Abstract
Background
Hypercoagulability seems to contribute to SARS‐CoV‐2 pneumonia pathogenesis. However, age and metabolic syndrome are potential confounders when assessing the value of coagulation biomarkers’ prediction of COVID‐19 outcomes. We assessed whether coagulation biomarkers, including factor VIII (FVIII) and von Willebrand factor (VWF) levels, measured at time of admission, were predictive of COVID‐19 adverse outcomes irrespective of age and major comorbidities associated with metabolic syndrome.
Methods
Blood was sampled at admission in 243 adult COVID‐19 patients for analysis of coagulation biomarkers including FVIII and VWF on platelet‐poor plasma. The association between baseline C‐reactive protein (CRP), activated partial thromboplastin time ratio, prothrombin time ratio, D‐dimers, fibrinogen, FVIII, VWF antigen (VWF:Ag), and FVIII/VWF:Ag ratio levels and adverse outcomes (increased oxygen requirements, thrombosis, and death at day 30) was assessed by regression analysis after adjustment on age, sex, body mass index (BMI), diabetes, and hypertension.
Results
In univariable regression analysis increased CRP (subdistribution hazard ratio [SHR], 1.68; 95% confidence interval [CI], 1.26‐2.23), increased fibrinogen (SHR, 1.32; 95% CI, 1.04‐1.68), and decreased FVIII/VWF:Ag ratio (SHR, 0.70; 95% CI, 0.52‐0.96) levels at admission were significantly associated with the risk of increased oxygen requirement during follow‐up. Leucocytes (SHR, 1.36; 95% CI, 1.04‐1.76), platelets (SHR,1.71; 95% CI, 1.11‐2.62), D‐dimers (SHR, 2.48; 95% CI, 1.66‐3.78), and FVIII (SHR, 1.78; 95% CI, 1.17‐2.68) were associated with early onset of thrombosis after admission. After adjustment for age, sex, BMI, hypertension, and diabetes, these associations were not modified.
Conclusion
Coagulation biomarkers are early and independent predictors of increased oxygen requirement in COVID‐19 patients.
Keywords: body mass index, factor VIII, oxygen, SARS‐CoV2, von Willebrand factor
Essentials
-
•
Von Willebrand factor (VWF) levels are associated with severity and oxygen need in COVID‐19 at admission.
-
•
Low factor VIII (FVIII)/VWF ratio at admission is predictive of increased oxygen requirements.
-
•
Coagulation biomarkers predict outcome independently of major comorbidities in COVID‐19.
-
•
FVIII is predictive of early thrombotic events irrespective of body mass index in COVID‐19.
Alt-text: Unlabelled Box
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is frequently associated with laboratory markers of hypercoagulability.1 These coagulation changes, mainly characterized by increased D‐dimers and fibrinogen levels, are generally observed in critically ill patients, especially those with hypoxemia reflecting inflammation.2 This increase in D‐dimers at admission, without signs of disseminated intravascular coagulation, has been reported to be associated with the risk of death.3., 4.
Evidence for an increased risk of thrombosis in COVID‐19 has been first identified through clinical manifestations of large vessel thrombosis. Venous thromboembolic events (VTE) and pulmonary embolism (PE) are reported in 20% to 30% of intensive care unit (ICU) patients5., 6., 7., 8. and other thrombotic complications, such as arterial thrombosis or thrombosis of central lines and in extracorporeal circuits, have also been described.6 All post mortem analyses have confirmed the high frequency of pulmonary vascular thrombosis in ventilated and non‐ventilated patients receiving thromboprophylaxis or not.9., 10. Post mortem histological data also show local direct vascular injury characterized by severe endothelial injury with intracellular SARS‐CoV‐2, widespread vascular thrombosis with microangiopathy and occlusion of alveolar capillaries, and angiogenesis.11., 12. Altogether, these findings suggest that COVID‐19‐induced hypercoagulability and inflammation result in both microangiopathy involved in multiple organ failure and macroangiopathy involved in large vessel thrombosis.13
An increased risk for more severe forms of COVID‐19 with ICU admission and death is associated with age, sex, body weight, hypertension, and diabetes.14., 15., 16. All these factors increase the risk for thrombotic disease in part through the generation of hypercoagulation. Von Willebrand factor (VWF) and factor VIII (FVIII) are also elevated in patients with COVID‐19.6., 7., 17., 18. FVIII and VWF are associated with inflammation and thrombotic risk but are also related to endothelial damage. Lung endothelium is a dominant source of circulating VWF, the levels of which might be differentially altered compared to FVIII, which is mainly synthetized in the liver.19 Investigations as to whether VWF and FVIII levels are related to outcome in COVID‐19 disease have not been reported yet. Whether the laboratory markers of hypercoagulability, including FVIII and VWF, and the risk of thrombosis in COVID‐19 are mainly explained by comorbidities or whether they independently reflect SARS‐CoV‐2–induced vascular damage has not yet been elucidated.
The objective of the study was to assess whether hypercoagulability markers in COVID‐19 patients, including FVIII and VWF levels, measured at admission to the emergency department were predictive of increased oxygen requirement and to evaluate the influence of major comorbidities, including age, sex, body weight, diabetes, and hypertension, on this relation. We also aimed to evaluate if these hypercoagulability markers were predictive of thrombotic events.
2. PATIENTS AND METHODS
2.1. Patients
Consecutive adult patients admitted for COVID‐19 infection were recruited from the emergency department (ED) of the Lille University Hospital between 20 March and 17 April 2020. Inclusion criteria were: individuals aged 18 years or older with either a positive COVID‐19 nasal or tracheal real‐time reverse transcriptase polymerase chain reaction (RT‐PCR) or with radiological signs of interstitial pneumonia on chest x‐rays or computed tomography (CT) scan and a high probability score according to the score for COVID‐19 of Liao et al.20 Following admission, all hospitalized patients received thromboprophylaxis as standard of care unless contra‐indicated. Patients admitted while treated with direct oral anticoagulant (DOAC) or vitamin K antagonists (VKA) were switched to curative heparin therapy. Ward patients received thromboprophylaxis with enoxaparin 4000 or 6000 IU once daily according to their body weight. ICU patients received enoxaparin or unfractionated heparin according to their renal status and the need for invasive procedures. In overweight and obese patients, the dosing regimen was adapted according to the European Society of Cardiology proposals.21 From 1 April onward, patients received thromboprophylaxis according to the GFHT/GIHP (French study group on thrombosis and haemostasis/French working group on perioperative haemostasis) proposals.22 Limitation, withholding, or withdrawal of life‐sustaining treatment in the ICU was based on French guidelines in compliance with French law.23 Deaths in this context were recorded to analyze the real contribution to death of SARS‐CoV‐2 infection.
2.2. Data collection
Epidemiological data, demographic information, past medical history and treatments, clinical data, and outcomes were prospectively collected from the hospital electronic medical records from emergency department admission to hospital discharge. Comorbidities including hypertension and diabetes were defined according to the presence of an antihypertensive or antidiabetic drug at baseline or according to the medical records. Body mass index (BMI) was measured upon admission. The clinical status of patients who were directly discharged home from the emergency department was assessed at day 30 by phone interview. The study was approved by the Institutional Review Board (N°CPP 20‐LILL‐02, NCT04341792) in strict compliance with the French reference methodology MR‐004 and informed consent was obtained from all participants.
2.3. Laboratory testing
For each subject, a 3 mL blood sample was collected at admission on a 0.109 mol/L trisodium citrate tube (BD Vacutainer®, BD Diagnostics). All hemostasis tests were performed on platelet poor plasma obtained after double centrifugation of citrate tubes at 2000 g for 15 minutes at room temperature. Assays included prothrombin time (PT), activated partial thromboplastin time (aPTT), fibrinogen, D‐dimer, FVIII, and VWF antigen (VWF:Ag) levels. The PT, aPTT, and fibrinogen assays were measured on a STA R Max® analyzer (Diagnostica Stago SAS) using STA Neoplastin R®, Triniclot® aPTT HS, T Coag®, and STA Liquid Fib® (Diagnostica Stago SAS). D‐dimer levels were measured in µg/mL fibrinogen equivalent units (FEU) using an immunoturbidimetric latex‐particle assay Liatest DDI‐Plus® on the STA R Max analyser (Diagnostica Stago SAS). The reading area is equal to the measuring area and range from 0.27 µg/mL (FEU) to 20 µg/mL (FEU). FVIII activity was measured by a one‐stage clotting aPTT‐based assay using Triniclot® aPTT HS, T Coag®, and factor VIII‐deficient plasma, on a Sysmex CS 2400 analyzer (Siemens Healthineers AG). VWF:Ag was measured using an immunoturbidimetric assay, LIAPHEN vWF:Ag (HYPHEN BioMed). Other lab tests including a complete blood count, C‐reactive protein (CRP), high sensitivity cardiac troponin, lactate dehydrogenase (LDH) levels, and ABO blood group were prospectively assessed by standard methods as part of patients’ care in the Biology and Pathology Center (CHU Lille).
2.4. Study outcomes
The primary outcome of the study was the increase in oxygen requirement defined as a need for re‐admission after discharge for a limitation of activities and/or a change in oxygen requirements (no oxygen, supplemental oxygen, non‐invasive ventilation or high‐flow oxygen, invasive mechanical ventilation, or extracorporeal membrane oxygenation) and/or death related to acute respiratory distress syndrome without limitation, withholding, or withdrawal of life‐sustaining treatment at day 30. This composite criterion includes any worsening of the respiratory status in patients and comprises different severity of illness without focusing on the most critically ill patients. The secondary outcomes were occurrence of any thromboembolic event (symptomatic PE, deep vein thrombosis [DVT], catheter‐related thrombosis, myocardial infarction [MI], or stroke) at day 30 and all‐cause mortality at day 30. The association between outcomes and the baseline values of leucocytes, lymphocytes, monocytes, platelets, CRP, aPTT ratio, PT ratio, D‐dimers, fibrinogen, FVIII, VWF:Ag, and FVIII/VWF:Ag ratio were evaluated.
2.5. Statistical analysis
Quantitative variables were expressed as means (standard deviation) in the case of normal distribution or medians (interquartile range [IQR]) otherwise. Normality of distributions was assessed using histograms and the Shapiro‐Wilk test. Categorical variables were expressed as numbers (percentage). Cumulative incidence of respiratory worsening and thrombotic events within 30 days of admission were estimated using the Kalbfleisch and Prentice method24 (by taking into account death related to limitation, withholding, or withdrawal of life‐sustaining treatment as competing event for respiratory worsening, and any death as competing event for thrombotic events). Cumulative incidence of all‐cause mortality was estimated using Kaplan‐Meier method.
Main biological markers (PaO2/FiO2 ratio, CRP, fibrinogen, D‐dimers, FVIII, VWF:Ag, FVIII/VWF:Ag) were compared according to admission type and oxygen requirements at admission by using one‐way analysis of variance (or Kruskall Wallis test according to normality of distribution); in case of significant difference, post hoc pairwise comparisons were done using linear contrast (or using Dunn's test).
We assessed the association of biological markers measured at admission to the ED with the occurrence of increase in oxygen requirements, thrombotic events, and all‐cause mortality within 30 days of admission using univariable and multivariable regression models. For biological markers with a skewed distribution, the log‐transformed values were used in regression models. For respiratory worsening and thrombotic events, we used Fine and Gray models before and after pre‐specified adjustment for age, sex, BMI, diabetes, and hypertension by treating death (related to limitation, withholding, or withdrawal of life‐sustaining treatment for increased oxygen requirement and all‐cause death for thrombotic events) as a competing event. For all‐cause mortality, we used Cox's proportional hazard models before and after adjustment for age, sex, BMI, diabetes and hypertension. We assessed the proportional hazard (PH) assumptions by examining the Schoenfeld residuals; in cases of PH departure, the association was modeled using time‐dependent coefficients. We assessed the log‐linearity assumptions by using restricted cubic spline functions;25 in cases of departure, the association was modeled using the quartiles of biomarker distributions. Strength of the associations were evaluated by deriving from regression models, the subhazard ratios (Fine and Gray models) or hazard ratios (Cox model) per one standard deviation increase in biological data as effect sizes (for blood group, effect sizes were calculated for O versus other blood groups).
Multivariate regression analyses were performed after handling missing data on biological markers and covariates using multiple imputation procedure. Imputation procedure was performed using regression‐switching approach26(chained equations with m = 10 obtained) under the missing at random assumption using all baseline characteristics (see Table 1 ), with a predictive mean matching method for quantitative variables and logistic regression model (binary, ordinal, or multinomial) for categorical variables. Estimates obtained in the different imputed data sets were combined using Rubin's rules.27
Table 1.
Patient clinical and biological characteristics at admission to the emergency department in the overall study population
| Characteristics | N | Values |
|---|---|---|
| Age, years (mean ± SD) | 243 | 63.9 ± 16.2 |
| Male sex | 243 | 155 (63.8) |
| Body mass index, kg/m2 (mean ± SD) | 200 | 28.0 ± 6.1 |
| Chronic medical illness | ||
| Diabetes | 243 | 56 (23.0) |
| Hypertension | 243 | 118 (48.6) |
| Chronic pulmonary disease | 243 | 38 (15.6) |
| Cardiopathy | 243 | 36 (14.8) |
| Myocardial infarction | 243 | 22 (9.1) |
| Stroke | 243 | 22 (9.1) |
| Hepatopathy | 243 | 5 (2.1) |
| Chronic renal failure | 243 | 17 (7.0) |
| Active cancer | 243 | 23 (9.5) |
| Immunocompromised | 242 | 11 (4.5) |
| Number of medical illness | 243 | |
| None | 79 (32.5) | |
| One | 65 (26.8) | |
| More than one medical illness | 99 (40.7) | |
| Illness characteristics | 243 | |
| Time from illness onset to admission, days | 8 (5‐11) | |
| Admission type | 243 | |
| Emergency department | 158 (65.0) | |
| Ward | 10 (4.1) | |
| ICU | 75 (30.9) | |
| Severity of respiratory illness at admission | ||
| Respiratory rate/min (mean SD) | 236 | 24.0 ± 5.9 |
| PaO2/FiO2 ratio (mmHg) | 236 | 357 (252‐448) |
| Oxygen requirement at admission | ||
| No oxygen | 243 | 74 (30.5) |
| Supplemental oxygen | 102 (42) | |
| Non‐invasive ventilation or high flow oxygenation | 20 (8.2) | |
| Invasive mechanical ventilation | 47 (19.3) | |
| Biological data | ||
| ABO blood group | 192 | |
| A | 192 | 85 (44.3) |
| AB | 11 (5.7) | |
| B | 19 (9.9) | |
| O | 77 (40.1) | |
| Leucocytes/mm3(mean ± SD) | 229 | 181 |
| Neutrophils/mm3 | 7674 ± 3749 | 5000 (3700‐7000) |
| Lymphocytes/mm3 (mean ± SD) | 182 | 1060 ± 611 |
| Monocytes/mm3 | 182 | 400 (300‐700) |
| Platelets, G/L (mean ± SD) | 238 | 228 ± 113 |
| Creatinine, mg/L | 227 | 8 (7‐11) |
| Lactate dehydrogenase, IU/L | 199 | 377 (286‐479) |
| Troponin, ng/L | 204 | 15.0 (7.5‐25.5) |
| CRP, mg/L | 227 | 69 (31‐126) |
| aPTTr | 211 | 1.13 (1.03‐1.23) |
| PTr | 211 | 1.10 (1.04‐1.16) |
| D‐dimers, µg/mL | 227 | 1.00 (0.70‐1.80) |
| Fibrinogen, g/L (mean ± SD) | 227 | 6.1 ± 1.6 |
| FVIII, IU/dL (mean ± SD) | 210 | 241 ± 96 |
| VWF:Ag, IU/dL (mean ± SD) | 212 | 361 ± 128 |
| FVIII/VWF:Ag ratio (mean ± SD) | 210 | 0.72 ± 0.27 |
Note
Values are number (%) or median (interquartile range) unless otherwise as indicated.
Abbreviations: aPTTr, activated partial thromboplastin time ratio; FVIII, factor VIII; ICU, intensive care unit; PTr, prothrombin time ratio; SD, standard deviation; VWF, von Willebrand factor.
Finally, the association of occurrence of thrombosis events with oxygen requirements and all‐cause mortality was investigated using Cox's proportional hazard regression models by treating the occurrence of thrombosis events as a time‐dependent covariate; hazard ratio associated with the time period with thrombosis events was derived as effect size.
Statistical testing was performed at the two‐tailed α level of 0.05. No correction for multiple testing was done regarding the exploratory nature of the present study and results should be interpreted with caution and as hypothesis generating. Data were analyzed using the SAS software package, release 9.4 (SAS Institute).
3. RESULTS
3.1. Clinical and biological characteristics on emergency department admission
Of the 303 patients with high probability or confirmed COVID‐19 admitted to the ED at our hospital from 20 March to 17 April 2020, 243 patients (155 men and 88 women) were included in the study with a median age of 63.9 years. Patients transferred to our hospital from another hospital were not included in the study (Figure S1 in supporting information). The proportion of patients with hypertension, diabetes, and overweight‐to‐obese (BMI > 25) was 48.6%, 23.0%, and 76.2% respectively. Other underlying comorbidities are detailed in Table 1. Thirty‐two patients were receiving antithrombotic treatment at baseline (DOAC, n = 19; VKA, n = 8; enoxaparin, n = 5).
Upon arrival in the ED, 30.9% of patients were directly admitted to the ICU and 65% were first admitted to the ED. Twenty‐three (9.4%) patients were directly discharged home from the ED after medical assessment for standardized ambulatory clinical follow‐up. All other patients were hospitalized in the medical ward dedicated to COVID‐19.
Median time from illness onset to admission was 8 (IQR, 5‐11) days. At admission, 169 (69.5%) patients required oxygen support and all had radiological signs of interstitial pneumonia on chest X‐rays or CT scan. Baseline median respiratory rate and PaO2/FiO2 were 24 (IQR, 20‐28) per min and 357 (IQR, 252‐448) mm Hg, respectively. In accordance with local guidelines to prevent overwhelming the virology lab, RT‐PCR testing was limited to COVID‐19 patients requiring hospitalization. RT‐PCR was positive for COVID‐19 in 220 (90.5%) patients at baseline.
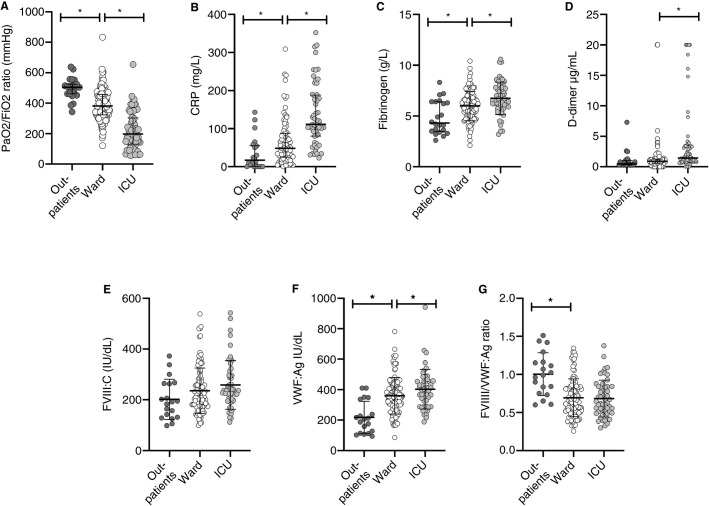
The values of the main biological markers at baseline are presented in Table 1. The PaO2/FiO2 ratio was significantly different according to the admission type with the lowest values for patients directly admitted to the ICU (outpatients, ward, and ICU, respectively 505 [461‐523], 381 [324‐458], 197 [130‐302], P < .001). Also depending on admission type (outpatient, ward, or ICU), CRP, D‐dimers, fibrinogen, and VWF levels were highest for patients directly admitted to the ICU (Figure 1 ). As RT‐PCR testing was in most cases limited to COVID‐19 patients requiring hospitalization, respiratory illness at admission was less severe in the RT‐PCR negative patient group despite the presence of radiological signs of pneumonia. Among the 23 patients without RT‐PCR testing, only 2 were admitted to the medical ward dedicated to COVID‐19 whereas the 21 others were outpatients. As shown in Figure 1A, outpatients had a higher PaO2/FiO2 and a lower respiratory rate (20.6 ± 5 versus 24.4 ± 6, P = .003) upon admission in ED and none of them needed oxygen supply during follow‐up.
FIGURE 1.
Baseline values of main biomarkers according to admission type. Scatter plots of (A) PaO2/FiO2 ratio, (B) C‐reactive protein, (C) fibrinogen, (D) D‐dimers, (E) factor VIII (FVIII), (F) von Willebrand factor antigen (VWF:Ag), and (G) FVIII/VWF:Ag ratio. Bars indicate median and interquartile range or means ± standard deviation as appropriate. ICU = intensive care unit. *P < .05.
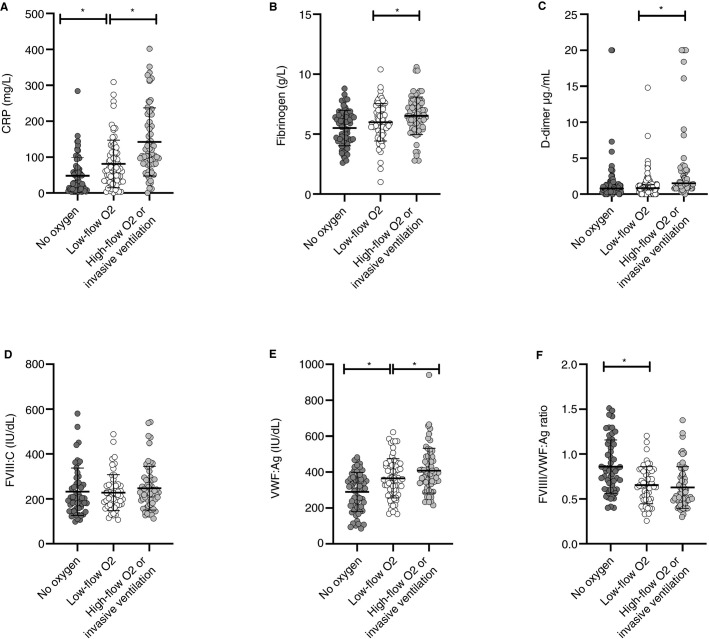
As shown in Figure 2 , CRP, fibrinogen, D‐dimers, and VWF:Ag levels were increased depending on oxygen requirements at admission, with the lowest levels for patients with no oxygen and the highest for patients requiring high‐flow oxygen or invasive ventilation. FVIII levels were not affected by oxygen requirements.
FIGURE 2.
Baseline values of main biomarkers according to oxygen requirements at admission to the emergency department. Scatter plots of (A) C‐reactive protein, (B) fibrinogen, (C) D‐dimers, (D) factor VIII (FVIII), (E) von Willebrand factor antigen (VWF:Ag) and (F) FVIII/VWF:Ag ratio. Bars indicate median and interquartile range or means ± standard deviation as appropriate. *P < .05
3.2. Increase in oxygen requirement and biomarkers
At the time of analysis (20 May 2020), all patients either died (n = 32) or reached the 30‐day follow‐up after admission. Respiratory worsening was observed in 71 patients (30‐day incidence, 29.2%; 95% CI, 23.6%‐35.0%) during follow‐up: 2 patients were re‐admitted for dyspnea, 59 required an escalation in oxygen supply (low flow oxygen, n = 13; non‐invasive ventilation or high flow oxygen devices, n = 9; invasive mechanical ventilation, n = 37) and 10 died from acute respiratory distress syndrome (ARDS) without context of limitation, withholding, or withdrawal of life‐sustaining treatment. In patients presenting an increase in oxygen requirement there was a significant decrease of PaO2/FiO2 ratio the day of increase in oxygen requirement compared to baseline values (172 [122‐233] versus 313 [185‐396], P < .01; Figure S2 in supporting information) Most of the increased oxygen requirement events (87.3%, n = 62) occurred in the first 10 days following admission (Figure S3A in supporting information). Among the 71 patients with aggravation according to the increase of oxygen requirements, we observed no significant difference in the timing of aggravation according to the severity of oxygen requirements (3 [1‐4] days for escalation of non‐invasive oxygen supply versus 3 [1‐5] days for the need of invasive ventilation); however, death from ARDS occurred at a significantly later stage (12 [5‐19] days, P = .001).
In univariable Fine and Gray regression analysis considering the 16 deaths in the context of limitation of life‐sustaining treatment as competing events, increased CRP (subdistribution hazard ratio [SHR], 1.68; 95% confidence interval [CI], 1.26‐2.23), increased fibrinogen (SHR, 1.32; 95% CI, 1.04‐1.68), and decreased FVIII/VWF:Ag ratio (SHR, 0.70; 95% CI, 0.52‐0.96) levels at admission were significantly associated with the risk of respiratory degradation during follow‐up (Table 2 ). After adjustment for age, sex, BMI, hypertension, and diabetes, these associations were not modified (Table 2). In multivariate analysis, the association between decreased lymphocytes and risk of increased oxygen requirement reached the significance level, with an adjusted SHR of 0.71 (95% CI, 0.50‐0.99). In conclusion, CRP, fibrinogen, the FVIII/VWF ratio, and reduced lymphocyte count were all independently associated with the increased need for oxygen support.
Table 2.
Associations of biological data with 30‐day increase of oxygenation requirements
| Biological data | 30‐day aggravation |
Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|---|---|
| No (n = 172) | Yes (=71) | SHR (95% CI) | P | SHR (95% CI) | P | |
| O blood group, n (%) | 56 (42.1) | 21 (35.6) | 0.78 (0.46‐1.32)b | .35 | 0.80 (0.46‐1.39)b | .44 |
| Leucocytes/mm3 | 7676 ± 3796 | 7667 ± 3663 | .31f | .26f | ||
| 0‐5 daysd | 1.12 (0.89‐1.40) | .16 | 1.13 (0.89‐1.43) | .32 | ||
| 6‐30 daysd | 0.65 (0.31‐1.33) | .24 | 0.66 (0.32‐1.34) | .24 | ||
| Neutrophils/mm3 | 4700 (3600‐6500) | 5350 (4400‐7500) | 1.29 (0.96‐1.74)c | .090 | 1.19 (0.88‐1.61)c | .24 |
| Lymphocytes/mm3 | 1109 ± 646 | 927 ± 485 | 0.74 (0.54‐1.01) | .061 | 0.71 (0.50‐0.99) | .041 |
| Monocytes/mm3 | 500 (300‐700) | 400 (300‐700) | 0.97 (0.75‐1.25)c | .81 | 0.91 (0.72‐1.16)c | .44 |
| Platelets, G/L | 236 ± 120 | 214 ± 98 | .073f | .28f | ||
| 0‐5 daysd | 0.94 (0.73‐1.21) | .62 | 0.95 (0.72‐1.24) | .69 | ||
| 6‐30 daysd | 0.66 (0.45‐0.94) | .023 | 0.66 (0.43‐1.01) | .057 | ||
| Creatinine, mg/L | 8 (7‐11) | 10 (7‐12) | 1.20 (0.95‐1.51)c | .12 | 1.10 (0.83‐1.47)c | .48 |
| Lactate dehydrogenase, IU/L | 359 (280‐476) | 426 (312‐508) | 1.22 (0.96‐1.54)c | .090 | 1.11 (0.87‐1.43)c | .38 |
| Troponine ng/L | 14 (7‐26) | 16 (9‐24) | .25f | .50f | ||
| <8 | 41 (28.1) | 10 (17.2) | 1.00 (ref.) | ‐ | 1.00 (ref.) | ‐ |
| 8‐14 | 33 (22.6) | 17 (29.3) | 1.90 (0.88‐4.04) | .098 | 1.16 (0.61‐2.18) | .65 |
| 15‐25 | 34 (23.3) | 18 (31.0) | 1.99 (0.93‐4.23) | .075 | 1.19 (0.60‐2.31) | .62 |
| >25 | 38 (26.0) | 13 (22.5) | 1.36 (0.61‐3.04) | .46 | 0.71 (0.33‐1.53) | .38 |
| C‐reactive protein, mg/L | 60 (25‐111) | 101 (56‐143) | 1.68 (1.26‐2.23)c | <.001 | 1.66 (1.25‐2.19)c | <.001 |
| aPTT | 1.13 (1.00‐1.26) | 1.13 (1.06‐1.23) | 1.02 (0.85‐1.23)c | .82 | 1.02 (0.84‐1.24)c | .85 |
| PTr | 1.09 (1.04‐1.16) | 1.10 (1.05‐1.18) | 1.15 (0.98‐1.35)c | .081 | 1.10 (0.89‐1.38)c | .34 |
| D‐dimers, µg/mL | 1.00 (0.70‐1.80) | 1.00 (0.80‐1.90) | 1.04 (0.77‐1.40)c | .80 | 1.01 (0.74‐1.38)c | .95 |
| Fibrinogen, g/L | 5.9 ± 1.7 | 6.4 ± 1.5 | 1.32 (1.04‐1.68) | .021 | 1.34 (1.04‐1.74) | .022 |
| Factor VIII, IU/dL | 244 ± 104 | 233 ± 79 | 0.93 (0.73‐1.17) | .51 | 0.89 (0.71‐1.13) | .34 |
| VWF:Age, IU/dL | 351 ± 141 | 381 ± 98 | .30f | .39f | ||
| <270 | 44 (28.6) | 9 (15.5) | 1.00 (ref.) | ‐ | 1.00 (ref.) | ‐ |
| 270‐354 | 35 (22.7) | 17 (29.3) | 2.09 (0.94‐4.60) | .068 | 1.71 (0.77‐3.78) | .18 |
| 355‐430 | 38 (24.7) | 16 (27.6) | 1.86 (0.83‐4.14) | .13 | 1.86 (0.87‐3.93) | .11 |
| >430 | 37 (24.0) | 16 (27.6) | 1.94 (0.86‐4.35) | .11 | 1.80 (0.82‐3.97) | .14 |
| FVIII/VWF:Ag ratio | 0.74 ± 0.27 | 0.64 ± 0.24 | 0.70 (0.52‐0.96) | .025 | 0.71 (0.51‐0.98) | .036 |
Note
Values are median (interquartile range) or means ± standard deviation. SubHazard ratios (SHRs) were calculated using Fine and Gray models taking into account the mortality in the context of withholding or withdrawal of life‐sustaining treatment (n = 16) as competing events and were expressed per one standard deviation unless otherwise as indicated.
Abbreviations: aPTTr, activated partial thromboplastin time ratio; CI, confidence interval; CRP, C‐reactive protein; FVIII, factor VIII activity; PTr, prothrombin time ratio; SD, standard deviation; SHR, subhazard ratio; VWF: Ag, von Willebrand factor antigen.
Adjusted for age, sex, body mass index, hypertension, and diabetes calculated after handling missing values (in biological and confounding factors) by multiple imputation.
SHR calculated for O versus others blood groups.
SHR calculated per one standard deviation in log‐transformed values.
Modeled with time‐dependent coefficients to accommodate deviation in proportional subhazard assumption.
Modeled as categorical variables based on quartiles to accommodate deviation in log linear relationship.
P‐value for overall effect calculated using a likelihood ratio test.
3.3. Thrombotic events and biomarkers
The cumulative incidence of 30‐day any thrombotic event was 12.8% (95% CI, 8.9%‐17.3%); there were 22 patients presenting PE (1 troncular, 3 lobar, 12 segmental, and 6 subsegmental), 4 DVT, 1 MI, 2 ischemic stroke, and 2 catheter‐related thrombosis of the jugular vein. The 31 thrombotic events occurred with a median time from hospital admission to thrombotic events of 8 (IQR, 1‐11) days (Figure S3B). As shown in Table 3 , the proportional subhazard assumptions for several biomarkers were not satisfied with a positive association with occurrence of thrombotic event during the first 5 days for leucocytes (SHR, 1.36; 95% CI, 1.04‐1.76), platelets (SHR, 1.71; 95% CI, 1.11‐2.62), D‐dimers (SHR, 2.48; 95% CI, 1.66‐3.78), FVIII (SHR, 1.78; 95% CI, 1.17‐2.68). Intriguingly a negative association with occurrence of thrombotic event after 5 days was observed for FVIII (SHR, 0.46; 95% CI 0.26‐0.82) and VIII/VWF:Ag ratio (SHR, 0.47; 95% CI, 0.25‐0.87). However, patients presenting a thrombotic event after 5 days had lower FVIII levels at baseline compared to patients presenting a thrombotic event before day 5 or no thrombotic event (190 ± 45, 314 ± 143, and 232 ± 82, respectively; P < .01). After 4 days, FVIII increased in patients presenting a thrombotic event after 5 days and remained stable in patients presenting early thrombosis and the difference between both groups was no longer significant (Figure S4 in supporting information). Time interval between symptom onset and thrombosis was longer in the group with early thrombotic event after admission when compared to late thrombotic event after admission, although not significant (11 [8‐12] days versus 9.5 [7‐11]).
Table 3.
Associations of biological data with 30‐day thrombotic events
| Biological data | 30‐day thrombotic events |
Unadjusted |
Adjusteda |
|||
|---|---|---|---|---|---|---|
| No (n = 212) | Yes (=31) | SHR (95% CI) | P | SHR (95% CI) | P | |
| O blood group, n (%) | 67 (41.6) | 10 (32.3) | 0.69 (0.32‐1.45)b | .32 | 0.76 (0.35‐1.61)b | .47 |
| Leucocytes/mm3 | 7542 ± 3784 | 8512 ± 3461 | .070e | .14e | ||
| 0‐5 daysd | 1.36 (1.04‐1.76) | .022 | 1.50 (1.11‐2.03) | .008 | ||
| 6‐30 daysd | 1.02 (0.72‐1.45) | .89 | 1.11 (0.74‐1.65) | .60 | ||
| Neutrophils/mm3 | 4900 (3600‐6500) | 6550 (5200‐8200) | 1.40 (1.06‐1.84)c | .018 | 1.42 (1.03‐1.95)c | .031 |
| Lymphocytes/mm3 | 1070 ± 633 | 974 ± 368 | 0.85 (0.60‐1.20) | .36 | 0.92 (0.60‐1.40) | .69 |
| Monocytes/mm3 | 400 (300‐700) | 400 (200‐800) | .086e | .35e | ||
| 0‐5 daysd | 1.47 (0.79‐2.73)c | .22 | 1.28 (0.53‐3.07)c | .57 | ||
| 6‐30 daysd | 0.72 (0.51‐1.02)c | .064 | 0.75 (0.50‐1.11)c | .15 | ||
| Platelets, G/L | 226 ± 112 | 246 ± 120 | .020e | .053e | ||
| 0‐5 daysd | 1.71 (1.11‐2.62) | .014 | 1.71 (1.10‐2.66) | .016 | ||
| 6‐30 daysd | 0.78 (0.52‐1.18) | .23 | 0.78 (0.51‐1.18) | .24 | ||
| Creatinine, mg/L | 8.5 (7‐11.5) | 8.0 (6‐11) | 0.84 (0.58‐1.21)c | .35 | 0.92 (0.62‐1.34)c | .64 |
| Lactate dehydrogenase, IU/L | 360 (280‐456) | 495 (383‐599) | 1.79 (1.36‐2.36)c | <.001 | 1.87 (1.36‐2.58)c | <.001 |
| Troponin, ng/L | 15.0 (7.5‐26.0) | 11.5 (7.5‐23.0) | 1.08 (0.69‐1.70)c | .73 | 1.35 (0.84‐2.15)c | .21 |
| C‐reactive protein, mg/L | 69 (31‐123) | 79 (28‐157) | 1.08 (0.71‐1.62)c | .73 | 1.20 (0.77‐1.86)c | .40 |
| aPTTr | 1.10 (1.03‐1.23) | 1.17 (1.03‐1.26) | 1.25 (0.89‐1.73)c | .19 | 1.10 (0.71‐1.72)c | .66 |
| PTr | 1.09 (1.04‐1.15) | 1.15 (1.08‐1.20) | 1.13 (0.94‐1.35)c | .17 | 1.10 (0.83‐1.46)c | .51 |
| D‐dimers, µg/mL | 1.00 (0.70‐1.60) | 1.60 (1.10‐4.20) | <.001e | <.001e | ||
| 0‐5 daysd | 2.48 (1.66‐3.70)c | <.001 | 2.90 (1.86‐4.50)c | <.001 | ||
| 6‐30 daysd | 1.01 (0.31‐3.30)c | .99 | 1.21 (0.33‐4.40)c | .77 | ||
| Fibrinogen, g/L | 6.1 ± 1.6 | 5.9 ± 1.8 | 0.90 (0.60‐1.32) | .31 | 0.96 (0.65‐1.41) | .84 |
| Factor VIII, IU/dL | 239 ± 91 | 251 ± 123 | <.001e | .007e | ||
| 0‐5 dd | 1.78 (1.17‐2.68) | .006 | 1.72 (1.15‐2.55) | .007 | ||
| 6‐30 daysd | 0.46 (0.26‐0.82) | .008 | 0.50 (0.27‐0.91) | .022 | ||
| VWF:Ag, IU/dL | 358 ± 131 | 381 ± 107 | 1.18 (0.90‐1.55) | .22 | 1.23 (0.92‐1.63) | .16 |
| FVIII/VWF:Ag ratio | 0.72 ± 0.27 | 0.67 ± 0.29 | .035e | .063e | ||
| 0‐5 daysd | 1.23 (0.69‐2.16) | .48 | 1.09 (0.61‐1.92) | .77 | ||
| 6‐30 daysd | 0.47 (0.25‐0.87) | .015 | 0.47 (0.26‐0.85) | .012 | ||
Note
Values are median (interquartile range) or means ± standard deviation. Subhazard ratios (SHRs) were calculated using Fine and Gray models taking into account the mortality (n = 27) as competing events and were expressed per one standard deviation unless otherwise as indicated.
Abbreviations: aPTTr, activated partial thromboplastin time ratio; CI, confidence interval; CRP, C‐reactive protein; FVIII, factor VIII activity; PTr, prothrombin time ratio; SD, standard deviation; SHR, subhazard ratio; VWF:Ag, von Willebrand factor antigen.
Adjusted for age, sex, body mass index, hypertension, and diabetes calculated after handling missing values (in biological and confounding factors) by multiple imputation.
SHR calculated for O versus others blood groups.
SHR calculated per one standard deviation in log‐transformed values.
Modeled with time‐dependent coefficients to accommodate deviation in proportional subhazard assumption.
P‐value for overall effect calculated using a likelihood ratio test.
Thromboembolic complications were significantly associated with a higher risk of increase in oxygen requirements (% per patients‐days exposed versus non‐exposed to thrombosis events: 1.9% versus 0.4%), with a hazard ratio (HR) 2.66 (95% CI, 1.26‐5.60). Most events occurred in ICU.
We also observed a positive association between thromboembolic events during the entire follow‐up period (without deviation to proportional subhazard assumptions) for neutrophils (SHR, 1.40; 95% CI, 1.06‐1.84), and lactate dehydrogenase (LDH; SHR 1.79; 95% CI, 1.36‐2.36). After adjustment for pre‐specified confounders, these associations were not modified (Table 3).
3.4. All‐cause mortality and biomarkers
The 30‐day mortality was 13.2% (95% CI, 9.2%‐17.8%; Figure S3C) with half of patients in a context of limitation, withholding, or withdrawal of life‐sustaining treatment, mainly limitation of invasive mechanical ventilation in case of worsening respiratory status. Thromboembolic complications were not significantly associated with all‐cause mortality (% per patients‐days exposed versus non‐exposed to thrombosis events: 0.8% versus 0.4%) with an HR of 2.07 (95% CI, 0.79‐5.43). However, the numbers of both thrombotic events and deaths were too low to achieve a significant difference. The results are presented in Table S4 in supporting information.
4. DISCUSSION
In this study, we provide evidence that coagulation biomarkers, including FVIII and VWF, at admission to the ED are associated with the severity of COVID‐19 and predict a higher risk of increase in oxygen requirements irrespective of age, sex, BMI, diabetes, and hypertension. Our results support the hypothesis that SARS‐CoV‐2–associated thromboinflammatory hypercoagulability could directly contribute to the underlying pulmonary pathogenesis.
The objective of this study was to assess whether COVID‐19 hypercoagulability and especially markers associated with inflammation and endothelial damage such as FVIII and VWF were associated with disease severity. To better understand this role of coagulation in SARS‐CoV‐2 pneumonia pathogenesis we aimed to adjust the predictive value of coagulation biomarkers to confounding factors such as major comorbidities. Moreover, we aimed to evaluate the severity according to oxygen requirements rather than only admission to ICU or death given that these outcomes associated with COVID‐19 are heavily influenced by the presence of other underlying comorbidities.
In our cohort, biomarkers that have already been associated with a poor outcome such as lymphopenia, elevated CRP, and fibrinogen were predictive of respiratory worsening.28., 29., 30. Reflecting the inclusion of all consecutive primary admissions irrespective of the initial severity, the initial admitting respiratory rate and PaO2/FiO2 were, although not completely normal, within a reasonable spectrum. However, we identified a gradual increase in VWF levels according to oxygen requirements at admission with only 10 patients presenting with normal VWF levels, all of them discharged from the ED with outpatient follow‐up. Unusually high circulating VWF levels have been reported in patients with severe COVID‐19 infection.6., 17. The vascular endothelium is emerging as a key target‐organ of SARS‐Cov‐2. SARS‐Cov‐2 infects target cells in the lung, heart, intestine, and kidney using the angiotensin converting enzyme 2 (ACE2) receptor, which is also widely expressed on endothelial cells. Recent autopsy findings suggest that SARS‐Cov‐2 infection induces widespread endothelial dysfunction and inflammation that could shift the endothelial balance toward a procoagulant state.11VWF endothelial expression is characterized by a vascular‐bed heterogeneity31., 32. with lung endothelial cells being the first source of circulating VWF.22 VWF expression and release from endothelial cell Weibel‐Palade bodies is also stimulated by hypoxia.33., 34. Hypoxia‐induced VWF upregulation is associated with the presence of thrombi in heart and lung vascular beds and promotes recruitment of leukocytes.35
In our cohort including COVID‐19 patients with varying degrees of illness, a decrease in FVIII/VWF ratio values on admission was associated with a higher risk of worsening respiratory status, as evidenced by an increase in oxygen requirements. This suggests that both inflammation and a SARS‐CoV‐2–induced endotheliopathy could contribute to lung damage pathogenesis. The different levels of FVIII and VWF as evaluated by their ratio could reflect a major synthesis and release of VWF in the lung due to inflammation, hypoxia, and direct SARS‐CoV‐2 destruction of endothelial cells, while the levels of FVIII are less increased because the liver is only exposed to pro‐inflammatory cytokines.
Age, male sex, increased BMI, and metabolic syndrome are significantly associated with an increased risk of severe forms and death from COVID‐19 infection.36 Importantly, the prediction of an increase in oxygen requirement remained significant after adjusting for these metabolic comorbidities, confirming the direct involvement of procoagulant changes in COVID‐19 pneumonia pathogenesis.
Thrombosis was associated with biomarkers reflecting inflammation including increased leucocyte, neutrophil, and platelet counts. We also observed an association with higher LDH, D‐dimers, and FVIII levels. In a recent meta‐analysis, an association between elevated LDH levels measured at earliest time point in hospitalization and worse outcomes was reported in patients with COVID‐1937 as observed in patients with Middle East respiratory syndrome (MERS).38 Several mechanisms may account for LDH increase in COVID‐19 including thrombotic microangiopathy, upregulation of the glycolytic pathway in a context of severe hypoxia, and direct cell damage because this intracellular enzyme is found in pneumocytes, the main target cell of SARS‐CoV‐2.
D‐dimers at admission or increasing D‐dimers over time have been associated with an increased risk of respiratory degradation, thrombosis, and death from COVID‐19 infection.2., 14., 39. Reports to date have not accounted for age or other major comorbidities that contribute to D‐dimer elevation as potential confounders in risk prediction. Of note, in our cohort the predictive value of D‐dimers on the occurrence of thrombosis remained significant after adjustment on major metabolic comorbidities associated with COVID‐19 and also risk factors for thrombosis such as BMI and age. Similarly, high FVIII levels at admission were also associated with an increased risk of early‐onset thrombosis (eg, in the 5 days following admission) independently of major comorbidities. Among coagulation factors, high levels of FVIII and VWF were the strongest identified risk factors and recently a causal role of these two proteins in thrombotic events has been suggested.40., 41. As we observed a secondary increase of FVIII in patients with late‐onset thrombosis we suspect that this factor can only predict events in a short time frame, and so should be closely and frequently monitored. VWF levels upon admission were not predictive of thrombotic events during follow‐up. This could suggest that the important increase in VWF observed in almost all patients related to endothelial injury in the lung, as reflected by the relation with the oxygen requirements at admission, is not a marker of patients at higher risk for thrombosis.
Altogether, these biological features make the link between SARS‐CoV2 infection and the severity of hypoxemia. Indeed activation of coagulation cascade leading to widespread thrombosis in the lung and endothelial damages are thought to be involved in the disruption of pulmonary vasoregulation especially the vasoconstriction secondary to alveolar hypoxia.42
The main strengths of our study are: (a) our study was prospective with a systematic follow‐up in all patients at day 30; (b) in order to prevent referral bias, this study was performed in COVID‐19 patients with radiological signs of pneumonia but with varying severity of illness; (c) adjustment for established risk factors for disease progression and death and accounting for limitation of life‐sustaining treatment as competing events for adverse outcomes are two other major strengths of the present study; (d) potential limitation, withholding, or withdrawal of life‐sustaining treatment in frail elderly patients is a potential confounder that should be taken into account as a competing event when assessing the association between biomarkers and the risk of adverse outcomes in COVID‐19 patients.43 In our study we considered death with limitation, withholding, or withdrawal of life‐sustaining treatment as a competing event for the evaluation of respiratory worsening meaning that death with respiratory failure was not considered as worsening when occurring in the context of limited, withheld, or withdrawn life‐sustaining treatment. However, this study has three main limitations. First, this was a single‐center study, second the thrombotic complications were not diagnosed through systematic screening with doppler ultrasound or CT pulmonary angiogram. The prevalence of VTE and PE was therefore probably underestimated because the access doppler ultrasound and CT pulmonary angiogram was limited in COVID‐19 patients hospitalized in ICU for practical reasons. Furthermore, as seasonal influenza outbreak was over in Europe at the start of the COVID‐19 pandemic, we could not include patients with interstitial pneumonia related to viruses other than SARS‐Cov2 as controls. It remains to be assessed whether the biomarkers associated with worse outcomes in our study are specific to COVID‐19 pneumonia or could be translated to pneumonia related to other viruses such as SARS, MERS, or influenza.
5. CONCLUSION
We provide evidence that levels of coagulation biomarkers including FVIII and VWF at time of admission to the ED are associated with the severity of COVID‐19 and predict risk of increased oxygen requirements irrespective of age, sex, BMI, diabetes, and hypertension.
CONFLICTS OF INTEREST
No disclosures relevant to the manuscript.
AUTHOR CONTRIBUTIONS
AR and SS designed the study, analyzed the data, and wrote the manuscript. FL, JG, MC, LC, AR, EJ, AD, KF, and ML collected clinical data. JL and AD performed the statistical analysis. EK, DG, PL, and JP provided critical input and analysis. All authors provided editorial review and assisted in writing the manuscript.
DATA AVAILABILITY STATEMENT
For original data, please contact sophiesusen@aol.com.
ACKNOWLEDGMENTS
The authors wish to thank Laureline Bourgeois, Aurélie Jospin, Catherine Marichez, Vincent Dalibard, Bénédicte Pradines, Sandrine Vanderziepe, all the biologists and technicians of the Hemostasis Department, and the Lille COVID Research Network (LICORNE) for their support during the COVID‐19 pandemic.
Agence Nationale de la RechercheI‐SITE ULNE / ANR‐16‐IDEX‐0004 ULNE
Footnotes
Manuscript handled by: Patricia Liaw
Final decision: Patricia Liaw, 14 August 2020
Funding informationThis study was supported by the French government through the Programme Investissement d’Avenir (I‐SITE ULNE/ ANR‐16‐IDEX‐0004 ULNE) managed by the Agence Nationale de la Recherche.
Supporting Information
Table S1,Fig S1‐S4
REFERENCES
- 1.Connors J.M., Levy J.H. COVID‐19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033–2040. doi: 10.1182/blood.2020006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klok F.A., Kruip M.J.H.A., van der Meer N.J.M., et al. Incidence of thrombotic complications in critically ill ICU patients with COVID‐19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Helms J., Tacquard C., Severac F., et al. High risk of thrombosis in patients with severe SARS‐CoV‐2 infection: a multicenter prospective cohort study. Intensive Care Med. 2020;46(6):1089–1098. doi: 10.1007/s00134-020-06062-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poissy J., Goutay J., Caplan M., et al. Pulmonary embolism in COVID‐19 patients: awareness of an increased prevalence. Circulation. 2020;142(2):184–186. doi: 10.1161/CIRCULATIONAHA.120.047430. [DOI] [PubMed] [Google Scholar]
- 8.Middeldorp S., Coppens M., van Haaps T.F., et al. Incidence of venous thromboembolism in hospitalized patients with COVID‐19. J Thromb. Haemost. 2020;18(8):1995–2002. doi: 10.1111/jth.14888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wichmann D., Sperhake J.‐.P., Lütgehetmann M., et al. Autopsy findings and venous thromboembolism in patients with COVID‐19. Ann Intern Med. 2020;173(4):268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lax S.F., Skok K., Zechner P., et al. Pulmonary arterial thrombosis in COVID‐19 with fatal outcome: results from a prospective, single‐center, clinicopathologic case series. Ann Intern Med. 2020;173(5):350–361. doi: 10.7326/M20-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varga Z., Flammer A.J., Steiger P., et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet. 2020;395(10234):1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ackermann M., Verleden S.E., Kuehnel M., et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl. J Med. 2020;383(2):120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Iba T., Levy J.H., Levi M., Connors J.M., Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. 2020;48(9):1358–1364. doi: 10.1097/CCM.0000000000004458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasselli G., Zangrillo A., Zanella A., et al. Baseline characteristics and outcomes of 1591 patients infected With SARS‐CoV‐2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323(16):1574. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York City Area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panigada M., Bottino N., Tagliabue P., et al. Hypercoagulability of COVID‐19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. 2020;18(7):1738–1742. doi: 10.1111/jth.14850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Escher R., Breakey N., Lämmle B. Severe COVID‐19 infection associated with endothelial activation. Thromb Res. 2020;190:62. doi: 10.1016/j.thromres.2020.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pan J., Dinh T.T., Rajaraman A., et al. Patterns of expression of factor VIII and von Willebrand factor by endothelial cell subsets in vivo. Blood. 2016;128(1):104–109. doi: 10.1182/blood-2015-12-684688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liao X., Wang B., Kang Y. Novel coronavirus infection during the 2019–2020 epidemic: preparing intensive care units‐the experience in Sichuan Province, China. Intensive Care Med. 2020;46(2):357–360. doi: 10.1007/s00134-020-05954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rocca B., Fox K.A.A., Ajjan R.A., et al. Antithrombotic therapy and body mass: an expert position paper of the ESC Working Group on Thrombosis. Eur. Heart J. 2018;39(19):1672–1686f. doi: 10.1093/eurheartj/ehy066. [DOI] [PubMed] [Google Scholar]
- 22.Susen S., Tacquard C.A., Godon A., et al. Prevention of thrombotic risk in hospitalized patients with COVID‐19 and hemostasis monitoring. Crit Care. 2020;24(1):364. doi: 10.1186/s13054-020-03000-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Société de réanimation de langue Limitation et arrêt des traitements en réanimation adulte. Actualisation des recommandations de la Société de réanimation de langue française. Réanimation. 2010;19(8):679–698. [Google Scholar]
- 24.Prentice R.L., Kalbfleisch J.D., Peterson A.V., et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34(4):541–554. [PubMed] [Google Scholar]
- 25.Harrell F.E., Lee K.L., Mark D.B. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 26.van Buuren S., Groothuis‐Oudshoorn K. Multivariate imputation by chained equations in R. J Stat Soft. 2011;45(3) [Google Scholar]
- 27.Rubin G.J., Donald B. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sons; Chichester, New York, Brisbane, Toronto, Singapore: 1987. [Google Scholar]
- 28.Guan W.‐.J., Ni Z.‐.Y., Hu Y., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogarty H., Townsend L., Ni Cheallaigh C., et al. COVID19 coagulopathy in Caucasian patients. Br J Haematol. 2020;189(6):1044–1049. doi: 10.1111/bjh.16749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ranucci M., Ballotta A., Di Dedda U., et al. The procoagulant pattern of patients with COVID‐19 acute respiratory distress syndrome. J Thromb Haemost. 2020;18(7):1747–1751. doi: 10.1111/jth.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nassiri M., Liu J., Kulak S., et al. Repressors NFI and NFY participate in organ‐specific regulation of von Willebrand factor promoter activity in transgenic mice. Arterioscler Thromb Vasc Biol. 2010;30(7):1423–1429. doi: 10.1161/ATVBAHA.110.206680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shirodkar A.V., St Bernard R., Gavryushova A., et al. A mechanistic role for DNA methylation in endothelial cell (EC)‐enriched gene expression: relationship with DNA replication timing. Blood. 2013;121(17):3531–3540. doi: 10.1182/blood-2013-01-479170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mojiri A., Nakhaii‐Nejad M., Phan W.‐.L., et al. Hypoxia results in upregulation and de novo activation of von Willebrand factor expression in lung endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(6):1329–1338. doi: 10.1161/ATVBAHA.113.301359. [DOI] [PubMed] [Google Scholar]
- 34.Pinsky D.J., Naka Y., Liao H., et al. Hypoxia‐induced exocytosis of endothelial cell Weibel‐Palade bodies. A mechanism for rapid neutrophil recruitment after cardiac preservation. J Clin Invest. 1996;97(2):493–500. doi: 10.1172/JCI118440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mojiri A., Alavi P., Lorenzana Carrillo M.A., et al. Endothelial cells of different organs exhibit heterogeneity in von Willebrand factor expression in response to hypoxia. Atherosclerosis. 2019;282:1–10. doi: 10.1016/j.atherosclerosis.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Guo T., Fan Y., Chen M., et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19) JAMA Cardiol. 2020;5(7):811. doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID‐19): a meta‐analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 38.Assiri A., Al‐Tawfiq J.A., Al‐Rabeeah A.A., et al. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect Dis. 2013;13(9):752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rietveld I.M., Lijfering W.M., le Cessie S., et al. High levels of coagulation factors and venous thrombosis risk: strongest association for factor VIII and von Willebrand factor. J Thromb Haemost. 2019;17(1):99–109. doi: 10.1111/jth.14343. [DOI] [PubMed] [Google Scholar]
- 41.Sabater‐Lleal M., Huffman J.E., de Vries P.S., et al. Genome‐wide association transethnic meta‐analyses identifies novel associations regulating coagulation factor VIII and von Willebrand factor plasma levels. Circulation. 2019;139(5):620–635. doi: 10.1161/CIRCULATIONAHA.118.034532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marini J.J., Gattinoni L. Management of COVID‐19 respiratory distress. JAMA. 2020;323(22):2329. doi: 10.1001/jama.2020.6825. [DOI] [PubMed] [Google Scholar]
- 43.Vincent J.‐.L., Taccone F.S. Understanding pathways to death in patients with COVID‐19. Lancet Resp Med. 2020;8(5):430–432. doi: 10.1016/S2213-2600(20)30165-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1,Fig S1‐S4
Data Availability Statement
For original data, please contact sophiesusen@aol.com.