Abstract
To optimize diagnostic workup of the current severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) pandemic, we systematically reviewed neurological and neuroradiological manifestations of SARS‐CoV‐2 and all other known human coronavirus species (HCoV). Which lessons can we learn? We identified relevant publications (until 26 July 2020) using systematic searches in PubMed, Web of Science, and Ovid EMBASE with predefined search strings. A total of 4571 unique publications were retrieved, out of which 378 publications were selected for in‐depth analysis by two raters, including a total of 17549 (out of which were 14418 SARS‐CoV‐2) patients. Neurological complications and associated neuroradiological manifestations are prevalent for all HCoVs (HCoV‐229E, HKU1, NL63, OC43, Middle East respiratory syndrome (MERS)‐CoV, SARS‐CoV‐1, and SARS‐CoV‐2). Moreover there are similarities in symptomatology across different HCoVs, particularly between SARS‐CoV‐1 and SARS‐CoV‐2. Common neurological manifestations include fatigue, headache, and smell/taste disorders. Additionally, clinicians need to be attentive for at least five classes of neurological complications: (1) Cerebrovascular disorders including ischemic stroke and macro/micro‐hemorrhages, (2) encephalopathies, (3) para‐/postinfectious immune‐mediated complications such as Guillain‐Barré syndrome and acute disseminated encephalomyelitis, (4) (meningo‐)encephalitis, potentially with concomitant seizures, and (5) neuropsychiatric complications such as psychosis and mood disorders. Our systematic review highlights the need for vigilance regarding neurological complications in patients infected by SARS‐CoV‐2 and other HCoVs, especially since some complications may result in chronic disability. Neuroimaging protocols should be designed to specifically screen for these complications. Therefore, we propose practical imaging guidelines to facilitate the diagnostic workup and monitoring of patients infected with HCoVs.
Introduction
Neurological complications of coronavirus disease 2019 (COVID‐19), caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), are emerging. Meanwhile, there are opportunities to learn from previous experiences with other human coronaviruses (HCoVs), among them SARS‐CoV‐1, triggering an epidemic in 2002, 1 and Middle East respiratory syndrome coronavirus (MERS‐CoV), responsible for an epidemic in 2012. 2 Moreover four additional HCoV species (HCoV‐229E, HCoV‐HKU1, HCoV‐NL63, and HCoV‐OC43) cause disease in humans, albeit typically with milder clinical courses. 3
While coronaviruses primarily target the human respiratory system, 4 they can also enter the central nervous system (CNS). This is evident from pre‐clinical research, where murine coronaviruses have been used to model human encephalitis for decades. 5 HCoVs also show neurotropism. SARS‐CoV‐1 6 and SARS‐CoV‐2 7 both use the cell membrane‐bound human angiotensin‐converting enzyme 2 for cellular entry, which is, among other tissues, expressed on vascular endothelial cells in the brain. 8 Yet, SARS‐CoV‐2 seems to have an even higher affinity to bind to this receptor compared to SARS‐CoV‐1. 9 SARS‐CoV‐1 and SARS‐CoV‐2 are also most related genetically, with 79% genome homology. 10 Therefore, it is not surprising that several studies have described neurological symptomatology in both SARS‐CoV‐1 and SARS‐CoV‐2 infections. 3 , 11 Neurological complications have also been described in other HCoVs. 12
The aim of this study was to systematically summarize neurological and neuroimaging manifestations of all known HCoVs in order to provide possibilities to predict short‐ and long‐term neurological complications of COVID‐19. The underlying hypothesis was that there are similarities, in particular between SARS‐CoV‐1 and SARS‐CoV‐2, that can help guide our current and future diagnostics of neurological complications to minimize the long‐term effects of the current SARS‐CoV‐2 pandemic.
Methods
We registered the study protocol in the International prospective register of systematic reviews (PROSPERO, CRD42020183405, https://www.crd.york.ac.uk/PROSPERO/) and used the Preferred Reporting Items for Systematic Reviews and Meta‐Analysis (PRISMA) Guidelines for reporting. 13
Search strategy
We searched for original studies/abstracts on coronavirus and neurological/neuroradiological manifestations published until 26 July 2020 in PubMed, Web of Science, and Ovid EMBASE. The search strings used in each of these databases are described in Table S1. Reviews on the topic were also scrutinized for additional references.
Eligibility criteria
We included publications that reported on any neurological and/or neuroradiological manifestations related to acute or prior coronavirus infection, that is, neurological symptoms/complications in coronavirus‐infected persons (we included studies reporting on any symptom associated with, but not necessarily specific for neurological diseases, e.g., headache, fatigue), neuropathology findings of coronavirus infections as well as neuroimaging findings associated with coronavirus infections. We excluded animal studies, records with non‐English abstracts and reviews.
Study selection and data extraction
Titles and abstracts of studies were independently screened for their relevance in the web‐based application Rayyan (https://rayyan.qcri.org/) by two reviewers (J.A. and B.V.I.) followed by full‐text screening. 14 The same reviewers independently extracted title, authors, publication year, study design, country, number of subjects per group, neurological symptoms/complications, neuropathological findings, and/or neuroimaging findings associated to acute or prior coronavirus infection. Any results of associations, including whether analyses were adjusted for confounders, were extracted. Discrepancies in findings were resolved by discussion among assessors, a third reviewer (T.G.) was consulted as needed.
Quality assessment
The quality of each study with more than 10 included subjects was assessed against pre‐defined criteria by the same two reviewers using the Newcastle‐Ottawa scale for evaluating risk of bias in non‐randomized studies and are further described in Table S2. 15
Data synthesis and analysis
Studies and results were qualitatively compared and summarized. We did not assess publication bias.
Results
Eligible publications
In total, 12195 original publications were retrieved from our comprehensive database search. An additional 13 publications were retrieved by screening relevant reviews. After abstract and title screening, 611 studies were eligible for full‐text search of which 371 (8.3% of deduplicated references) and an additional 7 abstract for which the English full‐text was not available were included in the qualitative synthesis (Fig. 1). All references are listed in the Data S1.
Figure 1.
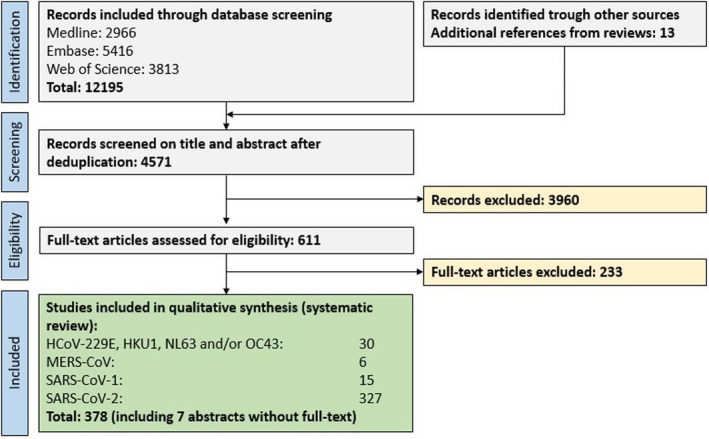
PRISMA flow chart depicting the study selection process. Abbreviations: HCoV, human coronavirus; MERS, Middle Eastern respiratory syndrome; SARS, severe acute respiratory syndrome.
Risk of bias assessment
We evaluated the risk of bias for all studies with more than 10 subjects. Most of the studies showed a low risk of bias for the selection domain, as well as for subunits 2 and 3 from the exposure domain, that is, if patients and controls were treated equally and if studies showed low exclusion rates, respectively (see Table S3). Many studies did not report on adjusting their statistical analyses for subject age, sex or other potential confounders (comparability domain), thus potentially introducing biases. Of note, a considerable proportion of studies was published as letters or editorials thus hampering a thorough risk of bias assessment.
General study characteristics
Of the eligible 378 publications, 30 publications investigated clinically more benign strains of HCoV, including a total of 961 subjects. The majority of these publications assessed HCoV OC43 (26 publications), followed by 229E (22), NL63 (7), and NKU1 (5). Six publications assessed MERS‐CoV, comprising a total of 341 patients, while 15 publications assessed SARS‐CoV‐1, including a total of 1829 cases. About 327 publications assessed SARS‐CoV‐2, with the majority being case reports (179). These studies included a total of 14418 SARS‐CoV‐2 patients (including 45 children) from 35 different countries. Study characteristics and main findings for every HCoV strain are summarized in Tables S4–S7.
Association of coronavirus infections with neurological symptoms and complications
Clinically milder infections with HCoVs: 229E, HKU1, NL63, and OC43
Acute infections
Findings from studies regarding the more clinically benign HCoVs are summarized in Table S4. Most studies assessed neurological manifestations of coronavirus infections in children. A retrospective cohort study from the USA investigated 1683 respiratory specimens from children and found that 84 of them (5%) were positive for coronavirus (229E, HKU1, NL63, and/or OC43); of these, 5 children (8%) had meningoencephalitis and/or febrile seizures. 16 A prospective multicenter study from China confirmed the association of coronavirus, particularly HKU1, with febrile seizures: 87 of 4181 consecutive patients (2.1%) were tested positive for coronavirus (229E, HKU1, NL63, and/or OC43). 17 Five of the 13 children (38%) positive for HKU1 had febrile seizures and one adult patient with OC43 had an aseptic meningitis. Two prospective studies from Slovenia investigating the same patient cohort found that 19 of the 278 children (7%) with febrile seizures were tested positive for coronavirus (mostly OC43: 12/19 patients, 63%). 18 , 19 Another prospective multicenter study from Turkey included 192 children with febrile seizures and found that HCoV OC43 is the single most common, yet still rare, cause for febrile seizures in children under 12 months (6% of children under 12 months with febrile seizures were positive for OC43). 20 Finally, five case reports in children described the following neurological complications associated with the detection of coronavirus: acute flaccid paralysis, acute disseminated encephalomyelitis (ADEM), Guillain–Barré syndrome (GBS) and two cases of fatal encephalitis (both in children with underlying immune deficiency), described in Table S4.
One retrospective cohort study from Finland assessed neurological complications associated with HCoV infections in adults. OC43 was detected in 28 out of 14000 sera from patients with infectious diseases. 21 Four of these patients suffered either meningitis, convulsions, headache, or vertigo.
Association of prior infections to neurological/psychiatric diseases
Findings are summarized in Table S8. An early case–control study from 1980 isolated infectious coronavirus particles from autopsy brain material from two MS patients (of the 13 MS patients and 12 controls). 22 Injection of 10% homogenate of the HCoV positive brain material (in saline solution) led to encephalitis in mice within 3–5 days. Several subsequent studies confirmed the presence of OC43 and/or 229E in different tissues including brain, serum and cerebrospinal fluid (CSF). 23 , 24 , 25 Yet, while some studies observed a higher prevalence of coronavirus antibodies in MS patients versus controls, most studies could not confirm this finding (Table S8).
Two studies have assessed the association of prior coronavirus infections with psychiatric symptoms. A case–control study that included 257 subjects with major depression or suicide attempt, found that seropositivity for coronavirus strain NL63 (as well as Influenza A and B) was associated with the history of mood disorders. 26 Another case–control study observed higher immunoglobulin G (IgG) titers toward different coronavirus species in patients with recent onset of psychotic symptoms as compared to controls. 27
MERS‐CoV
Findings are summarized in Table S5. Two larger retrospective case series from Saudi Arabia assessed symptomatology among MERS‐CoV patients. One multicenter study found that 71% of patients (185/227) reported some form of neurological symptoms. 28 The most common neurological symptoms were fatigue (108/185, 58%), headache (59, 31%), altered consciousness (53, 29%), and/or focal neurological deficits (10, 5%). Additionally, altered level of consciousness at time of diagnosis was a significant risk factor for death. The other study included 70 patients with confirmed MERS, of which the large majority had clinical symptoms (67/70, 96%). 29 The neurological symptomatology was similar; fatigue (29/67, 43%), confusion (18, 27%), headache (9, 13%), and/or seizures (6, 9%). Four smaller case series/case reports including a total of 10 patients described neurological complications in MERS‐HCoV patients, among them toxic/infectious neuropathy, Bickerstaff brainstem‐encephalitis with intracranial hemorrhage, ischemic stroke, critical illness neuropathy, brain stem dysfunction, and ADEM. Of note, eight of these patients had pre‐existing medical conditions, including diabetes type 2 and/or cardiovascular disease (Table S5).
SARS‐CoV‐1
Findings are summarized in Table S6. Two cohort studies assessed neurological manifestations of adult SARS‐CoV‐1 patients. One study comprised 206 SARS‐CoV‐1 cases, constituting all SARS‐CoV‐1 patients from Singapore. 30 Five of these patients (2.4%) suffered large artery cerebral strokes. It is noteworthy that only one of these patients had pre‐existing risk factors for cerebrovascular disease. The other cohort study included 1291 Chinese SARS‐CoV‐1 patients and investigated how cerebrocardiovascular disease affected SARS‐CoV‐1 disease outcomes (only abstract available in English). 31 Critical conditions and multi‐organ dysfunction occurred more frequently among SARS‐CoV‐1 patients with pre‐existing cerebrocardiovascular diseases (58% and 27%, respectively) compared to patients without underlying diseases (28% and 10%, respectively). One cross‐sectional study aimed at assessing neuropsychiatric symptoms during acute and convalescence phases of SARS‐CoV‐1 via a questionnaire sent out to 308 patients. 32 The response rate was 33% (102 patients); 65% of these patients had suffered neuropsychiatric symptoms during the convalescence phase of SARS‐CoV‐1. Steroid treatment during hospitalization was predictive for anxiety, depression, psychosis, and behavioral symptoms during both the acute and convalescence phases. Another Chinese study (only abstract available in English) including 173 SARS‐CoV‐1 patients confirmed high rates of neurological/neuropsychiatric symptoms in SARS‐CoV‐1 patients (53%). 33 The symptomatology comprised headache (67%), affective disorder (31%), dizziness (29%), anxiety (20%), reduced consciousness (10%), phobia (8%), depression (6%), unspecified mental disorder (5%), suicidal ideation (1%), seizures (1%), and focal neurological signs (0.6%). Several case reports, comprising a total of 11 patients, described neurological complications in SARS‐CoV‐1, among them critical illness neuro‐/myopathy, seizures, persistent sleeping difficulties, persistent anosmia, delirium, and generalized pain (Table S6).
Children
One cohort study assessed neurological manifestation of SARS‐CoV‐1 in children. 34 The study comprised 183 children with encephalitis out of which 22 (12%) were positive for SARS‐CoV‐1. Headache, seizures, and neck stiffness were common symptoms among SARS‐CoV‐1 positive children with encephalitis (10/22 [46%], 5/22 [23%] and 7/22 [32%], respectively). A total 16 of these children underwent neuroimaging of which 8 (50%) showed abnormal findings, among them abnormalities in the temporal lobe, in the periventricular region, and in the basal ganglia/thalamus. This study also measured different inflammatory proteins in serum and CSF; in matched serum‐CSF samples, interleukin (IL)‐6, IL‐8, and chemokine (C‐C motif) ligand 2 were increased in CSF compared to serum.
Neuropathology
Several studies addressed neuropathological changes of SARS‐CoV‐1. One relatively large study including findings from 18 autopsies (of which eight were confirmed SARS‐CoV‐1 infections pre‐mortem) detected SARS‐CoV‐1 infected neurons in the hypothalamus and cortex using in situ hybridization and electron microscopy. 35 Six of eight patients (75%) showed diffuse hypoxic neuronal injury. Interestingly, SARS‐CoV‐1 viral sequences and pathologic changes were unique to brains of SARS‐CoV‐1‐confirmed cases. A smaller case series including three patients who died from SARS‐CoV‐1 showed signs of systemic vasculitis engaging smaller brain vessels. 36 In one case with CT findings suggestive of ischemia, the histopathological exam showed signs of neuronal necrosis and glial cell hyperplasia. 37 One study including 5 SARS‐CoV‐1 patients found changes in cell numbers/staining intensities compared to controls in various endocrine cell pools of the adenohypophysis. 38 Finally, one study found myopathic changes in four of eight SARS‐CoV‐1 cases (50%) undergoing autopsy (in situ hybridization for SARS‐CoV‐1 in the skeletal muscle was negative though). 39
SARS‐CoV‐2
Neurological symptomatology
Findings are summarized in Table S7. Two large retrospective studies from Spain and China including 841 patients and 214 patients reported that 57% and 38% of patients had neurological manifestations, respectively. 40 , 41 Both studies also found that neurological symptoms were more common in patients with more severe disease courses.
Several common neurological symptoms among SARS‐CoV‐2 patients have been described in these studies, such as fatigue (44–64% of patients), 42 , 43 , 44 headache (8–14% of patients [up to 70% in 1 study] 45 ) 40 , 41 , 42 , 43 , 44 , 46 , 47 , dizziness (6%) 41 and confusion (0.9–9% of patients). 44 , 46 Smell and/or taste disorders are other symptoms reported by several studies, 45 , 48 , 49 having a prevalence of 39–88% in SARS‐CoV‐2 patients, 47 , 50 Two studies also observed that smell/taste disorders are the initial manifestation of SARS‐CoV‐2 infection in 36%/60% of patients. 41 , 50 A Spanish survey‐based study showed that smell disorders can persist for several weeks in about 1/3 of patients. 51 Also other symptoms may persist; an Italian study followed 143 patients for an average of 60 days after onset of SARS‐CoV‐2 symptoms, finding that 76 patients (53%) suffered persisting fatigue. 52
Neurological complications
Several studies reported on neurological complications of SARS‐CoV‐2, mostly in severe or critically ill patients. A case series from the UK included 43 SARS‐CoV‐2 patients who had been admitted to a specialized COVID neurology unit. 53 Based on symptomatology, patients could be classified into four major subgroups: (1) ischemic stroke (8 patients), (2) inflammatory CNS syndromes (para‐/postinfectious, 12 patients), (3) peripheral nervous system (PNS) syndromes such as GBS (7 patients), and (4) encephalopathies (10 patients).
Several larger studies have confirmed these classes of neurological complications in SARS‐CoV‐2. Cerebrovascular diseases were among the most commonly reported complications, including ischemic stroke, and cerebral hemorrhages. 41 , 54 , 55 , 56 , 57 , 58 , 59 . Studies from China, Spain and the US report that 1–1.6% of hospitalized patients were affected by cerebrovascular disease. 41 , 54 , 55 , 57 , 59 Yet, cardiovascular risk factors were pre‐existent in many of the stroke patients. 60 Ischemic brain lesions seem to be more common in more severe disease courses: In a Chinese study, 20% of deceased SARS‐CoV‐2 patients had signs of hypoxic encephalopathy compared to only 1% in patients who recovered. 43 Cerebral susceptibility abnormalities (i.e., microbleeds/‐thrombosis) were also described as a relatively common neuroimaging feature, particularly in atypical locations such as in the corpus callosum or juxtacortically. 61 , 62 , 63 , 64 Several cases of cerebral venous thromboses have been observed. 56 , 61
Inflammatory and/or demyelinating syndromes of both the central and the peripheral nervous system have been reported, among them GBS, 53 , 56 , 65 ADEM, 53 Miller‐Fisher syndrome, and facial nerve palsy (Table S7).
Encephalopathy and/or encephalitis are also reported by an increasing number of studies. An observational case series from France reported on neurological complications in intensive care unit patients: 13/58 of patients (22%) presented with encephalopathic features. 66 Brain MRI showed that 8/13 patients displayed leptomeningeal enhancement on post‐contrast T1‐weighted and fluid‐attenuated inversion recovery (FLAIR) sequences – a sign of leptomeningeal inflammation. Another French study found that 7/26 patients (27%) showed electroencephalogram (EEG)/MRI changes suggestive of encephalopathy. 64 Also, several cases of acute necrotizing encephalopathy (ANE) or posterior reversible encephalopathy syndrome (PRES) have been described. 67 , 68 A Turkish study showed that 12/27 patients (44%) showed abnormalities on brain MRI with signs suggestive of ischemia or menigoencephalitis. 69 Of note, only minimal evidence points to direct infection of neurons/glial cells by SARS‐CoV‐2 viral particles, as shown by mostly negative RT‐PCR analyses from CSF (Table S7). 53 , 64 , 66 Nevertheless, CSF from a small subset of patients with encephalitic features did test positive for SARS‐CoV‐2 RNA. 70 , 71
Seizures have been reported in SARS‐CoV‐2 patients; in a Chinese cohort, 4/78 hospitalized patients (0.5%) suffered at least one seizure. 40 This was mirrored in a Spanish cohort, where 6/841 hospitalized patients (0.7%) had at least one seizure. 41 However, a study focusing on seizure prevalence among SARS‐CoV‐2 patients did not observe any acute symptomatic seizures at all, despite seizure‐triggering metabolic changes such as electrolyte imbalance in 84/311 patients (27%). 72
Finally, several recent studies observed psychiatric complications in SARS‐CoV‐2 patients. An Italian survey‐based study observed sleep impairment as the most commonly reported neurological symptom (94/103 patients). 73 A prospective cross‐sectional study from the USA showed that impaired sense of smell was associated with anxiety and depressed mood. 74 Finally, a UK‐wide surveillance study recorded 23 patients with neuropsychiatric disorders, among them new‐onset psychosis (10 patients, 43%), neurocognitive syndrome (6 patients, 26%), and affective disorders (4 patients, 17%). 58
Children
In a study from the UK, 4 of the 27 children (15%) suffering multisystem inflammatory syndromes also developed neurological symptoms, including encephalopathy, brainstem/cerebellar signs, and reduced reflexes. MRI showed signal changes of the splenium in all patients, interpreted as a sign suggestive of an ongoing (para‐)infectious process. 75 However, virus was undetectable in CSF from 2/2 sampled children. All patients showed at least some degree of recovery by the end of the study. One case series from China included eight children with severe SARS‐CoV‐2 courses: 76 Of them, one child presented with headache and another with fatigue. Furthermore, cases of children with GBS, stroke and encephalitis have been described (Table S7).
Fluid biomarkers
In a Chinese study, patients with neurological symptoms had lower lymphocyte levels, platelet counts as well as higher blood urea nitrogen levels compared with those without neurological symptoms. 40 Blood neurofilament light chain (NfL) levels have been linked to SARS‐CoV‐2: SARS‐CoV‐2 status seems to be an independent predictor of serum NfL levels. Additionally, in severe SARS‐CoV‐2 patients, an early peak in plasma GFAP was followed by a slower increase in NfL, possibly reflecting a more acute involvement of astrocytes followed by a more prolonged neuro‐axonal degeneration.
Neuropathology
Several studies assessed neuropathological changes associated with SARS‐CoV‐2 infections: One case series including six SARS‐CoV‐2 patients found lymphocytic panencephalitis and meningitis and, in three patients aged <65 years, widespread petechial hemorrhages. 77 Interestingly, all patients also showed signs of brain stem encephalitis. Signs of perivenular inflammation/demyelination suggestive of ADEM have also been described. 53 , 78 A neuropathological assessment of 18 SARS‐CoV‐2 patients showed changes consistent with hypoxic injury. However, RT‐PCR and immunohistochemistry disclosed only minimal evidence of SARS‐ CoV‐2 infection. 79 Yet, one SARS‐CoV‐2 case in a patient with Parkinson’s disease showed electron dense particles suggestive of SARS‐CoV‐2 in both brain endothelial cells and neurons. SARS‐CoV‐2 RT‐PCR was positive in brain tissue but not in the CSF. 80
Comparison between SARS‐CoV‐1 and SARS‐CoV‐2
Direct comparison of neurological manifestations between SARS‐CoV‐1 and SARS‐CoV‐2 shows substantial similarities (Table 1). In both diseases, a considerable number of patients suffer neurological complications, in particular those with a more severe disease course. Fatigue, headache, smell/taste disorders and reduced consciousness are among the most commonly reported symptoms. For example, the SARS‐CoV‐2 patient shown in Figure 2, presented with altered consciousness to the intensive care unit. Ischemic/hypoxic pathology, including stroke as well as seizures, have been described in both diseases. Similarly, neuropsychiatric complications such as psychosis and mood disorders have been reported for both viral infections. Finally, immune‐mediated complications such as GBS, Miller‐Fisher syndrome, or polyneuritis cranialis have been described in both diseases.
Table 1.
Comparison between neurological manifestations in SARS‐CoV‐1 versus SARS‐CoV‐2, ordered according to evidence level.
| Evidence level | SARS‐CoV‐1 | SARS‐CoV‐2 |
|---|---|---|
| Prospective or retrospective cohort studies |
Symptoms:
|
Symptoms:
|
|
Complications:
|
Complications:
|
|
| Case‐control studies, large case series |
Symptoms:
|
Symptoms:
|
|
Complications:
|
Complications:
|
|
| Cross‐sectional studies |
|
|
| Case reports, small case series |
|
|
Abbreviations: ADEM, acute demyelinating encephalomyelitis; CNS, central nervous system; CoV, coronavirus; COVID‐19, coronavirus disease 2019; GBS, Guillain‐Barré Syndrome; PNS, peripheral nervous system; SARS, severe acute upper respiratory syndrome.
Figure 2.
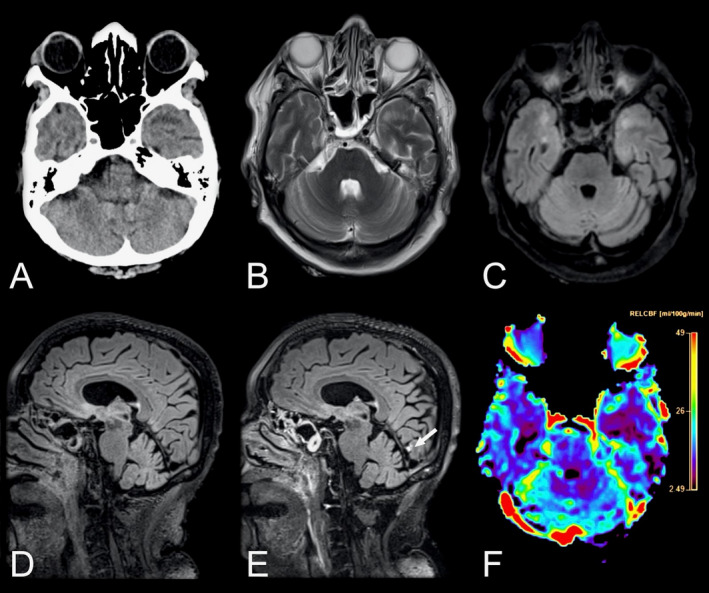
Neuroradiological findings in a 69‐year‐old female with COVID‐19 admitted to the ICU with altered consciousness. Axial non‐enhanced head CT scan of the brain (A) showing suspected hypodensities in the temporal poles. Brain MRI was performed 2 days later. Axial T2‐weighted images (B) and T2‐weighted FLAIR images (C) confirmed the finding. Pre‐ and post‐contrast sagittal T2‐weighted FLAIR images (D and E) in the same patient revealed a solitary focal leptomeningeal enhancement (arrow) by the medial aspect of left occipital lobe, otherwise not easily visible in T1‐weighted contrast‐enhanced sequences. The contrast‐enhanced perfusion map (F) further revealed reduced blood flow in both temporal lobes. Abbreviations: COVID‐19, coronavirus disease 2019; FLAIR, fluid attenuated inversion recovery; ICU, intensive care unit.
Discussion
The findings of this systematic review suggest that all HCoV strains are associated with neurological manifestations. Specifically, 229E, HKU1, NL63, and OC43 infections have been mainly studied in children, where they are associated with febrile seizures and meningitis. GBS, ADEM, and encephalitis have also been described in children. The less abundant evidence for MERS‐CoV suggests that fatigue, headache, and altered consciousness are common neurological manifestations. Stroke, critical‐illness polyneuropathy, and GBS have also been observed. Finally, SARS‐CoV‐1 and SARS‐CoV‐2, which are genetically highly related, 10 show substantial similarities regarding the prevalence and presentation of neurological manifestations; symptoms include fatigue, headache and smell/taste disorders. Complications that were observed among both SARS‐CoV‐1 and SARS‐CoV‐1 include cerebrovascular pathology, encephalopathy, autoimmune disorders such as GBS, meningoencephalitis, and seizures.
Findings in the context of existing evidence
Overall, HCoV infections show a similar symptom complex across all of the HCoV strains. These symptoms include fatigue and headache. However, shared symptomatology is not surprising since all respiratory virus infections (including rhinoviruses and influenzaviruses) can cause fatigue and headache. 81 Moreover these are highly unspecific symptoms that are commonly associated with neurological but also non‐neurological diseases. 82 Thus, these symptoms could arise without direct neuronal involvement of HCoV.
Of note, persistent fatigue has been reported in the recovery/convalescence phase of SARS‐CoV‐2. 52 This is reminiscent of persistent fatigue after Epstein‐Barr virus (EBV) infection. 83 Here, the most accepted hypothesis suggests a multifactorial pathogenesis including latent EBV infection, immune and/or neuroendocrine dysfunction as well as behavioral factors. It may be speculated if a similar etiopathogenesis could explain such persistent symptoms also with SARS‐CoV‐2.
It is evident that SARS‐CoV‐1 and SARS‐CoV‐2 not only share neurological symptomatology but also complications. This is not surprising since it has been shown recently that both SARS‐CoV‐1 and SARS‐CoV‐2 exploit the same membrane bound molecule angiotensin‐converting enzyme 2 as a cellular entry target. 7 This receptor is, among other tissues, expressed on vessel endothelial cells in the brain, which could serve as a viral entry point to the CNS. 8 Based on existing literature, five different syndromes can be delineated: (1) cerebrovascular disease, (2) encephalopathy, (3) para‐/postinfectious inflammatory CNS/PNS syndromes, (4) (meningo‐)encephalitis, and (5) neuropsychiatric complications.
First, ischemic stroke has been reported among both SARS‐CoV‐1 30 and SARS‐CoV‐2 (and anecdotally also for MERS‐CoV). 41 , 53 , 66 Presumable underlying factors contributing to CNS hypoxia are a prothrombotic state evidenced by high D‐dimer levels, which were observed among intensive care unit patients, 40 , 42 , 84 disseminated intravascular coagulation (DIC) 85 and/or systemic vasculitis. 35 , 36 Indeed, widespread endotheliitis has recently been demonstrated in several organs in SARS‐CoV‐2. 86 All these conditions facilitate systemic blood clotting. Furthermore, elderly patients, who were commonly reported being affected by stroke, are more likely to have risk factors for cerebrovascular disease. 87 Additionally, exclusively for SARS‐CoV‐2, intracranial hemorrhages and cerebral microbleeds have been reported, potentially provoked by endothelial damage/dysfunction. 53 , 64 , 77
Second, encephalopathies with clinical features of delirium have been observed among SARS‐CoV‐2 patients. 53 , 66 Many of these patients showed full clinical recovery. 53 Anecdotal evidence also indicates encephalopathic changes in SARS‐CoV‐1. 88 The underlying mechanisms for the encephalopathy is likely multifactorial, resulting from combinatorial effects of sepsis, hypoxia, and immune hyperstimulation (“cytokine storm”). 89
Third, both studies on SARS‐CoV‐1 and SARS‐CoV‐2 reported on GBS, an inflammatory demyelinating disease of the PNS. 65 , 90 , 91 Additionally, ADEM, an inflammatory demyelinating CNS disease has been reported among SARS‐CoV‐2 patients. 53 Anecdotal evidence also suggests a link of clinically less severe HCoV and MERS‐CoV to these diseases. They have a presumable autoimmune pathogenesis 92 and are thus less likely to be a direct consequence of neuronal infection by HCoV – despite the detection of HCoV RNA in CSF in a few reported cases, but rather represent a para‐infectious complication. 90 Additionally, other viral infections, such as cytomegaly or EBV, have been associated with GBS. 92 MS is another autoimmune disease whose potential association with HCoV infection has been substantially investigated. The conflicting results do not allow final conclusions on the role of HCoV in MS pathogenesis, even though the majority of evidence contradicts such an association.
Fourth, exclusively for SARS‐CoV‐2, few patients with clinical and imaging features of (meningo‐)encephalitis have been described (in children anecdotally also for HCoV‐229E, HKU1, NL63 and/or OC43). These cases were suggestive for primary HCoV CNS infection. 64 , 66 Yet, SARS‐CoV‐2 RNA in CSF was only detected in a handful of cases. 64 , 71
Fifth, a link between neuropsychiatric complications such as psychosis and mood disorders has been described for SARS‐CoV‐1, SARS‐CoV‐2, and clinically more benign HCoV. However, large cohort studies with adequate control groups are needed to address potential confounders, such as secondary consequences of pandemic lockdown measures.
It is noteworthy that SARS‐CoV‐2 RNA could not be retrieved from the CSF in the vast majority of patients from larger case series/cohort studies. 53 , 64 , 66 . Similarly, only few incidences of SARS‐CoV‐1 RNA detection in CSF have been described. 93 , 94 Neuropathological findings support this notion: only one study reported on electron dense particles in CNS neurons suggestive of SARS‐CoV‐2, though no RNA was detected in the CSF. 80 Failure to detect SARS‐CoV‐2 RNA in CSF could depend on different factors: first, the virus could be cell‐bound thereby spreading from cell‐to‐cell; second, virus particles have very low levels in CSF or third, endogenous CSF endo‐/exonucleases cleave SARS‐CoV‐2 RNA. 95 For the entry of HCoVs into the CNS, two major pathways have been proposed: 96 the hematogenous (via the blood–brain barrier) and neuronal retrograde pathway (via the cribriform plate/olfactory nerve). The exact pathway is yet still a matter of debate and more studies are necessary to dissect the specific CNS entry mechanisms of HCoVs. However, the absence of SARS‐CoV‐2 from the CNS might also indicate that a large share of neurological complications are secondary to the severe systemic manifestations of SARS‐CoV‐2, that is, from a hyperinflammation syndrome 89 , vasculopathy/coagulopathy, post‐infectious auto‐inflammation, and the effects of sepsis and hypoxia. 53
Recommendations for neuroimaging
The results of the current study highlight the need for adequate neuroimaging protocols in HCoV infections with neurological manifestation to be able to accurately detect encephalitis, leptomeningeal enhancement and vascular complications such as microbleeds, subarachnoid hemorrhage, and infarcts. Previous studies have mainly relied on CT and MRI without gadolinium‐based contrast agents. Cautiousness regarding the use of contrast agents in critically ill patients is naturally warranted, as is the use of ionizing radiation in children. For acute deterioration, a non‐enhanced head CT scan is likely the most adequate choice, while more subacute symptomatology or further investigation may necessitate a brain and/or spinal cord MRI. Based on the findings in this review and our emerging experience with SARS‐CoV‐2, we recommend the MRI protocols suggested in Table 2 to identify HCoV infection‐related neuroradiological manifestations. Examples of neuroimaging findings in SARS‐CoV‐2 from our clinical practice using the recommended protocols can be found in Figures 2 and 3.
Table 2.
Recommended brain MRI protocol for COVID‐19.
| COVID‐19 brain MRI protocol without GBCA | Potential findings |
|---|---|
| Sagittal 3D T2‐weighted FLAIR | White matter changes, encephalitis, infarcts, posterior reversible encephalopathy syndrome |
| Axial 3D SWI | Microbleeds, subarachnoid hemorrhage, cortical superficial siderosis |
| Axial 2D DWI | Infarcts, encephalitis |
| Axial 2D T2‐weighted imaging | White matter changes, encephalitis, infarcts, posterior reversible encephalopathy syndrome |
| Sagittal 3D T1‐weighted GRE IR/TSE | White matter changes, demyelination |
| Sagittal 3D T1‐weighted TSE | White matter changes, demyelination |
| Arterial TOF | Occlusions, stenosis, vasculitis |
| ASL perfusion | Perfusion abnormalities |
| COVID‐19 brain MRI protocol with GBCA | Potential findings |
|---|---|
| Sagittal 3D T2‐weighted FLAIR | White matter changes, encephalitis, infarcts, posterior reversible encephalopathy syndrome |
| Axial 3D SWI | Microbleeds, subarachnoid hemorrhage, cortical superficial siderosis |
| Axial 2D DWI | Infarcts, encephalitis |
| Sagittal 3D T1‐weighted GRE IR/TSE | White matter changes, demyelination |
| Contrast‐enhanced perfusion | Perfusion abnormalities |
| Axial 2D T2‐weighted imaging | White matter changes, encephalitis, infarcts, posterior reversible encephalopathy syndrome |
| Sagittal 3D T1‐weighted GRE IR/TSE | Blood‐brain‐barrier disruption, leptomeningeal enhancement, white matter changes, demyelination |
| Sagittal 3D T1‐weighted TSE | Blood‐brain‐barrier disruption, leptomeningeal enhancement |
| Sagittal 3D T2‐weighted FLAIR | Leptomeningeal enhancement |
| Arterial TOF | Occlusions, stenosis, vasculitis |
Abbreviations: ASL, arterial spin labelling; COVID‐19, coronavirus disease 2019; DWI, diffusion‐weighted imaging; FLAIR, fluid attenuated inversion recovery; GBCA, gadolinium‐based contrast agents; GRE, gradient echo; IR, inversion recovery; posterior reversible encephalopathy syndrome; SWI, susceptibility weighted imaging; TOF, time of flight; TSE, turbo spin echo.
Figure 3.
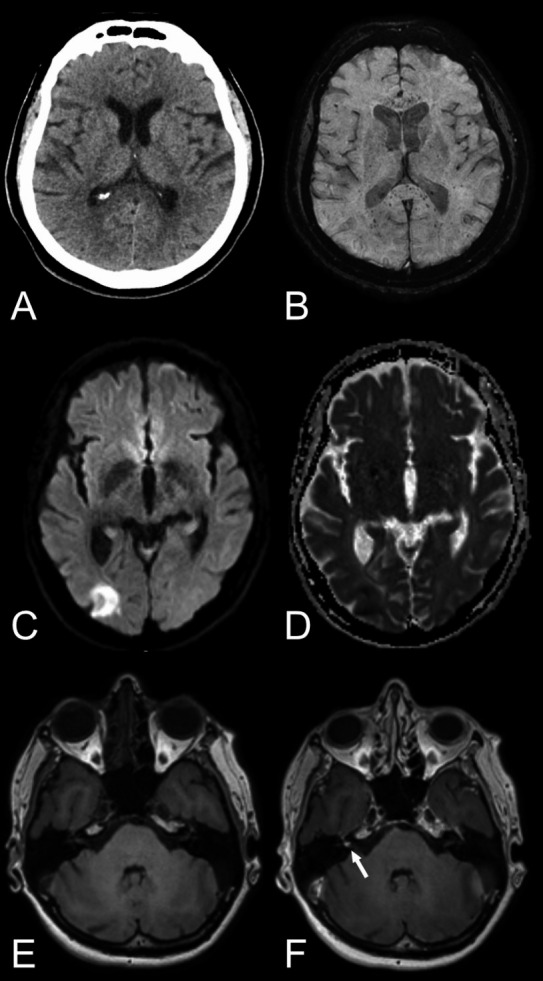
Various neuroradiological findings in COVID‐19 patients. First row: Axial non‐enhanced head CT, reported as normal (A) but brain MRI, including susceptibility‐weighted imaging (B) revealed numerous microbleeds with a predilection to the corpus callosum and especially the splenium of corpus callosum. The aforementioned case exemplifies the importance of susceptibility‐weighted imaging in the neuroimaging of COVID‐19 patients in means of MRI brain examination. Second row: Axial diffusion‐weighted imaging, b1000 (C) and ADC map (D) showing a cortical infarct in the right occipital lobe. Third row: Axial T1‐weighted images pre‐ and post contrast (E and F) depicting a focal enhancement of the right vestibular nerve. Abbreviations: ADC, apparent diffusion coefficient; COVID‐19, coronavirus disease 2019.
Limitations
Due to potential delay in publishing relevant findings, a significant proportion of evidence on SARS‐CoV‐2 may yet to be published. Nevertheless, the available evidence suggests striking similarities of neurological manifestations between SARS‐CoV‐1 and SARS‐CoV‐2 patients.
Conclusions
Our systematic review provides high level evidence that HCoVs, particularly SARS‐CoV‐1 and SARS‐CoV‐2, have a common ground on neurological symptomatology and complications. Particularly, clinicians treating these patients should be vigilant for at least five classes of neurological complications: (1) Cerebrovascular disorders including ischemic stroke and macro/micro‐hemorrhages, (2) encephalopathies, likely caused by combinatorial effects of sepsis, hypoxia, and immune hyperstimulation, (3) para‐/postinfectious immune‐mediated complications such as GBS and ADEM, (4) (meningo‐)encephalitis, potentially with concomitant seizures, and (5) neuropsychiatric complications such as mood disorders or psychosis. Thus, the clinical neuroimaging protocol, as herein recommended, should include appropriate MRI sequences for early diagnosis and monitoring of patients. Our results also underscore the need to further research and optimize treatment regarding modulation of the coagulation and immune systems in order to reduce the risk of neurological complications.
Author Contribution
Jesper Almqvist contributed to designed and conceptualized the study, data acquisition, analysis and interpretation, and drafting and revision of the manuscript. Tobias Granberg contributed to designed and conceptualized the study, data acquisition, analysis and interpretation, and drafting and revision of the manuscript. Antonios Tzortzakakis contributed to data interpretation and revision of the manuscript for intellectual content. Stefanos Klironomos contributed to data interpretation and revision of the manuscript for intellectual content. Evangelia Kollia contributed to data interpretation and revision of the manuscript for intellectual content. Claes Öhberg contributed to data interpretation and revision of the manuscript for intellectual content. Roland Martin contributed to data interpretation and revision of the manuscript for intellectual content. Fredrik Piehl contributed to data interpretation and revision of the manuscript for intellectual content. Russell Ouellette contributed to data interpretation and revision of the manuscript for intellectual content. Benjamin V. Ineichen contributed to designed and conceptualized the study, data acquisition, analysis and interpretation, and drafting of the manuscript.
Conflicts of Interest
This study was supported by the Center for Innovative Medicine (CIMED) and Region Stockholm. The authors report no disclosures relevant to the manuscript.
Ethical Approval
Reporting figures with examples from our own practice was approved by the Swedish Ethical Review Authority; informed consent was waived due to the retrospective nature of the retrospective case reports.
Supporting information
Table S1. Search string.
Table S2. Pre‐defined criteria Newcastle‐Ottawa scale for risk of bias.
Table S3. Risk of bias assessment.
Table S4. Summary of studies assessing neurological manifestations and complication in the more clinically mild HCoVs: HCoV‐229E, HKU1, NL63 and/or OC43 infections.
Table S5. Summary of studies assessing neurological manifestations and complication in MERS‐CoV infections.
Table S6. Summary of studies assessing neurological manifestations and complication in SARS‐CoV‐1 infections.
Table S7. Summary of studies assessing neurological manifestations and complication in SARS‐CoV‐2 infections.
Table S8. Association of coronaviruses with multiple sclerosis.
Data S1. Supplementary search string for medical data bases.
Data S2. Search string for Medline, Embase, and Web of Science.
Acknowledgments
The authors thank Francis Poulenc for help with data analysis and Ioannis Koupidis for help with figure panels.
Funding Statement
This work was funded by Center for Innovative Medicine grant .
References
- 1. Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med 2003;348:1953–1966. [DOI] [PubMed] [Google Scholar]
- 2. Widagdo W, Okba NMA, Stalin Raj V, Haagmans BL. MERS‐coronavirus: from discovery to intervention. One Health 2017;3:11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weiss SR, Navas‐Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev 2005;69:635–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coronaviruses MK. Coronaviruses: a comparative review. Current Topics in Microbiology and Immunology/Ergebnisse der Mikrobiologie und Immunitätsforschung. pp. 85–129. Springer, 1974. [Google Scholar]
- 5. MacNamara KC, Chua MM, Phillips JJ, Weiss SR. Contributions of the viral genetic background and a single amino acid substitution in an immunodominant CD8+ T‐cell epitope to murine coronavirus neurovirulence. J Virol 2005;79:9108–9118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li W, Moore MJ, Vasilieva N, et al. Angiotensin‐converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003;426:450–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yan R, Zhang Y, Li Y, et al. Structural basis for the recognition of SARS‐CoV‐2 by full‐length human ACE2. Science 2020;367:1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamming I, Timens W, Bulthuis M, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tai W, He L, Zhang X, et al. Characterization of the receptor‐binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol 2020;17:613–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Herman C, Mayer K, Sarwal A. Scoping review of prevalence of neurologic comorbidities in patients hospitalized for COVID‐19. Neurology 95 (2), 77–84 (2020. [DOI] [PubMed] [Google Scholar]
- 12. Desforges M, Le Coupanec A, Brison E, et al. Neuroinvasive and neurotropic human respiratory coronaviruses: potential neurovirulent agents in humans. Adv Exp Med Biol 2014;2014:75–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher D, Shamseer L, Clarke M, et al. Preferred reporting items for systematic review and meta‐analysis protocols (PRISMA‐P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wells GA, Tugwell P, O’Connell D, et al. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta‐analyses. 2015.
- 16. Dominguez SR, Robinson CC, Holmes KV. Detection of four human coronaviruses in respiratory infections in children: a one‐year study in Colorado. J Med Virol 81:1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lau SK, Woo PC, Yip CC, et al. Coronavirus HKU1 and other coronavirus infections in Hong Kong. J Clin Microbiol 44:2063–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jevšnik M, Steyer A, Pokorn M. The role of human coronaviruses in children hospitalized for acute bronchiolitis, acute gastroenteritis, and febrile seizures: a 2‐year prospective study. PLoS One 2016;11:e0155555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pokorn M, Jevšnik M, Petrovec M, et al. Respiratory and enteric virus detection in children: a prospective study comparing children with febrile seizures and healthy controls. J Child Neurol 2017;32:84–93. [DOI] [PubMed] [Google Scholar]
- 20. Carman KB, Calik M, Karal Y, et al. Viral etiological causes of febrile seizures for respiratory pathogens (EFES Study). Hum Vaccin Immunother 2019;15:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Riski H, Hovi T. Coronavirus infections of man associated with diseases other than the common cold. J Med Virol 1980;6:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Burks JS, Devald BL, Jankovsky LD, Gerdes JC. 2 Coronaviruses isolated from central nervous‐system tissue of 2 multiple‐sclerosis patients. Science 1980;209:933–934. [DOI] [PubMed] [Google Scholar]
- 23. Stewart JN, Mounir S, Talbot PJ. Human coronavirus gene expression in the brains of multiple sclerosis patients. Virology 191:502–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cristallo A, Gambaro F, Biamonti G, et al. Human coronavirus polyadenylated RNA sequences in cerebrospinal fluid from multiple sclerosis patients. New Microbiol 20:105–114. [PubMed] [Google Scholar]
- 25. Hovanec DL, Flanagan TD. Detection of antibodies to human coronaviruses 229E and OC43 in the sera of multiple sclerosis patients and normal subjects. Infect Immun 41:426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Okusaga O, Yolken RH, Langenberg P, et al. Association of seropositivity for influenza and coronaviruses with history of mood disorders and suicide attempts. J Affect Disord 130:220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Severance EG, Dickerson FB, Viscidi RP, et al. Coronavirus immunoreactivity in individuals with a recent onset of psychotic symptoms. Schizophr Bull 37:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Noorwali ASA, Turkistani AHM, Asiri SI, et al. Descriptive epidemiology and characteristics of confirmed cases of Middle East respiratory syndrome coronavirus infection in the Makkah Region of Saudi Arabia, March to June 2014. Ann Saudi Med 2015;35:203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saad M, Omrani AS, Baig K, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single‐center experience in Saudi Arabia. Int J Infect Dis 2014;29:301–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Umapathi T, Kor AC, Venketasubramanian N, et al. Large artery ischaemic stroke in severe acute respiratory syndrome (SARS). J Neurol 2004;251:1227–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hu SS, Yang YJ, Zhu ML, et al. Effects of underlying cerebrocardiovascular diseases on the incidence of critical conditions and multiple organs dysfunction syndrome in severe acute respiratory syndrome cases. Zhonghua Yi Xue Za Zhi 84:1257–125 9. [PubMed] [Google Scholar]
- 32. Sheng B, Cheng SKW, Lau KK, et al. The effects of disease severity, use of corticosteroids and social factors on neuropsychiatric complaints in severe acute respiratory syndrome (SARS) patients at acute and convalescent phases. Eur Psychiatry 2005;20:236–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tang YM, Zhang YY, Li JG, et al. Characteristics of neuropsychiatric impairment symptoms in patients with severe acute respiratory syndrome. Chin J Clin Rehabil 2005;9:208–209. [Google Scholar]
- 34. Li Y, Li H, Fan R, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology 2016;59:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gu J, Gong E, Zhang B, et al. Multiple organ infection and the pathogenesis of SARS. J Exp Med 2005;202:415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ding Y, Wang H, Shen H, et al. The clinical pathology of severe acute respiratory syndrome (SARS): a report from China. J Pathol 200:282–28 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu J, Zhong S, Liu J, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine MIG in pathogenesis. Clin Infect Dis 41:1089–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wei L, Sun S, Zhang J, et al. Endocrine cells of the adenohypophysis in severe acute respiratory syndrome (SARS). Biochem Cell Biol 88:723–7 30. [DOI] [PubMed] [Google Scholar]
- 39. Leung TW, Wong KS, Hui AC, et al. Myopathic changes associated with severe acute respiratory syndrome: a postmortem case series. Arch Neurol 62:1113–1117. [DOI] [PubMed] [Google Scholar]
- 40. Mao L, Jin H, Wang M, et al. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol 77:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Romero‐Sánchez CM, Díaz‐Maroto I, Fernández‐Díaz E, et al. Neurologic manifestations in hospitalized patients with COVID‐19: The ALBACOVID registry. Neurology 10, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ 368:m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan, China. Clin Infect Dis 71:762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lechien JR, Chiesa‐Estomba CM, Place S, et al. Clinical and epidemiological characteristics of 1,420 European patients with mild‐to‐moderate coronavirus disease 2019. J Intern Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 2020;395:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lechien JR, Chiesa‐Estomba CM, De Siati DR, et al. Olfactory and gustatory dysfunctions as a clinical presentation of mild‐to‐moderate forms of the coronavirus disease (COVID‐19): a multicenter European study. Eur Arch Otorhinolaryngol:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Galougahi MK, Ghorbani J, Bakhshayeshkaram M, et al. Olfactory bulb magnetic resonance imaging in SARS‐CoV‐2‐induced anosmia: the first report. Acad Radiol 27;892–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hopkins C, Surda P, Whitehead E, Kumar BN. Early recovery following new onset anosmia during the COVID‐19 pandemic–an observational cohort study. J Otolaryngol‐Head Neck Surg 2020;49:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beltrán‐Corbellini Á, Chico‐García JL, Martínez‐Poles J, et al. Acute‐onset smell and taste disorders in the context of Covid‐19: a pilot multicenter PCR‐based case‐control study. Eur J Neurol. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chiesa‐Estomba CM, Lechien JR, Radulesco T, et al. Patterns of smell recovery in 751 patients affected by the COVID‐19 outbreak. Eur J Neurol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA 324:603–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID‐19 neurology: clinical, radiological and laboratory findings. Brain 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jain R, Young M, Dogra S, et al. COVID‐19 related neuroimaging findings: a signal of thromboembolic complications and a strong prognostic marker of poor patient outcome. J Neurol Sci 2020; 414:116923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hernández‐Fernández F, Valencia HS, Barbella‐Aponte RA, et al. Cerebrovascular disease in patients with COVID‐19: neuroimaging, histological and clinical description. Brain 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Mahammedi A, Saba L, Vagal A, et al. Imaging in neurological disease of hospitalized COVID‐19 patients: an Italian multicenter retrospective observational study. Radiology 2020:201933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Merkler AE, Parikh NS, Mir S, et al. Risk of ischemic stroke in patients with coronavirus disease 2019 (COVID‐19) vs patients with influenza. JAMA Neurol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Varatharaj A, Thomas N, Ellul MA, et al. Neurological and neuropsychiatric complications of COVID‐19 in 153 patients: a UK‐wide surveillance study. Lancet Psychiatry 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Xiong W, Mu J, Guo J, et al. New onset neurologic events in people with COVID‐19 infection in three regions in China. Neurology 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 60. Pons‐Escoda A, Naval‐Baudín P, Majós C, et al. Neurologic involvement in COVID‐19: cause or coincidence? A neuroimaging perspective. Am J Neuroradiol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Chougar L, Shor N, Weiss N, et al. Retrospective observational study of brain magnetic resonance imaging findings in patients with acute SARS‐CoV‐2 infection and neurological manifestations. Radiology 2020:202422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Fitsiori A, Pugin D, Thieffry C, et al. Unusual microbleeds in brain MRI of Covid‐19 patients. J Neuroimaging 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Radmanesh A, Derman A, Lui YW, et al. COVID‐19 ‐associated diffuse leukoencephalopathy and microhemorrhages. Radiology 2020:202040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Kremer S, Lersy F, de Sèze J, et al. Brain MRI findings in severe COVID‐19: a retrospective observational study. Radiology 2020;16:202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Toscano G, Palmerini F, Ravaglia S, et al. Guillain‐Barré syndrome associated with SARS‐CoV‐2. N Engl J Med 2020;382:2574–2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Helms J, Kremer S, Merdji H, et al. Neurologic features in severe SARS‐CoV‐2 infection. N Engl J Med. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Scullen T, Keen J, Mathkour M, et al. Coronavirus 2019 (COVID‐19)‐associated encephalopathies and cerebrovascular disease: the New Orleans experience. World Neurosurg 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Coolen T, Lolli V, Sadeghi N, et al. Early postmortem brain MRI findings in COVID‐19 non‐survivors. Neurology 2020. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 69. Kandemirli SG, Dogan L, Sarikaya ZT, et al. Brain MRI findings in patients in the intensive care unit with COVID‐19 infection. Radiology 2020;201697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Virhammar J, Kumlien E, Fällmar D, et al. Acute necrotizing encephalopathy with SARS‐CoV‐2 RNA confirmed in cerebrospinal fluid. Neurology 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS‐Coronavirus‐2. Int J Infect Dis 94:55–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lu L, Xiong W, Liu D, et al. New‐onset acute symptomatic seizure and risk factors in Corona Virus Disease 2019: a retrospective multicenter study. Epilepsia. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Liguori C, Pierantozzi M, Spanetta M, et al. Subjective neurological symptoms frequently occur in patients with SARS‐CoV2 infection. Brain Behav Immun 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Speth MM, Singer‐Cornelius T, Oberle M, et al. Mood, anxiety and olfactory dysfunction in COVID‐19: evidence of central nervous system involvement? Laryngoscope 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Abdel‐Mannan O, Eyre M, Löbel U, et al. Neurologic and radiographic findings associated with COVID‐19 infection in children. JAMA Neurol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Sun D, Li H, Lu XX, et al. Clinical features of severe pediatric patients with coronavirus disease 2019 in Wuhan: a single center's observational study. World J Pediatr. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. von Weyhern CH, Kaufmann I, Neff F, Kremer M. Early evidence of pronounced brain involvement in fatal COVID‐19 outcomes. Lancet 2020;395:e109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Reichard RR, Kashani KB, Boire NA, et al. Neuropathology of COVID‐19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)‐like pathology. Acta Neuropathol 2020;140:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Solomon IH, Normandin E, Bhattacharyya S, et al. Neuropathological features of Covid‐19. N Engl J Med 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Paniz‐Mondolfi A, Bryce C, Grimes Z, et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). J Med Virol 2020;92:699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Davis BM, Foxman B, Monto AS, et al. Human coronaviruses and other respiratory infections in young adults on a university campus: prevalence, symptoms, and shedding. Influenza Other Respir Viruses 2018;12:582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Silberstein SD. Headache in clinical practice: Routledge 2018.
- 83. Eligio P, Delia R, Valeria G. EBV Chronic Infections. Mediterr J Hematol Infect Dis 2010;2:e2010022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 2020;18:844–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Terpos E, Ntanasis‐Stathopoulos I, Elalamy I, et al. Hematological findings and complications of COVID‐19. Am J Hematol 2020. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Varga Z, Flammer AJ, Steiger P, et al. Endothelial cell infection and endotheliitis in COVID‐19. Lancet 2020;395:1417–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Liu K, Pan M, Xiao Z, Xu X. Neurological manifestations of the coronavirus (SARS‐CoV‐2) pandemic 2019‐2020. J Neurol Neurosurg Psychiatry.(2020) [DOI] [PubMed] [Google Scholar]
- 88. Xu J, Zhong SQ, Liu JH, et al. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 41:1089–10 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Mehta P, McAuley DF, Brown M, et al. COVID‐19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yeh EA, Collins A, Cohen ME, et al. Detection of coronavirus in the central nervous system of a child with acute disseminated encephalomyelitis. Pediatrics 113:e73–e7 6. [DOI] [PubMed] [Google Scholar]
- 91. Tsai LK, Hsieh ST, Chao CC, et al. Neuromuscular disorders in severe acute respiratory syndrome. Arch Neurol 61:1669–16 73. [DOI] [PubMed] [Google Scholar]
- 92. Yuki N, Hartung H‐P. Guillain‐Barré syndrome. N Engl J Med 2012;366:2294–2304. [DOI] [PubMed] [Google Scholar]
- 93. Hung ECW, Chim SSC, Chan PKS, et al. Detection of SARS coronavirus RNA in the cerebrospinal fluid of a patient with severe acute respiratory syndrome. Clin Chem 2003;49:2108–2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Lau KK, Yu WC, Chu CM, et al. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis 10:342–34 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Li YC, Bai WZ, Hashikawa T. The neuroinvasive potential of SARS‐CoV2 may play a role in the respiratory failure of COVID‐19 patients. J Med Virol.(2020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Desforges M, Le Coupanec A, Dubeau P, et al. Human coronaviruses and other respiratory viruses: underestimated opportunistic pathogens of the central nervous system? Viruses 2020;12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search string.
Table S2. Pre‐defined criteria Newcastle‐Ottawa scale for risk of bias.
Table S3. Risk of bias assessment.
Table S4. Summary of studies assessing neurological manifestations and complication in the more clinically mild HCoVs: HCoV‐229E, HKU1, NL63 and/or OC43 infections.
Table S5. Summary of studies assessing neurological manifestations and complication in MERS‐CoV infections.
Table S6. Summary of studies assessing neurological manifestations and complication in SARS‐CoV‐1 infections.
Table S7. Summary of studies assessing neurological manifestations and complication in SARS‐CoV‐2 infections.
Table S8. Association of coronaviruses with multiple sclerosis.
Data S1. Supplementary search string for medical data bases.
Data S2. Search string for Medline, Embase, and Web of Science.


