Abstract
The Envelope protein (E) is one of the four structural proteins encoded by the genome of SARS‐CoV and SARS‐CoV‐2 Coronaviruses. It is an integral membrane protein, highly expressed in the host cell, which is known to have an important role in Coronaviruses maturation, assembly and virulence. The E protein presents a PDZ‐binding motif at its C‐terminus. One of the key interactors of the E protein in the intracellular environment is the PDZ containing protein PALS1. This interaction is known to play a key role in the SARS‐CoV pathology and suspected to affect the integrity of the lung epithelia. In this paper we measured and compared the affinity of peptides mimicking the E protein from SARS‐CoV and SARS‐CoV‐2 for the PDZ domain of PALS1, through equilibrium and kinetic binding experiments. Our results support the hypothesis that the increased virulence of SARS‐CoV‐2 compared to SARS‐CoV may rely on the increased affinity of its Envelope protein for PALS1.
Keywords: binding, envelope protein, kinetics, PALS1, PDZ, SARS‐CoV‐2
The recent spread of a novel coronavirus, SARS‐CoV‐2, causing coronavirus disease 2019 (COVID‐19), represents a global health emergency of inconceivable severity, characterized by a remarkable morbidity and mortality worldwide. Coronaviruses are enveloped RNA viruses that causes respiratory, enteric, hepatic, and neurological diseases. SARS‐CoV‐2 is closely related to the original SARS‐CoV, 1 the causal agent of the severe acute respiratory syndrome outbreaks in 2002 and 2003 in China. 2 , 3
Because of the unprecedented impact of SARS‐CoV‐2 pandemic infection on world's economy and health systems, it is of critical importance to describe the main features related to its infectivity, diagnosis and prognosis. Furthermore, in an effort to establish the mechanisms of infection and propagation of SARS‐CoV‐2, it might be particularly useful to compare its behavior to that observed for SARS‐CoV.
A common pathogenic mechanism of different human viruses lies in their ability to hijack the cellular protein–protein interaction pathways. This feature is exerted by displaying short linear motifs, interacting with cellular proteins. Among these types of protein–protein recognition modules, PDZ domains represent a frequent target of viral systems, ranging from HPV, adenoviruses, rabies to influenza. 4 , 5 These viruses display PDZ binding motifs (PBM) that bind specific cellular PDZ domain proteins, thereby jeopardizing the function of these proteins.
In the case of SARS‐CoV, a critical role has been suggested for the E protein, an integral membrane protein displaying a PBM in its C‐terminus. 6 , 7 In fact, a recombinant SARS‐CoV virus lacking E results in an attenuated virulence in vivo. Moreover, a reduced virulence may also be obtained by removing exclusively the PBM of the E protein, 8 highlighting a central role of the PDZ‐dependent viral targeting of host proteins. The importance of E protein PBM was demonstrated in SARS‐CoV infected cells, the deletion of the PBM resulting in the acquisition of an alternative PBM after several cell passages. 9 Among the cellular targets of the PBM of the E protein, it appears that PALS1 plays a pivotal role. 10 PALS1 is a PDZ containing polarity protein that is critical for the maintenance of epithelial polarity in mammals. The interaction between PALS1 and the E protein has been suggested to play a key role in the SARS‐CoV pathology, by compromising the integrity of the lung epithelia and therefore dramatically increasing viral dissemination. 10 , 11
Despite the role of the PBM of the E protein has been already established, there is to date no quantitative information about its binding to PALS1. Furthermore, the differences, if any, between SARS‐CoV and SARS‐CoV‐2 have not been studied in detail. The purpose of this study is therefore to provide a comparative quantitative assessment of these interactions. By comparing the binding equilibrium and kinetics of the PBM of SARS‐CoV and SARS‐CoV‐2 on the PDZ domain of PALS1, we show here that the mutation in SARS‐CoV‐2 results in an increased affinity between the partners relative to SARS‐CoV.
In order to investigate the binding between the PDZ domain of PALS1 and the PBM of SARS‐CoV and SARS‐CoV‐2, we synthesized two peptides mimicking their respective C‐termini (Figure 1). A peptide search in Uniprot for the two sequences confirmed that they are uniquely associated to the Envelope protein of Coronaviruses. Importantly, a recent investigation on 3,617 naturally occurring mutants of Sars‐CoV‐2 have identified only two variants in SARS‐CoV‐2 falling in the PBM of the envelope protein. 12 Indeed, these mutations were suggested to mistarget the interaction to PALS1 and, accordingly, to reduce replication and/or infectivity of the virus. In analogy to our previous work on PDZ domains, 13 , 14 to engineer a Förster Resonance Energy Transfer (FRET) upon binding, a dansyl group was covalently attached to the N‐terminus of the peptides and a single Trp variant was produced for the PDZ domain of PALS1 by mutating a Phe at position 318. The equilibrium binding transitions of the SARS‐CoV and SARS‐CoV‐2, measured in buffer sodium phosphate 50 mM, pH 7.2 at 25°C are reported in Figure 2.
FIGURE 1.
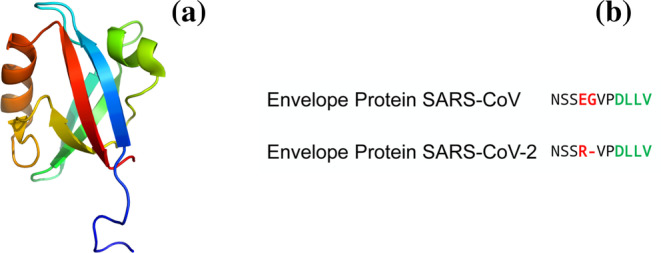
Structural features of the interaction between the E protein and PALS1. (a) Three‐dimensional structure of the PDZ domain of PALS1 (PDB: 4UU5); (b) comparison of the sequences of peptides mimicking the Envelope protein of SARS‐CoV and SARS‐CoV‐2. Residues not conserved are highlighted in red. The PDZ binding motifs are highlighted in green
FIGURE 2.
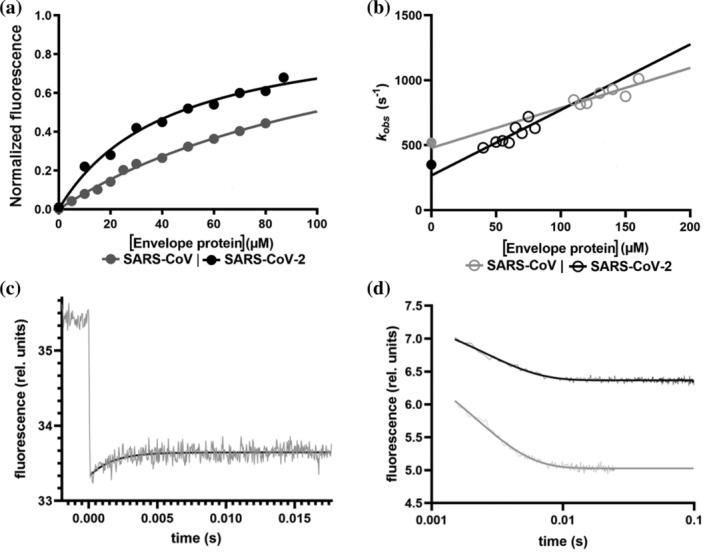
Comparing the binding of peptides mimicking the Envelope protein of SARS‐CoV and SARS‐CoV‐2 to the PDZ domain of PALS1. (a) Equilibrium binding titration monitored by FRET between PALS1 PDZ domain and dansylated peptides mimicking the Envelope protein of SARS‐CoV (in gray) and SARS‐CoV‐2 (in black). Lines are the best fit to a hyperbolic function. (b) Observed rate constants calculated from T‐jump kinetics at different concentrations of dansylated SARS‐CoV (gray empty circles) and SARS‐CoV‐2 (black empty circles) peptides. Lines represent the best fit to a linear function. Full gray and black circles represent the microscopic dissociation rate constant (k off) calculated from stopped‐flow displacement experiments (displacement traces fitted to a single exponential equation are reported in (d)—gray SARS‐CoV, black SARS‐CoV‐2). (c) Representative T‐jump experiment carried out in the presence of PALS1 PDZ and dansylated peptide mimicking the Envelope protein from SARS‐CoV‐2. The monitored reaction appears to be complete within ~4 ms. Fluorescence emission before and after the temperature jump is shown (in gray). Black line represents the best fit to a single exponential function
The calculated K d values for the two peptides are 130 ± 10 and 40 ± 10 μM, respectively, indicating that the naturally occurring mutation of SARS‐CoV‐2 increases the binding affinity for the PDZ domain of PALS1.
To further investigate the binding reactions, we performed temperature jump kinetic experiments, as the reaction is too fast to be examined by the stopped‐flow apparatus. In particular, by taking advantage of the fluorescence signal change upon binding, we carried out kinetic binding experiments by mixing a constant concentration of the PDZ domain (10 μM) with excess concentrations of peptides and subjecting each solution to a rapid increase of temperature of 9°C, from 8 to 17°C, using a fluorescence equipped capacitor‐discharge temperature‐jump instrument. A typical fluorescence transition is reported in Figure 2.
A pseudo‐first‐order plot of the observed rate constants for the SARS‐CoV and SARS‐CoV‐2 peptides is also reported in Figure 2. In agreement with the equilibrium data, it is evident that the mutation results in an increase of the binding rate constant, as mirrored by the slopes of the observed dependence, and a decrease of the dissociation rate constant, which can be estimated from the extrapolation at zero peptide concentration. To further substantiate the calculated values of the dissociation rate constants obtained from the analysis of the pseudo‐first‐order plots, we resorted to perform displacement experiments. 15 In particular, the preformed complex between the PDZ domain and each of the peptides was challenged with non‐dansylated peptides, acting as competitive PBMs. At large excess of displacing reactant, approaching quasi‐irreversibility (i.e., >10 times of the concentration of the complex), the measured dissociation rate constants were 520 ± 15 s−1 for the SARS‐CoV complex and 350 ± 10 s−1 for the SARS‐CoV‐2 complex, confirming that the natural mutation occurring in the PBM of the E protein of SARS‐CoV‐2 corresponds to both an increase in association and decreased in dissociation rate constants respectively.
Establishing the differences and similarities between SARS‐CoV and SARS‐CoV‐2 from a biochemical perspective might be of vital importance to understand SARS‐CoV‐2 and to design specific drugs to fight its pathogenesis, as exemplified by the structural studies on the protein spike. 16 , 17 Here we observe that SARS‐CoV‐2 displays an increased affinity for the PALS1, a binding event that appears to represent a critical step in compromising the lung epithelia and therefore in triggering viral infection. Indeed, whilst the interaction between PALS1 and the E protein has been already established as critical for the virulence of Sars‐CoV, no successful inhibitors have been obtained to date. Our experimental observations support the hypothesis that characteristic virulence of SARS‐CoV‐2 could rely at least in part on the strengthened interaction between the E protein and PALS1.
1. MATERIALS AND METHODS
1.1. Equilibrium binding experiments
Equilibrium binding titrations were performed on a Fluoromax single photon counting
spectrofluorometer (Jobin‐Yvon, NJ), by mixing a constant concentration of pseudo‐wild‐type PALS1 PDZ F318W (2 μM) versus increasing concentrations of dansylated peptides mimicking SARS‐CoV and SARS‐CoV‐2 Envelope proteins. Samples were excited at 280 nm and fluorescence emission spectra were collected between 300 and 400 nm. Experiments were performed in buffer sodium phosphate 50 mM pH 7.2 at 25°C.
1.2. T‐jump kinetic binding experiments
Relaxation binding experiments were performed using a Hi‐Tech PTJ‐64 capacitor‐discharge T‐jump apparatus (Hi‐Tech, Salisbury, UK). A constant concentration of pseudo‐wild‐type PALS1 PDZ F318W (10 μM) was mixed with dansylated peptides mimicking SARS‐CoV and SARS‐CoV‐2 Envelope proteins at different concentrations. Temperature was rapidly increased with a jump of ~9°C, from 8 to 17°C. Samples were excited at 296 nm and fluorescence was collected using a 320 nm cut‐off filter. Buffer used was sodium phosphate 50 mM pH 7.2 in the presence of NaCl 150 mM. Presence of NaCl in the buffer was required to ensure conductivity and temperature jump upon rapid discharge of the capacitor.
1.3. Stopped‐flow displacement experiments
Displacement experiments were performed using a single‐mixing SX‐18 stopped‐flow instrument (Applied Photophysics, Leatherhead, Surrey, UK). A preincubated complex of pseudo‐wild‐type PALS1 PDZ F318W (10 μM) and dansylated peptides mimicking Envelope proteins (100 μM for SARS‐CoV and 50 μM for SARS‐CoV‐2) were rapidly mixed with a high excess of non‐dansylated peptide. The excitation wavelength was 280 nm, and emission was collected using a 475‐nm cut‐off glass filter. For each acquisition, the average of at least five independent experiments was fitted to a single exponential equation.
AUTHOR CONTRIBUTIONS
Angelo Toto: Conceptualization; investigation; writing‐original draft; writing‐review and editing. Sana Ma: Investigation; methodology; writing‐review and editing. Francesca Malagrino: Investigation; methodology; writing‐review and editing. Lorenzo Visconti: Investigation; methodology; writing‐review and editing. Livia Pagano: Investigation; methodology; writing‐review and editing. Kristian Stromgaard: Conceptualization; funding acquisition; investigation; writing‐review and editing. Stefano Gianni: Conceptualization; funding acquisition; investigation; methodology; writing‐original draft; writing‐review and editing.
ACKNOWLEDGEMENTS
Work partly supported by grants from the Italian Ministero dell'Istruzione dell'Università e della Ricerca (Progetto di Interesse “Invecchiamento” to S.G.), Sapienza University of Rome (RP11715C34AEAC9B and RM1181641C2C24B9, RM11916B414C897E to S.G.), the Associazione Italiana per la Ricerca sul Cancro (Individual Grant ‐ MFAG 2016, 18701 to S.G.) and the Istituto Pasteur Italia (Teresa Ariaudo Research Project 2018, to A.T.). F.M. was supported by a fellowship from the FIRC ‐ Associazione Italiana per la Ricerca sul Cancro (Filomena Todini fellowship).
Toto A, Ma S, Malagrinò F, et al. Comparing the binding properties of peptides mimicking the Envelope protein of SARS‐CoV and SARS‐CoV‐2 to the PDZ domain of the tight junction‐associated PALS1 protein. Protein Science. 2020;29:2038–2042. 10.1002/pro.3936
Funding information the Istituto Pasteur Italia; Associazione Italiana per la Ricerca sul Cancro, Grant/Award Number: MFAG 2016, 18701; Sapienza University of Rome, Grant/Award Numbers: RM11916B414C897E, RM1181641C2C24B9, RP11715C34AEAC9B; Italian Ministero dell'Istruzione dell'Università e della Ricerca
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhong NS, Zheng BJ, Li YM, et al. Epidemiology and cause of severe acute respiratory syndrome (SARS) in Guangdong, People's Republic of China, in February, 2003. Lancet. 2003;362:1353–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N Engl J Med. 2003;348:1953–1966. [DOI] [PubMed] [Google Scholar]
- 4. Gutiérrez‐González LH, Santos‐Mendoza T. Viral targeting of PDZ polarity proteins in the immune system as a potential evasion mechanism. FASEB J. 2019;33:10607–10617. [DOI] [PubMed] [Google Scholar]
- 5. Barreda D, Sánchez‐Galindo M, López‐Flores J, et al. PDZ proteins are expressed and regulated in antigen‐presenting cells and are targets of influenza A virus. J Leukoc Biol. 2018;103:731–738. [DOI] [PubMed] [Google Scholar]
- 6. Thiel V, Ivanov KA, Putics Á, et al. Mechanisms and enzymes involved in SARS coronavirus genome expression. J Gen Virol. 2003;84:2305–2315. [DOI] [PubMed] [Google Scholar]
- 7. Torres J, Parthasarathy K, Lin X, Saravanan R, Kukol A, Liu DX. Model of a putative pore: The pentameric alpha‐helical bundle of SARS coronavirus E protein in lipid bilayers. Biophys J. 2006;91:938–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Castaño‐Rodriguez C, Honrubia JM, Gutiérrez‐Álvarez J, et al. Role of severe acute respiratory syndrome coronavirus viroporins E, 3a, and 8a in replication and pathogenesis. MBio. 2018;9:e02325–e02317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jimenez‐Guardeño JM, Regla‐Nava JA, Nieto‐Torres JL, et al. Identification of the mechanisms causing reversion to virulence in an attenuated SARS‐CoV for the design of a genetically stable vaccine. PLoS Pathog. 2015;11:e1005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Teoh K‐T, Siu Y‐L, Chan W‐L, et al. The SARS coronavirus E protein interacts with PALS1 and alters tight junction formation and epithelial morphogenesis. Mol Biol Cell. 2010;21:3838–3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. DeDiego ML, Nieto‐Torres JL, Jimenez‐Guardeño JM, et al. Coronavirus virulence genes with main focus on SARS‐CoV envelope gene. Virus Res. 2014;194:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hassan SS, Choudhury PP, Roy B. SARS‐CoV2 envelope protein: Non‐synonymous mutations and its consequences. Genomics. 2020;112:3890–3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Toto A, Pedersen SW, Karlsson OA, et al. Ligand binding to the PDZ domains of postsynaptic density protein 95. Protein Eng Des Sel. 2016;29:169–175. [DOI] [PubMed] [Google Scholar]
- 14. Toto A, Mattei A, Jemth P, Gianni S. Understanding the role of phosphorylation in the binding mechanism of a PDZ domain. Protein Eng Des Sel. 2017;30:1–5. [DOI] [PubMed] [Google Scholar]
- 15. Antonini E, Brunori M. Hemoglobin and myoglobin in their reactions with ligands. Amsterdam: North‐Holland, 1971. [Google Scholar]
- 16. Lan J, Ge J, Yu J, et al. Structure of the SARS‐CoV‐2 spike receptor‐binding domain bound to the ACE2 receptor. Nature. 2020;581:215–220. [DOI] [PubMed] [Google Scholar]
- 17. Zhai X, Sun J, Yan Z, et al. Comparison of severe acute respiratory syndrome coronavirus 2 spike protein binding to ACE2 receptors from human, pets, farm animals, and putative intermediate hosts. J Virol. 2020;94:e00831–e00820. [DOI] [PMC free article] [PubMed] [Google Scholar]


