In this study, we examine the incidence, location, and follow-up kidney function testing of hospitalized AKI episodes among children in a large integrated health care delivery system.
Abstract
Video Abstract
BACKGROUND:
Acute kidney injury (AKI) may lead to short- and long-term consequences in children, but its epidemiology has not been well described at a population level and outside of ICU settings.
METHODS:
In a large, diverse pediatric population receiving care within an integrated health care delivery system between 2008 and 2016, we calculated age- and sex-adjusted incidences of hospitalized AKI using consensus serum creatinine (SCr)–based diagnostic criteria. We also investigated the proportion of AKI detected in non-ICU settings and the rates of follow-up outpatient SCr testing after AKI hospitalization.
RESULTS:
Among 1 500 546 children, the mean age was 9.8 years, 49.0% were female, and 33.1% were minorities. Age- and sex-adjusted incidence of hospitalized AKI among the entire pediatric population did not change significantly across the study period, averaging 0.70 (95% confidence interval: 0.68–0.73) cases per 1000 person-years. Among the subset of hospitalized children, the adjusted incidence of AKI increased from 6.0% of hospitalizations in 2008 to 8.8% in 2016. Approximately 66.7% of AKI episodes were not associated with an ICU stay, and 54.3% of confirmed, unresolved Stage 2 or 3 AKI episodes did not have outpatient follow-up SCr testing within 30 days postdischarge.
CONCLUSIONS:
Community-based pediatric AKI incidence was ∼1 per 1000 per year, with two-thirds of cases not associated with an ICU stay and more than one-half not receiving early outpatient follow-up kidney function testing. Further efforts are needed to increase the systematic recognition of AKI in all inpatient settings with appropriate, targeted postdischarge kidney function monitoring and associated management.
What’s Known on This Subject:
Pediatric acute kidney injury (AKI) is associated with short- and long-term consequences in critically ill children, including increased mortality, hospitalizations, and progression of chronic kidney disease. However, its epidemiology has not been well described outside of critical care settings.
What This Study Adds:
We describe the burden of AKI in a community-based population of children within and outside critical care inpatient settings. Importantly, there appears to be opportunity to improve follow-up outpatient kidney function testing in pediatric care after unresolved, severe hospitalized AKI.
Acute kidney injury (AKI) is common among critically ill children, with researchers estimating, in recent studies, that 1 in 4 children admitted to ICUs experience AKI.1–3
Critically ill children with AKI have significantly higher short-term mortality1,3–8 and inpatient use, including ICU and hospital lengths of stay, than children without AKI.2–4,9,10 Longer-term outcomes associated with pediatric AKI in the ICU include increased mortality, rehospitalization, and progression to chronic kidney disease, as also observed in adults with AKI.11–13
Researchers have examined pediatric AKI outside of critical care settings in relatively few studies. Published estimates of AKI among hospitalized children range from 0.4% to 40%, depending on the definition of AKI used and type of patients studied.4,7,9,14 In existing studies on non–critically ill children, researchers have reported a risk of ∼30%, but those studies are limited to high-risk samples (eg, primary renal disease, exposure to nephrotoxic medications, or undergoing cardiac procedures) or contained varying definitions of AKI.15–20
The etiology of pediatric AKI can be highly multifactorial.21,22 In the absence of known preexisting kidney disease, awareness, detection and short-term monitoring of AKI episodes and their consequences may fall outside of nephrology services and into general pediatric care. Given AKI can be associated with both adverse short- and longer-term outcomes in children, earlier detection and systematic follow-up (particularly after an episode of severe AKI) may offer opportunities to improve clinical outcomes in this at-risk population.
In this study, we examine recent temporal trends in the incidence of hospitalized AKI among all children across critical and noncritical care settings and describe follow-up outpatient kidney function monitoring after AKI in a large, integrated health care delivery system.
Methods
Source Population and Study Sample
The source population was based within Kaiser Permanente Northern California (KPNC), an integrated health care delivery system currently providing comprehensive primary through tertiary care for >4.4 million members across 21 hospitals and >245 clinics. Its membership is highly representative of the local and statewide population in terms of age, sex, and race and/or ethnicity.23 Nearly all aspects of care are captured through KPNC’s electronic medical record system that is integrated across all practice settings. Members receive essentially all their care within KPNC facilities, with a low rate of outside referral for only selected care.
We constructed calendar year cohorts of all pediatric (age 1–17 years) patients from January 2008 to December 2016. We excluded patients from yearly cohorts who had an unknown sex, previous chronic dialysis, previous organ or bone marrow transplant, or death before January 1 of each year. All eligible pediatric patients on January 1 of each year were included in the respective calendar year cohort; therefore, most patients were included in multiple calendar year cohorts. Information on receipt of renal replacement therapy was obtained from a comprehensive health plan end-stage renal disease treatment registry.24
The study was approved by the KPNC Institutional Review Board. Waivers of informed consent were obtained because of the nature of the study.
Follow-up
Patients were censored in a given calendar year at initiation of chronic dialysis, organ transplant, death, health plan disenrollment, reaching the age of 18 years, or on December 31 of each year. Death was identified from administrative and hospital discharge databases, California death certificate files, and Social Security Administration vital status files.25,26
Identification of Hospitalized AKI
We ascertained episodes of hospitalized AKI and its severity using revised Kidney Disease: Improving Global Outcomes (KDIGO) criteria as follows: stage 1 (serum creatinine [SCr] increase of 1.5 to 1.9-fold above the baseline or an increase in SCr of ≥0.3 mg/dL within 48 hours during a hospitalization); stage 2 (SCr increase of 2.0–2.9-fold above baseline); stage 3 (SCr increase of more than threefold above the baseline, increase in SCr to ≥4.0 mg/dL, receipt of acute dialysis, or decrease in estimated glomerular filtration rate (eGFR) to <35 mL/min per 1.73m2).27 Urine output data were unavailable. The baseline SCr was defined as the most recent outpatient, nonemergency SCr measurement between 7 and 365 days before admission. The peak inpatient SCr was used for comparison with the baseline, and the eGFR was calculated from this value by using the modified bedside Schwartz equation.28 Height measured during the hospitalization was used in the Schwartz equation; otherwise, the closest height measurement within 90 days of the hospitalization was used. For hospitalizations with no available baseline SCr measurement, the baseline kidney function was back-calculated through the Schwartz equation by presuming a baseline eGFR of 120 mL/min per 1.73 m2, as previously described and validated.1,29 All SCr measurements were made using an assay traceable to a standard isotope dilution mass spectrometry reference procedure. AKI episodes were classified as resolved if the last SCr measurement before discharge after the qualifying AKI SCr measurement was ≤0.1 mg/dL above the baseline SCr measurement. In a sensitivity analysis, resolution of AKI was also defined as a reduction in SCr to below the AKI threshold (ie, <1.5-fold over the baseline or <0.3 mg/dL over the initial qualifying measurement).
Patient Characteristics
Demographic characteristics included age, sex, and self-reported race and/or ethnicity and were ascertained on January 1 of each year from electronic medical records. Serum creatinine concentration values were identified from comprehensive inpatient and outpatient health plan laboratory databases. Nonnetwork hospitalizations were not included because access to clinical data was unavailable.
Statistical Approach
Analyses were conducted by using SAS version 9.4 (SAS Institute, Inc, Cary, NC). We compared characteristics for patients with or without AKI and by AKI stage using analysis of variance or Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables. Among children with AKI, we present characteristics at the time of their first hospitalization with AKI. In children with no AKI during the study period, we present characteristics at the time of their first hospitalization. Therefore, all comparisons involved categories with unique children.
We separately calculated overall and stage-specific incidence of AKI per 1000 person-years and per 100 hospitalizations per year, directly standardized to the age and sex distribution of the 2008 population. To test for a temporal trend, we performed age- and sex-adjusted Poisson regression using a generalized estimating equations approach to evaluate the significance of an ordinal calendar year term.
We calculated the proportions of AKI detected in the ICU, detected outside the ICU but with a corresponding ICU stay during the hospitalization, and detected outside the ICU with no corresponding ICU stay. AKI was determined to be detected in ICU settings if the qualifying peak inpatient SCr measurement fell within the admission and discharge times of the ICU stay. We delineated patients whose qualifying peak SCr measurement was outside the ICU but who had an ICU stay, to account for cases in which AKI or another acute illness may have prompted ICU care. We also calculated the proportions of children with follow-up outpatient, nonemergency serum creatinine testing within 30, 60, 90, and 365 days after discharge from a hospitalization with AKI per year, excluding from the denominator those who died, who initiated chronic renal replacement therapy before each end point, or whose AKI resolved before discharge.
Results
Patient Characteristics
Between 2008 and 2016, we identified 1 500 546 eligible children (Supplemental Table 4). Across the study period, mean (SD) age on January 1 of each study year was 9.9 (4.9) years, and 49% were female (Table 1). From 2008 to 2016, we identified 3582 hospitalizations with AKI (7.4% of all hospitalizations), affecting 3171 children (0.2% of the pediatric population). Baseline SCr measurements were imputed for 2979 (83.2%) hospitalizations with AKI. Compared with children without AKI, children with AKI were more likely to be older, male, and Black (Table 2). Children with AKI were more likely to have a primary hospital diagnosis related to kidney disease, metabolic disease, cancer, and infectious disease; were less likely to be hospitalized for respiratory disease, musculoskeletal disease, and pregnancy; and had higher risks of in-hospital death (1.2% vs 0.0%, respectively; P <.001) and longer hospital length of stay (7.9 vs 3.8 days, respectively; P <.001; Table 2). More severe AKI was associated with younger age; female sex; being more likely to be hospitalized primarily with a kidney, infectious disease, or blood disorder-related diagnosis; longer hospital length of stay; and higher risks of inpatient and 30-day all-cause death (Table 2). Characteristics for all hospitalizations are shown in Supplemental Tables 5 and 6.
TABLE 1.
Characteristics of the Pediatric Population Aged 1 to 17 Years Old Within KPNC, 2008–2016
| Characteristics | 2008 (N = 720 131) | 2009 (N = 715 768) | 2010 (N = 710 451) | 2011 (N = 722 173) | 2012 (N = 730 261) | 2013 (N = 727 609) | 2014 (N = 719 901) | 2015 (N = 739 185) | 2016 (N = 764 549) |
|---|---|---|---|---|---|---|---|---|---|
| Age, y | |||||||||
| Mean (SD) | 9.9 (4.9) | 9.9 (4.9) | 9.9 (4.9) | 9.8 (4.9) | 9.8 (4.9) | 9.8 (4.9) | 9.8 (4.9) | 9.8 (4.9) | 9.8 (4.9) |
| Median (IQR) | 10.2 (5.7–14.3) | 10.1 (5.6–14.2) | 10.0 (5.6–14.2) | 10.0 (5.6–14.1) | 10.0 (5.6–14.1) | 10.0 (5.7–14.1) | 9.9 (5.7–14.1) | 9.9 (5.7–14.0) | 9.9 (5.6–14.0) |
| Age category, y, No. (%) | |||||||||
| 1–5 | 190 914 (26.5) | 192 566 (26.9) | 192 256 (27.1) | 195 595 (27.1) | 197 650 (27.1) | 195 831 (26.9) | 193 068 (26.8) | 198 103 (26.8) | 206 506 (27.0) |
| 6–10 | 204 026 (28.3) | 203 663 (28.5) | 203 103 (28.6) | 207 902 (28.8) | 211 060 (28.9) | 212 621 (29.2) | 213 019 (29.6) | 219 665 (29.7) | 227 116 (29.7) |
| 11–17 | 325 191 (45.2) | 319 539 (44.6) | 315 092 (44.4) | 318 676 (44.1) | 321 551 (44.0) | 319 157 (43.9) | 313 814 (43.6) | 321 417 (43.5) | 330 927 (43.3) |
| Female sex, No. (%) | 352 528 (49.0) | 350 403 (49.0) | 347 450 (48.9) | 353 068 (48.9) | 357 580 (49.0) | 356 295 (49.0) | 352 454 (49.0) | 362 071 (49.0) | 374 360 (49.0) |
| Race, No. (%) | |||||||||
| White | 260 444 (36.2) | 258 777 (36.2) | 258 731 (36.4) | 260 490 (36.1) | 263 386 (36.1) | 262 007 (36.0) | 258 575 (35.9) | 263 858 (35.7) | 269 148 (35.2) |
| Black | 63 341 (8.8) | 62 401 (8.7) | 60 983 (8.6) | 60 039 (8.3) | 59 421 (8.1) | 57 709 (7.9) | 55 661 (7.7) | 55 961 (7.6) | 56 272 (7.4) |
| Asian American and/or Pacific Islander | 110 304 (15.3) | 114 505 (16.0) | 118 605 (16.7) | 124 613 (17.3) | 130 580 (17.9) | 134 288 (18.5) | 136 507 (19.0) | 142 412 (19.3) | 148 632 (19.4) |
| American Indian | 3859 (0.5) | 3852 (0.5) | 3716 (0.5) | 3713 (0.5) | 3781 (0.5) | 3599 (0.5) | 3393 (0.5) | 3350 (0.5) | 3353 (0.4) |
| Multiracial | 45 294 (6.3) | 46 665 (6.5) | 47 773 (6.7) | 48 911 (6.8) | 50 554 (6.9) | 50 801 (7.0) | 50 348 (7.0) | 51 560 (7.0) | 53 065 (6.9) |
| Other and/or unknown | 236 889 (32.9) | 229 568 (32.1) | 220 643 (31.1) | 224 407 (31.1) | 222 539 (30.5) | 219 205 (30.1) | 215 417 (29.9) | 222 044 (30.0) | 234 079 (30.6) |
| Children with ≥1 hospitalization in Kaiser Permanente facility, No. (%) | 5096 (0.7) | 5315 (0.7) | 5474 (0.8) | 5368 (0.7) | 4876 (0.7) | 4347 (0.6) | 3942 (0.5) | 4694 (0.6) | 4244 (0.5) |
IQR, interquartile range.
TABLE 2.
Characteristics of Children Hospitalized Within KPNC, 2008–2016, by AKI Status and AKI Severity
| Characteristics | No AKI (N = 35 084) | AKI (N = 3171) | P | AKI Stage 1 (N = 2741) | AKI Stage 2 (N = 488) | AKI Stage 3 (N = 360) | P |
|---|---|---|---|---|---|---|---|
| Age, y | <.001 | <.001 | |||||
| Mean (SD) | 10.2 (5.5) | 12.7 (4.9) | 13.2 (4.7) | 11.5 (5.1) | 10.7 (5.3) | ||
| Median (IQR) | 11.4 (4.8–15.3) | 14.9 (10.1–16.4) | 15.2 (11.4–16.5) | 13.2 (7.5–16.0) | 12.2 (5.6–15.4) | ||
| Age category, y, No. (%) | <.001 | <.001 | |||||
| 1–5 | 10 502 (29.9) | 469 (14.8) | 315 (12.8) | 81 (19.1) | 73 (25.8) | ||
| 6–10 | 6421 (18.3) | 410 (12.9) | 276 (11.2) | 84 (19.8) | 50 (17.7) | ||
| 11–17 | 18 161 (51.8) | 2292 (72.3) | 1872 (76.0) | 260 (61.2) | 160 (56.5) | ||
| Female sex, No. (%) | 18 299 (52.2) | 1259 (39.7) | <.001 | 936 (38.0) | 182 (42.8) | 141 (49.8) | <.001 |
| Race, No. (%) | <.001 | .10 | |||||
| White | 14 007 (39.9) | 1290 (40.7) | 1005 (40.8) | 164 (38.6) | 121 (42.8) | ||
| Black | 3122 (8.9) | 464 (14.6) | 354 (14.4) | 80 (18.8) | 30 (10.6) | ||
| Asian American and/or Pacific Islander | 4900 (14.0) | 482 (15.2) | 370 (15.0) | 61 (14.4) | 51 (18.0) | ||
| American Indian | 187 (0.5) | 11 (0.4) | 9 (0.4) | 2 (0.5) | 0 (0.0) | ||
| Multiracial | 3321 (9.5) | 309 (9.7) | 247 (10.0) | 31 (7.3) | 31 (11.0) | ||
| Other and/or unknown | 9547 (27.2) | 615 (19.4) | 478 (19.4) | 87 (20.5) | 50 (17.7) | ||
| Year of first hospitalization, No. (%) | <.001 | .77 | |||||
| 2008 | 4684 (13.4) | 334 (10.5) | 250 (10.2) | 50 (11.8) | 34 (12.0) | ||
| 2009 | 4533 (12.9) | 374 (11.8) | 288 (11.7) | 54 (12.7) | 32 (11.3) | ||
| 2010 | 4506 (12.8) | 402 (12.7) | 305 (12.4) | 58 (13.7) | 39 (13.8) | ||
| 2011 | 4335 (12.4) | 365 (11.5) | 284 (11.5) | 51 (12.0) | 31 (11.0) | ||
| 2012 | 3838 (10.9) | 315 (9.9) | 248 (10.1) | 34 (8.0) | 31 (11.0) | ||
| 2013 | 3348 (9.5) | 324 (10.2) | 259 (10.5) | 33 (7.8) | 32 (11.3) | ||
| 2014 | 3054 (8.7) | 312 (9.8) | 252 (10.2) | 40 (9.4) | 20 (7.1) | ||
| 2015 | 3610 (10.3) | 366 (11.5) | 287 (11.7) | 49 (11.5) | 29 (10.3) | ||
| 2016 | 3181 (9.1) | 381 (12.0) | 290 (11.8) | 56 (13.2) | 35 (12.4) | ||
| Primary hospitalization diagnosis group, No. (%) | <.001 | <.001 | |||||
| Gastrointestinal | 6393 (18.2) | 519 (16.4) | 430 (17.5) | 57 (13.4) | 32 (11.3) | ||
| Psychiatric | 4956 (14.1) | 529 (16.7) | 497 (20.2) | 27 (6.4) | 5 (1.8) | ||
| Respiratory | 4804 (13.7) | 174 (5.5) | 135 (5.5) | 29 (6.8) | 10 (3.5) | ||
| Musculoskeletal | 4054 (11.6) | 147 (4.6) | 119 (4.8) | 14 (3.3) | 14 (5.0) | ||
| Nervous system | 2754 (7.9) | 226 (7.1) | 194 (7.9) | 16 (3.8) | 16 (5.7) | ||
| Pregnancy | 2150 (6.1) | 52 (1.6) | 41 (1.7) | 9 (2.1) | 2 (0.7) | ||
| Metabolic | 1417 (4.0) | 428 (13.5) | 292 (11.9) | 106 (24.9) | 30 (10.6) | ||
| Infectious | 817 (2.3) | 194 (6.1) | 108 (4.4) | 50 (11.8) | 36 (12.7) | ||
| Kidney | 569 (1.6) | 185 (5.8) | 90 (3.7) | 29 (6.8) | 66 (23.3) | ||
| Hematologic | 464 (1.3) | 74 (2.3) | 37 (1.5) | 12 (2.8) | 25 (8.8) | ||
| Circulatory | 321 (0.9) | 77 (2.4) | 60 (2.4) | 12 (2.8) | 5 (1.8) | ||
| Cancer | 256 (0.7) | 120 (3.8) | 83 (3.4) | 20 (4.7) | 17 (6.0) | ||
| Rehabilitation | 66 (0.2) | 24 (0.8) | 15 (0.6) | 5 (1.2) | 4 (1.4) | ||
| Other | 6063 (17.3) | 422 (13.3) | 362 (14.7) | 39 (9.2) | 21 (7.4) | ||
| Hospitalization length of stay, d | |||||||
| Mean (SD) | 3.8 (5.9) | 7.9 (15.6) | <.001 | 6.9 (14.0) | 9.5 (19.1) | 14.7 (20.6) | <.001 |
| Median (IQR) | 2 (1–4) | 4 (2–8) | <.001 | 4.0 (2.0–7.0) | 4.0 (2.0–9.0) | 8.0 (3.0–16.0) | <.001 |
| Deaths within hospitalization, No. (%) | 15 (0.0) | 36 (1.1) | <.001 | 11 (0.5) | 6 (1.4) | 19 (6.7) | <.001 |
| Deaths within 30 d of discharge, No. (%) | 4 (0.0) | 11 (0.4) | <.001 | 7 (0.3) | 0 (0.0) | 4 (1.4) | .004 |
For those with no AKI during the study period, characteristics at first hospitalization are presented. For those with AKI at any point, characteristics at the first hospitalization with AKI are presented. IQR, interquartile range.
Population- and Hospitalization-Based Incidence of AKI
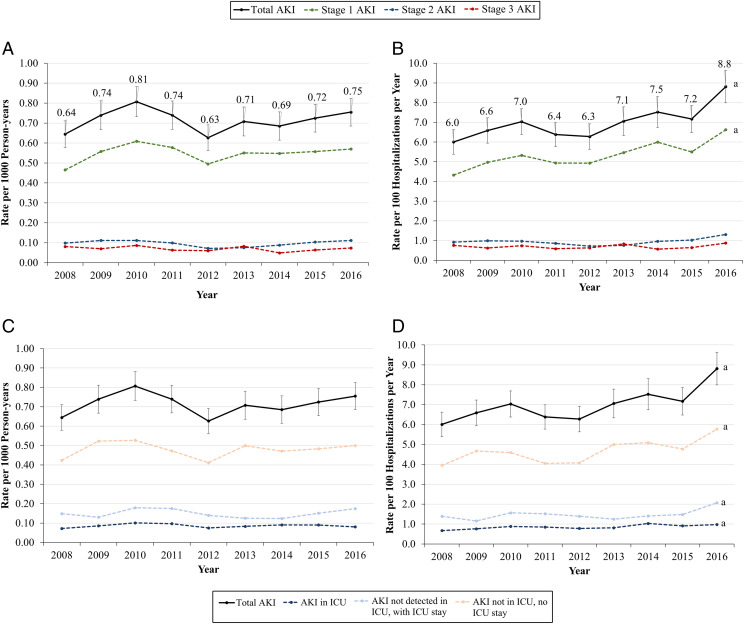
Crude rates of AKI are shown in Supplemental Tables 7 through 9. Population-level age- and sex-adjusted incidence of AKI did not change significantly over time, from 0.64 (95% confidence interval [CI]: 0.58–0.71) per 1000 person-years in 2008 to 0.75 (95% CI: 0.69–0.82) per 1000 person-years in 2016, with an average of 0.70 (95% CI: 0.68–0.73) cases per 1000 person-years across the study period (Fig 1A).
FIGURE 1.
Age- and sex-adjusted incidence of AKI, 2008–2016. A, Rate among all children, per 1000 person-years, by severity. B, Rate among hospitalized children, per 100 hospitalizations per year, by severity. C, Rate among all children, per 1000 person-years, by ICU status. D, Rate among hospitalized children, per hospitalizations per year, by ICU status. a Denotes trend P < .05.
Among the subset of hospitalized children, however, age- and sex-adjusted risk of AKI increased significantly from 6.0 (95% CI: 5.4–6.6) cases per 100 hospitalizations in 2008 to 8.8 (95% CI: 8.0–9.6) cases per 100 hospitalizations in 2016 (Fig 1B). This was largely driven by a significant increase in stage 1 AKI because the combined incidence of severe AKI (stages 2 and 3) remained similar at ∼1.6 cases per 100 hospitalizations per year (Fig 1B). No significant temporal trends were observed in sensitivity analyses in which stage 1 AKI ascertained with an imputed baseline were excluded (Supplemental Fig 3) or only hospitalizations with measured SCr values were included (Supplemental Fig 4).
ICU-Based Incidence of AKI
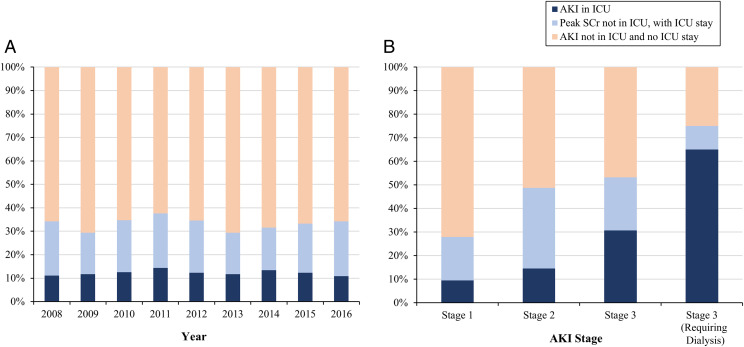
The community-level incidence of AKI in each care setting did not significantly change over time (Fig 1C). However, among the subset of hospitalized children, the incidence of AKI in all care settings increased significantly across the study period (Fig 1D). Among hospitalizations with AKI, the proportion with AKI detected in ICU settings averaged 12.3% each year from 2008 to 2018 (Fig 2A). AKI with no ICU stay contributed 66.7% of all AKI episodes (Fig 2A). Among AKI episodes detected in the ICU, the onset of AKI started before ICU admission in 28.6% of episodes. Among AKI detected outside of the ICU but with transfer to the ICU, the SCr remained elevated above AKI levels in 26.3% of episodes. Thus, the overall proportion of AKI episodes with SCr elevation meeting AKI criteria in ICU settings was 18.3%. Among 2393 AKI hospitalizations not associated with an ICU stay, 418 (17.7%) were classified as severe (Fig 2B).
FIGURE 2.
Distribution of AKI detection within ICU versus non-ICU settings among 3582 pediatric hospitalizations with AKI in KPNC, by year and AKI stage, 2008–2016. A, By year. B, By AKI severity.
Resolution of AKI and Follow-up Kidney Function Testing
Among 3582 hospitalized AKI episodes, 26.0% were discharged with SCr levels that returned to baseline, 28.2% were discharged with elevated SCr compared with baseline, and 45.7% did not have any inpatient SCr measurements after the value used to define AKI.
Follow-up SCr testing rates within 30, 60, 90, and 365 days postdischarge from a hospitalization with unresolved AKI did not change significantly from 2008 to 2016. On average, follow-up testing increased from 15.8% at 30 days to 28.5% at 365 days as well as across AKI stages (Table 3). Thirty-day follow-up testing was higher among severe, confirmed unresolved AKIs (45.7%) compared to severe resolved AKIs (33.0%), although more than one-half of these episodes did not receive a follow-up test (Table 3).
TABLE 3.
Follow-up SCr Testing After 3582 Pediatric Hospitalizations With AKI, by Year, AKI Stage, and Resolution of AKI, 2008–2016
| Strata | n | First Outpatient SCr Measurement After Discharge, No. (%) | |||
|---|---|---|---|---|---|
| ≤30 d | ≤60 d | ≤90 d | ≤365 d | ||
| Unresolved or unknown resolution of AKI, by year | 2651 | ||||
| 2008 | 251 | 41 (16.3) | 50 (19.9) | 56 (22.3) | 77 (30.7) |
| 2009 | 308 | 41 (13.3) | 52 (16.9) | 54 (17.5) | 85 (27.6) |
| 2010 | 337 | 58 (17.2) | 68 (20.2) | 70 (20.8) | 96 (28.5) |
| 2011 | 319 | 51 (16.0) | 64 (20.1) | 66 (20.7) | 95 (29.8) |
| 2012 | 265 | 48 (18.1) | 58 (21.9) | 61 (23.0) | 79 (29.8) |
| 2013 | 264 | 47 (17.8) | 54 (20.5) | 62 (23.5) | 78 (29.6) |
| 2014 | 279 | 39 (14.0) | 46 (16.5) | 54 (19.4) | 82 (29.4) |
| 2015 | 296 | 41 (13.9) | 46 (15.5) | 48 (16.2) | 75 (25.3) |
| 2016 | 332 | 52 (15.7) | 63 (19.0) | 70 (21.1) | 89 (26.8) |
| Unresolved or unknown resolution of AKI, by stage | 2651 | ||||
| Stage 1 | 2099 | 206 (9.8) | 266 (12.7) | 293 (14.0) | 455 (21.7) |
| Stage 2 | 306 | 77 (25.2) | 90 (29.4) | 97 (31.7) | 134 (43.8) |
| Stage 3 | 246 | 135 (54.9) | 145 (58.9) | 151 (61.4) | 167 (67.9) |
| Resolved AKI, by stage | 931 | ||||
| Stage 1 | 640 | 183 (28.6) | 211 (33.0) | 222 (34.7) | 285 (44.5) |
| Stage 2 | 182 | 45 (24.7) | 55 (30.2) | 59 (32.4) | 71 (39.0) |
| Stage 3 | 109 | 51 (46.8) | 57 (52.3) | 61 (56.0) | 70 (64.2) |
| Confirmed unresolved AKI, by stage | 1013 | ||||
| Stage 1 | 639 | 108 (16.9) | 137 (21.4) | 148 (23.2) | 205 (32.1) |
| Stage 2 | 203 | 65 (32.0) | 76 (37.4) | 79 (38.9) | 103 (50.7) |
| Stage 3 | 171 | 106 (62.0) | 111 (64.9) | 114 (66.7) | 121 (70.8) |
| Unknown resolution of AKI, by stage | 1638 | ||||
| Stage 1 | 1460 | 98 (6.7) | 129 (8.8) | 145 (9.9) | 250 (17.1) |
| Stage 2 | 103 | 12 (11.7) | 14 (13.6) | 18 (17.5) | 31 (30.1) |
| Stage 3 | 75 | 29 (38.7) | 34 (45.3) | 37 (49.3) | 46 (61.3) |
In sensitivity analyses using a less stringent definition of AKI resolution, 41.7% of AKI were resolved and 12.6% were unresolved before discharge. However, 30-day follow-up testing was only 53.1% among severe, confirmed unresolved AKIs by using this revised definition (Supplemental Table 10).
Discussion
In a diverse cohort of >1.5 million children receiving care across 21 medical centers in an integrated health care delivery system, we characterized the community-level and hospitalization-based incidences of AKI as defined using KDIGO SCr-based criteria. Our overall pediatric and hospitalized populations are highly similar to the US national population with a median age of 10 years, 49% female, a median hospital length of stay of 3.8 days, and similar hospital diagnoses in comparison to the 2012 Kids’ Inpatient Database.30,31 From 2008 to 2016, the incidence of hospitalized AKI in the pediatric population was stable at 0.70 cases per 1000 person-years; in contrast, the subset of pediatric hospitalizations affected by AKI increased from 6.0 per 100 hospitalizations to 8.8 per 100 hospitalizations. This increase may be explained by secular trends in managing more care outside the hospital to reduce the risk of hospital-related complications given our integrated care delivery structure, leading to more severe illness among children hospitalized in later years. The lower overall hospitalization rate in 2016 (0.5%) compared to 2008 (0.7%) accompanied by an increase in the proportion of hospitalizations with SCr measurements (55.6% to 68.4%) supports this hypothesis, given that SCr testing may be indicative of more severe illnesses. Furthermore, the incidence of AKI did not change among the subset of hospitalizations with SCr measurements over the study period, suggesting that the apparent increase in AKI is due to shifts in the underlying population of hospitalized children.
In several studies, researchers have reported on the incidence of pediatric AKI. Holmes et al19 reported an AKI incidence (using KDIGO criteria) among Welsh children of 1.37 per 1000 person-years. However, the authors included potential nonhospitalized AKI in this estimate, which accounted for ∼30% of AKI episodes among nonneonates in the study. Sutherland et al9 estimated the rate of AKI to be 0.39 per 100 hospitalizations among hospitalized children but relied on administrative diagnosis codes to identify AKI, which are known to have suboptimal accuracy compared to more objective SCr-based criteria. Among 13 914 noncritical hospitalized children, McGregor et al15 identified AKI by KDIGO criteria in 5% of all hospitalizations and 30% of hospitalizations with SCr measurements. This estimate is more consistent with our estimate of 7.0% and is expected to be slightly lower because of its focus on only non–critically ill patients.
Importantly, we found that 67% of AKI episodes were not associated with an ICU stay, representing a substantial proportion of hospitalized AKI that has not been the focus of previous studies of AKI in children.1–3,6,11,32 This proportion is consistent with the percentage of AKI not occurring in the ICU observed in the Kids’ Inpatient Database of 65.5%.9 Although the large majority of non-ICU AKIs in our study were low severity as expected, 18% were severe AKIs. Many of these AKI episodes could reflect clinically undetected AKI; however, up to nearly 1 in 5 cases of potentially undetected AKI were of presumed high AKI severity and may reflect a need for increased surveillance in selected hospitalized children. Further studies are necessary to determine any differences in etiology or outcomes of severe AKI episodes among non–critically ill patients.
Overall, postdischarge outpatient SCr testing after an episode of AKI was moderate in our cohort. On average, 15.8% of patients with a presumed unresolved AKI received a follow-up SCr test within 30 days after discharge and 28.5% within 365 days. This may be due, in part, to the discrepancy between AKI defined by using an imputed baseline SCr and clinically recognized AKI. Thirty-day follow-up testing rates across all AKI cases were much higher among those with a measured baseline SCr (57.7%) compared with those with an imputed baseline SCr (11.7%) (Supplemental Table 11). However, it is likely that children with a measured baseline may already be in the care of outpatient nephrology services and reflect a particularly high-risk population. In addition, many providers may choose not to test children in situations in which the child appears fully recovered clinically or performing a blood test is contraindicated. As expected, the proportion of patients with follow-up tests was significantly higher with increasing severity of AKI. However, even at one year after discharge, a follow-up SCr test was not found in more than one-third of severe AKI patients who did not appear resolved before discharge, regardless of the definition of AKI resolution used. In pediatric patients, previous studies and guidelines have suggested that AKI may contribute to long-term incidence or worsening chronic kidney disease, highlighting the need to consider more systematic follow-up kidney function testing and disease monitoring after episodes of AKI by pediatricians and other primary care providers, with potential referral to specialty care.12,27,33
Our study has several strengths. To our knowledge, ours is the first study of the community-level incidence of hospitalized AKI using KDIGO SCr-based criteria and an isotope dilution mass spectrometry-traceable SCr data over a contemporary study period. This is a distinct advantage over studies using diagnostic codes to identify AKI episodes because it allows for ascertainment of both clinically detected and uncoded AKI by using a standardized definition. Importantly, we were also able to characterize the location of the onset and peak of the AKI within a hospitalization and estimate AKI resolution using serial SCr measurements. Additional strengths include our large, demographically diverse population and our ability to systematically follow-up with patients to evaluate kidney function testing patterns during the first-year postdischarge.
Our findings should also be placed in the context of several limitations. Because our source population received care through an integrated health care delivery system, we were unable to capture clinical data for nonnetwork hospitalizations. Although these accounted for ∼20% of hospitalizations among the pediatric population over the study period, the vast majority of those patients are routinely transferred to KPNC facilities shortly after admission to complete their care. We lacked data on urine output, which would lead to an underestimate of AKI episodes.1,34 A measured baseline SCr was unavailable for 76% of all hospitalizations (83% of hospitalizations with AKI), as is expected among children; however, in children with a measured baseline SCr, the imputed SCr value was similar (median difference: −0.03 [interquartile range: −0.12 to 0.05] mg/dL), supporting the validity of the imputation approach. We were also unable to characterize the occurrence of AKI episodes occurring outside the hospital. Finally, our results may not be fully generalizable to uninsured patients, other geographic areas, or to all practice settings, because AKI incidence may be influenced by network-specific practice patterns.
Conclusions
We observed a community-based incidence of hospitalized AKI of 0.7 per 1000 person-years among children, with two-thirds of AKI episodes occurring outside critical care settings and approximately one-half of children with presumed severe AKI not receiving follow-up SCr testing within 90 days of discharge. To reduce the potentially longer-term consequences of AKI in children, further efforts should be made for the systematic recognition and awareness of AKI in all inpatient settings with appropriate, risk-based postdischarge follow-up kidney function monitoring by pediatricians and other pediatric primary care providers. Studies are also needed to examine the etiologies, long-term clinical outcomes, and more effective preventive and therapeutic strategies for pediatric AKI, especially in non–critically ill children.
Glossary
- AKI
acute kidney injury
- CI
confidence interval
- eGFR
estimated glomerular filtration rate
- KDIGO
Kidney Disease: Improving Global Outcomes
- KPNC
Kaiser Permanente Northern California
- SCr
serum creatinine
Footnotes
Mr Parikh designed the study, analyzed the data and created the figures and tables, and drafted the manuscript; Dr Go designed the study and drafted the manuscript; Ms Tan drafted the manuscript; Drs Salyer, Auron, Kim, and Ku provided critical feedback and revised the manuscript; and all authors approved the final version of the manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Partially supported by research grants (R01 DK101507, U01 DK08223) from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
COMPANION PAPER: A companion to this article can be found online at www.pediatrics.org/cgi/doi/10.1542/peds.2020-0880.
References
- 1.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators . Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017;376(1):11–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hessey E, Morissette G, Lacroix J, et al. . Healthcare utilization after acute kidney injury in the pediatric intensive care unit. Clin J Am Soc Nephrol. 2018;13(5):685–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selewski DT, Cornell TT, Heung M, et al. . Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensive Care Med. 2014;40(10):1481–1488 [DOI] [PubMed] [Google Scholar]
- 4.Sutherland SM, Byrnes JJ, Kothari M, et al. . AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol. 2015;10(4):554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Pinto LN, Goldstein SL, Schneider JB, Khemani RG. Association between progression and improvement of acute kidney injury and mortality in critically ill children. Pediatr Crit Care Med. 2015;16(8):703–710 [DOI] [PubMed] [Google Scholar]
- 6.Bailey D, Phan V, Litalien C, et al. . Risk factors of acute renal failure in critically ill children: a prospective descriptive epidemiological study. Pediatr Crit Care Med. 2007;8(1):29–35 [DOI] [PubMed] [Google Scholar]
- 7.Chang J-W, Jeng M-J, Yang L-Y, et al. . The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int. 2015;87(3):632–639 [DOI] [PubMed] [Google Scholar]
- 8.Plötz FB, Bouma AB, van Wijk JAE, Kneyber MCJ, Bökenkamp A. Pediatric acute kidney injury in the ICU: an independent evaluation of pRIFLE criteria. Intensive Care Med. 2008;34(9):1713–1717 [DOI] [PubMed] [Google Scholar]
- 9.Sutherland SM, Ji J, Sheikhi FH, et al. . AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol. 2013;8(10):1661–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneider J, Khemani R, Grushkin C, Bart R. Serum creatinine as stratified in the RIFLE score for acute kidney injury is associated with mortality and length of stay for children in the pediatric intensive care unit. Crit Care Med. 2010;38(3):933–939 [DOI] [PubMed] [Google Scholar]
- 11.Hessey E, Morissette G, Lacroix J, et al. . Long-term mortality after acute kidney injury in the pediatric ICU. Hosp Pediatr. 2018;8(5):260–268 [DOI] [PubMed] [Google Scholar]
- 12.Mammen C, Al Abbas A, Skippen P, et al. . Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis. 2012;59(4):523–530 [DOI] [PubMed] [Google Scholar]
- 13.Chawla LS, Eggers PW, Star RA, Kimmel PL. Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med. 2014;371(1):58–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Nie S, Zhang A, et al. . A new criterion for pediatric AKI based on the reference change value of serum creatinine. J Am Soc Nephrol. 2018;29(9):2432–2442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGregor TL, Jones DP, Wang L, et al. . Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: a retrospective observational study. Am J Kidney Dis. 2016;67(3):384–390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moffett BS, Goldstein SL. Acute kidney injury and increasing nephrotoxic-medication exposure in noncritically-ill children. Clin J Am Soc Nephrol. 2011;6(4):856–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zappitelli M, Moffett BS, Hyder A, Goldstein SL. Acute kidney injury in non-critically ill children treated with aminoglycoside antibiotics in a tertiary healthcare centre: a retrospective cohort study. Nephrol Dial Transplant. 2011;26(1):144–150 [DOI] [PubMed] [Google Scholar]
- 18.Lex DJ, Tóth R, Cserép Z, et al. . A comparison of the systems for the identification of postoperative acute kidney injury in pediatric cardiac patients. Ann Thorac Surg. 2014;97(1):202–210 [DOI] [PubMed] [Google Scholar]
- 19.Holmes J, Roberts G, May K, et al. ; Welsh Acute Kidney Injury Steering Group . The incidence of pediatric acute kidney injury is increased when identified by a change in a creatinine-based electronic alert. Kidney Int. 2017;92(2):432–439 [DOI] [PubMed] [Google Scholar]
- 20.Jenssen GR, Hovland E, Bangstad H-J, Nygård K, Vold L, Bjerre A. The incidence and aetiology of acute kidney injury in children in Norway between 1999 and 2008. Acta Paediatr. 2014;103(11):1192–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andreoli SP. Acute kidney injury in children. Pediatr Nephrol. 2009;24(2):253–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andreoli SP. Management of acute kidney injury in children: a guide for pediatricians. Paediatr Drugs. 2008;10(6):379–390 [DOI] [PubMed] [Google Scholar]
- 23.Gordon NP. Characteristics of Adult Members in Kaiser Permanente’s Northern California Region, as Estimated from the 2011 Adult Member Health Survey. Oakland, CA: Division of Research, Kaiser Permanente Medical Care Program; 2013 [Google Scholar]
- 24.Lo LJ, Go AS, Chertow GM, et al. . Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76(8):893–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arellano MG, Petersen GR, Petitti DB, Smith RE. The California Automated Mortality Linkage System (CAMLIS). Am J Public Health. 1984;74(12):1324–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C-Y. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. [published correction appears in N Engl J Med. 2004;18(4):4]. N Engl J Med. 2004;351(13):1296–1305 [DOI] [PubMed] [Google Scholar]
- 27.Kidney Disease: Improving Global Outcomes Acute Kidney Injury Work Group KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2(1):1–138 [Google Scholar]
- 28.Schwartz GJ, Muñoz A, Schneider MF, et al. . New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3(4):948–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Witt WP, Weiss AJ, Elixhauser A. Overview of Hospital Stays for Children in the United States, 2012: Statistical Brief #187 In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville, MD: Agency for Healthcare Research and Quality; 2014 [PubMed] [Google Scholar]
- 31.Leyenaar JK, Ralston SL, Shieh M-S, Pekow PS, Mangione-Smith R, Lindenauer PK. Epidemiology of pediatric hospitalizations at general hospitals and freestanding children’s hospitals in the United States. J Hosp Med. 2016;11(11):743–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71(10):1028–1035 [DOI] [PubMed] [Google Scholar]
- 33.Goldstein SL, Devarajan P. Progression from acute kidney injury to chronic kidney disease: a pediatric perspective. Adv Chronic Kidney Dis. 2008;15(3):278–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kellum JA, Sileanu FE, Murugan R, Lucko N, Shaw AD, Clermont G. Classifying AKI by urine output versus serum creatinine level. J Am Soc Nephrol. 2015;26(9):2231–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]