Summary
Background
Since the start of the COVID‐19 pandemic, there have been many scientific reports regarding gastrointestinal manifestations. Several reports indicate the possibility of viral shedding via faeces and the possibility of faecal‐oral transmission.
Aims
To critically assess the clinical relevance of testing stool samples and anal swabs and provide an overview of the potential faecal‐oral transmission of SARS‐CoV‐2.
Methods
A systematic literature search with MeSH terms was performed, scrutinising the Embase database, Google scholar, MEDLINE database through PubMed and The Cochrane Library, including articles from December 2019 until July 7 2020. Data were subsequently analysed with descriptive statistics.
Results
Ninety‐five studies were included in the qualitative analysis. 934/2149 (43%) patients tested positive for SARS‐CoV‐2 in stool samples or anal swabs, with positive test results up to 70 days after symptom onset. A meta‐analysis executed with studies of at least 10 patients revealed a pooled positive proportion of 51.8% (95% CI 43.8 ‐ 59.7%). Positive faecal samples of 282/443 patients (64%) remained positive for SARS‐CoV‐2 for a mean of 12.5 days, up to 33 days maximum, after respiratory samples became negative for SARS‐CoV‐2. Viable SARS‐CoV‐2 was found in 6/17 (35%) patients in whom this was specifically investigated.
Conclusions
Viral shedding of SARS‐CoV‐2 in stool samples occurs in a substantial proportion of patients, making faecal‐oral transmission plausible. Furthermore, detection in stool samples or anal swabs can persist long after negative respiratory testing. Therefore, stool sample or anal swab testing should be (re)considered in relation to decisions for isolating or discharging a patient.
Keywords: anal swab, Coronavirus, COVID‐19, faecal‐oral transmission, SARS‐CoV‐2, stool test
1. INTRODUCTION
Since December 2019, the world has been dealing with the outbreak of the novel Severe Acute Respiratory Syndrome CoronaVirus 2 (SARS‐CoV‐2) leading to Corona Virus Disease 2019 (COVID‐19) that emerged in Wuhan, China. The outbreak in this city led to a major world crisis, the COVID‐19 Pandemic. 1 , 2
SARS‐CoV‐2 is a non‐segmented positive‐sense RNA virus causing the third betacoronavirus outbreak of this century, which appears to have a higher transmission rate but is less deadly than the previous two; SARS‐CoV 2003 and Middle East Respiratory Syndrome (MERS) 2012. 3 , 4 Prior studies demonstrated that the genome sequence of SARS‐CoV‐2 is 79.5% identical to SARS‐CoV, whereas it shares 96.2% of its identity to the Coronavirus RaTG13 found in bats, but the intermediate reservoir has yet to be identified. 5
While patients infected with SARS‐CoV‐2 typically present with fever and respiratory symptoms, a rapidly increasing number of studies report patients presenting with a variety of gastrointestinal symptoms such as diarrhoea, vomiting and abdominal pain. 6
The established transmission route of SARS‐CoV‐2 is through respiratory droplets (aerosols), mainly during close person‐to‐person contact, 7 whereas numerous reports also mention the transmission by infected surfaces. Based on the spread through aerosols, the diagnosis of active COVID‐19 infection primarily relies on the detection of SARS‐CoV‐2 viral RNA in specimens from the upper respiratory tract (URT; nasopharyngeal and oropharyngeal cavity) and/or lower respiratory specimens (LRT; sputum and/or bronchoalveolar lavage). 8 , 9
Knowledge about SARS‐CoV‐2’s other potential routes of transmission and the significance of different methods of testing is relatively sparse, 10 partly as a result of the novelty of this virus. However, there is a growing body of studies in which SARS‐CoV‐2 RNA was detected in stool samples (including anal swabs) from COVID‐19 patients. 11 These findings support the possibility of a faecal‐oral route of transmission. Interestingly, stool tests seem to remain positive when respiratory tests are, or have become, negative. 12 , 13 , 14
A few articles have briefly reviewed the rapidly increasing body of knowledge on the potential for faecal‐oral transmission. 11 , 15 , 16
This study aims to (1) critically assess the clinical relevance of testing stool samples and anal swabs and (2) provide a critical overview of the available literature regarding the faecal‐oral transmission of SARS‐CoV‐2.
2. METHODS
2.1. Literature Search
This systematic literature search was performed following the PRISMA guidelines and conducted using the Embase database, Google scholar, The MEDLINE database through PubMed and The Cochrane Library from the outbreak in December 2019 until the 17 June 2020. The search strategy can be found in Online Supplement 1.
All articles were imported to Mendeley (version 1.17.6), and duplicates were removed. Extensive cross‐checking of reference lists of the included articles and other reviews was performed. As a result of the rapidly evolving research field concerning COVID −19, we also included journal pre‐proof articles.
2.2. Study selection
All articles were screened based on title and abstract. Studies were included when the following inclusion criteria were met:
Study population: Human COVID‐19 patients (both adult and paediatric patients) tested for COVID‐19 in gastrointestinal specimens (eg stool samples or anal swabs);
Study design: case reports/case series, cohort studies, case‐control studies and randomised controlled trials.
We excluded articles written before December 2019, when the article or abstract/outcomes were not available in English, Dutch or German and when the results or quality of data were ambiguous. Papers written in Chinese, of which the abstract contained sufficient data to provide answers to our research questions, were included for analysis and data extraction. We excluded articles in which follow‐up data were insufficient (ie when results of stool testing were not mentioned). Review articles were not included, however, reference lists were scrutinised for additional articles.
2.3. Data extraction
We collected the following data from the eligible original articles: study design, geographic location, study period, number of patients, age, types of tested specimens, number of tested specimens, methods of the performed tests, duration and prevalence of positive test results in different specimens, disease severity, gastrointestinal symptoms, endoscopic results, specific evidence supporting faecal‐oral transmission and remarkable patient/population characteristics.
Data were subsequently analysed with descriptive statistics. Relevant data were tabulated with a subdivision by study population size. All studies with population of at least 10 patients were included in the meta‐analysis.
2.4. Statistical analysis
A weighted pooled estimate of the proportion testing positive from the stool samples was calculated using the Freeman‐Tukey arcsine square root transformation under a random effects model. This analysis was undertaken using MedCalc® v19.4.0. The heterogeneity in the estimates between studies was statistically tested using Cochran's Q statistic and summarised as I2.
3. RESULTS
3.1. Search Strategy
The search strategy resulted in 300 articles suitable for title and abstract screening. After the exclusion of articles which met the exclusion criteria, we included a total of 95 articles for final analysis. Figure 1 shows details of the selection procedure.
Figure 1.
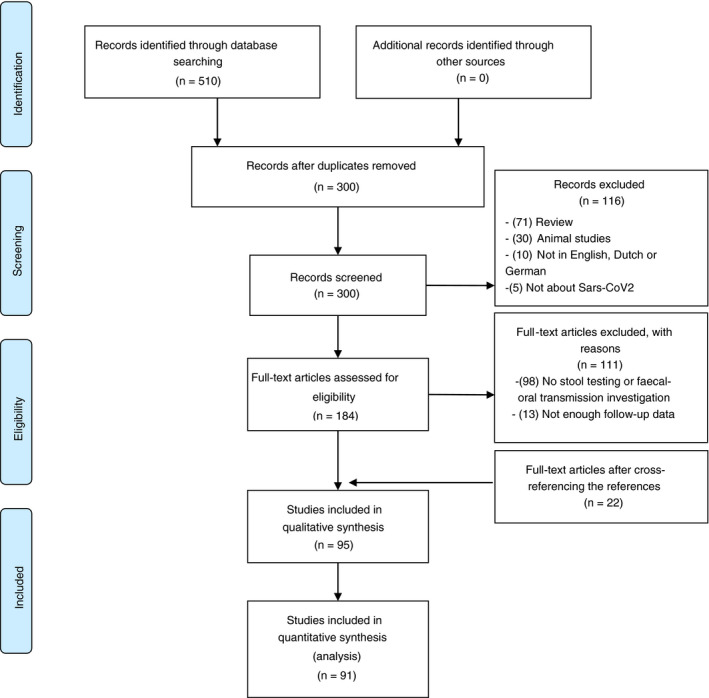
Flowchart of included articles
The majority of the included studies were performed in China (74 (77%)), other studies were conducted in Korea (6), Singapore (2), the United States of America (5), Italy (4), France (1), Germany (1), Thailand (1) and Austria (1). All included studies had a case report/case series design. In most study populations, the subpopulation on which stool and/or anal testing were conducted was considerably lower. In total, stool samples or anal swabs (from now on collectively named as GI specimens) from 2175 patients were tested for SARS‐CoV‐2 RNA. Four studies were included for qualitative analysis, but due to the lack of necessary (follow‐up) information, these studies were excluded before final quantitative analysis. 17 , 18 , 19 , 20 Therefore, 2149 patients were included for final analysis.
In 23 studies, only children were included, of which four studies did not specify the age of included children. In 43 studies, only adults were included, whereas 12 studies included both children and adults. In the remaining 17 studies, the range of age was not reported. Detailed study characteristics are depicted in Tables 1 and 2, with subdivision by study population size (Table 1: n < 10 and Table 2: n ≥ 10).
Table 1.
All studies with study population less than 10 Covid‐19 patients
| Study | Country of origin | Number of patients included |
Age of included patients Average ± (SD) or median (range) in years |
Type of GI specimens (S‐stool sample A‐anal swab) |
Number of positive patients (GI specimens) Npositive/Ntotal (%) CT mean (SD) |
COVID‐19 diagnosis based on |
Positive stool but (converted) Negative respiratory test Npositive S negative O/Ntotal positive S (%) |
Max. Duration positive stool (d) |
Time differences between negative respiratory test and negative stool test (d) Mean (range) |
|---|---|---|---|---|---|---|---|---|---|
| Cai 29 | China | 6 | 6.2 (0.25‐10.9) | S |
5/6 (83) |
URT and/or LRT a PCR | 5/5 | 30 | 12 (11‐18) |
| Tang 24 | China | 1 | 10 | S | 1/1 | NS | 1/1 | 25 | NS |
| Young 58 | Singapore | 8 | 47 (31‐73) | S | 4/8 (50) | NPS PCR | 1/4 (25) | NS |
4 |
| Chan 59 | China | 4 | 50 (10‐66) | S | 0 | NS | 0/4 | NA | NA |
| Kam 30 | Singapore | 1 | 0.5 | S | 1/1 | NPS PCR | NS | 9 | NA |
| Zhang Y 21 | China | 1 | NA | S | 1/1 | NS | NA | 15 | NA |
| Zhang JF 60 | China | 1 | 54 | S | 1 | NPS PCR | NS | 25 | NS |
| Zhang B 31 | China | 7 | 26 (0.83 ‐ 35) | A | 6/7 (86) | URT and/or LRT a PCR | 5/6 (83) | 44 | 21.3 (14‐31) |
| Holshue 38 | USA | 1 | 35 | S | 1/1 | NPS PCR | 0 | NA | NA |
| Park JY 32 | Korea | 1 | 10 | S | 1/1 | NS | 1/1 | 17 | 4 |
| China | NA | S | 7/7 | NS | 3/7 (43) | NS | 6 (3‐7) | ||
| China | 1 | Neonate | A | 1/1 | NS | 1/1 | NS | NS | |
| Zhang T 62 | China | 3 | 7.7 (6‐9) |
S |
3/3 | URT and/or LRT a PCR |
3/3 |
10 | 19 (17‐21) |
| Jiang 25 | China | 1 | 8 |
A |
1/1 | NS | 2/2 | 42 | NS |
| Li J 33 | Korea | 1 | 0.67 | A | 1/1 | NS | 1/1 | 14 | NS |
| Wu Y 63 | China | 9 | 26 ‐ 40 |
Maternal S |
1/9 | NPS PCR | NA | NA | NA |
| Lei 64 | China | 7 | 43.2 (14.0) | S | 4/7 (57) | URT and/or LRT a PCR | 2/4 (25) | 16 | 5 ‐ 6 |
| Xing YH 65 | China | 3 | 4.2 (1.5‐6) | S | 3/3 | URT and/or LRT a PCR | 3/3 | 30 | 16 (8‐20) |
| Fan 66 | China | 1 | 0.25 | A | 1/1 | NPS PCR | 1/1 | 28 | 14 |
| Chen Equation 67 | China | 1 | 34 | S | 0 | Clinical suspicion | NA | NA | NA |
| Chen L 26 | China | 1 | 25 | S | 1/1 | NS | 1/1 | 11 | NS |
| Lescure 34 | France | 5 | 47 (31‐80) | S | 2/5 (40) | URT and/or LRT a PCR | ½ (50) | 19 | 7 |
| Nicastri 68 | Italy | 1 | Late 20s | S | 1/1 | URT and/or LRT a PCR | 0/1 | 13 | NA |
| Peng 69 | China | 9 | 38.9 (27‐62) | A | 2/9 (22) | URT and/or LRT a PCR | NA | NA | NA |
| Song 70 | China | 1 | Middle aged | A | 0 | NS | NA | NA | NA |
| Tan LV 71 | China | 1 | 73 | A | 1/1 | NS | 1/1 | 23 | 7 |
| Xie C 72 | China | 9 | 34 (18‐62) | S | 8/9 (89) | URT PCR | NA | NA | NA |
| Thammathiwat 73 | Thailand | 1 | 58 | S | 1/1 (100) | NPS PCR | NA | NA | NA |
| Zou B 74 | China | 2 | 2, 13 | S | 2/2 (100) | Serology | 2/2 (100) | 24 | NS |
| Shen 75 | China | 7 | 51 (15‐88) | S | 6/7 (86) | URT PCR | NA | 29 | NA |
| Zhou Y 76 | China | 9 | 53 (37‐70) | S | 9/9 (100) | NPS PCR | NA | NA | NA |
| Chen X 77 | China | 1 | 7 | S | 1/1 (100) | NPS PCR | 0 | 5 | NA |
| Kim JY 78 | Korea | 2 | 35, 55 | S | 0/2 | NPS PCR | NA | NA | NA |
| Liu 79 | China | 9 | NS | S | 8/9 (89) | NPS PCR | 8/8 (100) | 46 | 23 |
| Wang Q 80 | China | 5 | 42 (35‐56) | S | 5/5 (100) | NPS PCR | NA | 30 | NA |
| Huang R 81 | China | 2 | 35, 54 | A | 1/2 (50) | URT PCR | NA | NA | NA |
| Zhou J 47 | China | 1 | 68 | S | 1/1 | NS | NA | NA | NA |
| Yin 82 | China | 8 | 54 (40 ‐ 72) | S | 8/8 | Laboratory confirmed | NA | NA | NA |
| Mao 83 | China | 1 | 1.2 | S | 1/1 | NPS PCR | NA | 28 | NA |
| Wang X 84 | China | 3 | 31.6 (24 ‐ 40) | S | 3/3 | URT PCR | 3/3 | 40 | 10.3 (11 ‐ 15) |
| Xu T 27 | China | 1 | NA | S | 1/1 | NS | 1/1 | NA | NS |
| Hu 85 | China | 3 | NA | S | 3/3 | NPS PCR | 2/3 (67) | 29 | 5‐15 |
| Tan Y 86 | China | 4 | 8 (3‐9) | S | 3/4 (75) | URT PCR | 1/3 (33) | 17 | 10 |
| Han 87 | Korea | 1 | 27d | S | 1/1 | NPS PCR | 1/1 | 18 | 1 |
| Xing Y 88 | China | 3 | NA | S | 3/3 | NPS PCR | 3/3 | NA | 16 (8‐20) |
| Cozzi 39 | Italy | 2 | 46‐71 | S | 1/2 | NPS PCR | 0 | NA | NA |
| Wang C 89 | China | 1 | 50 | S | 1/1 | NPS PCR | 1 | 35 | 22 |
| Wölfel 48 | Germany | 9 | NA | S | 8/9 (89) | NPS PCR | 6/8 (75) | NA | NS |
Abbreviations: URT, Upper respiratory tract; LRT, Lower respiratory tract; SP, Sputum; OS, Oral Sample; NPS, Nasopharyngeal Sample; TS, Throat Swab; NA, Not Applicable; GI, Gastrointestinal; PCR, polymerase chain reaction; SD, standard deviation; NS, not specified
World Health Organization Guidance recommends collection of upper respiratory tract (URT) specimens (nasopharyngeal and oropharyngeal) and, where clinical suspicion remains and URT specimens are negative, to collect specimens from the lower respiratory tract (LRT) when readily available (expectorated sputum, or endotracheal aspirate/bronchoalveolar lavage in ventilated patient).
Data extracted from abstract; full‐article only available in Chinese.
Table 2.
All studies with study population more than 9 Covid‐19 patients
| Study | Country of origin | Number of patients included |
Age of included patients Average ± SD/ median (range) in years |
Type of GI specimens (S‐stool sample A‐anal swab) |
Number of positive patients (GI specimens) Npositive/Ntotal (%) CT mean (SD) |
COVID‐19 diagnosis based on |
Positive stool but (converted) Negative respiratory test Npositive S negative O/Ntotal positive S (%) |
Max. Duration positive stool (d) |
Time differences between negative respiratory test and negative stool test (d) Mean (range) |
|---|---|---|---|---|---|---|---|---|---|
| Wang W 19 | China | 153 | 44 (5‐67) | S |
44/153 (29) |
“Based on symptoms and radiology and confirmed by SARS‐CoV‐2 detection” | Yesα | NA | NS |
| Zhang JC 40 | China | 14 | 41 (18‐87) | S | 5/14 (36) | NS |
3/5 (60) |
13 | NS |
| Zhang W 35 | China | 16 | NA | A | 10/16 (63) | NPS PCR | 6/10 (60) | NA | NS |
| Xiao F, Tang M 41 | China | 73 | 43 (0.83‐78) | S | 39/73 (53) | NPS PCR | 17/39 (44) | 12 | NS |
|
Kujawski 42 |
USA | 10 | 53 (21‐68) | S | 7/10 (70) | NPS, URT and/or LRT a PCR |
NS+ 3/7 (43) mean 3.3 (0‐5) days difference ORS+ 5/7 (71) mean 3.2 (0‐13) |
25 | NS |
| Ling 12 | China | 66 | 44.0 (34.0‐62.0) | S |
55/66 (82) |
NS | 43/55 (78) | 16 |
2.0 (1.0‐4.0) |
| Chen W 90 | China | 28 | NA | A | 11/28 (39) | NS | Yesα | Max. 13 | NS |
| Wu Yongjian 13 | China | 74 | 41.29 ± 3.14 | S |
41/74 (55) |
URT and/or LRT a PCR | 32/41 (78) | 47 | Mean: 11.2 (1‐ 33) |
| Xu Y 43 | China | 10 | 6.6 (0.17‐15) | A | 8/10 (80) | NPS PCR | 8/8 | 26 | 17 (2‐19) |
| Han 44 | China | 22 | 43.3 (27‐71) | S | 12/22 (55) | NS | NA | NA | NA |
| Chen Chen 36 | China | 19 | 36.5 (2‐64) | S | 12/19 (63) | NPS PCR | 9/12 (75) | 24 | 4.7 (1‐10) |
| Lin Lu 45 | China | 65 | 45.3 ± 18.3 | S | 31/65 (48) | NPS PCR | NA | NA | NA |
| Chen Y 14 | China | 42 | 51 (42.75‐62) | S | 28/42 (67) | URT and/or LRT a PCR | 18/28 (64) | 23 |
7 (6‐10) |
| Cheung 46 | China | 59 | 58.5 (22‐96) | S | 9/59 (15) | NS | NA | NA | NA |
| China | 13 | Children | S |
NA |
NS | Yesα | NA | 12 | |
| Wu J 20 | China | NS | 66.7 ± 9.1 years | A & S |
132 patients Total of tests: A+ 12/120 (10) S+ 24/244 (10) |
NPS PCR |
Yesα |
NS | NS |
| Ma X 37 | China | 27 |
6 children 4.7 (0.92‐9) 2 adults 33 and 39 |
S | 8/27 | NS | 8/8 | 35 | 14.6‐27.4 |
| Pan Y 91 | China | 17 | NA | S | 9/17 (53) | NS | NA | NA | NA |
| Lo 92 | China | 10 | 54 (27 ‐ 64) | S | 10/10 | URT and/or LRT a PCR | 2/10 (20) | 19 | 2 ‐ 3 |
| Xiao Fei, Sun J 49 | China | 28 | NA | S | 12/28 (43) | NS | NA | NA | NA |
| Yuan 28 | China | 78 |
Single A+ 6.2 (2.7‐8.3) Single TS+ 7.5 (3.3‐11.7) |
A |
41/78 (53) |
NPS PCR | 17/41 (41) | 23 | NS |
| Zhang N 22 | China | 12 | 48.0 (40‐62) | S | 10/12 (83) | NS | NS | 25 | 26 |
| Zuo 55 | China | 15 | 55 (44‐67.5) | S | 11/15 (73) | NS | NA | 37 | NA |
| Park S 93 | Korea | 36 | 26 (18‐57) | S | 2/46 (4) | URT and/or LRT a PCR | No | 50 | NA |
| Deng L 94 | China | 56 | >18 | S | 25/56 (45) | NPS PCR | 4/25 (16) | 7 | NS |
| Wu B, 95 b | China | 36 | 49 (17‐86) | S/A | 20/36 (56) | NS | NA | NA | NA |
| Guan 96 | China | 62 | 68 (44‐77) | S | 4/62 (6) | URT and/or LRT a PCR | 1/4 (25) | NA | NS |
| Szymczak 97 | USA | 77 | NA | S | 27/77 (35) | URT and/or LRT a PCR | NA | 33 | NA |
| Shi D 98 | China | 99 | 54 (IQR 39‐64) | S | 21/99 (21) | URT and/or LRT a PCR | NA | NA | NA |
| Mesoraca 99 | Italy | 15 | NA | S | 11/15 (73) | URT and/or LRT a PCR | 10/11 (89) | 40 | NS |
| Chen Z 100 | China | 32 | 9.5 (3mo – 18y) | S/A | 17/32 (53) | NPS PCR | NA | 65 | 13.1 |
| Guo 101 | China | 23 | 20‐62 | S | 11/23 (48) | NPS PCR | NA | NA | NA |
| Deng W 102 | China | 61 | 55 | S | 17/61 (28) | NPS PCR | 14/17 (82) | NA | 14 |
| Du 103 | China | 10 | 5 (1‐14) | S | 7/10 (70) | URT and/or LRT a PCR | 7/7 (100) | Median 34 | 25 |
| Hua 23 | China | 35 | 8 (0.25 ‐ 14) | S | 32/35 (91) | URT and/or LRT a PCR | NA | 70 | NA |
| Han 104 | Korea | 12 | 6.5 (0.01‐16) | S | 11/12 (92) | NPS PCR | NA | NA | NA |
| De Ioris 105 | Italy | 22 | 7 (0 ‐ 18) | S | 15/22 (68) | NPS PCR | 6/9 (67) | 14 | NS |
| Zhao 106 | China | 401 | NA | A | 80/401 (20) | Clinical suspicion | NA | 49 | NA |
| Perchetti 107 | USA | 20 | NA | S | 13/20 (65) | NPS PCR | NA | NA | NA |
| Lu 108 | USA | 28 | NA | S | 7/28 (25) | NS | NA | NA | NA |
| Wu Q 109 | China | 10 | 6 (0.10 ‐ 15.1) | S | 10/10 | NPS PCR | 8/10 | 23 | 11 (5 ‐ 23) |
| Zheng 110 | China | 96 | 55 (IQR 44.3‐64.8) | S | 57/96 (59) | URT and/or LRT a PCR | NA | 59 | NA |
| Yun 111 | China | 32 | 50 (IQR 37‐66) | S | 8/32 (25) | NPS PCR | NA | NA | NA |
| Effenberger 112 | Austria | 40 | NA | S | 12/40 (30) | NPS PCR | NA | NA | NA |
| Li Y 113 | China | 13 | 52.8 ± 20.2 | S | 5/13 (83) | NPS PCR | 2/5 (40) | 24 | 14‐15 |
| Huang J 114 | China | 33 | 47 (2 ‐ 84) | S | 30/33 (91) | NPS PCR | NA | NA | NA |
| Yongchen 115 | China | 15 | 37 (10 ‐ 37) | S | 5/15 (33) | NPS PCR | 4/5 (80) | 30 | 8 (2‐17) |
Abbreviations: URT, Upper respiratory tract; LRT, Lower respiratory tract; SP, Sputum; OS, Oral Sample; NPS, Nasopharyngeal Sample; TS, Throat Swab; NA, Not Applicable; GI, Gastrointestinal; PCR, polymerase chain reaction; SD, standard deviation; NS, not specified
World Health Organization Guidance recommends collection of upper respiratory tract (URT) specimens (nasopharyngeal and oropharyngeal) and, where clinical suspicion remains and URT specimens are negative, to collect specimens from the lower respiratory tract (LRT) when readily available (expectorated sputum, or endotracheal aspirate/bronchoalveolar lavage in ventilated patient).
Data extracted from abstract; full‐article only available in Chinese.
3.2. Test Characteristics
Seventeen (18%) studies tested SARS‐CoV‐2 presence in anal swabs and 81 (85%) in stool samples. In three studies, both specimens were tested. In all studies but one, real‐time reverse transcription polymerase chain reaction (RT‐PCR) was used to detect SARS‐CoV‐2. One study performed inoculation of stool suspension into Vero cells followed by virus detection through electron microscopy. 21
3.3. Outcomes
In 91/95 (96%) of the included studies, SARS‐CoV‐2 RNA was identified in GI specimens from at least one of the included patients (Tables 1 & 2). In total, 934 patients had one or more positive GI specimens (43%). A meta‐analysis performed on studies with at least 10 patients showed a pooled positive proportion of 51.8% (95%CI 43.8 ‐ 59.7%; Figure 2; Supplementary Table 1). It has to be mentioned that there is a significant amount of heterogeneity among the included studies, with an I2 of 91.9%. SARS‐CoV‐2 RNA was detected in GI specimens up to a maximum of 70 days after the onset of symptoms and 26 days after discharge from hospital. 22 , 23 In total, 42 studies reported the maximum days of GI specimen positivity after symptom onset or first positive test in any specimens, with a mean of 25.0 (range 3‐70) days after symptom onset.
Figure 2.
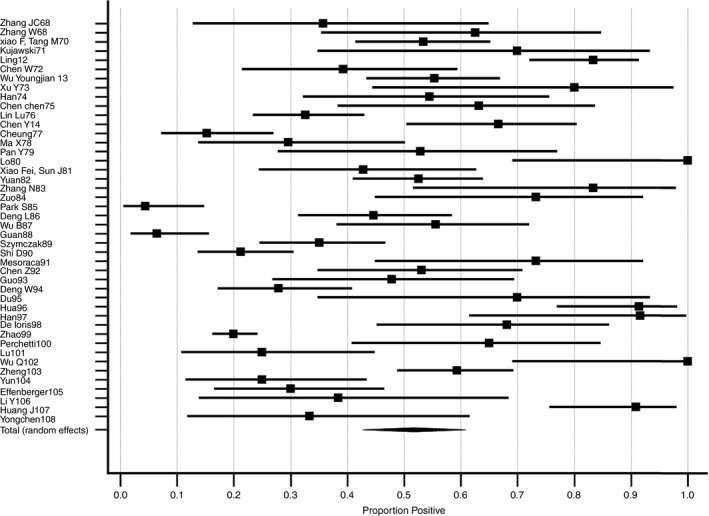
Meta‐analysis of included articles. The proportion positive shows the number of tests positive for SARS‐CoV‐2 divided by the total number of tests. Bars show 95% CI indeed. Data are further specified in Supplementary Table 1
In 22 patients (1%), infection with COVID‐19 would not have been diagnosed without GI specimens testing, meaning these patients had negative results in every other specimen type tested and would not have been confirmed as carriers of the virus otherwise. 19 , 24 , 25 , 26 , 27 , 28
Out of 54 studies with serial SARS‐CoV‐2 RNA test results for both respiratory and GI specimens, 49 (91%) studies reported persistently positive tests for SARS‐CoV‐2 RNA in GI specimens after respiratory specimens had become negative. Almost two thirds of the patients (282/443 (64%)) who had a positive GI specimen test had persistent positive GI specimen tests despite negative respiratory tests. The mean duration of positive GI testing after negative respiratory testing was 12.5 days. The maximum duration of positive GI testing after negative respiratory testing was 33 days. 13 Interestingly, several studies reported patients with ongoing positive GI specimen tests after hospital discharge.
Detectability of SARS‐CoV‐2 RNA depends on the type of specimen tested during different stages of the disease (eg respiratory or faecal sample). In most studies in which serial measurements took place, it was reported that viral RNA was more likely to be detected in respiratory tract samples during an early stage of the disease, whereas GI specimens were more likely to be positive later on during the disease. 12 , 13 , 14 , 22 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37
Twelve studies discussed the association between positive GI specimens and GI symptoms. 13 , 14 , 19 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 In all studies, the majority of patients with GI symptoms tested positive in GI specimens, but the association was not statistically significant in most studies. In the study by Han et al, it was observed that patients with GI symptoms were significantly more likely to test positive for SARS‐CoV‐2 in a stool test (P = 0.033). 44 Furthermore, Cheung et al found that the proportion of positive stool tests and the stool viral load was higher in patients with diarrhoea than without (P = 0.019 and 0.06 respectively). 46
In addition to the clinical symptoms and the positive GI specimens testing, two studies detected SARS‐CoV‐2 RNA in endoscopic specimens of the oesophagus, stomach, duodenum and rectum in 1/1 and 2/6 patients. 41 , 45 Viability of SARS‐CoV‐2 was investigated and detected in five studies, in which six patients (6/17 (35%)) had live active virus in their GI specimens using Vero cell testing. 19 , 21 , 47 , 48 , 49
4. DISCUSSION
In this study, we performed a systematic review of the rapidly expanding body of literature to assess the performance and accuracy of testing stool samples or anal swabs and investigate the potential faecal‐oral transmission of SARS‐CoV‐2. We conclude that the gastrointestinal tract is a potential shedding route of SARS‐CoV‐2 as all but four of the 95 studies with GI specimens testing found positive results of SARS‐CoV‐2 RNA by RT‐PCR in at least one of the patients. A pooled proportion of 51.4% of all included patients tested positive in GI specimens.
Viral RNA can be detected in GI specimens up to 70 days after onset of symptoms or after the first positive SARS‐CoV‐2 test in any specimen. After respiratory tests turned negative, GI samples stayed persistently positive up to a maximum of 33 days, implying that the virus may be actively replicating in the patient's gastrointestinal tract and that faecal–oral transmission might occur after viral clearance in the respiratory tract. Although we observed a relation of patients with gastrointestinal symptoms to be more likely to test positive for SARS‐CoV‐2, the absence of gastrointestinal symptoms is not a firm indicator for negative GI specimen tests.
While SARS‐CoV‐2 may be shedding through stool in a notable subset of patients, the detection of viral genetic material in stool does not necessarily imply that viable infectious virions are present in GI specimens or that the virus can or has spread through faecal transmission. Live SARS‐CoV‐2 was found in 6/17 (35%) of the patients in which this was specifically investigated. Isolation of live SARS‐CoV‐2 in cultured GI specimens underlines the possibility of faecal‐oral transmission through infected faeces.
Similar patterns of faecal‐oral transmission and the relevance of stool testing of other coronaviridae have been witnessed over the years. 50 , 51 The initial SARS‐CoV outbreak in the Amoy Gardens was primarily attributed to an airborne spread via inefficient sanitation and toilet ventilation systems. 52 , 53 Infection of the GI tract with the previous coronaviridae is proposed to be mediated via Angiotensin Converting Enzyme (ACE)‐2 receptors. ACE‐2 has also been identified as the host receptor that interacts with the viral spike protein to facilitate entry of SARS‐CoV‐2 into the host cell. 50 ACE‐2 receptors are highly expressed in the small intestine and the binding affinity of ACE‐2 receptors determine infectivity. As ACE‐2 modulates intestinal inflammation, SARS‐CoV‐2 may disrupt ACE‐2 function and result in GI shedding and symptoms, such as diarrhoea, vomiting and abdominal pain.
Furthermore, wastewater surveillance and wastewater‐based epidemiology are considered a complementary approach to estimate the presence and even the prevalence of COVID‐19 in communities, detecting SARS‐CoV‐2 in wastewater from households with infection. 54 Additionally, a recent study observed prolonged gut microbiome dysbiosis in COVID‐19 patients and its association with faecal SARS‐CoV‐2 virus shedding and disease severity, suggesting that SARS‐CoV‐2 infection may be associated with a more long‐lasting effect on the gut microbiome. 55
Besides the fact that the genome of both SARS‐CoV viridea and thus the shedding routes are very similar, SARS‐CoV‐2 also falls under the same shell disorder category as SARS‐CoV, and SARS‐CoV‐2 has the hardest outer shell within the entire corona family. The hardness of the outer shell could provide SARS‐CoV‐2 with greater resilience to conditions outside the body and in bodily fluid, as the harder shell will provide better protection. Chances of infection via indirect contact and airborne virus from faeces and bodily fluids are therefore higher and faecal‐oral transmission more likely. 56
The results of this study may have various consequences for the diagnosis, prognosis and spread of COVID‐19. First and foremost, worldwide the decision to isolate or discharge a patient is primarily based on relevant clinical symptoms, focusing on the respiratory tract, and (sequential) negative test results on respiratory specimens collected more than 24 hours apart. 57 We observed that in 64% of patients who tested positive for SARS‐CoV‐2 in GI specimens, their GI specimens remained positive for a mean of 12.5 days after respiratory samples became negative. As a result, a number of patients were discharged up to a month before the absence of SARS‐CoV‐2 in GI specimens could be guaranteed. The (additional) use of GI specimen testing may provide a more appropriate rationale for isolation and discharge.
A major concern could be continuing person‐to‐person transmission by the faecal‐oral route, which argues for closer attention to hand and sanitation hygiene. This should be considered when determining diagnosis and isolation policies.
In general the risk to health care professionals from patient exposure is well known, specifically in high aerosol‐generating procedures. Currently, medical management protocols include measures to mitigate the aerosol transmission risks from procedures related to respiratory tract. 8 Our analysis suggests that faecal‐oral transmission risk from gastrointestinal procedures such as colonoscopies or physical examination, should also be taken into account.
Determining whether a virus is viable using RNA detection by RT‐PCR is challenging. Limited studies have observed viable virus in stool and further research is needed to determine whether the irrefutable faecal shedding and the high and long‐lasting detection rate of viral RNA in GI specimens really indicates the likelihood of faecal‐oral transmission. Studies using fresh stool samples at later time points in patients with extended duration of GI specimen positivity are required to define transmission potential. Nevertheless, the importance of GI specimen tests for detection of SARS‐CoV‐2 in general, and even more in the longer term surveillance of infected patients, has been confirmed in our systematic review.
All included studies were observational case studies without control groups, based on a relatively small number of heterogeneous patients (I2 approximately 90%), and the timing of specimen collection has been largely inconsistent and unstandardised. In particular, evidence for viable virions in GI specimens is based on a small number of patients whose specimens were collected at different times over the course of illness or convalescence. This is not surprising, as most included studies are case reports or small case series of patients treated on the frontlines during the pandemic, in which adhering to standard research protocols is difficult. This generates the risk of bias in these kinds of studies, especially publication bias.
As a result, our analyses were based on relatively small patient groups (median 9; range 1‐401 patients) and inconsistent methods, parameters, sample timing, sample frequencies and study endpoints differing widely between the included studies, impeding comparisons and robust conclusions. In the early response to the emerging COVID‐19 outbreak, only respiratory specimens were required for the detection of SARS‐CoV‐2 according to initial clinical guidelines. A lot of studies, therefore, refrained from obtaining GI specimens from the patients during their first few days of hospitalisation or observation and could not determine whether respiratory and GI specimens were positive on RT‐PCR analysis simultaneously. Furthermore, the phenomenon that viral RNA of SARS‐CoV‐2 can remain positive in GI specimens after respiratory samples became negative was not identified in all studies. This resulted in inadequate (follow‐up) information, potentially causing a (outcome) measurement bias.
The sole four studies that reported no positive tests in GI specimens were all based on small sample size (1‐4 patients) and the testing was performed at an early stage of the disease course. Our review demonstrated that there seems a tendency for SARS‐CoV‐2 to be more detectable in the respiratory tract at an early stage of the disease and later on, more likely to be detected in GI specimens, which could explain the early negative testing.
Our review confirms that SARS‐Cov‐2 is commonly present in stool samples or anal swabs in which the virus can persist long after respiratory testing has become negative and that the virus may be viable. This suggests the possibility of faecal‐oral transmission and that stool sample or anal swab testing should be (re)considered in relation to decisions for isolating or discharging a patient.
AUTHORSHIP
Guarantor of the article: NdB.
Author contributions: AvD drafted the first version of the manuscript and performed the analyses. BM, MB and NdB critically revised the manuscript for important intellectual content. CF performed the statistical analyses. All authors approved to the final version of the manuscript.
Supporting information
Supplementary Material
ACKNOWLEDGEMENTS
Declaration of personal interests: AvD, BM, CF and MB have nothing to declare. NdB has served as a speaker for AbbVie and MSD and has served as consultant and principal investigator for TEVA Pharma BV and Takeda. He has received a (unrestricted) research grant from Dr Falk, TEVA Pharma BV, MLDS and Takeda
van Doorn AS, Meijer B, Frampton CMA, Barclay ML, de Boer NKH. Systematic review with meta-analysis: SARS-CoV-2 stool testing and the potential for faecal-oral transmission. Aliment Pharmacol Ther. 2020;52:1276–1288. 10.1111/apt.16036
As part of AP&T’s peer‐review process, a technical check of this meta‐analysis was performed by Dr Y Yuan. The Handling Editor for this article was Professor Jonathan Rhodes, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Zhu NA, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . WHO Director‐General’s opening remarks at the mission briefing on COVID‐19. https://www.who.int/dg/speeches/detail/who‐director‐general‐s‐opening‐remarks‐at‐the‐media‐briefing‐on‐covid‐19–‐11‐march‐2020. 2020.
- 3. Gorbalenya AE, Baker SC, Baric RS, et al. The species Severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;5:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rehman SU, Shafique L, Ihsan A, Liu Q. Evolutionary trajectory for the emergence of novel coronavirus SARS‐CoV‐2. Pathogens. 2020;9:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Guo Y‐R, Cao Q‐D, Hong Z‐S, et al. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID‐19) outbreak‐ A n update on the status. Military Medical. Research. 2020;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pan L, Mu MI, Yang P, et al. Clinical Characteristics of COVID‐19 Patients With Digestive Symptoms in Hubei, China. Am J Gastroenterol. 2020;115:766‐773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. World Health Organization W . Modes of transmission of virus causing COVID‐19: implications for IPC precaution recommendations. Sci Br. 2020. [Google Scholar]
- 8. World Health Organization . WHO Clinical management of severe acute respiratory infection (SARI) when COVID‐19 disease is suspected. Who. 2020. [Google Scholar]
- 9. World Health Organization W . Laboratory testing for coronavirus disease 2019 (COVID‐19) in suspected human cases. Interim Guid. 2020. [Google Scholar]
- 10. Gao QY, Chen YX, Fang JY. 2019 novel coronavirus infection and gastrointestinal tract. J Dig Dis. 2020;21:125‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amirian ES. Potential Fecal Transmission of SARS‐CoV‐2: Current Evidence and Implications for Public Health. Int J Infect Dis. 2020;2:363‐370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ling Y, Xu S‐B, Lin Y‐X, et al. Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl). 2020;133:1039‐1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS‐CoV‐2 viral RNA in faecal samples. Lancet. Gastroenterol Hepatol. 2020;5:434‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen Y, Chen L, Deng Q, et al. The presence of SARS‐CoV‐2 RNA in the feces of COVID‐19 patients. J Med Virol. 2020;92:833‐840. [DOI] [PubMed] [Google Scholar]
- 15. Dona D, Minotti C, Costenaro P, Da Dalt L, Giaquinto C. FECAL‐ORAL TRANSMISSION OF SARS‐COV‐2 IN CHILDREN: IS IT TIME TO CHANGE OUR APPROACH? Pediatr Infect Dis J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID‐19 and the possibility of faecal transmission. Aliment Pharmacol Ther. 2020;51:843‐851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang Z, Li G, Dai X, Liu G, Li G, Jie Y. Three cases of novel coronavirus pneumonia with viral nucleic acids still positive in stool after throat swab detection turned negative. Chinese J Dig. 2020. [Google Scholar]
- 18. Tan X, Huang J, Zhao F, Zhou Y, Li JQ, Wang XY. Clinical features of children with SARS‐CoV‐2 infection: an analysis of 13 cases from Changsha, China. Zhongguo Dang Dai Er Ke Za Zhi. 2020;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang W, Xu Y, Gao R, et al. Detection of SARS‐CoV‐2 in Different Types of Clinical Specimens. JAMA ‐ J Am Med Assoc. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wu J, Liu J, Li S, et al. Detection and analysis of nucleic acid in various biological samples of COVID‐19 patients. Travel Med Infect Dis. 2020;101673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang Y, Chen C, Zhu S, et al. Isolation of 2019‐nCoV from a Stool Specimen of a Laboratory‐Confirmed Case of the Coronavirus Disease 2019 (COVID‐19). China CDC Wkly. 2:123‐124. [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang N, Gong Y, Meng F, Bi Y, Yang P, Wang F. Virus shedding patterns in nasopharyngeal and fecal specimens of COVID‐19 patients. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hua C‐Z, Miao Z‐P, Zheng J‐S, et al. Epidemiological features and viral shedding in children with SARS‐CoV‐2 infection. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tang AN, Tong Z‐D, Wang H‐L, et al. Detection of Novel Coronavirus by RT‐PCR in Stool Specimen from Asymptomatic Child, China. Emerging Infectious Diseases. 2020;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jiang X, Luo M, Zou Z, Wang X, Chen C, Qiu J. Asymptomatic SARS‐CoV‐2 infected case with viral detection positive in stool but negative in nasopharyngeal samples lasts for 42 days. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chen L, Lou J, Bai Y, Wang M. COVID‐19 Disease With Positive Fecal and Negative Pharyngeal and Sputum Viral Tests. Am J Gastroenterol. 2020;115:790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu T, Huang R, Zhu LI, et al. Epidemiological and clinical features of asymptomatic patients with SARS‐CoV‐2 infection. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan C, Zhu H, Yang Y, et al. Viral loads in throat and anal swabs in children infected with SARS‐CoV‐2. Emerg. Microbes Infect. 2020;1‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cai J, Xu J, Lin D, et al. A Case Series of children with 2019 novel coronavirus infection: clinical and epidemiological features. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kam KQ, Yung CF, Cui L, et al. A Well Infant with Coronavirus Disease 2019 (COVID‐19) with High Viral Load. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhang B, Liu S, Dong Y, et al. Positive rectal swabs in young patients recovered from coronavirus disease 2019 (COVID‐19). J Infect. 2020;81:e49‐e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Park JY, Han MS, Park KU, Kim JY, Choi EH. First pediatric case of coronavirus disease 2019 in Korea. J Korean Med Sci. 2020;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li J, Feng J, Liu TH, Xu FC, Song GQ. An infant with a mild SARS‐CoV‐2 infection detected only by anal swabs: a case report. Braz J Infect Dis. 2020;24:247‐249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lescure F‐X, Bouadma L, Nguyen D, et al. Clinical and virological data of the first cases of COVID‐19 in Europe: a case series. Lancet Infect Dis. 2020;20:697‐706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang W, Du R‐H, Li B, et al. Molecular and serological investigation of 2019‐nCoV infected patients: implication of multiple shedding routes. Emerg. Microbes Infect. 2020;9:386‐389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen C, Gao G, Xu Y, et al. SARS‐CoV‐2–Positive Sputum and Feces After Conversion of Pharyngeal Samples in Patients With COVID‐19. Ann Intern Med. 2020;172:832‐834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma X, Su L, Zhang Y, Zhang X, Gai Z, Zhang Z. Do children need a longer time to shed SARS‐CoV‐2 in stool than adults? J Microbiol Immunol Infect. 2020;53:373‐376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cozzi E, Faccioli E, Marinello S, et al. COVID‐19 pneumonia in lung transplant recipients: report of two cases. Am J Transplant. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang JC, Bin WS, Xue YD. Fecal specimen diagnosis 2019 novel coronavirus–infected pneumonia. J Med Virol. 2020;92:680‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS‐CoV‐2. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kujawski SA, Wong KK, Collins JP, et al. First 12 patients with coronavirus disease 2019 (COVID‐19) in the United States. medRxiv. 2020. [DOI] [PubMed] [Google Scholar]
- 43. Xu YI, Li X, Zhu B, et al. Characteristics of pediatric SARS‐CoV‐2 infection and potential evidence for persistent fecal viral shedding. Nat Med. 2020;26:502‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Han C, Duan C, Zhang S, et al. Digestive Symptoms in COVID‐19 Patients With Mild Disease Severity: Clinical Presentation, Stool Viral RNA Testing, and Outcomes. Am J Gastroenterol. 2020;115:916‐923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lin LU, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS‐CoV‐2 infection. Gut. 2020;69:997‐1001. [DOI] [PubMed] [Google Scholar]
- 46. Cheung KS, Hung IF, Chan PP, et al. Gastrointestinal Manifestations of SARS‐CoV‐2 Infection and Virus Load in Fecal Samples from the Hong Kong Cohort and Systematic Review and Meta‐analysis. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhou J, Li C, Liu X, et al. Infection of bat and human intestinal organoids by SARS‐CoV‐2. Nat Med. 2020;26:1077‐1083. [DOI] [PubMed] [Google Scholar]
- 48. Wölfel R, Corman VM, Guggemos W, et al. Virological assessment of hospitalized patients with COVID‐2019. Nature. 2020;581:465‐469. [DOI] [PubMed] [Google Scholar]
- 49. Xiao F, Sun J, Xu Y, et al. Infectious SARS‐CoV‐2 in Feces of Patient with Severe COVID‐19. Emerg Infect Dis J. 2020;26:1920‐1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gu J, Han B, Wang J. COVID‐19: Gastrointestinal Manifestations and Potential Fecal–Oral Transmission. Gastroenterology. 2020;15:1518‐1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Goh GK‐M, Dunker AK, Uversky VN. Understanding Viral Transmission Behavior via Protein Intrinsic Disorder Prediction: Coronaviruses. J Pathog. 2012;2012:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu ITS, Li Y, Wong TW, et al. Evidence of Airborne Transmission of the Severe Acute Respiratory Syndrome Virus. N Engl J Med. 2004;350:1731‐1739. [DOI] [PubMed] [Google Scholar]
- 53. Leung WK, To KF, Chan PKS, et al. Enteric involvement of severe acute respiratory syndrome ‐ Associated coronavirus infection. Gastroenterology. 2003;125:1011‐1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Randazzo W, Truchado P, Cuevas‐Ferrando E, Simón P, Allende A, Sánchez G. SARS‐CoV‐2 RNA in wastewater anticipated COVID‐19 occurrence in a low prevalence area. Water Res. 2020;181:115942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zuo T, Zhang F, Lui GCY, et al. Alterations in Gut Microbiota of Patients With COVID‐19 During Time of Hospitalization. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Goh GKM, Dunker AK, Foster JA, et al. Shell disorder analysis predicts greater resilience of the SARS‐CoV‐2 (COVID‐19) outside the body and in body fluids. Microb Pathog. 2020;144:104177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Diagnosis and treatment plan of Corona Virus Disease 2019 (tentative sixth edition). Glob Health J. 2020;4:1‐5. 10.1016/j.glohj.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic Features and Clinical Course of Patients Infected with SARS‐CoV‐2 in Singapore. JAMA ‐ J Am Med Assoc. 2020;323:1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chan J‐W, Yuan S, Kok K‐H, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person‐to‐person transmission: a study of a family cluster. Lancet. 2020;395:514‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang JF, Yan K, Ye HH, Lin J, Zheng JJ, Cai T. SARS‐CoV‐2 turned positive in a discharged patient with COVID‐19 arouses concern regarding the present standard for discharge. Int J Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zeng LK, Tao XW, Yuan WH, Wang J, Liu X, Liu ZS. First case of neonate infected with novel coronavirus pneumonia in China. Zhonghua er ke za zhi = Chinese. J Pediatr. 2020. [DOI] [PubMed] [Google Scholar]
- 62. Zhang T, Cui X, Zhao X, et al. Detectable SARS‐CoV‐2 viral RNA in feces of three children during recovery period of COVID‐19 pneumonia. J Med Virol. 2020;92:909‐914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wu Y, Liu C, Dong L, et al. Viral Shedding of COVID‐19 in Pregnant Women. SSRN Electron J. 2020. [Google Scholar]
- 64. Lei Z, Cao H, Jie Y, et al. A cross‐sectional comparison of epidemiological and clinical features of patients with coronavirus disease (COVID‐19) in Wuhan and outside Wuhan, China. Travel Medicine and Infectious Disease. 2020;35:101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Xing Y‐H, Ni W, Wu Q, et al. Prolonged viral shedding in feces of pediatric patients with coronavirus disease 2019. J Microbiol Immunol Infect. 2020;53:473‐480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fan Q, Pan Y, Wu Q, et al. Anal swab findings in an infant with COVID‐19. Pediatr Investig. 2020;4:48‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Chen E‐Q, Wang L‐C, Tang G‐M, et al. Brief report of the first cured 2019‐nCoV pneumonia patient in West China Hospital. Eur J Clin Microbiol Infect Dis. 2020;39:1593‐1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Nicastri E, D’Abramo A, Faggioni G, et al. Coronavirus disease (COVID‐19) in a paucisymptomatic patient: Epidemiological and clinical challenge in settings with limited community transmission, Italy, February 2020. Eurosurveillance. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Peng L, Liu J, Xu W, et al. 2019 Novel Coronavirus can be detected in urine, blood, anal swabs and oropharyngeal swabs samples. medRxiv. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Song L, He M, Jia X. A case of SARS‐CoV‐2 carrier for 32 days with several times false negative nucleic acid tests. medRxiv. 2020. [Google Scholar]
- 71. Van TL, Ngoc NM, That BTT, et al. Duration of viral detection in throat and rectum of a patient with COVID‐19. medRxiv. 2020. [Google Scholar]
- 72. Xie C, Jiang L, Huang G, et al. Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis. 2020;93:264‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thammathiwat T, Tungsanga S, Tiankanon K, et al. A Case of Successful Treatment of Severe COVID‐19 Pneumonia with Favipiravir and Tocilizumab in Post‐kidney Transplant Recipient. Transpl Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zou B, Ma DI, Li Y, et al. Are They Just Two Children COVID‐19 Cases Confused With Flu? Front Pediatr. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Shen Y, Zheng F, Sun D, et al. Epidemiology and clinical course of COVID‐19 in Shanghai, China. Emerg. Microbes Infect. 2020;1‐28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Zhou Y, Ding N, Hu M, Yang G. The positive of stool test for SARS‐CoV‐2: a report of 9 cases in Changsha, outside Wuhan. China. Ann Transl Med. 2020;8:596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Chen X, Zou XJ, Xu Z. Serial computed tomographic findings and specific clinical features of pediatric COVID‐19 pneumonia: A case report. World J Clin Cases. 2020;8:2345‐2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kim JY, Ko J‐H, Kim Y, et al. Viral load kinetics of SARS‐CoV‐2 infection in first two patients in Korea. J Korean Med Sci. 2020;35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu P, Cai J, Jia R, et al. Dynamic surveillance of SARS‐CoV‐2 shedding and neutralizing antibody in children with COVID‐19. Emerging Microbes and Infections. 2020;9:1254‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang QX, Huang KC, Qi L, Zeng XH, Zheng SL. No infectious risk of COVID‐19 patients with long‐term fecal 2019‐nCoV nucleic acid positive. Eur Rev Med Pharmacol Sci. 2020;24:5772‐5777. [DOI] [PubMed] [Google Scholar]
- 81. Huang R, Zhao H, Wang J, Yan X, Shao H, Wu C. A family cluster of COVID‐19 involving an asymptomatic case with persistently positive SARS‐CoV‐2 in anal swabs. Travel Medicine and Infectious Disease. 2020;101745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yin S, Peng Y, Ren Y, et al. The implications of preliminary screening and diagnosis: Clinical characteristics of 33 mild patients with SARS‐CoV‐2 infection in Hunan, China. J Clin Virol. 2020;128:104397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Mao L‐J, Xu J, Xu Z‐H, et al. A child with household transmitted COVID‐19. BMC Infect Dis. 2020;20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Wang X, Zhou Y, Jiang N, Zhou Q, Ma WL. Persistence of intestinal SARS‐CoV‐2 infection in patients with COVID‐19 leads to re‐admission after pneumonia resolved. Int J Infect Dis. 2020;95:433‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hu Y, Shen L, Yao Y, Xu Z, Zhou J, Zhou H. A report of three COVID‐19 cases with prolonged viral RNA detection in anal swabs. Vol. 26, Clin Microbiol Infect. 2020;26:786‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tan Y‐P, Tan B‐Y, Pan J, Wu J, Zeng S‐Z, Wei H‐Y. Epidemiologic and clinical characteristics of 10 children with coronavirus disease 2019 in Changsha, China. J Clin Virol. 2019;2020:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Han MS, Seong MW, Heo EY, et al. Sequential analysis of viral load in a neonate and her mother infected with SARS‐CoV‐2. Clin Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Xing Y, Ni W, Wu Q, et al. Dynamics of faecal SARS‐CoV‐2 in infected children during the convalescent phase. J Infect. 2020;81:318‐356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chunli W, Liya H, Weiwei LU, et al. Clinical Characteristics of Pneumonia Patients of Long Courses Infected with SARS‐CoV‐2. SSRN Electron J. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Chen W, Lan Y, Yuan X, et al. Detectable 2019‐nCoV viral RNA in blood is a strong indicator for the further clinical severity. Emerging Microbes and Infections. 2020;9:469‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS‐CoV‐2 in clinical samples. Lancet Infect Dis. 2020;20:411‐412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lo IL, Lio CF, Cheong HH, et al. Evaluation of SARS‐CoV‐2 RNA shedding in clinical specimens and clinical characteristics of 10 patients with COVID‐19 in Macau. Int J Biol Sci. 2020;16:1698‐1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Park S‐K, Lee C‐W, Park D‐I, et al. Detection of SARS‐CoV‐2 in Fecal Samples from Patients with Asymptomatic and Mild COVID‐19 in Korea. Clin Gastroenterol Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Deng L, Li C, Zeng QI, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J Infect. 2020;81:e1‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Wu B, Yu T, Huang Z. Nucleic acid detection of fecal samples from confirmed cases of COVID‐19. Chin J Zoonoses. 2020. [Google Scholar]
- 96. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020. [Google Scholar]
- 97. Szymczak WA, Goldstein DY, Orner EP, et al. Utility of Stool PCR for the Diagnosis of COVID‐19: Comparison of Two Commercial Platforms. J Clin Microbiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Shi D, Wu W, Wang Q, et al. Clinical characteristics and factors associated with long‐term viral excretion in patients with SARS‐CoV‐2 infection: a single center 28‐day study. J Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Mesoraca A, Margiotti K, Viola A, Cima A, Sparacino D, Giorlandino C. Evaluation of SARS‐CoV‐2 viral RNA in fecal samples. Virol J. 2020;17:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Chen Z, Tong L, Zhou Y, et al. Childhood COVID‐19: a multicentre retrospective study. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol. Infect Dis. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Guo L, Zhao S, Li W, et al. Absence of SARS‐CoV‐2 in Semen of a COVID‐19 Patient Cohort. Andrology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Deng W, Guang T‐W, Yang M, et al. Positive results for patients with COVID‐19 discharged form hospital in Chongqing. China. BMC Infect Dis. 2020;20:429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Du W, Yu J, Liu X, Chen H, Lin L, Li Q. Persistence of SARS‐CoV‐2 virus RNA in feces: A case series of children. J Infect Public Health. 2020;13:926‐931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Han MS, Seong M‐W, Kim N, et al. Viral RNA Load in Mildly Symptomatic and Asymptomatic Children with COVID‐19, Seoul. Emerg Infect Dis. 2020;26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. De Ioris MA, Scarselli A, Ciofi degli Atti ML, et al. Dynamic Viral Severe Acute Respiratory Syndrome Coronavirus 2 RNA Shedding in Children: Preliminary Data and Clinical Consideration from a Italian Regional Center. J Pediatric Infect Dis Soc. 2020;9:366‐369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Zhao F, Yang Y, Wang Z, Li L, Liu L, Liu Y. The Time Sequences of Oral and Fecal Viral Shedding of Coronavirus Disease 2019 (COVID‐19) Patients. Gastroenterology. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Perchetti GA, Nalla AK, Huang M‐L, et al. Validation of SARS‐CoV‐2 detection across multiple specimen types. J Clin Virol. 2020;128:104438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Lu X, Wang L, Sakthivel SK, et al. US CDC Real‐Time Reverse Transcription PCR Panel for Detection of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020;26:1654‐1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wu Q, Xing Y, Shi L, et al. Coinfection and Other Clinical Characteristics of COVID‐19 in Children. Pediatrics. 2020;146:e20200961. [DOI] [PubMed] [Google Scholar]
- 110. Zheng S, Fan J, Yu F, et al. Retrospective cohort study. BMJ. 2020;2020:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Yun H, Sun Z, Wu J, Tang A, Hu M, Xiang Z. Laboratory data analysis of novel coronavirus (COVID‐19) screening in 2510 patients. Clin Chim Acta. 2020;507:94‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Effenberger M, Grabherr F, Mayr L, et al. Faecal calprotectin indicates intestinal inflammation in COVID‐19. Gut. 2020;69:1543‐1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li Y, Hu Y, Yu Y, et al. Positive result of Sars‐Cov‐2 in faeces and sputum from discharged patient with COVID‐19 in Yiwu, China. J Med Virol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Huang J, Mao T, Li S, et al. Long period dynamics of viral load and antibodies for SARS‐CoV‐2 infection: an observational cohort study. medRxiv. 2020. [Google Scholar]
- 115. Yongchen Z, Shen H, Wang X, et al. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID‐19 patients. Vol. 9, Emerging microbes & infections. 2020;9:833‐836. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


