The DART QI program provides 1 solution for reducing inappropriate outpatient antibiotic prescribing for respiratory infections nationally.
Abstract
Video Abstract
BACKGROUND:
One-third of outpatient antibiotic prescriptions for pediatric acute respiratory tract infections (ARTIs) are inappropriate. We evaluated a distance learning program’s effectiveness for reducing outpatient antibiotic prescribing for ARTI visits.
METHODS:
In this stepped-wedge clinical trial run from November 2015 to June 2018, we randomly assigned 19 pediatric practices belonging to the Pediatric Research in Office Settings Network or the NorthShore University HealthSystem to 4 wedges. Visits for acute otitis media, bronchitis, pharyngitis, sinusitis, and upper respiratory infection for children 6 months to <11 years old without recent antibiotic use were included. Clinicians received the intervention as 3 program modules containing online tutorials and webinars on evidence-based communication strategies and antibioti c prescribing, booster video vignettes, and individualized antibiotic prescribing feedback reports over 11 months. The primary outcome was overall antibiotic prescribing rates for all ARTI visits. Mixed-effects logistic regression compared prescribing rates during each program module and a postintervention period to a baseline control period. Odds ratios were converted to adjusted rate ratios (aRRs) for interpretability.
RESULTS:
Among 72 723 ARTI visits by 29 762 patients, intention-to-treat analyses revealed a 7% decrease in the probability of antibiotic prescribing for ARTI overall between the baseline and postintervention periods (aRR 0.93; 95% confidence interval [CI], 0.90–0.96). Second-line antibiotic prescribing decreased for streptococcal pharyngitis (aRR 0.66; 95% CI, 0.50–0.87) and sinusitis (aRR 0.59; 95% CI, 0.44–0.77) but not for acute otitis media (aRR 0.93; 95% CI, 0.83–1.03). Any antibiotic prescribing decreased for viral ARTIs (aRR 0.60; 95% CI, 0.51–0.70).
CONCLUSIONS:
This program reduced antibiotic prescribing during outpatient ARTI visits; broader dissemination may be beneficial.
What’s Known on This Subject:
Behavioral interventions including individualized clinician prescribing feedback can reduce inappropriate antibiotic prescribing in ambulatory settings. These interventions have not been previously paired with communication training and evidence-based education on antibiotic prescribing for childhood acute respiratory tract infections.
What This Study Adds:
In this multisite stepped-wedge cluster-randomized trial, the Dialogue Around Respiratory Illness Treatment intervention combined communication training, evidence-based antibiotic prescribing education, and individualized prescribing feedback, producing a 7% sustained reduction in the probability of antibiotic prescribing for acute respiratory infection visits.
Antibiotic prescribing for childhood acute respiratory tract infections (ARTIs) occurs at an estimated annual rate of 421 prescriptions per 1000 population, accounting for >70% of all antibiotics prescribed to ambulatory children.1,2 Approximately one-third of all antibiotic prescriptions for childhood ARTIs are likely inappropriate, accounting for >10 million potentially preventable antibiotic prescriptions for US children annually.2,3 Although recent data revealed that oral antibiotic prescriptions for all pediatric conditions decreased 13% from 2011 to 2016, the 2015 US Government action plan targets a 50% reduction in inappropriate outpatient antibiotic use by 2020.4,5
The American Academy of Pediatrics (AAP) and Infectious Diseases Society of America have published treatment guidelines outlining first- and second-line treatments for the following bacterial ARTIs: acute otitis media (AOM), sinusitis, and group A streptococcal pharyngitis.6–8 Audit of primary care provider antibiotic prescribing for ARTIs paired with individualized feedback reports previously reduced inappropriate antibiotic prescribing for bacterial ARTIs, although the reduction disappeared after feedback discontinuation.9,10 Behavioral interventions similarly reduced antibiotic treatment of adult viral ARTIs, but the effect waned within 12 months of the study conclusion.11,12 Internet-based communication skills training has reduced inappropriate antibiotic prescribing in adult health care settings.13,14 In previous work, authors using the methods of conversation analysis have also elucidated the provider communication best practices that are associated with decreased inappropriate antibiotic prescribing during pediatric ARTI visits.15–18 However, a comprehensive, large-scale intervention combining best practices in antibiotic prescribing feedback, behavioral interventions, and communication techniques used to reduce inappropriate antibiotic prescribing for pediatric ARTIs is lacking.
We developed the Dialogue Around Respiratory Illness Treatment (DART) quality improvement (QI) program, hypothesizing that pairing Internet-based communication skills training with individualized antibiotic prescribing audit and feedback would reduce overall antibiotic prescribing for ARTI.
Methods
Study Design, Participants, and Setting
We implemented the DART QI program using a cluster-randomized stepped-wedge clinical trial to maximize statistical power and allow each practice to receive the intervention through staggered implementation across 19 community-based primary care pediatric practices. Study data were collected from November 2015 through June 2018. All practices were recruited from 2 practice-based research networks: the AAP Pediatric Research in Office Settings (PROS) (n = 11 practices from 9 states) and the NorthShore University HealthSystem (n = 8 practices in the Chicago, IL, metropolitan area). Included practices used a common electronic health record (EHR) within their network. The practice was the unit of randomization. The NorthShore practices were allocated by random permutation (by C.Z.) to each of 4 wedges (2 practices each; Fig 1). The PROS practices were enrolled later and were similarly randomly allocated to wedges 2 to 4 (3–4 practices each). Written informed consent was obtained from pediatricians and pediatric nurse practitioners (“clinicians”; n = 57; 1–6 per practice) before random assignment. Enrolled clinicians received intervention modules according to their practice-assigned wedge. Two NorthShore clinicians saw patients at 2 study sites randomly assigned to different wedges. For both clinicians, all visits at either study site after their earliest intervention exposure were considered postintervention visits.
FIGURE 1.
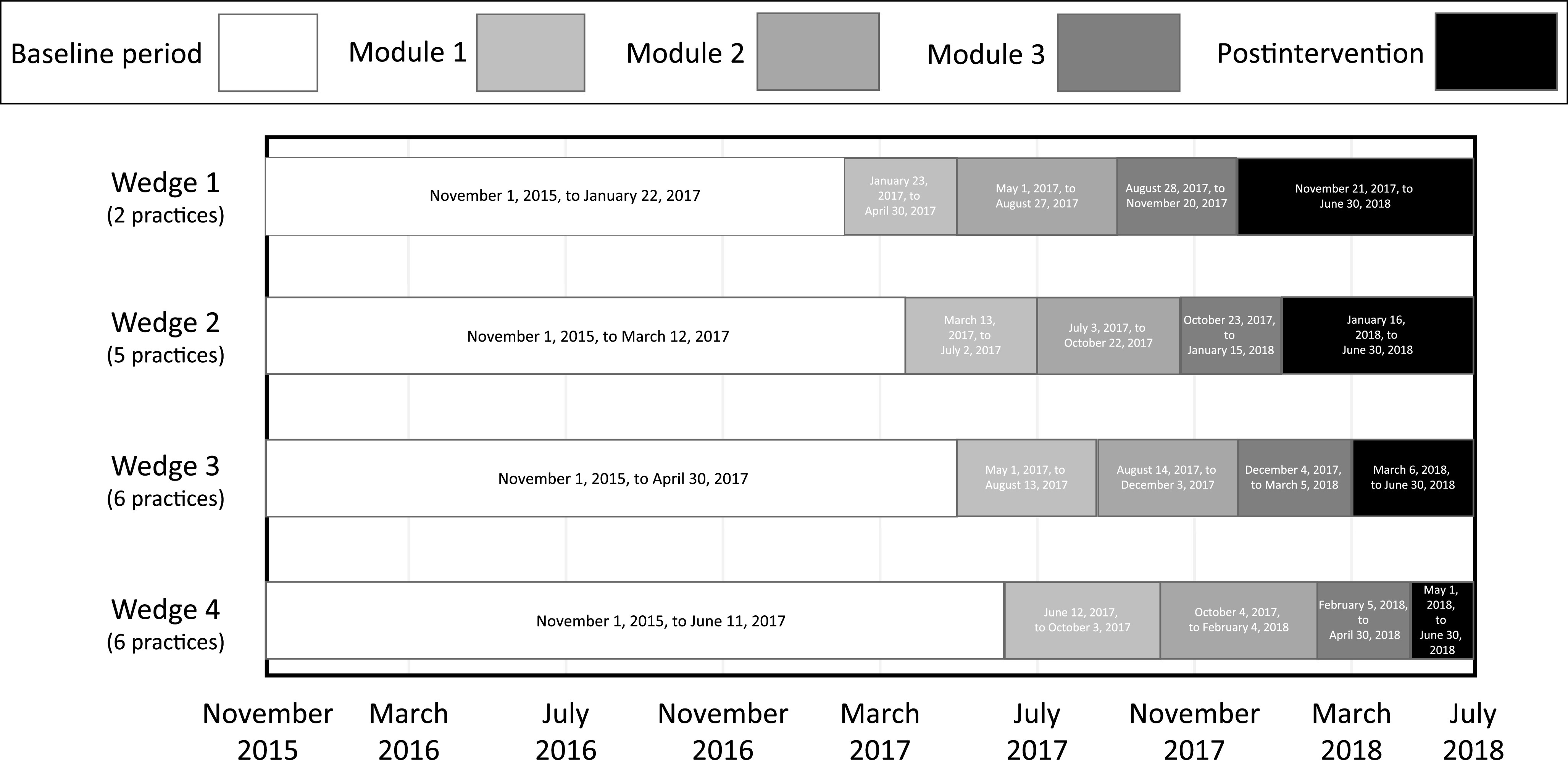
Study intervention and timing.
Study Visit Inclusion and Exclusion Criteria
Visits by children aged 6 months to <11 years of age with an International Classification of Diseases, 10th Revision (ICD-10) diagnosis code (Supplemental Table 5) for AOM, bronchitis, pharyngitis, sinusitis, or upper respiratory infection (URI) were included. Only oral antibiotics prescribed on the clinic visit date were included in prescribing measures for each ARTI. ARTI visits were excluded from prescribing measures if there were any concomitant non-ARTI bacterial diagnoses (Supplemental Table 5) or antibiotic prescriptions during the 30 days preceding the index visit (which might necessitate second-line prescribing). Visits by children with penicillin or cephalosporin antibiotic allergies were excluded from second-line prescribing measures.
Intervention
The DART QI program (1) was received by clinicians; (2) contained evidence-based online tutorials, webinars, booster video vignette sessions, and individualized antibiotic prescribing feedback reports; and (3) was received in 3 modules over an 11-month period (Fig 1). All DART QI program educational materials are available online.19
In module 1, clinicians viewed 25-minute online tutorials about best practices for both parent-clinician communication practices and antibiotic prescribing, participated in live or recorded 40-minute webinars on those topics, and received an individualized feedback report presenting antibiotic prescribing rates during ARTI visits in the baseline control period (see Outcomes section below). The theory of planned behavior20 underpinned development of the evidence-based communication tutorial, which aims to modify how providers frame treatment recommendations and follow-up plans for patients with ARTIs.15–17 We implemented 2 main strategies: (1) building subjective norms, self-awareness, and changing attitudes among the clinicians supporting the targeted communication and prescribing behaviors and (2) developing the skills to achieve these goals through modeling, practice, feedback, reinforcement, and building self-confidence. The evidence-based antibiotic prescribing tutorials were based on published guidelines for the diagnosis and management of each ARTI, including delayed prescribing techniques.6–8,21 Each individualized feedback report (Supplemental Fig 3) contained antibiotic prescribing rates for all ARTI combined (primary outcome) and for 5 secondary outcomes (described below). Rates were compared to a goal rate derived from the 20% of enrolled clinicians with the lowest antibiotic prescribing rates for those conditions, a form of peer-comparison feedback. Outlier feedback report results were investigated, and targeted validation was performed at providers’ requests.
In module 2, clinicians received two 5-minute online booster video vignettes recapping communication best practices and the second antibiotic prescribing feedback report, presenting prescribing rates during the module 1 participation period. In module 3, clinicians received 1 communication booster video vignette and the third and fourth antibiotic prescribing feedback reports, presenting prescribing rates during modules 2 and 3, respectively.
All enrolled clinicians received links to the Web-based tutorials, webinars, booster video vignettes, and individualized antibiotic prescribing feedback reports via e-mail. Study staff tracked clinician participation in the Web-based intervention components by determining if clinicians opened each online tutorial and completed embedded quiz questions at a passing rate of 80%. All enrolled pediatricians were offered American Board of Pediatrics Maintenance of Certification Part 4 credit for completing the DART QI program.
Data Collection
EHR data were used to collect patient-level covariates, assess visit-level ARTI antibiotic prescribing rates, and generate the DART QI program antibiotic prescribing feedback reports, primary, and secondary outcome measures. Enrolled clinicians provided usual care during the baseline control period until their wedge started the intervention (Fig 1). Ongoing data collection until June 2018 provided a planned additional 2- to 8-month postintervention sustainability period, during which practices no longer actively received the intervention nor were aware of data collection.
Outcomes
The primary outcome was the visit-level (as opposed to patient-level) antibiotic prescribing rate for all ARTIs during each study module, for which the denominator was all included ARTI visits during that module, and the numerator was ARTI visits during which antibiotic prescribing occurred. Because antibiotic appropriateness measures require symptom data difficult to extract from an EHR (eg, presence of severe otalgia during AOM), we selected antibiotic prescribing rather than appropriateness as the primary outcome. The 5 secondary outcomes included (1) visit-level antibiotic prescribing rates for viral ARTI, (2) visit-level prescribing rates for pharyngitis (streptococcal and nonstreptococcal combined), and second-line antibiotic prescribing rates for (3) AOM, (4) streptococcal pharyngitis, and (5) sinusitis.
Bronchitis, nonstreptococcal pharyngitis, and URI were considered viral ARTIs for which antibiotics are inappropriate. Bacterial ARTIs included AOM, streptococcal pharyngitis, and sinusitis. On the basis of published national guidelines,6–8 the goal for first-line prescribing for each bacterial ARTI was amoxicillin for AOM (except when diagnosed concurrently with conjunctivitis, for which amoxicillin-clavulanate was also considered as first line to treat Haemophilus influenzae otitis-conjunctivitis syndrome), penicillin or amoxicillin for streptococcal pharyngitis, and amoxicillin or amoxicillin-clavulanate for sinusitis. When multiple diagnoses were present, antibiotics were attributed in a hierarchical fashion first to sinusitis, then to AOM, and then to pharyngitis. Visits were only described as viral ARTI if no competing bacterial diagnoses were present. All systemic antibiotic prescriptions that did not meet the definition of first line were considered second line.
Statistical Analysis
The primary intention-to-treat (ITT) analysis included all study clinicians and used multivariable mixed-effects logistic regression clustered by both the clinician and practice to determine the effect of exposure to each DART module on the binary outcome of whether an antibiotic was prescribed during each ARTI visit, adjusted a priori for child age, sex, and race and/or ethnicity (factors previously associated with antibiotic prescribing).22,23 Each practice used standard approaches to collect race and/or ethnicity data, typically at the time of registration. Analysis was also adjusted for influenza season (November through March) and patient level of medical complexity by using the previously validated Pediatric Medical Complexity Algorithm version 3.0.24,25 In reporting the results, we converted the logistic regression odds ratios into adjusted rate ratios (aRRs) to facilitate interpretation and describe these results as increases or decreases in the likelihoods of antibiotic prescribing during ARTI visits.26,27 On the basis of the cluster-randomized design, all preintervention ARTI visits serve as controls. An additional model including a time period indicator used a likelihood ratio test to determine if changes in antibiotic prescribing over time were driven by secular trends.
We planned a priori to evaluate the primary and secondary outcomes among the subgroup of clinicians who actively participated in the entire intervention (ie, they watched all available tutorials, webinars, and boosters and received feedback reports) and those who did not (ie, only received feedback reports but did not engage fully with remaining intervention components). All analyses began after completion of the postintervention period in June 2018.
Power and sample size calculations were based on preliminary studies outlining national ARTI antibiotic prescribing rates and the effect of previous feedback interventions on improving antibiotic prescribing.3,9 Those calculations assumed 4 wedges of 5 practices each, a small −0.1% temporal effect, a practice random-effect SD of 5% and within-clinician random-effect SD of 10%, and that clinician ARTI antibiotic prescribing would follow a binomial distribution. Using linear mixed-effects regression on clinician-level prescribing rates, we estimated that 4 wedges, 4 practices per wedge, and 3 clinicians per practice would provide ∼93% power to detect an absolute decrease in antibiotic prescribing for all ARTI visits from 55% to 45% and an absolute decrease in second-line prescribing for bacterial ARTI from 27% to 17%.
This study was reviewed and approved by the Western, AAP, NorthShore University Health System, and Children’s Hospital of Philadelphia Institutional Review Boards. All analyses were conducted in R version 3.6.0.28
Results
Overall, 57 clinicians (50 pediatricians and 7 nurse practitioners) from 19 practices agreed to participate and were included in the analysis (Fig 2). The number of clinicians at each practice ranged from 1 to 6 (median of 3).
FIGURE 2.
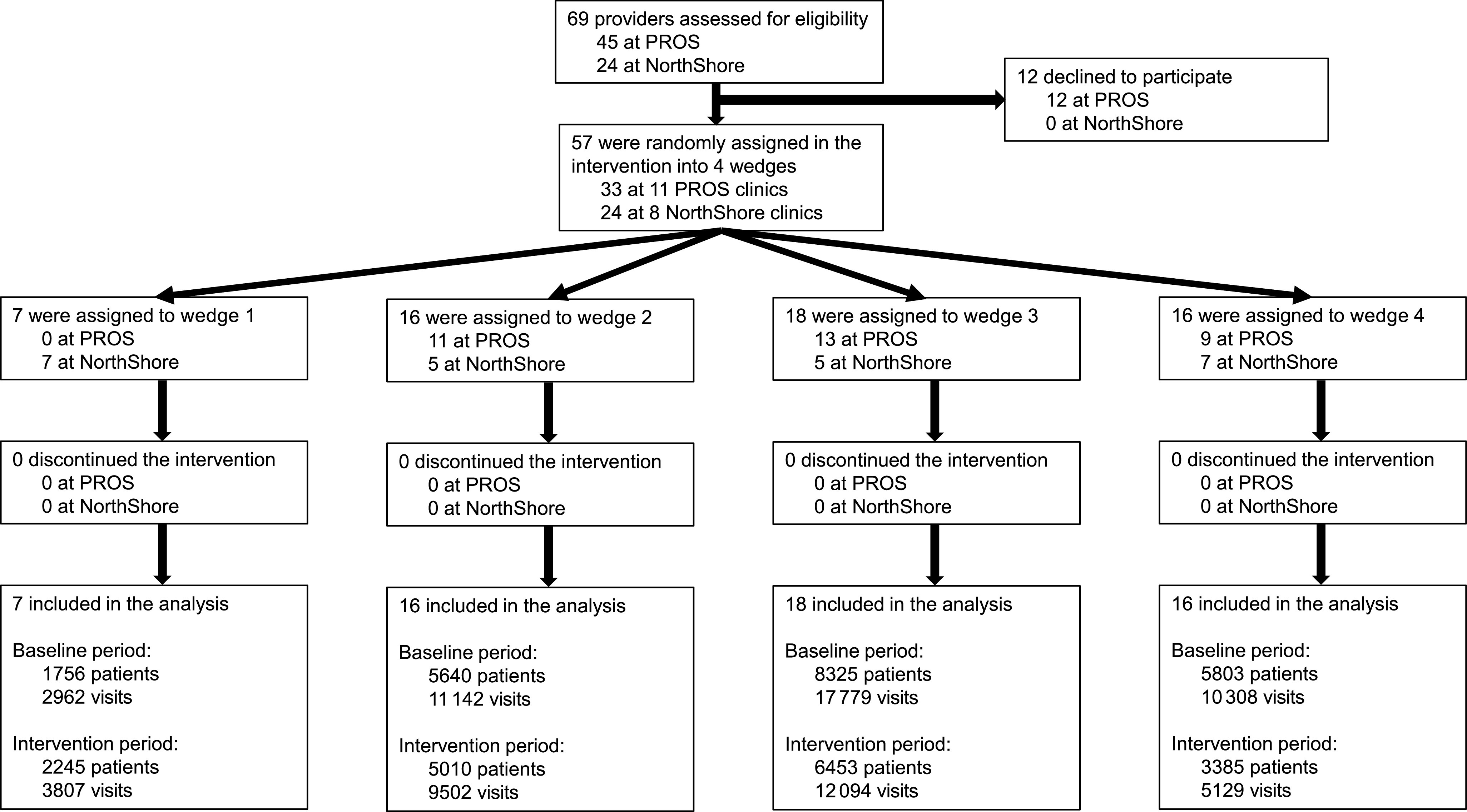
Study flow diagram.
Over the study period, 29 762 individual children (Table 1) experienced 72 723 total ARTI visits, with 13 764 (46.2%) children having 1 ARTI visit, 6387 (21.5%) having 2, and 9611 (32.3%) having ≥3 (range of 3–29). Among all 72 723 ARTI visits, 28 758 (39.5%) received antibiotics. A total of 447 children were excluded because of antibiotic use in the previous 30 days, and 1590 children were excluded because of concomitant bacterial infections during their ARTI visit.
TABLE 1.
ARTI Patient Demographics and Diagnoses by Study Time Period
| Demographics | Baseline | Module 1 | Module 2 | Module 3 | Postintervention |
|---|---|---|---|---|---|
| No. patients | 21 401 | 1936 | 2132 | 2211 | 2082 |
| Age, y, median (IQR) | 4.24 (1.86–6.84) | 3.83 (1.32–6.69) | 3.08 (1.02–6.07) | 3.41 (1.08–6.42) | 2.80 (0.95–6.07) |
| Female | 10 538 (49.2) | 959 (49.5) | 1038 (48.7) | 1069 (48.3) | 1028 (49.4) |
| Race | |||||
| White | 13 438 (62.8) | 1045 (54.0) | 1331 (62.4) | 1264 (57.2) | 1089 (52.3) |
| Black or African American | 470 (2.2) | 56 (2.9) | 45 (2.1) | 52 (2.4) | 52 (2.5) |
| Asian American or Pacific Islander | 797 (3.7) | 83 (4.3) | 98 (4.6) | 85 (3.8) | 120 (5.8) |
| Native American | 169 (0.8) | 6 (0.3) | 23 (1.1) | 17 (0.8) | 3 (0.1) |
| Mixed race | 292 (1.4) | 20 (1.0) | 28 (1.3) | 29 (1.3) | 15 (0.7) |
| Not available | 6235 (29.1) | 726 (37.5) | 607 (28.5) | 764 (34.6) | 803 (38.6) |
| Hispanic ethnicity | 4760 (22.2) | 372 (19.2) | 424 (19.9) | 436 (19.7) | 385 (18.5) |
| PMCA | |||||
| Nonchronic | 14 630 (68.4) | 1365 (70.5) | 1595 (74.8) | 1650 (74.6) | 1561 (75.0) |
| Noncomplex chronic | 4862 (22.7) | 410 (21.2) | 389 (18.2) | 423 (19.1) | 394 (18.9) |
| Complex chronic | 1909 (8.9) | 161 (8.3) | 148 (6.9) | 138 (6.2) | 127 (6.1) |
| Diagnoses | |||||
| All ARTI, n | 42 191 | 6287 | 7319 | 8166 | 8760 |
| Viral ARTI | 19 438 (46.1) | 2764 (44.0) | 3633 (49.6) | 4265 (52.2) | 4224 (48.2) |
| All pharyngitis | 12 190 (28.9) | 2146 (34.1) | 2023 (27.6) | 1765 (21.6) | 2340 (26.7) |
| AOM | 7461 (17.7) | 1006 (16.0) | 1131 (15.5) | 1530 (18.7) | 1684 (19.2) |
| Streptococcal pharyngitis | 4300 (10.2) | 781 (12.4) | 618 (8.4) | 567 (6.9) | 840 (9.6) |
| Sinusitis | 3102 (7.4) | 371 (5.9) | 532 (7.3) | 606 (7.4) | 512 (5.8) |
Data are presented as No. (%) unless otherwise specified. Module 1 contained online communication and antibiotic prescribing tutorials and webinars and an individualized antibiotic prescribing feedback report. Module 2 contained 2 online communication booster video vignettes and an antibiotic prescribing feedback report. Module 3 contained 1 communication booster video vignette and 2 antibiotic prescribing feedback reports. IQR, interquartile range.
Engagement With the Intervention
All clinicians received feedback reports. There were 41 (72%) clinicians at 17 practices who engaged actively with the intervention by viewing all online communication and prescribing tutorials and webinars, whereas 16 (28%) clinicians at 8 practices did not engage in all aspects of the intervention (14 clinicians viewed none of the online training materials, whereas 2 clinicians viewed at least 1 but not all online modules).
Primary Outcome
In the adjusted ITT analysis, the probability of antibiotic prescribing for all ARTI visits was lower during each module and during the 2- to 8-month postintervention period compared to the baseline control period (Table 2, Supplemental Fig 4). The probability of antibiotic prescribing for all ARTIs decreased 4% (95% confidence interval [CI], 1%–7%) in module 1, 16% (95% CI, 12%–19%) in module 2, 11% (95% CI, 8%–14%) in module 3, and 7% (95% CI, 4%–10%) during the postintervention period. Unadjusted antibiotic prescribing rates are reported in Supplemental Table 6.
TABLE 2.
Rate Ratios of Antibiotic Prescribing During All ITT Analysis Visits for ARTI Overall and by Condition (by Study Time Period)
| Condition | Measure | Baseline | Module 1 | Module 2 | Module 3 | Postintervention |
|---|---|---|---|---|---|---|
| aRR (95% CI) | ||||||
| ARTI overall | Any prescribing | Reference | 0.96 (0.93–0.99) | 0.84 (0.81–0.88) | 0.89 (0.86–0.92) | 0.93 (0.90–0.96) |
| Viral ARTI | Any prescribing | Reference | 0.63 (0.52–0.76) | 0.65 (0.55–0.78) | 0.64 (0.54–0.75) | 0.60 (0.51–0.70) |
| All pharyngitis | Any prescribing | Reference | 1.06 (1.01–1.12) | 0.90 (0.84–0.95) | 0.85 (0.80–0.91) | 0.96 (0.91–1.02) |
| AOM | Second-line prescribing | Reference | 1.01 (0.88–1.15) | 0.69 (0.60–0.80) | 0.96 (0.86–1.07) | 0.93 (0.83–1.03) |
| Streptococcal pharyngitis | Second-line prescribing | Reference | 0.71 (0.53–0.94) | 0.61 (0.46–0.82) | 0.46 (0.32–0.66) | 0.66 (0.50–0.87) |
| Sinusitis | Second-line prescribing | Reference | 0.75 (0.55–0.99) | 0.51 (0.38–0.68) | 0.66 (0.52–0.85) | 0.59 (0.44–0.77) |
Module 1 contained online communication and antibiotic prescribing tutorials and webinars and an individualized antibiotic prescribing feedback report. Module 2 contained 2 online communication booster video vignettes and an antibiotic prescribing feedback report. Module 3 contained 1 communication booster video vignette and 2 antibiotic prescribing feedback reports.
Secondary Outcomes
The probability of prescribing antibiotics for viral ARTI was significantly lower during each DART module and during the postintervention period compared to the baseline control period (Table 2). The probability of prescribing antibiotics for pharyngitis was significantly lower during modules 2 and 3 but not during module 1 or during the postintervention period. The probability of prescribing second-line antibiotics for AOM was only lower during module 2 compared to the baseline control period. However, for streptococcal pharyngitis and sinusitis, the probability of second-line antibiotic prescribing was significantly lower during each module and during the postintervention period. Additional models including a time period indicator revealed unchanged results for both the primary and secondary outcomes and demonstrated no significant secular trend in antibiotic prescribing rates.
Subgroup Analyses
Subgroup analyses revealed similar results to those of the ITT analyses for both engaged and less-engaged clinicians regarding the probability of antibiotic prescribing for all ARTIs, viral ARTI, and pharyngitis during all study periods compared to baseline (Tables 3 and 4). However, for second-line antibiotic prescribing, although results for engaged clinicians were similar to the ITT results, those for the less-engaged clinicians revealed a significantly increased probability of prescribing second-line antibiotics for AOM during the postintervention period compared to the baseline (increased 27% [7%–47%]; Table 4) and no change in the probability of prescribing second-line antibiotics for streptococcal pharyngitis or sinusitis across study periods (Table 4).
TABLE 3.
Rate Ratios of Antibiotic Prescribing During Visits to Fully Engaged Clinicians for ARTI Overall and by Condition (by Study Time Period)
| Condition | Measure | Baseline | Module 1 | Module 2 | Module 3 | Postintervention |
|---|---|---|---|---|---|---|
| aRR (95% CI) | ||||||
| ARTI overall | Any prescribing | Reference | 0.97 (0.93–1.01) | 0.84 (0.80–0.87) | 0.89 (0.86–0.93) | 0.92 (0.89–0.96) |
| Viral ARTI | Any prescribing | Reference | 0.73 (0.59–0.90) | 0.57 (0.46–0.72) | 0.61 (0.50–0.74) | 0.62 (0.52–0.74) |
| All pharyngitis | Any prescribing | Reference | 1.06 (0.99–1.12) | 0.87 (0.81–0.93) | 0.84 (0.78–0.91) | 0.96 (0.90–1.02) |
| AOM | Second-line prescribing | Reference | 1.02 (0.87–1.19) | 0.69 (0.58–0.81) | 0.87 (0.75–0.99) | 0.78 (0.67–0.90) |
| Streptococcal pharyngitis | Second-line prescribing | Reference | 0.74 (0.53–1.00) | 0.62 (0.45–0.84) | 0.37 (0.24–0.56) | 0.63 (0.46–0.87) |
| Sinusitis | Second-line prescribing | Reference | 0.64 (0.45–0.91) | 0.47 (0.34–0.66) | 0.60 (0.45–0.80) | 0.49 (0.35–0.69) |
Fully engaged clinicians watched all available tutorials, webinars, and boosters and received feedback reports.
TABLE 4.
Rate Ratios of Antibiotic Prescribing During Visits to Less-Engaged Clinicians for ARTI Overall and by Condition (by Study Time Period)
| Condition | Measure | Baseline | Module 1 | Module 2 | Module 3 | Postintervention |
|---|---|---|---|---|---|---|
| aRR (95% CI) | ||||||
| ARTI overall | Any prescribing | Reference | 0.96 (0.89–1.03) | 0.88 (0.81–0.95) | 0.87 (0.82–0.93) | 0.93 (0.87–0.99) |
| Viral ARTI | Any prescribing | Reference | 0.40 (0.26–0.61) | 0.85 (0.62–1.15) | 0.70 (0.53–0.93) | 0.53 (0.39–0.72) |
| All pharyngitis | Any prescribing | Reference | 1.09 (0.97–1.21) | 1.02 (0.89–1.16) | 0.87 (0.75–1.00) | 0.98 (0.87–1.09) |
| AOM | Second-line prescribing | Reference | 0.99 (0.77–1.24) | 0.70 (0.52–0.92) | 1.17 (0.99–1.38) | 1.27 (1.07–1.47) |
| Streptococcal pharyngitis | Second-line prescribing | Reference | 0.58 (0.29–1.12) | 0.65 (0.31–1.33) | 0.98 (0.51–1.83) | 0.72 (0.40–1.27) |
| Sinusitis | Second-line prescribing | Reference | 1.13 (0.65–1.83) | 0.63 (0.34–1.11) | 0.90 (0.54–1.42) | 0.96 (0.57–1.54) |
Less-engaged clinicians only received feedback reports but did not engage fully with remaining intervention components. Module 1 contained online communication and antibiotic prescribing tutorials and webinars and an individualized antibiotic prescribing feedback report. Module 2 contained 2 online communication booster video vignettes and an antibiotic prescribing feedback report. Module 3 contained 1 communication booster video vignette and 2 antibiotic prescribing feedback reports.
Discussion
In this stepped-wedge, cluster-randomized clinical trial including >70 000 ARTI visits in practices across 9 states, the DART QI program decreased the overall rate of antibiotic prescribing among all ARTI visits, and this effect was sustained in the 2- to 8-month postintervention period. The DART QI program resulted in sustained reductions in antibiotic prescribing during viral ARTI visits and sustained decreases in second-line antibiotic prescribing during streptococcal pharyngitis and sinusitis visits. However, the intervention did not result in sustained reductions in antibiotic prescribing during all pharyngitis visits nor in reduced second-line antibiotic prescribing for AOM.
With this study, we build off work demonstrating that educational interventions combined with quarterly individualized antibiotic prescribing feedback could reduce broad-spectrum antibiotic prescribing for patients with pneumonia and sinusitis.9 However, that intervention did not reduce broad-spectrum antibiotic prescribing for pharyngitis or any antibiotic prescribing for viral ARTIs. Additionally, follow-up data revealed that antibiotic prescribing improvements reversed immediately after the study conclusion, and study pediatricians later reported ignoring or distrusting their feedback reports.10,29
Other randomized trials in adults have revealed that behavioral and communication intervention strategies can improve ambulatory antibiotic prescribing. The Stemming the Tide of Antibiotic Resistance educational program involved topics such as provision of guidelines and video communication skills training and led to significant reductions in antibiotic prescribing for all diagnoses during the year after intervention exposure.13 A separate study including 246 practices from 8 European practice-based research networks revealed that Internet-based communication skills training alone reduced antibiotic prescribing rates for adults with ARTIs by 9%.14 Similar to the DART QI program, these interventions allowed clinicians to access online components and practice communication skills at convenient times, a critical flexibility for busy primary care clinicians.
The DART QI intervention combines professionally produced, evidence-based educational modules that can be viewed asynchronously at clinicians’ discretion with individual feedback reports that also contain a peer comparison element. The DART training videos remain freely available online to interested clinicians (including study participants for intervention sustainability), although study clinicians no longer receive feedback reports.20 The 7% reduction in antibiotic prescribing for all ARTIs, if extrapolated to all ambulatory ARTI visits to pediatricians nationally, would represent >1.5 million fewer antibiotic prescriptions for children with ARTI annually.3 The DART QI intervention also resulted in lasting improvements in antibiotic prescribing for viral ARTI, streptococcal pharyngitis, and sinusitis. When examining only those visits to clinicians engaged in all intervention components, the DART QI intervention appears to improve antibiotic prescribing for AOM as well, suggesting that the addition of evidence-based communication and antibiotic prescribing education may be important to improving prescribing for this condition.
There are several important limitations to this study. First, 2 study clinicians each practiced at multiple practices that were randomized to different wedges. In the analysis, we assigned all visits after their first intervention as postintervention visits, biasing the results comparing pre- and postintervention visits toward the null. Because most of the intervention was received individually, the possibility of these 2 clinicians contaminating the intervention in their later-randomized practice is unlikely. Clinicians could also have altered their antibiotic prescribing habits because of a Hawthorne effect after study commencement, but our use of a prolonged baseline antibiotic prescribing period helped mitigate this issue. Because this study generated antibiotic prescribing feedback reports on the basis of ICD-10 diagnosis codes, clinicians could alter their choice of diagnosis codes over time (eg, coding a visit as sinusitis rather than as URI to justify antibiotic prescribing), but the proportion of bacterial ARTI diagnoses did not increase in a consistent or clinically meaningful way to suggest that such code-shifting occurred (Table 1). Practices may be unable to generate their own feedback reports, limiting generalizability of and the ability to disseminate this intervention.
Because rapid streptococcal antigen testing was not routinely captured in the EHR of all practices, we could not include this as part of our streptococcal pharyngitis visit definition. Our inclusion criteria would miss children who received antibiotics within 30 days before at outside clinics (eg, urgent care centers); however, those children would generally receive second-line antibiotics for ARTI, thereby decreasing the apparent intervention effect. Likewise, the intervention effect on antibiotic prescriptions provided outside ARTI clinic visits, and the changes in the antibiotic prescribing rate for AOM were not evaluated. In this study, we only evaluated the effect of the DART QI intervention on primary care pediatricians and nurse practitioners, but it is unknown whether these results can be generalized to others who provide care for children with ARTIs (eg, family practice, emergency department, or urgent care clinicians). Lastly, the 2- to 8-month postintervention period may not represent the true long-term intervention durability.
Conclusions
The DART QI program reduced overall antibiotic prescribing during childhood ARTI visits, and this antibiotic prescribing reduction was sustained during the postintervention period. Providing online communication training and evidence-based antibiotic prescribing education in combination with individualized antibiotic prescribing feedback reports may help achieve national goals of reducing unnecessary outpatient antibiotic prescribing for children.
Acknowledgments
We thank the practices, pediatricians, and nurse practitioners that participated in the DART study. The NorthShore practices were as follows (alphabetical order, listed with permission): Deerfield, Illinois; Evanston, Illinois (Central); Evanston, Illinois (Davis); Glenview, Illinois; Gurnee, Illinois; Lincolnwood, Niles, Old Orchard, Illinois; Plaza Del Lago, Illinois; Vernon Hills, Illinois. The PROS practices were as follows (alphabetical order, listed with permission): Advanced Preventive Care Pediatrics (Bradenton, FL); All Star Pediatrics (Countryside, IL); A to Z Pediatric and Youth Healthcare (Addison, IL); Cornerstone Pediatrics (Seguin, TX); East End Pediatrics, PC (East Hampton, NY); Eureka Pediatrics (Eureka, CA); Hampton Pediatrics, PLLC (Southampton, NY); Paragould Pediatrics, PLLC (Paragould, AR); Pediatric Medicine of Wallingford, LLP (Wallingford, CT); Pediatric Partners of the Southwest (Durango, CO); Plateau Pediatrics (Crossville, TN).
Glossary
- AAP
American Academy of Pediatrics
- AOM
acute otitis media
- aRR
adjusted rate ratio
- ARTI
acute respiratory tract infection
- CI
confidence interval
- DART
Dialogue Around Respiratory Illness Treatment
- EHR
electronic health record
- ICD-10
International Classification of Diseases, 10th Revision
- ITT
intention-to-treat
- PROS
Pediatric Research in Office Settings
- QI
quality improvement
- URI
upper respiratory infection
Footnotes
Deidentified data limited to visit-based prescribing rates, patient characteristics, and dummy variables for clinic site will be shared. No protected health information for study participants will be shared. Data will be provided as a comma-separated values file with a data dictionary defining all variables included in the file and will be transferred by using a secure file transfer protocol. Additional tools will not be made available. The data will be made available after publication of the primary studies to researchers who provide a detailed methodologically sound proposal and data use agreement. Proposals should be submitted to Dr Mangione-Smith (Rita.M.Mangione-Smith@kp.org).
Dr Mangione-Smith conceptualized and designed the study, obtained funding, assisted with study execution, analyzed and interpreted the data, drafted the initial manuscript, and provided study supervision; Dr Kronman assisted with study design and execution and data analysis and interpretation and drafted the initial manuscript; Dr Zhou assisted with study design, performed statistical analysis of the data, and drafted the initial manuscript; Drs Gerber, Grundmeier, Heritage, and Robinson assisted with study design and execution, interpreted the data, and critically revised the manuscript; Dr Fiks, assisted with study design and execution, data acquisition, and data interpretation and critically revised the manuscript; Drs Shalowitz, Stout, Shone, and Wright and Ms Steffes assisted with study design and execution and data acquisition, critically revised the manuscript, and provided administrative, technical, and material support; Mr Burges, Mr Hedrick, and Ms Warren critically revised the manuscript and provided administrative, technical, and material support; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
This trial has been registered at www.clinicaltrials.gov (identifier NCT02943551).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by the National Institutes of Health R01 HD084547-01 to Dr Mangione-Smith (principal investigator). Additional infrastructure funding was provided by the American Academy of Pediatrics and the Health Resources and Services Administration of the US Department of Health and Human Services under UA6MC15585, National Research Network to Improve Child Health. The information, content, and/or conclusions are those of the authors and should not be construed as the official position or policy of, nor should any endorsements be inferred by the Health Resources and Services Administration, Department of Health and Human Services, or US Government. Additionally, the funders and/or sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the article; or decision to submit the article for publication. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Hersh AL, Shapiro DJ, Pavia AT, Shah SS. Antibiotic prescribing in ambulatory pediatrics in the United States. Pediatrics. 2011;128(6):1053–1061 [DOI] [PubMed] [Google Scholar]
- 2.Fleming-Dutra KE, Hersh AL, Shapiro DJ, et al. Prevalence of inappropriate antibiotic prescriptions among US ambulatory care visits, 2010-2011. JAMA. 2016;315(17):1864–1873 [DOI] [PubMed] [Google Scholar]
- 3.Kronman MP, Zhou C, Mangione-Smith R. Bacterial prevalence and antimicrobial prescribing trends for acute respiratory tract infections. Pediatrics. 2014;134(4). Available at: www.pediatrics.org/cgi/content/full/134/4/e956 [DOI] [PubMed] [Google Scholar]
- 4.King LM, Bartoces M, Fleming-Dutra KE, Roberts RM, Hicks LA. Changes in US outpatient antibiotic prescriptions from 2011-2016. Clin Infect Dis. 2020;70(3):370–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National action plan for combating antibiotic-resistant bacteria. Available at: https://www.cdc.gov/drugresistance/pdf/national_action_plan_for_combating_antibotic-resistant_bacteria.pdf. Accessed March 14, 2019
- 6.Lieberthal AS, Carroll AE, Chonmaitree T, et al. The diagnosis and management of acute otitis media [published correction appears in Pediatrics. 2014;133(2):346]. Pediatrics. 2013;131(3). Available at: www.pediatrics.org/cgi/content/full/131/3/e964 [DOI] [PubMed] [Google Scholar]
- 7.Chow AW, Benninger MS, Brook I, et al. ; Infectious Diseases Society of America . IDSA clinical practice guideline for acute bacterial rhinosinusitis in children and adults. Clin Infect Dis. 2012;54(8):e72–e112 [DOI] [PubMed] [Google Scholar]
- 8.Shulman ST, Bisno AL, Clegg HW, et al. ; Infectious Diseases Society of America . Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America [published correction appears in Clin Infect Dis. 2014;58(10):1496]. Clin Infect Dis. 2012;55(10):e86–e102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerber JS, Prasad PA, Fiks AG, et al. Effect of an outpatient antimicrobial stewardship intervention on broad-spectrum antibiotic prescribing by primary care pediatricians: a randomized trial. JAMA. 2013;309(22):2345–2352 [DOI] [PubMed] [Google Scholar]
- 10.Gerber JS, Prasad PA, Fiks AG, et al. Durability of benefits of an outpatient antimicrobial stewardship intervention after discontinuation of audit and feedback. JAMA. 2014;312(23):2569–2570 [DOI] [PubMed] [Google Scholar]
- 11.Meeker D, Linder JA, Fox CR, et al. Effect of behavioral interventions on inappropriate antibiotic prescribing among primary care practices: a randomized clinical trial. JAMA. 2016;315(6):562–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Linder JA, Meeker D, Fox CR, et al. Effects of behavioral interventions on inappropriate antibiotic prescribing in primary care 12 months after stopping interventions. JAMA. 2017;318(14):1391–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Butler CC, Simpson SA, Dunstan F, et al. Effectiveness of multifaceted educational programme to reduce antibiotic dispensing in primary care: practice based randomised controlled trial. BMJ. 2012;344:d8173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Little P, Stuart B, Francis N, et al. ; GRACE Consortium . Effects of internet-based training on antibiotic prescribing rates for acute respiratory-tract infections: a multinational, cluster, randomised, factorial, controlled trial. Lancet. 2013;382(9899):1175–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mangione-Smith R, Elliott MN, Stivers T, McDonald LL, Heritage J. Ruling out the need for antibiotics: are we sending the right message? Arch Pediatr Adolesc Med. 2006;160(9):945–952 [DOI] [PubMed] [Google Scholar]
- 16.Stivers T. Non-antibiotic treatment recommendations: delivery formats and implications for parent resistance. Soc Sci Med. 2005;60(5):949–964 [DOI] [PubMed] [Google Scholar]
- 17.Mangione-Smith R, Zhou C, Robinson JD, Taylor JA, Elliott MN, Heritage J. Communication practices and antibiotic use for acute respiratory tract infections in children. Ann Fam Med. 2015;13(3):221–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sidnell J, Stivers T. The Handbook of Conversation Analysis. Hoboken, NJ: John Wiley & Sons; 2012 [Google Scholar]
- 19.Interactive Medical Training Resources, University of Washington Dialogue Around Respiratory Illness Treatment. Available at: https://www.uwimtr.org/dart/. Accessed July 8, 2020
- 20.Ajzen I, Madden TJ. Prediction of goal-directed behavior: attitudes, intentions, and perceived behavioral control. J Exp Soc Psychol. 1986;22(5):453–474 [Google Scholar]
- 21.Hersh AL, Jackson MA, Hicks LA; American Academy of Pediatrics Committee on Infectious Diseases . Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics. 2013;132(6):1146–1154 [DOI] [PubMed] [Google Scholar]
- 22.Gerber JS, Prasad PA, Localio AR, et al. Racial differences in antibiotic prescribing by primary care pediatricians. Pediatrics. 2013;131(4):677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hicks LA, Bartoces MG, Roberts RM, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9):1308–1316 [DOI] [PubMed] [Google Scholar]
- 24.Simon TD, Cawthon ML, Stanford S, et al. ; Center of Excellence on Quality of Care Measures for Children with Complex Needs (COE4CCN) Medical Complexity Working Group. Pediatric Medical Complexity Algorithm: a new method to stratify children by medical complexity. Pediatrics. 2014;133(6). Available at: www.pediatrics.org/cgi/content/full/133/6/e1647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon TD, Haaland W, Hawley K, Lambka K, Mangione-Smith R. Development and validation of the Pediatric Medical Complexity Algorithm (PMCA) version 3.0. Acad Pediatr. 2018;18(5):577–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Yu KF. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690–1691 [DOI] [PubMed] [Google Scholar]
- 27.Grant RL. Converting an odds ratio to a range of plausible relative risks for better communication of research findings [published correction appears in BMJ. 2014;348:g2124]. BMJ. 2014;348:f7450. [DOI] [PubMed] [Google Scholar]
- 28.R Core Team R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2019. Available at: https://www.R-project.org/. Accessed July 8, 2020
- 29.Szymczak JE, Feemster KA, Zaoutis TE, Gerber JS. Pediatrician perceptions of an outpatient antimicrobial stewardship intervention. Infect Control Hosp Epidemiol. 2014;35(suppl 3):S69–S78 [DOI] [PubMed] [Google Scholar]


