Abstract
Anemia commonly aggravates the severity of respiratory diseases, whereas thus far, few studies have elucidated the impact of anemia on coronavirus disease 2019 (COVID‐19). The aim of this study was to evaluate the clinical characteristics of patients with anemia, and to further explore the relationship between anemia and the severity of COVID‐19. In this single‐center, retrospective, observational study, a total of 222 confirmed patients admitted to Wuhan Ninth Hospital from 1 December 2019 to 20 March 2020 were recruited, including 79 patients with anemia and 143 patients without anemia. Clinical characteristics, laboratory findings, disease progression and prognosis were collected and analyzed. Risk factors associated with the severe illness in COVID‐19 were established by univariable and multivariable logistic regression models. In our cohort, compared to patients without anemia, patients with anemia were more likely to have one or more comorbidities and severe COVID‐19 illness. More patients demonstrated elevated levels of C‐reactive protein (CRP), procalcitonin (PCT) and creatinine in anemia group. Levels of erythrocyte sedimentation rate, D‐dimer, myoglobin, T‐pro brain natriuretic peptide (T‐pro‐BNP) and urea nitrogen in patients with anemia were significantly higher than those without. In addition, the proportion of patients with dyspnea, elevated CRP, and PCT was positively associated with the severity of anemia. The odd ratio of anemia related to the severe condition of COVID‐19 was 3.47 (95% confidence interval [CI]: 1.02‐11.75; P = .046) and 3.77 (95% CI: 1.33‐10.71; P = .013) after adjustment for baseline date and laboratory indices, respectively. Anemia is an independent risk factor associated with the severe illness of COVID‐19, and healthcare professionals should be more sensitive to the hemoglobin levels of COVID‐19 patients on admission. Awareness of anemia as a risk factor for COVID‐19 was of great significance.
Keywords: anemia, COVID‐19, hemoglobin, risk factor, severe illness
Highlights
Anemia is an independent risk factor associated with the severe illness of COVID‐19.
Covid‐19 patients with anemia exhibit severe inflammation response, which is positively associated with the severity of anemia.
Covid‐19 patients with anemia are older and exhibit severe organ injuries including poorer lung function, more serious myocardial injury and renal dysfunction.
Abbreviations
- ACE2
angiotensin‐converting enzyme 2
- ALP
alkaline phosphatase
- ALT
glutamic‐pyruvic transaminase
- AST
glutamic oxiracetam transaminase
- BUN
urea nitrogen
- CI
confidence interval
- CK‐MB
creatine kinase‐MB
- CKD
chronic kidney disease
- COPD
chronic obstructive pulmonary disease
- COVID‐19
coronavirus disease 2019
- CRP
C‐reactive protein
- CVD
chronic cardiovascular disease
- ESR
erythrocyte sedimentation rate
- FiO2
fraction of inspiration O2
- GGT
gamma glutamyl transpeptidase
- IQR
inter‐quartile range
- LDH
lactate dehydrogenase
- MERS‐CoV
Middle East respiratory syndrome coronavirus
- 2019‐nCoV
2019 novel coronavirus
- OR
odd ratio
- PaO2
partial pressure of oxygen
- PCT
procalcitonin
- RBC
red blood cell count
- RR
respiratory rate
- RT‐PCR
reverse‐transcription polymerase chain reaction
- SaO2
oxygen saturation
- SARS
severe acute respiratory syndrome
- SARS‐CoV
severe acute respiratory syndrome coronavirus
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus‐2
- SpO2
oxyhemoglobin saturation
- T‐pro‐BNP
T‐pro brain natriuretic peptide
- WBC
white blood cell count
- WHO
World Health Organization
1. BACKGROUND
Since the end of December 2019, clusters of cases of unexplained pneumonia linked to Huanan seafood market exposure have been reported in Wuhan, China. A novel member of the coronavirus family was identified in samples of bronchoalveolar lavage fluid from patients in Wuhan Jinyintan Hospital, 1 which was named severe acute respiratory syndrome coronavirus‐2 (SARS‐CoV‐2). 2 Based on next‐generation sequencing data, it has been shown that SARS‐CoV‐2 is similar to the SARS‐CoV and Middle East respiratory syndrome coronavirus, with 79% and 50% sequence identity, respectively. 3 Moreover, laboratory findings, together with the clinical manifestation of 2019 novel coronavirus‐infected pneumonia, are analogous to what has been described in cases of SARS. 4 Notably, person‐to‐person transmission was confirmed among close contacts. 5 Thus far, the outbreak has rapidly spread to over the world, and the number of confirmed cases continues to grow.
Anemia commonly aggravates the severity of respiratory diseases, and several studies have suggested that the prevalence of anemia was associated with poor outcomes and increased mortality in patients with community‐acquired pneumococcal pneumonia. 6 , 7 In 99 coronavirus disease 2019 (COVID‐19) patients transferred to Jinyintan Hospital, 51% of patients showed a decreasing tendency in hemoglobin levels. 8 In a study on 1099 laboratory‐confirmed COVID‐19 cases, it was shown that severe patients had significantly lower hemoglobin levels than those diagnosed as nonsevere cases. It should be noted that the decline in hemoglobin was more pronounced in patients who reached to the composite endpoint incorporating admission to the intensive care units (ICUs), or mechanical ventilation, or death, thus indicating that low hemoglobin levels might be related to poor progression and prognosis. 9 Therefore, anemia could possibly be a risk factor for severe disease in COVID‐19.
In this study, we aimed to evaluate the impact of anemia on the clinical course of COVID‐19 patients. We particularly focused on the differences between anemic patients and nonanaemic patients. We sought to reveal the relationship between anemia and the severity of COVID‐19 pneumonia, with the aim of contributing to the early recognition of disease severity and to extend the understanding of anemia in COVID‐19 patients.
2. METHOD
2.1. Study design and patient involvement
This retrospective, single‐center, observational study was performed at the Wuhan Ninth Hospital from 1 December 2019 to 20 March 2020. Patients admitted to Wuhan Ninth Hospital and diagnosed with 2019 novel coronavirus (2019‐nCoV)‐infected pneumonia according to the World Health Organization (WHO) interim guidance were enrolled in this study. Patients were excluded if: (a) they were younger than 18 years old; (b) had missing data on hemoglobin levels and outcomes. Finally, 222 patients were included. The study was approved by The Ethics Commission of Wuhan University People's Hospital (wdry2020‐k064), and written informed consent was obtained from all participants before enrollment.
2.2. Definitions
Based on the Diagnosis and Treatment Guideline of 2019 New Coronavirus Pneumonia issued by the Chinese National Health Committee (Trail Version Seven), confirmed patients in our study were classified as nonsevere or severe type. Patients were defined as being severe cases when they meet one of the following criteria: (a) respiratory distress, respiratory rate ≥30 times/minute; (b) oxyhemoglobin saturation (SpO2) less than 93% at rest; (c) partial pressure of oxygen/fraction of inspiration O2 (PaO2/FiO) ≤300 mm Hg; (d) have respiratory failure and need for mechanical assistance; shock; “extra pulmonary” organ failure, ICU is needed. Otherwise, the patients were diagnosed as nonsevere cases.
Based on the WHO definitions, anemia was defined as hemoglobin level of less than 120 g/L in women and less than 130 g/L in men. Based on hemoglobin value, anemia severity was categorized as mild (110‐119 g/L for women and 110‐129 g/L for men), moderate (80‐109 g/L) or severe (<80 g/L). 10
2.3. Data collection
We recorded demographic information, signs and symptoms, comorbidities, routine laboratory examinations, and outcomes. All of the information was extracted from electronic medical records, or through direct communication with patients and healthcare providers. Two physicians independently reviewed the data, and a third researcher decided whether there was any difference in data collection between the two primary reviewers. Patients’ hemoglobin levels were collected upon admission to the hospital and then patients were identified as either being anemic or not.
2.4. Laboratory measurements
Respiratory specimens were collected from patients suspected of being infected with 2019‐nCoV. Laboratory testing of the virus was performed using next‐generation sequencing or real‐time reverse‐transcription polymerase chain reaction in the clinical laboratory in Wuhan, as previously described. 9 Only laboratory‐confirmed patients were included in the study. Clinical laboratory tests were performed using conventional methods upon hospital admission, including laboratory assessments consisting of routine blood tests, coagulation profiles, inflammation profiles, cardiac function, liver function, and renal function. Routine blood tests were performed within 24 hours after admission. The first time of laboratory values for each patient were recorded in our study.
2.5. Statistical analysis
Continuous variables were presented as the median and interquartile range (IQR) and compared with t test or one‐way analysis of variance tests if they were normally distributed; otherwise, the Mann‐Whitney U test was used. Categorical variables were described as percentages and frequency rates, and compared by χ 2 test or Fisher's exact test, as appropriate. Univariate logistic regression analysis was adopted to evaluate independent risk factors related to disease severity. Variables were entered into a multivariate logistic regression model by a backward elimination procedure. SPSS (version 26.0) and R (version 3.5.3) were used for all statistical analyzes. A two‐sided α value of less than .05 was considered to indicate statistical significance. Statistical diagrams were drawn using GraphPad Prism (version 8.3.1) and R (version 3.5.3).
3. RESULTS
3.1. Demographic and clinical characteristics of COVID‐19 patients
A total of 375 patients were admitted to hospital, and 153 cases were excluded due to having negative reverse‐transcription polymerase chain reaction results (n = 144), age less than 18 (n = 3), missing data on hemoglobin levels (n = 3). Finally, a total of 222 cases, including 202 nonsevere patients and 20 severe patients, were included in our study. Among 222 patients, three died. Baseline characteristics are described in Table 1. The median age of the patients was 55 years (IQR: 42‐66 years); there were 80 (36.0%) male and 142 (64.0%) women. Overall, 81 (36.5%) patients had one or more comorbidities, of which hypertension (28.6%) was the most common one, followed by diabetes (12.2%), cardiovascular disease (CVD) (7.7%), and chronic obstructive pulmonary disease (COPD) (3.6%). The most common symptom at the onset of the illness was fever (59.5%), followed by cough (54.1%), expectoration (23.0%), weakness (18.0%), chest pain (15.3%), and dysponea (13.5%). A minority of patients initially presented with diarrhea (9.9%), myalgia (1.8%), and pharyngula (1.4%). Based on the guideline described previously, disease severity was graded as severe or nonsevere, respectively. As reported by previous studies, compared with nonsevere patients, severe cases were significantly older (54 vs 65 years, P = .004) and significantly more likely to suffer from underlying disorders (31.7% vs 85.0%, P = .000), such as hypertension, CVD, and COPD.
Table 1.
The baseline characteristics of COVID‐19 patients between severe group and nonsevere group
| Disease severity | ||||
|---|---|---|---|---|
| No./total no. (%) | All patients (n = 222) | Nonsevere (n = 202) | Severe (n = 20) | P value |
| Age, median (IQR), y | 55 (42‐66) | 54 (41‐66) | 65 (57‐81) | .004 |
| Sex—no., % | .699 | |||
| Male | 80 (36.0) | 72 (35.6) | 8 (40.0) | |
| Female | 142 (64.0) | 130 (64.4) | 12 (60.0) | |
| Signs and symptoms—no., % | ||||
| Fever | 132 (59.5) | 117 (57.9) | 15 (75.0) | .138 |
| Cough | 120 (54.1) | 108 (53.5) | 12 (60.0) | .576 |
| Expectoration | 51 (23.0) | 45 (22.3) | 6 (30.0) | .434 |
| Pharyngula | 3 (1.4) | 3 (1.5) | 0 (0.0) | 1.000 |
| Dyspnea | 30 (13.5) | 24 (11.9) | 6 (30.0) | .024 |
| Chest pain | 34 (15.3) | 30 (14.9) | 4 (20.0) | .542 |
| Myalgia | 4 (1.8) | 4 (2.0) | 0 (0.0) | 1.000 |
| Diarrhea | 22 (9.9) | 21 (10.4) | 1 (5.0) | .441 |
| Weakness | 40 (18.0) | 37 (18.3) | 3 (15.0) | .713 |
| Comorbidities—no., % | ||||
| Any comorbidities | 81 (36.5) | 64 (31.7) | 17 (85.0) | .000 |
| Hypertension | 63 (28.4) | 51 (25.2) | 12 (60.0) | .001 |
| Diabetes | 27 (12.2) | 25 (12.4) | 2 (10.0) | .756 |
| CKD | 3 (1.4) | 2 (1.0) | 1 (5.0) | .248 |
| CVD | 17 (7.7) | 11 (5.4) | 6 (30.0) | .000 |
| COPD | 8 (3.6) | 5 (2.5) | 3 (15.0) | .004 |
| Death | 3 (1.4) | 0 (0.0) | 3 (15.0) | .001 |
| Mechanical ventilation | 2 (0.9) | 0 (0.0) | 2 (10.0) | .008 |
Note: Data are presented as no./total no. (%) and median (IQR), P values were calculated by the Mann‐Whitney U test, t test, χ 2 test, or Fisher's exact test, as appropriate. P values denoted the comparison between severe and nonsevere groups.
Abbreviations: COVID‐19, coronavirus disease 2019; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, chronic cardiovascular disease; IQR, interquartile range.
3.2. Laboratory measurement of COVID‐19 patients
Laboratory tests of COVID‐19 patients were conducted at the time of admission, and the results are shown in Table 2. Patients with COVID‐19 presented with an inflammatory response combined with functional impairment of major organs. Differences were observed between the subgroups. Compared with nonsevere patients, some indices of coagulation, cardiac, renal, and liver function, such as D‐dimer, prothrombin time, lactate dehydrogenase (LDH), urea nitrogen (BUN), glutamic oxiracetam transaminase, total bilirubin, were significantly elevated in severe patients (all P < .05). Moreover, inflammatory biomarkers such as C‐reactive protein (CRP), procalcitonin (PCT), erythrocyte sedimentation rate (ESR), and neutrophil counts were higher in severe patients than in nonsevere cases. Notably, 79 (35.6%) patients met the diagnostic criteria for anemia. Consistent with previous research, in severe patients, hemoglobin levels showed a significant decline when compared to nonsevere patients (128 vs 111.5 g/L, P = .002). Further, significantly more patients in the severe group met the diagnostic criteria for anemia (32.2% vs 70.0%, P = .001), which is of great significance but can easily be ignored.
Table 2.
Laboratory findings of COVID‐19 patients on admission between severe group and nonsevere group
| Disease severity | ||||
|---|---|---|---|---|
| Median (IQR) | All patients (n = 222) | Nonsevere (n = 202) | Severe (n = 20) | P value |
| Blood routine | ||||
| White blood cell count, ×109/L | 6.2 (5.2‐7.5) | 6.2 (5.2‐7.5) | 6.4 (5.7‐8.4) | .346 |
| Lymphocyte count, ×109/L | 1.7 (1.3‐2.2) | 1.8 (1.4‐2.2) | 0.87 (0.71‐1.5) | .000 |
| Neutrophil count, ×109/L | 3.7 (3‐5) | 3.6 (3‐4.8) | 5.2 (3.5‐7.1) | .014 |
| Monocyte count, ×109/L | 0.47 (0.37‐0.57) | 0.47 (0.37‐0.56) | 0.48 (0.36‐0.61) | .884 |
| Eosinophil count, ×109/L | 0.08 (0.05‐0.16) | 0.09 (0.06‐0.16) | 0.03 (0.01‐0.08) | .000 |
| Basophils count, ×109/L | 0.01 (0.01‐0.02) | 0.01 (0.01‐0.02) | 0.01 (0‐0.02) | .050 |
| Red blood cell count, ×109/L | 4.3 (3.9‐4.6) | 4.3 (3.9‐4.6) | 3.8 (3.6‐4.2) | .002 |
| Hemoglobin, g/L | 127.0 (116.0‐137.0) | 128 (118‐137) | 111.5 (104‐128.3) | .002 |
| Hematocrit | 39.0 (35.7‐41.6) | 39.1 (36.3‐41.7) | 34.1 (31.9‐39.7) | .003 |
| Platelet count, ×109/L | 228.0 (183.0‐279.8) | 229.5 (188‐280.8) | 193.0 (169.5‐267.3) | .106 |
| PaO2, mm Hg | 92.5 (77.5‐138.8) | 89.0 (76.0‐131.0) | 95.0 (86.5‐186.5) | .292 |
| SaO2, % | 98.0 (97.0‐99.0) | 98.0 (97.0‐98.3) | 98.0 (97.0‐100.0) | .291 |
| Coagulation function | ||||
| Prothrombin time, s | 11 (10.7‐11.6) | 11 (10.6‐11.5) | 11.4 (11.1‐12.2) | .013 |
| Activated partial thrombin time, s | 27.4 (25.3‐30.2) | 27.4 (25.3‐30.1) | 28 (25.3‐33.4) | .510 |
| D‐dimer, μg/mL | 0.5 (0.3‐0.63) | 0.5 (0.3‐0.6) | 0.9 (0.5‐1.4) | .000 |
| Inflammation profile | ||||
| C‐reactive protein, mg/L | 1.9 (1.5‐3.9) | 1.8 (1.5‐3) | 46.8 (2.8‐76) | .000 |
| Procalcitonin, ng/mL | 0.02 (0.01‐0.07) | 0.02 (0.01‐0.06) | 0.06 (0.02‐0.46) | .005 |
| Blood lactic acid, mmol/L | 1.4 (1‐1.9) | 1.3 (1‐1.9) | 1.6 (1‐2) | .769 |
| ESR, mm/h | 11 (6.5‐23.5) | 10 (6‐21) | 42 (22.3‐73) | .005 |
| Cardiac function | ||||
| Myoglobin, ng/mL | 18 (11.2‐35.4) | 18 (11.2‐34.1) | 21.5 (10‐42.4) | .719 |
| CK‐MB, μg/mL | 2.6 (1‐7.5) | 2.6 (1.1‐8.5) | 2.5 (1‐4) | .476 |
| LDH, U/L | 178 (145.3‐211.8) | 173 (144.5‐203.5) | 261 (204‐395.5) | .000 |
| T‐pro‐BNP, pg/mL | 120 (16‐1422) | 88 (15‐489.8) | 524 (31.3‐1876.5) | .242 |
| Liver function | ||||
| ALT, U/L | 18 (13‐30.8) | 18 (13‐30) | 27 (11.8‐32.5) | .465 |
| AST, U/L | 22.5 (18‐28) | 22 (18‐28) | 31.5 (17.8‐48.3) | .027 |
| Total bilirubin, μmol/L | 10.1 (8.3‐13.3) | 9.8 (8.2‐13.1) | 14.5 (10.4‐18.5) | .001 |
| ALP, U/L | 83 (70‐102) | 83 (70‐101) | 79 (69.5‐103.3) | .704 |
| GGT, U/L | 20 (12.3‐30.8) | 18.5 (12‐28) | 27.5 (18.8‐40.3) | .064 |
| Renal function | ||||
| BUN, mmol/L | 4.4 (3.7‐5.8) | 4.3 (3.7‐5.6) | 5.9 (3.6‐7.5) | .044 |
| Creatinine ≥133 μmol/L—no., % | 6 (2.7) | 3 (1.5) | 3 (15.0) | .000 |
| Uric acid, μmol/L | 289 (240‐353) | 289 (236‐349) | 296 (259.5‐400.5) | .351 |
Note: Data are presented as no./total no. (%) and median (IQR), P values were calculated by the Mann‐Whitney U test, t test, χ 2 test, or Fisher's exact test, as appropriate. P values denoted the comparison between severe and nonsevere groups.
Abbreviations: ALP, alkaline phosphatase; ALT, glutamic‐pyruvic transaminase; AST, glutamic oxiracetam transaminase; BUN, urea nitrogen; CK‐MB, creatine kinase‐MB; COVID‐19, coronavirus disease 2019; ESR, erythrocyte sedimentation rate; GGT, gamma‐glutamyl transpeptidase; LDH, lactate dehydrogenase; PaO2, partial pressure of oxygen; SaO2, oxygen saturation; T‐pro‐BNP, T‐pro brain natriuretic peptide.
3.3. Comparisons between patients with anemia and without anemia
The diagnosis and severity of anemia were established based on the WHO definitions. 10 Hemoglobin levels of patients with and without anemia were 134 and 112 g/L, respectively (P = .000) (Figure 1A). In our study, the prevalence of severe illness in the anemic group was significantly higher than that in the nonanaemic group (8.1% vs 17.7%, P = .001) (Figure 1B). Tables 3 and 4 present the differences between the subgroups. Compared with patients without anemia, patients with anemia were older and more likely to have chronic kidney disease (0.0% vs 3.8%), CVD (3.5% vs 15.2%), and COPD (0.0% vs 10.1%) (all P < .05). Neither sex nor symptoms were significantly different between patients with anemia and those without. In terms of laboratory testing, COVID‐19 patients with anemia were predisposed to more severe inflammatory responses, coagulation disorders, and organ injuries. More patients demonstrated elevated levels of CRP (8.5% vs 24.7%), and PCT (1.3% vs 15.6%) in the anemic group (all P < .05) (Figure 1C,D). Beyond that, patients with anemia showed significantly higher levels of ESR, D‐dimer, myoglobin, T‐pro‐BNP, and BUN (all P < .05) (Figure 1E). Further, most indices of blood routine including white blood cell count (WBC), lymphocyte, neutrophils, eosinophils, red blood cell count (RBC), hematocrit, and platelet count were prominently lower in the anemic group compared to the nonanaemic group (all P < .05) (Figure 1F).
Figure 1.
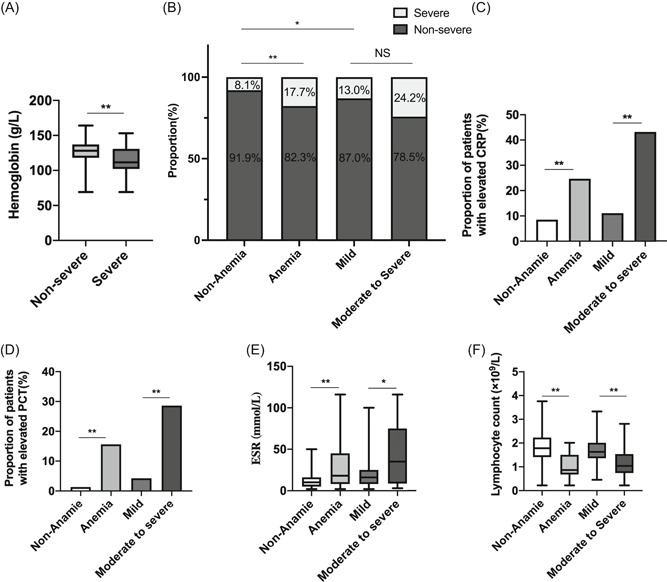
A, Hemoglobin levels between severe and nonsevere group. B, Prevalence of clinical subtypes of COVID‐19 severity among patients with and without anemia as well as patients with different severity of anemia. C‐F, Significant laboratorial findings including C‐reactive protein, erythrocyte sedimentation rate, and lymphocyte count among patients with and without anemia as well as patients with different severity of anemia. *P < .05; **P < .01; ***P < .001. COVID‐19, coronavirus disease 2019
Table 3.
The baseline characteristics of COVID‐19 patients with or without anemia on admission
| Anemia | Severity of anemia | |||||
|---|---|---|---|---|---|---|
| No./total no. (%) | No (n = 143) | Yes (n = 79) | P value | Mild (n = 46) | Moderate to severe (n = 33) | P value |
| Age, median (IQR), y | 51 (39.5‐63) | 64 (47.5‐74) | .000 | 63 (45.5‐68) | 66 (49‐78) | .146 |
| Sex—no., % | .471 | .946 | ||||
| Male | 54 (37.8) | 26 (32.9) | 15 (32.6) | 11 (33.3) | ||
| Female | 89 (62.2) | 53 (67.1) | 31 (67.4) | 22 (66.7) | ||
| Signs and symptoms—no., % | ||||||
| Fever | 86 (60.1) | 46 (58.2) | .781 | 26 (56.5) | 20 (60.6) | .717 |
| Cough | 79 (55.2) | 41 (51.9) | .632 | 23 (50.0) | 18 (54.4) | .690 |
| Expectoration | 37 (25.9) | 14 (17.7) | .167 | 8 (17.4) | 6 (18.2) | .928 |
| Pharyngula | 2 (1.4) | 1 (1.3) | 1.000 | 1 (2.2) | 0 (0.0) | 1.000 |
| Dyspnea | 19 (13.3) | 11 (13.9) | .894 | 3 (6.5) | 8 (24.2) | .025 |
| Chest pain | 22 (15.4) | 12 (15.2) | .969 | 7 (15.2) | 5 (15.2) | .994 |
| Myalgia | 3 (2.1) | 1 (1.3) | 1.000 | 1 (2.2) | 0 (0.0) | 1.000 |
| Diarrhea | 12 (8.4) | 10 (12.7) | .308 | 8 (17.4) | 2 (6.1) | .135 |
| Weakness | 25 (17.5) | 15 (19.0) | .780 | 8 (17.4) | 7 (21.2) | .669 |
| Comorbidities—no., % | ||||||
| Any comorbidities | 43 (30.1) | 38 (48.1) | .008 | 19 (41.3) | 19 (57.6) | .153 |
| Hypertension | 39 (27.3) | 24 (30.4) | .623 | 13 (28.3) | 11 (33.3) | .629 |
| Diabetes | 15 (10.5) | 12 (15.2) | .305 | 5 (10.9) | 7 (21.2) | .207 |
| CKD | 0 (0.0) | 3 (3.8) | .044 | 1 (2.2) | 2 (6.1) | .568 |
| CVD | 5 (3.5) | 12 (15.2) | .002 | 5 (10.9) | 7 (21.2) | .207 |
| COPD | 0 (0.0) | 8 (10.1) | .000 | 5 (10.9) | 3 (9.1) | 1.000 |
| Death | 0 (0.0) | 3 (3.8) | .044 | 1 (2.2) | 2 (6.1) | .568 |
| Mechanical ventilation | 0 (0.0) | 2 (2.5) | .126 | 1 (2.2) | 1 (3.0) | 1.000 |
Note: Data are presented as no./total no. (%) and median (IQR), P values were calculated by the Mann‐Whitney U test, t test, χ 2 test, or Fisher's exact test, as appropriate. P values denoted the comparison between anemic and nonanaemic groups, the comparison between patients with mild anemia and patients with moderate to severe anemia, respectively.
Abbreviations: COVID‐19, coronavirus disease 2019; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CVD, chronic cardiovascular disease; IQR, interquartile range.
Table 4.
The laboratory findings of COVID‐19 patients with or without anemia on admission
| Anemia | Severity of anemia | |||||
|---|---|---|---|---|---|---|
| Median (IQR) | No (n = 143) | Yes (n = 79) | P value | Mild (n = 46) | Moderate to severe (n=33) | P value |
| Blood routine | .078 | |||||
| White blood cell count, ×109/L | 6.6 (5.5‐7.7) | 5.8 (4.7‐6.8) | .001 | 5.9 (5.2‐6.8) | 5.1 (3.6‐6.8) | .000 |
| Lymphocyte count, ×109/L | 1.8 (1.4‐2.3) | 1.5 (1‐1.9) | .000 | 1.6 (1.4‐2) | 1 (0.8‐1.5) | .000 |
| Neutrophil count, ×109/L | 4.1 (3.2‐5.2) | 3.4 (2.6‐4.3) | .007 | 3.5 (3‐4.1) | 3.4 (2.3‐5.2) | .496 |
| Monocyte count, ×109/L | 0.48 (0.38‐0.58) | 0.45 (0.34‐0.56) | .125 | 0.46 (0.36‐0.56) | 0.44 (0.31‐0.55) | .315 |
| Eosinophil count, ×109/L | 0.1 (0.06‐0.16) | 0.07 (0.04‐0.14) | .039 | 0.08 (0.06‐0.16) | 0.04 (0.02‐0.08) | .012 |
| Basophils count, ×109/L | 0.01 (0.01‐0.02) | 0.01 (0.01‐0.02) | .926 | 0.01 (0.01‐0.02) | 0.01 (0.01‐0.02) | .051 |
| Red blood cell count, ×109/L | 4.4 (4.2‐4.7) | 3.8 (3.6‐3.9) | .000 | 3.9 (3.7‐4.1) | 3.6 (3.2‐3.8) | .000 |
| Hemoglobin, g/L | 134 (127‐140) | 112 (104‐117) | .000 | 116 (113‐118) | 101 (86‐106) | .000 |
| Hematocrit | 40.9 (39‐42.4) | 34.4 (32.4‐36.4) | .000 | 36 (34.6‐37.1) | 32.2 (28.5‐32.6) | .000 |
| Platelet count, ×109/L | 233 (198.5‐283.5) | 210 (169‐260.5) | .037 | 230 (181.5‐269) | 197 (151‐240) | .033 |
| PaO2, mm Hg | 87.5 (77.5‐103.5) | 95 (83‐163.8) | .099 | 155.5 (93.5‐238.5) | 80 (65.8‐103.3) | .002 |
| SaO2,% | 98 (96‐98) | 98 (97‐99) | .138 | 98.5 (97‐100) | 97.5 (95‐98) | .014 |
| Coagulation function | ||||||
| Prothrombin time, s | 10.9 (10.6‐11.4) | 11.3 (10.8‐11.9) | .002 | 11 (10.7‐11.6) | 11.6 (11.3‐12.3) | .003 |
| Activated partial thrombin time, s | 27.4 (25.4‐29.9) | 27.6 (25.1‐31.8) | .726 | 27.9 (25.6‐30.2) | 27.2 (24.8‐32.1) | .830 |
| D‐dimer, μg/mL | 0.4 (0.3‐0.5) | 0.5 (0.4‐0.93) | .003 | 0.5 (0.4‐0.6) | 0.8 (0.45‐2.3) | .011 |
| Inflammation profile | ||||||
| C‐reactive protein, mg/L | 1.9 (1.5‐3.5) | 1.9 (1.5‐8.2) | .476 | 1.9 (1.5‐3.5) | 1.9 (1.5‐8.2) | .318 |
| ≥10 mg/L—no., % | 12 (8.5) | 19 (24.7) | .001 | 5/45 (11.1) | 14/32 (43.8) | .001 |
| Procalcitonin, ng/mL | 0.01 (0.01‐0.05) | 0.04 (0.01‐0.15) | .011 | 0.01 (0.01‐0.05) | 0.04 (0.01‐0.15) | .060 |
| ≥0.5 ng/mL—no., % | 1 (1.3) | 7 (15.6) | .002 | 1/24 (4.2) | 6/21 (28.6) | .024 |
| Blood lactic acid, mmol/L | 1.4 (1.2‐2) | 1.2 (1‐1.8) | .316 | 1.6 (1.1‐1.8) | 1.1 (0.9‐1.8) | .295 |
| ESR, mm/h | 10 (5‐15) | 18 (8‐45) | .006 | 16 (8‐25) | 35 (9.5‐73) | .040 |
| Cardiac function | ||||||
| Myoglobin, ng/mL | 12.2 (8.9‐20.2) | 28.5 (15.3‐38.6) | .028 | 23.1 (15.4‐40.2) | 30.5 (17.3‐38) | .940 |
| CK‐MB, μg/mL | 2 (1‐9) | 3.1 (2‐5.2) | .565 | 3.4 (2.1‐7) | 3.1 (2.1‐4.1) | .622 |
| LDH, U/L | 178.5 (147.3‐206.3) | 177.5 (143.8‐212) | .788 | 177 (144.5‐206.5) | 186 (145.5‐228.5) | .442 |
| T‐pro‐BNP, pg/mL | 16 (15‐85) | 513 (81.5‐2894) | .000 | 309 (112.8‐900) | 1243.5 (47.3‐5943.3) | .275 |
| Liver function | ||||||
| ALT, U/L | 19 (14.5‐33.5) | 17 (11‐26.5) | .002 | 17.5 (11.3‐28.8) | 14 (10‐22) | .230 |
| AST, U/L | 23 (19‐28.5) | 21 (18‐28) | .255 | 21 (18‐28) | 20 (17‐28) | .992 |
| Total bilirubin, μmol/L | 10.2 (8.5‐13.5) | 9.5 (8.2‐13.2) | .408 | 9.1 (7.7‐12.1) | 10.6 (8.5‐14.7) | .116 |
| ALP, U/L | 87 (72‐102.8) | 79 (67.5‐100) | .070 | 80 (70.5‐98.8) | 73 (64‐102) | .426 |
| GGT, U/L | 21 (14‐31.5) | 16 (11.5‐27) | .025 | 17.5 (12‐24) | 15 (11‐33) | .877 |
| Renal function | ||||||
| BUN, mmol/L | 4.3 (3.7‐5.1) | 5.1 (3.8‐7.2) | .011 | 4.2 (3.7‐6.2) | 5.9 (3.8‐8.3) | .096 |
| Creatinine ≥133 μmol/L—no., % | 1 (0.7) | 5 (6.3) | .023 | 1 (2.2) | 4 (12.1) | .155 |
| Uric acid, μmol/L | 292 (250.8‐359) | 276 (230.5‐336.5) | .257 | 293.5 (236.5‐342.8) | 261 (216‐320) | .167 |
Note: Data are presented as no./total no. (%) and median (IQR), P values were calculated by the Mann‐Whitney U test, t test, χ 2 test, or Fisher's exact test, as appropriate. P values denoted the comparison between anemic and nonanaemic groups, the comparison between patients with mild anemia and patients with moderate to severe anemia, respectively.
Abbreviations: ALP, alkaline phosphatase; ALT, glutamic‐pyruvic transaminase; AST, glutamic oxiracetam transaminase; BUN, urea nitrogen; CK‐MB, creatine kinase‐MB; COVID‐19, coronavirus disease 2019; ESR, erythrocyte sedimentation rate; GGT, gamma‐glutamyl transpeptidase; LDH, lactate dehydrogenase; PaO2, partial pressure of oxygen; SaO2, oxygen saturation; T‐pro‐BNP, T‐pro brain natriuretic peptide.
3.4. Differences between patients with anemia in various severity
The severity of anemia was established based on the WHO definitions. 10 Among the 222 patients in our study, 46 patients were classified as having mild anemia, whereas 29 and 4 patients were classified as having moderate and severe anemia, respectively. Hemoglobin levels of the three groups were 116, 103, and 72 g/L, respectively. Compared with the mild anemia group, patients with moderate to severe anemia were more likely to present with dyspnea (24.2% vs 6.5%, P = .025), and had lower levels of PaO2 and SaO2 than patients with mild anemia (both P < .05), while no significant difference was found in the age, sex, comorbidities, proportion of severe patients, and mortality between the anemia subtypes (as is shown in Table 3, Figure 1B, and Table S1). For laboratory indices, we found that the severity of anemia was positively associated with inflammatory responses and coagulation disorders, whereas no significant relationship with organ injuries was observed. The prevalence of CRP, PCT beyond the normal range, and elevated levels of ESR and D‐dimer, were prominently higher in patients with moderate to severe anemia compared to patients with mild anemia (Figure 1C,D). Moreover, the absolute values of WBC, lymphocyte count (Figure 1E), eosinophils, RBC, platelet count, hematocrit, SO2, and PO2 gradually and significantly decreased as the anemia grade increased (all P < .05).
3.5. Associations between anemia and severe illness of COVID‐19
To assess whether anemia is a risk factors for the severe illness of COVID‐19, logistic regression analysis was performed. Based on the recent studies and our statistical results, some variables among the baseline data and laboratory findings were included in the logistic regression model. As summarized in Figure 2 and Table S2, in univariate analysis, baseline data including age ≥60 years, anemia, any com comorbidities, hypertension, CVD, COPD, and laboratory indices containing CRP ≥10 mg/L, LDH ≥250 U/L, and D‐dimer ≥0.5 mg/L, Creatinine ≥133 μmol/L were significantly associated with the increased disease severity in patients with COVID‐19. We further screened and selected the variables to be included in the multivariable logistic regression model. The multivariable analysis indicated that anemia remained significant as an independent risk factor for patients with severe COVID‐19, even after adjusting for baseline data (odd ratio [OR]: 3.47; 95% confidence interval [CI]: 1.02‐11.75; P = .046) and laboratory indexes (OR: 3.77; 95% CI: 1.33‐10.71; P = .013). However, anemia showed an insignificant relationship with the overall mortality of COVID‐19 patients in univariate analysis (P = .996), possibly because of the limited death toll in our cohort.
Figure 2.
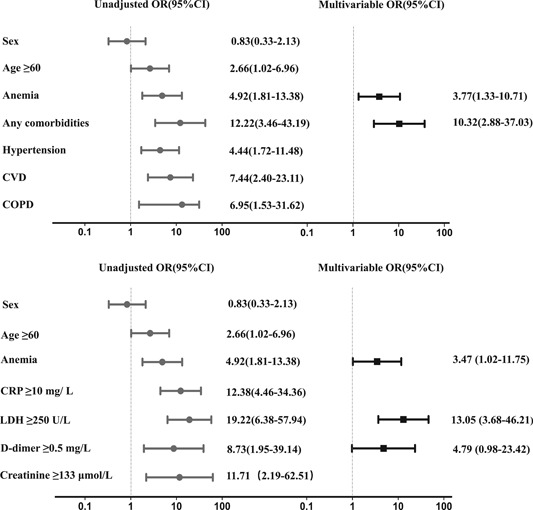
Univariate and multivariate logistic analysis associated with severe illness in COVID‐19. Odds ratios were calculated by univariate and multivariate logistic regression. The x‐axis is on a log scale. Variables with P < .05 were defined as potential risk factors and included in multivariate regression analysis by a backward elimination procedure. COPD, chronic obstructive pulmonary disease; COVID‐19, coronavirus disease 2019; CRP, C‐reactive protein; CVD, chronic cardiovascular disease; LDH: lactate dehydrogenase
4. DISCUSSION
We reported 222 patients with COVID‐19 in this cohort. The clinical and laboratory features of COVID‐19 patients were similar to those in other series. 11 In this retrospective cohort study, we mainly identified that COVID‐19 patients with anemia were more likely to develop severe conditions. It was shown that more patients died in the anemic group when compared to nonanaemiac group, however, none significant relationship was found between anemia and death in univariate regression analysis. Comorbidities were more commonly seen in patients with anemia. In addition, anemic patients were older and had a higher risk of severe inflammatory responses and organ injuries. Moreover, the severity of anemia was positively and strongly associated with more serious inflammatory responses. Our research also demonstrated that anemia is an independent risk factor associated with severe illness of COVID‐19.
Anemia is common among patients suffering from pneumonia, with nearly 7% to 12% in community‐acquired pneumonia and 31.8% in severe influenza A. 6 , 11 Zhou et al 12 in which 191 patients were enrolled, found that the frequency of anemia in COVID‐19 patients was 15%. In a cohort of 267 patients with severe acute respiratory syndrome, 16% had anemia at presentation, whereas the incidence increased to 53% during hospitalization. 13 In our study, the prevalence of anemia in hospitalized COVID‐19 patients was up to 35.5%, which is much higher than that reported by Zhou et al 12 Due to the limited literature on anemia among COVID‐19 patients, the accurate prevalence of anemia in patients with COVID‐19 remains unclear.
Anemia commonly aggravates the severity of respiratory diseases, and it has been documented that respiratory diseases combined with anemia are associated with poor outcomes and increased mortality. 6 , 7 Hitherto, few research has noted the clinical characteristics of COVID‐19 patients with anemia as well as the direct correlation between anemia and disease severity in patients with COVID‐19. It is worth noting the clinical characteristics in patients with and without anemia as well as in patients with different severities of anemia. Our study is the first investigation that exclusively and systematically focuses on anemia in COVID‐19 patients. We first described the clinical and laboratory characteristics of COVID‐19 patients with anemia and then further evaluated the impact of anemia on patients with COVID‐19.
The physiological mechanisms of the direct correlation observed in our cohort between anemia and COVID‐19 severity remained elusive. Previous investigations have revealed that anemic patients had poorer lung function than nonanaemic patients. 14 Additionally, it is well acknowledged that anemia and low hemoglobin could decrease oxygen delivery. Therefore, it is plausible to speculate that COVID‐19 patients with anemia were more susceptible to severe illness due to worse pulmonary function and poor tissue oxygenation. Despite the lack of significant differences in lung function‐related parameters between anemic patients and nonanaemic patients in our study, patients with moderate to severe anemia presented a prominently higher proportion of dyspnea symptoms and lower levels of PaO2 and SaO2 than patients with mild anemia. As shown in the recent autopsy reports of COVID‐19 patients, macrophages, neutrophils, and lymphocytes were observed in alveolar cavities. 15 Since the increasing degree of neutrophilic infiltration was more evident in patients with anemia, an elevated neutrophilic count might indicate serious pulmonary infiltration of inflammation, which might further degrade lung function.
In our study, myocardial injury and renal dysfunction were more remarkable in patients with anemia. The anemic group showed higher NT‐proBNP levels and a higher proportion of elevated creatinine cases compared to the nonanaemic group. A possible explanation for the underlying mechanism of anemia‐induced organ injuries is a progressive reduction in blood oxygen content and limited tissue oxygen delivery. 16 Indeed, it has been reported that the median NT‐proBNP concentration in patients with anemia was significantly higher than in those without anemia. 17 , 18 Guo et al 19 found that patients with underlying myocardial injury were more likely to have cardiac dysfunction during the course of COVID‐19, whereas cardiac dysfunction was significantly associated with fatal outcome of COVID‐19. Additionally, Pei et al 20 reported that renal complications in COVID‐19 were associated with poor mortality. Thus, myocardial injury and renal dysfunction might potentially contribute to the greater risk of severe condition in anemic patients with COVID‐19.
Iron requirements are essential to sustain hemoglobin synthesis, and these requirements are mostly satisfied by the iron recycling of senescent erythrocytes by macrophages. 21 Angiotensin‐converting enzyme 2, the well‐established receptor of SARS‐CoV‐2, was confirmed to be expressed in macrophages. 22 , 23 SARS‐CoV‐2 triggers macrophages to produce interleukin (IL)‐6, the essential driver of “cytokine storm syndrome.” 24 At the same time, IL‐6 increased hepcidin levels, causing iron‐restricted erythropoiesis and anemia of inflammation via the IL‐6/STAT3 pathway. 25 Although cytokines were not evaluated in our cohort, several studies have demonstrated that IL‐6 levels were significantly higher in severe patients than in nonsevere patients, and were also strikingly associated with the severity of COVID‐19. 26 , 27 In our study, we observed that a significantly larger percentage of patients in anemia group had elevated inflammation‐related indicators (eg, PCT, CRP). In addition, the severe anemia group showed a higher proportion of patients with elevated PCT and CRP levels than the mild anemia group. Therefore, it was rational to hypothesize that more severe inflammatory responses may explain why patients with anemia were more susceptible to a severe disease course in COVID‐19.
Anemia has been an independent risk factor for adverse outcomes in various diseases, including pneumonia, stroke, and heart failure. 28 , 29 It has been proven that pneumonia patients with anemia are at greater risk of poor outcomes and nosocomial infections in community‐acquired pneumonia, and influenza A. 6 , 7 , 30 In the study by Zhou et al, 12 COVID‐19 patients with anemia were more susceptible to death, nonsurvival group showed a higher proportion of patients with anemia when compared to survival group (26% vs 11%, P = .0094). In line with previous findings, anemia was an independent risk factor related to severe illness in COVID‐19 in our cohort. It has been shown 31 that comorbidities (eg, hypertension, CVD, and COPD) and old age are strong predictors of poor outcomes in COVID‐19. Our results also showed that hypertension, CVD, COPD, and old age were associated with greater COVID‐19 severity. It should be noted that, after adjustment for these risk factors, anemia still had a significant adverse impact on the clinical course of COVID‐19. Besides, anemia was still identified as a risk factor even after adjusting for laboratory findings of CRP ≥10 mg/L, LDH ≥250 U/L, D‐dimer ≥0.5 mg/L, and Creatinine ≥133 μmol/L in the multivariate logistic regression model. Therefore, healthcare professionals should be more sensitive to the hemoglobin level of COVID‐19 patients on admission. Awareness of anemia as a risk factor for COVID‐19 was of great significance.
Several limitations of this study should be acknowledged. First, only 20 patients with severe illness were included in our cohort, thus the interpretation of our findings might be limited by the relatively small sample size. Second, the diagnosis of anemia was made based on the levels of hemoglobin on admission, the exact cause and duration of anemia, however, remained unclear. Thus, it is difficult to verify whether that SARS‐CoV‐2 has a direct role in anemia, as well as if patients have anemia of chronic disease. Third, we had no information on the hemoglobin levels before admission and dynamic hemoglobin levels during hospitalization. Therefore, whether deterioration of COVID‐19 occurs along with persistently declined anemia is a question that merits further study.
5. CONCLUSION
COVID‐19 patients with anemia showed a higher rate of comorbidities, more severe inflammatory responses, and organ injuries when compared with the nonanaemic controls. The degree of inflammatory responses in COVID‐19 patients was positively associated with the severity of anemia. Moreover, anemia was an independent risk factor associated with severe illness in COVID‐19.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
ZY‐T analyzed the data and drafted the manuscript; MY‐L and JY‐W enrolled the patients in the project and collected the data; JX helped in the draft of the article; WC and ZT‐Y helped enrolled the patients in the project; XM‐X and LL helped collected the data; RW‐C and JY‐X helped in the data analysis. HM‐W edited the manuscript; JL‐L designed the research and revised the article. All authors read and approved the final manuscript.
Supporting information
Supporting information
Supporting information
ACKNOWLEDGMENTS
We would like to thank all the patients and guardians of those patients for their participation in the study. We would also like to acknowledge the professors and colleagues from Shanghai Jiao Tong University and including XiaoLing Xi (Ruijin hospital), Bin Tang (Ruijin hospital), YaNan Sun (Ruijin hospital), Yi Cheng (Ruijin hospital), QiHan Wu (Ruijin hospital). This study was supported by the National Natural Science Foundation of China (8177010121) and (8197010190) awarded to JL‐L, Medical‐engineering Cross Fundation of Shanghai Jiao Tong University “2019‐nCoV research project” (YG2020YQ30) awarded to JL‐L.
Tao Z, Xu J, Chen W, et al. Anemia is associated with severe illness in COVID‐19: A retrospective cohort study. J Med Virol. 2021;93:1478–1488. 10.1002/jmv.26444
Contributor Information
Mingyu Liu, Email: 1347110395@qq.com.
Jingyi Wu, Email: ajbriankevin@hotmail.com.
Huiming Wang, Email: rm000301@whu.edu.cn.
Jialin Liu, Email: ljl11243@rjh.com.cn.
REFERENCES
- 1. Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu N, Zhang D, Wang W, et al. Novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coronaviridae Study Group of the International Committee on Taxonomy Of V. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol, 2020(5):536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaki AM, Van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N Engl J Med. 2012;367:1814‐1820. [DOI] [PubMed] [Google Scholar]
- 5. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reade MC, Weissfeld L, Angus DC, Kellum JA, Milbrandt EB. The prevalence of anemia and its association with 90‐day mortality in hospitalized community‐acquired pneumonia. BMC Pulm Med. 2010;10:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Doshi SM, Rueda AM, Corrales‐Medina VF, Musher DM. Anemia and community‐acquired pneumococcal pneumonia. Infection. 2011;39:379‐383. [DOI] [PubMed] [Google Scholar]
- 8. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guan WJ, Zheng‐yi NI, Hu Y, et al. China Medical Treatment Expert Group for Covid‐19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. World Health Organization : Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. http://www.who.int/vmnis/indicators/haemoglobin_zh.pdf
- 11. Zhang JJ, Dong X, Cao YY, et al. Clinical characteristics of 140 patients infected with SARS‐CoV‐2 in Wuhan, China. Allergy. 2020;75(7):1730‐1741. [DOI] [PubMed] [Google Scholar]
- 12. Zhou F, Yu T, Du RH, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi KW, Chau TN, Tsang O, et al. Outcomes and prognostic factors in 267 patients with severe acute respiratory syndrome in Hong Kong. Ann Intern Med. 2003;139:715‐723. [DOI] [PubMed] [Google Scholar]
- 14. Von Drygalski A, Biller J. Anemia in cystic fibrosis: incidence, mechanisms, and association with pulmonary function and vitamin deficiency. Nutr Clin Pract. 2008;23:557‐563. [DOI] [PubMed] [Google Scholar]
- 15. Wang C, Xie J, Zhao L, et al. Alveolar macrophage dysfunction and cytokine storm in the pathogenesis of two severe COVID‐19 patients. EBioMedicine. 2020;57:102833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mistry N, Mazer CD, Sled JG, et al. Red blood cell antibody‐induced anemia causes differential degrees of tissue hypoxia in kidney and brain. Am J Physiol‐Reg I. 2018;314:R611‐R622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hogenhuis J, Voors AA, Jaarsma T, et al. Anaemia and renal dysfunction are independently associated with BNP and NT‐proBNP levels in patients with heart failure. Eur J Heart Fail. 2007;9:787‐794. [DOI] [PubMed] [Google Scholar]
- 18. Willis MS, Lee ES, Grenache DG. Effect of anemia on plasma concentrations of NT‐proBNP. Clin Chim Acta. 2005;358:175‐181. [DOI] [PubMed] [Google Scholar]
- 19. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pei G, Zhang Z, Peng J, et al. Renal involvement and early prognosis in patients with COVID‐19 pneumonia. J Am Soc Nephrol. 2020;31:1157‐1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell. 2017;168:344‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abassi Z, Knaney Y, Karram T, Heyman SN. The lung macrophage in SARS‐CoV‐2 infection: a friend or a foe? Front Immunol. 2020;11:1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and Is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID‐19: interleukin‐6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Camaschella C, Nai A, Silvestri L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica. 2020;105:260‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mcelvaney OJ, Mcevoy N, Mcelvaney OF, et al. Characterization of the inflammatory response to severe COVID‐19 illness. Am J Respir Crit Care Med. 2020;202:812‐821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID‐19 inpatients in Wuhan. J Allergy Clin Immunol. 2020;146(1):110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wei CC, Zhang ST, Tan G, Zhang SH, Liu M. Impact of anemia on in‐hospital complications after ischemic stroke. Eur J Neurol. 2018;25:768‐774. [DOI] [PubMed] [Google Scholar]
- 29. Tim Goodnough L, Comin‐Colet J, Leal‐Noval S, et al. Management of anemia in patients with congestive heart failure. Am J Hematol. 2017;92:88‐93. [DOI] [PubMed] [Google Scholar]
- 30. Moschovis PP, Banajeh S, Macleod WB, et al. Childhood anemia at high altitude: risk factors for poor outcomes in severe pneumonia. Pediatrics. 2013;132:E1156‐E1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Du RH, Liang LR, Yang CQ, et al. Predictors of mortality for patients with COVID‐19 pneumonia caused by SARS‐CoV‐2: a prospective cohort study. Eur Respir J. 2020;55:2000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Supporting information


