Abstract
Being a pandemic and having a high global case fatality rate directed us to assess the evidence strength of hydroxychloroquine efficacy in treating coronavirus disease‐2019 (COVID‐19) arising from clinical trials and to update the practice with the most reliable clinical evidence.
A comprehensive search was started in June up to 18 July, 2020 in many databases, including PubMed, Embase, and others. Of 432 studies found, only six studies fulfilled the inclusion criteria, which includes: clinical trials, age more than 12 years with nonsevere COVID‐19, polymerase chain reaction‐confirmed COVID‐19, hydroxychloroquine is the intervention beyond the usual care. Data extraction and bias risk assessment were done by two independent authors. Both fixed‐effect and random‐effect models were utilized for pooling data using risk difference as a summary measure. The primary outcomes are clinical and radiological COVID‐19 progression, severe acute respiratory syndrome coronavirus‐2 clearance in the pharyngeal swab, and mortality. The secondary outcomes are the adverse effects of hydroxychloroquine.
Among 609 COVID‐19 confirmed patients obtained from pooling six studies, 294 patients received hydroxychloroquine and 315 patients served as a control. Hydroxychloroquine significantly prevents early radiological progression relative to control with risk difference and 95% confidence interval of −0.2 (−0.36 to −0.03). On the other hand, hydroxychloroquine did not prevent clinical COVID‐19 progression, reduce 5‐day mortality, or enhance viral clearance on days 5, 6, and 7. Moreover, many adverse effects were reported with hydroxychloroquine therapy. Failure of hydroxychloroquine to show viral clearance or clinical benefits with additional adverse effects outweigh its protective effect from radiological progression in nonsevere COVID‐19 patients. Benefit‐risk balance should determine the hydroxychloroquine use in COVID‐19.
Keywords: COVID‐19, hydroxychloroquine, mortality, Progression, viral clearance
Highlights
Hydroxychloroquine does not reduce mortality or save COVID‐19 patients live.
Hydroxychloroquine does not promote a clinical improvement of SARS CoV‐2 infected patients.
Hydroxychloroquine Fails to accelerate clearance of new coronavirus.
Hydroxychloroquine increases adverse events specially diarrhea without proven clinical benefits.
Meta‐analysis of clinical trials failed to show clear evidence, large randomized trial is required.
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19), also known as severe acute respiratory syndrome coronavirus‐2 (SARS CoV‐2), 1 was first recognized in Wuhan city in China in December 2019. 2 Shortly after, it was declared a pandemic by World Health Organization (WHO). 3 Coronaviruses belong to the family of Coronaviridae, which are enveloped viruses with a single‐strand RNA. 4 Before discovering COVID‐19, two important coronaviruses were discovered a few years ago; severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome. 5 To date, the global case fatality rate of hospitalized patients with COVID‐19 infection is estimated to be 6.5%. 6 In Italy, the estimated case fatality rate is 7.2%, 7 which is higher than that reported in China (2.3%). 8
The pathogenesis of COVID‐19 was explained by cytokine storm, 9 reduction in ACE2 expression, 10 and activation of complement pathways‐induced microvascular injury and thrombosis. 11
Despite lack of strong evidence, some agents are proposed to improve clinical outcomes of COVID‐19 based on their mechanisms of action, in vitro activity against SARS CoV‐2, low‐quality observational studies, or small interventional studies. These agents include hydroxychloroquine (HCQ), chloroquine, lopinavir/ritonavir, ivermectin, favipiravir, tocilizumab, colchicine, interferons, macrolides, and so forth. 12 Also remdesivir is a promising broad spectrum anti‐coronavirus agent. 13 On 1 May, 2020, the Food Drug Administration issued an emergency use authorization for remdesivir to be used in severe COVID‐19 patients. 14 The emergency use authorization was also issued for HCQ in March and revoked in June 2020 due to safety and efficacy concerns. 15
HCQ is a weak base 4‐aminoquinoline, developed in 1946 as an antimalarial agent, which is a safer derivative than chloroquine. 16 The antiviral activity of HCQ against viral diseases such as human immunodeficiency virus and SARS was studied many years ago. 17 It also showed in vitro activity against SARS CoV‐2 by inhibiting viral entry through targeting early endosomes and endolysosomes. 18 Moreover, HCQ could modulate the immune response and reduce proinflammatory cytokines, 19 which are important inducers of acute respiratory distress syndrome. 20 Few retrospective observational studies reported some benefits in treating COVID‐19 patients, where HCQ decreased mortality and IL‐6 level, 21 decreased case fatality rate, 22 and improved patient survival. 23 On the other hand, other observational studies reported no benefits and more frequent side effects while using HCQ. 24 , 25 , 26 The same controversies are found in the randomized clinical trials (RCT) that investigated HCQ efficacy in COVID‐19. In addition, HCQ is one of the most widely used agents for treating COVID‐19 infection despite insufficient supporting evidence. A few numbers of meta‐analyses investigating this subject were conducted. However, they were criticized for some flaws addressed and discussed afterward.
Therefore, there is an urgency to conduct a systematic review and meta‐analysis including all available clinical trials that meet the prespecified inclusion criteria. The objectives are to summarize the efficacy of HCQ use in COVID‐19 relative to control based on available clinical trials indicated by all possible improvements of the disease and to pool all short‐term possible side effects related to HCQ therapy in COVID‐19 patients.
2. METHOD
The Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) Statement were followed to improve reporting the present systematic review. 27
2.1. Protocol and registration
The protocol was registered in the International Prospective Reregister of Systematic Review with registration number CRD42020195886 in June 2020.
2.2. Eligibility criteria
The inclusion criteria for the eligible studies for systematic review and meta‐analysis include: clinical trials either randomized or not. COVID‐19 patients more than 12 years. Infected with SARS CoV‐2 and had a polymerase chain reaction (PCR) confirmation test; the test should be based on nasopharyngeal or oropharyngeal swab. Nonsevere infection (mild and moderate) based on clinical assessment by each study. The treatment arm is HCQ ± usual treatment that was given according to each hospital and was not proven to be anti‐COVID‐19. Control group is only on the usual treatment. Outcomes: any clinical outcomes or drug‐related side effects during the follow‐up period.
2.3. Information sources
The following databases were used for studies identification: PubMed, Embase, Cochrane, Google Scholar, ClinicalTrial.gov, ProQuest, Science direct, Chinese Clinical Trial Registry (ChiCTR), and medRxiv. The search started in June and continued through July 2020 to track all new studies.
2.4. Search strategy
The advanced search was used in different databases with limitation to clinical trials and fields of title and abstract without other limitations. The synonyms applied in search terms were SARS or COVID and HCQ or plaquenil. Three researchers independently underwent comprehensive searching and identified certain studies after removing duplicated ones.
2.5. Study selection
According to the PRISMA flow diagram (Figure 1), 28 the selection of eligible studies for meta‐analysis from identified ones was conducted by two researchers through three steps; abstracts screening for relevant studies, full‐text articles assessment for eligibility, and effect measures assessment for quantitative synthesis. Disagreements were resolved by discussion among the authors.
Figure 1.
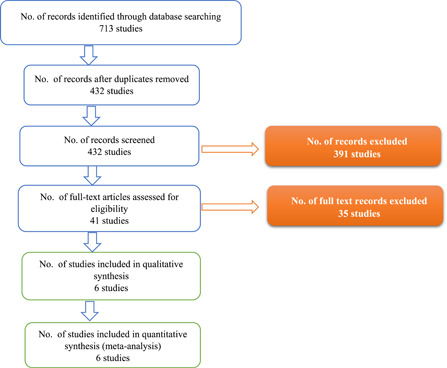
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses flow chart of the included studies in the qualitative and quantitative synthesis
2.6. Data collection process
“Data collection form for intervention reviews: RCTs and non‐RCTs” developed by Cochrane was used for data extraction. 29 Numbers were extracted directly from text and tables and indirectly from graphs using Getdata graph digitizer version 2.26.0.20. 30 Data extraction was done by three independent authors.
2.7. Variables definition
There are three types of variables; (a) independent variable is HCQ therapy; (b) dependent variables include viral clearance in the pharyngeal swab, clinical progression (increase in the baseline severity), radiological progression, adverse effects, and mortality; (c) confounders include usual treatment that varied among studies, age, sex, disease onset, and different HCQ doses.
2.8. Risk of bias in the included studies and across the studies
The Cochrane Risk of Bias Tools to assess the bias risk were followed. 31 It includes six domains: selection bias, reporting bias, performance bias, detection bias, attrition bias, and other sources of bias. The Consolidated Standards of Reporting Trials were also utilized. 32 The risk of bias was assessed by three authors and a final consensus was done. Publication bias could not be assessed because of the low number of the included studies. Sensitivity analysis was performed after removing the low‐quality studies.
2.9. Synthesis of the quantitative results
The principal summary measures were risk difference (RD) for the outcomes, odds ratio (OR) for gender, and mean difference (MD) for age with 95% confidence interval (CI) to compare between HCQ arm and control arm using RevMan version 5.4. Statistical heterogeneity was tested using the Q statistic and quantified with I 2 value. Each of fixed‐effect and random‐effect models was used to pool the effect sizes according to the heterogeneity of each outcome. 33 Mantel Hazel method and inverse variance method were used for dichotomous data and continuous data, respectively. All time point meta‐analysis was used to summarize the result of viral clearance at each available time point.
3. RESULTS
3.1. Study selection
PRISMA flow diagram in Figure 1 shows that 432 studies were identified after the removal of duplications, 391 studies were removed after screening titles and abstracts based on their relevance, and 35 studies were removed after assessment of full article for eligibility based on the inclusion criteria. The remaining six studies were included in the systematic review and meta‐analysis. Some studies were excluded because of their retrospective design, 34 did not recruit PCR‐confirmed cases, 35 , 36 or recruited less than 12‐year‐old patients. 36
3.2. Study characteristics
The population of the included studies had nonsevere COVID‐19 except two severe patients in Tang et al's 37 study. The disease severity definition slightly varied by the studies. It was based on the Chinese guidelines in three studies, 37 , 38 , 39 , 40 based on WHO clinical progression scale 41 in Mitjà et al's study, 42 required ICU admission in Gautret et al's 43 study, or who was mechanically ventilated/had comorbid conditions in Barbosa et al's study. 44 The disease onset before HCQ treatment varied from 1 and 4 days in three studies 42 , 43 , 44 to 16 days in another study. 37
HCQ regimens varied among the studies; only three studies used loading doses of 800 and 1200 mg/d. 37 , 42 , 44 Maintenance daily doses of 200, 400, 600, 800 mg were also used according to each study (Table 1).
Table 1.
Characteristics of the included studies
| Study ID | Mean age ± SD (male %) | HCQ arm (follow‐up) | Usual treatment that was given to all patients as required | Outcomes (combinable and not combinable) | Events HCQ (control) |
|---|---|---|---|---|---|
| Chen et al 38 | HCQ: | 400 mg/d for 5 d (7 d) | O2 therapy, interferon‐alpha, lopinavir/ritonavir, antibiotics, and supportive treatment | Viral clearance | 13/15 (14/15) |
| 50.5 ± 3.8 (60%) | HCQ side effects | 4/15 (3/15) | |||
| Clinical progression | 1/15 (0/15) | ||||
| Control: | |||||
| 46.7 ± 3.6 (80%) | Radiological progression | 5/15 (7/15) | |||
| Barbosa et al 44 | HCQ: | 400 mg LD BID for 1‐2 d then 200‐400 mg/d for a total 5 d (7 d) | O2 therapy | Rate of intubation | 7/17 (2/21) |
| 59.76 ± 18.92 (46.9%) | Change in Respiratory | 0.76 ± 0.83 (0.24 ± 0.7) | |||
| Support level: mean ± SD | |||||
| Control: | |||||
| 64.00 ± 15.92 (71%) | Change in lymphocyte count | 0.8 ± 0.46 (1 ± 0.49) | |||
| Mortality | 2/17 (1/21) | ||||
| Gautret et al 43 | HCQ: | 200 mg TID for 10 d (14 d) | Symptomatic treatment and antibiotics | Viral clearance | 14/20 (2/16) |
| 51.2 ± 18.7 (45%) | Clinical progression | 3/26a (0/16) | |||
| Mortality | 1/26a (0/16) | ||||
| Control: | |||||
| 37.3 ± 24.0 (37.5%) | |||||
| Tang et al 37 | HCQ: | 1200 mg/d LD for 3 d, then 800 mg daily for 2‐3 wk (4 wk) | Some antiviral agents, antibiotics, and corticosteroids | Viral clearance | 60/70 (65/80) |
| 48.0 ± 14.1 (56%) | Disease progression | 1/70 (0/80) | |||
| Mortality | 0/70 (0/80) | ||||
| Control: | |||||
| 44.1 ± 15 | All adverse effects | 21/70 (7/80) | |||
| (53%) | |||||
| Chen et al 39 | HCQ: | 400 mg/d for 5 d (5 d) | O2 therapy, antiviral agents, antibacterial agents, and immunoglobulin, ±corticosteroids | Clinical progression | 0/31 (4/31) |
| 44.1 ± 16.1 (45.2%) | Radiological progression | 2/31 (9/31) | |||
| Control: | Radiological improvement | 25/31 (17/31) | |||
| 45.2 ± 14.7 (48.3%) | |||||
| Fever: days ± SD | 2.2 ± 0.4 (3.2 ± 1.3) | ||||
| Cough: days ± SD | 2 ± 0.2 (3.1 ± 1.5) | ||||
| Adverse effects | 2/31 (0/31) | ||||
| Mitjà et al 42 | HCQ: | 800 mg/d LD for 1 day, then 400 mg daily for 6 d | Usual care | Viral load reduction (log10 copies/mL): mean ± SE (day 3) | −1.41 ± 0.15 (−1.41 ± 0.14) |
| 41.6 ± 12.4 (27.9%) | |||||
| Viral load reduction (log10 copies/mL): mean ± SE (day 7) | −3.44 ± 0.19 (−3.37 ± 0.18) | ||||
| Control: | |||||
| 41.7 ± 12.6 (34.4%) | (28 d) | ||||
| Hospitalization | 8/136 (11/154) | ||||
| Adverse effects (absolute) | 282 (23) |
Abbreviations: BID, twice daily; HCQ, hydroxychloroquine; LD, loading dose; O2, oxygen; SD, standard deviation; SE, standard error; TID, trice daily.
Denominator is the initial sample size in HCQ arm.
Usual treatment was given to all patients according to needs and varied widely among the studies. It included supportive care, symptomatic treatment, steroids, antibiotics, and antivirals 37 , 38 , 39 , 42 , 43 (Table 1). The two groups were comparable in all mentioned baseline factors in each included study.
3.3. Risk of bias within studies
The risk of bias is summarized in Figure 2. The study design of Barbosa et al 44 was included as it emulated clinical trial design and the recall bias was unlikely. It was obtained as an under‐reviewing article from the New England journal of medicine in June 2020 and considered a low‐quality study. The study of Tang et al 37 did not show attrition, at least during the first weeks of the follow‐up, but randomization was violated by moving some patients between the groups and changing in baseline factors. Two of the six included studies are of low quality after all authors' agreement, which are Barbosa et al 44 and Gautret et al. 45 The study of Chen et al 39 has been registered in the Chinese Clinical Trial Registry since February 2020 with the unique identifier of ChiCTR2000029559. It has been cited more than 238 times, although it has not been published yet.
Figure 2.
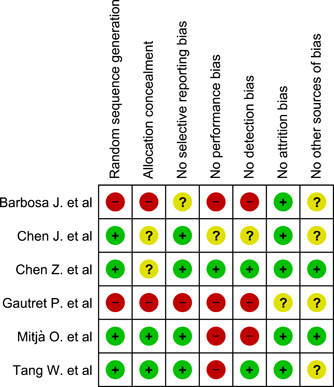
Risk of bias assessment of the included six studies; “+” in the green circles: low risk; “−” in the red circles: high risk; “?” in the yellow circles: unknown
3.4. Synthesis of results
Among 609 COVID‐19 confirmed patients obtained from pooling six studies, 294 patients received HQC and 315 patients served as a control (results of the individual studies are summarized in Table 1). The pooled age was significantly higher in the HCQ‐treated group compared to control with MD of 2.13 and 95% CI of (0.42‐3.85). The pooled gender did not differ between the groups, where the OR of males in the HQC‐treated group relative to control was 0.81 (0.58‐1.13).
3.4.1. Efficacy
Viral clearance in pharyngeal swab at three time points pooled from three studies 37 , 38 , 43 using all time point meta‐analysis and fixed‐effect model had a nonsignificant RD of 0.04 (−0.1 to 0.18), 0.06 (−0.08 to 0.2), −0.12 (−0.26 to 0.02) on days 5, 6, 7, respectively. Heterogeneity was significant on days 5 and 6 (χ 2 = 10.16, P = .001, I 2 = 90%; χ 2 = 17.37, P < .0001, I 2 = 94%, respectively). The RD on days 5 and 6 using the random‐effect model was 0.19 (−0.33 to 0.7) and 0.25 (−0.38 to 0.88), respectively (Figure 3). Early computed tomography (CT)‐based radiological progression (within 5‐7 days) pooled from two studies 38 , 39 using the fixed‐effect model had a significant RD of −0.2 (−0.36 to −0.03) which favored HCQ. Heterogeneity was not significant (χ 2 = 0.23, P = .63) (Figure 4). Early clinical progression (within 5‐7 days) was not statistically significant between the two groups. RD pooled from four studies 38 , 39 , 43 , 44 using fixed‐effect model was 0.06 (−0.03 to 0.15), while in random‐effect model it was 0.07 (−0.10 to 0.24). Heterogeneity was significant (χ 2 = 12.6, P = .006, I 2 = 76%). It was identified as a rate of intubation in Barbosa et al's 44 study and the rate of ICU admissions in Gautret et al's 43 study (Figure 4). Clinical progression within 28 days was not statistically significant between the two groups. RD pooled from two studies 37 , 42 using the fixed‐effect model was −0.00 (−0.04 to 0.04). Heterogeneity was not significant (χ 2 = 0.96, P = .33). It was identified as a rate of hospitalization in Mitjà et al's 42 study and a progression from mild to moderate or severe in Tang et al's 37 study (Figure 4). Five‐day mortality was not statistically significant between the two groups. RD pooled from four studies 37 , 42 , 43 , 44 using the fixed‐effect model was 0.01 (−0.01 to 0.03). Heterogeneity was not significant (χ 2 = 2.47, P = .48) (Figure 4). Twenty‐eight days mortality was not statistically significant between the two groups. RD pooled from two studies 37 , 42 using the fixed‐effect model was 0.00 (−0.01 to 0.01). Heterogeneity was not significant (χ 2 = 0.00, P = 1) (Figure 4).
Figure 3.
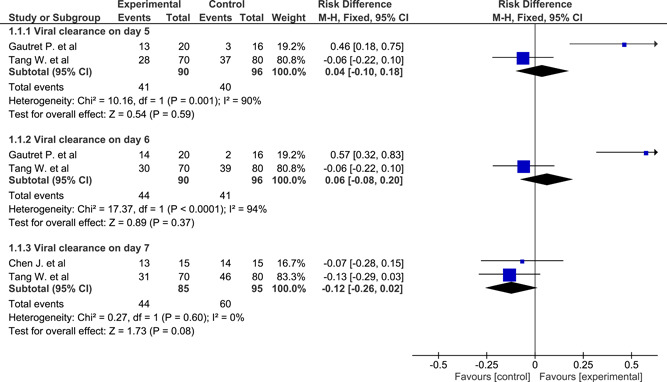
Forest plot of viral clearance at three time points using the fixed‐effect model and risk difference with 95% confidence interval
Figure 4.
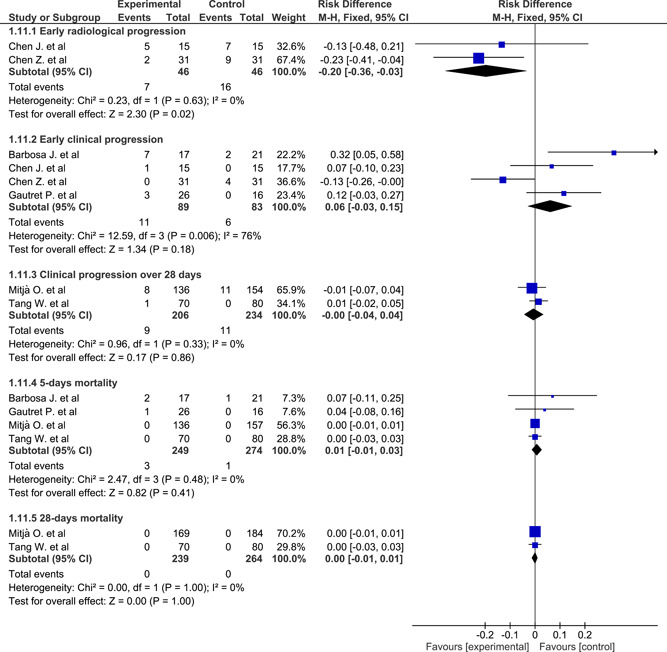
Forest plot of radiological and clinical progression and mortality using the fixed‐effect model and risk difference with 95% confidence interval
3.4.2. Safety
Gastrointestinal adverse effects pooled from three studies 37 , 38 , 42 using the fixed‐effect model had a significant RD of 0.59 (0.55 to 0.64) which favored control, while using the random‐effect model gave a nonsignificant RD of 0.36 (−0.21 to 0.64). Heterogeneity was significant (χ 2 = 264, P < .001). The three studies with different follow‐up periods; 7 and 28 days were combined based on that the gastrointestinal side effects of HCQ are more likely to occur early and with high doses. 46 The gastrointestinal adverse effects included nausea, vomiting, diarrhea, abdominal bloating and discomfort, and decreased appetite (Figure 5). Dermatological adverse effects pooled from three studies 37 , 39 , 42 using the fixed‐effect model had a significant RD of 0.05 (0.02 to 0.08) which favored control. Heterogeneity was not significant (χ 2 = 3.99, P = .14). They included skin rash and flush. (Figure 5). Cardiac adverse effects over a 28‐day follow‐up period pooled from two studies 37 , 42 using fixed‐effect model had a nonsignificant RD of 0.01 (−0.01 to 0.03), while in the random‐effect model it was 0.02 (−0.05 to 0.09). Heterogeneity was significant (χ 2 = 6.35, P = .01). They included sinus bradycardia, hypertension, and orthostatic hypotension. There were no arrhythmias detected among the studies (Figure 5). CNS adverse effects pooled from three studies 37 , 39 , 42 using the fixed‐effect model had a significant RD of 0.23 (0.18 to 0.28) which favored control, while in the random‐effect model, it was not significant 0.13 (−0.20 to 0.46). Heterogeneity was significant (χ 2 = 158, P < .0001). They included blurred vision, headache, drowsiness, and metallic test (Figure 5).
Figure 5.
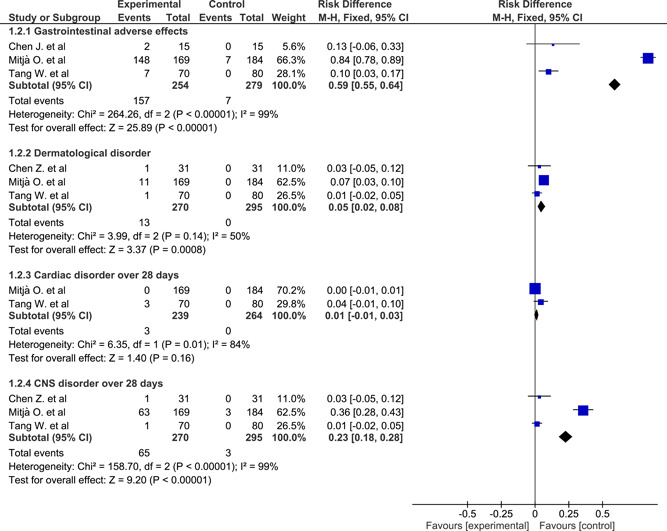
Forest plot of adverse effects of hydroxychloroquine among patients with coronavirus disease‐2019 using the fixed‐effect model and risk difference with 95% confidence interval
3.5. Sensitivity analysis
After excluding the two low‐quality studies, 44 , 45 the same analyses on the applicable outcomes were performed. No difference was observed between the two analyses on the progression, mortality, and viral clearance.
4. DISCUSSION
HCQ therapy in COVID 19 is still a matter of debate among healthcare providers. 47 It was introduced early in the pandemic based on early studies. 48 Meanwhile, numerous observational and interventional studies raised concerns about the safety of the drug and even prematurely terminated due to serious cardiac side effects. 24 , 49 , 50 , 51
Sensitive indicators for a possible efficacy of anti‐COVID‐19 drugs should rely on the improvement in the disease clinical course and modification in possible causes of the related mortality. Radiological abnormalities of the lung could be a good measure of drug efficacy. Lung abnormalities on chest CT in patients with COVID‐19 changed gradually from ground‐glass opacities on the first days to an increase in the crazy‐paving pattern after 1 week, then became consolidated on day 10 and started to resolve after 2 weeks of the disease course. 52 The ability of a drug to prevent disease progression from mild/moderate to severe has been targeted as a reliable efficacy measure. 53 Accordingly, it could inhibit the pathophysiological pathways of the virus. The disease severity was defined by WHO as SpO2 < 94% on room air, including those who require any form of supplemental oxygen. 54 Viral clearance is of clinical importance as it correlated with the clinical and biochemical outcomes, 55 but may underestimate the immunomodulators effect including HCQ. 12 , 56 On the other hand, low rates of mortality were reported among nonsevere COVID‐19, large number is required to get enough power to show a significant difference. 57 Mortality may not be a sensitive indicator among those with nonsevere COVID‐19.
The present meta‐analysis targeted nonsevere COVID‐19 patients to assess the efficacy and safety of HCQ based on the available evidence. In addition, the minimal age of the inclusion criteria was expanded to 12 years to add more studies. HCQ was used in the RECOVERY trial for infants more than 6 months without concerns, 58 but it could not be included in this review as it also had no PCR‐based confirmation test.
The present study offers moderate‐quality evidence built on five clinical trials and one quasi‐trial. The meta‐analysis investigated five measurable objective outcomes, two of them showed statistical significance; chest CT progression and incidence of some adverse drug effects. However, clinical progression, viral clearance at three time points, and 5‐day mortality did not differ between the two groups. All time points meta‐analysis to summarize the effect size on 5, 6, and 7 days was performed to get more accurate results. 59
The chest CT‐based disease assessment was performed on days 0 and 6 to evaluate the disease progression. 39 It depended on pneumonia absorption on CT and weather it was absorbed by more or less 50%, it also depended on pneumonia absorption on CT. 38
The clinical progression definition was consistent in three studies 37 , 38 , 39 which included increasing in the disease severity from nonsevere to severe, while in the other three studies, it was the requirement for mechanical ventilation, 44 ICU admission, 43 or hospitalization. 42
The authors faced a significant heterogeneity in viral clearance on days 5 and 6, where the study of Gautret et al's 45 had a different effect size direction to the other two studies. Low quality of the study might be the cause. Heterogeneity was also found in a disease progression as a result of the deference in the definition of clinical progression among the included studies. Heterogeneity was obvious among the studies in the occurrence of adverse effects. The study of Mitjà et al 42 reported more gastrointestinal, dermatological, and neurological adverse effects than Tang et al's 37 during the 28‐day follow‐up period, although it used lower HCQ dose for shorter treatment course.
The heterogeneity in the disease severity assessment among the included studies was addressed in this meta‐analysis. Common severity assessment tools were found in four studies; one of them 42 followed WHO Clinical Progression Scale which defined severe cases as at least hospitalized patients and received oxygen by noninvasive ventilation or high flow, while the other three 37 , 38 , 39 followed the Chinese Center for Disease Control and Prevention guidelines that defined severe cases as having dyspnea, respiratory frequency ≥30/min, blood oxygen saturation ≥ 93%, partial pressure of arterial oxygen to fraction of inspired oxygen ratio <300, and/or lung infiltrates >50% within 24 to 48 hours. The other two studies 43 , 44 lacked a well‐defined disease severity definition.
Adverse effects of HCQ are common; it includes gastrointestinal, cutaneous, central nervous system, ocular, cardiological, and hematological adverse effects. 60 A significant dermatological, gastrointestinal, and neurological adverse effects with HCQ therapy were pooled by the fixed‐effect model in the present meta‐analysis. However, the random‐effect model gave a very wide nonsignificant 95% CI because of the heterogeneity. The reported side effects did not result in a significant withdrawal or attrition bias. On the other hand, no cardiac arrythmias were reported along the 28 days in the studies and the other cardiac adverse effects were not significant.
A meta‐analysis first done by Sarma et al 61 consisted of three studies of the present meta‐analysis reported the same significant effect size of the radiological progression in the present meta‐analysis. On the other hand, the author combined days 5 and 6 viral clearance, mortality/progression, and all possible side effects in three variables that gave inconsistent results with us. In addition, wrong denominators for viral clearance and death/worsening were observed in effect size pooling. 61 The second meta‐analysis conducted by Singh et al 62 summarized mortality and viral clearance with HCQ use from seven comparative studies either clinical trial or observational studies. It found a significant pooled mortality associated with HCQ use. It is criticized for combining 6‐, 7‐, 28‐day viral clearance from three studies in one effect size. 37 , 38 , 43 The third preprint meta‐analysis conducted by Shamshirian et al 63 included 18 comparative studies for quantitative synthesis. The study found a higher mortality rate, adverse drug effects, and more radiological improvement associated with HCQ. The fourth preprint meta‐analysis by Amani et al 64 tried to investigate only among controlled trials but suffered from fallacies such as failure of combinability between CT progression and CT improvement, wrong denominators in extracting data for viral clearance and clinical progression from Tang et al, 37 and invalid combinability between different time‐based outcomes.
Large RCT with sufficient power is required with a longer follow‐up period, it should report more sensitive outcomes stratified by the disease severity and based on the proposed mechanisms of action of HCQ to improve the clinical course of COVID‐19.
5. LIMITATIONS
A lot of limitations faced the investigators due to conflicts between the included trials, high level of heterogeneity which is present among some studies methodologies and outcomes such as COVID‐19 severity definitions and the background treatment. The low number of studies with relatively small sample size and low quality is also another challenge.
6. CONCLUSION
There are no tangible beneficial effects of adding HCQ to the treatment of patients suffering from nonsevere PCR‐confirmed COVID‐19 infection. Reducing the chest CT progression by HCQ was neither sufficient to reduce the early mortality nor promote the early clinical progression more than the usual therapy used. Its use was accompanied with a significant incidence of adverse effects without any effect on viral clearance.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
HKE was responsible for studies search, data extraction, data analysis, and final revision of the manuscript. MAE helped in studies searching and data extraction and was responsible for manuscript writing. MGE helped in data searching, data extraction, and manuscript writing. AHE was responsible for the final revision of the manuscript and the work.
Elsawah HK, Elsokary MA, Elrazzaz MG, Elshafie AH. Hydroxychloroquine for treatment of nonsevere COVID‐19 patients: Systematic review and meta‐analysis of controlled clinical trials. J Med Virol. 2021;93:1265–1275. 10.1002/jmv.26442
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available. 37 , 38 , 39 , 42 , 43 , 44
REFERENCES
- 1. Gorbalenya AE, Baker SC, Baric RS, et al. The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nature Microbiol. 2020;5(4):536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu R, Han H, Liu F, et al. Positive rate of RT‐PCR detection of SARS‐CoV‐2 infection in 4880 cases from one hospital in Wuhan, China, from Jan to Feb 2020. Clin Chim Acta. 2020;505:172‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cucinotta D, Vanelli M. WHO declares COVID‐19 a pandemic. Acta Bio‐Med. 2020;91(1):157‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Weiss SR, Navas‐Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol Biol Rev. 2005;69(4):635‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Su S, Wong G, Shi W, et al. Epidemiology, genetic recombination, and pathogenesis of coronaviruses. Trends Microbiol. 2016;24(6):490‐502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pormohammad A, Ghorbani S, Khatami A, et al. Global comparison of influenza type A and B with COVID‐19: a systematic review and meta‐analysis on clinical, laboratory, and radiographic findings. Lab, Radiograph Findings. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Onder G, Rezza G, Brusaferro S. Case‐fatality rate and characteristics of patients dying in relation to COVID‐19 in Italy. JAMA. 2020;323(18):1775‐1776. [DOI] [PubMed] [Google Scholar]
- 8. Novel CPERE. The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID‐19) in China. CCDC Weekly. 2020;2(8):113‐122. [PMC free article] [PubMed] [Google Scholar]
- 9. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020;395(10223):497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Klhůfek J. The role of angiotensin‐converting enzyme 2 in the pathogenesis of COVID‐19: the villain or the hero? Acta Clin Belg. 2020;27:1‐8. [DOI] [PubMed] [Google Scholar]
- 11. Magro C, Mulvey JJ, Berlin D, et al. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID‐19 infection: a report of five cases. Transl Res. 2020;220:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID‐19): a review. JAMA. 2020;323(18):1824‐1836. [DOI] [PubMed] [Google Scholar]
- 13. Al‐Tawfiq JA, Al‐Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID‐19. Travel Med Infect Dis. 2020;34:101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Aschenbrenner DS. Remdesivir receives emergency use authorization for severely Ill patients with COVID‐19. Am J Nurs. 2020;120(7):26. [DOI] [PubMed] [Google Scholar]
- 15. Lenzer J. Covid‐19: US gives emergency approval to hydroxychloroquine despite lack of evidence. BMJ. 2020;369:m1335. [DOI] [PubMed] [Google Scholar]
- 16. Browning DJ. Pharmacology of chloroquine and hydroxychloroquine. Hydroxychloroquine and Chloroquine Retinopathy. New York, NY: Springer; 2014:35‐63. [Google Scholar]
- 17. Savarino A, Di Trani L, Donatelli I, Cauda R, Cassone A. New insights into the antiviral effects of chloroquine. Lancet Infect Dis. 2006;6(2):67‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu J, Cao R, Xu M, et al. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS‐CoV‐2 infection in vitro. Cell Discov. 2020;6(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Willis R, Seif A, McGwin G Jr, et al. Effect of hydroxychloroquine treatment on pro‐inflammatory cytokines and disease activity in SLE patients: data from LUMINA (LXXV), a multiethnic US cohort. Lupus. 2012;21(8):830‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Park WY, Goodman RB, Steinberg KP, et al. Cytokine balance in the lungs of patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;164(10):1896‐1903. [DOI] [PubMed] [Google Scholar]
- 21. Yu B, Wang DW, Li C. Hydroxychloroquine application is associated with a decreased mortality in critically ill patients with COVID‐19. MedRxiv. 2020. [Google Scholar]
- 22. Million M, Lagier J‐C, Gautret P, et al. Full‐length title: early treatment of COVID‐19 patients with hydroxychloroquine and azithromycin: a retrospective analysis of 1061 cases in Marseille, France. Travel Med Infect Dis. 2020;35:101738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Novales FJM, Ramírez‐Olivencia G, Estébanez M, et al. Early hydroxychloroquine is associated with an increase of survival in COVID‐19 patients: an observational study. Preprint. 2020. 10.20944/preprints202005.0057.v2 [DOI] [Google Scholar]
- 24. Rosenberg ES, Dufort EM, Udo T, et al. Association of treatment with hydroxychloroquine or azithromycin with in‐hospital mortality in patients with COVID‐19 in New York state. JAMA. 2020;323(24):2493‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Magagnoli J, Narendran S, Pereira F, et al. Outcomes of hydroxychloroquine usage in United States veterans hospitalized with Covid‐19. Med Clin Adv. 2020. 10.1016/j.medj.2020.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Geleris J, Sun Y, Platt J, et al. Observational study of hydroxychloroquine in hospitalized patients with Covid‐19. New Engl J Med. 2020;382:2411‐2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8(5):336‐341. [DOI] [PubMed] [Google Scholar]
- 28. Stovold E, Beecher D, Foxlee R, Noel‐Storr A. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. System Rev. 2014;3(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. The Cochran Collaboration . Data Collection Forms for Intervention Reviews: RCTs and non‐RCTs . Version 3. 2014.
- 30. Fedorov S. GetData Graph Digitizer version 2.24 . 2002. www-getdata-graph-digitizer-com. Accessed May 3, 2020.
- 31. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials: the CONSORT statement. JAMA. 1996;276(8):637‐639. [DOI] [PubMed] [Google Scholar]
- 33. Higgins JP, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. Hoboken, NJ: John Wiley & Sons; 2019. [Google Scholar]
- 34. Mahevas M, Tran V‐T, Roumier M, et al. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID‐19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. MedRxiv. 2020. 10.1101/2020.04.10.20060699 [DOI] [Google Scholar]
- 35. Esper RB, da Silva RS, Teiichi F, et al. Empirical Treatment With Hydroxychloroquine and Azithromycin for Suspected Cases of COVID‐19 Followed‐Up by Telemedicine. 2020. [Google Scholar]
- 36. Torjesen I. Covid‐19: Hydroxychloroquine does not benefit hospitalised patients, UK trial finds. BMJ. 2020;369:m2263. [DOI] [PubMed] [Google Scholar]
- 37. Tang W, Cao Z, Han M, et al. Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ. 2020;369:m1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen J, Liu D, Liu L, et al. A pilot study of hydroxychloroquine in treatment of patients with common coronavirus disease‐19 (COVID‐19). J Zhejiang Unit. 2020;49(2):215‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chen Z, Hu J, Zhang Z, et al. Efficacy of hydroxychloroquine in patients with COVID‐19: results of a randomized clinical trial. MedRxiv. 2020. 10.1101/2020.03.22.20040758 [DOI] [Google Scholar]
- 40. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID‐19) outbreak in China: summary of a report of 72 314 cases from the Chinese Center for Disease Control and Prevention. JAMA. 2020;323(13):1239‐1242. [DOI] [PubMed] [Google Scholar]
- 41. Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID‐19 clinical research. Lancet Infect Dis. 2020;20(8):E192‐E197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mitjà O, Corbacho‐Monné M, Ubals M, et al. Hydroxychloroquine for early treatment of adults with mild Covid‐19: a randomized‐controlled trial. Clin Infect Dis. 2020. 10.1093/cid/ciaa1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Barbosa J, Kaitis D, Freedman R, Le K, Lin X. Clinical outcomes of hydroxychloroquine in hospitalized patients with COVID‐19: a quasi‐randomized comparative study. N Engl J Med. 2020. [Google Scholar]
- 45. Gautret P, Lagier J‐C, Parola P, et al. Clinical and microbiological effect of a combination of hydroxychloroquine and azithromycin in 80 COVID‐19 patients with at least a six‐day follow up: a pilot observational study. Travel Med Infect Dis. 2020;34:101663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rynes R. Antimalarial drugs in the treatment of rheumatological diseases. Rheumatology. 1997;36(7):799‐805. [DOI] [PubMed] [Google Scholar]
- 47. Badgujar KC, Badgujar AB, Patil VP, Dhangar DV. Hydroxychloroquine for COVID‐19: a review and a debate based on available clinical trials/case studies. J Drug Del Therap. 2020;10(3):304‐311. [Google Scholar]
- 48. Colson P, Rolain J‐M, Lagier J‐C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID‐19. Int J Antimicrob Agents. 2020;55:105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chorin E, Dai M, Shulman E, et al. The QT interval in patients with COVID‐19 treated with hydroxychloroquine and azithromycin. Nature Med. 2020;26:808‐809. [DOI] [PubMed] [Google Scholar]
- 50. Chorin E, Wadhwani L, Magnani S, et al. QT interval prolongation and torsade de pointes in patients with COVID‐19 treated with Hydroxychloroquine/azithromycin. Heart Rhythm. 2020;S1547‐5271(20):30435‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Borba M, de Almeida Val F, Sampaio VS, et al. Chloroquine diphosphate in two different dosages as adjunctive therapy of hospitalized patients with severe respiratory syndrome in the context of coronavirus (SARS‐CoV‐2) infection: Preliminary safety results of a randomized, double‐blinded, phase IIb clinical trial (CloroCovid‐19 Study). MedRxiv. 2020;3(4):e208857. 10.1001/jamanetworkopen.2020.8857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shi H, Han X, Jiang N, et al. Radiological findings from 81 patients with COVID‐19 pneumonia in Wuhan, China: a descriptive study. The Lancet Infect Dis. 2020;20:425‐36. 10.1016/S1473-3099(20)30086-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou D, Dai S‐M, Tong Q. COVID‐19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75(7):1667‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. World Health Organization . Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID‐19 Disease is Suspected: Interim Guidance . 2020.
- 55. Yuan J, Zou R, Zeng L, et al. The correlation between viral clearance and biochemical outcomes of 94 COVID‐19 infected discharged patients. Inflam Res. 2020;69:599‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Alijotas‐Reig J, Esteve‐Valverde E, Belizna C, et al. Immunomodulatory therapy for the management of severe COVID‐19. Beyond the anti‐viral therapy: A comprehensive review. Autoimmun Rev. 2020;19(7):102569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ruan Q, Yang K, Wang W, Jiang L, Song J. Clinical predictors of mortality due to COVID‐19 based on an analysis of data of 150 patients from Wuhan, China. Intens Care Med. 2020;46(5):846‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wilkinson E. RECOVERY trial: the UK COVID‐19 study resetting expectations for clinical trials. BMJ. 2020;369:m1626. [DOI] [PubMed] [Google Scholar]
- 59. Peters JL, Mengersen KL. Meta‐analysis of repeated measures study designs. J Eval Clin Pract. 2008;14(5):941‐950. [DOI] [PubMed] [Google Scholar]
- 60. Tang C, Godfrey T, Stawell R, Nikpour M. Hydroxychloroquine in lupus: emerging evidence supporting multiple beneficial effects. Intern Medi J. 2012;42(9):968‐978. [DOI] [PubMed] [Google Scholar]
- 61. Sarma P, Kaur H, Kumar H, et al. Virological and clinical cure in COVID‐19 patients treated with hydroxychloroquine: a systematic review and meta‐analysis. J Med Virol. 2020;92(7):776‐785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Singh AK, Singh A, Singh R, Misra A. Hydroxychloroquine in patients with COVID‐19: a systematic review and meta‐analysis. Diab Metabol Synd. 2020;14(4):589‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Shamshirian A, Hessami A, Heydari K, et al. Hydroxychloroquine versus COVID‐19: a periodic systematic review and meta‐analysis. MedRxiv. 2020. 10.1101/2020.04.14.20065276 [DOI] [Google Scholar]
- 64. Amani B, Khanijahani A, Amani B. Hydroxychloroquine plus standard care compared with the standard care alone in COVID‐19: a meta‐analysis of randomized controlled trials. MedRxiv. 2020. 10.1101/2020.06.05.20122705 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are openly available. 37 , 38 , 39 , 42 , 43 , 44


