Abstract
Background
Despite descriptions of various cardiovascular manifestations in patients with coronavirus disease 2019 (COVID‐19), there is a paucity of reports of new onset bradyarrhythmias, and the clinical implications of these events are unknown.
Methods
Seven patients presented with or developed severe bradyarrhythmias requiring pacing support during the course of their COVID‐19 illness over a 6‐week period of peak COVID‐19 incidence. A retrospective review of their presentations and clinical course was performed.
Results
Symptomatic high‐degree heart block was present on initial presentation in three of seven patients (43%), and four patients developed sinus arrest or paroxysmal high‐degree atrioventricular block. No patients in this series demonstrated left ventricular systolic dysfunction or acute cardiac injury, whereas all patients had elevated inflammatory markers. In some patients, bradyarrhythmias occurred prior to the onset of respiratory symptoms. Death from complications of COVID‐19 infection occurred in 57% (4/7) patients during the initial hospitalization and in 71% (5/7) patients within 3 months of presentation.
Conclusions
Despite management of bradycardia with temporary (3/7) or permanent leadless pacemakers (4/7), there was a high rate of short‐term morbidity and death due to complications of COVID‐19. The association between new‐onset bradyarrhythmias and poor outcomes may influence management strategies for acutely ill patients with COVID‐19.
Keywords: bradyarrhythmia, COVID‐19, leadless pacemakers, pacing strategies, temporary pacing
1. INTRODUCTION
Various cardiovascular complications have been described in patients with coronavirus disease 2019 (COVID‐19), and with it a concern for a higher mortality. 1 Recent reports have described acute cardiac injury, 2 , 3 cardiogenic shock, 3 electrocardiographic (ECG) changes, 1 , 4 right ventricular dysfunction, 5 thromboembolic complications, 6 and tachyarrhythmias. 7 Ventricular arrhythmias have been reported as the first clinical manifestation of COVID‐19. 8 However, reports of severe bradyarrhythmias have been rare, mostly anecdotal, and likely underreported. 9 The clinical implications of new onset bradyarrhythmias in patients with COVID‐19 are unknown, and the approach to management is debatable without understanding short‐ and long‐term outcomes, and the risk to health care providers who are involved with urgent invasive procedures.
Herein, we report seven cases of patients admitted to our health system in Suffolk County, New York during the initial 6‐week period of peak COVID‐19 incidence, who presented with or developed severe bradyarrhythmias during hospitalization. The management strategies and short‐term outcomes including in‐hospital mortality are described.
2. METHODS
We included all patients with confirmed COVID‐19 admitted between March 15 and April 30, 2020 to two affiliated hospitals who required pacing support for severe bradycardia. Patients were excluded if their bradycardia required only medication adjustment, was identified only at the time of death, or if an alternate etiology of bradycardia was suspected. Clinical data were obtained from the electronic medical record. All testing and treatment, including permanent and temporary pacemaker implantations, were performed at the discretion of the treating physicians.
The Northwell Health Institutional Review Board approved this case series as minimal‐risk research using data collected for routine clinical practice and waived the requirement for informed consent. The initial characteristics of 5700 patients from Northwell are presented elsewhere 2 ; this case series presents in‐depth cardiac results not in that article.
3. RESULTS
Between March 15 and April 30, 2020, seven patients with confirmed COVID‐19 and severe bradycardia requiring acute intervention were retrospectively identified. Demographic and clinical characteristics of these patients are shown in Table 1. An overview of each patient's presentation and clinical course is presented in Table 2.
TABLE 1.
Clinical characteristics on presentation
| Parameter | Overall (n = 7) |
|---|---|
| Demographics | |
| Age (years) | 64 ± 9.6 |
| Male gender | 42.9% (3) |
| Residential care facility resident | 28.6% (2) |
| Obese (BMI >30) | 28.6% (2) |
| Cardiovascular risk factors | |
| Hypertension | 71.4% (5) |
| Hypercholesterolemia | 14.3% (1) |
| Diabetes mellitus | 85.7% (6) |
| Hepatic disease/cirrhosis | 28.6% (2) |
| Cerebrovascular disease/stroke prior to admission | 14.3% (1) |
| Chronic kidney disease | 14.3% (1) |
| Baseline conduction disease | |
| RBBB | 28.6% (2) |
| LBBB | 0% (0) |
| LAHB+RBBB | 14.3% (1) |
| First‐degree AVB | 14.3% (1) |
| Prior sinus node dysfunction | 0% (0) |
| Symptoms on hospital presentation | |
| Dyspnea | 57.2% (4) |
| Fever | 57.2% (4) |
| Chest pain | 28.6% (2) |
| Dizziness | 42.9% (3) |
| Medications | |
| Beta‐blocker | 28.6% (2) |
| ACE‐inhibitor or angiotensin receptor blocker | 14.3% (1) |
| Aspirin | 28.6% (2) |
| Hydroxychloroquine | 71.4% (5) |
| Actemra | 28.6% (2) |
| Anakinra | 14.3% (1) |
| Convalescent plasma | 14.3% (1) |
| Laboratory values | |
| Troponin (n = 5) | |
| Initial | 0.046 ± 0.103 |
| Peak | 0.08 ± 0.112 |
| CRP (n = 6) | |
| Initial | 15.3 ± 16.1 |
| Peak | 22 ± 13.8 |
| Ferritin (n = 5) | |
| Initial | 1109 ± 337 |
| Peak | 5017 ± 6955 |
| D‐dimer (n = 5) | |
| Initial | 2938 ± 4085 |
| Peak | 7636 ± 6986 |
| AST | |
| Initial | 51 ± 31 |
| Peak | 971 ± 2276 |
| ALT | |
| Initial | 31 ± 9 |
| Peak | 540 ± 1189 |
| Potassium | |
| Initial | 4.6 ± 0.8 |
| Minimum | 3.6 ± 0.9 |
| Peak | 5.2 ± 1.1 |
| Magnesium | |
| Initial | 2.1 ± 0.4 |
| Minimum | 1.9 ± 0.5 |
| Peak | 2.6 ± 0.6 |
TABLE 2.
Patient‐specific clinical events and outcomes
| Patient | Presenting symptoms/diagnosis | Bradycardia present on admission | Preexisting conduction disorder | COVID‐19 symptoms (noncardiac) | Bradycardia event (No. of days after admission) | Intervention(No. of days after admission) | Outcome at end of hospitalization(No. of days after admission) |
|---|---|---|---|---|---|---|---|
| #1 | Dizziness, weakness, acute CVA | CHB | First‐degree AV block | Dyspnea with pulmonary infiltrates found on CXR day 21 of hospitalization | CHB with narrow complex escape rhythm (0) | Leadless pacemaker implant (2) | Death (26) |
| #2 | Fall | CHB | RBBB, LAFB | Fever and hypoxia starting day 2 of hospitalization | CHB with wide complex escape rhythm (0) | Leadless pacemaker implant (0) | Death (6) |
| #3 | Unilateral facial droop, abdominal pain | None | Leftward axis only | Fever, cough starting day 1 of hospitalization | Sinus bradycardia and intermittent CHB with junctional escape rhythm (15) | Temporary pacemaker (26) |
Discharged (40) No permanent pacing required |
| #4 | Dizziness, fever, dyspnea, pleuritic chest pain | 2:1 AV block | RBBB | Fever, chills, cough, dyspnea, and pleuritic chest pain on presentation | 2:1 AV block (0) | Leadless pacemaker implant (2) |
Discharged (15) No permanent pacing required |
| #5 | Myalgias, cough, dyspnea, and pleuritic chest pain | None | None | Myalgias, cough, dyspnea, pleuritic chest pain | Sinus pauses up to 10 seconds (30) | Semipermanent pacemaker implant (34) and leadless pacemaker implant (58) |
Discharged to long‐term acute care facility (48) Death due to further complications (90) |
| #6 | Myalgias, cough, dyspnea, fever, anorexia | None | None | Myalgias, cough, dyspnea, fever, anorexia on presentation | Sinus pauses up to 17 seconds and sinus arrest requiring resuscitation (29) | Semipermanent pacemaker implant (29) | Death (30) |
| #7 | hypoxemic respiratory failure, ARDS, encephalopathy, unresponsiveness | None | None | Hypoxemic respiratory failure, ARDS, encephalopathy, unresponsiveness on presentation | Sinus pause of 36 seconds and new RBBB with right axis deviation (4) | Leadless pacemaker implant (5) | Death (7) |
Symptomatic high‐degree heart block was present on initial presentation in three of seven patients (43%); in two patients, respiratory symptoms developed later in their hospitalization (despite chest radiography suggestive of viral pneumonia at presentation), and the diagnosis of COVID‐19 was made after permanent pacemaker implantation (Figure 1). Four patients developed sinus arrest or paroxysmal high‐degree atrioventricular (AV) block lasting between 10 and 37 seconds documented on inpatient telemetry.
FIGURE 1.
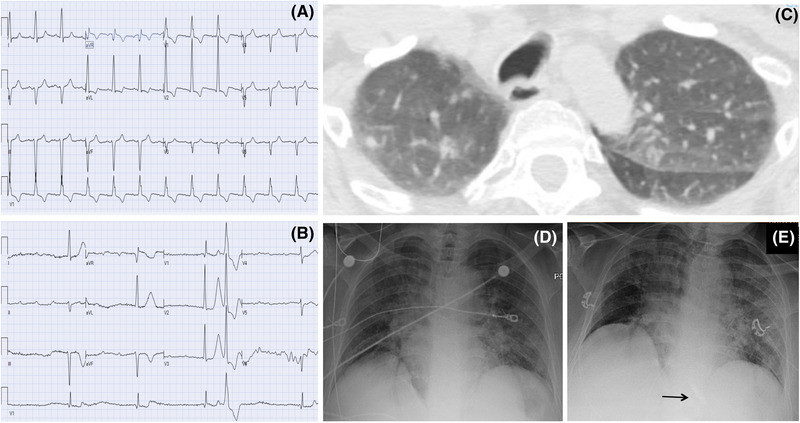
A patient presenting with AV block and imaging findings consistent with COVID‐19 prior to development of pulmonary symptoms. A, Baseline ECG prior to admission. B, ECG on presentation demonstrating third‐degree AV block. C, CT on admission with incidental findings of ground‐glass nodular opacities in the lung apices. D and E, Chest X‐ray from admission, and following leadless pacemaker implant (arrow) showing progressive bilateral pulmonary infiltrates [Color figure can be viewed at wileyonlinelibrary.com]
No patients in this series had preexisting cardiac disease. No significant QT prolongation was noted at baseline or during hospitalizations, although preexisting conduction disease was present in three patients. Echocardiography performed during hospitalization, or within 6 months prior, revealed normal left ventricular systolic function in all patients (left ventricular ejection fraction 67.5 ± 14.3%). Severe pulmonary hypertension and right ventricular dysfunction were identified in two patients. There was no evidence of acute coronary syndrome or documented thromboembolism. Respiratory symptoms and radiographic evidence of viral pneumonia were ultimately found in all patients.
Laboratory findings demonstrated significantly elevated inflammatory markers in all patients. No significant electrolyte or thyroid function abnormalities were observed. Cardiac troponin levels (measured with the Roche troponin T assay) were normal in three patients (≤0.01 ng/mL), not measured in two patients, and mildly elevated in two patients who had concomitant renal insufficiency: patient #1 had end‐state renal disease (ESRD) and peak troponin of 0.23 ng/mL, and patient #5 had a peak troponin of 0.17 ng/mL in the setting of acute renal failure and peak creatinine (>6 mg/dL) (Table 1). No patients demonstrated ischemic ECG changes apart from bradycardia or conduction disturbances. Two patients received new rate‐slowing medication prior to the bradyarrhythmia events; however, this was not considered to be the primary factor precipitating bradycardia in either case. Five patients received hydroxychloroquine during their hospitalization, four prior to their first recorded bradycardia episode.
Due to perceived life‐threatening bradycardia, all patients received temporary (3/7) or permanent pacemakers (4/7). One patient (patient #5) who received a temporary pacemaker after the initial bradycardia event subsequently received a leadless pacemaker due to recurrent bradycardia. In all five patients receiving permanent pacemakers, a leadless pacemaker (Medtronic Micra) was implanted. No operating staff involved in these procedures subsequently developed COVID‐19.
Mortality in this cohort was high, as five of seven (71%) patients died within 3 months following admission. Four of the seven patients (57%) died during the initial hospitalization, occurring 17.3 ± 12.5 days following admission and 8.3 ± 10.7 days after the first identified bradyarrhythmia. All mortalities were considered complications of COVID‐19 infection, including three patients with hypoxemic respiratory failure, and one who developed fevers, lethargy, and hypoglycemia prior to expiration, presumed to be due to COVID‐19 sepsis. Another patient had a prolonged hospital course with multisystem organ failure ultimately requiring tracheostomy and feeding tube placement, and suffered further complications following discharge to a long‐term care facility prompting withdrawal of supportive care and subsequent expiration on day 90 following initial hospital admission. In the two patients who survived, one had an acute stroke and the other had transient severe encephalopathy during their hospitalizations.
4. DISCUSSION
In this series of seven patients with COVID‐19 and severe bradyarrhythmias, short‐term mortality and morbidity was high, despite prompt management of bradycardia with pacing support. All patients had elevation of inflammatory markers and multisystem organ involvement, without acute cardiac injury or associated cardiomyopathy. In some patients, bradyarrhythmias presented prior to the onset of respiratory symptoms.
The mechanism of cardiac involvement during COVID‐19 remains speculative. Acute bradycardia may be due to direct SARS‐CoV‐2 infiltration of myocardial cells and the dedicated conduction system, aggravation of preexisting conduction disease during acute illness, pulmonary injury leading to hypoxia and secondary bradycardia, or collateral damage from inflammatory system activation and cytokine storm. 10 SARS‐CoV‐2 may also activate the angiotensin‐converting enzyme 2 (ACE2) receptor, 10 as ACE2 expression has been demonstrated specifically in sino‐atrial nodal cells, 11 and ACE2 overexpression has been associated with conduction disturbances. 12 In addition, the SARS‐CoV causing the 2002 outbreak was associated with sinus bradycardia in up to 15% patients. 10 , 13
Adverse effects of medications, including chloroquine and hydroxychloroquine, may also contribute to conduction system dysfunction. Long‐term chloroquine use has been shown to increase Purkinje fiber refractory period and action potential duration, resulting in AV nodal and infra‐Hisian conduction disturbance, 14 most commonly fascicular block. 15 Five patients in this series were treated with hydroxychloroquine, although this treatment preceded the development of bradycardia only in four patients.
All patients received temporary or permanent pacing. A leadless pacemaker was implanted in all patients clinically deemed to require permanent pacing. Leadless pacemaker implantation may be an attractive option in patients with active COVID‐19, as the risk of cardiac and intravascular infection may be lower, and the risk of viral transmission to operating staff may be mitigated by the percutaneous approach. However, despite pacing support, outcomes were poor, with four of seven patients dying during initial hospitalization, and five of seven dying within 3 months due to complications of COVID‐19.
5. CONCLUSION
Bradyarrhythmias may occur as part of the clinical course in patients with COVID‐19. In our experience, acute bradycardia was associated with elevation of inflammatory markers and high short‐term mortality rates, despite lack of coexistent cardiomyopathy or acute cardiac injury. Management decisions and resource allocation should take into account the potential for adverse outcomes regardless of invasive pacing support, as well as potential risks to health care staff during intervention on these acutely ill patients.
AUTHOR CONTRIBUTIONS
Study concept and design: Chinitz, Goyal, Epstein, Gruberg, and Gandotra. Analysis and interpretation of data: Chinitz, Harding, Gruberg, and Gandotra. Drafting of the manuscript: Chinitz, Harding, and Goyal. Critical revision of the manuscript for important intellectual content: Gruberg, Gandotra, Epstein, and Ong. Statistical analysis: Chinitz and Harding. Approval of the article: Chinitz, Goyal, Epstein, Gruberg, Gandotra, Harding, Veseli, Jadonath, Ong, and Maccaro. Data collection: Chinitz, Goyal, Veseli, Jadonath, Maccaro, and Epstein. All authors had access to the data and a role in preparing the manuscript.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
ACKNOWLEDGMENT
We would like to acknowledge the contributions of the Northwell Health COVID‐19 Research Consortium.
Chinitz JS, Goyal R, Harding M, et al. Bradyarrhythmias in patients with COVID‐19: Marker of poor prognosis? Pacing Clin Electrophysiol. 2020;43:1199–1204. 10.1111/pace.14042
REFERENCES
- 1. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA. 2020;5(7):802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Richardson S, Hirsch J, Narasimham M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID‐19 in the New York city area. JAMA. 2020;323(20):2052‐2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bangalore S, Sharma A, Slotwiner A, et al. ST‐Segment elevation in patients with covid‐19 —a case series. N Engl J Med. 2020;382(25):2478‐2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Argulian E, Sud K, Vogel B, et al. Right ventricular dilation in hospitalized patients with COVID‐19 infection. JACC Cardiovasc Imaging. 2020. 10.1016/j.jcmg.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bikdeli B, Madhavan MV, Jimenez D, et al. COVID‐19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow‐up. J Am Coll Cardiol. 2020;75(23):2950‐2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inciardi RM, Lupi L, Zaccone G, et al. Cardiac involvement in a patient with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5(7):819‐824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kir D, Mohan C, Sancassani R. Heart break ‐ an unusual cardiac manifestation of coronavirus disease 2019 (COVID‐19). JACC Case Rep. 2020;2(9):1252‐1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kochi A, Tagliari AP, Forelo B, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID‐19. J Cardiovasc Electrophysiol. 2020;21:1003‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferreira AJ, Moraes PL, Foureauz G, Andrade AB, Santos RA, Almeida AP. The angiotensin‐ (1‐7)/Mas receptor axis is expressed in sinoatrial node cells of rats. J Histochem Cytochem. 2011;59:761‐768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Danilczyk U, Penninger JM. Angiotensin‐converting enzyme II in the heart and the kindey. Circ Res. 2006;98:463‐471. [DOI] [PubMed] [Google Scholar]
- 13. Yu CM, Wong RS, Wu ED, et al. Cardiovascular complications of severe acute respiratory syndrome. Postgrad Med J. 2006;82:140‐144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ratliff NB, Estes ML, McMahon JT, Myles JL. Chloroquine‐induced cardiomyopathy. Arch Pathol Lab Med. 1988;112:578‐614. [PubMed] [Google Scholar]
- 15. Verny C, de Gennes C, Sebastian P, et al. Heart conduction disorders in long‐term treatment with chloroquine: two new cases. Presse Med. 1992;2:800‐804. [PubMed] [Google Scholar]


