Abstract
Aims
Data on the impact of COVID‐19 in chronic heart failure (CHF) patients and its potential to trigger acute heart failure (AHF) are lacking. The aim of this work was to study characteristics, cardiovascular outcomes and mortality in patients with confirmed COVID‐19 infection and a prior diagnosis of heart failure (HF). Further aims included the identification of predictors and prognostic implications for AHF decompensation during hospital admission and the determination of a potential correlation between the withdrawal of HF guideline‐directed medical therapy (GDMT) and worse outcomes during hospitalization.
Methods and results
Data for a total of 3080 consecutive patients with confirmed COVID‐19 infection and follow‐up of at least 30 days were analysed. Patients with a previous history of CHF (n = 152, 4.9%) were more prone to the development of AHF (11.2% vs. 2.1%; P < 0.001) and had higher levels of N‐terminal pro brain natriuretic peptide. In addition, patients with previous CHF had higher mortality rates (48.7% vs. 19.0%; P < 0.001). In contrast, 77 patients (2.5%) were diagnosed with AHF, which in the vast majority of cases (77.9%) developed in patients without a history of HF. Arrhythmias during hospital admission and CHF were the main predictors of AHF. Patients developing AHF had significantly higher mortality (46.8% vs. 19.7%; P < 0.001). Finally, the withdrawal of beta‐blockers, mineralocorticoid receptor antagonists and angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers was associated with a significant increase in in‐hospital mortality.
Conclusions
Patients with COVID‐19 have a significant incidence of AHF, which is associated with very high mortality rates. Moreover, patients with a history of CHF are prone to developing acute decompensation after a COVID‐19 diagnosis. The withdrawal of GDMT was associated with higher mortality.
Keywords: COVID‐19, Heart failure, NT‐proBNP, Drug withdrawal, Mortality, Morbidity
Heart failure in COVID‐19 patients: prevalence, incidence and prognostic implications.

Introduction
Coronavirus disease 2019 (COVID‐19), which accounts for the ongoing pandemic and is responsible for substantial morbidity and mortality around the globe, has proven to be a multisystemic condition with frequent cardiac manifestations. Cardiovascular (CV) disease and classic CV risk factors are common comorbidities in COVID‐19 patients and have been associated with poor outcomes. 1
In addition, heart failure (HF) is one of the leading causes of morbidity and mortality worldwide and should not be left unattended. Previous knowledge of other respiratory tract infections, such as influenza, has proven virus potential to trigger decompensation in HF patients. 2 Recent research has focused on the impact of the COVID‐19 pandemic on hospitalization rates, incidence and characteristics of patients treated in specialized HF units, 3 but data on the prevalence, incidence and prognostic implications of HF in patients with a confirmed diagnosis of SARS‐CoV‐2 infection are still lacking.
The aim of this work was to study characteristics, CV outcomes and mortality rates in patients with confirmed COVID‐19 infection and a prior diagnosis of HF. This work also focused on identifying predictors for and prognostic implications of HF decompensation during hospital admission and explored whether there is an association between the withdrawal of guideline‐directed medical therapy (GDMT) and worse outcomes during hospitalization.
Methods
Study design and participants
All consecutive patients with a clinical suspicion of COVID‐19 infection presenting at the emergency department of a tertiary hospital in Madrid, in the region of Spain most affected by COVID‐19, between 1 March and 20 April 2020, were screened. Patients were included in the study only if COVID‐19 infection was confirmed by RNA reverse‐transcriptase polymerase chain reaction (RT‐PCR) assay from nasopharyngeal swab specimens. The study specifically aimed to include patients for whom follow‐up data for a period of at least 30 days from microbiological diagnosis were available. Therefore, data for patients who were alive but whose diagnoses dated from less than 30 days from the start of analysis were excluded from the present analyses. This study was approved by the institutional review board at the study centre. Requirements for individual written informed consent were waived in line with legal standards for national health care emergency situations.
Data collection
Epidemiological, demographic, clinical, laboratory, treatment and outcome data were extracted from electronic medical records for index and subsequent hospital admissions using a standardized electronic data collection form. In addition, the central regional health care record system, which collects information and medical reports from all public hospitals and primary health care centres in Madrid, was reviewed for additional information and follow‐up data. All data were thoroughly reviewed by a team of 13 cardiologists. Any disagreements regarding data classification were reviewed by the whole team and final decisions were made by consensus. Special care was given to the identification of baseline CV profiles, clinical outcomes and, specifically, diagnoses of acute HF (AHF).
Study definitions
Chronic HF (CHF) was defined as a history of previous congestive decompensation or a diagnosis of left ventricular (LV) systolic dysfunction [LV ejection fraction (LVEF) of <40%]. AHF refers to the rapid onset or worsening of symptoms and/or signs of HF during the study period. Because of the difficulty in distinguishing between respiratory and cardiac causes of dyspnoea in COVID‐19, AHF events were adjudicated on a case‐by‐case basis by consensus among all investigators. Decisions were based on all clinical information available for each patient and included: a codified HF diagnosis; the description of serial physical examinations in the electronic medical record; radiological tests (chest radiography and computed tomography); echocardiographic studies, and N‐terminal pro brain natriuretic peptide (NT‐proBNP) levels according to the recommended cut‐off values of the Heart Failure Association of the European Society of Cardiology (ESC) for the diagnosis of AHF (>450 pg/mL in patients aged <50 years, >900 pg/mL in patients aged 50–75 years, and >1800 pg/mL in patients aged >75 years). 4
The categorization of echocardiographic measurements of LV systolic function were based on published recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. 5 Bleeding events were defined as specified in the Thrombolysis in Myocardial Infarction (TIMI) bleeding classification. 6
In order to minimize the risk for selection bias caused by severe illness (i.e. hypotension caused by septic shock), patients in whom medication was withdrawn were defined as those who had received chronic treatment with angiotensin‐converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), beta‐blockers or mineralocorticoid receptor antagonists (MRAs) before hospital admission and who did not receive any further dose after hospital admission, as confirmed by the central pharmacy's computerized information system, irrespective of their clinical status.
Statistical analysis
Categorical variables are shown as rates and percentages, and continuous variables as the mean ± standard deviation (SD) or median and interquartile range (IQR) as appropriate. Means for continuous variables were compared using independent group t‐tests when data were normally distributed; otherwise, Mann–Whitney tests were performed. The normality of distributions was assessed using the Shapiro–Wilk test. Proportions for categorical variables were compared using the chi‐squared test or Fisher's exact test, as appropriate. Survival during follow‐up was assessed using Kaplan–Meier analysis and, when appropriate, the log‐rank test. The associations of CHF and AHF with mortality during follow‐up were studied using a Cox proportional hazard model accounting for relevant covariates (age, sex, CV risk factors, coronary heart disease, chronic kidney disease and cerebrovascular disease). Stepwise logistic regression was used to develop a predictive model for AHF during admission, using as candidate variables those which were statistically significant in the univariate analysis. All data were analysed using STATA Version 14.2 (StataCorp LLC, College Station, TX, USA). A two‐sided P‐value of <0.05 was considered to indicate differences of statistical significance in all analyses.
Results
During the study period, 3080 patients with confirmed COVID‐19 infection were found to fulfil the study selection criteria and were ultimately included in the present analysis (Figure 1 ). The mean ± SD age of the cohort was 62.3 ± 20.3 years and 1689 (54.8%) were male. A total of 626 patients (20,3%) died during a median follow‐up of 59 days (IQR 50–66 days). The median length of time from SARS‐CoV‐2 diagnosis to death was 6 days (IQR 3–12 days; range: 0–53 days).
Figure 1.
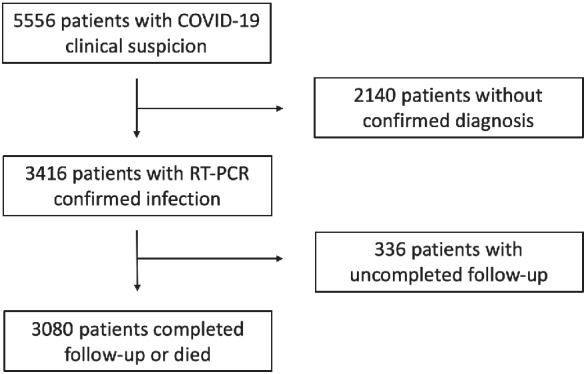
Study flow COVID‐19, coronavirus disease 2019; RT‐PCR, reverse‐transcriptase polymerase chain reaction.
COVID‐19 patients with vs. those without chronic heart failure
A total of 152 patients with CHF (4.9% of those with positive RT‐PCR) were studied. Of those, 98 (64.5%) had some degree of LV systolic dysfunction prior to COVID‐19 diagnosis. The remaining 54 had normalized LVEF after the initiation of GDMT or had HF with preserved ejection fraction with other significant echocardiographic abnormalities such as LV hypertrophy or moderate‐to‐severe valvular disease. Baseline patient characteristics are shown in Table 1 . Patients with a history of CHF were older (mean ± SD 81.9 ± 11.9 years vs. 61.2 ± 20.1 years; P < 0.001) and had a higher CV risk profile, as well as different forms of atherosclerotic disease (coronary, cerebrovascular and peripheral). As expected, they were more frequently being treated with CV medications. With respect to the prescription of dedicated COVID‐19 drugs, patients in the CHF group more often received hydroxychloroquine (85.5% vs. 77.0%; P = 0.014), but less often received tocilizumab and azithromycin.
Table 1.
Baseline characteristics, drug therapy, vital signs, laboratory data and clinical outcomes in COVID‐19 patients with and without a previous history of chronic heart failure
| Variable | All patients (n = 3080) | Non‐CHF patients (n = 2928) | CHF patients (n = 152) | P‐value |
|---|---|---|---|---|
| Baseline characteristics and coexisting disorder | ||||
| Age, years, mean ± SD | 62.3 ± 20.3 | 61.2 ± 20.1 | 81.9 ± 11.9 | <0.001 |
| Male sex, n (%) | 1689 (54.8%) | 1595 (54.5%) | 94 (61.8%) | 0.075 |
| Hypertension, n (%) | 1322 (43.1%) | 1193 (40.9%) | 129 (86.0%) | <0.001 |
| Diabetes, n (%) | 559 (18.3%) | 501 (17.2%) | 58 (39.5%) | <0.001 |
| Dyslipidaemia, n (%) | 1103 (37.1%) | 995 (35.2%) | 108 (75.0%) | <0.001 |
| Smoking habit, n (%) | 304 (9.9%) | 273 (9.3%) | 31 (20.3%) | <0.001 |
| Obesity, n (%) | 430 (14.0%) | 397 (13.6%) | 33 (21.7%) | 0.005 |
| Peripheral artery disease, n (%) | 199 (6.5%) | 157 (5.4%) | 42 (28.6%) | <0.001 |
| Ischaemic stroke, n (%) | 187 (6.1%) | 155 (5.3%) | 32 (21.8%) | <0.001 |
| Coronary artery disease, n (%) | 199 (6.5%) | 155 (5.3%) | 44 (29.7%) | <0.001 |
| Atrial fibrillation/flutter, n (%) | 269 (8.7%) | 190 (6.5%) | 79 (52.0%) | <0.001 |
| PM/ICD, n (%) | 53 (1.7%) | 37 (1.3%) | 16 (10.5%) | <0.001 |
| COPD, n (%) | 236 (7.7%) | 191 (6.5%) | 45 (29.6%) | <0.001 |
| Chronic kidney disease, n (%) | 180 (5.8%) | 141 (4.8%) | 39 (25.7%) | <0.001 |
| Cancer, n (%) | 301 (9.8%) | 273 (9.3%) | 28 (18.4%) | <0.001 |
| Baseline cardiovascular drug therapy, n (%) | ||||
| Therapeutic anticoagulation | 309 (10.1%) | 223 (7.7%) | 86 (57.7%) | <0.001 |
| Antiplatelet | 440 (14.3%) | 390 (13.3%) | 50 (32.9%) | <0.001 |
| ACEI or ARB | 1005 (32.6%) | 915 (31.3%) | 90 (59.2%) | <0.001 |
| MRA | 93 (3.0%) | 46 (1.6%) | 47 (30.9%) | <0.001 |
| Sacubitril–valsartan | 13 (0.4%) | 2 (0.1%) | 11 (7.2%) | <0.001 |
| Beta‐blocker | 407 (13.2%) | 313 (10.7%) | 94 (61.8%) | <0.001 |
| Diuretics | 628 (20.4%) | 521 (17.8%) | 107 (70.4%) | <0.001 |
| SGLT2 inhibitors | 43 (1.4%) | 37 (1.3%) | 6 (4.0%) | <0.001 |
| Digoxin | 22 (0.7%) | 12 (0.4%) | 10 (6.6%) | <0.001 |
| Statin | 878 (28.5%) | 782 (26.7%) | 96 (63.2%) | <0.001 |
| Antiarrhythmic | 22 (0.7%) | 19 (0.7%) | 3 (2.0%) | 0.059 |
| Vital signs at hospital presentation | ||||
| SBP, mmHg, mean ± SD | 129.0 ± 21.4 | 129.0 ± 21.3 | 129.4 ± 23.9 | 0.804 |
| Heart rate, bpm, mean ± SD | 93.6 ± 19.7 | 94.3 ± 19.5 | 82.7 ± 20.3 | <0.001 |
| First oxygen saturation, %, mean ± SD | 92.2 ± 6.3 | 92.3 ± 6.2 | 90.4 ± 7.4 | <0.001 |
| First oxygen saturation receiving oxygen, n (%) | 284 (10.4%) | 248 (9.6%) | 36 (25.2%) | <0.001 |
| First chest radiography, n (%) | ||||
| Without infiltrates | 821 (27.8%) | 776 (27.7%) | 45 (30.6%) | 0.132 |
| Unilateral infiltrates | 592 (20.1%) | 572 (20.4%) | 20 (13.6%) | |
| Bilateral infiltrates | 1537 (52.1%) | 1455 (51.9%) | 82 (55.8%) | |
| Laboratory data, mean ± SD | ||||
| Median eGFR, mL/min/1.73 m2 | 75.0 ± 21.0 | 76.6 ± 19.7 | 47.9 ± 23.2 | <0.001 |
| Median haemoglobin, g/dL | 13.5 ± 1.8 | 13.6 ± 1.7 | 12.7 ± 2.2 | <0.001 |
| Highest ferritin, ng/dL | 1481 ± 6298 | 1494 ± 6445 | 1226 ± 2220 | 0.730 |
| Highest D‐dimer, ng/mL | 9351 ± 32 509 | 9475 ± 33 038 | 7040 ± 20 125 | 0.468 |
| Highest troponin, ng/L | 456 ± 5301 | 306 ± 2646 | 4331 ± 23 952 | <0.001 |
| Highest NT‐proBNP, pg/mL | 6067 ± 16 730 | 4726 ± 13 530 | 16 802 ± 30 726 | <0.001 |
| Highest fibrinogen, mg/dL | 820 ± 282 | 820 ± 282 | 825 ± 274 | 0.854 |
| Highest CRP, mg/L | 131.5 ± 109.3 | 131.1 ± 110.2 | 138.0 ± 92.1 | 0.464 |
| Highest IL‐6, pg/mL | 311.4 ± 591.8 | 310.6 ± 590.6 | 339.1 ± 650.6 | 0.840 |
| Antimicrobial and immunomodulatory agents against COVID‐19, n (%) | ||||
| Hydroxychloroquine | 2383 (77.4%) | 2253 (77.0%) | 130 (85.5%) | 0.014 |
| Lopinavir/ritonavir | 319 (10.4%) | 303 (10.4%) | 16 (10.5%) | 0.944 |
| Azithromycin | 1404 (45.6%) | 1347 (46.0%) | 57 (37.5%) | 0.040 |
| Tocilizumab | 227 (7.4%) | 223 (7.6%) | 4 (2.6%) | 0.022 |
| Systemic glucocorticoid | 444 (14.4%) | 419 (14.3%) | 25 (16.5%) | 0.465 |
| Clinical outcomes, n (%) | ||||
| Hospital admission | 2191 (72.1%) | 2054 (71.1%) | 137 (92.0%) | <0.001 |
| Clinical diagnosis of AHF | 77 (2.5%) | 60 (2.1%) | 17 (11.2%) | <0.001 |
| Pulmonary embolism | 76 (2.5%) | 75 (2.6%) | 1 (0.7%) | 0.140 |
| Thrombotic event | 116 (3.8%) | 113 (3.9%) | 3 (2.0%) | 0.234 |
| Major bleeding | 22 (0.8%) | 21 (0.8%) | 1 (0.7%) | 0.642 |
| No major bleeding | 66 (2.5%) | 61 (2.5%) | 5 (3.8%) | |
| Atrial fibrillation/flutter during admission | 87 (2.8%) | 81 (2.8%) | 6 (4.0%) | 0.392 |
| Ventricular arrhythmias during admission | 11 (0.4%) | 10 (0.3%) | 1 (0.7%) | 0.524 |
| Critical care admission | 182 (6.1%) | 180 (6.4%) | 2 (1.4%) | 0.013 |
| Mechanical ventilation | 173(5.8%) | 171 (6.1%) | 2 (1.4%) | 0.017 |
| Death | 626 (20.5%) | 552 (19.0%) | 74 (48.7%) | <0.001 |
ACEI, angiotensin‐converting enzyme inhibitor; AHF, acute heart failure; ARB, angiotensin‐receptor blocker; CHF, chronic heart failure; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; IL‐6, interleukin‐6; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N terminal pro brain natriuretic peptide; PM, pacemaker; SBP, systolic blood pressure; SD, standard deviation; SGLT2, sodium‐glucose co‐transporter 2.
Patients in the CHF group had a significantly lower estimated glomerular filtration rate (47.9 ± 23.2 mL/min/1.73m2 vs. 76.6 ± 19.7 mL/min/1.73m2; P < 0.001) and haemoglobin level (12.7 ± 2.2 g/dL vs. 13.6 ± 1.7 g/dL; P < 0.001). No differences were found in proinflammatory makers (ferritin, fibrinogen, C‐reactive protein, interleukin‐6) or in D‐dimer. However, peak NT‐proBNP (16 802 ± 30 726 pg/mL vs. 4726 ± 13 530 pg/mL; P < 0.001) and high‐sensitivity (hs) troponin I (4331 ± 23 952 ng/L vs. 306 ± 2646 ng/L; P < 0.001) levels were significantly higher during hospital admission.
Patients with CHF were more prone to the development of clinical signs suggestive of AHF, as well as elevation of NT‐proBNP above the AHF cut‐off value. Patients in the CHF group developed numerically more thrombotic events than those in the non‐CHF group, but the numbers were small and without significant differences (2.0% vs. 3.9%; P = 0.234). However, CHF patients received significantly more chronic therapeutic anticoagulant therapy [n = 86 (57.7%) vs. n = 223 (7.7%); P < 0.001].
Subjects in the CHF group were less frequently admitted to the intensive care unit (ICU) (1.4% vs. 6.4%; P = 0.013) and had a higher mortality rate during follow‐up (48.7% vs. 19.0%; P < 0.001) (Figure 2A ). However, after adjustment for other relevant covariates, CHF did not reach the statistical significance required to be identified as an independent predictor of mortality (Supplementary material online, Table S1 ).
Figure 2.
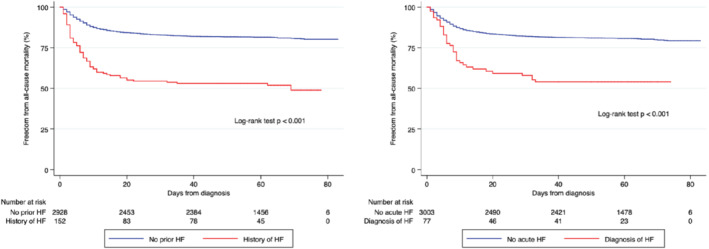
(A) Survival analysis showing significant differences (P < 0.001) between patients with and without chronic heart failure (HF). (B) Kaplan–Meier survival curves stratified by clinical diagnosis of acute HF showing significant differences (P < 0.001) in mortality.
Development of acute heart failure in COVID‐19 patients
During the study period, 77 patients (2.5%) received a diagnosis of AHF (Table 2 ). Of these, 47 had documented abnormal NT‐proBNP levels according to the recommended cut‐off value for a diagnosis of AHF; NT‐proBNP levels were not determined in the remaining 30 patients during hospital admission (Supplementary material online, Table S2 ). Qualitative information regarding point‐of‐care echocardiographic examinations was available for 31 AHF patients, of whom 12 had some degree of LV systolic dysfunction, 17 had other pathological findings (such as significant valvular heart disease, pericardial effusion or right ventricular dysfunction) and two were reported as without significant abnormalities.
Table 2.
Baseline characteristics, drug therapy, vital signs, laboratory data and clinical outcomes in patients with COVID‐19 with and without the development of acute heart failure (AHF) during admission
| Variable | All patients (n = 3080) | Non‐AHF patients (n = 3003) | AHF patients (n = 77) | P‐value |
|---|---|---|---|---|
| Baseline characteristics and coexisting disorders | ||||
| Age, years, mean ± SD | 62.3 ± 20.3 | 61.8 ± 20.3 | 78.6 ± 12.6 | <0.001 |
| Male sex, n (%) | 1689 (54.8%) | 1647 (54.9%) | 42 (54.6%) | 0.958 |
| Hypertension, n (%) | 1322 (42.9%) | 1260 (42.0%) | 62 (80.5%) | <0.001 |
| Diabetes, n (%) | 559 (18.2%) | 532 (17.7%) | 27 (35.1%) | <0.001 |
| Dyslipidaemia, n (%) | 1103 (35.8%) | 1056 (35.2%) | 47 (61.0%) | <0.001 |
| Smoking habit, n (%) | 304 (9.9%) | 296 (9.9%) | 8 (10.4%) | 0.877 |
| Obesity, n (%) | 430 (14.0%) | 413 (13.8%) | 17 (22.1%) | 0.037 |
| Peripheral artery disease, n (%) | 199 (6.5%) | 187 (6.2%) | 12 (15.6%) | 0.001 |
| Ischaemic stroke, n (%) | 187 (6.1%) | 170 (5.7%) | 17 (22.1%) | <0.001 |
| Coronary artery disease, n (%) | 199 (6.5%) | 190 (6.3%) | 9 (11.7%) | 0.051 |
| Atrial fibrillation/flutter, n (%) | 269 (8.7%) | 246 (8.2%) | 23 (29.9%) | <0.001 |
| Chronic heart failure, n (%) | 152 (4.9%) | 135 (4.50) | 17 (22.1) | <0.001 |
| PM/ICD, n (%) | 53 (1.7%) | 49 (1.6%) | 4 (5.2%) | 0.042 |
| COPD, n (%) | 236 (7.7%) | 217 (7.2%) | 19 (24.7%) | <0.001 |
| Chronic kidney disease, n (%) | 180 (5.8%) | 165 (5.5%) | 15 (19.5%) | <0.001 |
| Cancer, n (%) | 301 (9.8%) | 289 (9.6%) | 12 (15.6%) | 0.115 |
| Baseline cardiovascular drug therapy, n (%) | ||||
| Therapeutic anticoagulation | 309 (10.0%) | 284 (9.5%) | 25 (32.5%) | <0.001 |
| Antiplatelet | 440 (14.3%) | 420 (14.0%) | 20 (26.0%) | 0.003 |
| ACEI or ARB | 1005 (32.6%) | 963 (32.1%) | 42 (54.6%) | <0.001 |
| MRA | 93 (3.0%) | 83 (2.8%) | 10 (13.0%) | <0.001 |
| Sacubitril–valsartan | 13 (0.4%) | 11 (0.4%) | 2 (2.6%) | 0.040 |
| Beta‐blocker | 407 (13.2%) | 376 (12.5%) | 31 (40.3%) | <0.001 |
| Diuretics | 628 (20.4%) | 588 (19.6%) | 40 (52.0%) | <0.001 |
| SGLT2 inhibitors | 43 (1.4%) | 41 (1.4%) | 2 (2.6%) | 0.292 |
| Digoxin | 22 (0.7%) | 21 (0.7%) | 1 (1.3%) | 0.428 |
| Statin | 878 (28.5%) | 837 (27.9%) | 41 (53.3%) | <0.001 |
| Antiarrhythmic | 22 (0.7%) | 19 (0.6%) | 3 (3.9%) | 0.016 |
| Vital signs at hospital presentation | ||||
| SBP, mmHg, mean ± SD | 129.0 ± 21.4 | 128.9 ± 21.2 | 132.0 ± 27.4 | 0.229 |
| Heart rate, b.p.m., mean ± SD | 93.6 ± 19.7 | 93.8 ± 19.6 | 86.7 ± 22.1 | 0.002 |
| First oxygen saturation, %, mean ± SD | 92.2 ± 6.3 | 92.3 ± 6.2 | 89.3 ± 7.6 | <0.001 |
| First oxygen saturation receiving oxygen, n (%) | 284 (9.2%) | 268 (8.9%) | 16 (20.8%) | 0.001 |
| First chest radiography, n (%) | ||||
| Without infiltrates | 821 (26.7%) | 809 (26.9%) | 12 (15.6%) | 0.052 |
| Unilateral infiltrates | 592 (19.2%) | 576 (19.2%) | 16 (20.8%) | |
| Bilateral infiltrates | 1537 (49.9%) | 1489 (49.6%) | 48 (62.3%) | |
| Laboratory data, mean ± SD | ||||
| Median eGFR, mL/min/1.73 m2 | 75.4 ± 21.3 | 75.8 ± 21.1 | 60.4 ± 24.9 | <0.001 |
| Median haemoglobin, g/dL | 13.5 ± 1.8 | 13.5 ± 1.8 | 12.7 ± 2.1 | <0.001 |
| Highest ferritin, ng/dL | 1481 ± 6298 | 1481 ± 6400 | 1481 ± 1744 | 1.000 |
| Highest D‐dimer, ng/mL | 9351 ± 32 508 | 9281 ± 32 674 | 11 580 ± 26 887 | 0.001 |
| Highest troponin, ng/L | 456 ± 5300 | 458 ± 5403 | 417 ± 1683 | 0.963 |
| Highest NT‐proBNP, pg/mL | 6067 ± 16 730 | 5469 ± 16 118 | 10 508 ± 20 374 | <0.001 |
| Highest fibrinogen, mg/dL | 820 ± 281 | 818 ± 282 | 878 ± 273 | 0.267 |
| Highest CRP, mg/L | 131.5 ± 109.3 | 130.4 ± 108.9 | 167.9 ± 115.6 | 0.004 |
| Highest IL‐6, pg/mL | 311.4 ± 591.8 | 310.3 ± 598.3 | 339.1 ± 400.0 | 0.125 |
| Antimicrobial and immunomodulatory agents against COVID‐19, n (%) | ||||
| Hydroxychloroquine | 2383 (77.4%) | 2313 (77.0%) | 70 (90.9%) | 0.004 |
| Lopinavir/ritonavir | 319 (10.4%) | 314 (10.5%) | 5 (6.5%) | 0.343 |
| Azithromycin | 1404 (45.6%) | 1362 (45.4%) | 42 (54.6%) | 0.132 |
| Tocilizumab | 227 (7.4%) | 220 (7.3%) | 7 (9.1%) | 0.558 |
| Systemic glucocorticoid | 444 (14.4%) | 421 (14.0%) | 23 (29.9%) | <0.001 |
| Clinical outcomes, n (%) | ||||
| Hospital admission | 2191 (71.1%) | 2115 (70.4%) | 76 (98.7%) | <0.001 |
| Pulmonary embolism | 76 (2.5%) | 72 (2.4%) | 4 (5.2%) | 0.120 |
| Thrombotic event | 116 (3.8%) | 111 (3.7%) | 5 (6.5%) | 0.212 |
| Major bleeding | 22 (0.7%) | 21 (0.7%) | 1 (1.3%) | 0.045 |
| Non‐major bleeding | 66 (2.1%) | 61 (2.0%) | 5 (6.5%) | |
| Atrial fibrillation/flutter during admission | 87 (2.8%) | 76 (2.5%) | 11 (14.3%) | <0.001 |
| Ventricular arrhythmias during admission | 11 (0.4%) | 10 (0.3%) | 1 (1.3%) | 0.243 |
| Critical care admission | 182 (5.9%) | 176 (5.9%) | 6 (7.8%) | 0.497 |
| Mechanical ventilation | 173 (5.6%) | 168 (5.6%) | 5 (6.5%) | 0.623 |
| Death | 626 (20.3%) | 590 (19.7%) | 36 (46.8%) | <0.001 |
ACEI, angiotensin‐converting enzyme inhibitor; AHF, acute heart failure; ARB, angiotensin‐receptor blocker; COPD, chronic obstructive pulmonary disease; CRP, C‐reactive protein; eGFR, estimated glomerular filtration rate; ICD, implantable cardioverter defibrillator; IL‐6, interleukin‐6; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N terminal pro brain natriuretic peptide; PM, pacemaker; SBP, systolic blood pressure; SD, standard deviation; SGLT2, sodium–glucose co‐transporter 2.
The mean ± SD patient age (78.6 ± 12.6 vs. 61.8 ± 20.3; P < 0.001) and CV risk profile (exception made for the proportion of smokers) were higher in the AHF group. Other associated comorbidities and treatment with CV medications were also more prevalent in this group, although numbers for both were lower than those seen in the CHF group (Table 1 ). Interestingly, the vast majority of patients who developed AHF did not have a history of CHF (n = 60, 77.9%). Patients in the AHF group had more severe presentations of COVID‐19, as indicated by lower levels of oxygen saturation at admission (89.3 ± 7.6% vs. 92.3 ± 6.2%; P < 0.001) and a higher need for supplementary oxygen (20.8% vs. 8.9%; P = 0.001), and showed a trend towards more frequent bilateral pneumonia.
As expected, peak NT‐proBNP was higher in AHF patients (10 508 ± 20 374 pg/mL vs. 5469 ± 16 118 pg/mL; P < 0.001). With respect to other laboratory findings, significant differences were observed in C‐reactive protein and D‐dimer. Although hs troponin I was mildly elevated in both groups, its levels did not differ significantly (417 ± 1683 ng/L vs. 458 ± 5403 ng/L; P = 0.963).
Patients with AHF were more likely to receive hydroxychloroquine and systemic corticosteroids, and underwent more frequent hospital admission (98.7% vs. 70.4%; P < 0.001), atrial arrhythmias (14.3% vs. 2.5%; P < 0.001) and bleeding events. Indeed, mortality in this group was significantly higher (46.8% vs. 19.7%; P < 0.001 for the log‐rank test) (Figure 2B ). However, no differences between the groups regarding mechanical ventilation or ICU admission were noted. After adjustment for relevant covariates, the development of AHF did not reach the threshold of significance required to be considered as an independent predictor of mortality during follow‐up [hazard ratio (HR) 1.40, 95% confidence interval (CI) 0.98–1.98; P = 0.062] (Supplementary material online, Table S3 ).
Stepwise logistic regression techniques were used to develop a model for the prediction of AHF during hospital admission (Table 3 ). This model illustrated that advanced age [odds ratio (OR) 1.28 (95% CI 1.16–1.41) per 5‐year increase)], atrial arrhythmias during admission (OR 4.64, 95% CI 2.19–9.83), CHF (OR 2.51, 95% CI 1.33–4.76), bleeding events (OR 1.60, 95% CI 0.98–2.62) and chronic obstructive pulmonary disease (COPD) (OR 2.51, 95% CI 1.40–4.49) were independently associated with AHF after COVID‐19 diagnosis.
Table 3.
Univariate and multivariate logistic regression model for the prediction of acute heart failure during follow‐up in COVID‐19 patients
| Variable | Non‐adjusted | Adjusted | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | SE | P‐value | OR (95% CI) | SE | P‐value | |
| Age (per 5‐year increase) | 1.33 (1.23–1.45) | 0.06 | <0.001 | 1.28 (1.16–1.41) | 0.06 | <0.001 |
| Atrial arrhythmias during admission | 6.42 (3.26–12.64) | 2.22 | <0.001 | 4.64 (2.19–9.83) | 1.78 | <0.001 |
| Chronic heart failure | 6.02 (3.42–10.60) | 1.74 | <0.001 | 2.51 (1.33–4.76) | 0.82 | 0.005 |
| Bleeding during admission | 1.77 (1.11–2.80) | 0.42 | 0.016 | 1.60 (0.98–2.62) | 0.40 | 0.061 |
| COPD | 4.21 (2.46–7.19) | 1.15 | <0.001 | 2.51 (1.40–4.49) | 0.75 | 0.002 |
CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio; SE, standard error.
Withdrawal of guideline‐directed medical therapy and mortality
Among the 152 patients with CHF, 90 (59.2%), 94 (61.8%) and 47 (30.9%) received GDMT with ACEIs/ARBs, beta‐blockers and MRAs, respectively, for HF with reduced ejection fraction or HF with recovered LVEF. These chronic prescriptions were not associated with worse outcomes after adjustment for relevant covariates (Supplementary material online, Table S4 ).
With respect to the impact of withdrawal of GDMT, 32 (35.6%), 15 (16.0%) and 22 (46.8%) patients discontinued ACEIs, beta‐blockers and MRAs, respectively, during their hospital stay. The differences between patients who discontinued HF drugs at admission and those who did not are presented in (Supplementary material online, Table S4). Statistically significant differences were identified only with respect to the prevalence of baseline hypertension, treatment with MRAs, systolic blood pressure at admission and use of lopinavir/ritonavir. Survival analysis using the log‐rank test showed that discontinuation of GDMT was associated with a higher risk for in‐hospital death (Figure 3 ).
Figure 3.
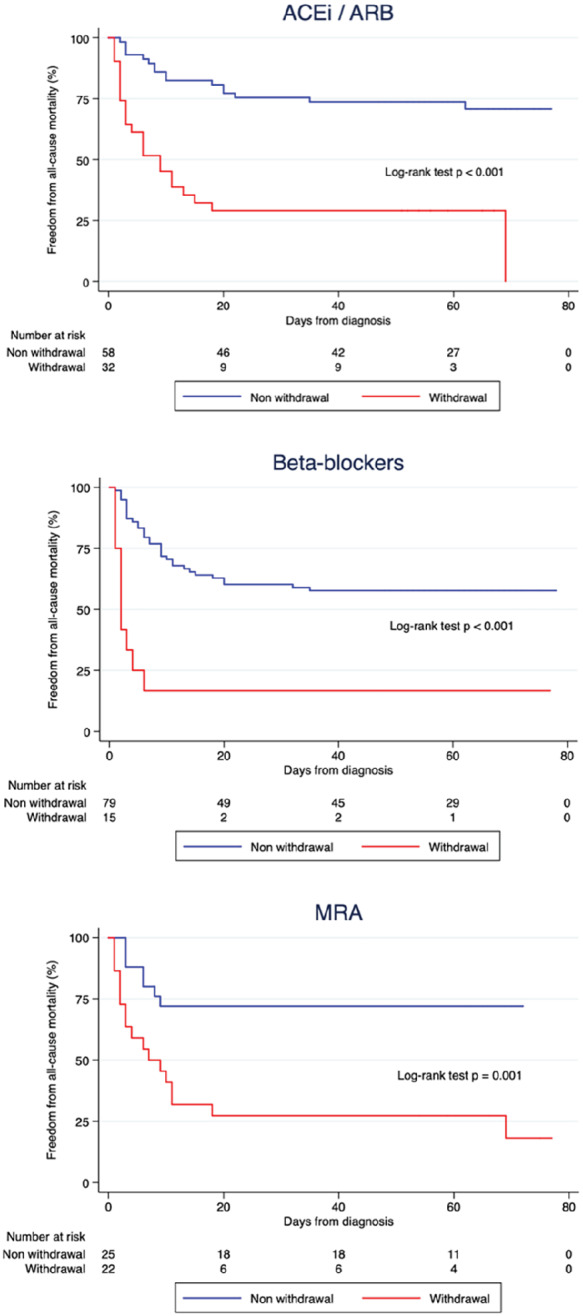
Kaplan–Meier survival analysis in patients receiving chronic treatment with (A) angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs), (B) beta‐blockers and (C) mineralocorticoid receptor antagonists (MRAs) showed that patients discontinuing these drugs at the time of admission had worse survival during follow‐up.
These findings were subsequently assessed among the corresponding subgroup of patients receiving each type of GDMT using multivariable Cox regression adjusting for relevant covariates (Supplementary material online, Tables S5 – S7 ). This analysis confirmed that the withdrawal of, ACEIs/ARBs (HR 4.50, 95% CI 2.14–9.48), beta‐blockers (HR 4.15, 95% CI 1.61–10.71) and MRAs (HR 3.36, 95% CI 1.15–9.89) were independent predictors of mortality. Indeed, the number of HF drugs discontinued during hospital admission was significantly associated with increasing risk for in‐hospital death (Supplementary material online, Figure S1 and Table S8 ). However, in this clinical context, the present group was unable to identify a higher incidence of AHF in patients in whom GDMT was withdrawn [ORs 1.10 (95% CI 0.24–4.92), 0.48 (95% CI 0.13–1.85) and 0.48 (95% CI 0.14–1.63) for ACEI/ARB, beta‐blocker and MRA discontinuation, respectively].
Discussion
The present work studied the prevalence and prognostic implications of HF in a large cohort of 3080 consecutive COVID‐19 patients. The first key finding was that patients with CHF were at significant risk for acute decompensation after COVID‐19 diagnosis. Furthermore, the development of clinical HF in this series was noteworthy and associated with poor outcomes.
Chronic heart failure and COVID‐19
The potential of SARS‐CoV‐2 to produce myocardial injury, along with the impaired cardiopulmonary reserve and poorer baseline characteristics of patients with CHF, may result in higher mortality and more frequent acute decompensation. Indeed, data from smaller cohorts show poor outcomes in patients with previous cardiac disease, although details regarding baseline CV conditions were not reported. 7 , 8
In the present series, CHF patients showed a significantly worse clinical picture at first medical contact, with subsequently higher all‐cause mortality. In addition, CHF patients were less frequently admitted to the ICU (2.1% vs. 8.0%; P = 0.037) and less often received mechanical ventilation (2.1% vs. 7.6%; P = 0.046). This apparent paradox illustrates the difficulties of allocating medical resources during the pandemic. The need to maximize clinical benefit led physicians to give priority to those patients with higher chances of surviving with a reasonable life expectancy. 9 Therefore, a significant number of CHF patients with advanced age and comorbidities may have had limited access to critical care units and this may be one of the contributing causes of the higher mortality in this population.
With regard to other in‐hospital complications and given the significant concerns of a higher likelihood of thromboembolic disease in HF patients with COVID‐19, it is remarkable that only three of the CHF patients in the present cohort had a thrombotic event during admission. Indeed, no differences in thrombotic or inflammatory biomarkers were observed between groups, but these findings may also be related to the higher prevalence of chronic therapeutic anticoagulation among CHF patients.
Development of acute heart failure in COVID‐19 patients
A total of 77 patients (2.5%) developed clinical features of AHF during the study period. HF has been described after respiratory infections and pneumonia 10 and, in this context, a meta‐analysis of 25 studies on non‐COVID‐19 pneumonia reported major cardiac complications in one‐quarter of the patients included, of which AHF was the most prevalent (14%). 11 However, the incidence of AHF in patients with diseases caused by coronaviruses, such as severe acute respiratory syndrome (caused by SARS‐CoV‐1) and Middle East respiratory syndrome (caused by MERS‐CoV), was not addressed in the medical literature prior to 2020 and remains unknown.
In this large cohort of 3080 consecutive patients with a high prevalence of pneumonia at admission, the incidence of major CV complications is significant. Yet, extraordinary measures focused on reducing the exposure of health care workers to the virus (i.e. simplified physical examinations or restrictive criteria for the indication of non‐invasive imaging tests as recommended by international scientific societies 4 , 8 , 12 , 13 ) may have limited the ability to detect incident CV disease. In addition, the overlapping clinical and radiological presentations of both COVID‐19 and HF create an undeniable barrier to the proper diagnosis of this condition. 8 , 12 , 13 A low threshold for suspicion of COVID‐19 has been advocated in CHF patients, 8 and the present group suggests that a similar recommendation should apply with regard to AHF in COVID‐19 patients.
Further, information regarding the diagnostic and prognostic roles of biomarkers such as NT‐proBNP in this clinical context is lacking. Historically, studies such as TOPCAT 14 have supported the value of NT‐proBNP and it has in recent years played a growing role in the standardization of both the definition of HF and the inclusion criteria of major clinical trials. 15 This cardiac biomarker has proven useful for the diagnosis of AHF in patients presenting with dyspnoea and no previous history of HF, especially if other imaging techniques such as transthoracic echocardiography are not readily available. 4 Such a scenario certainly resembles many in the COVID‐19 pandemic.
The proportion of patients developing AHF was higher in the CHF group (11.2% vs. 1.8%; P < 0.001), yet the vast majority of AHF cases (77.9%) developed in patients without a history of HF. This supports the potential of SARS‐CoV‐2 to induce myocardial damage. 16 Indeed, mildly elevated hs troponin I was identified in patients both with and without AHF. This is in line with recent research showing raised myocardial native T1, T2 and late‐gadolinium enhancement in a large proportion of unselected patients recovered from COVID‐19. 17 The pathophysiological mechanisms of these observations are not yet fully understood, but various authors have raised concerns about an intense proinflammatory cytokine‐modulated reaction, 16 prothrombotic state 18 and endothelial dysfunction, 19 as well as plaque rupture. Interestingly, a recent pathological investigation showed particles consistent with SARS‐CoV‐2 in the myocardial endothelial compartment, but not in the myocytes, and there was no evidence of lymphocytic myocarditis. 20
Mortality rates per year in patients admitted for AHF approach 17%. 21 Although non‐COVID‐19 pneumonia presenting with a concomitant major cardiac complication results in a non‐negligible five‐fold rise in mortality, 11 the mortality rates observed in patients who develop AHF during the course of COVID‐19 infection are dramatic (46.8% vs. 19.7%; P < 0.001).
Although the main cause of death in the present population was respiratory failure directly related to viral pneumonia, 1 it is undeniable that other CV comorbidities may coexist and not only in the context of left‐sided HF. Right ventricular dysfunction caused by either the so‐called lung‐restricted vascular immunopathology associated with COVID‐19 22 or thromboembolic disease 23 may also underlie abnormally high levels of NT‐proBNP, proinflammatory markers and, of course, D‐dimer.
In the context of the COVID‐19 pandemic, the issue of whether a more liberal determination of cardiac biomarkers might improve the early diagnosis and management of AHF and other CV complications should be prospectively investigated.
Multivariable analysis confirmed that a history of CHF, the presence of COPD and older age, all three of which are variables associated with a poorer baseline clinical profile, were independent predictors of AHF during hospital admission for COVID‐19. Bleeding, a marker of patient vulnerability that usually requires volume resuscitation and administration of blood products, was also independently associated with this complication. However, the strongest predictor of AHF in these patients was the development of atrial arrhythmias during hospital admission. Atrial fibrillation was by far the most common arrhythmia in the present series. Its effects may be mediated by multiple and well‐known mechanisms, such as loss of atrial mechanical contraction, poor rate control, impaired diastolic filling, irregular R‐R intervals and neurohormonal activation. 24 Better knowledge of the conditions related to the development of AHF may promote the early identification of high‐risk patients who may benefit from more dedicated cardiac monitoring and early referral to a multidisciplinary HF management team.
Withdrawal of chronic heart failure therapies
Despite published recommendations from several scientific societies, many patients and physicians chose to stop chronic GDMT. The results of in vitro and animal SARS‐Cov‐2 models showing a greater potential of infection due to an overexpression of the ACE2 enzyme could not be reproduced in real‐life Chinese cohorts 25 or other large and recently published series. 26 , 27 Moreover, recent research including a meta‐analysis 25 , 28 showed that chronic use of renin–angiotensin–aldosterone system (RAAS) inhibitors is actually associated with a reduction in in‐hospital mortality, although none of these studies were originally designed to examine this specific issue. Ongoing randomized clinical trials 29 are expected to address the specific hypothesis that the withdrawal of RAAS inhibitors in the general population of COVID‐19 patients may significantly impact survival.
In the context of CHF, the withdrawal of GDMT in patients with dilated cardiomyopathy who have recovered normal LVEF was specifically explored in TRED‐HF. 30 In this study, up to 40% of patients had a relapse in the form of either clinical HF or worsened LVEF.
In order to avoid selection bias (i.e. patients discontinuing GDMT as a result of COVID‐19‐related progressive clinical deterioration), GDMT withdrawal was defined as the absence of any dose received during hospital admission of HF medication in patients with CHF treatment. The present findings should be interpreted cautiously in view of the observational nature of this study, but they strongly suggest that the withdrawal of ACEIs/ARBs, beta‐blockers and MRAs is associated with higher mortality and argue in favour of the maintenance of these treatments as long as individual benefit is expected. Thus, even if severe presentations of COVID‐19 may require the temporary reduction or withdrawal of ACEIs/ARBs as a result of haemodynamic or renal deterioration, 8 all efforts should be made at discharge to restore GDMT that has been proven to favourably impact the clinical course of HF.
Limitations
This is an observational, retrospective and single‐centre study with the inherent limitations of this type of design. Its data reflect the scenario at the beginning of the pandemic in Spain, a time when data were even more scarce than in the present situation. This may result in diagnostic and therapeutic differences with current international recommendations. The study group acknowledges that NT‐proBNP was not routinely performed in every COVID‐19 patient, and that institutional policies focused on avoiding SARS‐CoV‐2 transmission may have limited its capacity to detect AHF and other CV complications.
Conclusions
COVID‐19 patients have a significant incidence of AHF, which is associated with poor outcomes. Patients with CHF are also at high risk and are vulnerable to acute decompensation after COVID‐19 diagnosis. The withdrawal of ACEIs/ARBs, beta‐blockers and MRAs in patients with CHF was associated with higher mortality during follow‐up.
Supporting information
Figure S1: Kaplan‐Meier survival analysis stratified on the number of HF drugs discontinued at the time of admission
Table S1 Univariate and multivariate analysis of chronic heart failure as a predictor of mortality accounting for relevant covariates
Table S2. Univariate and multivariate analysis of acute heart failure as a predictor of mortality accounting for relevant covariates
Table S3 Univariate and multivariate analysis of the relationship between prior use of guideline‐directed medical therapy and mortality accounting for relevant covariates
Table S4. Baseline characteristics, drug therapy, vital signs, laboratory data and clinical outcomes according to the withdrawal or not of CHF medications.
Table S5. Univariate and multivariate analysis of the relationship between withdrawal of ACEi/ARB and mortality accounting for relevant covariates
Table S6. Univariate and multivariate analysis of the relationship between withdrawal of BB and mortality accounting for relevant covariates
Table S7. Univariate and multivariate analysis of the relationship between withdrawal of MRA and mortality accounting for relevant covariates
Table S8. Univariate and multivariate analysis of the relationship between the number of discontinued GDMT medications and mortality accounting for relevant covariates
Acknowledgements
The authors thank Sylvia‐Merino, MD, for reviewing the manuscript and Inmaculada Haro and Milagros Lopez‐Nieto, from the study institution's IT department, for their support in extracting electronic medical records.
Conflict of interest: J.L.L.S. reports grants from Bayer, Pfizer, Menarini, Sanofi, Merk, Boehringer Ingelheim and Amgen, outside the submitted work. J.L.M. reports grants and personal fees from Bayer, Correvio, Daiichi‐Sankyo and Sanofi, outside the submitted work. All other authors have no conflicts of interest to declare.
Contributor Information
Juan R. Rey, Email: cardcovid@arritmias.net.
for the CARD‐COVID Investigators:
Jose L. Merino, Juan Caro‐Codon, Sergio Castrejon‐Castrejon, Angel M. Iniesta, Marcel Martinez‐Cossiani, Carlos Merino, Lorena Martin‐Polo, Luis A. Martinez, Irene Marco, Jose M. Garcia‐Veas, Laura Rodriguez‐Sotelo, Sandra O. Rosillo, Jose L. Lopez‐Sendon, Juan R. Rey, Juan Jose Rios, Jose R. Arribas, Francisco Arnalich, Concepción Prados, Rodolfo Alvarez‐Sala, Manuel Quintana, Abelardo García de Lorenzo, Francisco Reinoso, Angelica Rivera, Rosario M. Torres, Julio Garcia‐Rodriguez, Luis Gonzalez‐Valle, Alicia Herrero, Alberto Borobia, and Antonio Buño
References
- 1. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panhwar MS, Kalra A, Gupta T, Kolte D, Khera S, Bhatt DL, Ginwalla M. Effect of influenza on outcomes in patients with heart failure. JACC Heart Fail 2019;7:112–117. [DOI] [PubMed] [Google Scholar]
- 3. Bromage DI, Cannatà A, Rind IA, Gregorio C, Piper S, Shah AM, McDonagh TA. The impact of COVID‐19 on heart failure hospitalization and management: report from a heart failure unit in London during the peak of the pandemic. Eur J Heart Fail 2020;22:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, Lainscak M, Filippatos G, Seferovic PM, Meijers WC, Bayes‐Genis A, Mueller T, Richards M, Januzzi JL Jr. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail 2019;21:715–731. [DOI] [PubMed] [Google Scholar]
- 5. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233–271. [DOI] [PubMed] [Google Scholar]
- 6. Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 2011;123:2736–2747. [DOI] [PubMed] [Google Scholar]
- 7. Inciardi RM, Adamo M, Lupi L, Cani DS, Di Pasquale M, Tomasoni D, Italia L, Zaccone G, Tedino C, Fabbricatore D, Curnis A, Faggiano P, Gorga E, Lombardi CM, Milesi G, Vizzardi E, Volpini M, Nodari S, Specchia C, Maroldi R, Bezzi M, Metra M. Characteristics and outcomes of patients hospitalized for COVID‐19 and cardiac disease in Northern Italy. Eur Heart J 2020;41:1821–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang Y, Coats AJS, Zheng Z, Adamo M, Ambrosio G, Anker SD, Butler J, Xu D, Mao J, Khan MS, Bai L, Mebazaa A, Ponikowski P, Tang Q, Ruschitzka F, Seferovic P, Tschöpe C, Zhang S, Gao C, Zhou S, Senni M, Zhang J, Metra M. Management of heart failure patients with COVID‐19: a joint position paper of the Chinese Heart Failure Association & National Heart Failure Committee and the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 2020;22:941–956. [DOI] [PubMed] [Google Scholar]
- 9. Emanuel EJ, Persad G, Upshur R, Thome B, Parker M, Glickman A, Zhang C, Boyle C, Smith M, Phillips JP. Fair allocation of scarce medical resources in the time of Covid‐19. N Engl J Med 2020;382:2049–2055. [DOI] [PubMed] [Google Scholar]
- 10. Corrales‐Medina VF, Musher DM, Shachkina S, Chirinos JA. Acute pneumonia and the cardiovascular system. Lancet 2013;381:496–505. [DOI] [PubMed] [Google Scholar]
- 11. Corrales‐Medina VF, Suh KN, Rose G, Chirinos JA, Doucette S, Cameron DW, Fergusson DA. Cardiac complications in patients with community‐acquired pneumonia: a systematic review and meta‐analysis of observational studies. PLoS Med 2011;8:e1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. European Society of Cardiology . ESC guidance for the diagnosis and management of CV disease during the COVID‐19 pandemic. https://www.escardio.org/Education/COVID‐19‐and‐Cardiology/ESC‐COVID‐19‐Guidance (23 Septermber 2020).
- 13. Skulstad H, Cosyns B, Popescu BA, Galderisi M, Salvo GD, Donal E, Petersen S, Gimelli A, Haugaa KH, Muraru D, Almeida AG, Schulz‐Menger J, Dweck MR, Pontone G, Sade LE, Gerber B, Maurovich‐Horvat P, Bharucha T, Cameli M, Magne J, Westwood M, Maurer G, Edvardsen T. COVID‐19 pandemic and cardiac imaging: EACVI recommendations on precautions, indications, prioritization, and protection for patients and healthcare personnel. Eur Heart J Cardiovasc Imaging 2020;21:592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Myhre PL, Vaduganathan M, Claggett BL, Anand IS, Sweitzer NK, Fang JC, O'Meara E, Shah SJ, Desai AS, Lewis EF, Rouleau J, Pitt B, Pfeffer MA, Solomon SD. Association of natriuretic peptides with cardiovascular prognosis in heart failure with preserved ejection fraction: secondary analysis of the TOPCAT randomized clinical trial. JAMA Cardiol 2018;3:1000–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ibrahim NE, Burnett JC Jr, Butler J, Camacho A, Felker GM, Fiuzat M, O'Connor C, Solomon SD, Vaduganathan M, Zile MR, Januzzi JL Jr. Natriuretic peptides as inclusion criteria in clinical trials: a JACC heart failure position paper. JACC Heart Fail 2020;8:347–358. [DOI] [PubMed] [Google Scholar]
- 16. Guo T, Fan Y, Chen M, Wu X, Zhang L, He T, Wang H, Wan J, Wang X, Lu Z. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020;5:811–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, Shchendrygina A, Escher F, Vasa‐Nicotera M, Zeiher AM, Vehreschild M, Nagel E. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID‐19). JAMA Cardiol 2020;5:265–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rey JR, Caro‐Codón J, Pineda DP, Luis Merino J, Iniesta ÁM, Luis López‐Sendón J. Arterial thrombotic complications in hospitalized patients with COVID‐19. Rev Esp Cardiol (Engl Ed) 2020;73:769–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid‐19. N Engl J Med 2020;383:120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fox SE, Li G, Akmatbekov A, Harbert JL, Lameira FS, Brown JQ, Vander Heide RS. Unexpected features of cardiac pathology in COVID‐19 infection. Circulation 2020;142:1123–1125. [DOI] [PubMed] [Google Scholar]
- 21. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, Urso R, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors AA, Nielsen OW, Zannad F, Tavazzi L; Heart Failure Association of the European Society of Cardiology (HFA) . EURObservational Research Programme: regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail 2013;15:808–817. [DOI] [PubMed] [Google Scholar]
- 22. McGonagle D, O'Donnell J, Sharif K, Emery P, Bridgewood C. Immune mechanisms of pulmonary intravascular coagulopathy in COVID‐19 pneumonia. Lancet Rheumatol 2020;2:e437–e445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fauvel C, Weizman O, Trimaille A, Mika D, Pommier T, Pace N, Douair A, Barbin E, Fraix A, Bouchot O, Benmansour O, Godeau G, Mecheri Y, Lebourdon R, Yvorel C, Massin M, Leblon T, Chabbi C, Cugney E, Benabou L, Aubry M, Chan C, Boufoula I, Barnaud C, Bothorel L, Duceau B, Sutter W, Waldmann V, Bonnet G, Cohen A, Pezel T; Critical Covid‐19 France Investigators . Pulmonary embolism in COVID‐19 patients: a French multicentre cohort study. Eur Heart J 2020;41:3058–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, Wang TJ, Levy D, Benjamin EJ, Ho JE. Response to letter regarding article, ‘Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction’. Circulation 2016;133:e962–e963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang P, Zhu L, Cai J, Lei F, Qin JJ, Xie J, Liu YM, Zhao YC, Huang X, Lin L, Xia M, Chen MM, Cheng X, Zhang X, Guo D, Peng Y, Ji YX, Chen J, She ZG, Wang Y, Xu Q, Tan R, Wang H, Lin J, Luo P, Fu S, Cai H, Ye P, Xiao B, Mao W, Liu L, Yan Y, Liu M, Chen M, Zhang XJ, Wang X, Touyz RM, Xia J, Zhang BH, Huang X, Yuan Y, Rohit L, Liu PP, Li H. Association of inpatient use of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with mortality among patients with hypertension hospitalized with COVID‐19. Circ Res 2020;126:1671–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin–angiotensin–aldosterone system blockers and the risk of Covid‐19. N Engl J Med 2020;382:2431–2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, Katz SD, Fishman GI, Kunichoff D, Chen Y, Ogedegbe G, Hochman JS. Renin–angiotensin–aldosterone system inhibitors and risk of Covid‐19. N Engl J Med 2020;382:2441–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gao C, Cai Y, Zhang K, Zhou L, Zhang Y, Zhang X, Li Q, Li W, Yang S, Zhao X, Zhao Y, Wang H, Liu Y, Yin Z, Zhang R, Wang R, Yang M, Hui C, Wijns W, McEvoy JW, Soliman O, Onuma Y, Serruys PW, Tao L, Li F. Association of hypertension and antihypertensive treatment with COVID‐19 mortality: a retrospective observational study. Eur Heart J 2020;41:2058–2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lopes RD, Macedo AVS, de Barros E, Silva PGM, Moll‐Bernardes RJ, Feldman A, D'Andréa Saba Arruda G, de Souza AS, de Albuquerque DC, Mazza L, Santos MF, Salvador NZ, Gibson CM, Granger CB, Alexander JH, de Souza OF; BRACE CORONA Investigators . Continuing versus suspending angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) – the BRACE CORONA Trial. Am Heart J 2020;226:49–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Halliday BP, Wassall R, Lota AS, Khalique Z, Gregson J, Newsome S, Jackson R, Rahneva T, Wage R, Smith G, Venneri L, Tayal U, Auger D, Midwinter W, Whiffin N, Rajani R, Dungu JN, Pantazis A, Cook SA, Ware JS, Baksi AJ, Pennell DJ, Rosen SD, Cowie MR, Cleland JGF, Prasad SK. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED‐HF): an open‐label, pilot, randomised trial. Lancet 2019;393:61–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Kaplan‐Meier survival analysis stratified on the number of HF drugs discontinued at the time of admission
Table S1 Univariate and multivariate analysis of chronic heart failure as a predictor of mortality accounting for relevant covariates
Table S2. Univariate and multivariate analysis of acute heart failure as a predictor of mortality accounting for relevant covariates
Table S3 Univariate and multivariate analysis of the relationship between prior use of guideline‐directed medical therapy and mortality accounting for relevant covariates
Table S4. Baseline characteristics, drug therapy, vital signs, laboratory data and clinical outcomes according to the withdrawal or not of CHF medications.
Table S5. Univariate and multivariate analysis of the relationship between withdrawal of ACEi/ARB and mortality accounting for relevant covariates
Table S6. Univariate and multivariate analysis of the relationship between withdrawal of BB and mortality accounting for relevant covariates
Table S7. Univariate and multivariate analysis of the relationship between withdrawal of MRA and mortality accounting for relevant covariates
Table S8. Univariate and multivariate analysis of the relationship between the number of discontinued GDMT medications and mortality accounting for relevant covariates


