ABSTRACT
Objective
Pregnant women can be infected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), yet the incidence of perinatal infection is low. We hypothesized that this could be related to low expression of the membrane receptor for SARS‐CoV‐2, angiotensin‐converting enzyme 2 (ACE2), in the fetoplacental unit. We evaluated protein expression of ACE2 at various gestational ages in both placentae and fetal organs from pregnancies not infected with SARS‐CoV‐2.
Methods
In May 2020, using samples from a registered biobank, we performed immunohistochemical analysis for ACE2 in tissue samples from fetal organs and placentae from five cases of second‐ or third‐trimester medical termination of pregnancy in healthy women (performed between 15 and 38 weeks' gestation), as well as a further two placentae, one from a 7‐week spontaneous miscarriage in a non‐infected woman and one from a symptomatic pregnant woman positive for SARS‐CoV‐2 delivered by Cesarean section at 34 weeks. Samples were paraffin‐embedded and organ tissues included kidney, brain, lung, intestinal tract, heart and testis. Matching tissues (kidney, intestinal tract, lung and testis) from autopsies of four 8‐year‐old children were tested as controls. Tissue sections were incubated with rabbit monoclonal anti‐ACE2, and protein expression of ACE2 was detected by immunohistochemistry.
Results
ACE2 expression was detected in fetal kidney, rectum and ileum samples from 15 weeks onwards and in the pediatric controls. It was barely detectable in fetal lung samples at 15 + 5 weeks' gestation and not detectable thereafter, and, in the pediatric controls, ACE2 was detectable only in type‐2 pneumocytes. No ACE2 expression was found in the cerebral ependymal or parenchymal tissues or in cardiac tissues. ACE2 was expressed in placental syncytiotrophoblast and cytotrophoblast samples, but not in the amnion, from 7 weeks onwards. The intensity and distribution of ACE2 staining in the placenta from the symptomatic SARS‐CoV‐2 woman was similar to that in the non‐infected placentae.
Conclusions
Marked placental expression of ACE2 provides a rationale for vertical transmission at the cellular level. Absence of ACE2 expression in the fetal brain and heart is reassuring regarding the risk of congenital malformation. Clinical follow‐up of infected pregnant women and their children is needed to validate these observations. © 2020 International Society of Ultrasound in Obstetrics and Gynecology
Keywords: ACE2, COVID‐19, fetal organs, placenta, protein expression, SARS‐CoV‐2, vertical transmission
Short abstract
Linked article: There is a comment on this article by Vivanti et al. Click here to view the Correspondence.
CONTRIBUTION —
What are the novel findings of this work?
We found marked expression of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2)‐specific receptor, angiotensin‐converting enzyme 2 (ACE2), in the placenta and fetal bowel and kidneys, but not in the fetal brain and heart, in non‐infected pregnancies across gestation.
What are the clinical implications of this work?
The placental findings provide a rationale for the vertical transmission of SARS‐CoV‐2 at the cellular level. The absence of ACE2 expression in the fetal brain and heart is reassuring regarding the risk of congenital malformation, as organs with absent ACE2 expression should not be the target of direct virus‐related insult.
INTRODUCTION
Pregnant women can be infected by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), and the possibility of vertical transmission of the virus to the fetus is the subject of heated debate 1 , 2 , 3 , 4 , 5 , 6 . The entry of SARS‐CoV‐2 into target cells is enabled by the presence of angiotensin‐converting enzyme 2 (ACE2) on the membrane of the host cell. The viral spike protein is primed by the transmembrane cellular protease serine 2 (TMPRSS2), enabling membrane fusion and viral entry 2 , 7 . TMPRSS2 has a broader distribution than does ACE2, suggesting that ACE2, rather than TMPRSS2, may be a limiting factor for viral entry 8 . Assuming it follows a typical pathway and transmits vertically in the usual way, we hypothesized that the low incidence of perinatal infection might relate to a relatively low expression of ACE2 in the placenta and organs targeted by the virus.
We aimed to evaluate protein expression of ACE2 at various gestational ages in both placentae and fetal organs from pregnancies not infected with SARS‐CoV‐2. We also evaluated ACE2 expression in the placenta of a pregnant woman infected with SARS‐CoV‐2, in order to assess whether expression was modified in the context of SARS‐CoV‐2 infection.
METHODS
Immunohistochemical analysis for ACE2 was performed on fetal and placental tissue samples from a biobank
authorized by the National Biomedical Agency. All women had given informed consent for the use of fetal and placental specimens for research purposes.
Fetal and placental tissues
In May 2020, we retrieved organ tissue and placental samples from five cases of medical termination of pregnancy in healthy, non‐hypertensive women. Data from these five cases are presented in Table 1. Our three inclusion criteria for these cases were as follows. (1) The different stages of development of the fetoplacental unit should be represented. We thus chose fetuses/placentae of different gestational ages: three cases from the second trimester (one from the beginning (15 + 5 weeks), one from the middle (20 + 1 weeks) and one from the end (27 + 5 weeks)) and two cases from the third trimester (one from the beginning (29 + 4 weeks; olfactory mucosa only) and one from the end (38 + 1 weeks)). (2) The fetuses should not have extracerebral organ damage or anomalies on fetopathological examination (in particular lung, kidney, digestive tract and heart). The placenta should be eutrophic. It was impossible to include fetuses with no sign of brain damage, since the reason for medical termination of pregnancy at such late stages was most often related to late discovery of a brain abnormality that could affect the neurodevelopmental prognosis. (3) The reason for termination of the pregnancy should not be that the fetus had a congenital infection.
Table 1.
Characteristics of five cases of termination of pregnancy (TOP)
| Prenatal indication for TOP | Cytogenetic CGH‐array | GA (weeks) | Birth weight (g) | Fetal sex | Fetal pathology exam | Placental tissue exam |
|---|---|---|---|---|---|---|
| Maternal distress | NP | 15 + 5 | 73 (AGA) | Female | Normal | Normal |
| PPROM and anhydramnios | NP | 20 + 1 | 328 (AGA) | Male | Normal | Normotrophic placenta, histological appearance consistent with GA, extrachorionic configuration with peripheral fibrinohemorrhagic changes |
| Brain anomalies | Normal | 27 + 5 | 1175 (AGA) | Female | Ischemic‐hemorrhagic rearrangements, with ventriculomegaly, corpus callosal dysgenesis, bilateral occipital polymicrogyria, cleft and hypoplasia of vermis | Normal |
| Chromosomal anomaly | Deletion 15q13.3 (0.4 Mb) | 29 + 4 | 1077 (AGA) | Female | Normal brain anatomy except for hypoplasia of posterior vermis | Eutrophic, discrete hypoxic–ischemic‐related alterations |
| Hydrocephaly | Normal | 38 + 1 | 3539 (AGA) | Male | Post‐hemorrhagic triventricular dilatation with hypoxic–ischemic white‐matter lesion foci | Normal |
AGA, appropriate‐for‐gestational age; CGH‐array, array‐based comparative genomic hybridization; GA, gestational age at delivery; NP, not performed; PPROM, preterm prelabor rupture of membranes.
A further two placentae were also analyzed. One was from a 7‐week spontaneous miscarriage in a 33‐year‐old woman with no sign of septic abortion. Ultrasound examination (dating scan) showed an aborted pregnancy and the patient requested surgical management by endouterine suction. The results of placental histological analysis were normal and there were no signs of intervillitis. The other was from a symptomatic pregnant woman who was positive on SARS‐CoV‐2 reverse transcription polymerase chain reaction (RT‐PCR) testing, and delivered by Cesarean section at 34 weeks. The placenta tested negative for SARS‐CoV‐2 by RT‐PCR.
Tissue samples were paraffin‐embedded and included kidneys, brain, lungs, intestinal tract, heart, testes, cornea, conjunctiva and olfactory mucosa. From the registered human tissue collection of the pathology department of the Hôpital Necker‐Enfants Malades, we selected as controls matching tissue samples (kidney, intestinal tract, lung and testis), without histological lesion in the analyzed sections, taken during autopsy from four 8‐year‐old children. The aim of choosing pediatric cases was to obtain positive controls to visualize the expected cell type and subcellular ACE2 in different organs known to express ACE2.
Immunohistochemistry for ACE2
An automated BOND‐III IHC stainer (Leica Biosystems, Buffalo Grove, IL, USA) was used for detection of protein expression of ACE2 by immunohistochemistry. Briefly, 4‐µm sections (7‐µm sections for brain samples) of paraffin‐embedded tissue were deparaffinized, rehydrated and submitted to heat‐induced antigen retrieval (H1, PH6 device, Leica Biosystems). Tissue sections were incubated with rabbit monoclonal anti‐ACE2 (Abcam, [EPR4435 (2)]), diluted 1/200 v/v, for 30 min. The primary antibody was visualized using diaminobenzidine, and counterstained with hematoxylin using the Leica Biosystems BOND detection kit. Sections processed with replacement of the primary antibody by tris‐buffered saline were used as a negative control.
RESULTS
Immunostaining for ACE2 in fetal and 8‐year‐old control organs (Figures 1 and 2)
Figure 1.
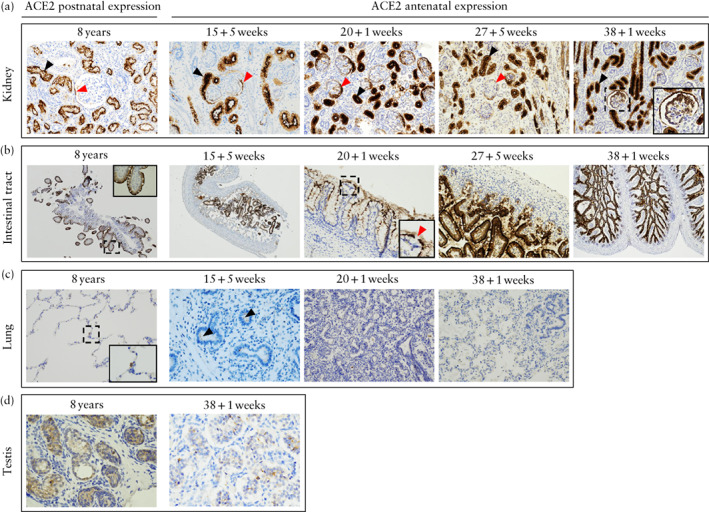
Expression of SARS‐CoV‐2‐specific receptor, angiotensin‐converting enzyme 2 (ACE2), in fetal kidney (a), intestinal tract (b), lung (c) and testis (d), compared with those of 8‐year‐old control patients, as detected by immunohistochemistry using anti‐ACE2 antibodies. (a) Light microscopy (original magnification, ×200) showing strong ACE2 expression across gestation in kidney, within proximal tubule (black arrows) and glomeruli (red arrows), with parietal cell and podocyte expression. (b) Light microscopy (×40, ×40, ×400, ×200, ×40) showing ACE2 expression across gestation in intestinal tract (rectum and ileum). Red arrow indicates goblet cell with no ACE2 expression surrounded by enterocytes positive for ACE2 expression. (c) Light microscopy (×200, ×400, ×400, ×400) showing ACE2 expression in lung. 8‐year‐old control patient showed positive ACE2 staining in type‐2 pneumocyte. 15 + 5‐week fetus showed weak apical expression in apical membrane in alveolar epithelium (arrows), and there was no clear ACE2 expression after this gestational age. (d) Light microscopy (×400) showing weak expression within fetal testis at 38 + 1 weeks.
Figure 2.
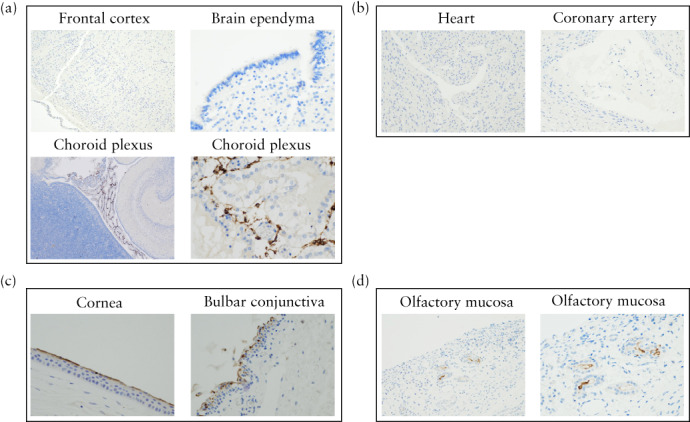
Expression of SARS‐CoV‐2‐specific receptor, angiotensin‐converting enzyme 2 (ACE2), in fetal brain (a), heart (b), eye (c) and olfactory mucosa (d), as detected by immunohistochemistry using anti‐ACE2 antibodies. (a) Fetal brain at 20 + 1 weeks. Top left: light microscopy (original magnification, ×100) revealed no ACE2 expression in frontal cortex. Top right: light microscopy (×400) revealed no ACE2 expression in brain ependyma. Bottom left and right: light microscopy (×40 and ×400) showed ACE2 expression in choroid plexus. (b) Heart at 38 + 1 weeks. Light microscopy (×400) showed no expression of ACE2 in heart or coronary artery. (c) Eye at 38 + 1 weeks. Light microscopy (×400) showed ACE2 expression in apical membrane in cornea and bulbar conjunctiva. (d) Olfactory mucosa at 29 + 4 weeks. Light microscopy (×400, ×200) showed staining in Bowman's gland.
Protein expression of ACE2 was detected in fetal testis, kidneys, rectum and ileum, from 15 + 5 weeks' onwards. Similar results were found in pediatric controls. The fetal olfactory mucosa, and the cornea and bulbar conjunctiva, each showed ACE2 protein expression in only one sample: the former at 29 + 4 and the latter at 38 + 1 weeks. Regarding the precise location of ACE2 immunostaining, in the testis, there was membrane and cytosolic staining within the seminiferous tubules. In the kidneys, there was apical membrane staining of the proximal convoluted tubule in the kidney cortex, with mild cytosolic expression due to the protein synthesis pathway. There was also ACE2 expression in membrane podocytes and in parietal epithelial cells within the glomeruli. In the intestinal tract there was apical membrane staining of enterocytes but none within the goblet cells. In the olfactory mucosa sample which showed protein expression of ACE2, this was located in Bowman's gland, and in the eye samples there was apical membrane staining within the cornea and conjunctiva epithelium.
Immunohistochemical staining indicating expression of ACE2 was barely detectable in the lungs at 15 + 5 weeks and was not found at later gestational ages. This weak staining was in the apical membrane within type‐2 pneumocytes and was not present at all in type‐1 pneumocytes. In the pediatric controls, ACE2 protein expression was also detectable only in type‐2 pneumocytes.
Expression of ACE2 was not found in the cortex and parenchyma or the ependyma of the brain, and was limited to the choroid plexuses. Cytosolic and membrane staining for ACE2 was positive in the inner stromal core of the choroid plexus villi, possibly corresponding to stroma cells. These non‐epithelial cells had fewer spindle‐shaped nuclei than did the endothelial cells, and were negative for actin and CD 34 staining. Thus, these cells were potentially not vascular endothelial cells, CD34 being quite specific to this lineage; nor were they myocyte or fibroblast cells, being actin‐negative.
Neither cardiac tissue nor coronary endothelial cells showed ACE2 staining.
Immunostaining for ACE2 in placentae (Figure 3)
Figure 3.
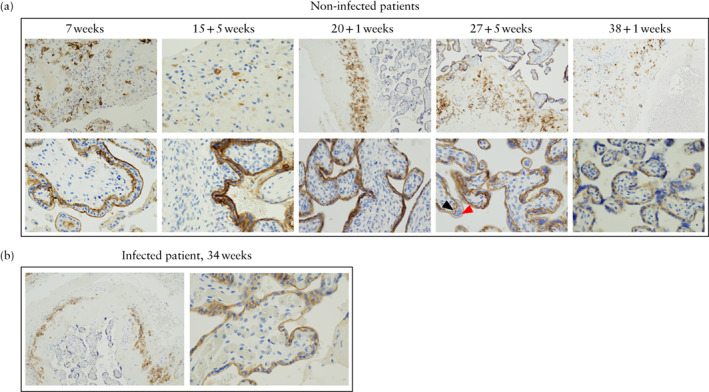
Comparison of expression of SARS‐CoV‐2‐specific receptor, angiotensin‐converting enzyme 2 (ACE2), in placental tissue of non‐infected (a) and SARS‐CoV‐2‐infected (b) patients, as detected by immunohistochemistry using anti‐ACE2 antibodies. (a) Placentae of non‐infected patients. Top row: light microscopy (original magnification, ×100) of basal plate revealed ACE2 expression in intermediate trophoblasts. Bottom row: light microscopy (×400) of placental villi showed ACE2 expression in cytotrophoblast (black arrow) and syncytiotrophoblast (red arrow) tissue. (b) ACE2 expression in placenta of SARS‐CoV‐2‐infected patient, showing similar staining to that in placentae of non‐infected patients.
Staining indicating expression of ACE2 was detectable in all non‐infected placentae, including as early as 7 weeks, within both syncytiotrophoblast and cytotrophoblast tissue, but not in the vascular endothelium. In the basal plate, there was staining in the intermediate trophoblast membrane and, in the placental villi, there was apical membrane staining in both syncytiotrophoblast and cytotrophoblast tissue. ACE2 expression was not detected in the amnion. ACE2 staining in the placenta of the symptomatic pregnant woman was of the same intensity and distribution as that in the non‐infected cases.
DISCUSSION
Main findings
Protein expression of ACE2 was marked in kidney and gastrointestinal tract samples from all fetuses, but not in brain ependyma or parenchyma. Protein expression of ACE2 was also marked in placentae both from an infected woman and from non‐infected women throughout pregnancy, but not in the amnion. These results help to provide insight into the likelihood, and potential pathways and morbidity, of vertical transmission of SARS‐CoV‐2, providing a unique anatomical demonstration across gestation of the distribution of its specific receptor, ACE2, in fetal organs and placentae.
Interpretation
Proven neonatal infection with SARS‐CoV‐2 within 48 hours of birth has raised the possibility of perinatal infection 1 , 9 . Our findings support that SARS‐CoV‐2 is able to cross into the placenta at any gestational age. This may occur either by blood‐borne transmission across the maternal–fetal interface or through ascending vertical transmission of the virus 5 , 10 . In SARS‐CoV‐2 infection, viremia is detectable in only around 1% of symptomatic adults, and it could not be demonstrated in any infected neonates 10 , which makes blood‐borne transmission unlikely. In symptomatic, infected pregnant women, viral RNA could not be amplified from amniotic fluid 2 or vaginal secretions 11 . This makes ascending infection through an intact amnion unlikely. Furthermore, ACE2 is not expressed in the amnion. RT‐PCR in placentae has yielded mainly negative results 2 , with the exception of a few cases following Cesarean section 9 , 12 , and the diagnosis of chorioamnionitis related to SARS‐CoV‐2 has also been suggested 13 . Ascending colonization and infection of the placenta following prolonged rupture of the amniotic membranes therefore appears the most likely pathway for vertical transmission of SARS‐CoV‐2.
In the event of fetal infection with SARS‐CoV‐2, our data are reassuring regarding the risk of fetal complications. We found no expression of ACE2 in the brain or ependyma. It therefore appears unlikely that the virus would cause direct neurological sequelae such as can be observed, for example, in cytomegalovirus infection. ACE2 was markedly expressed in the fetal kidneys, which could affect amniotic fluid production and kidney development. ACE2 was also expressed in the fetal intestinal epithelium, which makes SARS‐CoV‐2 a candidate for causing enterocolitis, a condition broadly associated with perinatal infection and showing hyperechoic bowel on antenatal ultrasound examination. ACE2 expression in the lungs is known to involve mainly type‐2 pneumocytes 2 . This is compatible with our observed transient expression at 15 + 5 weeks, at the end of the pseudoglandular phase of lung development. The absence of ACE2 expression in lung tissue in the third trimester is compatible with the scarcity of related respiratory symptoms found in infected neonates. The absence of ACE2 immunostaining in all cardiac tissues suggests that the heart is not a direct target of the virus but rather may be exposed indirectly to the pathogenicity of coronavirus disease (COVID‐19), for example with deregulated inflammation through the myocardial effects of cytokine storms 14 . Finally, ACE2 expression was found in one case in the Bowman's glands of the olfactory mucosa involved in olfaction. This could contribute to our understanding of olfactory defects in COVID‐19 patients 15 .
Recent work of Vivanti et al. 9 described a proven case of congenital infection with SARS‐CoV‐2 associated with neurological manifestations. The authors reported that the neonate showed signs of white‐matter injury and provided insights into fetal neurological manifestations in the setting of SARS‐CoV‐2 infection. However, we think that the white‐matter injury identified by magnetic resonance imaging (MRI) lacked specificity and could have been triggered by any of several causes, such as hypoxia, immune injury or endothelial cell activation (complement dysregulation, pro‐inflammatory cytokines). Indeed, there was limited evidence for a direct effect of the virus on the brain: (1) the newborn had several punctures of cerebrospinal fluid (CSF), all of which were negative for SARS‐CoV‐2; (2) no parenchymatous lesions (which can be observed for congenital cytomegalovirus, toxoplasmosis and Zika virus infections) were observed on MRI; (3) all other brain tests (electroencephalogram, brain ultrasound) were normal. Finally, even in this one case describing neurological disorder in the neonatal period, there was little cause to believe that SARS‐CoV‐2 would provide long‐term neurological sequelae: (1) the neurological examination returned to normal in the absence of any medical intervention; (2) the newborn was discharged from hospital after a length of stay that was standard for a premature infant; (3) the neurological examination was normal at 2 months of age; and (4) this is the first and only case in the literature reporting neurological effects in children born to infected mothers. The postnatal data of this case of proven congenital infection mostly support our histological data: direct placental infection by SARS‐CoV‐2, detection of the virus in the nasopharynx and feces, normal cardiac function in the newborn, a normal lung ultrasound examination with no respiratory signs and absence of the virus in the CSF.
Strengths and limitations
Our study provides unique in‐vivo human anatomical mapping of the distribution of the SARS‐CoV‐2 receptor, ACE2. It is a strength that we achieved this at a variety of gestational ages, with fetal organ tissue samples ranging between 15 and 38 weeks, and placental samples ranging between 7 and 38 weeks.
It is a potential limitation that we did not study the expression of TMPRSS2, a cofactor of ACE2. However, its broader distribution suggests that it may not be a limiting factor for viral entry 8 . Furthermore, the multibasic cleavage site in the spike protein of SARS‐CoV‐2 is activated by ubiquitously expressed proprotein convertases, including furin, suggesting that membranous proteases other than TMPRSS2 may trigger virus entry 16 .
Conclusion
The marked placental expression of the SARS‐CoV‐2‐specific receptor, ACE2, and its absence from the amnion suggest that ascending vertical transmission could occur mainly following rupture of the amniotic membranes. The absence of ACE2 expression in the fetal brain, lungs and heart is reassuring regarding the risk of congenital malformation and the morbidity of perinatal infection. However, placenta‐mediated fetal morbidity, including chorioamnionitis‐related prematurity and growth restriction, should be investigated in follow‐up studies of infected pregnancies.
Linked article: There is a comment on this article by Vivanti et al. Click here to view the Correspondence.
REFERENCES
- 1. Zeng L, Xia S, Yuan W, Yan K, Xiao F, Shao J, Zhou W. Neonatal Early‐Onset Infection With SARS‐CoV‐2 in 33 Neonates Born to Mothers With COVID‐19 in Wuhan, China. JAMA Pediatr 2020; 174: 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lamouroux A, Attie‐Bitach T, Martinovic J, Leruez‐Ville M, Ville Y. Evidence for and against vertical transmission for SARS‐CoV‐2 (COVID‐19). Am J Obstet Gynecol 2020; 223: 91.e1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dong L, Tian J, He S, Zhu C, Wang J, Liu C, Yang J. Possible Vertical Transmission of SARS‐CoV‐2 From an Infected Mother to Her Newborn. JAMA 2020; 323: 1846–1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kimberlin DW, Stagno S. Can SARS‐CoV‐2 Infection Be Acquired In Utero?: More Definitive Evidence Is Needed. JAMA 2020; 323: 1788–1789. [DOI] [PubMed] [Google Scholar]
- 5. Algarroba GN, Rekawek P, Vahanian SA, Khullar P, Palaia T, Peltier MR, Chavez MR, Vintzileos AM. Visualization of SARS‐CoV‐2 virus invading the human placenta using electron microscopy. Am J Obstet Gynecol 2020; 223: 275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poon LC, Yang H, Kapur A, Melamed N, Dao B, Divakar H, McIntyre HD, Kihara AB, Ayres‐de‐Campos D, Ferrazzi EM, Di Renzo GC, Hod M. Global interim guidance on coronavirus disease 2019 (COVID‐19) during pregnancy and puerperium from FIGO and allied partners: Information for healthcare professionals. Int J Gynaecol Obstet 2020; 149: 273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hoffmann M, Kleine‐Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu N‐H, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS‐CoV‐2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020; 181: 271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, Talavera‐López C, Maatz H, Reichart D, Sampaziotis F, Worlock KB, Yoshida M, Barnes JL. SARS‐CoV‐2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020; 26: 681–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vivanti AJ, Vauloup‐Fellous C, Prevot S, Zupan V, Suffee C, Do Cao J, Benachi A, De Luca D. Transplacental transmission of SARS‐CoV‐2 infection. Nat Commun 2020; 11: 3572–3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS‐CoV‐2 in Different Types of Clinical Specimens. JAMA 2020; 323: 1843–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Qiu L, Liu X, Xiao M, Xie J, Cao W, Liu Z, Morse A, Xie Y, Li T, Zhu L. SARS‐CoV‐2 Is Not Detectable in the Vaginal Fluid of Women With Severe COVID‐19 Infection. Clin Infect Dis 2020; 71: 813–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Penfield CA, Brubaker SG, Limaye MA, Lighter J, Ratner AJ, Thomas KM, Meyer J, Roman AS. Detection of SARS‐COV‐2 in Placental and Fetal Membrane Samples. Am J Obstet Gynecol MFM 2020; 2: 100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Favre G, Pomar L, Musso D, Baud D. 2019‐nCoV epidemic: what about pregnancies? Lancet 2020; 395: e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Viner RM, Whittaker E. Kawasaki‐like disease: emerging complication during the COVID‐19 pandemic. Lancet 2020; 395: 1741–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Butowt R, Bilinska K. SARS‐CoV‐2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem Neurosci 2020; 11: 1200–1203. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann M, Kleine‐Weber H, Pöhlmann S. A Multibasic Cleavage Site in the Spike Protein of SARS‐CoV‐2 Is Essential for Infection of Human Lung Cells. Mol Cell 2020; 78: 779–784.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]


