Abstract
Objectives
In patients with suspected coronavirus disease 2019 (COVID‐19) consulting primary care (PC) centers, clinical criteria may not be sensitive enough to detect many cases in which complications first occur. We intended to assess whether lung ultrasound (LUS) examinations performed by PC physicians are a useful tool to detect lung injury and may help in decisions about hospital referral.
Methods
This study included 61 patients with moderate symptoms suggesting COVID‐19 who were evaluated with LUS by PC physicians and then referred to a hospital during the current pandemic peak in Madrid. We analyzed association of a simple self‐designed LUS severity scale (grade 0, normal; grade 1, multiple separated B‐lines, pleural irregularity, or both; and grade 2, coalescent B‐lines, consolidations, pleural effusion, or a combination thereof) with the main outcome indicating adequacy of hospital referral, and also with chest x‐ray (CXR) findings.
Results
The proposed LUS severity scale was significantly associated with the main outcome of appropriate referral (P = 0.001): the higher the scale, the higher the percentage of adequate referrals. The LUS scale was also associated with a CXR severity scale (P = 0.034). The presence of coalescent B‐lines was the only independent LUS finding significantly associated with the appropriate‐referral outcome (P =0 .008) and also with a higher probability of hospital admission (P = 0.02) and with several CXR findings.
Conclusions
This study supports the use of LUS in PC as a tool to assess patients with suspected COVID‐19. Its use can reduce uncertainty during clinical evaluations of moderate patients, facilitate early detection of lung involvement, allow early appropriate referral, and avoid unnecessary referral.
Keywords: coronavirus disease 2019, COVID‐19, primary care, ultrasound
Abbreviations
- CI
confidence interval
- COVID‐19
coronavirus disease 2019
- CXR
chest x‐ray
- ED
emergency department
- LUS
lung ultrasound
- OR
odds ratio
- PC
primary care
- SARS‐CoV‐2
severe acute respiratory syndrome coronavirus 2
- Spo 2
peripheral oxygen saturation
- US
ultrasound
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection presents as a mild condition in most patients, but some cases develop a lung disease, named coronavirus disease 2019 (COVID‐19), with potential evolution to respiratory failure. 1 In Spain, very accessible primary care (PC) deals with mild case evaluations and monitoring. According to scientific evidence, criteria to decide on hospital referral are not yet clearly established. The use of only clinical symptoms and signs may not be sensitive enough to detect many cases with initial complications. Furthermore, treatments such as hydroxychloroquine and antivirals cannot be prescribed to all patients suspected of having COVID‐19 in PC just based on anamnesis and the physical examination, as they can be associated with several potentially important adverse reactions. In addition, most PC physicians cannot order a chest x‐ray (CXR) examination or urgent blood tests in their own PC center, so the patient would go to another center and could contribute to virus spreading. Therefore, excessive referral of patients to a hospital for an imaging test could contribute to the collapse of emergency services. Identifying patients when complications begin to occur before becoming severe turns into a priority for PC physicians. Having a decision support tool capable of reducing this uncertainty could be relevant in the current pandemic context.
Most PC centers in Madrid have ultrasound (US) devices, but US has only been included as a diagnostic tool in PC during the last few years, and most physicians still do not use it because of their lack of experience. Lung ultrasound (LUS) has previously demonstrated its usefulness in assessing different respiratory problems, such as pneumonia, pleural effusion, and pneumothorax. 2 Recently, its usefulness for COVID‐19 in hospital settings has also been demonstrated. 3 , 4 As far as we know, LUS may not yet have been evaluated in PC for patients with suspected COVID‐19.
Lung US findings in COVID‐19 are described as the following: a discontinuous, thickened, and irregular pleural line; B‐lines in a variety of patterns, including focal, multifocal, and coalescent; subpleural consolidations (small multifocal, nontranslobar, and translobar); rarely, localized pleural effusion; and the appearance of A‐lines in previously affected areas during the recovery phase. 4 , 5 Higher sensitivity of LUS than CXR has been reported for lung diseases with a peripheral distribution. 6 Lung US might be able to identify lung lesions before the development of hypoxemia in COVID‐19, and its usefulness in symptomatic patients in PC has been recently suggested. 6 Furthermore, an LUS examination is easy and quick to perform (it may take a few minutes when performed by an experienced sonographer), and just brief training is required to achieve the basic ability. 7 These reasons make us consider LUS performed by PC physicians as an optimal tool that may help them decide whether to refer patients with suspected SARS‐CoV‐2 infection to the emergency department (ED).
The main aim of this study was to assess whether LUS findings in patients with suspected COVID‐19 attended a PC center (without access to urgent CXR or blood tests in the same center) to help physicians make better decisions about hospital referral. Secondary objectives were to describe clinical, LUS and CXR findings of a series of individuals with suspected COVID‐19 who required face‐to‐face care by PC physicians and to study the association of LUS findings obtained by PC physicians with CXR findings obtained at a hospital. To our knowledge, this was the first study developed in PC that assessed LUS usefulness in patients with suspected COVID‐19.
Materials and Methods
Patient Enrollment
This study included 61 patients with a clinical suspicion of COVID‐19 who required face‐to‐face care in 3 PC centers in Madrid (region with a high incidence of SARS‐CoV‐2 infection) between March 15 and April 15, 2020, in the current pandemic context. This study was performed in accordance with the Declaration of Helsinki and approved by the Ethics and Research Committee of the Puerta de Hierro University Hospital, Majadahonda, Madrid. All adult participants provided their informed consent to participate in this study.
Following a PC protocol for COVID‐19 in Madrid, because of a shortage of personal protective equipment, only a single physician attended patients with suspected COVID‐19 face to face each workday in each PC center (rotationally). Thus, each of the 5 researchers recruiting patients for this study attended in‐person consultations on a single workday every 1 to 2 weeks, so each recruited patients on 2 to 4 workdays. (On the rest of the days, physicians who attended in‐person COVID‐19 consultations did not use LUS, as they were not used to using it.) Patients were included if there was a clinical suspicion of COVID‐19. Patients were excluded for any of the following circumstances: if they had mild signs and symptoms in a triage before the face‐to‐face evaluation (not evaluated in person), if they had a severe clinical condition after the face‐to‐face evaluation that ensured the need for hospital referral (LUS examination not performed), and if LUS findings were normal, and the conclusion of the physician after the face‐to‐face clinical evaluation was a good clinical condition (not referred to the hospital). This process and criteria for patient inclusion and exclusion are summarized in Figure 1. Thus, this study included “selected” patients with moderate signs and symptoms who attended PC in person and underwent an LUS examination and were then referred to the hospital for further tests (CXR and blood tests) and consideration for hospital admission or specific treatment.
Figure 1.
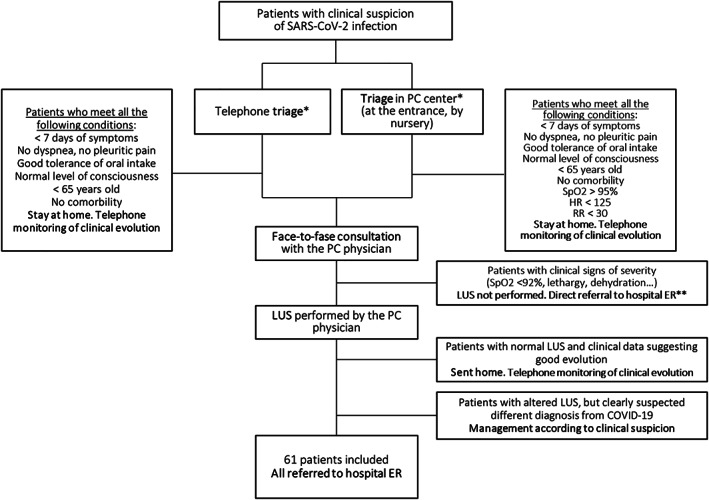
Patient flow diagram describing inclusion and exclusion criteria. Included patients were selected by triage and exclusion criteria. *Both systems of triage are established in the Madrid PC protocol for treatment of patients with suspected COVID‐19 infection. **Lung US results would not change the clinical decision of hospital referral. HR indicates heart rate; and RR, respiratory rate.
Procedure and Data Collection
Clinical and US evaluations of patients were conducted by 4 family physicians and a PC pediatrician with LUS experience over the last 5 years. Each physician, wearing personal protective equipment, attended patients in a specific area designated for those with symptoms suggestive of COVID‐19.
The US devices used were MyLab 6 (convex transducer; Esaote SpA, Genoa, Italy) and Butterfly iQ (Hitachi Medical Systems, Tokyo, Japan) systems. The US scanning technique followed the usual procedure for performing LUS examinations, 6 , 8 , 9 with the patient in a sitting position, fully exploring posterior, lateral, and anterior areas of both hemithoraxes. For safety, the provider stood behind the patient the whole time.
Clinical and LUS data were collected prospectively by each physician. Data on CXR and management in the ED were collected 1 day later from the hospital's electronic medical record. Data were recorded anonymously.
Variables and Outcomes
The main analyzed variable was a LUS severity scale, which was self‐designed and based on others currently published 2 , 8 , 9 , 10 but simplified and adapted to PC aims. For using LUS in PC, we propose 3 degrees of LUS severity. The grade in each patient was established by the physician who performed the LUS examination and determined by the presence of the following findings anywhere in the whole thorax: grade 0: normal; grade 1, multiple separated B‐lines, pleural irregularity, or both (no grade 2 findings); and grade 2, coalescent B‐lines, consolidations, pleural effusion, or a combination thereof (Figure 2).
Figure 2.
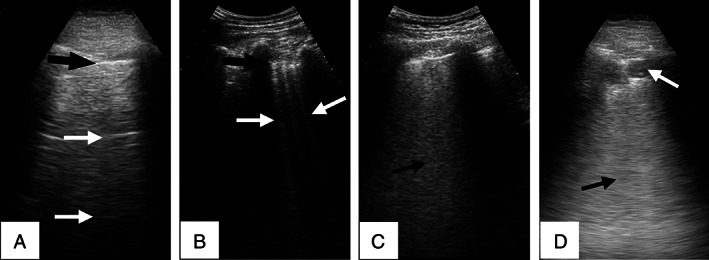
Images of COVID‐19 LUS findings representative of the 3 grades of the proposed LUS severity scale. A, Grade 0 findings: normal LUS A‐line pattern, with a well‐defined pleural line (black arrow) and parallel A‐lines (white arrows). B, Grade 1 findings: multiple separated B‐lines (white arrows) and an irregular pleural line (black arrow). C and D, Grade 2 findings: coalescent B‐lines shown as a light beam or white lung (C, arrow) and subpleural consolidation, shown as a superficial hypoechoic area (D, white arrow), followed in depth by a white lung artifact (D, black arrow).
The main outcome was a dichotomous variable that determined whether the hospital referral was “appropriate” depending on whether treatment of the patient after going to the ED was different from that of the nonreferred patients. It distinguished between 2 kinds of patients: (1) appropriate referral, consisting of patients who benefited from hospital referral because they were admitted or discharged with specific treatment such as hydroxychloroquine or antivirals (in our setting, these drugs cannot be prescribed at PC centers); and (2) discharged without specific treatment, consisting of patients who did not benefit from hospital referral.
Among secondary study variables, we use a simple CXR severity scale, also self‐designed, based on literature. 1 It included the following 3 categories (each patient's grade was established from the radiologist's report on the CXR examination performed at the hospital): grade A, normal; grade B, peribronchial thickening, an interstitial pattern, a ground glass pattern, or a combination thereof, without consolidations; and grade C, consolidations (regardless of other findings).
Statistics
A statistical association analysis was performed with the following tests: for qualitative variables, the χ2 test or, for small samples (<20% of expected frequencies of <5 in the contingency table), the Yates correction (2 × 2 table) or likelihood ratio (larger tables), accepting the most conservative result; for quantitative variables, the Student t test (if a normal distribution according to the Kolmogorov–Smirnov‐Lilliefors test) or Mann–Whitney U test (if not a normal distribution). A significance level of P < .05 was established. If a significant association between 2 dichotomous qualitative variables was found, the odds ratio (OR) and its 95% confidence interval (CI) are shown. The analyses were prespecified and executed by the SPSS version 24.0 statistical package (IBM Corporation, Armonk, NY).
Results
Characteristics of the 61 included patients are shown in Table 1. Both main variables (the proposed LUS severity scale and the appropriate‐referral outcome) were significantly associated (Table 2). The higher the scale, the higher the percentage of adequate referrals. There was good differentiation among the 3 categories of the LUS scale, with significant differences between them (Table 2). Looking for confounding factors, the study of the association of clinical variables with the main outcome of appropriate referral showed its association with only peripheral oxygen saturation (Spo 2) and the presence of pathologic auscultation, but the strength of the association was less powerful for Spo 2 (OR, 5.4) and auscultation (OR, 3.81) than for pathologic LUS findings (OR, 13.33; Table 2). Furthermore, more than half of patients with Spo 2 of greater than 95% were appropriately referred, and there were 5 admissions among them (13.2%), whereas no hospitalizations occurred in those with normal LUS findings. Only a single patient with pathologic auscultation (wheezing only) had normal LUS findings (and also had normal CXR findings).
Table 1.
Description of Patients Included in the Study (n = 61)
| Characteristic | Value | ||
|---|---|---|---|
| Clinical data (evaluated at PC) | |||
| Age, y | Mean ± SD: 52.9 ± 14.0 | Range: 26–87 | |
| Symptoms before LUS, d | Median (IQR): 7 (4–13) | Range: 1–27 | |
| n | % | ||
| Sex | Male | 32 | 52.% |
| Female | 29 | 47.5 | |
| Fever | 48 | 78.7 | |
| Cough | 38 | 62.3 | |
| Dyspnea | 23 | 37.7 | |
| Pleuritic pain | 12 | 19.7 | |
| HR >125 beats/min | 3 | 4.9 | |
| RR >30 breaths/min | 0 | 0.0 | |
| Hemoptysis | 0 | 0.0 | |
| Hypotension (SBP <90 and/or DBP <60 mm Hg) | 0 | 0.0 | |
| Depressed level of consciousness | 0 | 0.0 | |
| Inability of adequate oral intake (ie, severe vomiting or diarrhea) | 0 | 0 | |
| Spo2 | >95% | 38 | 62.3 |
| 92%–95% | 23 | 37.7 | |
| Auscultation | Normal | 37 | 60.7 |
| Crackles | 19 | 31.1 | |
| Hypoventilation | 3 | 4.9 | |
| Wheezing | 1 | 1.6 | |
| Rhonchi | 1 | 1.6 | |
| LUS data (obtained at PC) | |||
| Severity scale | 0. Normal LUS (A‐line pattern) | 6 | 9.8 |
|
1. Multiple separated B‐lines, irregular pleural line (no grade 2 findings) |
12 | 19.7 | |
| 2. Coalescent B‐lines, consolidation, mild pleural effusion | 43 | 70.5 | |
| Individual LUS findings (not exclusive; several can be found in the same patient) | Coalescent B‐lines | 33 | 54.1 |
| Multiple separated B‐lines | 28 | 45.9 | |
| Consolidation | 19 | 31.1 | |
| Irregular pleural line | 17 | 27.9 | |
| Mild pleural effusion | 4 | 6.6 | |
| Location of LUS findings | Unifocal | 11 | 18.0 |
| Multifocal unilateral | 4 | 6.6 | |
| Bilateral | 40 | 65.6 | |
| CXR data (obtained at hospital ED) | |||
| Severity scale | A. Normal CXR | 22 | 36.1 |
| B. Peribronchial thickening, interstitial pattern, ground glass pattern | 19 | 31.1 | |
| C. Consolidations | 20 | 32.8 | |
| Location of radiographic findings | Unilateral | 14 | 23.0 |
| Bilateral | 25 | 41.0 | |
| Destination after ED care | |||
| Hospital admission | 15 | 24.6 | |
| Discharged from ED with specific treatment | 26 | 42.6 | |
| Discharged from ED without specific treatment | 20 | 32.8 | |
DBP indicates diastolic blood pressure; HR, heart rate; IQR, interquartile range; RR, respiratory rate; and SBP, systolic blood pressure.
Table 2.
Association Analysis of the Main Outcome of Appropriate Referral With LUS and Clinical Variables
| Main Outcome, Appropriate Referral | Total, | POR (95% CI) | |||
|---|---|---|---|---|---|
| Admission or Discharge With Specific Treatment, | Discharge Without Specific Treatment, | ||||
| Characteristic | n (%) | n (%) | n (%) | ||
| LUS data (obtained at PC) | |||||
| LUS severity scale | 0. Normal LUS (A‐line pattern) | 1 (16.7) | 5 (83.3) | 6 (100) | p = .001 |
|
1. Multiple separated B‐lines, irregular pleural line (no grade 2 findings) |
5 (41.7) | 7 (58.3) | 14 (100) | ||
| 2. Coalescent B‐lines, consolidation, mild pleural effusion | 35 (81.4) | 8 (18.6) | 43 (100) | ||
| Pathologic LUS (grade 1 or 2) vs normal LUS (grade 0) | Grade 1 or 2 | 40 (72.7) | 15 (27.3) | 55 (100) | .021333 (1.43–123.69) |
| Grade 0 | 1 (16.7) | 5 (83.3) | 6 (100) | ||
| Grade 2 vs grade 1 | Grade 2 | 35 (81.4) | 8 (18.6) | 43 (100) | .018613 (1.54–24.37) |
| Grade 1 | 5 (41.7) | 7 (58.3) | 12 (100) | ||
| Grade 2 vs Grade 0 or 1 | Grade 2 | 35 (81.4) | 8 (18.6) | 43 (100) | <.001875 (2.52–30.39) |
| Grade 0 or 1 | 6 (33.3) | 12 (66.7) | 18 (100) | ||
| Coalescent B‐lines (as independent LUS finding) | Present | 27 (81.8) | 6 (18.2) | 33 (100) | .008 4.5 (1.42–14.27) |
| Absent | 14 (50) | 14 (50) | 28 (100) | ||
| Location of LUS findings | Bilateral | 30 (75) | 10 (25) | 40 (100) | .537 |
| Unilateral | 10 (66.7) | 5 (33.3) | 15 (100) | ||
| Clinical data (at PC evaluation) | |||||
| Sex | Male | 22 (68.8) | 10 (31.3) | 32 (100) | .788 |
| Female | 19 (65.5) | 10 (34.5) | 29 (100) | ||
| Fever | Yes | 35 (72.9) | 13 (27.1) | 48 (100) | .136 |
| No | 6 (46.2) | 7 (53.8) | 13 (100) | ||
| Cough | Yes | 24 (63.2) | 14 (36.8) | 38 (100) | .386 |
| No | 17 (73.9) | 6 (26.1) | 23 (100) | ||
| Dyspnea | Yes | 14 (60.9) | 9 (39.1) | 23 (100) | .412 |
| No | 27 (71.1) | 11 (28.9) | 38 (100) | ||
| Pleuritic pain | Yes | 7 (58.3) | 5 (41.7) | 12 (100) | .698 |
| No | 34 (69.4) | 15 (30.6) | 49 (100) | ||
| HR | >125 beats/min | 1 (33.3) | 2 (66.7) | 3 (100) | .515 |
| ≤125 beats/min | 40 (69.0) | 18 (31.0) | 58 (100) | ||
| Spo 2 | 92%–95% | 20 (87) | 3 (13) | 23 (100) | .01154 (1.37–21.27) |
| >95% | 21 (55.3) | 17 (44.4) | 38 (100) | ||
| Auscultation | Pathologic | 20 (83.3) | 4 (16.7) | 19 (100) | .031381 (1.09–13.37) |
| Normal | 21 (56.8) | 16 (43.2) | 37 (100) | ||
| Age, y | Mean ± SD | 54.2 ± 15.6 | 50.3 ± 10 | 52.9 ± 14.0 | .313 |
| Symptoms before LUS, d | Median (IQR) | 7 (4–10.5) | 8.5 (5.3–14.8) | 7 (4–13) | .313 |
HR indicates heart rate; and IQR, interquartile range.
Individually, the presence of coalescent B‐lines was the only LUS finding significantly associated with the appropriate‐referral outcome: 27 of 33 (81.8%) patients with coalescent B‐lines were appropriately referred compared to 14 of 28 (50%) of patients without coalescent B‐lines (P = .008; OR, 4.5; 95% CI, 1.42–14.27). The presence of coalescent B‐lines was also independently associated with a higher rate of hospital admission: 36.4% of patients with coalescent B‐lines were admitted compared to 10.7% of patients without this US finding (P = .02; OR, 4.76; 95% CI, 1.18–19.15).
There was a significant association between the proposed LUS severity scale and the CXR severity scale (Table 3): the higher the grade of US involvement, the higher the grade of radiologic involvement. Once again, the presence of coalescent B‐lines turned out to be the only independent finding associated with a higher grade of the CXR severity scale as well as a higher probability of having CXR consolidations or bilateral damage (Table 3).
Table 3.
Analysis of the Association Between the LUS and CXR Severity Scales and Between the Presence of Coalescent B‐Lines and CXR Findings
| CXR Severity Scale, n (%) | Total, n (%) | |||||
|---|---|---|---|---|---|---|
| Characteristic | A. Normal CXR | B. Peribronchial Thickening, Interstitial Pattern, Ground Glass Pattern | C. Consolidations | |||
| LUS data (obtained at PC) | P | |||||
| LUS severity score | 0. Normal LUS (A‐line pattern) | 4 (66.7) | 2 (33.3) | 0 (0.0) | 6 (100) | .034 |
|
1. Multiple separated B‐lines, irregular pleural line (no grade 2 findings) |
7 (58.3) | 4 (33.3) | 1 (8.3) | 12 (100) | ||
| 2. Coalescent B‐lines, consolidation, mild pleural effusion | 11 (25.6) | 13 (30.2) | 19 (44.2) | 43 (100) | ||
| Coalescent B‐lines (as independent LUS finding) | Present | 6 (18.2) | 10 (30.3) | 17 (51.5) | 33 (100) | .001 |
| Absent | 16 (57.1) | 9 (32.1) | 3 (10.7) | 28 (100) | ||
| CXR: normal/pathologic | P‐ (Sen, Spe) OR (95% CI) | |||||
| Normal (Grade A) | Pathologic findings (Grade B or C) | |||||
| Coalescent B‐lines (as independent LUS finding) | Present | 6 (18.2) | 27 (81.8) | 33 (100) |
.002 (69.2%, 72.7%) 6 (1.88–19.12) |
|
| Absent | 16 (57.1) | 12 (42.9) | 28 (100) | |||
| CXR: consolidations | P‐ OR (95% CI) | |||||
| Absent (Grade A or B) | Present(Grade C) | |||||
| Coalescent B‐lines (as independent LUS finding) | Present | 16 (48.5) | 17 (51.5) | 33 (100) |
.001 8.85 (2.23–35.14) |
|
| Absent | 25 (89.3) | 3 (10.7) | 28 (100) | |||
| Location of CXR findings | P‐ OR (95% CI) | |||||
| Bilateral | Unilateral | |||||
| Coalescent B‐lines (as independent LUS finding) | Present | 20 (60.6) | 13 (39.4) | 33 (100%) |
.001 7.08 (2.15–23.33) |
|
| Absent | 5 (17.9) | 23 (82.1) | 28 (100%) | |||
Sen indicates sensitivity; and Spe, Specificity.
Discussion
In this study, we evaluated the usefulness of LUS examinations performed by PC physicians during the epidemic peak of COVID‐19 in Madrid. The study population was selected, and a LUS examination was only performed when clinical concerns about hospital referral appeared. Although several systematic reviews have suggested the usefulness of US in PC, 11 , 12 , 13 , 14 we are unaware of the existence of studies on LUS in PC (and specifically in COVID‐19).
On the basis of LUS findings described in recent publications,5,8–10,15 we created a simple LUS severity scale, which is easy to apply for PC physicians and the result of which is linked to the main outcome variable of appropriate referral (admission or discharge with specific treatment) versus discharge without specific treatment. Our results suggest that, in patients with suspected non–clinically severe COVID‐19 attending a PC center, the presence of grade 2 LUS abnormalities confirms the need for a hospital referral (81.4% of appropriate referrals), whereas normal LUS findings allow the avoidance of referral with enough safety (only 1 patient with normal LUS findings received specific medical treatment, and none were admitted). In patients showing grade 1 findings, the uncertainty is greater, although as referral was also appropriate in 41.7% of patients, we suggest considering hospital referral (or at least performing further studies as a CXR examination and blood tests if easily accessible) until more evidence is available (being able to individualize the decision based on clinical data; Figure 3). Some studies in a hospital setting have proposed LUS as a tool that would allow for the distinction between low‐risk (negative LUS findings) and high‐risk (positive LUS findings) patients, suggesting its usefulness in PC for acute cases. 2 , 6 , 16 Although we are not aware of other similar studies that evaluated LUS in PC, our results support this suggestion.
Figure 3.
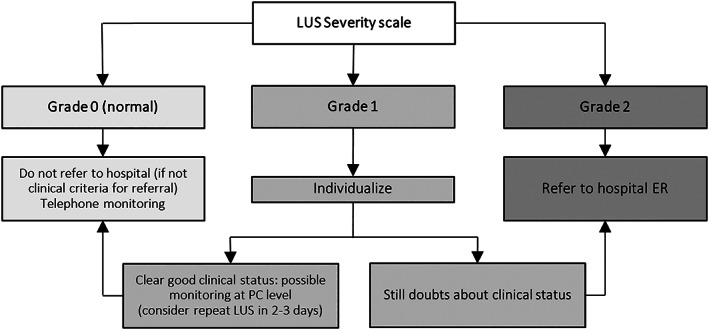
Proposed algorithm based on LUS findings to guide decision making in PC of moderate patients in the same clinical scenario as patients included in this study, following exclusion criteria shown in Figure 1.
We found a clearly significant association between the LUS severity scale and the CXR severity scale. Even so, the correspondence was not absolute, and up to 32.7% of patients with grade 1 or 2 LUS findings had normal CXR findings. These results agree with other studies. Lung US can identify changes in the physical state of superficial lung tissue, which correlate with histopathologic findings and can be identified on computed tomography but remain hidden in a large percentage of CXR examinations. 3 , 6 It has also been proposed that LUS can detect lung lesions before hypoxemia. 6 A recent study showed that 58% of the CXR findings obtained from symptomatic patients (SARS‐CoV‐2 positivity confirmed) who attended an ED were normal, and they were normal or only mildly abnormal in 89% of global patients. 17 Thus, as CXR has lower sensitivity compared to computed tomography, 17 but LUS has good sensitivity and agreement with computed tomography, 3 pathologic LUS findings with normal CXR findings could be explained by the better sensitivity for LUS than CXR.
Our results suggest special relevance of coalescent B‐lines as an independent finding, as they were strongly linked to an appropriate referral, a higher frequency of admission, higher CXR severity, and specific CXR findings (Table 3). This LUS finding, recently named “light beam” 9 , 15 or “white lung,” 8 although not specific to COVID‐19, has been described as frequent in several of the reviewed hospital‐based studies. 4 , 8 , 9 An ongoing multicenter study found this US sign in 97% (48 of 49) of patients with confirmed COVID‐19 pneumonia. 9
We found that abnormal lung auscultation was associated with the main outcome of appropriate referral, but the presence of LUS abnormalities had a clearly stronger association with this outcome. Furthermore, all patients with altered auscultation (except 1 patient with wheezing) had altered LUS findings. Lung US has recently been proposed as the first tool to rule out the presence of lung damage, avoiding auscultation on suspicion of COVID‐19, because of the risk of touching the face and ears or interfering with personal protective equipment elements, as well as the risk of virus spreading by using the stethoscope with other uninfected patients. 17 , 18 Our results support this approach.
We consider that these results are applicable and support using LUS in the PC setting for assessment of patients with suspected SARS‐CoV‐2 infection who have a mild or moderate clinical picture. Lung US examinations are easy to learn and perform. 7 The possibility of extending LUS among PC physicians is feasible, as long as there is a US device in the center, which is common, at least in our setting. Expanding the use of handheld devices will favor its application, even in a patient's home, also minimizing the virus‐spreading risk, as they would probably be used only by a single physician.
As we have shown the utility of LUS in PC for the COVID‐19 scenario, we suggest that it could possibly also be useful for patients' follow‐ups (discharged patients after hospital admission and those who did not require admission). This could allow safer hospital discharges.
Limitations of this study included the small number of cases, and that implies limitations to extrapolating the conclusions to the rest of the population. Furthermore, because in our setting we do not have the possibility to perform CXR examinations and urgent blood tests in our own PC centers, it could be difficult to completely extrapolate these results to other settings in which these diagnostic tools are easily accessible. However, COVID‐19 is a recently emerging disease, and this study provides substantial applicable results for its management in PC, even in different PC settings, where the results can guide decisions about performing further diagnostic studies. As another limitation, we include patients with suspected COVID‐19, but confirmatory microbiological tests were not performed on some of them. Nevertheless, we analyzed patients with suspected COVID‐19, not clinical treatment of patients with confirmed infection. On the other hand, we only studied patients referred to the hospital, as they were the only ones in which our main outcome variable could be evaluated. Therefore, we lacked CXR data and information of the potential hospital treatment of patients with normal LUS findings and a milder clinical picture. Future studies will be needed for a better understanding of that kind of clinical course.
In conclusion, this study provides evidence that supports the use of LUS by PC physicians as a tool to assess patients with suspected SARS‐CoV‐2 infection. Its use can reduce uncertainty during treatment of patients with mild‐to‐moderate symptoms, helping in early detection of lung involvement (and consequent hospital referral) and making it possible to avoid referring patients with a low likelihood of benefit and therefore reducing pressure at hospital emergency services.
Dr Calvo‐Cebrián is a member of the Ultrasound Working Group of the Madrid Society of Family and Community Medicine and the Ultrasound Working Group of the Spanish Society of Family and Community Medicine. Dr Alonso‐Roca is a member of the Ultrasound Working Group of the Madrid Society of Family and Community Doctors and the Ultrasound Working Group of the Spanish Society of Family and Community Doctors. Dr Rodriguez‐Contreras is a collaborator member of the Ultrasound Working Group of the Spanish Association of Primary Care Pediatrics. Drs Rodríguez‐Pascual and Calderín‐Morales are members of the Ultrasound Working Group of the Madrid Society of Family and Community Doctors. All of the authors of this article have reported no disclosures.
References
- 1. Guan W, Ni Z, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020; 382:1708–1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Volpicelli G, Elbarbary M, Blaivas M, et al. International evidence‐based recommendations for point‐of‐care lung ultrasound. Intensive Care Med 2012; 38:577–591. [DOI] [PubMed] [Google Scholar]
- 3. Lu W, Zhang S, Chen B, et al. A clinical study of noninvasive assessment of lung lesions in patients with coronavirus disease‐19 (COVID‐19) by bedside ultrasound. Ultraschall Med 2020; 41:300–307. [DOI] [PubMed] [Google Scholar]
- 4. Huang Y, Wang S, Liu Y, et al. A preliminary study on the ultrasonic manifestations of peripulmonary lesions of non‐critical novel coronavirus pneumonia (COVID‐19) [published online ahead of print February 26, 2020]. SSRN. doi: 10.2139/ssrn.3544750. [DOI] [Google Scholar]
- 5. Peng QY, Wang XT, Zhang LN. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019–2020 epidemic. Intensive Care Med 2020; 46:849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lung ultrasound during the COVID‐19 pandemic? J Ultrasound Med 2020; 39:1459–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vetrugno L, Bove T, Orso D, et al. Our Italian experience using lung ultrasound for identification, grading and serial follow‐up of severity of lung involvement for management of patients with COVID‐19. Echocardiography 2020; 37:625–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Soldati G, Smargiassi A, Inchingolo R, et al. Proposal for international standardization of the use of lung ultrasound for patients with COVID‐19: a simple, quantitative, reproducible method. J Ultrasound Med 2020; 39:1413–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Volpicelli G, Lamorte A, Villén T. What's new in lung ultrasound during the COVID‐19 pandemic. ? Intensive Care Med 2020; 46:1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith MJ, Hayward SA, Innes SM, Miller ASC. Point‐of‐care lung ultrasound in patients with COVID‐19: a narrative review. Anaesthesia 2020; 75:1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorensen B, Hunskaar S. Point‐of‐care ultrasound in primary care: a systematic review of generalist performed point‐of‐care ultrasound in unselected populations. Ultrasound J 2019; 11:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Andersen CA, Holden S, Vela J, Rathleff MS, Jensen MB. Point‐of‐care ultrasound in general practice: a systematic review. Ann Fam Med 2019; 17:61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bhagra A, Tierney DM, Sekiguchi H, Soni NJ. Point‐of‐care ultrasonography for primary care physicians and general internists. Mayo Clin Proc 2016; 91:1811–1827. [DOI] [PubMed] [Google Scholar]
- 14. Calvo Cebrián A, López García‐Franco A, Short Apellaniz J. Point of care ultrasound in primary care: is it a high resolution tool [in Spanish]? Aten Primaria 2018; 50:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Volpicelli G, Gargani L. Sonographic signs and patterns of COVID‐19 pneumonia. Ultrasound J 2020; 12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Buonsenso D, Piano A, Raffaelli F, Bonadia N, de Gaetano Donati K, Franceschi F. Point‐of‐care lung ultrasound findings in novel coronavirus disease‐19 pnemoniae: a case report and potential applications during COVID‐19 outbreak. Eur Rev Med Pharmacol Sci 2020; 24:2776–2780. [DOI] [PubMed] [Google Scholar]
- 17. Weinstock MB, Echenique A, Russell JW, et al. Chest x‐ray findings in 636 ambulatory patients with COVID‐19 presenting to an urgent care center: a normal chest x‐ray is no guarantee. J Urgent Care Med 2020; 14:13–18. [Google Scholar]
- 18. Buonsenso D, Pata D, Chiaretti A. COVID‐19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med 2020; 8:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]


