Abstract
Coronavirus (SARS‐CoV‐2) outbreak leading to the coronavirus disease (Covid‐19) has become a global pandemic. Patients with Cystic fibrosis are considered of major risk, as respiratory tract infections are more severe than in the general population, with a higher risk of complications and a negative impact on lung function. The performance of physical exercise is considered as key for its well‐known general benefits and also as a complementary method to help airway clearance. Therefore, physical exercise is also considered as key in the therapeutic strategy during the quarantine period. However, the impossibility to perform exercise with appropriate prescription and monitoring is of considerable worry to health care professionals. Thus, alternative strategies, such as online measures to monitor this therapy and, consequently, to achieve a safe and effective dose are highly needed. Exercise regimens should include strength and endurance, as well as balance and flexibility exercises. Patients are highly encouraged to participate in exercise programs to maintain fitness and exercise should be continued during the quarantine period. This commentary provides a summary of the main effects and benefits of physical exercise, as well as the main recommendations for its adequate execution, including exercise modality, frequency, intensity, and volume.
Keywords: COVID‐19, Cystic fibrosis, exercise, quarantine
1. INTRODUCTION
Coronavirus (SARS‐CoV‐2) outbreak leading to the coronavirus disease (Covid‐19) has started last December 2019 in China and the infection has spread throughout the world, despite the strategies adopted to stop this epidemiological phenomenon. Four months later, Covid‐19 has become a global pandemic with more than 14 043.176 cases diagnosed as of 19th July (2020). According to the Covid‐19 pandemic global information, some characteristics of the population at higher risk of Covid‐19 have been identified, including age over 55 to 60 years old, hypertension, diabetes, or presence of associated cardiovascular and respiratory diseases. Thus, patients with Cystic fibrosis (CF) are considered of major risk, as respiratory tract infections are more severe than in the general population, with a higher risk of complications and negative impact on lung function. During the 2009 influenza pandemic, the influenza A (H1N1) virus caused substantial morbidity in patients with CF. 1 Now, individuals with CF and their families are at the boundary of important new therapies that are expected to significantly change their health status and face an unprecedented global pandemic with several possible unknown effects. Preliminary information suggests that the majority of patients with CF are performing an excellent work to prevent SARS‐CoV‐2 infection and should remain dedicated to this task, as significant work has been performed in the past few months to collect data and to better understand the main factors that affect the severity of Covid‐19 in people with CF.
Following recommendations, many families of patients with CF adopted the lockdown strategy before the official alarm state situation announced by the different governments. This action has led to a low incidence of Covid‐19 among CF patients and, as of 13th April 2020, from approximately 63 000 patients diagnosed with CF in different countries worldwide (ie, Australia, Canada, France, Spain, Ireland, Netherlands, New Zealand, UK, and United States), only 48 patients have been infected with SARS‐CoV‐2 (46 adults and 2 children), with only one death, which is believed to be associated with expected complications of the disease, rather than the Covid‐19 (data from Cosgriff et al 2 and the Spanish Society of Pediatric Pulmonology, 2020). Thus, the main concern of the CF units is that treatments maintain their appropriate effectiveness at this situation and that multidisciplinary reviews can be restored in an orderly manner and with the necessary changes to adapt to this new reality. The performance of physical exercise is considered as key for its well‐known general benefits. In addition, it is also a complementary tool to help airway clearance when combined with physiotherapy. 3 , 4 Exercise as a complement to physiotherapy significantly increases airway clearance more than physiotherapy and exercise alone. 4 There is also evidence indicating additional beneficial effects of exercise on muscular fitness (ie, muscle mass and function), body composition and cardiorespiratory health. 5 , 6 , 7 , 8 , 9 Furthermore, exercise—aerobic or a combination of aerobic and strength—could help improve 5 , 7 , 8 or mitigate the annual decline 5 of forced vital capacity, forced expiratory volume in the first second and/or peak functional capacity (VO2peak). Taken together, the growing evidence indicates that exercise in patients with CF is an important therapeutic tool in reducing morbidity and mortality rates 10 and should be considered part of the standard of care. 11 , 12
Both respiratory impairment and muscle weakness limit exercise capacity, which gradually leads to exercise intolerance, increasing muscle cachexia and decreasing mucus elimination, worsening lung function. Individuals with CF are characterized by a hyperinflammatory phenotype, and exercise has been suggested to exert antiinflammatory effects. Therefore, specific exercise programs can be used to modify the course of chronic inflammatory and infectious diseases, contributing to minimize the dysfunction of the immune system. 13 In addition, patients with CF do not express the cystic fibrosis transmembrane conductance regulator (CFTR) protein in the muscle, 14 generating homeostatic, metabolic and contractile problems at the cellular level, and excessive fatigue of the diaphragm at the central level. 14 , 15 The consequences of these alterations are a greater degree of exercise intolerance, decreased strength, muscular atrophy, 16 and longer hospitalization rates. 17 CFTR is also an important regulator of cellular inflammatory homeostasis, and its absence has been found to be associated with increased nuclear factor kappa B (NF‐kB), 18 leading to chronic inflammation and excessive inflammatory responses. Since exercise‐induced peroxisome proliferator‐activated receptor gamma coactivator 1‐alpha can limit NF‐kB activity and increase Peroxisome proliferator‐activated receptor gamma (PPAR‐γ) activity, regular training programs can restore abnormalities in NF‐kB and PPAR‐γ levels (Figure 1). Therefore, exercise can be used as a strategy to reduce muscle catabolism, inflammation, and, consequently, increase muscle function.
Figure 1.
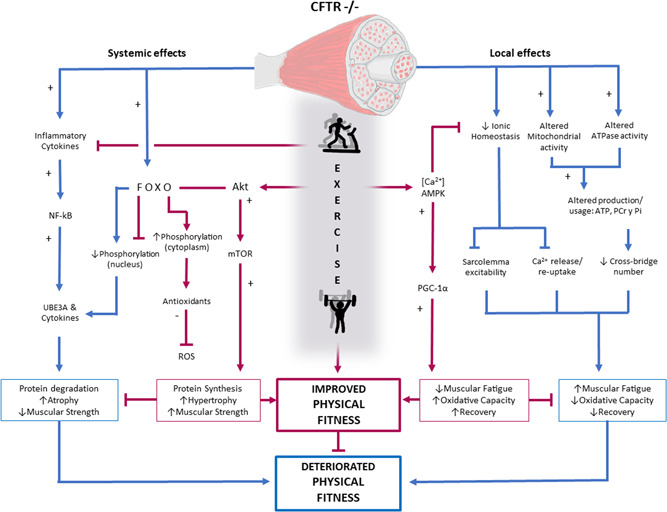
Schematic of the systemic and local effects of CFTR−/− with and without exercise. Akt, protein kinase B; ATP, adenosine triphosphate; [Ca2+], calcium ion; CFTR, Cystic fibrosis transmembrane conductance regulator gene; FOXO, forkhead box protein O; mTOR: mammalian target of rapamycin; NF‐kB, nuclear factor kappa B; PGC‐1α, peroxisome proliferator‐activated receptor γ coactivator 1 α; PCr, phosphocreatine; Pi, inorganic phosphate; ROS, reactive oxygen species; UBE3A, ubiquitin‐protein ligase E3A [Color figure can be viewed at wileyonlinelibrary.com]
Considering that individuals with CF and their families have put considerable effort to maintain good health, that a great adherence to treatment is needed, and that physical exercise is a key therapeutic strategy during the quarantine period, the impossibility to perform an exercise with appropriate prescription and monitoring is of considerable worry to health care professionals. Thus, alternative strategies, such as online measures to monitor this therapy and, consequently, to achieve a safe and effective dose is highly needed, although participation in any exercise program should be recommended and guided by a CF specialist. Exercise regimens should include strength and endurance, as well as balance and flexibility exercises, following the principles of both ACSM 19 and EACPR 20 for exercise training.
Therefore, patients are highly encouraged to participate in exercise programs to maintain fitness and to continue exercising during the quarantine period, always following the CF team recommendations. Additionally, it is important to define the correct dose of exercise through the characteristics of each of the components of the program. These recommendations include oxygen saturation (SpO2) monitoring during exercise (ie, pulse oximetry) to adjust for optimum SpO2 levels, when exercise‐induced desaturation is present. Patients with moderate to severe CF or abnormal SpO2 responses during exercise may also require supplemental oxygen and a closest supervision 21 , 22 to reduce the risk of hypoxemia and maximize the benefits of exercise. 22 A decision algorithm for exercise prescription is also provided as Figure 2.
Figure 2.
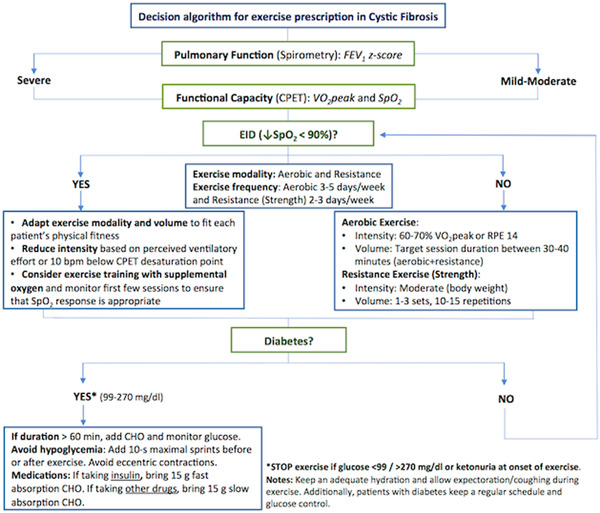
Decision algorithm for exercise prescription in patients with Cystic fibrosis, ↓: decreased. bpm, beats per minute; CHO, carbohydrate; CPET, cardiopulmonary exercise testing; EID, exercise‐induced desaturation; FEV1, forced expiratory volume in the first second; RPE: rate of perceived exertion; SpO2: oxygen saturation; VO2peak, peak oxygen consumption [Color figure can be viewed at wileyonlinelibrary.com]
2. EXERCISE MODALITY
Evidence suggests that both aerobic and resistance training, as well as a combined program, may have positive effects for individuals with CF. 23 Considering the muscle abnormalities 14 described for patients with CF, a combined aerobic and resistance training should be considered. Activities including balance and flexibility exercises are also desirable. Children and adolescents should also benefit from using active videogames as a complement for their daily physical exercise program. 24 In severe patients, adapted exercise modalities to fit each patient's physical fitness should be considered.
3. EXERCISE FREQUENCY
The usual frequency recommended for exercise is between three and five sessions per week. Considering the exceptional quarantine situation and that exercise must also help airway clearance in CF, we believe patients should target to perform exercise between 5 and 7 days per week, according to their clinical status and professional advisement.
4. EXERCISE INTENSITY
The exercise intensity recommended is moderate (≅60% of VO2peak), which could be monitored by targeting heart rate (HR) between 60% and 85% of the maximum HR. 25 If HR monitoring is not possible, there is evidence showing that exercise intensity may also be self‐regulated by using the OMNI scale in children with CF 26 or targeting 14 in the dyspnea Borg scale for adults.
5. EXERCISE VOLUME
Exercise sessions between 30 and 40 minutes, combining aerobic and resistance training, are suggested. A target of at least 150 to 300 minutes per week should be pursued.
6. EXAMPLES OF HOME EXERCISES
If specific equipment or materials for training are not available, home objects (chair, steps, packs of food, bags), as well as the use of their own bodyweight, may be used to help performing resistance exercises. Aerobic exercise may also be performed if ergometers are not available, using short walk/running movements inside the house, jumping, skipping rope, running on the spot, and stepping over obstacles. If more specific guidance is needed, the research group on Exercise, Health, and Biomarkers (EsBIDA—European University of Madrid) together with the CF unit of Niño Jesus Hospital and the Spanish CF Federation have developed the initiative #stayhomeactive (#QuédateenCasaActivo), in which illustrative videos may be found (https://drive.google.com/drive/folders/1ImnxAQ70XgT3pImSUpJh0SQRjkMJtM7I?usp=sharing). The project is hosted at the Federation's website (https://fibrosisquistica.org/).
7. SPECIAL RECOMMENDATIONS
Considering the particularities of the CF disease, a few especial recommendations should also be considered. Hydration is of great importance and patients should drink flavored sodium chloride‐containing fluids above thirst levels to prevent hyponatremic dehydration. Those with diabetes mellitus may require additional carbohydrates during prolonged exercise. 27 If possible, when exercise‐induced desaturation is present, SpO2 should be monitored and desaturations reported and discussed with a health professional. In addition, airway clearance performed before the exercise session may lead to improved ventilatory dynamics during exercise and contribute to increase performance. 28 For severe patients, exercise intensity, frequency, and volume should be individually targeted, and responses monitored at least weekly by a CF specialist.
8. CONCLUDING REMARKS
The treatment of patients with CF requires a multidisciplinary approach where physiotherapy is a key component. 29 , 30 , 31 Exercise, as a complement to physiotherapy, helps to increase airway clearance and physical fitness. 3 , 9 Furthermore, exercise in patients with CF has shown to provide additional positive effects on body composition, 9 , 13 , 14 bone heath, 32 , 33 and inflammation. 13 , 14 Therefore, the addition or maintenance of exercise in the standard treatment of CF, following the recommendations from CF specialists, may have considerable short‐ and long‐term benefits, especially in a situation of reduced social mobility and confinement. The development of an active lifestyle, through exercise early in life, may help in the management of diabetes, maintenance and/or improvement of lung function, muscle mass, bone health, and quality of life. However, exercise “dose” (ie, intensity, volume, frequency, and modality) must be appropriate for age, severity level, and physical fitness, as well as recommended/supervised by the CF multidisciplinary team.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
ACKNOWLEDGMENTS
The authors would like to thank Federación Española de Fibrosis Quística for the support. MVFD would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)—finance code 001, and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Fernandez‐del‐Valle M, Donadio MVF, Pérez‐Ruiz M. Physical exercise as a tool to minimize the consequences of the Covid‐19 quarantine: An overview for cystic fibrosis. Pediatric Pulmonology. 2020;55:2877–2882. 10.1002/ppul.25041
REFERENCES
- 1. Bucher J, Boelle PY, Hubert D, et al. Lessons from a French collaborative case—control study in cystic fibrosis patients during the 2009 A/H1N1 influenza pandemy. BMC Infect Dis. 2016;16:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cosgriff R, Ahern S, Bell SC, et al. A multinational report to characterise SARS‐CoV‐2 infection in people with cystic fibrosis. J Cyst Fibros. 2020;19:3‐358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Elbasan B, Tunali N, Duzgun I, Ozcelik U. Effects of chest physiotherapy and aerobic exercise training on physical fitness in young children with cystic fibrosis. Ital J Pediatr. 2012;38:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McIlwaine M. Chest physical therapy, breathing techniques and exercise in children with CF. Paediatr Respir Rev. 2007;8(1):8‐16. [DOI] [PubMed] [Google Scholar]
- 5. Schneiderman‐Walker J, Pollock SL, Corey M, et al. A randomized controlled trial of a 3‐year home exercise program in cystic fibrosis. J Pediatr. 2000;136(3):304–310. [DOI] [PubMed] [Google Scholar]
- 6. Selvadurai HC, Blimkie CJ, Meyers N, Mellis CM, Cooper PJ, Van Asperen PP. Randomized controlled study of in‐hospital exercise training programs in children with cystic fibrosis. Pediatr Pulmonol. 2002;33(3):194‐200. [DOI] [PubMed] [Google Scholar]
- 7. Sosa ES, Groeneveld IF, Gonzalez‐Saiz L, et al. Intrahospital weight and aerobic training in children with cystic fibrosis: a randomized controlled trial. Randomized Control Trial Med Sci Sport Exerc. 2012;44(1):2‐11. [DOI] [PubMed] [Google Scholar]
- 8. Santana‐Sosa E, Gonzalez‐Saiz L, Groeneveld IF, et al. Benefits of combining inspiratory muscle with “whole muscle” training in children with cystic fibrosis: a randomised controlled trial. Br J Sports Med. 2014;48(20):1513‐1517. [DOI] [PubMed] [Google Scholar]
- 9. Gupta S, Mukherjee A, Lodha R, et al. Effects of exercise intervention program on bone mineral accretion in children and adolescents with cystic fibrosis: a randomized controlled trial. Indian J Pediatr. 2019;86(11):987‐994. [DOI] [PubMed] [Google Scholar]
- 10. Vendrusculo F, Heinzmann‐filho J, da Silva J, Perez Ruiz M. Peak oxygen uptake and mortality in cystic fibrosis: systematic review and meta‐analysis. Respir Care. 2018;64(C):1‐8. [DOI] [PubMed] [Google Scholar]
- 11. Cystic Fibrosis F, Borowitz D, Robinson KA, et al. Cystic fibrosis foundation evidence‐based guidelines for management of infants with cystic fibrosis. J Pediatr. 2009;155(6 suppl.):73‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smyth AR, Bell SC, Bojcin S, et al. European cystic fibrosis society standards of care: best practice guidelines. J Cyst Fibros. 2014;13(S1):23‐42. [DOI] [PubMed] [Google Scholar]
- 13. Pbvdw L, Gerardus H, Arets M, Ent CKVD, Beekman JM. Infection, inflammation and exercise in cystic fibrosis. Respir Res. 2013;14(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Divangahi M, Balghi H, Danialou G, et al. Lack of CFTR in skeletal muscle predisposes to muscle wasting and diaphragm muscle pump failure in cystic fibrosis mice. PLoS Genet. 2009;5(7):e1000586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Antigny F, Girardin N, Raveau D, Frieden M, Becq F, Vandebrouck C. Dysfunction of mitochondria Ca2+ uptake in cystic fibrosis airway epithelial cells. Mitochondrion. 2009;9(4):232‐241. [DOI] [PubMed] [Google Scholar]
- 16. Gruet M, Decorte N, Mely L, Vallier J. Skeletal muscle contractility and fatigability in adults with cystic fibrosis. J Cyst Fibros. 2016;15(1):e1‐e8. [DOI] [PubMed] [Google Scholar]
- 17. Pérez M, Groeneveld IF, Santana‐Sosa E, et al. Aerobic fitness is associated with lower risk of hospitalization in children with cystic fibrosis. Pediatr Pulmonol. 2014;49(7):641‐649. [DOI] [PubMed] [Google Scholar]
- 18. Dekkers JF, van der Ent CK, Kalkhoven E, Beekman JM. PPARγ as a therapeutic target in cystic fibrosis. Trends Mol Med. 2012;18(5):283‐291. [DOI] [PubMed] [Google Scholar]
- 19. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine . Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults. Med Sci Sport Exerc. 2011;43(7):1334‐1359. [DOI] [PubMed] [Google Scholar]
- 20. Vanhees L, Geladas N, Hansen D, et al. Importance of characteristics and modalities of physical activity and exercise in the management of cardiovascular health in individuals with cardiovascular risk factors: recommendations from the EACPR. Part II. Eur J Prev Cardiol. 2012;19(5):1005‐1033. [DOI] [PubMed] [Google Scholar]
- 21. McKone EF, Barry SC, FitzGerald MX, Gallagher CG. The role of supplemental oxygen during submaximal exercise in patients with cystic fibrosis. Eur Respir J. 2002;20(1):134‐142. [DOI] [PubMed] [Google Scholar]
- 22. Elphick HE, Mallory G. Oxygen therapy for cystic fibrosis. Cochrane Database Syst Rev. 2013;2013(7):003884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radtke T, Nevitt SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database Syst Rev. 2017;11(6):CD002768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Campos NE, Heinzmann‐Filho JP, Becker NA, et al. Evaluation of the exercise intensity generated by active video gaming in patients with cystic fibrosis and healthy individuals. J Cyst Fibros. 2020;19:S1569‐S1993. [DOI] [PubMed] [Google Scholar]
- 25. Hebestreit H, Kriemler S, Radtke T. Exercise for all cystic fibrosis patients: is the evidence strengthening? Curr Opin Pulm Med. 2015;21(6):591‐595. [DOI] [PubMed] [Google Scholar]
- 26. Higgins LW, Robertson RJ, Kelsey SF, et al. NIH public access. Pediatr Pulmonol. 2013;48(5):497‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Philpott J, Houghton K, Luke A. Physical activity recommendations for children with specific chronic health conditions: juvenile idiopathic arthritis, hemophilia, asthma, and cystic fibrosis. Clin J Sport Med. 2010;15(4):213‐218. [DOI] [PubMed] [Google Scholar]
- 28. Vendrusculo FM, Johnstone Z, Dhouieb E, Donadio MVF, Cunningham S, Urquhart DS. Airway clearance physiotherapy improves ventilatory dynamics during exercise in patients with cystic fibrosis: a pilot study. Arch Dis Child. 2019;104(1):37‐42. [DOI] [PubMed] [Google Scholar]
- 29. Wilson LM, Morrison L, Robinson KA. Airway clearance techniques for cystic fibrosis: an overview of Cochrane systematic reviews. Cochrane Database Syst Rev. 2019;2019(1):011231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warnock L, Gates A. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database Syst Rev. 2015;2015(12):001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McIlwaine M, Button B, Nevitt SJ. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database Syst Rev. 2019;11:CD003147. 10.1002/14651858.CD003147.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hind K, Truscott JG, Conway SP. Exercise during childhood and adolescence: a prophylaxis against cystic fibrosis‐related low bone mineral density? Exercise for bone health in children with cystic fibrosis. J Cyst Fibros. 2008;7(4):270‐276. [DOI] [PubMed] [Google Scholar]
- 33. Tejero García S, Giráldez Sánchez MA, Cejudo P, et al. Bone health, daily physical activity, and exercise tolerance in patients with cystic fibrosis. Chest. 2011;140(2):475‐481. [DOI] [PubMed] [Google Scholar]


