Abstract
To inform seroepidemiological studies, we characterized the IgG‐ responses in COVID‐19 patients against the two major SARS‐CoV‐2 viral proteins, spike (S) and nucleocapsid (N). We tested 70 COVID‐19 sera collected up to 85 days post‐symptom onset and 230 non‐COVID‐19 sera, including 27 SARS sera from 2003. Although the average SARS‐CoV‐2 S and N‐IgG titers were comparable, N‐responses were more variable among individuals. S‐ and N‐assay specificity tested with non‐COVID‐19 sera were comparable at 97.5% and 97.0%, respectively. Therefore, S will make a better target due to its lower cross‐reactive potential and its' more consistent frequency of detection compared to N.
Keywords: antibodies, epidemiology, nucleocapsid, SARS‐CoV‐2, serology, spike
1. INTRODUCTION
Seroepidemiological studies are urgently needed to understand the true incidence of coronavirus disease 2019 (COVID‐19) and gauge the level of population immunity. Since such studies typically involve screening a large number of sera, the assays of choice should be highly sensitive, high‐throughput, and safe. Enzyme‐linked immunoassays (EIA), particularly those using recombinant proteins, although less specific than neutralization assays, have the advantage of being high‐throughput, safe, and easy to standardize.
The major antibody responses after coronavirus infections are directed against the Spike (S) and Nucleocapsid (N) proteins. 1 During Severe Acute Respiratory Syndrome Coronavirus‐1 (SARS) infections, anti‐N antibodies appeared earlier and were subject to higher cross‐reactivity than anti‐S antibodies, while anti‐S antibodies were better correlated to neutralization activity. 2 Aside from the zoonotic origin SARS and Middle‐East Respiratory Syndrome (MERS) coronavirus, there are four human strains of coronaviruses (reviewed in 3 ) and seroprevalence to any of these viruses in older adults can be greater than 90%. 4 Thus, the COVID‐19 serological assays should be sensitive and specific enough to discriminate responses from other coronaviruses.
As there are a number of commercial kits available to detect SARS‐CoV‐2 antibodies based on the S and N proteins, we wanted to understand the longitudinal kinetics of the COVID‐19 antibody responses to both proteins and the specificity of the S‐ and N‐ELISA‐based assays to facilitate future seroepidemiological studies.
2. MATERIALS AND METHODS
2.1. Ethics statement
The ethics committee of The FAHGMU (Ethics No. 2020‐85) and Dongguan's People's Hospital (KYKT2020‐005‐A1) has approved the use of patient's samples for this study.
2.2. Serum source
Seventy sera collected between days 0 and 85 post‐symptom onset were obtained from 31 laboratory‐confirmed COVID‐19 cases (aged 26 to 82 years old, median = 58) admitted to The First Affiliated Hospital of Guangzhou Medical University (FAHGMU) (N = 18) and Dongguan People's Hospital (N = 13). Patients were confirmed infected based on positive nucleic acid testing according to China's National Guidelines.
The non‐COVID‐19 sera panel consist of sera from 80 healthy elderly (between 60 to 89 years old) that were collected in 2015, 5 28 adults and 30 children with laboratory‐confirmed influenza at FAHGMU and 35 adults and 30 children that submitted sera for non‐respiratory illness testing at an independent clinical diagnostic laboratory. Thirty archived sera from patients infected with SARS‐CoV during the 2003 outbreak in Guangdong were screened for activity, and 27 were included in our study.
2.3. SARS‐CoV Spike and Nucleocapsid IgG ELISA
The archived SARS sera were tested for SARS‐CoV spike (Sc) and nucleocapsid (Nc)‐specific IgG antibodies using an ELISA kit that was provided by Autobio Diagnostics Co. Ltd (Zhengzhou, China).
2.4. SARS‐CoV‐2 Spike and Nucleocapsid IgG ELISA
Recombinant SARS‐CoV‐2 S (encompassing the extracellular domain, S1 and S2 subunits) and N proteins (Sino Biological Inc, China) were used to coat 96‐well plates at 0.5μg/ml overnight at 4°C. After washing and blocking, serially diluted sera (at a starting dilution of 1:100) were added to the plate and incubated for 2 hours at 37°C. Plates were washed and added with an anti‐human IgG horseradish peroxidase‐conjugated secondary antibody (Sigma). Colorimetric reaction was developed using 3,3′,5,5′‐Tetramethylbenzidine (TMB) substrate (Gcbio Technologies, China), stopped using 0.5 mol/L sulfuric acid and the absorbance read at 450 nm. Endpoint titers were determined to be the last reciprocal dilution with a positive/negative optical density (O.D) ratio ≥2.
2.5. Data analyses
Continuously variable data were log‐transformed, and geometric mean titers were used to describe the average titers. Differences between groups or time points were analyzed by ANOVA. Correlations between antibody titers were tested using Pearson's correlation test, with P‐values of <.05 considered statistically significant. All graphs and statistical testing were performed using Prism version 8 (GraphPad Software).
3. RESULTS
3.1. Kinetics and cross‐reactivity of the antibody responses to SARS‐CoV‐2 S and N proteins in laboratory‐confirmed COVID‐19 patients
Within the first 2 weeks of symptom onset, N‐ and S‐IgG were both present above detection threshold in 7 of the 15 (47%) sampled sera (Figure 1A,B, Table S1). By the third week, however, S‐specific IgG titers were detected in 100% of sera, compared to 94% in which N‐specific IgG titers were detected. Of the 55 sera samples that were collected after 2 weeks of symptom onset, 100% had detectable S‐specific IgG titers and 96% had detectable N‐specific IgG titers. The average S‐ and N‐IgG titers peaked around week four (day 22 to 28), with the average titer against N being slightly higher than S and decreased by 0.6 and 0.7 logs, respectively, by week 12. There was greater variability in the N‐specific IgG response, as evident in the higher coefficient of variation (CV) for the binned N‐specific IgG titers compared to the S‐specific IgG titers (Table S1). Eleven of the ICU patients from the FAHGMU have previously been reported 6 to have higher ratios of N/S‐specific IgG compared to mild patients within the first 2 weeks post‐symptom onset. This was also observed by week four in our study (Figure 1C,D), although this difference was no longer apparent by week 12, suggesting that the antibody dynamics may have changed according to clinical severity. Overall, there was a moderate correlation (r = .485, P < .0001) between S‐ and N‐ specific IgG titers (Figure 1E).
Figure 1.
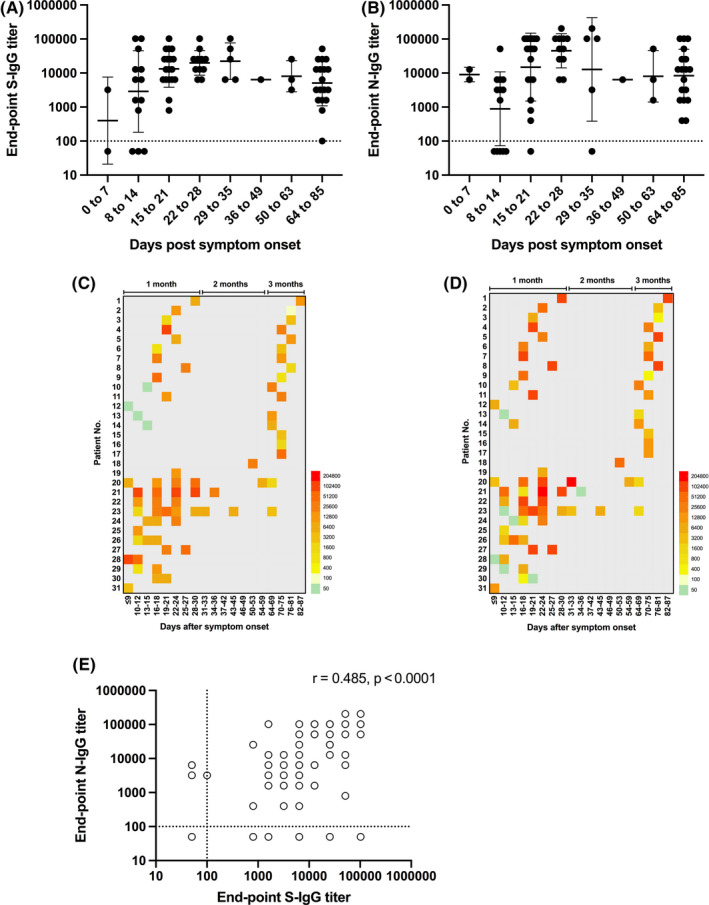
Kinetics of antibody responses against SARS‐CoV‐2 spike, S and nucleocapsid, N proteins in laboratory‐confirmed COVID‐19 patients. Endpoint IgG titers against the SARS‐CoV‐2 (A) spike, S and (B) nucleocapsid, N protein at different times post‐symptom onset in COVID‐19 sera. Individual endpoint IgG titers against the (C) S and (D) N protein at different times post‐symptom onset. (E) Correlation between S‐ and N‐IgG titers were tested by Pearson's correlation, with P < .05 considered significant
3.2. Cross‐reactivity between SARS‐CoV‐1 with SARS‐CoV‐2 antigens
Since the S and N proteins of SARS‐CoV‐2 and SARS‐CoV‐1 share 77% and 94% sequence homology, respectively, 7 we examined the cross‐reactivity between COVID‐19 and SARS sera against both these proteins. We selected 19 COVID‐19 sera to test its reactivity against the SARS‐Sc and SARS‐Nc protein. We plotted the endpoint SARS‐CoV‐2 S or N‐IgG titers against O.D value when measured using the SARS‐Sc and SARS‐Nc ELISA kits. There was a poor correlation (r = .269) between the S‐IgG titer and the Sc‐O.D readout (Figure 2A). In contrast, there was a good correlation between the N‐IgG titer and the Nc–O.D (r = .703, P < .001) readout (Figure 2B), suggesting that N is antigenically more conserved between the two viruses compared to S.
Figure 2.
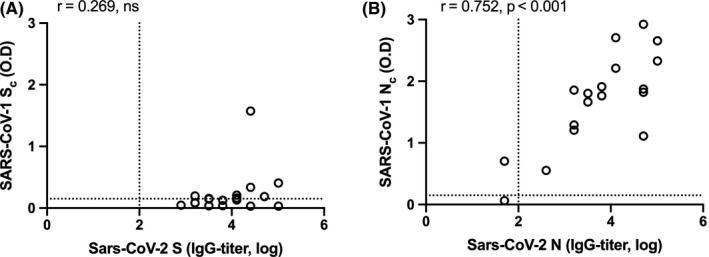
Correlation between COVID‐19 antibody reactivity to SARS‐CoV and SARS‐CoV‐2 proteins. The reactivity of 18 sera obtained from COVID‐19 patients against (A) the SARS‐CoV‐2 spike (S) and the SARS‐CoV spike (Sc) proteins, and (B) the SARS‐CoV‐2 nucleocapsid (N) and the SARS‐CoV nucleocapsid (Nc) proteins are shown. Log‐transformed IgG titers are shown on the Y‐axes and the optical density (O.D) values measured in the ELISAs are shown on the X‐axes. Correlation between spike and nucleocapsid IgG titers was tested by Pearson's correlation, with P < .05 considered significant
In a reciprocal manner, of the 30 SARS sera we retrieved, we confirmed the presence of SARS‐CoV spike (Sc) and nucleocapsid (Nc)‐specific IgG antibodies in 11 and 16 samples, respectively. The remaining three samples were negative for both SARS‐Sc and SARS‐Nc. In the 11 SARS sera that were IgG‐Sc‐positive, eight (73%) sera had IgG titers to SARS‐CoV‐2 S that were between 1,600 and 12,800. In the 16 SARS sera that were IgG‐Nc‐positive, 10 (63%) sera had IgG titers between 800 and 12,800 to SARS‐CoV‐2 N protein (Figure 3, Table S1). This suggest that SARS sera will cross‐react with SARS‐CoV‐2 S and N proteins.
Figure 3.
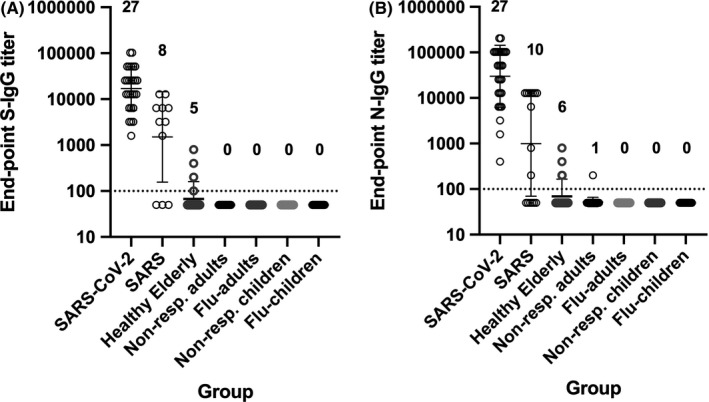
Cross‐reactivity of non‐COVID‐19 sera. Endpoint IgG titers against the SARS‐CoV‐2 spike (A) and nucleocapsid (B) proteins detected in non‐COVID‐19 serum samples. The single highest titer in COVID‐19 sera collected after day 14 post‐symptom onset (N = 27) were included for reference. For the SARS group, only SARS sera that were positive for its homologous protein were tested, that is 11 SARS‐Sc‐positive sera were tested against SARS‐CoV‐2 S, and 16 SARS‐Nc positive were tested against SARS‐CoV‐2 N. Other groups consisted of: 80 healthy elderly, 35 adults (non‐respiratory testing), 28 influenza‐confirmed adults, 30 children (non‐respiratory testing), and 30 influenza‐confirmed children. Number of positive samples per total sera are indicated above the graphs
3.3. Cross‐reactivity of non‐COVID‐19 antibody responses
We next examined the potential for non‐specific cross‐reactivity in non‐COVID‐19 sera to SARS‐CoV‐2 S and N with. Five elderly (2.5%) samples had detectable titers against S while six samples (2.6%, five elderly and one adult) and had titers against N (Figure 3, Table S1). Excluding SARS sera, the specificity of the S‐ and N‐based ELISAs was 97.0% and 97.5%, respectively.
4. DISCUSSION
Studies on the antibody kinetics to SARS‐CoV‐2 have generally focused on responses within 3 weeks of symptom onset, with the longest time examined being 50 days post‐symptom onset. In our study, we found that S‐ and N‐IgG peaked at week four and were detectable at comparable titers in most COVID‐19 patients up to 3 months post‐symptom onset. This observation is similar to that observed in SARS and MERS patients, 2 , 9 although in some studies N‐specific IgG appeared earlier compared to S. 10 , 11 Follow‐up studies with MERS and SARS‐CoV patients showed that antigen‐specific IgG remain detectable in most patients for up to 3 years, with the likelihood of positivity correlating well with the initial disease severity. 12 , 13 Similarly, a challenge study with the human coronavirus 229E also showed that virus‐specific IgG and IgA peak by week three post‐inoculation. 14 Taken together, coronavirus‐specific IgG can be reliably detected by week three after symptom onset.
A limitation to our study was that our COVID‐19 sera were from severely ill patients who were hospitalized and not discharged due to continued positivity for viral RNA as per national guidelines. The kinetics of responses reported here may not reflect the responses of patients with less severe infections, since the magnitude and profile of antibody responses can be influenced by disease or infection severity. 6 , 15 , 16 Indeed, some asymptomatic COVID‐19 cases do not have detectable antibody responses even a month after confirmation of infection. 8 , 17 Thus, the association between the severity of infection and the stability of the ensuing antibody response should be systematically studied to enable accurate interpretation of seroprevalence data. Another limitation is that we were not able to analyze the degree of cross‐reactivity and non‐specificity due to human coronaviruses (hCoV). To compensate for this, we screened a larger number of non‐COVID‐19 sera with an unclear history of hCoV infection and detected SARS‐CoV‐2 S‐ and N‐IgG in a small proportion of individuals. Our assay specificity appears to be in the range of other published studies, suggesting minimal cross‐reactivity with human coronaviruses. 18 , 19 , 20 Although this specificity range might still present a problem in areas of low disease prevalence, a hierarchical testing approach that includes a secondary validation test, such as neutralization assays, can be adopted to achieve both sensitivity and specificity. 15 , 21 Despite the comparable titers and assay specificity, our data indicate that S will make a better target due to its lower cross‐reactive potential and its slightly more consistent frequency of detection compared to N. Furthermore, its functional importance 15 , 19 , 22 will add value in using it as a serological target in any population studies.
CONFLICT OF INTEREST
All authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Cheng Xiao: Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Supervision (lead); Writing‐original draft (supporting); Writing‐review & editing (supporting). Shiman Ling: Data curation (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Resources (lead); Writing‐original draft (supporting); Writing‐review & editing (supporting). Minshan Qiu: Investigation (supporting); Resources (supporting); Writing‐review & editing (supporting). Zhenxuan Deng: Investigation (supporting); Resources (supporting); Writing‐review & editing (supporting). Liping Chen: Investigation (supporting); Resources (supporting); Writing‐review & editing (supporting). Airu Zhu: Investigation (supporting); Resources (supporting); Writing‐review & editing (supporting). Yi Chen: Investigation (supporting); Methodology (supporting); Resources (supporting); Writing‐review & editing (supporting). Yong Liu: Investigation (supporting); Resources (supporting); Writing‐review & editing (supporting). Xia Lin: Investigation (supporting); Methodology (supporting); Resources (supporting); Writing‐review & editing (supporting). Fangmei Lin: Investigation (supporting); Methodology (supporting); Writing‐review & editing (supporting). Qiubao Wu: Investigation (supporting); Resources (supporting); Writing‐review & editing (supporting). Lihan Shen: Investigation (supporting); Resources (lead); Writing‐review & editing (supporting). Feng Ye: Investigation (supporting); Resources (lead); Supervision (supporting); Writing‐review & editing (supporting). Xiaoqing Liu: Investigation (supporting); Resources (lead); Supervision (supporting); Writing‐review & editing (supporting). Yimin Li: Investigation (supporting); Resources (lead); Supervision (supporting); Writing‐review & editing (supporting). Jincun Zhao: Investigation (supporting); Resources (lead); Supervision (supporting); Writing‐review & editing (supporting). Zifeng Yang: Investigation (supporting); Resources (lead); Supervision (supporting); Writing‐review & editing (supporting). Benjamin Cowling: Investigation (supporting); Methodology (supporting); Resources (lead); Writing‐review & editing (supporting). Richard John Webby: Conceptualization (supporting); Methodology (supporting); Writing‐original draft (supporting); Writing‐review & editing (supporting). Mark Zanin: Conceptualization (lead); Formal analysis (lead); Investigation (lead); Methodology (lead); Project administration (lead); Resources (equal); Supervision (equal); Writing‐original draft (lead); Writing‐review & editing (lead). Sook‐San Wong: Conceptualization (lead); Formal analysis (lead); Funding acquisition (lead); Investigation (lead); Methodology (lead); Project administration (equal); Resources (equal); Supervision (equal); Writing‐original draft (lead); Writing‐review & editing (lead).
AUTHOR CONTRIBUTIONS
SSW, MPZ, and RW: Conceiving and designing of the study. CX, SML, XL, and FML: Laboratory assays and analysis of the data. AZ, LC, MQ, ZD, QW, YC, RL, LS, FY, BC, YL, JZ, and YZ: Collecting and processing of the clinical samples. SSW, MPZ, and RW: Writing of the manuscript.
Supporting information
Table S1
ACKNOWLEDGEMENTS
We thank the medical staff and participants of the study. We also thank Autobio Diagnostics Ltd, Zhengzhou, for donating the SARS S‐ and N‐IgG ELISA kits.
Xiao C, Ling S, Qiu M, et al. Human post‐infection serological response to the spike and nucleocapsid proteins of SARS‐CoV‐2. Influenza Other Respi Viruses. 2021;15:7–12. 10.1111/irv.12798
Cheng Xiao and Shiman Ling should be considered joint first‐authors.
Mark Zanin and Sook‐San Wong should be considered joint senior‐authors.
Funding information
This study was funded by the High‐Level University Talent Construction Program of Guangzhou Medical University and by China Evergrande Group‐2020GIRHHMS01.
REFERENCES
- 1. Qiu M, Shi Y, Guo Z, et al. Antibody responses to individual proteins of SARS coronavirus and their neutralization activities. Microbes Infect. 2005;7(5–6):882‐889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tan YJ, Goh PY, Fielding BC, et al. Profiles of antibody responses against severe acute respiratory syndrome coronavirus recombinant proteins and their potential use as diagnostic markers. Clin Diagn Lab Immunol. 2004;11(2):362‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Corman VM, Muth D, Niemeyer D, Drosten C. Hosts and sources of endemic human coronaviruses. Adv Virus Res. 2018;100:163‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gorse GJ, Donovan MM, Patel GB. Antibodies to coronaviruses are higher in older compared with younger adults and binding antibodies are more sensitive than neutralizing antibodies in identifying coronavirus‐associated illnesses. J Med Virol. 2020;92(5):512‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cowling BJ, Xu C, Tang F, et al. Cohort profile: the China Ageing REespiratory infections Study (CARES), a prospective cohort study in older adults in Eastern China. BMJ Open. 2017;7(10):e017503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sun B, Feng Y, Mo X, et al. Kinetics of SARS‐CoV‐2 specific IgM and IgG responses in COVID‐19 patients. Emerg Microbes Infect. 2020;9(1):940‐948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wu F, Zhao S, Yu B, et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang G, Nie S, Zhang Z, Zhang Z. Longitudinal change of SARS‐Cov2 antibodies in patients with COVID‐19. J Infect Dis. 2020;222(2):183‐188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Park WB, Perera RA, Choe PG, et al. Kinetics of serologic responses to MERS coronavirus infection in humans, South Korea. Emerg Infect Dis. 2015;21(12):2186‐2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Woo PC, Lau SK, Wong BH, et al. Longitudinal profile of immunoglobulin G (IgG), IgM, and IgA antibodies against the severe acute respiratory syndrome (SARS) coronavirus nucleocapsid protein in patients with pneumonia due to the SARS coronavirus. Clin Diagn Lab Immunol. 2004;11(4):665‐668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhao J, Wang W, Wang W, et al. Comparison of immunoglobulin G responses to the spike and nucleocapsid proteins of severe acute respiratory syndrome (SARS) coronavirus in patients with SARS. Clin Vaccine Immunol. 2007;14(7):839‐846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cao WC, Liu W, Zhang PH, Zhang F, Richardus JH. Disappearance of antibodies to SARS‐associated coronavirus after recovery. N Engl J Med. 2007;357(11):1162‐1163. [DOI] [PubMed] [Google Scholar]
- 13. Choe PG, Perera R, Park WB, et al. MERS‐CoV antibody responses 1 year after symptom onset, South Korea, 2015. Emerg Infect Dis. 2017;23(7):1079‐1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Callow KA, Parry HF, Sergeant M, Tyrrell DA. The time course of the immune response to experimental coronavirus infection of man. Epidemiol Infect. 1990;105(2):435‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Perera RA, Mok CK, Tsang OT, et al. Serological assays for severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), March 2020. Eurosurveillance. 2020;25(16):e2000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Qu J, Wu C, Li X, et al. Profile of Immunoglobulin G and IgM Antibodies Against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020:ciaa489 10.1093/cid/ciaa489 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lee YL, Liao CH, Liu PY, et al. Dynamics of anti‐SARS‐Cov‐2 IgM and IgG antibodies among COVID‐19 patients. J Infect. 2020;81(2):e55‐e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao R, Li M, Song H, et al. Early detection of SARS‐CoV‐2 antibodies in COVID‐19 patients as a serologic marker of infection. Clin Infect Dis. 2020:ciaa523 10.1093/cid/ciaa523 [Epub ahead of print]. [DOI] [Google Scholar]
- 19. Okba NMA, Muller MA, Li W, et al. Severe acute respiratory syndrome coronavirus 2‐specific antibody responses in coronavirus disease 2019 patients. Emerg Infect Dis. 2020;26(7):1478‐1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Beavis KG, Matushek SM, Abeleda APF, et al. Evaluation of the EUROIMMUN Anti‐SARS‐CoV‐2 ELISA Assay for detection of IgA and IgG antibodies. J Clin Virol. 2020;129:e104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Meyer B, Drosten C, Muller MA. Serological assays for emerging coronaviruses: challenges and pitfalls. Virus Res. 2014;194:175‐183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu L, Wei Q, Lin Q, et al. Anti‐spike IgG causes severe acute lung injury by skewing macrophage responses during acute SARS‐CoV infection. JCI Insight. 2019;4(4):e123158. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


