Abstract
Since the new coronavirus known as 2019‐nCoV (severe acute respiratory syndrome coronavirus 2, SARS‐CoV‐2) has widely spread in Wuhan, China, with severe pneumonia, scientists and physicians have made remarkable efforts to use various options such as monoclonal antibodies, peptides, vaccines, small‐molecule drugs and interferon therapies to control, prevent or treatment infections of 2019‐nCoV. However, no vaccine or drug has yet been confirmed to completely treat 2019‐nCoV. In this review, we focus on the use of potential available small‐molecule drug candidates for treating infections caused by 2019‐nCoV.
Keywords: 2019‐nCoV, infection, small‐molecule drugs
1. INTRODUCTION
In December 2019, the novel coronavirus disease known as 2019‐nCoV (severe acute respiratory syndrome‐related coronavirus SARS‐CoV‐2) has suddenly spread in Wuhan, China, and affected more than 200 countries (Figure 1) with over 6 600 000 confirmed cases (Figure 2 and over 397 000 confirmed death worldwide as (Figure 2) of 8 June 2020. The outbreak was declared a Public Health Emergency of International Concern on 30 January 2020. 1
FIGURE 1.
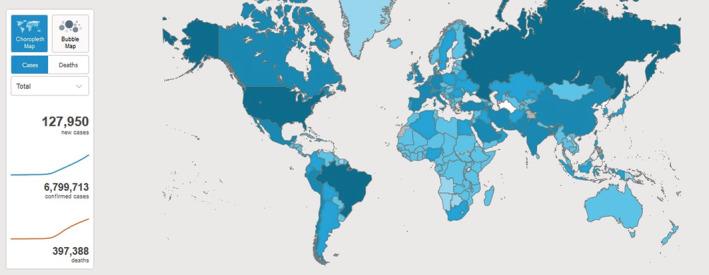
2019‐nCoV global distribution (Map was extracted from World Health Organization website)
FIGURE 2.
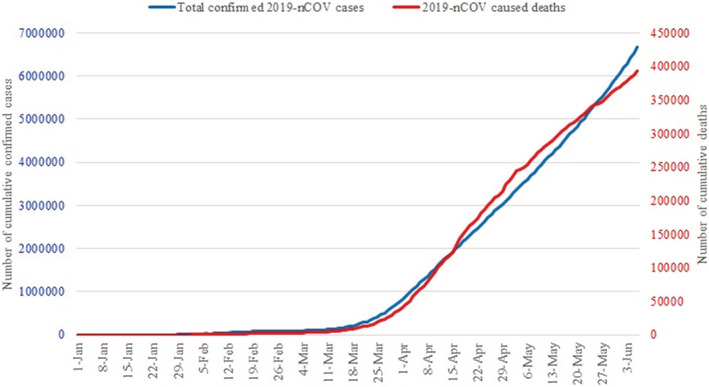
2019‐nCoV total confirmed cases and associated deaths diagrams (Data were gathered from World Health Organization website)
Until now, the virus had a lot of adverse and far‐reaching effects not only on the global economy but also on millions of people' life because of mandatory isolations/quarantines. 2 , 3 , 4 Generally, coronaviruses are known as relatively heavy and large viruses with enveloped positive sense single‐stranded RNA genome and categorized into four classes as alpha, beta, gamma and delta. More information about these classes and coronaviruses structure are beyond the scope of this review and have been already described in literatures. 4 , 5 , 6 Although there are no Food and Drug Administration (FDA)‐approved drugs to prevent or treat COVID‐19, we herein highlighted recent advances in the use of the pharmaceutical care of some frequently used small‐molecule drugs in patients with 2019‐nCoV. Table 1 showed their chemical structures and ClinicalTrials ID.
TABLE 1.
Chemical structures and ClinicalTrials ID of discussed drugs
| Drug name | Structure | ClinicalTrials ID (Phases) |
|---|---|---|
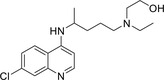
| Hydroxychloroquine |
|
NCT04329611 (III) NCT04329832 (II) NCT04329923 (II) NCT04351620 (I) NCT04334382 (III) NCT04344444 (III) NCT04307693 (II) NCT04345692 (III) NCT04331834 (III) NCT04332094 (II) NCT04342221 (III) NCT04347980 (III) NCT04261517 (III) NCT04325893 (III) NCT04328467 (III) NCT04316377 (IV) NCT04308668 (III) |
| Lopinavir |
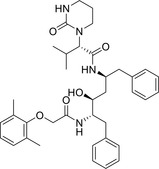
|
NCT04330690 (II) |
| Ritonavir |
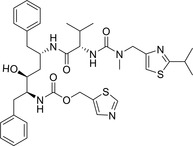
|
NCT04328285 (III) NCT04330690 (II) NCT04307693 (II) NCT04345276 (IV) |
| Remdesivir |
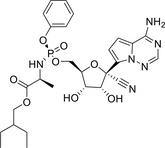
|
NCT04292730 (III) NCT04315948 (III) NCT04321616 (III) NCT04280705 (III) |
| Arbidol |
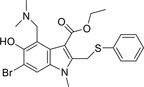
|
Not found |
| Favipiravir |
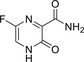
|
NCT04310228 (Not Applicable) |
2. CHLOROQUINE AND HYDROXYCHLOROQUINE
The synthesis of chloroquine (CQ) backs to 1943 in Germany. During World War II, it was administered for the prophylaxis and treatment of malaria. After approving by the World Health Organization as an essential medicine, 7 it was found to be useful to treatment of other diseases such as skin diseases, sarcoidosis, extra intestinal amoebiasis, chronic Q fever and rheumatoid disorders. In 1955, one important analogue of CQ known as hydroxychloroquine (HCQ) was employed for the treatment of rheumatologic and dermatologic diseases. 8 This anti‐malarial agent has become a mainstay of anti‐inflammatory treatment, given its relative low cost and favourable safety profile compared with other disease‐modifying anti‐rheumatic drugs. Retinal toxicity remains a well‐known side effect of the long‐term use of HCQ and its predecessor CQ. 9 It should be highlighted that the efficacy and safety of these drugs for treatment of SARS‐CoV‐2 (the new virus causing COVID‐19) pneumonia remains unclear. 10 However, according to the previous studies, the so‐called drugs inhibit coronavirus via a series of steps. 11 In one report, Yao et al 12 studied the antiviral and prophylactic property of CQ and HCQ using SARS‐CoV‐2 infected Vero cells. In this research, they could successfully predict drug concentrations using physiologically based pharmacokinetic model that was obtained from previous published clinical trial data. Experimental results and EC50 values showed that in vitro anti‐SARS‐Cov‐2 activity of HCQ is better than CQ. Although according to the unpublished clinical trial, CQ exhibited the therapeutic effect in SARS‐Cov‐2‐infected patients, this research demonstrated that HCQ with remarkable antiviral and prophylactic performance and tolerable safety profile may be used as a promising drug against SARS‐Cov‐2. In another interesting study, Zhou et al compared the therapeutic property of HCQ and CQ for the treatment of SARS‐CoV‐2 infection. 13 As it could be seen from Figure 3, they explained fully the antiviral mechanisms of CQ and HCQ.
FIGURE 3.
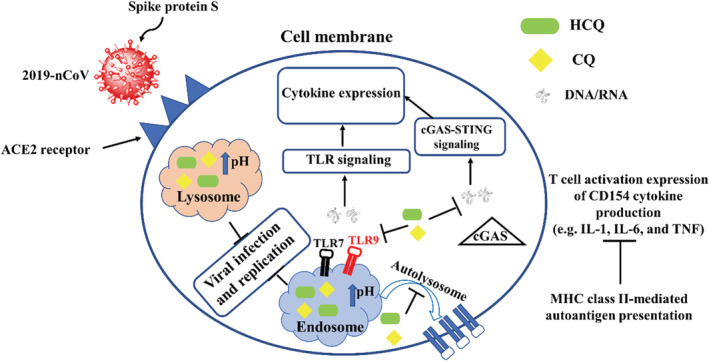
A cartonic illustration of the antiviral mechanism of HCQ and CQ. CQ, chloroquine; HCQ, hydroxychloroquine
Based on Figure 3, they concluded that HCQ could act as a better therapeutic agent than CQ for the treatment of the so‐called infection in china. For supporting their claim, they also suggested three major reasons as follow: (a) HCQ could decrease CD154 expression in T‐cells, leading to reduce the progression of 2019‐nCOV, (b) HCQ had a remarkable antiviral effect at both pre‐ and post‐infection stages and (c) compared to CQ, HCQ has some advantages such as low side effects, low cost and safe in pregnancy. As HCQ has been found to be efficient on the treatment of 2019‐nCoV in vitro, massive clinical trials aiming at evaluating the effect of HCQ on 2019‐nCoV have been developed (Table 1). For example, Gautret et al started to carried out a non‐randomized clinical trial in order to assess the effect of HCQ on the small group of patients with 2019‐nCoV infections. Although their study had some important limitations such as small sample size and limited long‐term outcome follow‐up, but the obtained results especially using HCQ with azithromycin were useful and could open a new window to fight this emerging viral infection before finding its vaccine in the future. 14 Liu et al evaluated the antiviral effect of HCQ against SARS‐CoV‐2 infection in comparison to CQ in vitro. The cytotoxicity of the two drugs in Vero E6 cells was determined by CCK‐8 assays. Vero E6 cells were treated with different doses of either compound or with PBS in the controls for 1 hour and then infected with SARS‐CoV‐2 at MOIs of 0.01, 0.02, 0.2 and 0.8. They predict that the drug has a good potential to combat the disease. This possibility awaits confirmation by clinical trials. 4
3. LOPINAVIR AND RITONAVIR
Lopinavir (LPV) is a protease inhibitor which is rapidly metabolized in vitro. However, co‐administration with ritonavir (RTV; an inhibitor of the cytochromeP450 3A isoenzyme) inhibits LPV metabolism, significantly increasing plasma concentrations of the drug and affording high and consistent levels of LPV. Thus, a co‐formulation of LPV and RTV (LPV/RTV) has been developed for clinical use. 15 , 16 For instance, Cao et al conducted a randomized, controlled, open‐label trial involving hospitalized adult patients with confirmed SARS‐CoV‐2 infection. Patients were randomly assigned in a 1:1 ratio to receive either LPV‐RTV (400 and 100 mg, respectively) twice a day for 14 days, in addition to standard care, or standard care alone. In conclusion, they found that LPV‐RTV treatment did not significantly accelerate clinical improvement, reduce mortality or diminish throat viral RNA detectability in patients with serious Covid‐19. 17 In one case clinical trial report, Lim et al investigated the therapeutic effect of LPV/RTV on a 54‐year‐old Korean man who lived in Wuhan, China, and entered Korea on 20 January 2020. At the first days, he had no major illness and his all tests such as Leptospira, Hantan virus, Tsutsugamushi, Malaria, M tuberculosis, human immunodeficiency virus (HIV) and venereal disease research laboratory were negative. On days 5 and 7, he had fever and dry cough, respectively. Real‐time polymerase chain reactoion (RT‐PCR) test confirmed that he had 2019‐nCoV and transmitted this virus to other persons. Treating the patient using two tablets (LPV 200 mg/RTV 50 mg) on day 10 of illness, β‐coronavirus viral load started to decrease and no detectable or little coronavirus titres have been observed. These findings demonstrated that LPV/RTV could be suggested to relatively high risk groups of 2019‐nCoV pneumonia (elderly patients or patients with underlying diseases) from the early stage. 18 In a study by Wang et al, four 2019‐nCoV patients including three males with the age of 32, 19 and 63 and one female with the age of 63 were given antiviral treatment including LPV/RTV (LPV 400 mg/RTV 100 mg), arbidol and Shufeng Jiedu Capsule (SFJDC, a traditional Chinese medicine) between 6 and 15 days. In addition, all patients were given antibiotic treatment and started on supplemental oxygen, delivered by nasal cannula after admission to hospital. After treatment, three patients showed significant improvement in pneumonia associated symptoms, two of whom were confirmed to be COVID‐19 negative and discharged, and one of whom was negative for the virus at the first test. 19 One comprehensive epidemiological and clinical study was done on 99 patients with 2019‐nCoV in Wuhan, China, by Chen et al. From drug treatment point of view, 75 (76%) patients received antiviral treatment, including oseltamivir (75 mg every 12 hours, orally), 500 mg LPV, 500 mg RTV and the intravenous administration of 0.25 g ganciclovir for 3 to 14 days. Most patients were given antibiotic treatment. Twenty‐five (25%) patients were treated with a single antibiotic and 45 (45%) patients were given combination therapy. By the end of 25 January, 31 (31%) patients had been discharged and 11 (11%) patients had died. All other patients stayed in hospital. From died patients, eight patients had lymphopenia, seven had bilateral pneumonia, five were older than 60 years, three had hypertension and one was a heavy smoker. 20
4. REMDESIVIR
Remdesivir, originally developed for the treatment of Ebola virus. Remdesivir is a phosphoramidate nucleotide analogue prodrug that is metabolized to a triphosphate form in cells and has been known as a broad inhibitor of RNA viruses, including filo‐, pneumo‐, paramxyo‐ and coronaviruses. 21 Remdesivir has shown great effectiveness against a number of coronaviruses with IC50 values of 0.1 M in human airway epithelial cell models of coronavirus infection. Remdesivir is currently being used as an antiviral therapy to treat SARS‐CoV‐2 infection. 22 Wang et al evaluated the antiviral efficiency of five FDA‐approved drugs including ribavirin, penciclovir, nitazoxanide, nafamostat, CQ and two broad spectrum antiviral drugs remdesivir (GS‐5734) and favipiravir (FPV; T‐705) against a clinical isolate of 2019‐nCoV in vitro. Amongst them, two compounds namely remdesivir and CQ potently blocked virus infection at low‐micromolar concentration. Their time‐of addition assay showed remdesivir functioned at a stage post virus entry, which is in agreement with its putative antiviral mechanism as a nucleotide analogue. The preliminary data showed that remdesivir also inhibited virus infection efficiently in a human cell line (human liver cancer Huh‐7 cells), which is sensitive to 2019‐nCoV. As these compounds have been used in human patients with a safety track record and shown to be effective against various ailments, they should be assessed in human patients suffering from the novel coronavirus disease. 23 In one theoretical study, Chang et al used molecular docking to repurpose HIV protease inhibitors and nucleoside analogues for COVID‐19 using docking scores calculated by AutoDock Vina and RosettaCommons. Their results suggested that indinavir and remdesivir possessed the best docking scores, and the comparison of the docking sites of the two drugs revealed a near perfect dock in the overlapping region of the protein pockets. As for remdesivir, the docking site is perfectly located in the NTP binding motif, which is expected to block the replication of RNA sequence. Because both drugs have been used in clinical practices with limited toxicity, we recommend that they should be taken into consideration whilst treating for COVID‐19. 24 With these advantageous, some clinical trials have been planned to evaluate the safety and efficacy of remdesivir against 2019‐nCoV respiratory disease.
5. ARBIDOL
Arbidol (known as umifenovir) is a broad‐spectrum antiviral compound approved in Russia and China for prophylaxis and can effectively block the fusion of influenza virus with host cells. 25 Arbidol not only stimulated overexpression of endogenous interferon against virus replication but also enhanced the phagocytic function of macrophages, as well as activated the natural killer cells. 26 This drug does not have remarkable side effects and successfully is patented for SARS treatment. 27 , 28 These promising futures encouraged scientists and physicians to evaluate its efficacy against 2019‐nCoV. For example, Deng et al compare arbidol and LPV/RTV (LPV/r) treatment for patients with COVID‐19 with LPV/r only. Patients were given oral arbidol and LPV/r in the combination group and oral LPV/r only in the monotherapy group for 5 to 21 days. Specifically, arbidol was given at a dose of 200 mg every 8 hours and LPV (400 mg)/RTV (100 mg) orally every 12 hours until coronavirus is detected negative by RT‐PCR for tree times. The administration period is about 5 to 21 days. They explored 16 patients who received oral arbidol and LPV/r in the combination group and 17 who oral LPV/r only in the monotherapy group, and both initiated after diagnosis. Their study shows that oral arbidol and LPV/r in the combination group is associated with a significant elevated negative conversion rate of coronavirus' test in 7 and 14 day, compared with LPV/r only in the monotherapy group. 29
6. FAVIPIRAVIR
Favipiravir is a novel antiviral compound that selectively and potently inhibits the RNA‐dependent RNA polymerase of influenza and many other RNA viruses. As described below, it has been found to inhibit all serotypes and strains of influenza A, B and C viruses against which it has been tested, including those resistant to currently approved neuraminidase inhibitors. 30 It is also active against a number of arena‐, bunya‐ and flaviviruses, both in vitro and in rodent models, and it has shown potent in vitro activity against members of the alphavirus, paramyxovirus and norovirus families. Cai et al conducted an open‐label and nonrandomized study with the Chinese Clinical Trial Registry (ID: ChiCTR2000029600) to investigate the effects of FPV vs LPV/RTV, IFN‐α for the treatment of COVID‐19. 31 Standard care included oxygen inhalation, oral or intravenous rehydration, electrolyte correction, antipyretics, analgesics, and antiemetic drugs. Changes in chest computed tomography (CT), viral clearance and drug safety were compared between the two groups. For the 35 patients enrolled in the FPV arm and the 45 patients in the control arm, all baseline characteristics were comparable between the two arms. The FPV arm showed significant improvement in chest imaging compared with the control arm, with an improvement rate of 91.43% vs 62.22% (P = .004). On day 14 after treatment, the improvement rates of the chest CT changes in the group with viral clearance within 7 days of treatment were significantly higher than those of the patients with viral clearance after 7 days of treatment.
7. CONCLUSION
During last several months, a lot of efforts have been devoted to prevent the spread of 2019‐nCoV in all over the world. Unfortunately, there are no any specific pharmacological treatments to date for 2019‐nCoV. Despite this fact, most of scientists around the world have moved at an unprecedented speed to use the mentioned‐drugs for treatment of 2019‐nCoV. Although some available drugs were employed against other coronaviruses like SARS and Ebola in the past, at first scientists must evaluate the efficiency of drugs against 2019‐nCoV in vitro and then transfer from bench to bed. This review provides an overview of ongoing works and developments for using of some available therapeutic agents for treatment of 2019‐nCoV. Conversely, in this report, we summarized a list of small molecule drugs that have been used frequently for treatment of 2019‐nCoV.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
Rahimkhoei V, Jabbari N, Nourani A, Sharifi S, Akbari A. Potential small‐molecule drugs as available weapons to fight novel coronavirus (2019‐nCoV): A review. Cell Biochem Funct. 2021;39:4–9. 10.1002/cbf.3576
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Velavan TP, Meyer CG. The COVID‐19 epidemic. Trop Med Int Health. 2020;25(3):278‐280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coronaviridae Study Group of the International CSG . The species severe acute respiratory syndrome‐related coronavirus: classifying 2019‐nCoV and naming it SARS‐CoV‐2. Nat Microbiol. 2020;1:536‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kupferschmidt K, Cohen J. Will novel virus go pandemic or be contained? Science. 2020;367:610‐611. [DOI] [PubMed] [Google Scholar]
- 4. Wang X, Cao R, Zhang H, et al. The anti‐influenza virus drug, arbidol is an efficient inhibitor of SARS‐CoV‐2 in vitro. Cell Discovery. 2020;6(1):1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li Z, Tomlinson AC, Wong AH, et al. The human coronavirus HCoV‐229E S‐protein structure and receptor binding. Elife. 2019;8:e51230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirchdoerfer RN, Cottrell CA, Wang N, et al. Pre‐fusion structure of a human coronavirus spike protein. Nature. 2016;531(7592):118‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsang AC, Ahmadi S, Hamilton J, et al. The diagnostic utility of multifocal electroretinography in detecting chloroquine and hydroxychloroquine retinal toxicity. Am J Ophthalmol. 2019;206:132‐139. [DOI] [PubMed] [Google Scholar]
- 8. Cortegiani A, Ingoglia G, Ippolito M, Giarratano A, Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID‐19. J Crit Care. 2020;57:279‐283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:1‐12. [DOI] [PubMed] [Google Scholar]
- 10. Colson P, Rolain J‐M, Lagier J‐C, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID‐19. Int J Antimicrob Agents. 2020;55:105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID‐19 associated pneumonia in clinical studies. Biosci Trends. 2020;14:72‐73. [DOI] [PubMed] [Google Scholar]
- 12. Yao X, Ye F, Zhang M, et al. In vitro antiviral activity and projection of optimized dosing Design of Hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). Clin Infect Dis. 2020;71(15):732‐739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhou D, Dai S‐M, Tong Q. COVID‐19: a recommendation to examine the effect of hydroxychloroquine in preventing infection and progression. J Antimicrob Chemother. 2020;75:1667‐1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gautret P, Lagier J‐C, Parola P, et al. Hydroxychloroquine and azithromycin as a treatment of COVID‐19: results of an open‐label non‐randomized clinical trial. Int J Antimicrob Agents. 2020;56:105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15. Sheahan TP, Sims AC, Leist SR, et al. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS‐CoV. Nat Commun. 2020;11(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ouedraogo HG, Matteelli A, Sulis G, et al. Pharmacokinetics of plasma lopinavir and ritonavir in tuberculosis–HIV co‐infected African adult patients also receiving rifabutin 150 or 300 mg three times per week. Ann Clin Microbiol Antimicrob. 2020;19(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cao B, Wang Y, Wen D, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid‐19. N Engl J Med. 2020;382:1787‐1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lim J, Jeon S, Shin H‐Y, et al. Case of the index patient who caused tertiary transmission of COVID‐19 infection in Korea: the application of lopinavir/ritonavir for the treatment of COVID‐19 infected pneumonia monitored by quantitative RT‐PCR. J Korean Med Sci. 2020;35(6):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends. 2020;14:64‐68. [DOI] [PubMed] [Google Scholar]
- 20. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507‐513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hassan HA, Diebold SS, Smyth LA, Walters AA, Lombardi G, Al‐Jamal KT. Application of carbon nanotubes in cancer vaccines: achievements, challenges and chances. J Control Release. 2019;297:79‐90. [DOI] [PubMed] [Google Scholar]
- 22. Li Y‐N, Su Y. Remdesivir attenuates high fat diet (HFD)‐induced NAFLD by regulating hepatocyte dyslipidemia and inflammation via the suppression of STING. Biochem Biophys Res Commun. 2020;526(2):381‐388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang M, Cao R, Zhang L, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019‐nCoV) in vitro. Cell Res. 2020;30(3):269‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chang Y‐C, Tung Y‐A, Lee K‐H, et al. Potential therapeutic agents for COVID‐19 based on the analysis of protease and RNA polymerase docking. Preprint. 2020. https://www.preprints.org/manuscript/202002.0242/v2 [Google Scholar]
- 25. Wang Y, Zhu L‐Q. Pharmaceutical care recommendations for antiviral treatments in children with coronavirus disease 2019. World J Pediatr. 2020;16:271‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rosa SGV, Santos WC. Clinical trials on drug repositioning for COVID‐19 treatment. Rev Panam Salud Publica. 2020;44:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Vankadari N. Arbidol: a potential antiviral drug for the treatment of SARS‐CoV‐2 by blocking the trimerization of viral spike glycoprotein? Int J Antimicrob Agents. 2020;56(2):105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. JI X‐G, ZHAO Y‐H, ZHANG M, ZHAO J‐H, WANG J‐Y. The experimental study of the anti‐SARS‐CoV effect of Arbidole [J]. Pharmaceut J Chin People's Liberation Army. 2004;4:1‐4. [Google Scholar]
- 29. Deng L, Li C, Zeng Q, et al. Arbidol combined with LPV/r versus LPV/r alone against Corona virus disease 2019: a retrospective cohort study. J Infect. 2020;81:e1‐e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shiraki K, Daikoku T. Favipiravir, an anti‐influenza drug against life‐threatening RNA virus infections. Pharmacol Ther. 2020;209:107512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cai Q, Yang M, Liu D, et al. Experimental treatment with Favipiravir for COVID‐19: an open‐label control study. Engineering. 2020. https://www.sciencedirect.com/science/article/pii/S2095809920300631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


