THE CORONAVIRUS INFECTION OUTBREAKS
This century has seen the emergence of three novel β coronavirus (CoV), namely the severe acute respiratory syndrome CoV (SARS-CoV-1), the Middle East respiratory syndrome CoV (MERS-CoV) and, most recently, the novel SARS-CoV-2 (COVID-19) that cause severe human diseases and death. Between November 2002 and August 2003, the global SARS-CoV-1 spread to 32 countries with a mortality rate of 10.87%. Between April and December 2012, the MERS-CoV spread to 27 countries affecting 2,496 individuals and causing 868 deaths1. The COVID-19 virus spread even more rapidly constituting a global pandemic with over 6.5 million individuals infected and nearly 400,000 deaths worldwide as of June 20202.
The spread of the 2003 SARS outbreak was primarily nosocomial largely affecting healthcare workers caring for sick individuals2. However, SARs-CoV-2 spreads by contact with individuals in the early phase of illness accounting for the higher transmissibility. Critically, high percentages of COVID-19 patients with mild symptoms are often missed making the potential for unknown contact much higher. In addition, the short serial interval (4–9 days) and the varied reproduction number (R0) of the COVID-19 virus across the globe supports the potential for transmission by asymptomatic carriers1.
DIAGNOSIS OF COVID-19
Extensive research is ongoing to develop tests that would allow healthcare professionals and public health workers to detect and isolate people with COVID-19 infection including asymptomatic people to contain the spread of the disease. The current gold standard for COVID-19 diagnosis is real-time polymerase chain reaction (RT-PCR) detection of SARS-CoV-2 from nasopharyngeal swabs. Acceptable alternative sample includes a nasal mid-turbinate swab by healthcare workers or onsite self-collection using a flocked tapered swab. However, several studies report a low sensitivity of these samples for detecting SARS-CoV-23,4. In addition, close contact at the time of collection increases the exposure risk to healthcare workers4.
SALIVA IS A HIGHLY SENSITIVE SPECIMEN FOR SARS-COV-2/COVID-19 DETECTION
A high concordance between the saliva and the nasopharyngeal swabs as specimens for laboratory diagnosis of respiratory viruses including the SARS-CoV-1 is well established3. Earlier this year, during the initial outbreak in China, To et al.5 assessed the saliva of 12 nasopharyngeal swab-confirmed COVID-19 patients between 0 and 7 days of hospitalization for SARS-CoV-2 as determined by RT-PCR. They reported a median viral load in the first saliva samples of 3.3 × 106 copies/mL with a range of 9.9 × 102 and 1.2 × 108 copies/mL5. More recently, a study at the Yale New Haven Hospital compared the saliva and paired nasopharyngeal swabs from 38 severely diseased COVID-19 patients. The SARS-CoV-2 titer from the saliva was significantly higher than that from the nasopharyngeal swabs4. Significantly, preliminary reports support the use of saliva for detection of pre-symptomatic or asymptomatic individuals albeit at lower titers of SARS-COV-2 (104–105 copies/mL)4,6.
Temporal profile of SARS-CoV2 in saliva
In general, the viral titer in a biospecimen is expected to decrease with recovery from disease. However, longitudinal testing of nasopharyngeal swabs for SARS-CoV-2 yielded many false–negative results, with positive reports following previous negative reports. In contrast, in a pilot study by Wyllie et al.4, serial saliva samples from 12 severely ill COVID-19 patients exhibited progressively decreasing SARS-CoV-2 titers. Interestingly, To et al. reported that in infected and recovered COVID-19 patients, the saliva was positive for SARS-CoV-2 even at 25 days after the first symptoms and that the titer was higher in the post-recovery saliva of patients who had experienced severe disease3,6.
Oral epithelial cells as reservoirs of SARS-CoV-2
The initial step of COVID-19 infection is its entrance into human cells. Several studies suggest that similar to SARS-CoV-1, SARS-CoV-2 exploits the angiotensin converting enzyme 2 (ACE2) as receptor to gain access to target cells. The spike protein of SARS-CoV-2 envelope binds the membrane bound ACE2, and the strength of this interaction as the initial step of attachment determines the susceptibility to infection. In humans, ACE2 is predominantly observed in epithelial cells in contact with the environment, although expression has also been reported in internal organs including kidney and heart. In general, the mode of transmission and the distribution of ACE2 expression in different tissues of the human body influence the route of infection, symptoms and outcome of COVID-197. Thus, the spread by droplets and the abundant ACE2 expression in the alveolar epithelial cells of the lungs supports the high incidence of respiratory pathology in COVID-19 patients.
Based on the analyses of two public RNA sequence databases, Xu et al.8 reported that the ACE2 was differentially expressed in the oral mucosa in different sites, with the highest expression in the tongue and floor of the mouth. They confirmed the high expression of ACE2 in oral epithelial cells by single cell transcriptome analysis of normal oral mucosal biopsies. Interestingly, a high expression of ACE2 has also been reported in the granular cells and the ductal epithelial cells of the salivary glands9. In this context, it is interesting to note that Chen et al.10 reported high titers of SARS-CoV2 in pure saliva collected from salivary gland duct of severely ill COVID-19 patients.
Collectively, these findings support a potential role for oral and salivary gland epithelial cells as COVID-19 reservoirs or as initial sites of infection. We and others have shown that the saliva is a rich source of viable oral epithelial cells11. Viral shedding by infected epithelial cells could contribute to the higher sensitivity of COVID-19 detection in the saliva of asymptomatic, pre-symptomatic and post-recovery individuals.
Oral-fecal transmission of SARS-CoV-2
Emerging clinical data suggest that a portion of COVID-19 patients may present with abdominal discomfort, diarrhea and other gastrointestinal symptoms. SARS-CoV-2 virus has been consistently detected in feces of COVID-19 patients. Because the rectal epithelial cells express high levels of ACE2, it is suggested that the viral entry, replication and shedding from these cells account for the high titers of SARS-CoV2 in feces12. In this context, oral–fecal communication and self-inoculation have been suggested as alternative mechanisms for the high titers of the COVID-19 virus in saliva.
SIGNIFICANCE FOR DENTAL HEALTH CARE PROFESSIONALS
Risk of direct transmission
Direct contact by respiratory droplets or aerosols is the most widely accepted mode of COVID-19 transmission. Use of rotary or surgical instruments such as handpieces, ultrasonic scalers or air–water syringes in routine dental practice generates visible spatter and droplets of saliva and blood13. The distance the droplets travel depends on the size with larger droplets tending to settle quickly. Very small droplets (<5 µM) may evaporate, become droplet nuclei or aerosol and could contribute to the airborne transmission of infections14. The number and size of saliva droplets generated not only vary with the dental procedure but also among people, suggesting heterogeneous transmission potential. Contamination of clinical gowns and inner surfaces of masks worn by dental hygienists with salivary droplets has been reported following ultrasonic cleaning. Although the surgical masks protect the mucous membrane of the mouth and nose from the spatter, they do not provide complete protection from airborne infections13.
Positive airborne transmission is dependent on the duration of stability of the infectious agent in aerosols and the susceptibility of the individual in the path of the aerosols. The observed stability of airborne SARS-CoV-1 by RT-PCR and in viable cultures suggests transmission of coronaviruses by short- and long-range aerosols15,16. As of now, little is known about the stability of COVID-19 virus in saliva droplets or aerosols. A study from the University of Nebraska Medical Center demonstrated that the SARS-CoV-2 genome was not only detectable in air samples from rooms of COVID-19 patients, but also in 66.7% of air samples obtained from hallways outside the patients’ rooms14. Using Bayesian regression model van Doremalen et al.16 compared the stability of COVID-19 virus and SARS-CoV-1 in aerosols. They found that much like the SARS-CoV-1, the COVID-19 virus remained viable in aerosols throughout the 3-hour period of experimental duration. Taken together, these observations substantiate the high risk for direct airborne transmission of the COVID-19 virus to dental healthcare professionals (Figure 1).
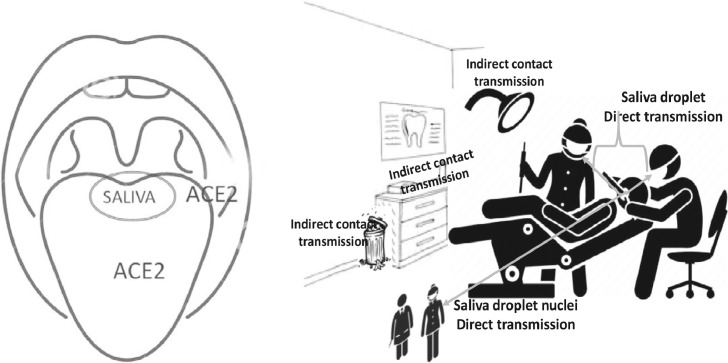
Fig. 1.
Saliva and COVID-19. The COVID-19 virus is detected in saliva of severely ill patients at higher sensitivity, as well as in asymptomatic carriers. The oral epithelial cells and the salivary gland ductal epithelial cells that express ACE2, the receptor for the COVID-19 virus, could function as cellular reservoirs for the virus. Dental healthcare professionals and co-workers are at risk for direct transmission of COVID-19 virus. The virus remains stable on inanimate surfaces for variable periods of time ranging from a few minutes to few days. Inadvertent transmission can occur by contaminating the instruments, work-surfaces and cabinets by hands soiled with saliva that repeatedly contact equipment in the operatory.
Indirect contact transmission
Clusters of COVID-19 infection in meat processing plants and church attenders as well as the success of social distancing in reducing the infection suggest additional modes of transmission of the airborne virus. Respiratory droplets are generated by talking, breathing, sneezing and coughing. Often large droplets with infectious agents deposit on hard surfaces and became a source of transmission. Indeed, during previous outbreaks, the SARS-CoV-1 and the MERS-CoV have been shown to survive on dry metal, ceramic or plastic surfaces for unusually long periods (4–6 days) at room temperature. Further, the SARS-CoV1 exhibited a dose response, being stable on disposable plastic for a short period of 1 hour following low dose inoculation (104 dose/mL) but survived for 2 days at higher inoculation concentration (106 dose/mL)15.
The COVID-19 virus is expected to share similar features of stability as SARS-CoV1 and MERS-CoV. Recently, Liu et al.17 analysed 35 aerosol samples from patient areas and medical staff areas in two hospitals in Wuhan, China for SARS-CoV2 by digital polymerase chain reaction. They observed that the droplets deposited on the surfaces of intensive care workstations tested positive for the virus. van Doremalen et al.16 evaluated the stability in a more controlled environment and found that much like SARS-CoV-1, the laboratory created COVID-19 aerosols remained stable on stainless steel and plastic surfaces for 72 hours.
In clinical dental settings, digital contamination, that is hands soiled with saliva that repeatedly contact operatory equipment and return to the patient's mouth during treatment, can easily increase the area of contaminated surfaces. This will in turn increase the chances of transmission to all personnel with access to the operatory as well as enhance the risk of cross-contamination between patients for an extended period (Figure 1).
CONCLUSIONS
To contain the COVID-19 pandemic, it is essential to find methods that can be used by a wide range of health care professionals to identify the virus. The less potential contagious nature of the collection process, the ease of collection and the convenience of frequent collection for real-time monitoring makes saliva an excellent specimen for home-based collection for epidemiological investigations. With respect to COVID-19, the use of saliva offers the added advantages of greater sensitivity and potential for detection at an early stage of infection.
However, the advantages from a diagnostic perspective also reflect the potential risk to dental professionals from saliva from infected patients. Although not validated in COVID-19 patients, but by extension from studies of SARS-CoV-1 studies, it is suggested that using antimicrobial mouthrinses such as chlorhexidine, hydrogen peroxide or sodium hypochlorite solutions could reduce the viral load in saliva droplets and reduce the risk of direct transmission. Because large saliva droplets could deposit on inanimate surfaces, changing the personal protective equipment including the clinical gown, gloves, masks, protective eye wear and face shield between patients, as well as decontamination of the work surfaces in the clinic, could reduce the risk of indirect contact transmission.
FUNDING
None.
AUTHORS' CONTRIBUTIONS
MS, concept and writing. TPT, writing and review. BC, review. DTZ, review.
CONFLICT OF INTEREST
The authors have no conflict of interest in relation to this work.
REFERENCES
- 1.Meo SA, Alhowikan AM, Al-Khlaiwi T, et al. Novel coronavirus 2019-nCoV: prevalence, biological and clinical characteristics comparison with SARS-CoV and MERS-CoV. Eur Rev Med Pharmacol Sci. 2020;24:2012–2019. doi: 10.26355/eurrev_202002_20379. [DOI] [PubMed] [Google Scholar]
- 2.Wilder-Smith A, Chiew CJ, Lee VJ. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect Dis. 2020;20:e102–e107. doi: 10.1016/S1473-3099(20)30129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang WK, Chen SY, Liu IJ, et al. Detection of SARS-associated coronavirus in throat wash and saliva in early diagnosis. Emerg Infect Dis. 2004;10:1213–1219. doi: 10.3201/eid1007.031113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wyllie AL, Fournier J, Casanovas-Massana A, et al. Saliva is more sensitive for SARS-CoV-2 detection in COVID-19 patients than nasopharyngeal swabs. medRxiv. 2020 2004.2016. 20067835. [Google Scholar]
- 5.To KK, Tsang OT, Chik-Yan Yip C, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.To KK, Tsang OT, Leung WS, et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: an observational cohort study. Lancet Infect Dis. 2020;20:565–574. doi: 10.1016/S1473-3099(20)30196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hamming I, Timens W, Bulthuis ML, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203:631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H, Zhong L, Deng J, et al. High expression of ACE2 receptor of 2019-nCoV on the epithelial cells of oral mucosa. Int J Oral Sci. 2020;12:8. doi: 10.1038/s41368-020-0074-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu L, Wei Q, Alvarez X, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol. 2011;85:4025–4030. doi: 10.1128/JVI.02292-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L, Zhao J, Peng J, et al. Detection of 2019-nCoV in saliva and characterization of oral symptoms in COVID-19 patients. SSRN Elect J. 2020 doi: 10.2139/ssrn.3557140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janardhanam SB, Zunt SL, Srinivasan M. Quality assessment of saliva bank samples. Biopreserv Biobank. 2012;10:282–287. doi: 10.1089/bio.2011.0039. [DOI] [PubMed] [Google Scholar]
- 12.Xiao F, Tang M, Zheng X, et al. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158 doi: 10.1053/j.gastro.2020.02.055. 1831–1833.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harrel SK, Molinari J. Aerosols and splatter in dentistry: a brief review of the literature and infection control implications. J Am Dent Assoc. 2004;135:429–437. doi: 10.14219/jada.archive.2004.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santarpia JL, Rivera DN, Herrera V. Transmission potential of SARS-CoV2 in viral shedding observed at the University of Nebraska Medical Center. MedRxiv. 2020 [Google Scholar]
- 15.Geller C, Varbanov M, Duval RE. Human coronaviruses: insights into environmental resistance and its influence on the development of new antiseptic strategies. Viruses. 2012;4:3044–3068. doi: 10.3390/v4113044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Doremalen N, Bushmaker T, Morris DH, et al. Aerosol and surface stability of SARS-CoV-2 as compared with SARS-CoV-1. N Engl J Med. 2020;382:1564–1567. doi: 10.1056/NEJMc2004973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu Y, Ning Z, Chen Y, et al. Aerodynamic characteristics and RNA concentration of SARS-CoV-2 aerosol in Wuhan hospitals during COVID-19 outbreak. bioRxiv. 2020 2020.2003. 2008.982637. [Google Scholar]