Neurological manifestations in some coronavirus disease 2019 (COVID‐19) patients and the neuroinvasive potential of its causative agent, the newly discovered severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), have increasingly attracted the attention of the neuroscience community. Less obvious from a neurologic perspective is the impact of the gastrointestinal (GI) abnormalities caused by SARS‐CoV‐2 infection, notably an imbalance of the gut microbiome (dysbiosis) and intestinal inflammation, on gut‐brain axis homeostasis and central nervous system (CNS) disorders. Dysbiosis, impaired intestinal barrier integrity and colon inflammation are important factors that have been associated with the pathogenesis of Parkinson's disease (PD) and other neurological disorders. Indeed, several evidences support the hypothesis that PD first begins in the gut and then spreads to the CNS, which is corroborated by GI manifestations commonly preceding the onset of movement‐related symptoms. In this context, an important question arising is whether COVID‐19 might represent a risk factor for PD.
Some patients with COVID‐19 exhibit significantly lower microbial diversity, with increased abundance of opportunistic pathogens and a decreased population of protective bacteria, 1 which could explain the occurrence of diarrhea and colon inflammation. The entry receptor for SARS‐CoV‐2, angiotensin‐converting enzyme 2 (ACE‐2), is highly expressed in small intestinal enterocytes, in which it plays a crucial role in the composition of the gut microbiome. 2 ACE‐2 is responsible for the renin‐angiotensin system (RAS) balance, and its dysfunction has been associated with PD pathogenesis. For instance, hyperactivation of RAS was reported to exacerbate microglia‐mediated inflammation and oxidative stress, which may contribute to degeneration of dopamine neurons in PD. 3 In a scenario of hyperinflammation (“cytokine storm”), as reported in critically ill COVID‐19 patients, increased proinflammatory cytokines and gut dysbiosis may compromise intestinal barrier integrity, causing elevation of circulating lipopolysaccharides (LPS), which eventually might trigger microglial activation and neuroinflammation (Fig. 1).
The loss of dopaminergic neurons in the substantia nigra pars compacta and the intraneuronal accumulation of aggregates of the protein α‐synuclein (aSyn), called Lewy bodies, are the main histopathological hallmarks of PD. In this respect, LPS can stimulate the formation of deposits of aSyn in enteric nerves, 4 and, importantly, aSyn pathology in colon tissue of PD patients seems to occur prior to the onset of motor symptoms. 5 Interestingly, treatment with a specific gut bacterium that is markedly increased in PD mouse models was sufficient to provoke selective death of dopamine neurons and motor deficits in mice, accompanied by neuroinflammation and accumulation of aggregates of aSyn in both colon and brain. 6 Additionally, aSyn seems to play an important role in immune cell activation in the GI system (likely via a chemoattractant activity and stimulation of dendritic cell maturation), in which the expression of the protein can be induced following viral infection, 7 potentially contributing to the formation of aSyn aggregates in enteric nervous system. Although COVID‐19 infection has not been linked so far to any specific long‐term neurological disorder, the data outlined‐above argue in favor of further investigations of the impact of SARS‐CoV‐2 infection on the incidence of PD and other neurological disorders.
FIG. 1.
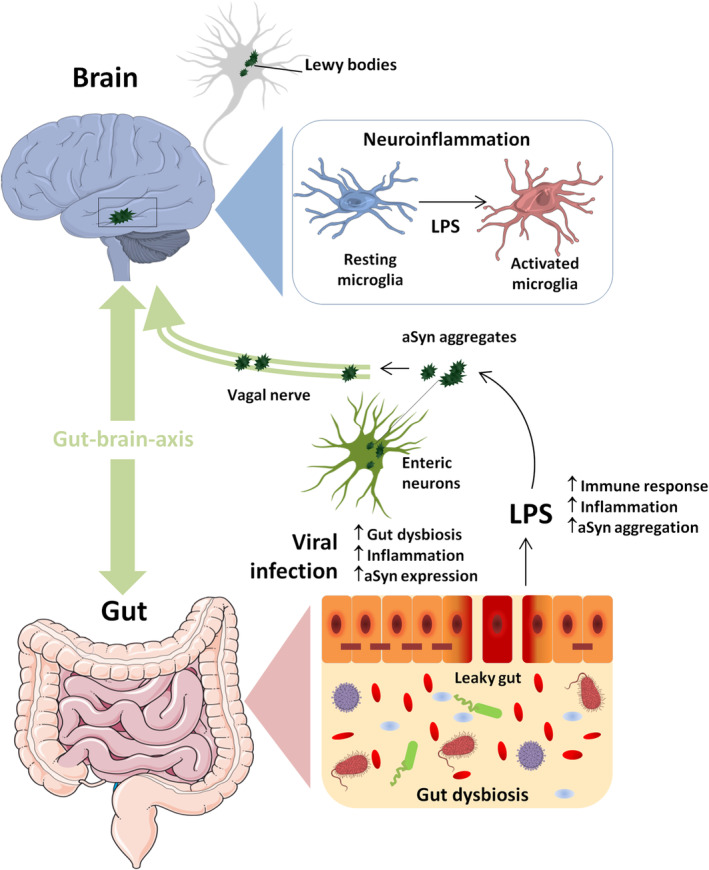
Viral infection‐induced gut microbiome imbalance, neuroinflammation and aSyn aggregation. Viral infection might promote gut dysbiosis and intestinal inflammation, which can lead to impaired mucosal integrity and release of LPS that, in turn, might stimulate the formation of deposits of aSyn in enteric nerves and neuroinflammation via microglial activation. Viral infection may also induce an increase in aSyn expression in enteric neurons as part of the immune response to the infection, contributing to the formation of aSyn aggregates that eventually may migrate from the intestine to the brain via the vagal nerve. This figure was created using Servier Medical Art templates, which are licensed under a Creative Commons Attribution 3.0 Unported License; https://smart.servier.com. [Color figure can be viewed at wileyonlinelibrary.com]
Acknowledgments
The author is grateful to the Carlos Chagas Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) and the Brazilian National Council for Scientific and Technological Development (CNPq).
Relevant conflicts of interests/financial disclosures: Nothing to report.
Funding agencies: Carlos Chagas Foundation for Research Support of the State of Rio de Janeiro (FAPERJ) and the Brazilian National Council for Scientific and Technological Development (CNPq).
References
- 1. Gu S, Chen Y, Wu Z, et al. Alterations of the gut microbiota in patients with COVID‐19 or H1N1 influenza. Clin Infect Dis. 2020;ciaa709. doi: 10.1093/cid/ciaa709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hashimoto T, Perlot T, Rehman A, et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012;487:477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Labandeira‐Garcia JL, Rodriguez‐Pallares J, Dominguez‐Meijide A, et al. Dopamine‐angiotensin interactions in the basal ganglia and their relevance for Parkinson's disease. Mov Disord 2013;28:1337–1342. [DOI] [PubMed] [Google Scholar]
- 4. Kelly LP, Carvey PM, Keshavarzian A, et al. Progression of intestinal permeability changes and alpha‐synuclein expression in a mouse model of Parkinson's disease. Mov Disord 2014;29:999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH. Is alpha‐synuclein in the colon a biomarker for premotor Parkinson's disease? Evidence from 3 cases. Mov Disord 2012;27:716–719. [DOI] [PubMed] [Google Scholar]
- 6. Choi JG, Kim N, Ju IG, et al. Oral administration of Proteus mirabilis damages dopaminergic neurons and motor functions in mice. Sci Rep 2018;8:1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stolzenberg E, Berry D, Yang D, et al. A role for neuronal alpha‐synuclein in gastrointestinal immunity. J Innate Immun 2017;9:456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]


