Abstract
Sepsis is defined as life‐threatening organ dysfunction caused by a deregulated immune host response to infection. The emergence of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has highlighted this multifactorial and complex syndrome. The absence of specific treatment neither against SARS‐CoV‐2 nor against acute respiratory distress syndrome (ARDS), the most serious stage of this infection, has emphasized the need to find alternative treatments. Several therapeutics are currently being tested, including mesenchymal stromal cells. These cells, already used in preclinical models of ARDS, sepsis, and septic shock and also in a few clinical trials, appear well‐tolerated and promising, but many questions remain unanswered.
Keywords: acute respiratory distress syndrome, COVID‐19, mesenchymal stromal/stem cells, sepsis, Wharton's Jelly
Significance statement.
Sepsis is defined as life‐threatening organ dysfunction caused by a deregulated immune host response to infection. The emergence of the severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has highlighted this multifactorial and complex syndrome. The absence of specific treatment neither against SARS‐CoV‐2 nor against acute respiratory distress syndrome (ARDS), the most serious stage of this infection, has emphasized the need to find alternative treatments. Several therapeutics are currently being tested, including, mesenchymal stromal cells. These cells, already used in preclinical models of ARDS, sepsis, and septic shock and also in a few clinical trials, appear well‐tolerated and promising.
1. MESENCHYMAL STEM/STROMAL CELLS
Mesenchymal stem/stromal cells (MSCs) were first described in the 1970s by Friedenstein's team in bone marrow (BM). 1 Such cells with a fibroblastic morphology were subsequently observed in many tissues like adipose tissue (AT), dental pulp, menstrual blood, or extra‐embryonic source such as Wharton's Jelly (WJ) or placenta, 2 these latter sources currently represent nearly 30% of clinical MSC products. 3 In 2006, the International Society for Cellular Therapy (ISCT) defined MSCs as (a) adherent to the cell culture plastic; (b) able to differentiate into osteocytes, chondrocytes, and adipocytes; and (c) with a phenotype that is positive for the CD73 CD90 CD105 mesenchymal markers and negative for the CD34 CD45 HLA‐DR hematopoietic markers. 4 According to ISCT, if the expression “Mesenchymal stem cells” is mainly indicating a stem cell population with self‐renewal and differentiation potential, the term “mesenchymal stromal cells” is used to design bulk unfractionated populations with secretory, immunomodulatory, and homing properties. 2 , 5 As these latter properties are of great interest, capitalized in nearly 25% of current clinical trials, “MSCs” will from now on refer to MSCs. 6 Mesenchymal stem/stromal cells can modulate both innate and adaptive immunity by cell contacts, secretion of cytokines, chemokines, growth factors, and release of extracellular vesicles (EV). 7 , 8 Regarding adaptive immunity, they can decrease the cytotoxicity of T lymphocytes, impair their proliferation but also their activation by inhibiting dendritic cell maturation and inducing regulatory T cells. In an inflammatory context, they inhibit the proliferation of B lymphocytes and their production of immunoglobulins. Regarding innate immunity, they notably decrease the production of reactive oxygen species by neutrophils and promote the M2 anti‐inflammatory phenotype of macrophages. 9
MSCs influence the phenotype of immune cells and their cytokine secretions. However, this immunomodulation seems to depend on the inflammatory context. In 2013, Bernardo and Fibbe described the existence of two MSC phenotypes depending on the inflammatory context and the stimulation of their Toll Like Receptor (TLR). 10 MSCs can have immunosuppressive or immunostimulatory properties. In a cell anergy context, they could exhibit a pro‐inflammatory phenotype, MSC1, making it possible to reduce apoptosis and promote T‐cell survival. 11 On the other hand, in case of inflammation, they could adopt an immunosuppressive and anti‐inflammatory phenotype, called MSC2. This change in phenotype depending on the inflammatory environment suggests new opportunities for therapeutic uses.
Surprisingly, these immunomodulatory cells whose behavior is modulated by the inflammatory context, appear little impacted by immune cells. As they lack MHC class II antigens and CD80/CD86 costimulatory molecules, they do not activate T cells but they induce T‐cell anergy. As they weakly express MHC class I antigens, they are rarely targeted by NK cells. Thus, they can be used in an allogeneic setting without considering HLA compatibility thanks to their low immunogenicity. However, an instant blood mediated inflammatory reaction generated by systemically infused MSCs has been recently described and results from the triggering of the host innate immune cascade systems, such as complement and coagulation. The intensity of this reaction is dependent from the sources of MSCs and related to the expression of procoagulant tissue factor (TF). 3 Therefore, MSC therapy can be considered in the context of vital emergency pathologies such as sepsis or septic shock, provided special attention is paid to controlling the risk of their potential procoagulant activity. 12
2. MSCs AND SEPSIS INCLUDING THE CONTEXT OF COVID‐19
Since the emergence of the SARS‐CoV‐2, sepsis was put in the spotlight. Sepsis, a leading cause of admission to intensive care units, is a major socioeconomic burden all over the world. It is defined as life‐threatening organ dysfunction caused by a dysregulated immune host response to infection. 13 , 14 Although sepsis induces a high mortality rate, there is currently no specific treatment for this complex syndrome, associating cytokine storm, excessive inflammation, but also immunosuppression and lymphocytopenia. 15 , 16 , 17 , 18 Modulating inflammation while limiting the concomitant immunosuppression would certainly be the main therapeutic issue. Thus, MSCs, which pro‐ or anti‐inflammatory behavior is induced by the inflammatory environment, seem promising candidates to treat sepsis (Figure 1). Currently, several preclinical studies have reported the protective action of MSCs in sepsis murine models and in its ultimate stage, septic shock. 19 MSCs improve survival, decrease organ failure, increase bacterial clearance, modulate cytokine production, and improve renal, pulmonary, liver, cardiac, and muscular functions. 20 , 21 , 22 , 23 These benefic actions appear to be closely related to the strong immunomodulatory properties of MSCs. By reducing ambient inflammation and favoring anti‐inflammatory cytokines and mediators, they limit organ damages. 24 , 25 , 26 , 27 These findings were found in small but also in large animal sepsis models. 28 , 29 However, the design of the studies being very variable (severity of sepsis, time of treatment, MSC tissue source, dosage, etc.), some results may be contradictory. Although our work has shown that MSCs are effective in murine and swine septic shock models, improving survival, organ failure, and bacterial load, we did not observe any MSC impact on inflammatory secreted factors, unlike other studies. 28 , 30 Similarly, in a porcine sepsis model, Horak et al did not find any impact of BM‐MSCs on inflammation. 31 However, they did not observe either any beneficial effect of MSCs on organ failures, unlike us. Despite the report of contradictory results, a recent meta‐analysis of 29 animal studies, including 1266 animals, demonstrated that MSC therapy was related to a significant lower mortality rate. 32 Moreover, cell tracing studies performed in rodents exhibited that MSCS distribute to a variety of tissues after IV infusion. They were found in the lung early after administration, then in the liver and the spleen. 33 However, the rationale for extrapolating data from small animals to humans remains weak. In addition to the classic questions about the correlation of animal models and human physiology, it should not be forgotten that the xenogenic context can strongly impact the results of the tested therapy, especially in the context of cell therapy. Animal‐derived MSCs are not a solution either: the product evaluated in animals would not match with the drug used in humans. Finally, only clinical studies will make it possible to assess the promising effect of MSCs during sepsis. 34 , 35
FIGURE 1.
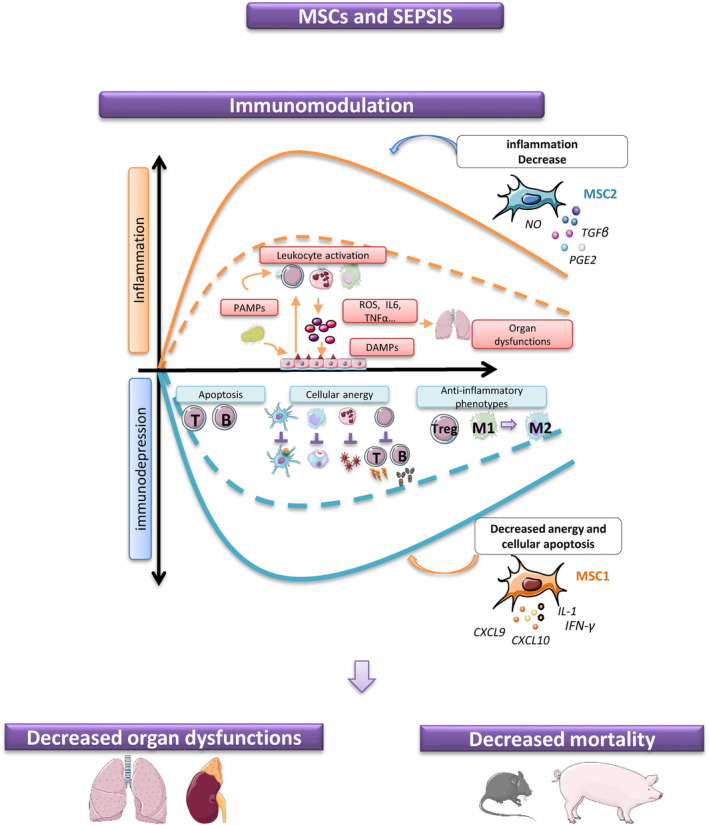
Sepsis is a dysfunction of the immune system with the concomitant presence of pro‐inflammatory and anti‐inflammatory states. A postinfection immunosuppressive state is evidenced by the development of anergy: increase in lymphocyte apoptosis, alteration of immune cell functions with less antigen presentation by dendritic cells, and promotion of reactive oxygen species (ROS) neutrophil production or phagocytic activities and anti‐inflammatory phenotypes. To be effective, therapeutic management should be able to adapt to this inflammatory context and at the same time, improve organ failures and survival. Mesenchymal stromal cell (MSC) capacities to switch to MSC1 or MSC2 phenotypes can be interesting in sepsis indication
Less than 12 clinical studies related to MSC infusion for sepsis and its main complications, acute respiratory distress syndrome (ARDS) and septic shock, have already been published (Table 1). These trials, mainly phase I, report good tolerance and the absence of major adverse effects related to the infusion of MSCs. McIntyre et al reported a pilot dose‐escalation study including nine patients with septic shock. Regardless of MSC dose (from 1 to 3 × 106 MSCs/kg), no serious adverse effects have been observed. 38 A phase I clinical trial published in 2014 has demonstrated that infusion of allogeneic AT‐MSCs as a treatment of ARDS was safe and well tolerated. 36 Similarly, Wilson et al demonstrated in a dose‐escalation study that infusion of allogeneic BM‐MSCs did not generate any adverse event. 37 Yip et al also reported in nine patients that a single dose of UC‐MSCs in ARDS was well tolerated regardless the tested doses (1, 5, and 10 × 106 MSCs/kg). 43 Recently, Chen et al observed that a multiple intravenous infusion (3 or 4) of 1 × 106 menstrual blood‐derived MSCs/kg was well tolerated in patients with moderate to severe H7N9 induced ARDS. 44 The overall safety of UC‐MSCs has also been reported in a systematic review of 93 clinical trials published up to 2017 in different indications: 72% of the trials mentioned safety and good tolerance to UC‐MSCs infusion, regardless the route, whereas 28% occasionally reported mild adverse events like headache, fever, dizziness, and local pain, all of them resolutive in few days. 33
TABLE 1.
Published clinical trials of mesenchymal stromal cell (MSC) infusion in the sepsis context
| References | Indication | Phase | Patient number | MSC source | Dose |
|---|---|---|---|---|---|
| Zheng 2014 36 | ARDS | Phase I |
6 patients in control group 6 patients in experimental group |
Allogeneic AT‐MSCs | 1 × 106 cells/kg |
| Wilson 2015 37 | ARDS | Phase I | 9 patients | Allogeneic BM‐MSCs | Dose‐escalation 1 × 106, 5 × 106, or 10 × 106 cells/kg |
| McIntyre 2017 38 | Septic shock | Phase I |
9 patients in MSC group 21 patients in an observational cohort |
Allogeneic BM‐MSCs | Dose‐escalation 0.5 × 106, 1 × 106, or 3 × 106 cells/kg |
| Gennadiy 2018 39 | Septic shock with severe neutropenia | Phase I/II |
15 patients in control group 15 patients in experimental group |
Allogeneic BM‐MSCs | 1 × 106 cells/kg |
| He 2018 40 | Severe sepsis | Phase I |
15 patients in historical case‐matched comparison group 15 patients in experimental group |
Allogeneic UC‐MSCs | Dose‐escalation 1 × 106, 2 × 106, or 3 × 106 cells/kg |
| Perlee 2018 41 | Healthy subjects received LPS intravenously (2 ng/kg) 1 hour after the end of MSC infusion | Phase I | 32 healthy subjects | Allogeneic AT‐MSCs | Dose‐escalation 0.25 × 106, 1 × 106, or 4 × 106 cells/kg |
| Matthay 2019 42 | ARDS | Phase IIa |
20 patients in control group 40 patients in experimental group |
Allogeneic BM‐MSCs | 10 × 106 cells/kg |
| Yip 2020 43 | ARDS | Phase I | 9 patients | Allogeneic UC‐MSCs | Dose‐escalation 1 × 106, 5 × 106, or 10 × 106 cells/kg |
| Chen 2020 44 | H7N9‐induced ARDS | Phase I |
44 patients in control group 17 patients in experimental group |
Allogeneic menstrual blood‐derived MSCs | 3 or 4 intravenous infusion of MSC 1 × 106 cells/kg |
| Liang 2020 45 | SARS‐CoV‐2 induced ARDS |
Case report Critical ill context |
1 patient | Allogeneic UC‐MSCs | 3 doses of 50.106 MSC |
| Leng 2020 46 | SARS‐CoV‐2 induced ARDS | Phase I |
3 patients in observational group 7 patients in experimental group |
Allogeneic UC‐MSCs | 1 × 106 cells/kg |
Abbreviations: ARDS, acute respiratory distress syndrome; AT‐MSCs, adipose‐derived MSCs; BM‐MSCs, bone marrow‐derived MSCs; UC‐MSCs, umbilical cord‐derived MSCs.
Finally, two recent studies reported encouraging results in phase II clinical trials. The first one, the START study (phase IIa) including patients with ARDS, reported after MSC infusion a significant improvement of Angiopoietin 2, a biomarker of endothelial failure. However, no improvement in survival was observed. 42 The authors attributed the absence of significant clinical effects to the high cell‐viability variability according to the cell batches (35%‐86%). They specified that a larger study would be necessary to conclude on a clinical benefit of MSCs. The second one, the RUMCESS study, reported hemodynamic stabilization, vasopressor withdrawal, attenuation of respiratory failure, and shortening of the neutropenia period in 15 neutropenic patients with septic shock treated with BM‐MSCs compared to a control group. 39
The occurrence of the SARS‐CoV‐2 infection, whose severe form is sepsis, may bring soon many answers on the clinical effect of MSCs. Until now, only a few teams and clinical trials were interested in the use of these cells during sepsis. However, the pandemic, in the absence of any specific treatment, has led to intense research for innovative therapeutics and to many changes regarding MSCs. In a few weeks, up to 30 clinical trials have been declared on ClinicalTrial.gov. Currently, two Chinese publications have reported encouraging results regarding UC‐MSC infusions in the indication of COVID‐19. The first one reported a compassionate use of UC‐MSCs in a 65‐year‐old patient requiring mechanical ventilation and with multiple organ failures. Three successive doses of 50.106 MSCs were administered 3 days apart. One day after the second infusion, an improvement in vital signs was noted and mechanical ventilation could be withdrawn. Two days after the third infusion, all the biological parameters returned to normal values and the patient could leave the intensive care unit. No side effects have been observed. 45
The second study described a 14‐day comparison among seven patients treated by the infusion of a single dose of 1.106 MSC/kg and a control group of three patients, all of them presenting a mild to critically severe SARS‐CoV‐2 infection. No adverse events were noted in the treated group. Normalizations of oxygen saturation levels, inflammation markers, and tissue damage were observed, with a pulmonary improvement observed on CT. 46
Although no adverse event was reported in those two studies, we have to keep in mind that critically ill COVID‐19 patients are in a systemic procoagulant state at high risk for disseminated intravascular coagulation, thromboembolism, and thrombotic multi‐organ failure. Regarding the previously mentioned procoagulant potential of MSCs, it may be of interest to include TF expression in MSC characterization, to prefer the intramuscular route to the intravenous one, to supplement cells with buffer containing HSA and low dose of anticoagulants and finally to prepare the patient with an anticoagulation protocol. 47
3. WHICH MSCs, WHEN, AND HOW?
The increase in clinical trials will certainly provide some answers but the variability in the procedures may be detrimental to interpretation. MSC tissue sources, route and number of infusions, time of administration, dosage, but also the use of the cells or their EV vary between trials.
Currently, MSC doses range from 0.4 × 106 to 42 × 106 MSC/kg in 1 to 5 infusions every 2 days in clinical trials listed in the indication for COVID‐19. 34 Kabat et al reported in a meta‐analysis of various clinical trials—the intravenous route being the most frequent—a median IV dose of 100 × 106 MSC/patient/dose and a minimum effective dose of 70 to 90 × 106 MSC/patient/dose. 48 The minimum dose effective per patient and trial has never been above 190 × 106 MSCs, whereas efficacy has never been reported below 70 × 106 MSCs. Effect depending on the dose has also been reported in an ARDS context by Wilson et al who observed better results with the highest dosage. 37 Although it is not yet known if a same dose administrated in a single or multiple infusions has similar efficacy, some studies promote the repetition of administrations arguing that MSCs generate a transient clinical effect. 39 , 43
Time of infusion is also a concern. Many preclinical studies report MSC administration at an early stage of sepsis or even prophylactic context. However, this is not feasible in a clinical context. In addition, reluctance to infuse them at early disease stages is often observed.
MSC tissue source is also a concern. Although most clinical trials listed in the indication of sepsis and ARDS now report the preferential use of UC‐MSCs and especially from WJ, heterogeneity of practices remains. In our experience, WJ is the best source of MSCs in the indication of sepsis. At least as effective as BM‐MSCs, WJ‐MSCs have a major advantage: their accessibility. 30 Sepsis potentially concerns a large population of patients and will require large‐scale production capacity to provide enough doses of MSCs, assuming the efficiency of MSCs is demonstrated. Thus, the use of WJ as a source of MSCs seems obvious. The accessibility and abundance of this tissue source but also the ease of production due to their great proliferative capacities make it the privileged tissue source. 49 However, the problem of large‐scale production remains. Research on animals requiring a small amount of cells and the high cost of reagents have led to the development of low‐scale production processes, often incompatible with industrialization. MSCs are mainly generated in 2D cultures: the cells migrate and adhere to the plastic of the culture flasks and proliferate until confluence. Thus, production capacities depend on the size of these flasks and the capacity of the cell therapy units to manage the cluttering. Currently, industrialization of 2D models begins with the marketing of bioreactors. Bioreactor increases cell culture surface and limits steric hindrance. Another large‐scale production strategy has also arisen last recent years: the 3D model, consisting in using microspheres, suspended in culture medium, on which cells adhere and expand. The number of microspheres can be potentially very important and the quantity of harvested cells also. However, this technique may change MSC characteristics and cells have to be tested and compared to 2D expanded MSCs to avoid any lack of efficiency.
The use of MSC extra‐vesicles instead of cells themselves is also an unsolved question. In sepsis indication, several preclinical and clinical studies demonstrated the protective effect of MSC exosomes. 50 , 51 , 52 Exosomes are small EV approximately 100 nm wide and are formed by the endosomal system of the cell. They harbor molecules including proteins, RNA, metabolites, or lipid membrane components. Secreted by MSCs and obtained by purification of the medium, exosomes exhibit anti‐inflammatory and immunomodulatory capacities. 53 However, although the production of standardized homogeneous batches would be an improvement compared to whole MSCs, the main advantage of the whole cells would be lost. The strength of MSCs relies on their main drawback: to be a “living drug”: they are able to change their phenotype (MSC1 or MSC2) according to their environment. The use of EV instead of cells induces the loss of adaptability capacities. As sepsis remains complex and multifactorial, with concomitant inflammation and lymphopenia as seen in SARS‐CoV‐2 infection, is it really possible to do without this main benefit?
Currently, intensivists do not have available predictive sepsis biomarkers. If this were the case, priming of MSCs to turn them toward an MSC1 or MSC2 phenotype would become of interest, allowing a more personalized and standardized treatment.
4. CONCLUSION
In conclusion, MSCs appear to be promising therapeutics in the indication of sepsis. Clinical trials in the COVID‐19 will provide a lot of answers about the use of MSCs but may also be fatal to their use, in the absence of proof of efficacy. It will then be necessary to remember that COVID‐19 is not the main etiology of sepsis, at the risk of putting aside their very promising use in bacterial sepsis due especially to their important antibacterial capacities.
CONFLICT OF INTEREST
The authors declared no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
C.L.: conception and design, collection and/or assembly of data (bibliography and clinical trials), and manuscript writing; S.G.: collection and/or assembly of data (bibliography and clinical trials) and final approval of manuscript; C.H.: conception and design, collection and/or assembly of data (bibliography and clinical trials); D.B.: conception and design, supervision of collection and/or assembly of data (bibliography and clinical trials), manuscript editing and writing, and final approval of manuscript.
Laroye C, Gibot S, Huselstein C, Bensoussan D. Mesenchymal stromal cells for sepsis and septic shock: Lessons for treatment of COVID‐19. STEM CELLS Transl Med. 2020;9:1488–1494. 10.1002/sctm.20-0239
REFERENCES
- 1. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea‐pig bone marrow and spleen cells. Cell Prolif. 1970;3:393‐403. [DOI] [PubMed] [Google Scholar]
- 2. Viswanathan S, Shi Y, Galipeau J, et al. Mesenchymal stem versus stromal cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell committee position statement on nomenclature. Cytotherapy. 2019;21:1019‐1024. [DOI] [PubMed] [Google Scholar]
- 3. Moll G, Ankrum JA, Kamhieh‐Milz J, et al. Intravascular mesenchymal stromal/stem cell therapy product diversification: time for new clinical guidelines. Trends Mol Med. 2019;25:149‐163. [DOI] [PubMed] [Google Scholar]
- 4. Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315‐317. [DOI] [PubMed] [Google Scholar]
- 5. Gao F, Chiu SM, Motan DAL, et al. Mesenchymal stem cells and immunomodulation: current status and future prospects. Cell Death Dis. 2016;7:e2062‐e2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Olsen TR, Ng KS, Lock LT, Ahsan T, Rowley JA. Peak MSC—are we there yet? Front Med. 2018;5 :178–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383‐396. [DOI] [PubMed] [Google Scholar]
- 8. Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol. 2008;8:726‐736. [DOI] [PubMed] [Google Scholar]
- 9. Guerrouahen BS, Sidahmed H, Al Sulaiti A, et al. Enhancing mesenchymal stromal cell immunomodulation for treating conditions influenced by the immune system. Stem Cells Int. 2019;2019:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bernardo ME, Fibbe WE. Mesenchymal stromal cells: sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392‐402. [DOI] [PubMed] [Google Scholar]
- 11. Le Burel S, Thepenier C, Boutin L, et al. Effect of mesenchymal stromal cells on T cells in a septic context: immunosuppression or immunostimulation? Stem Cells Dev. 2017;26:1477‐1489. [DOI] [PubMed] [Google Scholar]
- 12. Caplan H, Olson SD, Kumar A, et al. Mesenchymal stromal cell therapeutic delivery: translational challenges to clinical application. Front Immunol. 2019;10 :1645–1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315:801‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shankar‐Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third international consensus definitions for sepsis and septic shock (Sepsis‐3). JAMA. 2016;315:775‐787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Iskander KN, Osuchowski MF, Stearns‐Kurosawa DJ, et al. Sepsis: multiple abnormalities, heterogeneous responses, and evolving understanding. Physiol Rev. 2013;93:1247‐1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Primer. 2016;2:16045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hotchkiss RS, Monneret G, Payen D. Sepsis‐induced immunosuppression: from cellular dysfunctions to immunotherapy. Nat Rev Immunol. 2013;13:862‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wujtewicz M, Dylczyk‐Sommer A, Aszkiełowicz A, Zdanowski S, Piwowarczyk S, Owczuk R. COVID‐19 – what should anaethesiologists and intensivists know about it? Anaesthesiol Intensive Ther. 2020;52:34‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laroye C, Gibot S, Reppel L, Bensoussan D. Mesenchymal stromal/stem cells: a new treatment for sepsis and septic shock?: MSCs in the treatment for sepsis and septic shock. Stem Cells. 2017;35:2331‐2339. [DOI] [PubMed] [Google Scholar]
- 20. Zhu Y, Xu L, Collins JJP, et al. Human umbilical cord mesenchymal stromal cells improve survival and bacterial clearance in neonatal sepsis in rats. Stem Cells Dev. 2017;26:1054‐1064. [DOI] [PubMed] [Google Scholar]
- 21. Zhao X, Liu D, Gong W, et al. The toll‐like receptor 3 ligand, poly(I:C), improves immunosuppressive function and therapeutic effect of mesenchymal stem cells on sepsis via inhibiting MiR‐143: poly(I:C) improves MSCs immune function. Stem Cells. 2014;32:521‐533. [DOI] [PubMed] [Google Scholar]
- 22. Alcayaga‐Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, Khoury M. Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther. 2015;6:199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mei SHJ, Haitsma JJ, Dos Santos CC, et al. Mesenchymal stem cells reduce inflammation while enhancing bacterial clearance and improving survival in sepsis. Am J Respir Crit Care Med. 2010;182:1047‐1057. [DOI] [PubMed] [Google Scholar]
- 24. Németh K, Leelahavanichkul A, Yuen PST, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin‐10 production. Nat Med. 2009;15:42‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Condor JM, Rodrigues CE, Sousa Moreira RD, et al. Treatment with human Whartons Jelly‐derived mesenchymal stem cells attenuates sepsis‐induced kidney injury, liver injury, and endothelial dysfunction. Stem Cells Translational Medicine. 2016;5:1048‐1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rocheteau P, Chatre L, Briand D, et al. Sepsis induces long‐term metabolic and mitochondrial muscle stem cell dysfunction amenable by mesenchymal stem cell therapy. Nat Commun. 2015;6:10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tan L, Huang Y, Pan X, et al. Administration of bone marrow stromal cells in sepsis attenuates sepsis‐related coagulopathy. Ann Med. 2016;48:235‐245. [DOI] [PubMed] [Google Scholar]
- 28. Laroye C, Jérémie L, Amir B, et al. Clinical‐grade mesenchymal stem cells derived from umbilical cord improve septic shock in pigs. Intensive Care Med Exp. 2018;6:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rojas M, Xu J, Woods CR, et al. Bone marrow‐derived mesenchymal stem cells in repair of the injured lung. Am J Respir Cell Mol Biol. 2005;33:145‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Laroye C, Boufenzer A, Jolly L, et al. Bone marrow vs Wharton's Jelly mesenchymal stem cells in experimental sepsis: a comparative study. Stem Cell Res Ther. 2019;10:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Horak J, Nalos L, Martinkova V, et al. Evaluation of mesenchymal stem cell therapy for sepsis: a randomized controlled porcine study. Front Immunol. 2020;11:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun X‐Y, Ding X‐F, Liang H‐Y, et al. Efficacy of mesenchymal stem cell therapy for sepsis: a meta‐analysis of preclinical studies. Stem Cell Res Ther. 2020;11:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Can A, Celikkan FT, Cinar O. Umbilical cord mesenchymal stromal cell transplantations: a systemic analysis of clinical trials. Cytotherapy. 2017;19:1351‐1382. [DOI] [PubMed] [Google Scholar]
- 34. Khoury M, Cuenca J, Cruz FF, Figueroa FE, Rocco PRM, Weiss DJ. Current status of cell‐based therapies for respiratory virus infections: applicability to COVID‐19. Eur Respir J. 2020;55:2000858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Keane C, Jerkic M, Laffey JG. Stem cell‐based therapies for sepsis. Anesthesiology. 2017;127:1017‐1034. [DOI] [PubMed] [Google Scholar]
- 36. Zheng G, Huang L, Tong H, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose‐derived mesenchymal stem cells: a randomized, placebo‐controlled pilot study. Respir Res. 2014;15:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wilson JG, Liu KD, Zhuo H, et al. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. McIntyre LA, Stewart DJ, Mei SHJ, et al. Cellular immunotherapy for septic shock (CISS): a phase I clinical trial. Am J Respir Crit Care Med. 2017;197:337‐347. [DOI] [PubMed] [Google Scholar]
- 39. Gennadiy G, Polina M, Elena P, et al. The results of the single center pilot randomized Russian clinical trial of mesenchymal stromal cells in severe neutropenic patients with septic shock (RUMCESS). Int J Blood Res Disord. 2018;5 :33–41. [Google Scholar]
- 40. He X, Ai S, Guo W, et al. Umbilical cord‐derived mesenchymal stem (stromal) cells for treatment of severe sepsis: a phase 1 clinical trial. Transl Res. 2018;199:52‐61. [DOI] [PubMed] [Google Scholar]
- 41. Perlee D, van Vught LA, Scicluna BP, et al. Intravenous infusion of human adipose mesenchymal stem cells modifies the host response to lipopolysaccharide in humans: a randomized, single‐blind, parallel group, placebo controlled trial: mesenchymal stem cells in human endotoxemia. Stem Cells. 2018;36:1778‐1788. [DOI] [PubMed] [Google Scholar]
- 42. Matthay MA, Calfee CS, Zhuo H, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154‐162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yip H‐K, Fang W‐F, Li Y‐C, et al. Human umbilical cord‐derived mesenchymal stem cells for acute respiratory distress syndrome. Crit Care Med. 2020;48:e391‐e399. [DOI] [PubMed] [Google Scholar]
- 44. Chen J, Hu C, Chen L, et al. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza a (H7N9) infection: a hint for COVID‐19 treatment. Engineering. 2020;S2095809920300370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liang B, Chen J, Li T, et al. Clinical remission of a critically ill COVID‐19 patient treated by human umbilical cord mesenchymal stem cells. ChinaXiv. 2020;13–chinaXiv:202002.00084v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leng Z, Zhu R, Hou W, et al. Transplantation of ACE2‐ mesenchymal stem cells improves the outcome of patients with COVID‐19 pneumonia. Aging Dis. 2020;11:216‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moll G, Drzeniek N, Kamhieh‐Milz J, Geissler S, Volk HD, Reinke P. MSC therapies for COVID‐19: importance of patient coagulopathy, thromboprophylaxis, cell product quality and mode of delivery for treatment safety and efficacy. Front Immunol. 2020;11 :1091–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kabat M, Bobkov I, Kumar S, Grumet M. Trends in mesenchymal stem cell clinical trials 2004‐2018: is efficacy optimal in a narrow dose range? Stem Cells Translational Medicine. 2020;9:17‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Laroye C, Gauthier M, Antonot H, Decot V, Reppel L, Bensoussan D. Mesenchymal stem/stromal cell production compliant with good manufacturing practice: comparison between bone marrow, the gold standard adult source, and Wharton's Jelly, an Extraembryonic source. J Clin Med. 2019;8:2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhu Y, Feng X, Abbott J, et al. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin‐induced acute lung injury in mice: MSC MV attenuates ALI in part through KGF. Stem Cells. 2014;32:116‐125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Monsel A, Zhu Y, Gennai S, et al. Therapeutic effects of human mesenchymal stem cell–derived microvesicles in severe pneumonia in mice. Am J Respir Crit Care Med. 2015;192:324‐336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sengupta V, Sengupta S, Lazo A, et al. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID‐19. Stem Cells Dev. 2020;29:747‐754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Elahi FM, Farwell DG, Nolta JA, Anderson JD. Preclinical translation of exosomes derived from mesenchymal stem/stromal cells. Stem Cells. 2020;38:15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]


