Abstract
Background
In patients with Covid‐19, myocardial injury and increased inflammation are associated with morbidity and mortality. We designed a proof‐of‐concept randomized controlled trial to evaluate whether treatment with canakinumab prevents progressive respiratory failure and worsening cardiac dysfunction in patients with SARS‐CoV2 infection, myocardial injury, and high levels of inflammation.
Hypothesis
The primary hypothesis is that canakiumab will shorten time to recovery.
Methods
The three C study (canakinumab in Covid‐19 Cardiac Injury, NCT04365153) is a double‐blind, randomized controlled trial comparing canakinumab 300 mg IV, 600 mg IV, or placebo in a 1:1:1 ratio in hospitalized Covid‐19 patients with elevations in troponin and C‐reactive protein (CRP). The primary endpoint is defined as the time in days from randomization to either an improvement of two points on a seven category ordinal scale or discharge from the hospital, whichever occurs first up to 14 days postrandomization. The secondary endpoint is mortality at day 28. A total of 45 patients will be enrolled with an anticipated 5 month follow up period.
Results
Baseline characteristics for the first 20 randomized patients reveal a predominantly male (75%), elderly population (median 67 years) with a high prevalence of hypertension (80%) and hyperlipidemia (75%). CRPs have been markedly elevated (median 16.2 mg/dL) with modest elevations in high‐sensitivity troponin T (median 21 ng/L), in keeping with the concept of enrolling patients with early myocardial injury.
Conclusions
The three C study will provide insights regarding whether IL‐1β inhibition may improve outcomes in patients with SARS‐CoV2 associated myocardial injury and increased inflammation.
Keywords: canakinumab, Covid‐19, myocardial injury, SARS‐CoV‐2
1. INTRODUCTION
In December 2019, a novel coronavirus (SARS‐CoV‐2) resulted in an outbreak of pneumonia in Wuhan, China (coronavirus disease 2019, Covid‐19), which has since become a global pandemic. 1 , 2 Many therapies have been evaluated, 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 but few have been shown to improve outcomes includingremdesivir 9 , 10 and dexamethasone. 12 Given the disease burden, effective treatments are urgently required. Even though the predominant manifestation of Covid‐19 is a respiratory illness, concomitant cardiovascular complications result in substantial morbidity and mortality. 13
Acute cardiac injury, defined as a troponin above the 99%, may occur in up to a quarter of patients, though the incidence depends on the severity of illness and underlying comorbidities of the hospitalized population. 14 , 15 , 16 In many patients, cardiac dysfunction may contribute to mortality, often in combination with respiratory failure, 17 as patients with elevated troponins are more likely to have acute respiratory distress syndrome (ARDS) and require mechanical ventilation. 18 , 19 An elevated troponin may confer a mortality as high as 50%, and similarly, patients with abnormal N‐terminal pro‐brain natriuretic peptides (NT‐proBNP) have a dismal prognosis. 19 , 20 , 21
In patients who succumb to Covid‐19, the troponin may continue to rise throughout the illness, a pattern distinct from the typical rise and fall after an ischemic insult. 19 Moreover, patients with elevated troponins have higher levels of CRP. The correlation, though modest in magnitude, is similar to the correlation between troponin and NT‐proBNP. 19 , 21 These observations suggest that certain patients develop a heightened inflammatory state that perpetuates nonischemic myocardial injury. These biomarker changes have been described as a “cytokine storm” and appear strongly related to adverse outcomes. Antagonizing the inflammatory state by attenuating the deleterious cytokine response therefore has the potential to improve clinical outcomes. 22
Canakinumab is a fully human monoclonal antibody neutralizing IL‐1β with linear dose‐dependent pharmacokinetics and a long elimination half‐life of 26 days. 23 Canakinumab is approved to treat auto‐inflammatory diseases such as cryopyrin‐associated periodic syndromes (CAPS) and Familial Mediterranean Fever. 24 , 25 , 26 In a large randomized trial of patients with atherosclerotic disease and increased inflammation, treatment with canakinumab led to a lower rate of recurrent cardiovascular events. 25
Among patients with sepsis and organ dysfunction or heightened inflammation, a subgroup analysis of a randomized controlled trial showed improved survival with IL‐1 receptor antagonism. 27 , 28 From a safety perspective, high doses of IL‐1 receptor antagonism have not been associated with adverse events when utilized in trials of severe sepsis. 29 In patients with Covid‐19, a recent retrospective cohort study in Italy suggested that IL‐1 antagonism may be a promising target. Patients treated with high‐dose anakinra (5 mg/kg twice daily for median duration of 9 days), an IL‐1 receptor antagonist, had lower CRP and improved respiratory function when compared to standard therapy alone or low‐dose anakinra (100 mg twice daily). 30 Of note, rates of bacteremia were similar between the high‐dose anakinra group (4 of 29 patients or 14%) and the standard treatment group (2 of 16 patients or 13%). 30 Similarly, in a recent case‐control study, ten patients treated with 300 mg of canakinumab demonstrated improvement in oxygentation. 31 However, randomized controlled trials are needed, especially in a disease where the natural history is not well defined, and standard treatments are rapidly changing.
The three C study (canakinumab in Covid‐19 cardiac injury, NCT04365153) is unique in that it exclusively evaluates a population proven to be at high risk, patients with Covid‐19, myocardial injury and increased inflammation. We aim to assess whether canakinumab prevents progressive respiratory failure and cardiac dysfunction. Our primary hypothesis is that treatment with canakinumab will shorten time to recovery. In the setting of a favorable signal, this study will enable the design and conduct of a larger more definitive clinical trial.
2. METHODS
2.1. Study overview
The three C Study is a prospective, IRB approved, blinded randomized‐controlled Phase II study designed to evaluate whether treatment with canakinumab prevents progressive heart and respiratory failure in patients with Covid‐19 associated myocardial injury and increased inflammation. The trial is enrolling in hospitals across the Cleveland Clinic Health System and is being conducted in compliance with the study protocol, the Declaration of Helsinki, and good clinical practice as defined by the international conference on harmonization. Prior to patient participation, written informed consent will be obtained from each patient or their legally authorized representative. The trial is supported by an investigator‐initiated grant to the primary investigator and the design and conduct approved by the FDA.
2.2. Study population
The three C study plans to enroll a total of 45 patients. The first patient was randomized on 28 April 2020, and the study will be completed in approximately 7 months. Key inclusion and exclusion criteria are summarized in Table 1. In brief, the study will include hospitalized patients with Covid‐19 on standard therapy who have evidence of myocardial injury as well as clinical and biological markers of a heightened inflammatory syndrome. Patients must have a documented upper respiratory tract specimen positive for Covid‐19, a troponin >99% upper reference range, a NT‐proBNP or BNP greater than upper reference limit, and a CRP >50 mg/L (>5 mg/dL). At our hospitals, the fifth generation Roche Troponin T assay is used, and a value ≥12 ng/L is considered abnormal. 32 Although the majority of patients will likely have severe pneumonia, defined as tachypnea, severe respiratory distress, or SpO2 </= 93% on room air, because initial myocardial injury in the absence of severe pneumonia has been reported, severe pneumonia is not required for inclusion. Important exclusion criteria include myocardial infarction (MI) according to the fourth universal definition of MI as a cause for the troponin elevation, 33 uncontrolled systemic bacterial infection, mechanical ventilation for greater than 48 hours, or hemodynamic instability requiring mechanical circulatory support.
TABLE 1.
Key inclusion and exclusion criteria
| Inclusion criteria |
| Hospitalized due to Covid‐19 infection with positive Covid‐19 test within 5 days of enrollment |
| Documented SARS‐CoV2 acute myocardial injury: Defined as upper respiratory tract specimen positive for Covid‐19 AND troponin greater than 99% upper reference range without signs or symptoms of acute myocardial ischemia |
| NT‐proBNP or BNP greater than upper reference limit |
| Receiving current standard therapy |
| C‐reactive protein (CRP) > 50 mg/L |
| Exclusion criteria |
| Alternative explanation for troponin elevation (Type I or Type II MI according to fourth Universal Definition of Myocardial Infarction, which in addition to a rise and fall of troponin above the 99% upper reference limit, includes symptoms of acute myocardial ischemia, new ischemic ECG changes, development of pathologic Q waves, and imaging evidence of damage in a pattern consistent with an ischemic etiology) |
| Chronic Systolic Heart Failure with EF < 35% |
| Age < 18 years‐old |
| Uncontrolled systemic bacterial or fungal infection |
| Concomitant viral infection (eg, Influenza or other respiratory virus) |
| Pregnant. Breast‐feeding women are eligible with the decision to continue or discontinue breast‐feeding during therapy taking into account the risk of infant exposure, the benefits of breast‐feeding to the infant, and benefits of treatment to the mother. |
| On mechanical circulatory support |
| On mechanical ventilation for greater than 48 hours |
| Resuscitated cardiac arrest |
| Has a known hypersensitivity to canakinumab or any of its excipients |
| Neutrophil count <1000/mm3 |
| Has a history of myeloproliferative disorder or active malignancy receiving chemotherapy |
| Known active tuberculosis or history of incompletely treated tuberculosis |
| Current treatment with immunosuppressive agents |
| Chronic prednisone use >10 mg/daily (for more than 3 weeks prior to admission) |
| Has a history of solid‐organ or bone marrow transplant |
| Severe preexisting liver disease with clinically significant portal hypertension |
| End‐stage renal disease on chronic renal replacement therapy |
| Enrollment in another investigational study using immunosuppressive therapy |
| In the opinion of the investigator and clinical team, should not participate in the study |
| If male and sexually active, must have documented vasectomy or must practice birth control and not donate sperm during the study and for 3 months after study drug administration. |
| Women of child‐bearing potential, defined as all women physiologically capable of becoming pregnant, unless they are using highly effective methods of contraception during dosing of investigational drug. Such methods include: |
| Total abstinence (when this is in line with the preferred and usual lifestyle of the subject. Periodic abstinence (eg, calendar, ovulation, symptothermal, postovulation methods) and withdrawal are not acceptable methods of contraception |
| Female sterilization (have had surgical bilateral oophorectomy with or without hysterectomy), total hysterectomy, or bilateral tubal ligation at least 6 weeks before taking study treatment. In case of oophorectomy alone, only when the reproductive status of the woman has been confirmed by follow up hormone level assessment |
| Male sterilization (at least 6 months prior to screening). For female subjects on the study, the vasectomized male partner should be the sole partner for that subject |
| Use of oral, (estrogen and progesterone), injected or implanted hormonal methods of contraception or placement of an intrauterine device (IUD) or intrauterine system (IUS), or other forms of hormonal contraception that have comparable efficacy (failure rate < 1%), for example hormone vaginal ring or transdermal hormone contraception |
Abbreviations: COVID‐19, coronavirus disease 2019; EF, ejection fraction; SARS‐CoV2, severe acute respiratory syndrome coronavirus 2.
2.3. Study conduct
Enrolled patients will be randomized using a 1:1:1 allocation ratio. Randomization will be stratified by hospital and whether or not the patient is intubated at the time of enrollment. The study design is illustrated in Figure 1. Overall, 15 subjects will receive 600 mg intravenous (IV) canakinumab, 15 subjects will receive 300 mg IV canakinumab, and 15 patients will receive placebo infusion. Doses up to 600 mg have been safely tolerated in prior phase III trial in CAPS patients. 26 Thus, both 300 mg and 600 mg are being evaluated in this Phase II study. Concomitant medications including antipyretics, antibiotics related to secondary infections, antivirals related to Covid‐19, corticosteroids, use of convalescent plasma, and other immunosuppressive agents will be collected at baseline and daily thereafter until Day 7, at Day 14, Day 21, and Day 28. Importantly, enrolled patients are eligible to receive Covid‐19 therapies as deemed clinically appropriate irrespective of their participation in the three C study.
FIGURE 1.
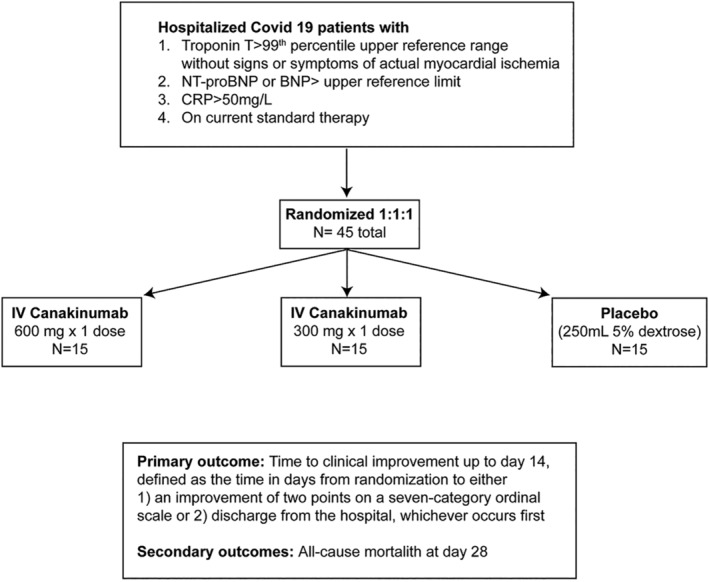
Trial design
After enrollment and treatment with randomized therapy (day 0), the ordinal scale will be assessed daily until discharge and also at day 14, day 21, day 28, day 90 and day 150. Subsequent encounters after the initial visit may include either in‐person or telehealth encounters after the patient has been discharged.
The three C study includes a data monitoring committee (DMC) that functions independently of all other individuals associated with the conduct of the clinical trial, including the investigators at the five participating sites (Supplementary Appendix). At defined intervals, the DMC assesses the progress of the trial, safety data, and critical efficacy variables to recommend whether to continue, modify, or terminate the trial. Specific details regarding the DMC are described in a separate charter.
2.4. Study outcomes
2.4.1. Primary outcomes
The primary efficacy outcome is the time to clinical improvement up to day 14, defined as the time in days from randomization to either an improvement of two points on a seven category ordinal scale (Table 2 ) or discharge from the hospital, whichever occurs first. The ordinal scale consists of disease severity categories ranging from patients discharged from the hospital with resumption of normal activities to spectrum of noninvasive (nasal cannula, high‐flow oxygen) and invasive (ECMO, mechanical ventilation) support to death. This ordinal scale has been simplified from the original model proposed by the World Health Organization. Specifically, the category uninfected (no clinical or virological evidence of infection) has been removed as this assessment may be difficult to document definitively. In addition, the categories of ventilation and ventilation plus additional organ support have been combined. 34
TABLE 2.
Ordinal scale for assessing endpoints
| Not hospitalized with resumption of normal activities |
| Not hospitalized but unable to resume normal activities |
| Hospitalized, not requiring supplemental oxygen |
| Hospitalized, requiring supplemental oxygen |
| Hospitalized, requiring nasal high‐flow oxygen, noninvasive mechanical ventilation or both |
| Hospitalized, requiring ECMO, invasive mechanical ventilation, or both |
| Death |
Abbreviations: ECMO, extracorporeal membrane oxygenation.
2.4.2. Secondary outcomes
The secondary endpoint is all cause mortality at day 28. Exploratory endpoints include the need for mechanical ventilation in nonintubated patients, the duration of mechanical ventilation in intubated patients, clinical status according to the ordinal scale up to day 28, mortality at day 90 and 150, length of intensive care unit and hospitalization stay, need for escalation to mechanical circulatory support, trends in PaO2/FiO2 up to day 28 or until discharge, change in Sequential Organ Failure Assessment (SOFA) scores up to day 28 or until discharge, changes in troponin and inflammatory markers (CRP and ferritin), changes in left ventricular ejection fraction (measured at day −3‐0, day 14, and day 90), change in NT‐proBNP or BNP at day 14 if still hospitalized, and time to negative SARS‐CoV2 respiratory sample. Of note, laboratory testing and follow‐up echocardiograms will be performed according to standard of care, assessed opportunistically, and therefore will not be uniform across all patients, as the focus of the trial is on clinical outcomes.
2.5. Statistical analysis
Based on prior reports of poor prognosis in patients who have SARS‐CoV‐2 infection and an elevated troponin in terms of high likelihood of requiring mechanical ventilation, developing ARDS, and death, we estimate that a minority in the control group will demonstrate clinical improvement by day 14. Estimates of efficacy of canakinumab in this patient population are not established. Therefore, no inferential statistics are planned a priori. The results from this proof‐of‐concept study will provide important data regarding effect size to inform feasibility of larger definitive studies. The primary efficacy analysis will be based on an intention‐to‐treat analysis. The primary efficacy comparison will be between patients receiving canakinumab 600 mg IV vs placebo. In the absence of a clear dosage response, the two canakinumab doses will be consolidated and compared to placebo in an exploratory analysis. The time to clinical improvement will be assessed daily until discharge, and for the purposes of the primary endpoint, failure to reach clinical improvement or death before day 14 is considered as right‐censored at day 14. The time to clinical improvement will be assessed with a log‐rank test between canakinumab and placebo. This “recovery rate ratio” is similar to the hazard ratio in survival analysis, but for the beneficial outcome of clinical improvement. Therefore, a “recovery rate ratio” greater than one indicates an improvement with canakinumab, and this analysis plan is aligned with prior randomized studies in patients with Covid‐19. 10 Given that patients censored at day 14 can have either failure of clinical improvement or death, an assessment of death by treatment arm is also important, and the secondary outcome is death at day 28. Of note, we plan to analyze the primary endpoint after all patients have reached day 14 to expeditiously determine whether a larger study should be undertaken. In addition to analysis of the entire cohort, we also plan to perform subgroup analyses of patients who are and who are not intubated at the time of randomization, as these may represent distinction populations. Similarly, in patients who are not intubated at the time of study infusion, an important subgroup analysis will include need for subsequent mechanical ventilation. Given that we do not plan to perform inferential statistics, data for endpoints will be displayed as point estimates with 95% confidence intervals. None of these estimates or intervals should be regarded as definitive for treatment effect.
Demographic and background characteristic variables will be summarized using descriptive summary statistics. Continuous variables will be summarized using n, mean, SD, median, Q1 (25%), Q3 (75%), minimum, and maximum. Categorical variables will be summarized using frequency and percentage.
2.6. Support
The three C study is an investigator‐initiated study conducted by the Cleveland clinic and C5 research with support from Novartis.
3. RESULTS
The baseline characteristics of the first 20 randomized patients are shown in Table 3. Patients are elderly, predominantly male, and nearly half are African‐American. Comorbidities are common with most having hypertension and hyperlipidemia, and about half having diabetes mellitus. A majority has dyspnea as a presenting symptom, and a minority have been intubated at the time of enrollment. CRP and ferritin have been markedly abnormal, whereas high‐sensitivity troponin T and NT‐pro‐BNP have generally been modestly elevated, in keeping with the concept of enrolling patients with early myocardial injury to prevent further deterioration. Left ventricular ejection fractions have been predominantly preserved with a median of 55%, minimum of 35%, and maximum of 65%.
TABLE 3.
Baseline Characteristics of the first 20 randomized patients
| Age (years) | 67.0 (60.9,74.0) |
| Male | 15 (75.0) |
| African‐American | 9 (45.0) |
| Caucasian | 10 (50.0) |
| Body mass index | 28.7 (25.8,41.5) |
| Diabetes mellitus | 9 (45.0) |
| Hypertension | 16 (80.0) |
| Hyperlipidemia | 15 (75.0) |
| Coronary artery disease | 6 (30.0) |
| Stroke | 2 (10.0) |
| Atrial fibrillation or flutter | 3 (15.0) |
| Chronic obstructive pulmonary disease | 3 (15.0) |
| Chronic kidney disease | 6 (30.0) |
| Current or former smoker | 7 (35.0) |
| Time from symptom onset to randomization (days) | 8.5 (6.0,14.0) |
| Dyspnea | 15 (75) |
| Temperature | 37.3 (36.8,37.9) |
| Hospitalized requiring invasive mechanical ventilation | 4 (20.0) |
| Hospitalized requiring nasal high‐flow oxygen or noninvasive ventilation, or both | 6 (30.0) |
| Hospitalized requiring supplemental oxygen | 8 (40.0) |
| Hospitalized, not requiring supplemental oxygen | 2 (10.0) |
| High sensitivity troponin T (ng/L) (reference range < 12 ng/L) | 21.0 (14.5,53.0) |
| N‐terminal pro B‐type natriuretic peptide (pg/mL) (reference range < 125 pg/mL) | 313.0 (208.0,819.0) |
| C reactive protein (mg/dL) (reference range 0.0‐0.4 mg/dL) | 16.2 (10.2, 20.8) |
| Ferritin (ng/mL)(reference range 14.7‐205.1 ng/mL) | 1012.2 (589.4,1962.5) |
| D‐dimer (ng/mL)(reference range < 500 ng/mL) | 1400 (730,1940) |
| Left ventricular ejection fraction (%) | 55 (55,60) |
Note: Expressed as n (%) or median (quartile 1, quartile 3).
4. DISCUSSION
Emerging evidence suggests that those with severe or critical Covid‐19 disease may develop a dysregulated immune response resembling a cytokine storm, which leads to a higher mortality. 3 , 17 , 22 Furthermore, the presence of myocardial injury with elevated troponin and NT‐proBNP correlates with increased inflammation with higher levels of CRP and portends worse outcomes. 19 , 20 , 21 With destruction of host cells and release of SARS‐CoV2 virus, the innate immune response is systemically activated. 35 , 36 A central feature of this response is activation of the NOD‐, LRR‐, and pyrin domain‐containing protein 3 (NLRP3) inflammasome, which subsequently leads to production and systemic release of IL‐1β. Viral activation of the NLRP3 inflammasome is well‐known and has also been previously described with SARS‐CoV, a similar pathogen in the coronavirus family. 37
Il‐1β has been considered the “apical” cytokine of the innate immune response and drives further cytokine and chemokine production as well as activation of macrophages. 38 In addition, IL‐1β induces its own production as well as synthesis of IL‐6, and this cascade leads to pyroptosis (inflammatory‐mediated cell death). 39 This deleterious process may result in vascular inflammation, endothelial dysfunction, and myocardial injury. In such Covid‐19 patients with evidence of myocardial injury and increased inflammation, canakinumab, an IL‐1β antagonist, may be a promising therapeutic option to attenuate the dysregulated immune response (Figure 2). Putative consequences of this systemic inflammatory response include oxygen supply‐demand mismatch in a vulnerable patient population, generation of a prothrombotic milieu, and activation of innate immune cells within preexisting atheroma. 40 , 41
FIGURE 2.
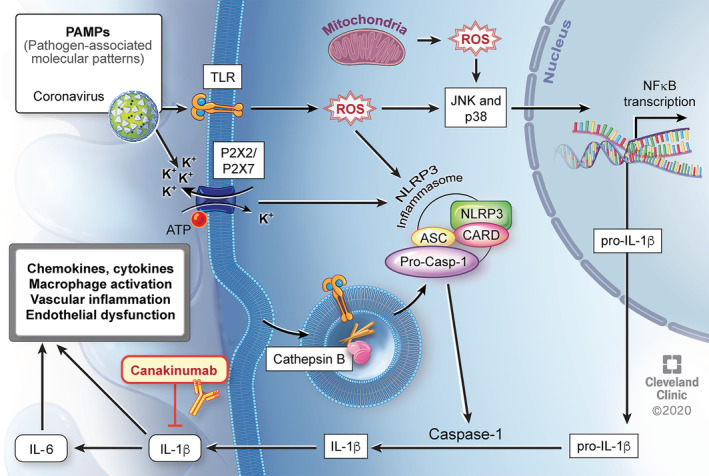
Putative mechanism of SARS‐CoV2 associated myocardial injury with increased inflammation and possible beneficial effect of canakinumab. SARS‐CoV2 acts as an initial PAMP recognized by innate immunity receptors at the cell surface or inside the cell. These receptors are integrated into the inflammasome. Signaling via ROS also leads to NF‐κB activation with increased transcription of pro‐IL‐1β. Inflammasome‐mediated cleavage of pro‐IL‐1β leads to systemic release of active IL‐1β. IL‐1β drives its own expression and production of other chemokines and cytokines, including IL‐6, all resulting in further macrophage activation and potentially contributing to vascular inflammation, endothelial dysfunction, and myocardial injury. Canakinumab may attenuate this response by blocking IL‐1β. ATP, adenosine triphosphate, CARD, caspase activation and recruitment domain, IL, interleukin, JNK, Jun amino‐terminal kinase, K+, potassium ion, NF‐κB, nuclear factor‐kappa B, NLRP3, nucleotide‐binding oligomerization domain‐like receptor pyrin domain‐containing 3, PAMP, pathogen‐associated molecular patterns, ROS, reactive oxygen species, TLR, Toll‐like receptors
Of note, these mechanisms of myocardial injury appear more likely than direct viral cardiotoxicity. In an autopsy study of 39 consecutive cases, high SARS‐CoV2 viral loads were found in 41.0%. 42 These patients had higher levels of cytokines, but there was no increase in inflammatory cells to suggest overt myocarditis. In patients with myocardial injury who have recovered from Covid‐19, abnormalities on cardiac magnetic resonance imaging (CMR) are also common despite overall normal cardiac function. 43 Moreover, in a cohort study of 100 patients with recovered Covid‐19 and CMR, 60% had evidence of ongoing myocardial inflammation, suggesting an opportunity for therapeutic intervention. 44
Currently, randomized controlled trial (RCT) evidence of potential treatments for Covid‐19 is lacking but a number of trials targeting the innate immune response are underway. In addition to canakinumab, RCTs with multiple other immune‐modulating agents including tocilizumab (NCT04320615), sarilumab (NCT04327388), anakinra (NCT04364009), lenzilumab (NCT04351152), mavrilimumab (NCT04399980), and colchicine (NCT04322682) are currently enrolling. Although other studies have investigated troponin as a biomarker endpoint, 45 to the best of our knowledge, the three C study is unique in requiring myocardial injury for inclusion. Patients are also required to have increased systemic inflammation, and this combination identifies a patient population at particularly high risk. In the absence of a vaccine, a sustained burden of disease related to Covid‐19 is expected, 46 , 47 , 48 and in the setting of a favorable signal, this study will enable the design and conduct of a larger more definitive clinical trial.
5. CONCLUSIONS
This blinded randomized controlled trial is designed as a proof of concept study to demonstrate whether IL‐1β antagonism can dampen the deleterious autoinflammatory response to SARS‐CoV2 infection in patients with myocardial injury and heightened inflammation. In evaluating this hypothesis, the Three C study will help inform whether targeting inappropriate activation of the innate immune system should be investigated in larger clinical trials to improve survival in patients with Covid‐19 and myocardial injury.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
FUNDING INFORMATION
Investigator‐initiated study with funding from Novartis.
Appendix A. SUPPLEMENTARY APPENDIX
Data Monitoring Committee.
Chair: Brian Mandell, MD.
James Young, MD.
Steven Gordon, MD.
Herbert Wiedemann, MD.
Katherine E Wolski, MPH
Participating Sites and Investigators:
Cleveland Clinic Main Campus:
Venu Menon, MD.
Calvin Sheng, MD.
Ossama Abou Hassan, MD.
Tom Wang, MD.
Cleveland Clinic Hillcrest Hospital:
Robier Aguillon‐Prada, MD.
Ravi Sunderkrishnan, MD.
Cleveland Clinic Fairview Hospital:
Siddharth Dugar, MD.
Narendrakumar Alappan, MD.
Cleveland Clinic Marymount Hospital:
Debasis Sahoo, MD.
Alice Goyanes, MD.
Cleveland Clinic Florida Weston:
Jamie Hernandez‐Montfort, MD.
Carla McWilliams, MD.
David Wolinsky, MD.
Sheng CC, Sahoo D, Dugar S, et al. Canakinumab to reduce deterioration of cardiac and respiratory function in SARS‐CoV‐2 associated myocardial injury with heightened inflammation (canakinumab in Covid‐19 cardiac injury: The three C study). Clin Cardiol. 2020;43:1055–1063. 10.1002/clc.23451
REFERENCES
- 1. Covid CDC, Team R. Severe outcomes among patients with coronavirus disease 2019 (COVID‐19)—United States, february 12–march 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. World Health Organization . Coronavirus disease 2019 (COVID‐19): situation report, 72 2020.
- 3. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 4. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020. [DOI] [PubMed] [Google Scholar]
- 5. Goyal P, Choi JJ, Pinheiro LC, et al. Clinical characteristics of Covid‐19 in New York city. New England J Med. 2020;382:2372‐2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodriguez‐Morales AJ, Cardona‐Ospina JA, Gutiérrez‐Ocampo E, et al. Latin American Network of Coronavirus Disease 2019‐COVID‐19 Research (LANCOVID‐19). Electronic address: https://www.lancovid.org. Clinical, laboratory and imaging features of COVID‐19: a systematic review and meta‐analysis. Travel Med Infect Dis. 2020;34:101623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with COVID‐19: an open‐label, randomized, controlled trial. MedRxiv. 2020. [Google Scholar]
- 8. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. New England J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grein J, Ohmagari N, Shin D, et al. Compassionate use of remdesivir for patients with severe Covid‐19. New England J Med. 2020;382:2327‐2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beigel JH, Tomashek KM, Dodd LE, et al. Remdesivir for the treatment of Covid‐19 — preliminary report. New England J Med. 2020. [DOI] [PubMed] [Google Scholar]
- 11. Li L, Zhang W, Hu Y, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life‐threatening COVID‐19: a randomized clinical trial. JAMA. 2020;324:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Horby P, Lim WS, Emberson JR, et al. Dexamethasone in hospitalized patients with Covid‐19 ‐ preliminary report. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zheng Y‐Y, Ma Y‐T, Zhang J‐Y, Xie X. COVID‐19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259‐260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gao C, Wang Y, Gu X, et al. Community‐Acquired Pneumonia—China Network. Association between cardiac injury and mortality in hospitalized patients infected with avian influenza a (H7N9) virus. Crit Care Med. 2020;48(4):451‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan China. Intensive care med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study. BMJ. 2020;368m1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gao L, Jiang D, Wen XS, et al. Prognostic value of NT‐proBNP in patients with severe COVID‐19. Respir Res. 2020;21(1):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Guo T, Fan Y, Chen M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID‐19: consider cytokine storm syndromes and immunosuppression. The Lancet. 2020;395(10229):1033‐1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chakraborty A, Tannenbaum S, Rordorf C, et al. Pharmacokinetic and pharmacodynamic properties of canakinumab, a human anti‐interleukin‐1β monoclonal antibody. Clin Pharmacokinet. 2012;51(6):e1‐e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lachmann HJ, Kone‐Paut I, Kuemmerle‐Deschner JB, et al. Canakinumab in CAPS Study Group. Use of canakinumab in the cryopyrin‐associated periodic syndrome. New England J Med. 2009;360(23):2416‐2425. [DOI] [PubMed] [Google Scholar]
- 25. Ridker PM, Everett BM, Thuren T, et al. CANTOS Trial Group. Antiinflammatory therapy with canakinumab for atherosclerotic disease. New England J Med. 2017;377(12):1119‐1131. [DOI] [PubMed] [Google Scholar]
- 26. Kuemmerle‐Deschner JB, Hachulla E, Cartwright R, et al. Two‐year results from an open‐label, multicentre, phase III study evaluating the safety and efficacy of canakinumab in patients with cryopyrin‐associated periodic syndrome across different severity phenotypes. Ann Rheum Dis. 2011;70(12):2095‐2102. [DOI] [PubMed] [Google Scholar]
- 27. Fisher CJ, Dhainaut J‐FA, Opal SM, et al. Recombinant human interleukin 1 receptor antagonist in the treatment of patients with sepsis syndrome: results from a randomized, double‐blind, placebo‐controlled trial. JAMA. 1994;271(23):1836‐1843. [PubMed] [Google Scholar]
- 28. Shakoory B, Carcillo JA, Chatham WW, et al. Interleukin‐1 receptor blockade is associated with reduced mortality in sepsis patients with features of the macrophage activation syndrome: re‐analysis of a prior phase III trial. Crit Care Med. 2016;44(2):275‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Granowitz EV, Porat R, Mier JW, et al. Pharmacokinetics, safety and immunomodulatory effects of human recombinant interleukin‐1 receptor antagonist in healthy humans. Cytokine. 1992;4(5):353‐360. [DOI] [PubMed] [Google Scholar]
- 30. Cavalli G, De Luca G, Campochiaro C, et al. Interleukin‐1 blockade with high‐dose anakinra in patients with COVID‐19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatol. 2020;2:e325‐e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ucciferri C, Auricchio A, Di Nicola M, et al. Canakinumab in a subgroup of patients with COVID‐19. The Lancet Rheumatol. 2020;2:e457‐ee458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mueller C, Giannitsis E, Christ M, et al. Multicenter evaluation of a 0‐hour/1‐hour algorithm in the diagnosis of myocardial infarction with high‐sensitivity cardiac troponin T. Ann Emerg Med. 2016;68(1):76‐87.e74. [DOI] [PubMed] [Google Scholar]
- 33. Thygesen K, Alpert JS, Jaffe AS, et al. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. 2018;72(18):2231‐2264. [DOI] [PubMed] [Google Scholar]
- 34. WHO . R&D Blueprint COVID‐19 Therapeutic Trial Synopsis. http://www.who.int/blueprint/priority‐diseases/key‐action/novel‐coronavirus/en/.
- 35. Turner AJ, Hiscox JA, Hooper NM. ACE2: from vasopeptidase to SARS virus receptor. Trends Pharmacol Sci. 2004;25(6):291‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hoffmann M, Kleine‐Weber H, Schroeder S, et al. SARS‐CoV‐2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271‐280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shi C‐S, Nabar NR, Huang N‐N, Kehrl JH. SARS‐coronavirus open Reading frame‐8b triggers intracellular stress pathways and activates NLRP3 inflammasomes. Cell Death Discov. 2019;5:101‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ridker PM. From C‐reactive protein to interleukin‐6 to interleukin‐1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118(1):145‐156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dinarello CA. Interleukin‐1 in the pathogenesis and treatment of inflammatory diseases. Blood J American Soc Hematol. 2011;117(14):3720‐3732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105(9):1135‐1143. [DOI] [PubMed] [Google Scholar]
- 41. Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID‐19 in Wuhan. Clinic Infect Diseases. 2020;71(15):762–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Linder D, Fitzek A, Brauninger H, et al. Association of cardiac infection with SARS‐CoV2 in confimred COVID‐19 autopsy cases. JAMA Cardiol. 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Knight DS, Kotecha T, Razvi Y, et al. COVID‐19: Myocardial injury in survivors. Circulation. https://doi.org:0.1161/CIRCULATIONAHA.120.049252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Puntmann VO, Carerj L, Wieters I, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Deftereos SG, Giannopoulos G, Vrachatis DA, et al. on behalf of the GRECCO‐19 investigators Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: the GRECCO‐19 randomized clinical trial. JAMA Netw Open. 2020;3(6):e2013136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Shim E, Tariq A, Choi W, Lee Y, Chowell G. Transmission potential and severity of COVID‐19 in South Korea. Int J Infect Dis. 2020;93:339‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kucharski AJ, Russell TW, Diamond C, et al. Early dynamics of transmission and control of COVID‐19: a mathematical modelling study. Lancet Infect Dis. 2020;20:553‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swerdlow DL, Finelli L. Preparation for possible sustained transmission of 2019 novel coronavirus: lessons from previous epidemics. JAMA. 2020;323(12):1129‐1130. [DOI] [PubMed] [Google Scholar]


