178 [DOI] [PubMed] [Google Scholar]
- Lee, S. K. , Park, Y. J. , Ko, M. J. , Wang, Z. , Lee, H. Y. , Choi, Y. W. , & Bae, Y.‐S. (2015). A novel natural compound from garlic (Allium sativum L.) with therapeutic effects against experimental polymicrobial sepsis. Biochemical and Biophysical Research Communications, 464(3), 774–779. 10.1016/j.bbrc.2015.07.031 [DOI] [PubMed] [Google Scholar]
- Lee, Y.‐H. , Lee, S. J. , Lee, M. K. , Lee, W.‐Y. , Yong, S. J. , & Kim, S.‐H. (2016). Serum selenium levels in patients with respiratory diseases: A prospective observational study. Journal of Thoracic Disease, 8(8), 2068–2078. 10.21037/jtd.2016.07.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung, P.‐O. , Lee, H.‐H. , Kung, Y.‐C. , Tsai, M.‐F. , & Chou, T.‐C. (2013). Therapeutic effect of C‐phycocyanin extracted from blue green algae in a rat model of acute lung injury induced by lipopolysaccharide. Evidence‐Based Complementary and Alternative Medicine: ECAM, 2013, 916590. 10.1155/2013/916590, 1, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, C. , Yu, Y. , Li, W. , Liu, B. , Jiao, X. , Song, X. , … Qin, S. (2017). Phycocyanin attenuates pulmonary fibrosis via the TLR2‐MyD88‐NF‐κB signaling pathway. Scientific Reports, 7, 5843. 10.1038/s41598-017-06021-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Feng, M. , Sun, R. , Li, Z. , Hu, L. , Peng, G. , … Liu, J. (2020). Andrographolide ameliorates bleomycin‐induced pulmonary fibrosis by suppressing cell proliferation and myofibroblast differentiation of fibroblasts via the TGF‐β1‐mediated Smad‐dependent and ‐independent pathways. Toxicology Letters, 321, 103–113. 10.1016/j.toxlet.2019.11.003 [DOI] [PubMed] [Google Scholar]
- Li, S.‐W. , Yang, T.‐C. , Wan, L. , Lin, Y.‐J. , Tsai, F.‐J. , Lai, C.‐C. , & Lin, C.‐W. (2012). Correlation between TGF‐β1 expression and proteomic profiling induced by severe acute respiratory syndrome coronavirus papain‐like protease. Proteomics, 12(21), 3193–3205. 10.1002/pmic.201200225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W.‐W. , Wang, T.‐Y. , Cao, B. , Liu, B. , Rong, Y.‐M. , Wang, J.‐J. , … Liu, Y.‐X. (2019). Synergistic protection of matrine and lycopene against lipopolysaccharide‐induced acute lung injury in mice. Molecular Medicine Reports, 20(1), 455–462. 10.3892/mmr.2019.10278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, X.‐H. , Xiao, T. , Yang, J.‐H. , Qin, Y. , Gao, J.‐J. , Liu, H.‐J. , & Zhou, H.‐G. (2018). Parthenolide attenuated bleomycin‐induced pulmonary fibrosis via the NF‐κB/snail signaling pathway. Respiratory Research, 19(1), 111. 10.1186/s12931-018-0806-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao, Q.‐J. , Ye, L.‐B. , Timani, K. A. , Zeng, Y.‐C. , She, Y.‐L. , Ye, L. , & Wu, Z.‐H. (2005). Activation of NF‐κB by the full‐length nucleocapsid protein of the SARS coronavirus. Acta Biochimica et Biophysica Sinica, 37(9), 607–612. 10.1111/j.1745-7270.2005.00082.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnakoski, R. , Reshamwala, D. , Veteli, P. , Cortina‐Escribano, M. , Vanhanen, H. , & Marjomäki, V. (2018). Antiviral agents from fungi: Diversity, mechanisms and potential applications. Frontiers in Microbiology, 9, 2325. 10.3389/fmicb.2018.02325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippi, G. , Plebani, M. , & Henry, B. M. (2020). Thrombocytopenia is associated with severe coronavirus disease 2019 (COVID‐19) infections: A meta‐analysis. Clinica Chimica Acta, 506, 145–148. 10.1016/j.cca.2020.03.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R.‐M. , & Gaston Pravia, K. A. (2010). Oxidative stress and glutathione in TGF‐β‐mediated fibrogenesis. Free Radical Biology & Medicine, 48(1), 1–15. 10.1016/j.freeradbiomed.2009.09.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, R.‐M. , & Desai, L. P. (2015). Reciprocal regulation of TGF‐β and reactive oxygen species: A perverse cycle for fibrosis. Redox Biology, 6, 565–577. 10.1016/j.redox.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Yin, S. , & Li, G. (1997). Effects of selenium supplement on acute lower respiratory tract infection caused by respiratory syncytial virus. Zhonghua Yu Fang Yi Xue Za Zhi [Chinese Journal of Preventive Medicine], 31(6), 358–361. [PubMed] [Google Scholar]
- Liu, Y. , Song, M. , Zhu, G. , Xi, X. , Li, K. , Wu, C. , & Huang, L. (2017). Corynoline attenuates LPS‐induced acute lung injury in mice by activating Nrf2. International Immunopharmacology, 48, 96–101. 10.1016/j.intimp.2017.04.029 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Li, Y. , Li, N. , Teng, W. , Wang, M. , Zhang, Y. , & Xiao, Z. (2016). TGF‐β1 promotes scar fibroblasts proliferation and transdifferentiation via up‐regulating MicroRNA‐21. Scientific Reports, 6(1), 1–9. 10.1038/srep32231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, J. , He, R. , Sun, P. , Zhang, F. , Linhardt, R. J. , & Zhang, A. (2020). Molecular mechanisms of bioactive polysaccharides from Ganoderma lucidum (Lingzhi), a review. International Journal of Biological Macromolecules, 150, 765–774. 10.1016/j.ijbiomac.2020.02.035 [DOI] [PubMed] [Google Scholar]
- Lv, H. , Liu, Q. , Wen, Z. , Feng, H. , Deng, X. , & Ci, X. (2017). Xanthohumol ameliorates lipopolysaccharide (LPS)‐induced acute lung injury via induction of AMPK/GSK3β‐Nrf2 signal axis. Redox Biology, 12, 311–324. 10.1016/j.redox.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv, H. , Yu, Z. , Zheng, Y. , Wang, L. , Qin, X. , Cheng, G. , & Ci, X. (2016). Isovitexin exerts anti‐inflammatory and anti‐oxidant activities on lipopolysaccharide‐induced acute lung injury by inhibiting MAPK and NF‐κB and activating HO‐1/Nrf2 pathways. International Journal of Biological Sciences, 12(1), 72–86. 10.7150/ijbs.13188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan, N. , Rawal, S. , Verma, M. , Poddar, M. , & Alok, S. (2013). A phytopharmacological overview on Ocimum species with special emphasis on Ocimum sanctum . Biomedicine & Preventive Nutrition, 3(2), 185–192. 10.1016/j.bionut.2012.08.002 [DOI] [Google Scholar]
- Malmström, J. , Lindberg, H. , Lindberg, C. , Bratt, C. , Wieslander, E. , Delander, E.‐L. , … Marko‐Varga, G. (2004). Transforming growth factor‐β1 specifically induce proteins involved in the myofibroblast contractile apparatus. Molecular & Cellular Proteomics, 3(5), 466–477. 10.1074/mcp.M300108-MCP200 [DOI] [PubMed] [Google Scholar]
- Mansouri, M. T. , Rajabi Vardanjani, H. , Hemmati, A. A. , Reza Tabandeh, M. , Rezaie, A. , Pashmforosh, M. , & Ahmadi Angali, K. (2019). Zingerone attenuates Bleomycin‐induced pulmonary fibrosis in rats. Jundishapur Journal of Natural Pharmaceutical Products; Kowsar., 14(1), e80098. 10.5812/jjnpp.80098 [DOI] [Google Scholar]
- Mao, Q.‐Q. , Xu, X.‐Y. , Cao, S.‐Y. , Gan, R.‐Y. , Corke, H. , Beta, T. , & Li, H.‐B. (2019). Bioactive compounds and bioactivities of ginger (Zingiber officinale roscoe). Food, 8(6), Article 185, 1–21. 10.3390/foods8060185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marefati, N. , Eftekhar, N. , Kaveh, M. , Boskabadi, J. , Beheshti, F. , & Boskabady, M. H. (2018). The effect of Allium cepa extract on lung oxidant, antioxidant, and immunological biomarkers in ovalbumin‐sensitized rats. Medical Principles and Practice, 27(2), 122–128. 10.1159/000487885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mediratta, P. K. , Sharma, K. K. , & Singh, S. (2002). Evaluation of immunomodulatory potential of Ocimum sanctum seed oil and its possible mechanism of action. Journal of Ethnopharmacology, 80(1), 15–20. 10.1016/s0378-8741(01)00373-7 [DOI] [PubMed] [Google Scholar]
- Menon, V. P. , & Sudheer, A. R. (2007). Antioxidant and anti‐inflammatory properties of curcumin. Advances in Experimental Medicine and Biology, 595, 105–125. 10.1007/978-0-387-46401-5_3 [DOI] [PubMed] [Google Scholar]
- Michalik, M. , Wójcik‐Pszczoła, K. , Paw, M. , Wnuk, D. , Koczurkiewicz, P. , Sanak, M. , … Madeja, Z. (2018). Fibroblast‐to‐myofibroblast transition in bronchial asthma. Cellular and Molecular Life Sciences, 75(21), 3943–3961. 10.1007/s00018-018-2899-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuguchi, S. , Takemura, S. , Minamiyama, Y. , Kodai, S. , Tsukioka, T. , Inoue, K. , … Suehiro, S. (2006). S‐allyl cysteine attenuated CCl4‐induced oxidative stress and pulmonary fibrosis in rats. BioFactors (Oxford, England), 26(1), 81–92. 10.1002/biof.5520260108 [DOI] [PubMed] [Google Scholar]
- Mizumoto, K. , Kagaya, K. , Zarebski, A. , & Chowell, G. (2020). Estimating the asymptomatic proportion of coronavirus disease 2019 (COVID‐19) cases on board the diamond princess cruise ship, Yokohama, Japan, 2020. Eurosurveillance, 25(10), Article 2000180. 10.2807/1560-7917.ES.2020.25.10.2000180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal, S. , Varma, S. , Bamola, V. D. , Naik, S. N. , Mirdha, B. R. , Padhi, M. M. , … Mahapatra, S. C. (2011). Double‐blinded randomized controlled trial for immunomodulatory effects of Tulsi (Ocimum sanctum Linn.) leaf extract on healthy volunteers. Journal of Ethnopharmacology, 136(3), 452–456. 10.1016/j.jep.2011.05.012 [DOI] [PubMed] [Google Scholar]
- Moselhy, S. S. , & Ali, H. K. H. (2009). Hepatoprotective effect of cinnamon extracts against carbon tetrachloride induced oxidative stress and liver injury in rats. Biological Research, 42(1), 93–98. 10.4067/S0716-97602009000100009 [DOI] [PubMed] [Google Scholar]
- Muszyńska, B. , Grzywacz‐Kisielewska, A. , Kała, K. , & Gdula‐Argasińska, J. (2018). Anti‐inflammatory properties of edible mushrooms: A review. Food Chemistry, 243, 373–381. 10.1016/j.foodchem.2017.09.149 [DOI] [PubMed] [Google Scholar]
- Nathens, A. B. , Neff, M. J. , Jurkovich, G. J. , Klotz, P. , Farver, K. , Ruzinski, J. T. , … Maier, R. V. (2002). Randomized, prospective trial of antioxidant supplementation in critically ill surgical patients. Annals of Surgery, 236(6), 814–822. 10.1097/00000658-200212000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, T. C. , Cruz, M. A. , & Carcillo, J. A. (2015). Thrombocytopenia‐associated multiple organ failure and acute kidney injury. Critical Care Clinics, 31(4), 661–674. 10.1016/j.ccc.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie, Y. , Yu, K. , Li, B. , Hu, Y. , Zhang, H. , Xin, R. , … Chai, G. (2019). S‐allyl‐l‐cysteine attenuates bleomycin‐induced pulmonary fibrosis and inflammation via AKT/NF‐κB signaling pathway in mice. Journal of Pharmacological Sciences, 139(4), 377–384. 10.1016/j.jphs.2019.03.002 [DOI] [PubMed] [Google Scholar]
- Ojha, A. , Nandi, D. , Batra, H. , Singhal, R. , Annarapu, G. K. , Bhattacharyya, S. , … Guchhait, P. (2017). Platelet activation determines the severity of thrombocytopenia in dengue infection. Scientific Reports, 7, Article 41697. 10.1038/srep41697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omrani, A. S. , Al‐Tawfiq, J. A. , & Memish, Z. A. (2015). Middle East respiratory syndrome coronavirus (MERS‐CoV): Animal to human interaction. Pathogens and Global Health, 109(8), 354–362. 10.1080/20477724.2015.1122852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzarini, E. , Dwikat, M. , Mariano, S. , Vergallo, C. , & Dini, L. (2014). Administration dependent antioxidant effect of Carica papaya seeds water extract. Evidence‐Based Complementary and Alternative Medicine: ECAM, 2014, 2014–2013. 10.1155/2014/281508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, H. S. , Kim, S. R. , & Lee, Y. C. (2009). Impact of oxidative stress on lung diseases. Respirology, 14(1), 27–38. 10.1111/j.1440-1843.2008.01447.x [DOI] [PubMed] [Google Scholar]
- Pattanaik, d. s. , Hota, D. , Prabhakar, P. , & Pandhi, P. (2009). Effect of simultaneous administration of piperine on plasma concentration of carbamazepine. Phytotherapy Research, 12, 264–269. [Google Scholar]
- Pattanayak, P. , Behera, P. , Das, D. , & Panda, S. K. (2010). Ocimum sanctum Linn. A reservoir plant for therapeutic applications: An overview. Pharmacognosy Reviews, 4(7), 95–105. 10.4103/0973-7847.65323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiris, J. S. M. , Yuen, K. Y. , Osterhaus, A. D. M. E. , & Stöhr, K. (2003). The severe acute respiratory syndrome. The New England Journal of Medicine, 349(25), 2431–2441. 10.1056/NEJMra032498 [DOI] [PubMed] [Google Scholar]
- Polansky, H. , & Lori, G. (2020). Coronavirus (COVID‐19), first indication of efficacy of Gene‐Eden‐VIR/Novirin in SARS‐CoV‐2 infections. International Journal of Antimicrobial Agents, 55, 105971. 10.1016/j.ijantimicag.2020.105971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poursalehi, H. R. , Samareh Fekri, M. , Sharifi Far, F. , Mandegari, A. , Izadi, A. , Mahmoodi, R. , … Samareh Fekri, M. (2018). Early and late preventive effect of Nigella sativa on the bleomycin‐induced pulmonary fibrosis in rats: An experimental study. Avicenna Journal of Phytomedicine, 8(3), 263–275. [PMC free article] [PubMed] [Google Scholar]
- Prashanth Goud, M. , Bale, S. , Pulivendala, G. , & Godugu, C. (2019). Therapeutic effects of Nimbolide, an autophagy regulator, in ameliorating pulmonary fibrosis through attenuation of TGF‐β1 driven epithelial‐to‐mesenchymal transition. International Immunopharmacology, 75, 105755. 10.1016/j.intimp.2019.105755 [DOI] [PubMed] [Google Scholar]
- Punithavathi, D. , Venkatesan, N. , & Babu, M. (2000). Curcumin inhibition of bleomycin‐induced pulmonary fibrosis in rats. British Journal of Pharmacology, 131(2), 169–172. 10.1038/sj.bjp.0703578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu, Y.‐L. , Cheng, X.‐N. , Bai, F. , Fang, L.‐Y. , Hu, H.‐Z. , & Sun, D.‐Q. (2018). Aucubin protects against lipopolysaccharide‐induced acute pulmonary injury through regulating Nrf2 and AMPK pathways. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 106, 192–199. 10.1016/j.biopha.2018.05.070 [DOI] [PubMed] [Google Scholar]
- Rabenau, H. F. , Cinatl, J. , Morgenstern, B. , Bauer, G. , Preiser, W. , & Doerr, H. W. (2005). Stability and inactivation of SARS coronavirus. Medical Microbiology and Immunology, 194(1), 1–6. 10.1007/s00430-004-0219-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani, A. H. , shabrmi, F. M. A. , & Aly, S. M. (2014). Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. International Journal of Physiology, Pathophysiology and Pharmacology, 6(2), 125–136. [PMC free article] [PubMed] [Google Scholar]
- Ramadan, N. , & Shaib, H. (2019). Middle East respiratory syndrome coronavirus (MERS‐CoV): A review. Germs, 9(1), 35–42. 10.18683/germs.2019.1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranasinghe, P. , Ranasinghe, P. , Abeysekera, W. P. K. M. , Premakumara, G. A. S. , Perera, Y. S. , Gurugama, P. , & Gunatilake, S. B. (2012). In vitro erythrocyte membrane stabilization properties of Carica papaya L. leaf extracts. Pharmacognosy Research, 4(4), 196–202. 10.4103/0974-8490.102261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao, P. V. , & Gan, S. H. (2014). Cinnamon: A multifaceted medicinal plant. Evidence‐Based Complementary and Alternative Medicine: Ecam, 2014, 642942. 10.1155/2014/642942, 1, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravimohan, S. , Kornfeld, H. , Weissman, D. , & Bisson, G. P. (2018). Tuberculosis and lung damage: From epidemiology to pathophysiology. European Respiratory Review: An Official Journal of the European Respiratory Society, 27(147), 27. 10.1183/16000617.0077-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues da Silva, M. , Schapochnik, A. , Peres Leal, M. , Esteves, J. , Bichels Hebeda, C. , Sandri, S. , … Lino‐dos‐Santos‐Franco, A. (2018). Beneficial effects of ascorbic acid to treat lung fibrosis induced by paraquat. PLoS One, 13(11), e0205535. 10.1371/journal.pone.0205535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, F. A. d. P. , Santos, A. D. d. C. , de Medeiros, P. H. Q. S. , Prata, M. d. M. G. , Santos, T. C. d. S. , da Silva, J. A. , … Havt, A. (2018). Gingerol suppresses sepsis‐induced acute kidney injury by modulating methylsulfonylmethane and dimethylamine production. Scientific Reports, 8(1), 1–10. 10.1038/s41598-018-30522-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothe, C. , Schunk, M. , Sothmann, P. , Bretzel, G. , Froeschl, G. , Wallrauch, C. , … Hoelscher, M. (2020). Transmission of 2019‐nCoV infection from an asymptomatic contact in Germany. The New England Journal of Medicine, 382(10), 970–971. 10.1056/NEJMc2001468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini, A. , Sharma, S. , & Chhibber, S. (2009). Induction of resistance to respiratory tract infection with Klebsiella pneumoniae in mice fed on a diet supplemented with tulsi (Ocimum sanctum) and clove (Syzgium aromaticum) oils. Journal of Microbiology, Immunology, and Infection = Wei Mian Yu Gan Ran Za Zhi, 42(2), 107–113. [PubMed] [Google Scholar]
- Salata, C. , Calistri, A. , Parolin, C. , & Palù, G. (2019). Coronaviruses: A paradigm of new emerging zoonotic diseases. Pathogens and Disease, 77, Article ftaa006. 10.1093/femspd/ftaa006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem, A. M. , Rani, S. , & Daniel, S. (2019). Effectiveness of Tulsi leaves and turmeric in steam inhalation to relieve symptoms of common cold. International Journal of Nursing & Midwifery Research, 6(2), 45–51. [Google Scholar]
- Sato, N. , Zhang, Q. , Ma, C.‐M. , & Hattori, M. (2009). Anti‐human immunodeficiency virus‐1 protease activity of new lanostane‐type triterpenoids from Ganoderma sinense. Chemical & Pharmaceutical Bulletin, 57(10), 1076–1080. 10.1248/cpb.57.1076 [DOI] [PubMed] [Google Scholar]
- Sener, G. , Topaloğlu, N. , Sehirli, A. O. , Ercan, F. , & Gedik, N. (2007). Resveratrol alleviates bleomycin‐induced lung injury in rats. Pulmonary Pharmacology & Therapeutics, 20(6), 642–649. 10.1016/j.pupt.2006.07.003 [DOI] [PubMed] [Google Scholar]
- Shahabi, R. , Anissian, A. , Javadmoosavi, S. A. , & Nasirinezhad, F. (2019). Protective and anti‐inflammatory effect of selenium nano‐particles against bleomycin‐induced pulmonary injury in male rats. Drug and Chemical Toxicology, 1–9. 10.1080/01480545.2018.1560466 [DOI] [PubMed] [Google Scholar]
- Shahwar, D. , Raza, M. A. , Shafiq‐Ur‐Rehman, Abbasi, M. A. , & Atta‐Ur‐Rahman. (2012). An investigation of phenolic compounds from plant sources as trypsin inhibitors. Natural Product Research, 26(12), 1087–1093. 10.1080/14786419.2011.559637 [DOI] [PubMed] [Google Scholar]
- Shang, A. , Cao, S.‐Y. , Xu, X.‐Y. , Gan, R.‐Y. , Tang, G.‐Y. , Corke, H. , … Li, H.‐B. (2019). Bioactive compounds and biological functions of garlic (Allium sativum L.). Food, 8(7), Article 246, 1–31. 10.3390/foods8070246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shariati, S. , Kalantar, H. , Pashmforoosh, M. , Mansouri, E. , & Khodayar, M. J. (2019). Epicatechin protective effects on bleomycin‐induced pulmonary oxidative stress and fibrosis in mice. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie, 114, 108776. 10.1016/j.biopha.2019.108776 [DOI] [PubMed] [Google Scholar]
- Shariatpanahi, Z. V. , Taleban, F. A. , Mokhtari, M. , & Shahbazi, S. (2010). Ginger extract reduces delayed gastric emptying and nosocomial pneumonia in adult respiratory distress syndrome patients hospitalized in an intensive care unit. Journal of Critical Care, 25(4), 647–650. 10.1016/j.jcrc.2009.12.008 [DOI] [PubMed] [Google Scholar]
- Sharma, A. , Fölster‐Holst, R. , Kassir, M. , Szepietowski, J. , Jafferany, M. , Lotti, T. , & Goldust, M. (2020). The effect of quarantine and isolation for COVID‐19 in general population and dermatologic treatments. Dermatologic Therapy, e13398. 10.1111/dth.13398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirpoor, A. , Gharalari, F. H. , Rasmi, Y. , & Heshmati, E. (2017). Ginger extract attenuates ethanol‐induced pulmonary histological changes and oxidative stress in rats. Journal of Biomedical Research, 31(6), 521–527. 10.7555/JBR.31.20160151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoba, G. , Joy, D. , Joseph, T. , Majeed, M. , Rajendran, R. , & Srinivas, P. S. (1998). Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers. Planta Medica, 64(4), 353–356. 10.1055/s-2006-957450 [DOI] [PubMed] [Google Scholar]
- Shou, Q.‐Y. , Fu, H.‐Y. , Zhang, L.‐Z. , Cai, Y.‐Q. , Chen, F.‐M. , & Chen, M.‐L. (2012). Study on treatment effect and mechanism of Hirsutella sinensis mycelium in idiopathic pulmonary fibrosis in rats. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China Journal of Chinese Materia Medica, 37(23), 3618–3623. [PubMed] [Google Scholar]
- Shuman, K. , Raju, R. , & Jogdeo, B. (2018). Effectiveness of steam inhalation with Tulsi leaves among children with upper respiratory tract infection. International Journal of Applied Research, 4(7), 80–83. [Google Scholar]
- Sillapachaiyaporn, C. , Nilkhet, S. , Ung, A. T. , & Chuchawankul, S. (2019). Anti‐HIV‐1 protease activity of the crude extracts and isolated compounds from Auricularia polytricha. BMC Complementary and Alternative Medicine, 19(1), 351. 10.1186/s12906-019-2766-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons, G. , Bertram, S. , Glowacka, I. , Steffen, I. , Chaipan, C. , Agudelo, J. , … Pöhlmann, S. (2011). Different host cell proteases activate the SARS‐coronavirus spike‐protein for cell‐cell and virus‐cell fusion. Virology, 413(2), 265–274. 10.1016/j.virol.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, M. , Singhi, S. , & Walia, B. N. (1990). Evaluation of steam therapy in acute lower respiratory tract infections: A pilot study. Indian Pediatrics, 27(9), 945–951. [PubMed] [Google Scholar]
- Singhal, T. (2020). A review of coronavirus Disease‐2019 (COVID‐19). Indian Journal of Pediatrics, 87, 281–286. 10.1007/s12098-020-03263-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, M. R. , Gangireddy, S. R. , Narala, V. R. , Hogaboam, C. M. , Standiford, T. J. , Christensen, P. J. , … Reddy, R. C. (2010). Curcumin inhibits fibrosis‐related effects in IPF fibroblasts and in mice following bleomycin‐induced lung injury. American Journal of Physiology‐Lung Cellular and Molecular Physiology, 298(5), L616–L625. 10.1152/ajplung.00002.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasa, A. , Tosounidou, S. , & Gordon, C. (2017). Increased incidence of gastrointestinal side effects in patients taking hydroxychloroquine: A brand‐related issue? The Journal of Rheumatology, 44(3), 398. 10.3899/jrheum.161063 [DOI] [PubMed] [Google Scholar]
- Srivastava, R. M. , Singh, S. , Dubey, S. K. , Misra, K. , & Khar, A. (2011). Immunomodulatory and therapeutic activity of curcumin. International Immunopharmacology, 11(3), 331–341. 10.1016/j.intimp.2010.08.014 [DOI] [PubMed] [Google Scholar]
- Steiropoulos, P. , Boglou, P. , Ntolios, P. , Tzouvelekis, A. , Xanthoudaki, M. , Kouratzi, M. , Koulelidis, A. , Nena, E. , Froudarakis, M. E. , & Bouros, D. (2014). Platelet activation indices in patients with idiopathic pulmonary fibrosis. European Respiratory Journal, 44 (Suppl 58), Article P3769. https://erj.ersjournals.com/content/44/Suppl_58/P3769 [DOI] [PubMed] [Google Scholar]
- Subapriya, R. , & Nagini, S. (2005). Medicinal properties of neem leaves: A review. Current Medicinal Chemistry. Anti‐Cancer Agents, 5(2), 149–156. 10.2174/1568011053174828 [DOI] [PubMed] [Google Scholar]
- Subenthiran, S. , Choon, T. C. , Cheong, K. C. , Thayan, R. , Teck, M. B. , Muniandy, P. K. , … Ismail, Z. (2013). Carica papaya leaves juice significantly accelerates the rate of increase in platelet count among patients with dengue fever and dengue haemorrhagic fever [Research Article]. Evidence‐Based Complementary and Alternative Medicine, 2013, 1–7. 10.1155/2013/616737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suleria, H. A. R. , Butt, M. S. , Anjum, F. M. , Saeed, F. , & Khalid, N. (2015). Onion: Nature protection against physiological threats. Critical Reviews in Food Science and Nutrition, 55(1), 50–66. 10.1080/10408398.2011.646364 [DOI] [PubMed] [Google Scholar]
- Sun, C.‐Y. , Xu, L.‐Q. , Zhang, Z.‐B. , Chen, C.‐H. , Huang, Y.‐Z. , Su, Z.‐Q. , … Su, Z.‐R. (2016). Protective effects of pogostone against LPS‐induced acute lung injury in mice via regulation of Keap1‐Nrf2/NF‐κB signaling pathways. International Immunopharmacology, 32, 55–61. 10.1016/j.intimp.2016.01.007 [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Zhang, J. , Yan, Y. , Chi, M. , Chen, W. , Sun, P. , & Qin, S. (2011). The protective effect of C‐phycocyanin on paraquat‐induced acute lung injury in rats. Environmental Toxicology and Pharmacology, 32(2), 168–174. 10.1016/j.etap.2011.04.008 [DOI] [PubMed] [Google Scholar]
- Suwannarach, N. , Kumla, J. , Sujarit, K. , Pattananandecha, T. , Saenjum, C. , & Lumyong, S. (2020). Natural bioactive compounds from Fungi as potential candidates for protease inhibitors and immunomodulators to apply for coronaviruses. Molecules, 25(8), 1800. 10.3390/molecules25081800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai, D. Y. H. (2007). Pharmacologic treatment of SARS: Current knowledge and recommendations. Annals of the Academy of Medicine, Singapore, 36(6), 438–443. [PubMed] [Google Scholar]
- Tan, Z.‐X. , Chen, Y.‐H. , Xu, S. , Qin, H.‐Y. , Zhang, C. , Zhao, H. , & Xu, D.‐X. (2016). Calcitriol inhibits bleomycin‐induced early pulmonary inflammatory response and epithelial–mesenchymal transition in mice. Toxicology Letters, 240(1), 161–171. 10.1016/j.toxlet.2015.10.022 [DOI] [PubMed] [Google Scholar]
- Tang, B. , Xia, F. , Tang, S. , Bragazzi, N. L. , Li, Q. , Sun, X. , … Wu, J. (2020). The effectiveness of quarantine and isolation determine the trend of the COVID‐19 epidemics in the final phase of the current outbreak in China. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases., 95, 288–293. 10.1016/j.ijid.2020.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, H. , Gao, L. , Mao, J. , He, H. , Liu, J. , Cai, X. , … Wu, T. (2016). Salidroside protects against bleomycin‐induced pulmonary fibrosis: Activation of Nrf2‐antioxidant signaling, and inhibition of NF‐κB and TGF‐β1/Smad‐2/‐3 pathways. Cell Stress & Chaperones, 21(2), 239–249. 10.1007/s12192-015-0654-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang, H. , He, H. , Ji, H. , Gao, L. , Mao, J. , Liu, J. , … Wu, T. (2015). Tanshinone IIA ameliorates bleomycin‐induced pulmonary fibrosis and inhibits transforming growth factor‐beta‐β‐dependent epithelial to mesenchymal transition. The Journal of Surgical Research, 197(1), 167–175. 10.1016/j.jss.2015.02.062 [DOI] [PubMed] [Google Scholar]
- Thomas‐Rüddel, D. , Winning, J. , Dickmann, P. , Ouart, D. , Kortgen, A. , Janssens, U. , & Bauer, M. (2020). Coronavirus disease 2019 (COVID‐19): Update for anesthesiologists and intensivists March 2020. Der Anaesthesist. 10.1007/s00101-020-00760-3 [DOI] [PubMed] [Google Scholar]
- Tian, S. , Hu, W. , Niu, L. , Liu, H. , Xu, H. , & Xiao, S.‐Y. (2020). Pulmonary pathology of early‐phase 2019 novel coronavirus (COVID‐19) pneumonia in two patients with lung cancer. Journal of Thoracic Oncology, 15, 704. 10.1016/j.jtho.2020.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian, S.‐L. , Yang, Y. , Liu, X.‐L. , & Xu, Q.‐B. (2018). Emodin attenuates Bleomycin‐induced pulmonary fibrosis via anti‐inflammatory and anti‐oxidative activities in rats. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 24, 1–10. 10.12659/msm.905496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari, V. , Darmani, N. A. , Yue, B. Y. J. T. , & Shukla, D. (2010). In vitro antiviral activity of neem (Azardirachta indica L.) bark extract against herpes simplex virus type‐1 infection. Phytotherapy Research: PTR, 24(8), 1132. 10.1002/ptr.3085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukioka, T. , Takemura, S. , Minamiyama, Y. , Mizuguchi, S. , Toda, M. , & Okada, S. (2017). Attenuation of Bleomycin‐induced pulmonary fibrosis in rats with S‐Allyl cysteine. Molecules, 22(4), 543. 10.3390/molecules22040543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar, R. S. , Surya, D. , & Nalini, N. (2004). Antioxidant efficacy of black pepper (Piper nigrum L.) and piperine in rats with high fat diet induced oxidative stress. Redox Report: Communications in Free Radical Research, 9(2), 105–110. 10.1179/135100004225004742 [DOI] [PubMed] [Google Scholar]
- Wang, C.‐Y. , Lu, C.‐Y. , Li, S.‐W. , Lai, C.‐C. , Hua, C.‐H. , Huang, S.‐H. , … Lin, C.‐W. (2017). SARS coronavirus papain‐like protease up‐regulates the collagen expression through non‐Samd TGF‐β1 signaling. Virus Research, 235, 58–66. 10.1016/j.virusres.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, D. , Hu, B. , Hu, C. , Zhu, F. , Liu, X. , Zhang, J. , … Peng, Z. (2020). Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA, 323(11), 1061–1069. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, L. , Zhang, P. , Li, X. , Zhang, Y. , Zhan, Q. , & Wang, C. (2019). Low‐molecular‐weight fucoidan attenuates bleomycin‐induced pulmonary fibrosis: Possible role in inhibiting TGF‐β1‐induced epithelial–mesenchymal transition through ERK pathway. American Journal of Translational Research, 11(4), 2590–2602. [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Cao, R. , Zhang, L. , Yang, X. , Liu, J. , Xu, M. , … Xiao, G. (2020). Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Research, 30(3), 269–271. 10.1038/s41422-020-0282-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M. , Zhao, D. , Spinetti, G. , Zhang, J. , Jiang, L.‐Q. , Pintus, G. , … Lakatta, E. G. (2006). Matrix metalloproteinase 2 activation of transforming growth factor‐β1 (TGF‐β1) and TGF‐β1–type II receptor signaling within the aged arterial wall. Arteriosclerosis, Thrombosis, and Vascular Biology, 26(7), 1503–1509. 10.1161/01.ATV.0000225777.58488.f2 [DOI] [PubMed] [Google Scholar]
- Wang, W. , Ye, L. , Ye, L. , Li, B. , Gao, B. , Zeng, Y. , … She, Y. (2007). Up‐regulation of IL‐6 and TNF‐α induced by SARS‐coronavirus spike protein in murine macrophages via NF‐κB pathway. Virus Research, 128(1), 1–8. 10.1016/j.virusres.2007.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y.‐L. , Guo, X.‐Y. , He, W. , Chen, R.‐J. , & Zhuang, R. (2017). Effects of alliin on LPS‐induced acute lung injury by activating PPARγ. Microbial Pathogenesis, 110, 375–379. 10.1016/j.micpath.2017.07.019 [DOI] [PubMed] [Google Scholar]
- Weiss, S. R. , & Navas‐Martin, S. (2005). Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiology and Molecular Biology Reviews, 69(4), 635–664. 10.1128/MMBR.69.4.635-664.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO . (2019). WHO | Middle East respiratory syndrome coronavirus (MERS‐CoV), Eastern Mediterranean Regional Office (EMRO), Cairo, Egypt: World Health Organization. Retrieved from http://www.who.int/emergencies/mers-cov/en/ [Google Scholar]
- WHO . (2020a). Clinical management of severe acute respiratory infection when COVID‐19 is suspected, WHO Global: World Health Organization. Retrieved from https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected [Google Scholar]
- WHO . (2020b). Coronavirus disease (COVID‐19)—Events as they happen. Rolling updates on coronavirus disease (COVID‐19) – World Health Organization. Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/events-as-they-happen
- WHO . (2020c). Infection prevention and control during health care when novel coronavirus (nCoV) infection is suspected, WHO Global: World Health Organization. Retrieved from https://www.who.int/publications-detail/infection-prevention-and-control-during-health-care-when-novel-coronavirus-(ncov)-infection-is-suspected-20200125 [Google Scholar]
- WHO . (2020d). Novel coronavirus (2019‐nCoV) situation reports, WHO Global: World Health Organization. Retrieved from https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports [Google Scholar]
- Wilder‐Smith, A. , & Freedman, D. O. (2020). Isolation, quarantine, social distancing and community containment: Pivotal role for old‐style public health measures in the novel coronavirus (2019‐nCoV) outbreak. Journal of Travel Medicine, 27(2), Article taaa020. 10.1093/jtm/taaa020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintergerst, E. S. , Maggini, S. , & Hornig, D. H. (2006). Immune‐enhancing role of vitamin C and zinc and effect on clinical conditions. Annals of Nutrition & Metabolism, 50(2), 85–94. 10.1159/000090495 [DOI] [PubMed] [Google Scholar]
- Wu, Y. , Ho, W. , Huang, Y. , Jin, D.‐Y. , Li, S. , Liu, S.‐L. , … Zheng, Z.‐M. (2020). SARS‐CoV‐2 is an appropriate name for the new coronavirus. The Lancet, 395(10228), 949–950. 10.1016/S0140-6736(20)30557-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, L. , Wang, Q. , Hou, Y. , Zhao, J. , Li, M. , Xu, D. , & Zeng, X. (2020). The Chinese herb Tripterygium wilfordii hook F for the treatment of systemic sclerosis‐associated interstitial lung disease: Data from a Chinese EUSTAR center. Clinical Rheumatology, 39(3), 813–821. 10.1007/s10067-019-04784-y [DOI] [PubMed] [Google Scholar]
- Ying, Z. , Li, M. , Qin, H. , Jingtao, L. , Yulong, C. , Runqing, J. , Sisi, S. , & Yi, Z. (2016). [In vitro anti‐HIV‐1 activity of cordyceps sinensis extracts]. Bing Du Xue Bao = Chinese Journal of Virology; Bing Du Xue Bao, 32(4), 417–422. https://pubmed.ncbi.nlm.nih.gov/29979545/ [PubMed] [Google Scholar]
- You, X.‐Y. , Xue, Q. , Fang, Y. , Liu, Q. , Zhang, C. F. , Zhao, C. , … Xu, X.‐H. (2015). Preventive effects of Ecliptae Herba extract and its component, ecliptasaponin A, on bleomycin‐induced pulmonary fibrosis in mice. Journal of Ethnopharmacology, 175, 172–180. 10.1016/j.jep.2015.08.034 [DOI] [PubMed] [Google Scholar]
- Yu, X. , & Yang, R. (2020). COVID‐19 transmission through asymptomatic carriers is a challenge to containment. Influenza and Other Respiratory Viruses, 14, 474–475. 10.1111/irv.12743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, D. , Liu, B. , Cao, B. , Wei, F. , Yu, X. , Li, G.‐F. , … Wang, P.‐L. (2017). Synergistic protection of Schizandrin B and Glycyrrhizic acid against bleomycin‐induced pulmonary fibrosis by inhibiting TGF‐β1/Smad2 pathways and overexpression of NOX4. International Immunopharmacology, 48, 67–75. 10.1016/j.intimp.2017.04.024 [DOI] [PubMed] [Google Scholar]
- Zhang, D. , Huang, C. , Yang, C. , Liu, R. J. , Wang, J. , Niu, J. , & Brömme, D. (2011). Antifibrotic effects of curcumin are associated with overexpression of cathepsins K and L in bleomycin treated mice and human fibroblasts. Respiratory Research, 12(1), 154. 10.1186/1465-9921-12-154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Penninger, J. M. , Li, Y. , Zhong, N. , & Slutsky, A. S. (2020). Angiotensin‐converting enzyme 2 (ACE2) as a SARS‐CoV‐2 receptor: Molecular mechanisms and potential therapeutic target. Intensive Care Medicine, 46(4), 586–590. 10.1007/s00134-020-05985-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, H. , Zhou, P. , Wei, Y. , Yue, H. , Wang, Y. , Hu, M. , … Du, R. (2020). Histopathologic changes and SARS–CoV‐2 immunostaining in the lung of a patient with COVID‐19. Annals of Internal Medicine, 172, 629–632. 10.7326/M20-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, K. , Si, X.‐P. , Huang, J. , Han, J. , Liang, X. , Xu, X.‐B. , … Wang, J.‐H. (2016). Preventive effects of Rhodiola rosea L. on Bleomycin‐induced pulmonary fibrosis in rats. International Journal of Molecular Sciences, 17(6), Article 879. 10.3390/ijms17060879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Cai, Y. , Zhang, W. , & Chen, X. (2018). Quercetin ameliorates pulmonary fibrosis by inhibiting SphK1/S1P signaling. Biochemistry and Cell Biology = Biochimie et Biologie Cellulaire, 96(6), 742–751. 10.1139/bcb-2017-0302 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. E. (2009). Non‐smad pathways in TGF‐β signaling. Cell Research, 19(1), 128–139. 10.1038/cr.2008.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, Z. , Zhou, J. , Song, D. , Sun, Y. , Liao, C. , & Jiang, X. (2017). Gastrodin protects against LPS‐induced acute lung injury by activating Nrf2 signaling pathway. Oncotarget, 8(19), 32147–32156. 10.18632/oncotarget.16740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao, L. , Wang, X. , Chang, Q. , Xu, J. , Huang, Y. , Guo, Q. , … Wang, J. (2010). Neferine, a bisbenzylisoquinline alkaloid attenuates bleomycin‐induced pulmonary fibrosis. European Journal of Pharmacology, 627(1–3), 304–312. 10.1016/j.ejphar.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Zhu, H. , Huang, C. , Wang, W. , Zhan, X. , & Fan, X. (2011). Effects of andrographolide on the concentration of cytokines in BALF and the expressions of type I and III collagen mRNA in lung tissue in bleomycin‐induced rat pulmonary fibrosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi = Chinese Journal of Cellular and Molecular Immunology, 27(7), 725–729. [PubMed] [Google Scholar]
- Zhu, T. , Zhang, W. , Xiao, M. , Chen, H. , & Jin, H. (2013). Protective role of andrographolide in bleomycin‐induced pulmonary fibrosis in mice. International Journal of Molecular Sciences, 14(12), 23581–23596. 10.3390/ijms141223581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang, M. , Jiang, H. , Suzuki, Y. , Li, X. , Xiao, P. , Tanaka, T. , … Hattori, T. (2009). Procyanidins and butanol extract of cinnamomi cortex inhibit SARS‐CoV infection. Antiviral Research, 82(1), 73–81. 10.1016/j.antiviral.2009.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Beneficial properties of various bacterial, algal, fungal and plant species against the COVID‐19 related symptoms. Overview shows the antifibrotic, antioxidant, anti‐inflammatory properties, histopathological and other lung properties of algal, fungal, plant and other phytochemicals (↑ up arrow represents the increase and ↓ down arrow represents decrease of a specific parameter) (Abbreviations used are given at the end of the table).


