Abstract
Acute sialadenitis may be caused by viruses, including coronaviruses. Although there are anecdotal reports of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) salivary gland infections, there have been no well‐documented cases of sialadenitis in patients with COVID‐19 described in the literature. We report a case of parotitis and submandibular gland sialadenitis, as well as an isolated case of parotitis, in two patients with concurrent SARS‐CoV‐2 infections. Computed tomography imaging demonstrated parotid and submandibular gland enlargement with heterogenous enhancement and attenuation, consistent with sialadenitis. Medical management was sufficient for successful resolution of the acute sialadenitis. Laryngoscope, 130:2595–2597, 2020
Keywords: COVID‐19, severe acute respiratory syndrome coronavirus 2, sialadenitis, parotitis, submandibular gland, parotid gland
INTRODUCTION
COVID‐19 has rapidly spread worldwide since first identified in December 2019 in Wuhan, China. Caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the disease has since been declared a pandemic. COVID‐19 often first presents with otolaryngological symptoms, including cough, rhinorrhea, sore throat, dyspnea, anosmia, and dysgeusia.
High viral loads of SARS‐CoV‐2 have been consistently found in saliva specimens of patients with COVID‐19. 1 Animal studies have shown that SARS‐CoV, the related coronavirus that caused the early 2000s global SARS outbreak, can infect epithelial cells lining salivary gland ducts. 2 Although these results suggest the possibility of a SARS‐CoV‐2 salivary gland infection, there is a paucity of reports in the literature documenting this phenomenon. 3 , 4 Herein, we report a case of parotitis and submandibular gland sialadenitis, as well as an isolated case of parotitis, in two patients with documented SARS‐CoV‐2 infections. Institutional review board approval was granted for this study (AAAT0206: Neuroimaging Manifestations of COVID‐19).
CASE REPORTS
Case 1
An 88‐year‐old female was admitted for failure to thrive and poor oral intake since a recent family member death. Physical examination demonstrated right preauricular swelling and pain; no purulent drainage from Stensen's duct could be expressed for cultures. Past medical history included diabetes, hypertension, hypothyroidism, pernicious anemia, autoimmune cirrhosis, and diffuse large B cell lymphoma (treated with chemotherapy). Laboratory testing was notable for a positive COVID‐19 real‐time polymerase chain reaction (RT‐PCR) test result (nasopharyngeal swab), mild leukocytosis with lymphopenia and neutrophilic predominance, and blood cultures that grew Micrococcus luteus.
Computed tomography (CT) imaging of the neck with intravenous (IV) contrast demonstrated an enlarged right parotid gland with heterogeneous enhancement and attenuation, as well as surrounding fat stranding and fascial thickening consistent with overlying cellulitis and fasciitis (Fig. 1). No fluid collection or obstructing sialolith was identified. Chest CT demonstrated peripheral ground‐glass opacities and interstitial thickening consistent with COVID‐19 pneumonia.
Fig. 1.
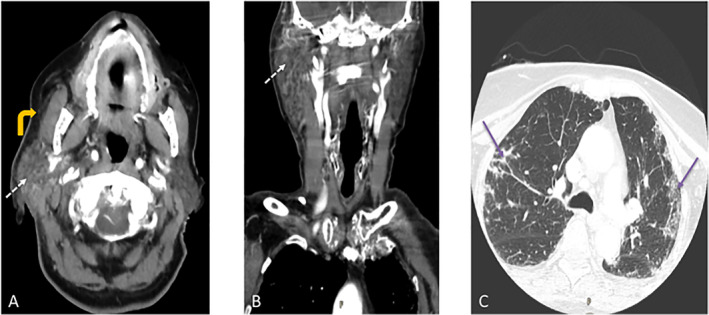
Case 1. Contrast‐enhanced computed tomography (CT) of the soft tissues of the neck. (A) Axial and (B) coronal images show enlarged right parotid gland with heterogeneous enhancement and attenuation (thin dotted white arrows). There is surrounding fat stranding and fascial thickening consistent with adjacent cellulitis (curved yellow arrow). There is no sialolith or fluid collection to suggest abscess. (C) Axial CT image of the chest from the same study demonstrate bilateral upper lobe patchy and peripheral areas of ground glass opacification (thin purple arrows) consistent with COVID‐19 pneumonia. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
The patient was treated with empiric intravenous antibiotics and supportive therapy. Over the next several days, the patient demonstrated improvement in right‐sided facial swelling and pain, resolution of leukocytosis, and was transitioned to oral antibiotics upon discharge. At outpatient follow‐up, the patient had no further complaints of parotitis.
Case 2
A 64‐year‐old male was admitted for hyponatremia in the setting of diarrhea and diuretic use and several‐day history of worsening bilateral preauricular swelling accompanied by fever. Physical examination was notable for bilateral preauricular and submandibular swelling consistent with parotid and submandibular gland enlargement, respectively; no purulent drainage could be expressed from Stensen's or Wharton's ducts with parotid or submandibular gland massage. No decreased neck range of motion, difficulty breathing, or stridor were noted. Past medical history was notable for hypertension, diabetes, smoking, gastroesophageal reflux disease, and Warthin's tumor (deferred surgery). Laboratory testing was notable for a positive COVID‐19 RT‐PCR test result (nasopharyngeal swab), severe leukocytosis with lymphopenia and neutrophilic predominance, and positive mumps virus immunoglobulin (Ig)G test consistent with immunization status and/or prior infection.
CT imaging of the neck with IV contrast demonstrated enlarged parotid glands with areas of heterogeneous attenuation and enhancement, as well as surrounding fat stranding and fascial thickening compatible with overlying cellulitis and fasciitis (Fig. 2). No fluid collection or obstructing sialolith was identified. CT also demonstrated retropharyngeal edema without rim enhancement as well as enlargement and heterogeneous enhancement of the submandibular glands, with the left greater than the right. Chest CT demonstrated peripheral ground glass opacities consistent with COVID‐19 pneumonia. Bilateral intraparotid lesions consistent with known Warthin's tumor were also visualized. These lesions were stable compared to previous CT imaging (performed 1 week prior for evaluation of facial trauma after a fall), which demonstrated multiple bilateral intraparotid lesions, with the largest on the right representing 2.8 × 2.2 cm in axial dimension; no swelling or acute inflammatory changes of parotid or submandibular glands were evident.
Fig. 2.

Case 2. Contrast‐enhanced computed tomography (CT) of the soft tissues of the neck. (A, B) Axial and (C) coronal CT images show enlarged parotid glands with areas of heterogeneous enhancement and attenuation (thin dotted white arrows) with surrounding fat stranding and fascial thickening compatible with adjacent cellulitis and fasciitis (curved yellow arrows). There is enlargement and enhancement of the left submandibular gland consistent with acute sialadenitis (thin long red arrow). There is a mass in the right parotid gland compatible with known, stable Warthin tumors (short green arrows). Left‐sided Warthin's tumor was also stable (not shown). There is no sialolith or fluid collection to suggest abscess. There is a low attenuation retropharyngeal effusion without rim enhancement to suggest abscess (thick yellow arrow). [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
The patient was treated with empiric intravenous antibiotics, sialogogues, warm compresses/massages to the parotid region, and hydration. Over his 10‐day course of admission, he demonstrated clinical improvement in bilateral facial swelling and pain, as well as decreased inflammatory markers. Patient was transitioned to oral antibiotics upon discharge. At outpatient follow‐up, the patient exhibited near‐complete resolution of bilateral facial swelling; surgical excision of the right‐sided Warthin's tumor was recommended.
DISCUSSION
Acute sialadenitis is caused by bacterial infection, viral infection, non‐infectious inflammatory processes (e.g., sarcoidosis) and immune‐mediated processes (Sjogren syndrome). Viruses causing salivary infection include paramyxovirus (i.e., mumps), influenza A, parainfluenza virus, human immunodeficiency virus, and coronavirus. The findings of parotitis and submandibular sialadenitis in two patients with documented SARS‐CoV‐2 infection by RT‐PCR testing suggests that acute, nonsuppurative sialadenitis is a possible manifestation of COVID‐19. CT findings suggest that the infection involves the entire gland without frank abscess formation. COVID‐19 sialadenitis responded to medical management with complete resolution.
Viral transmission into the salivary glands most commonly occurs through hematogenous spread, though retrograde ductal migration may occur with decreased salivary flow in the dehydrated patient. Animal studies have demonstrated SARS‐CoV tropism for epithelial cells lining salivary gland ducts. 2 Moreover, high viral loads of SARS‐CoV‐2 have been consistently found in saliva specimens. 1 Coronaviruses have been detected in saliva samples with high diagnostic concordance with nasopharyngeal specimens, widely considered the standard for diagnostic testing of respiratory viruses. 5 However, it should be noted that saliva contains secretions not only from the salivary glands, but also the nasopharynx and the lungs. These findings in toto suggest that SARS‐CoV‐2 directly infects salivary glands.
There are two reports in the literature related to SARS‐CoV‐2 salivary gland infections. One reported a case of parotitis in a COVID‐19–negative patient quarantined with family members (all COVID‐19 positive on RT‐PCR testing). The patient was initially COVID‐19 negative on RT‐PCR testing, but weakly positive on IgG testing several weeks after onset of symptoms. 3 Another report described three patients with COVID‐19 infection with intraparotid lymphadenitis on magnetic resonance imaging and with parotitis‐type symptoms. However, they did not have primary parotitis. 4 Thus, the current two cases represent the first documented cases of COVID‐19–related parotid and submandibular gland sialadenitis.
CONCLUSION
This case report describes a case of parotitis and submandibular gland sialadenitis, as well as an isolated case of parotitis, in two patients with concurrent, documented SARS‐CoV‐2 infection on RT‐PCR testing, suggesting that acute, nonsuppurative sialadenitis is a possible early manifestation of COVID‐19.
Editor's Note: This Manuscript was accepted for publication on August 18, 2020.
Anil K. Lalwani, MD, serves on the advisory board of Advanced Bionics, MED EL, and Spiral Therapeutics.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1. To KKW, Tsang OTY, Yip CYC, et al. Consistent detection of 2019 novel coronavirus in saliva. Clin Infect Dis 2020;71:841–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu L, Wei Q, Alvarez X, et al. Epithelial cells lining salivary gland ducts are early target cells of severe acute respiratory syndrome coronavirus infection in the upper respiratory tracts of rhesus macaques. J Virol 2011;85:4025–4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Capaccio P, Surgery N, Pignataro L, et al. Acute parotitis: a possible precocious clinical manifestation of SARS‐CoV‐2 infection? Otolaryngol Head Neck Surg 2020;163:182–183. [DOI] [PubMed] [Google Scholar]
- 4. Lechien JR, Chetrit A, Chekkoury‐Idrissi Y, et al. Parotitis‐like symptoms associated with COVID‐19, France, March–April 2020. Emerg Infect Dis 2020;26:2270–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. To KK, Lu L, Yip CC, et al. Additional molecular testing of saliva specimens improves the detection of respiratory viruses. Emerg Microbes Infect 2017;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]


