Abstract
High salt intake increases blood pressure, and dietary salt intake has been clearly demonstrated to be associated with hypertension incidence. Japanese people consume higher amounts of salt than Westerners. It has been reported that miso soup was one of the major sources of daily salt intake in Japanese people. Adding salt is indispensable to make miso, and therefore, in some cases, refraining from miso soup is recommended to reduce dietary salt intake. However, recent studies using salt-sensitive hypertensive models have revealed that miso lessens the effects of salt on blood pressure. In other word, the intake of miso dose not increase the blood pressure compared to the equivalent intake of salt. In addition, many clinical observational studies have demonstrated the absence of a relationship between the frequency of miso soup intake and blood pressure levels or hypertension incidence. The mechanism of this phenomenon seen in the subjects with miso soup intake has not been fully elucidated yet. However, in basic studies, it was found that the ingredients of miso attenuate sympathetic nerve activity, resulting in lowered blood pressure and heart rate. Therefore, this review focused on the differences between the effects of miso intake and those of the equivalent salt intake on sympathetic nerve activity, blood pressure, and heart rate.
Keywords: Miso, Hypertension, Sympathetic nerve activity, Blood pressure, Heart rate
Introduction
Elevated blood pressure is an important reason for increased morbidity and mortality [1, 2]. Both basic and clinical studies have shown that high salt intake increases the blood pressure [3–5]. A well-known study called The INTERSALT study revealed that dietary salt intake is related to the blood pressure level, after adjusting for confounding factors [6]. In addition, high salt intake is associated with a higher risk of cardiovascular disease [7], and dietary changes, which included reduction in salt intake, were reported to be associated with lower blood pressure. Therefore, reducing salt intake has been established as a standard method to prevent and treat hypertension [8–10], with patients instructed to refrain from salt intake for the prevention and treatment of hypertension.
Recently, the role of sympathetic nerve activity (SNA) in the pathogenesis of salt-induced hypertension has gained emphasis [11, 12]. The sympathetic nervous system, which modulates SNA, is well known to play an important role in the regulation of blood pressure and heart rate [13, 14]. Although the precise mechanisms remain unclear, several factors are reported to contribute to salt-induced sympathoexcitation. For example, in Dahl salt-sensitive rats (Dahl-S rats) and spontaneously hypertensive rats (SHR), which are known to be salt-sensitive hypertensive rats, higher dietary intake of salt increased the sodium concentration in the cerebrospinal fluid (CSF[Na]) [15]. The increase in CSF[Na] activates the neurons located in the cardiovascular center, resulting in sympathoexcitation [16]. Brain mineralocorticoid receptor (MR), epithelial sodium channels, and angiotensin type 1 receptor (AT1R) also contribute to salt-induced sympathoexcitation [17–21]. The activation of these pathways occurs only in salt-sensitive models, but not in salt-resistant models [15]. In contrast, intra-cerebroventricular (ICV) infusion of high-sodium artificial CSF (aCSF) increases SNA, blood pressure, and heart rate, even in salt-resistant models [22]. Collectively, these results indicate that the sympathetic nervous system plays an important role in salt-induced hypertension by modulating SNA. In addition, it has been clarified that an increase in SNA, particularly in renal SNA, plays an important role in salt-induced hypertension [23].
Compared with the Westerners, Japanese people consume higher amounts of salt [24] with miso soup and pickles being the major sources of daily salt intake in the Japanese [25]. Miso is a traditional Japanese soybean paste made from fermented soybeans. It is often prepared into a soup and is one of the major components of Japanese-style cooking. One serving of miso soup contains 1–2 g of salt, and the addition of salt is indispensable to miso preparation. Therefore, in some cases, it is recommended to refrain from miso soup to reduce dietary salt intake. However, recent studies using salt-sensitive hypertensive models have demonstrated that the intake of miso does not increase SNA, blood pressure, and heart rate compared with the equivalent intake of salt. Therefore, this review focused on the differences between the effects of miso intake and those of the equivalent salt intake on SNA, blood pressure, and heart rate.
Expectations for soybean products, a major constituent of Japanese-style diet
International interest in the Japanese-style diet has increased because of its health benefits. In fact, some reports have demonstrated that a Japanese-style diet reduces total mortality [26] and is associated with a longer survival time [27]. A recent qualitative systematic review study has evaluated the Japanese-style diet and has categorized its dietary characteristics [28]. This study segregated Japanese dietary constituents into 16 categories and revealed that the top 3 categories are soybeans/soybean-derived products, seafood, and vegetables, followed by rice and miso soup. Miso is also a soybean product. Therefore, soybean products are considered to contribute to the health benefits of Japanese-style diets.
Specifically, miso is a fermented soybean product. Fermentation is reported to improve the quality of soybeans, resulting in increased digestibility, enhanced nutrition, and increased isoflavone content [29]. Therefore, fermented soybean products are expected to have greater health benefits than nonfermented soybean products. In fact, a recent study has revealed that the intake of fermented soy products, but not nonfermented soy products, is inversely associated with developing high blood pressure in subjects with normal blood pressure [30]. Because miso, which is the focus of this review, is a traditional Japanese fermented soybean product, it is expected to have a significant impact on suppressing the development of high blood pressure.
Miso intake in basic studies
Many studies have revealed that habitual dietary intake of miso soup can prevent the worsening of hypertension caused by high salt intake in genetically salt-sensitive models. One study reported that the habitual dietary intake of miso soup attenuates the salt-induced blood pressure elevation compared with the equivalent intake of salt in Dahl-S rats, with the magnitude of blood pressure reduction caused by habitual dietary intake of miso soup reaching 35 mmHg [31]. Another study reported that the estimated decreases in blood pressure caused by habitual dietary intake of miso corresponded to 30% decrease in salt loading [32]. This study also revealed the natriuresis and diuresis through the dopamine system by habitual dietary intake of miso. In addition, the attenuation of the salt-induced blood pressure elevation observed in habitual dietary intake of miso soup was also demonstrated in stroke-prone SHR, which are also genetically salt-sensitive rats [33]. Miso intake was also confirmed to be associated with alleviation of organ damage and have protective effects against stroke [32–34].
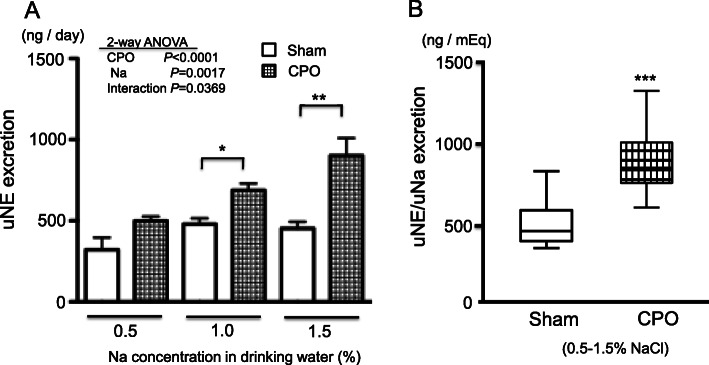
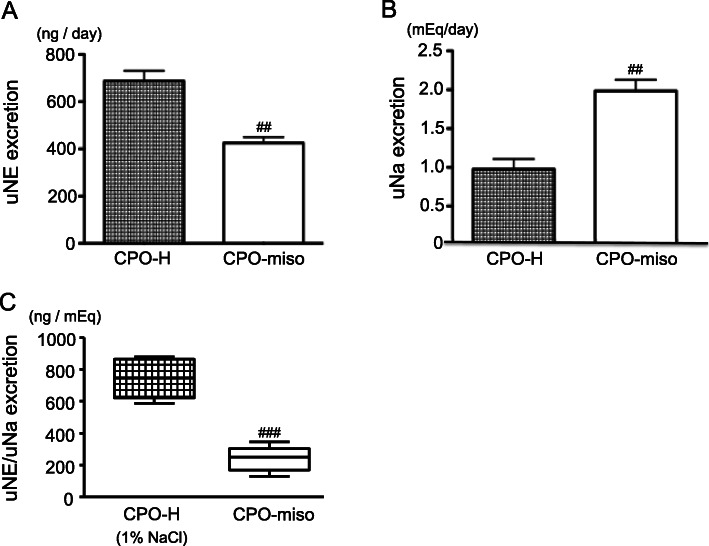
These effects observed in habitual dietary intake of miso soup were also confirmed using non-genetically salt-sensitive model. Non-genetic salt-sensitivity is acquired via abdominal aortic banding in mice [17]. The aortic banding causes chronic pressure overload (CPO) in the left side of the heart, resulting in left ventricular hypertrophy. In mice with CPO (CPO-mice), the brain hypothalamic MR- AT1R pathway gets activated [18–20]. Activation of this pathway combined with high salt intake leads to an increase in SNA, concomitant with further activation of this pathway. Figure 1 shows SNA evaluated on the basis of 24-h urinary norepinephrine (uNE) excretion [35], which increased according to the salt concentration of drinking water in CPO-mice but not in sham-operated control mice (Sham-mice) (Fig. 1a). The ratio of uNE/24-h urinary sodium (uNa) excretion, indicating uNE excretion per uNa excretion of 1 mEq, as a marker of SNA in response to salt intake was greater in CPO-mice than in Sham-mice (Fig. 1b). These results demonstrated that CPO-mice have acquired non-genetic salt sensitivity. In contrast, habitual dietary intake of miso soup suppressed the activation of the brain hypothalamic MR-AT1R pathway in CPO-mice, even though miso soup contained a measurable amount of salt [35]. In CPO-mice with miso soup consumption, uNE excretion was low (Fig. 2a), whereas uNa excretion was high (Fig. 2b) when compared with CPO-mice with high-salt water consumption. Therefore, the ratio of uNE/uNa excretion ratio was found to be lower in CPO-mice with miso soup consumption than in those with high-salt water consumption (Fig. 2c). In this study, heart rates were also lower in CPO-mice fed with miso soup than in CPO-mice fed with high salt water (499 ± 11 vs 535 ± 14 bpm) [35]. These results show that habitual dietary intake of miso soup can attenuate salt-induced sympathoexcitation and blood pressure elevation, despite the increased amount of salt intake.
Fig. 1.
a SNA evaluated on the basis of uNE excretion in each group. *p < 0.05, **p < 0.01 Sham versus CPO, n = 8 for each. Open bar graphs indicate Sham; square-block bar graph indicates CPO. b The ratio of uNE/uNa excretion, as a marker of SNA in response to salt intake. ***p < 0.001 Sham versus CPO, n = 24 for each. Open bar graphs indicate Sham; square-block bar graph indicates CPO. SNA, sympathetic nerve activity; Sham, sham-operated control mice; CPO, chronic pressure overload mice; uNE, 24-h urinary norepinephrine; uNa, 24-h urinary sodium
Fig. 2.
a SNA evaluated on the basis of uNE excretion in each group. ##p = 0.001, CPO-H versus CPO-miso, n = 8 for each. Open bar graphs indicate CPO-miso; square-block bar graph indicates CPO-H. b Na intake in terms of uNa excretion in each group. ##p = 0.001, CPO-H versus CPO-miso, n = 8 for each. Open bar graphs indicate CPO-miso; square-block bar graph indicates CPO-H. c The ratio of uNE/uNa excretion, as a marker of SNA in response to salt intake. ###p < 0.001, CPO-H versus CPO-miso, n = 8 for each. Open bar graphs indicate CPO-miso; square-block bar graph indicates CPO-H. SNA, sympathetic nerve activity; CPO-H, chronic pressure overload mice with high salt water; CPO-miso, chronic pressure overload mice with miso soup; uNE, 24-h urinary norepinephrine; uNa, 24-h urinary sodium
Miso intake in clinical studies
Increased miso soup intake is expected to cause hypertension, particularly in subjects with salt-sensitivity, because of increased salt intake. However, previous studies have demonstrated that the frequency of miso soup intake was not associated with high levels of blood pressure. One study reported that two daily servings of miso soup for 3 months did not affect the blood pressure of subjects with normotension or stage I hypertension [36]. In addition, a community-based prospective study reported that an increased intake of miso soup is associated with a reduced incidence of cardiovascular disease [37]. Intake of fermented soy products, such as miso and natto, was reported to be inversely associated with the development of high blood pressure in Japanese adults with normotension [30]. The results of these observational studies show that habitual miso soup intake has no effect on the levels of blood pressure or hypertension incidence in subjects with normotension or mild hypertension.
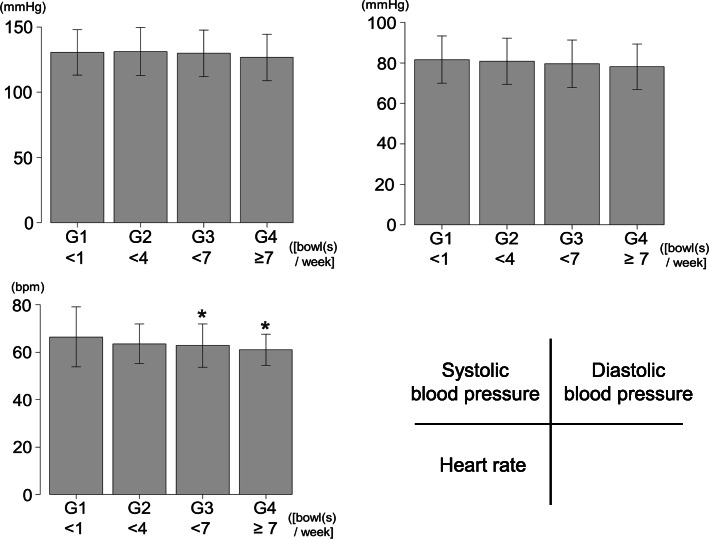
Recently, it was reported that heart rate in subjects with habitual miso soup intake was lower than in those without it [38]. Figure 3 shows the levels of blood pressure and heart rate in each group, with four groups stratified according to the frequency of miso soup intake. There was no association between the frequency of miso soup intake and the levels of blood pressure. However, the heart rate of subjects who reported a high frequency of miso soup intake was lower than that of subjects who reported a lower frequency of miso soup intake. A multivariable analysis adjusting for a priori identified covariates revealed that the frequency of miso soup intake independently affects heart rate but not blood pressure [38].
Fig. 3.
Blood pressure levels and heart rates in each group. G1, Group 1 (< 1 bowl of miso soup/week); G2, Group 2 (< 4 bowls of miso soup/week); G3, Group 3 (< 7 bowls of miso soup/week); G4, Group 4 (≥ 7 bowls of miso soup/week). *p < 0.05 versus G1
It is well known that the occurrence of sudden death due to acute cardiovascular diseases, such as acute myocardial infarction and cerebral infarction, increases in winter [39, 40]. The prevalence of hypertension also increases in winter [41] and the seasonal variations in SNA contribute to the increase in blood pressure in winter [42]. High SNA is well known to be associated with poor prognosis resulting from worsening cardiovascular disease [43–45]. Therefore, attenuation of SNA, particularly in the winter, is thought to have a significant impact on the prevention and treatment of cardiovascular diseases. A study evaluated the relationship between habitual miso soup intake and the levels of blood pressure or heart rate of Japanese adults and categorized the study into four seasons [46]. The heart rate of the participants who seldom consumed miso soup was higher in winter than in the other seasons. However, the heart rate of those who frequently had miso soup was not high in winter compared with that in the other seasons. Heart rate is modulated by autonomic nerve balance and is correlated with SNA; thus, it is a useful marker of SNA [47]. Therefore, habitual miso soup intake may be useful for preventing cardiovascular disease via the attenuation of enhanced SNA in winter.
Effects of miso on sympathetic nerve system
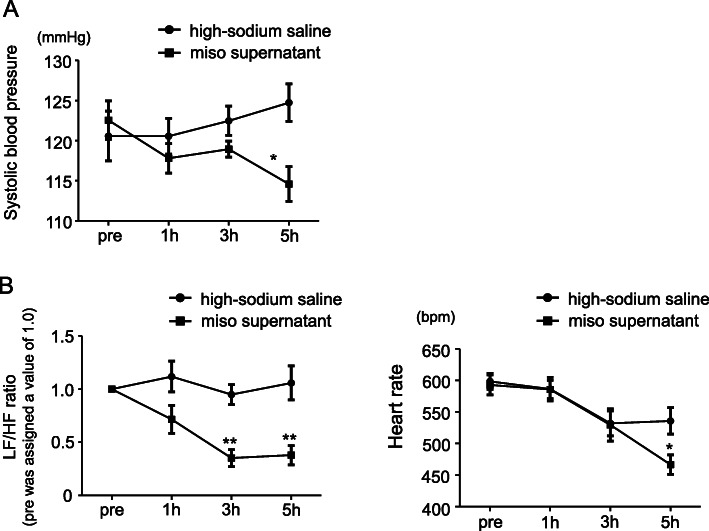
The relationship between miso intake and SNA has been clearly demonstrated in a previous study [48]. As shown in Fig. 4, intraperitoneal (IP) injection of high-sodium saline did not change the blood pressure in mice without salt-sensitivity (i.e., normal mice). However, the IP injection of miso supernatant with sodium level equivalent to high-sodium saline decreased the blood pressure (Fig. 4a). In this study, heart rate was evaluated using electrocardiogram (ECG), and SNA was also evaluated using ECG time-frequency analysis. As shown in Fig. 4b, the low frequency (LF)/high frequency (HF) ratio and the heart rate did not change on IP injection of high-sodium saline, but both the LF/HF ratio and heart rate decreased on IP injection of miso supernatant. Low LF/HF ratio indicates low SNA. Therefore, systemic administration of miso supernatant decreases SNA in normal mice. As shown in Fig. 5, in CPO-mice (i.e., non-genetically salt-sensitive mice), the LF/HF ratio increased on IP injection of high-sodium saline, indicating salt-induced sympathoexcitation. These results are consistent with the salt sensitivity in CPO-mice. Interestingly, IP injection of the miso supernatant did not increase the LH/HF ratio even when the sodium level was the same as the high-sodium saline. These results indicate the cancelation of salt-induced sympathoexcitation by systemic administration of miso supernatant. In fact, a recent study demonstrated that the decrease in blood pressure due to miso intake in subjects with high-normal blood pressure or stage I hypertension may be caused by deactivation of the adrenergic nervous system [49].
Fig. 4.
a Systolic blood pressure in normal mice after IP injection of high-sodium saline or miso supernatant. n = 5 for each. *p < 0.05 high-sodium saline versus miso supernatant. b LF/HF ratio (left) and heart rate (right) in normal mice evaluated via ECG. The LF/HF ratio before the injection was assigned a value of 1.0. n = 9 for each. *p < 0.05, **p < 0.01 high-sodium saline versus miso supernatant. IP, intraperitoneal; LF, low frequency power of heart rate variability; HF, high frequency power of heart rate variability
Fig. 5.
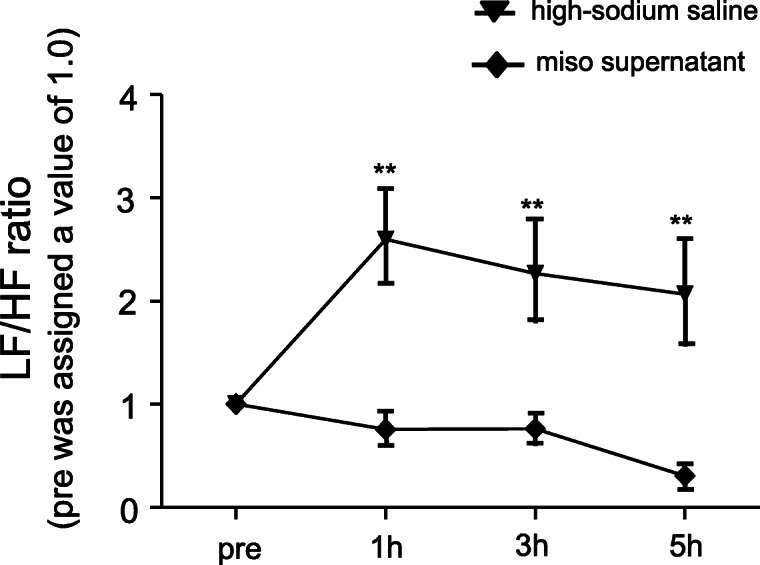
LF/HF ratio in CPO-mice evaluated via ECG. The LF/HF ratio before the injection was assigned a value of 1.0. n = 5 for each. **p < 0.01 high-sodium saline versus miso supernatant. LF, low frequency power of heart rate variability; HF, high frequency power of heart rate variability
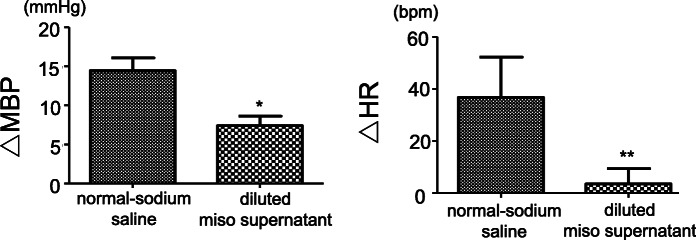
SNA is modulated by the sympathetic nervous system located in the brain. The possibility that the ingredients of miso work directly in the brain has also been demonstrated in a previous study [48]. Increase in CSF[Na] is known to increase blood pressure and heart rate [22]. As shown in Fig. 6, the degree of increases in blood pressure and heart rate produced by the ICV infusion of high-sodium aCSF was smaller in mice pre-treated with diluted miso supernatant (0.14 M sodium) than in those pre-treated with normal-sodium saline (0.14 M sodium) [48]. The results of this study indicate that the ingredients of miso work in the brain to modulate SNA and to attenuate salt-induced blood pressure and heart rate elevation.
Fig. 6.
Changes in mean blood pressure (left) and heart rate (right) after high-sodium saline ICV infusion. n = 5 for each. *p < 0.05, **p < 0.01 high-sodium saline versus miso supernatant. ICV, intra-cerebroventricular
The mechanisms involved in attenuated salt-induced sympathoexcitation following habitual dietary intake of miso soup have not been fully understood. As mentioned above, miso is a soybean product. Therefore, isoflavone may be involved in the inhibitory effects of miso on SNA, blood pressure, and heart rate [50, 51]. The results from previous studies have revealed that inhibition of the renin-angiotensin system (RAS) plays an important role in the effects caused by miso intake. The RAS in the brain modulates SNA and contributes to the pathogenesis of hypertension [52, 53]. Interestingly, fermented soybeans, including miso, are known to block the angiotensin-converting enzyme (ACE) activity [54, 55]. The active ingredient of miso that has ACE inhibitory activity was reported to be a small peptide [33]. Therefore, such small peptides with anti-ACE inhibitory effects might work directly in several organs, including the brain. In fact, it was revealed that the habitual intake of miso soup decreases AT1R expression in the brain of CPO-mice [35]. Therefore, inhibition of brain RAS is thought to be one of the mechanisms involved in attenuated salt-induced sympathoexcitation caused by miso intake.
Reduction of oxidative stress may be another key player in the attenuated salt-induced sympathoexcitation caused by miso intake. The decrease in brain oxidative stress due to miso intake was reported in a study using Dahl-S rats [56]. Brain oxidative stress is well known to be associated with blood pressure elevation via sympathoexcitation [57–59]. Furthermore, there is the complex interaction between RAS, oxidative stress, and SNA. For example, oxidative stress generated by RAS activation in the brain is reported to contribute to sympathoexcitation and the pathogenesis of cardiovascular disease [60]. Therefore, reduction of both RAS activity and oxidative stress in the brain may be involved in attenuated salt-induced sympathoexcitation in response to habitual dietary intake of miso soup.
Effects of miso on other molecular mechanisms which regulate blood pressure
The attenuation of the salt-induced blood pressure elevation by miso intake may be caused by vasodilation and natriuresis independent of SNA. In fact, it was reported that RAS activation causes vasoconstriction through enhanced myosin light chain kinase expression [61]. In addition, previous studies revealed the relationship between RAS and vascular injury characterized by endothelial dysfunction, structural remodeling, and inflammation [62]. Oxidative stress is also associated with the pathogenesis of hypertension via the deterioration of vascular function [63, 64]. Furthermore, the RAS in the kidney is also known to contribute to blood pressure regulation via modulation of sodium homeostasis. Specifically, AT1R expression in the renal proximal tubule is reported to regulate sodium homeostasis. Increases in AT1R expression in the proximal tubule leads to blood pressure elevation [65]. Oxidative stress also contributes to renal tubular transport including sodium transport [66]. There are complex relationships between RAS and oxidative stress in various organs that are involved in blood pressure regulation. Therefore, the reduction of both RAS activity and oxidative stress in several organs, such as the brain, blood vessels, or kidney, may be involved in the attenuated salt-induced hypertension following the habitual dietary intake of miso soup.
Perspectives
This review article summarizes the effects of miso intake on SNA, blood pressure, and heart rate. Because miso contains some amount of salt, habitual miso intake is expected to increase blood pressure. However, previous basic studies clearly demonstrated that miso intake attenuates salt-induced sympathoexcitation and blood pressure elevation in the salt-sensitive hypertensive models. In addition, it was also demonstrated that miso intake lowers SNA in normal models. Basic animal experiments have shown the direct effects of miso intake on SNA, blood pressure, and heart rate. Collectively, these findings support that miso has a potential to lower SNA, blood pressure, and heart rate.
The results obtained from animal experiments cannot be directly applied to clinical settings. Previous clinical studies failed to demonstrate the relationship between the frequency of miso intake and blood pressure levels. However, the heart rate, which is a useful marker of SNA, was lower in subjects with frequent miso soup consumption than in those with infrequent miso soup consumption, particularly in the winter. This may mean that the results of clinical studies and basic studies do not contradict each other. However, data are inadequate to conclude the effects of miso on blood pressure and heart rate in humans. The subjects with high miso soup consumption are thought to have the traditional Japanese dietary pattern. If so, they may consume more vegetables, seaweeds, and fish as well as miso. Therefore, the beneficial effects observed in the subjects with high miso soup consumption may be a result of not only miso, but also other foods. As such, interventional studies are necessary to establish the effects of miso on blood pressure and heart rate in human. In addition, there are other concerns with the interpretation of the findings of the previous studies. First, the precise mechanisms involved in the lowering of SNA, blood pressure, and heart rate following miso intake have not been fully understood. The miso ingredients that have inhibitory effects on SNA, blood pressure, and heart rate remain unclear. Second, the appropriate dietary intake of miso remains unclear. Third, previous studies demonstrated the safety of habitual miso soup intake in subjects with normotension or stage I hypertension, but not in those with stage II or III hypertension. Also, the safety of habitual miso intake in subjects with significant salt sensitivity remains unclear. Further studies will be needed to clarify these points.
Conclusion
The intake of miso soup does not increase blood pressure and heart rate compared with the equivalent intake of salt, probably in part due to the lowering of SNA.
Abbreviations
- ACE
Angiotensin-converting enzyme
- aCSF
Artificial CSF
- AT1R
Angiotensin type 1 receptor
- CPO
Chronic pressure overload
- CSF[Na]
Sodium concentration in the cerebrospinal fluid
- Dahl-S rats
Dahl salt-sensitive rats
- ECG
Electrocardiogram
- HF
High frequency
- ICV
Intra-cerebroventricular
- IP
Intraperitoneal
- LF
Low frequency
- MR
Mineralocorticoid receptor
- RAS
Renin-angiotensin system
- Sham-mice
Sham-operated control mice
- SHR
Spontaneously hypertensive rats
- SNA
Sympathetic nerve activity
- uNa
Urinary sodium
- uNE
Urinary norepinephrine
Author’s contributions
KI conceptualized and drafted the paper. The author read and approved the final manuscript.
Funding
Not applicable
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The author declares no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kannel WB. Role of blood pressure in cardiovascular morbidity and mortality. Prog Cardiovasc Dis. 1974;17:5–24. doi: 10.1016/0033-0620(74)90034-6. [DOI] [PubMed] [Google Scholar]
- 2.Smirk FH. The prognosis of untreated and treated hypertension and advantages of early treatment. Am Heart J. 1972;83:825–840. doi: 10.1016/0002-8703(72)90216-5. [DOI] [PubMed] [Google Scholar]
- 3.Feng W, Dell’Italia LJ, Sanders PW. Novel paradigms of salt and hypertension. J Am Soc Nephrol. 2017;28:1362–1369. doi: 10.1681/ASN.2016080927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garfinkle MA. Salt and essential hypertension: pathophysiology and implication for treatment. J Am Soc Hypertens. 2017;11:385–391. doi: 10.1016/j.jash.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 5.Grillo A, Salvi L, Coruzzi P, Salvi P, Parati G. Sodium intake and hypertension. Nutrients. 2019;11:E1970. doi: 10.3390/nu11091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stamler J. The INTERSALT study: background, findings, and implications. Am J Clin Nutr. 1997;65(Suppl):626S–642S. doi: 10.1093/ajcn/65.2.626S. [DOI] [PubMed] [Google Scholar]
- 7.Takachi R, Inoue M, Shimazu T, Sasazuki S, Ishihara J, Sawada N, Yamaji T, Iwasaki M, Iso H, Tsubono Y, Tsugane S, Japan Public Health Center-based Prospective Study Group Consumption of sodium and salted foods in relation to cancer and cardiovascular disease: the Japan Public Health Center-based prospective study. Am J Clin Nutr. 2010;91:456–464. doi: 10.3945/ajcn.2009.28587. [DOI] [PubMed] [Google Scholar]
- 8.Frisoli TM, Schmieder RE, Grodzicki T, Messerli FH. Salt and hypertension: is salt dietary reduction worth the efforts? Am J Med. 2012;125:433–439. doi: 10.1016/j.amjmed.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 9.Wang M, Moran AE, Liu J, Qi Y, Xie W, Tzong K, Zhao D. A meta-analysis of effect of dietary salt restriction in blood pressure in Chinease adults. Glob Heart. 2015;10:291–299. doi: 10.1016/j.gheart.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graudal NA, Hubeck-Graudal T, Jurgens G. Effects of low sodium diet versus high sodium diet in blood pressure, renin, aldosterone, catecholamines, cholesterol, and triglyceride. Cochrane Database Syst Rev. 2017;4:CD004022. doi: 10.1002/14651858.CD004022.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stocker SD, Madden CJ, Sved AF. Excess dietary salt intake alters the excitability of sympathetic networks. Physiol Behav. 2010;100:519–524. doi: 10.1016/j.physbeh.2010.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujita M, Fujita T. The role of CNS in salt-sensitive hypertension. Curr Hypertens Rep. 2013;15:390–394. doi: 10.1007/s11906-013-0358-z. [DOI] [PubMed] [Google Scholar]
- 13.Voora R, Hinderliter AL. Modulation of sympathetic overactivity to treat resistant hypertension. Curr Hypertens Rep. 2018;20:92. doi: 10.1007/s11906-018-0893-8. [DOI] [PubMed] [Google Scholar]
- 14.Zhou JJ, Ma HJ, Shao JY, Pan HL, Li DP. Impaired hypothalamic regulation of sympathetic outflow in primary hypertension. Neurosci Bull. 2019;35:124–132. doi: 10.1007/s12264-018-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang BS, Van Vliet BN, Leenen FH. Increases in CSF [Na+] precede the increases in blood pressure in Dahl S rats and SHR on a high-salt diet. Am J Physiol Heart Circ Physiol. 2004;287:H1160–H1166. doi: 10.1152/ajpheart.00126.2004. [DOI] [PubMed] [Google Scholar]
- 16.Adrogué HJ, Madias NE. Sodium and potassium in the pathogenesis of hypertension: focus on the brain. Curr Opin Nephrol Hypertens. 2017;26:106–113. doi: 10.1097/MNH.0000000000000301. [DOI] [PubMed] [Google Scholar]
- 17.Ito K, Hirooka Y, Sunagawa K. Acquisition of brain Na sensitivity contributes to salt-induced sympathoexcitation and cardiac dysfunction in mice with pressure overload. Circ Res. 2009;104:1004–1011. doi: 10.1161/CIRCRESAHA.108.188995. [DOI] [PubMed] [Google Scholar]
- 18.Ito K, Hirooka Y, Sunagawa K. Blockade of mineralocorticoid receptors improves salt-induced left-ventricular systolic dysfunction through attenuation of enhanced sympathetic drive in mice with pressure overload. J Hypertens. 2010;28:1449–1458. doi: 10.1097/hjh.0b013e328338bb37. [DOI] [PubMed] [Google Scholar]
- 19.Nakano M, Hirooka Y, Matsukawa R, Ito K, Sunagawa K. Mineralocorticoid receptors/epithelial Na(+) channels in the choroid plexus are involved in hypertensive mechanisms in stroke-prone spontaneously hypertensive rats. Hypertens Res. 2013;36:277–284. doi: 10.1038/hr.2012.174. [DOI] [PubMed] [Google Scholar]
- 20.Ito K, Hirooka Y, Nakano M, Honda N, Matsukawa R, Sunagawa K. Role of hypothalamic angiotensin type 1 receptors in pressure overload-induced mineralocorticoid receptor activation and salt-induced sympathoexcitation. Hypertens Res. 2013;36:513–519. doi: 10.1038/hr.2012.221. [DOI] [PubMed] [Google Scholar]
- 21.Wang HW, Huang BS, Chen A, Ahmad M, White RA, Leenen FH. Role of brain aldosterone and mineralocorticoid receptors in aldosterone-salt hypertension in rats. Neuroscience. 2016;314:90–105. doi: 10.1016/j.neuroscience.2015.11.055. [DOI] [PubMed] [Google Scholar]
- 22.Huang BS, White RA, Ahmad M, Jeng AY, Leenen FH. Central infusion of aldosterone synthase inhibitor prevents sympathetic hyperactivity and hypertension by central Na+ in Wister rats. Am J Phys Regul Integr Comp Phys. 2008;295:R166–R172. doi: 10.1152/ajpregu.90352.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fujita T. Mechanism of salt-sensitive hypertension: focus on adrenal and sympathetic nervous systems. J Am Soc Nephrol. 2014;25:1148–1155. doi: 10.1681/ASN.2013121258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D, Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE) Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open. 2013;3:e003733. doi: 10.1136/bmjopen-2013-003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wakasugi M, James Kazama J, Narita I. Associations between the intake of miso soup and Japanese pickles and the estimated 24-hour urinary sodium excretion: a population-based cross sectional study. Intern Med. 2015;54:903–910. doi: 10.2169/internalmedicine.54.4336. [DOI] [PubMed] [Google Scholar]
- 26.Okada E, Nakamura K, Ukawa S, Wakai K, Date C, Iso H, Tamakoshi A. The Japanese food score and risk of all-cause, CVD and cancer mortality: the Japan Collaborative Cohort Study. Br J Nutr. 2018;120:464–471. doi: 10.1017/S000711451800154X. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Tomata Y, Sugawara Y, Tsuduki T, Tsuji I. The Japanese dietary pattern is associated with longer disability-free survival time in the general elderly population in the Ohsaki Cohort 2006 Study. J Nutr. 2019;149:1245–1251. doi: 10.1093/jn/nxz051. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki N, Goto Y, Ota H, Kito K, Mano F, Joo E, Ikeda K, Inagaki N, Nakayama T. Characteristics of the Japanese diet described in epidemiologic publications: a qualitative systematic review. J Nutr Sci Vitaminol. 2018;64:129–137. doi: 10.3177/jnsv.64.129. [DOI] [PubMed] [Google Scholar]
- 29.Jayachandran M, Xu B. A insight into the health benefits of fermented soy products. Food Chem. 2019;271:362–371. doi: 10.1016/j.foodchem.2018.07.158. [DOI] [PubMed] [Google Scholar]
- 30.Nozue M, Shimazu T, Sasazuki S, Charvat H, Mori N, Mutoh M, Sawada N, Iwasaki M, Yamaji T, Inoue M, Kokubo Y, Yamagishi K, Iso H, Tsugane S. Fermented soy product intake is inversely associated with the development of high blood pressure: the Japan Public Health Center-based Prospective Study. J Nutr. 2017;147:1749–1756. doi: 10.3945/jn.117.250282. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe H, Kashimoto N, Kajimura J, Kamiya K. A miso (Japanese soybean paste) diet conferred greater protection against hypertension than a sodium chloride diet in Dahl salt-sensitive rats. Hypertens Res. 2006;29:731–738. doi: 10.1291/hypres.29.731. [DOI] [PubMed] [Google Scholar]
- 32.Yoshinaga M, Toda N, Tamura Y, Terakado S, Ueno M, Otsuka K, Numabe A, Kawabata Y, Uehara Y. Japanese traditional miso soup attenuates salt-induced hypertension and its organ damage in Dahl salt-sensitive rats. Nutrition. 2012;28:924–931. doi: 10.1016/j.nut.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 33.Tomari HS, Uchikawa M, Yamazaki A, Hirabayashi S, Yamakawa S, Kitagawa M, Yamada M, Itou S, Yamamoto T, Uehara Y. Newly manufactured Marukome MK-34-1 miso with angiotensin-converting enzyme inhibitory activity and its antihypertensive effects in genetic hypertensive rat models. Hypertens Res. 2019;42:790–800. doi: 10.1038/s41440-018-0197-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe H, Sasatani M, Doi T, Masaki T, Satoh K, Yoshizumi M. Protective effects of Japanese soybean paste (Miso) on stroke in stroke-prone spontaneously hypertensive rats (SHRSP) Am J Hypertens. 2017;8(31):43–47. doi: 10.1093/ajh/hpx129. [DOI] [PubMed] [Google Scholar]
- 35.Ito K, Hirooka Y, Sunagawa K. Miso (Japanese soybean paste) soup attenuates salt-induced sympathoexcitation and left ventricular dysfunction in mice with chronic pressure overload. Fukuoka Acta Medica. 2014;105:48–56. [PubMed] [Google Scholar]
- 36.Kitagawa M, Itoh K, Yamada M, Koike S, Yamamoto T, Uehara Y. Long term intake of miso soup unaffected blood pressure in subjects with normal or stage I hypertension-double blind comparative interventional trial. Jpn Pharmacol Ther. 2016;44:1601–1612. [Google Scholar]
- 37.Kokubo Y, Iso H, Ishihara J, Okada K, Inoue M, Tsugane S, JPHC Study Group Association of dietary intake of soy, beans, and isoflavones with risk of cerebral and myocardial infarction in Japanese populations: the Japan Public Health Center-based (JPHC) study cohort I. Circulation. 2007;116:2553–2262. doi: 10.1161/CIRCULATIONAHA.106.683755. [DOI] [PubMed] [Google Scholar]
- 38.Ito K, Miyata K, Mohri M, Origuchi H, Yamamoto H. The effects of the habitual consumption of miso soup on the blood pressure and heart rate of Japanese adults: a cross-sectional study of a health examination. Intern Med. 2017;56:23–29. doi: 10.2169/internalmedicine.56.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arntz HR, Willich SN, Schreiber C, Brüggemann T, Stern R, Schultheiss HP. Diurnal, weekly and seasonal variation of sudden death. Population-based analysis of 24,061 consecutive cases. Eur Heart J. 2000;21:315–320. doi: 10.1053/euhj.1999.1739. [DOI] [PubMed] [Google Scholar]
- 40.Manfredini R, Fabbian F, Pala M, Tiseo R, De Giorgi A, Manfredini F, Malagoni AM, Signani F, Andreati C, Boari B, Salmi R, Imberti D, Gallerani M. Seasonal and weekly patterns of occurrence of acute cardiovascular disease: dose a gender difference exist? J Women's Health. 2011;20:1663–1668. doi: 10.1089/jwh.2011.2734. [DOI] [PubMed] [Google Scholar]
- 41.Sinha P, Taneja DK, Singh NP, Saha R. Seasonal variation in prevalence of hypertension: implications for interpretation. Indian J Public Health. 2010;54:7–10. doi: 10.4103/0019-557X.70537. [DOI] [PubMed] [Google Scholar]
- 42.Sharma BK, Sagar S, Sood GK, Varma S, Kalra OP. Seasonal variations of arterial pressure in normotensive and essential hypertensives. Indian Heart J. 1990;42:66–72. [PubMed] [Google Scholar]
- 43.Floras JS. Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. J Am Coll Cardiol. 2009;54:375–385. doi: 10.1016/j.jacc.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 44.Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. doi: 10.1016/j.jacc.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 45.Florea VG, Cohn JN. The autonomic nervous system and heart failure. Circ Res. 2014;114:1815–1826. doi: 10.1161/CIRCRESAHA.114.302589. [DOI] [PubMed] [Google Scholar]
- 46.Ito K, Miyata K, Mohri M, Origuchi H, Yamamoto H. Relationship between miso soup consumption and blood pressure or heart rate of Japanese adults: a cross-sectional study divided into four seasons. JJCDP. 2017;52:82–93. doi: 10.2169/internalmedicine.56.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grassi G, Vailati S, Bertinieri G, Seravalle G, Stella ML, Dell'Oro R, Mancia G. Heart rate as marker of sympathetic activity. J Hypertens. 1998;16:1635–1639. doi: 10.1097/00004872-199816110-00010. [DOI] [PubMed] [Google Scholar]
- 48.Ito K, Hirooka Y, Sunagawa K. Japanese soybean paste miso lessens sympathovagal imbalance and attenuates brain sodium sensitivity in mice with pressure overload. Fukuoka Acta Medica. 2015;106:54–63. [PubMed] [Google Scholar]
- 49.Kondo H, Sakuyama Tomari H, Yamakawa S, Kitagawa M, Yamada M, Itou S, Yamamoto T, Uehara Y. Long-term intake of miso soup decreases nighttime blood pressure in subjects with high-normal blood pressure or stage I hypertension. Hypertens Res. 2019. 10.1038/s41440-019-0304-9. [DOI] [PMC free article] [PubMed]
- 50.Huikuri HV, Pikkujämsä SM, Airaksinen KE, Ikäheimo MJ, Rantala AO, Kauma H, Lilja M, Kesäniemi YA. Sex-related differences in autonomic modulation of heart rate in middle-age subjects. Circulation. 1996;94:122–125. doi: 10.1161/01.cir.94.2.122. [DOI] [PubMed] [Google Scholar]
- 51.Pamidimukkala J, Taylor JA, Welshons WV, Lubahn DB, Hay M. Estrogen modulation of baroreflex function in conscious mice. Am J Phys Regul Integr Comp Phys. 2003;284:R983–R989. doi: 10.1152/ajpregu.00761.2001. [DOI] [PubMed] [Google Scholar]
- 52.Sagara Y, Hirooka Y, Nozoe M, Ito K, Kimura Y, Sunagawa K. Pressor response induced by central angiotensin II is mediated by activation of Rho/Rho-kinase pathway via AT1 receptors. J Hypertens. 2007;25:399–406. doi: 10.1097/HJH.0b013e328010b87f. [DOI] [PubMed] [Google Scholar]
- 53.Marc Y, Llorens-Cortes C. The role of the brain renin-angiotensin system in hypertension: implications for new treatment. Prog Neurobiol. 2011;95:89–103. doi: 10.1016/j.pneurobio.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 54.Inoue K, Gotou T, Kitajima H, Mizuno S, Nakazawa T, Yamamoto N. Release of antihypertensive peptides in miso paste during its fermentation, by the addition of casein. J Biosci Bioeng. 2009;108:111–115. doi: 10.1016/j.jbiosc.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 55.De Leo F, Panarese S, Gallerani R, Ceci LR. Angiotensin converting enzyme (ACE) inhibitory peptides: production and implementation of functional food. Curr Pharm Des. 2009;15:3622–3643. doi: 10.2174/138161209789271834. [DOI] [PubMed] [Google Scholar]
- 56.Du DD, Yoshinaga M, Sonoda M, Kawakubo K, Uehara Y. Blood pressure reduction by Japanese traditional Miso is associated with increased diuresis and natriuresis through dopamine system in Dahl salt-sensitive rats. Clin Exp Hypertens. 2014;36:359–366. doi: 10.3109/10641963.2013.827702. [DOI] [PubMed] [Google Scholar]
- 57.Nagae A, Fujita M, Kawarazaki H, Matsui H, Ando K, Fujita T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation. 2009;119:978–986. doi: 10.1161/CIRCULATIONAHA.108.824730. [DOI] [PubMed] [Google Scholar]
- 58.Fujita M, Ando K, Kawarazaki H, Kawarasaki C, Muraoka K, Ohtsu H, Shimizu H, Fujita T. Sympathoexcitation by brain oxidative stress mediates arterial pressure elevation in salt-induced chronic kidney disease. Hypertension. 2012;59:105–112. doi: 10.1161/HYPERTENSIONAHA.111.182923. [DOI] [PubMed] [Google Scholar]
- 59.Kishi T, Hirooka Y, Kimura Y, Ito K, Shimokawa H, Takeshita A. Increased reactive oxygen species in rostral ventrolateral medulla contribute to neural mechanisms of hypertension in stroke-prone spontaneously hypertensive rats. Circulation. 2004;109:2357–2362. doi: 10.1161/01.CIR.0000128695.49900.12. [DOI] [PubMed] [Google Scholar]
- 60.Chan SH, Chan JY. Angiotensin -generated reactive oxygen species in brain and pathogenesis of cardiovascular disease. Antioxid Redox Signal. 2013;19:1074–1084. doi: 10.1089/ars.2012.4585. [DOI] [PubMed] [Google Scholar]
- 61.Han YJ, Hu WY, Piano M, de Lanerolle P. Regulation of myosin light chain kinase expression by angiotensin II in hypertension. Am J Hypertens. 2008;21:860–865. doi: 10.1038/ajh.2008.199. [DOI] [PubMed] [Google Scholar]
- 62.Montezano AC, Nguyen Dinh Cat A, Rios FJ, Touyz RM. Angiotensin II and vascular injury. Curr Hypertens Rep. 2014;16:431. doi: 10.1007/s11906-014-0431-2. [DOI] [PubMed] [Google Scholar]
- 63.Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 64.Sinha N, Dabla PK. Oxidative stress and antioxidants in hypertension-a current review. Curr Hypertens Rev. 2015;11:132–142. doi: 10.2174/1573402111666150529130922. [DOI] [PubMed] [Google Scholar]
- 65.van Haaster MC, McDonough AA, Gurley SB. Blood pressure regulation by the angiotensin type 1 receptor in the proximal tubule. Curr Opin Nephrol Hypertens. 2018;27:1–7. doi: 10.1097/MNH.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gonzalez-Vicente A, Hong NJ, Garvin JL. Effects of reactive oxygen species on renal tubular transport. Am J Physiol Ren Physiol. 2019 Jun 19. 10.1152/ajprenal.00604.2018. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable