Abstract
Purpose
To evaluate ultrasound signs of coronavirus disease‐19 (COVID‐19) pneumonia in symptomatic healthcare professionals and to correlate those changes with clinical findings.
Methods
All patients underwent real‐time polymerase chain reaction (RT‐PCR), lung ultrasound (LUS) and clinical evaluation on the same day. In each of the 12 areas evaluated in the LUS, the LUS signs were scored to generate the aeration score.
Results
A total of 409 participants had positive PCR, with a median age of 41 (35‐51) years. All participants had clinical symptoms, with cough in 84.1%, fever in 69.7%, and dyspnea in 36.2% of cases. In the LUS, 72.6% of participants had B‐lines >2, 36.2% had coalescent B‐lines, and 8.06% had subpleural consolidations. The median aeration score was 3 (2‐7). The aeration score differed significantly regarding the presence of cough (P = .002), fever (P = .001), and dyspnea (P < .0001). The finding of subpleural consolidations in the LUS showed significant differences between participants with or without dyspnea (P < .0001).
Conclusions
In healthcare professionals with COVID‐19, LUS plays a key role in the characterization of lung involvement. Although B‐lines are the most common ultrasound sign, subpleural consolidations are those that most impact the respiratory condition.
Keywords: COVID‐19, dyspnea, lung ultrasound, novel coronavirus, pneumonia
1. INTRODUCTION
In December 2019, the first cases of pneumonia of unknown origin at the time were described in the city of Wuhan, the capital of Hubei province, China. 1 One day later, the RNA of the virus of the order Nidovirales, from the family Coronaviridae and subfamily Betacoronavirus, was identified as being responsible for the cases. 2 As the disease spread across all continents, the disease was declared a pandemic by the World Health Organization on March 11, 2020 and was named coronavirus disease‐19 (COVID‐19). It is caused by a new coronavirus known as severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). 3 Currently, the strategy for the diagnosis of COVID‐19 pneumonia is based on the combination of a history of exposure, clinical characteristics and real‐time polymerase chain reaction (RT‐PCR) of samples obtained from oropharyngeal or nasopharyngeal swabs, bronchoalveolar lavage or tracheal aspirate, followed by imaging tests. 4
Imaging methods such as chest X‐ray, lung ultrasound (LUS), and computed tomography (CT) have a role in the diagnosis, prognosis, monitoring, and treatment of COVID‐19 pneumonia. However, the benefits of these different methods need to be balanced with the available resources, the level of technical knowledge of the personnel, the unique logistic configurations, the risk of infection of the involved operators, and the decontamination of the equipment used. 5 , 6 LUS has considerably evolved in recent years in terms of its theoretical and operational aspects, and as a consequence, its clinical application has become increasingly widespread. One of the main characteristics of LUS is the ability to define changes that affect the tissue‐air ratio on the lung surface. 7 LUS, although not used in the diagnosis of COVID‐19, can be used by physicians at the point of care or in intensive care units, where a rapid assessment of the pulmonary condition may be necessary. 8 In fact, this method seems promising as an imaging method for comprehensive and first‐line use in suspected or diagnosed COVID‐19 pneumonia. LUS as a diagnostic method has some advantages for the imaging of COVID‐19 pneumonia because it is a mobile, fast, noninvasive, and portable technology that can be used in various environments, including in screening tents or improvised hospitals. 5
Given that LUS can identify changes in the lung tissue that correlate with CT and histopathology findings, the role of LUS may be relevant in the context of the COVID‐19 pandemic. 7 LUS uses artifacts and findings in the lung periphery, and initial reports show that abnormal ultrasound signs are common in patients with COVID‐19. Characteristically abnormal signs on LUS in individuals being screened for COVID‐19 may identify those that require additional assessment or closer observation even before obtaining the RT‐PCR results. In addition to screening COVID‐19 cases for possible lung involvement, LUS plays an important role in the management of SARS‐CoV‐2 cases, allowing rapid diagnosis of COVID‐19 pneumonia and its possible evolution to SARS. 8 , 9 In this exam, the spectrum of the imaging manifestations of COVID‐19 pneumonia on admission includes thickening and irregularity of the pleural line, a variety of B‐line patterns and subpleural consolidations, and pleural effusion is rare. 10 , 11 Thus, the present study aims to evaluate the ultrasound signs of COVID‐19 pneumonia in symptomatic healthcare professionals in the prehospital phase and, secondarily, to correlate these changes with clinical findings.
2. MATERIALS AND METHODS
This was a cross‐sectional observational study that evaluated 1604 symptomatic healthcare professionals seen in screening tents installed in the courtyard of Piquet Carneiro Policlinic, State University of Rio de Janeiro, Rio de Janeiro, Brazil, between March 27 and April 9, 2020. These healthcare professionals were evaluated hierarchically by nurses and then by physicians. Prior to LUS, each participant underwent an RT‐PCR test for COVID‐19 and was asked about the presence of some signs and symptoms, including cough, fever, and dyspnea. The inclusion criteria were symptomatic patients ≥18 years old of both sexes. Individuals with a negative RT‐PCR test for COVID‐19 in nasopharyngeal swabs (n = 882) and those with LUS with normal signs (n = 313) were excluded. LUS was performed only in individuals who had at least one respiratory symptom, persistent fever in the last 3 days and/or pulse oximetry below 95% (Figure 1).
FIGURE 1.
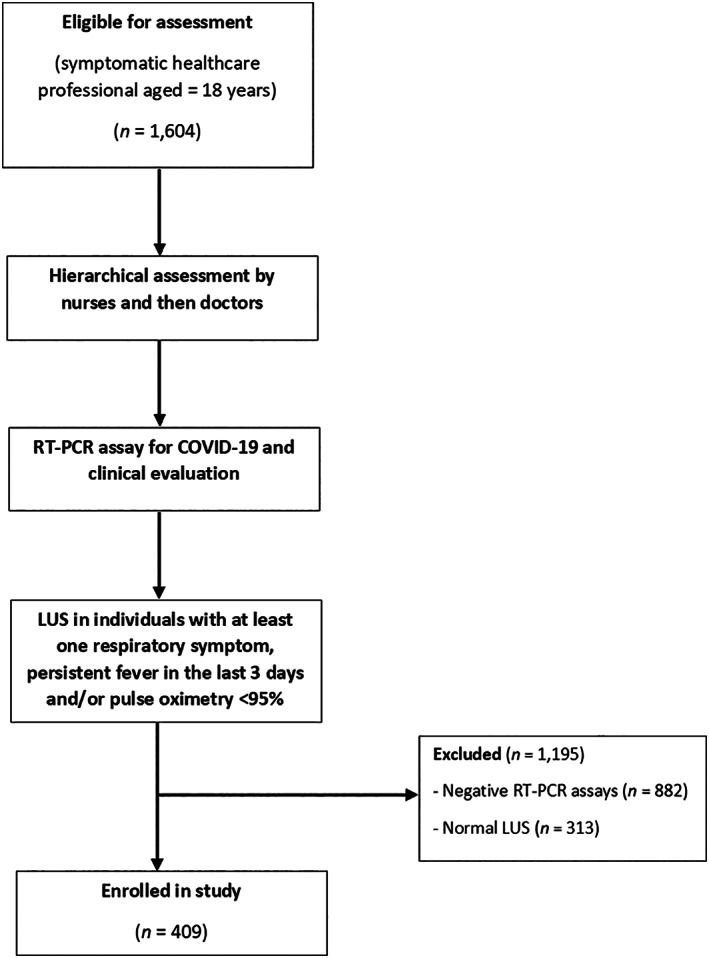
Flowchart for evaluating study participants
LUS exams were performed by a team of six clinicians with experience in the method, and each exam was performed by two doctors, obtaining consensus among them in cases of divergence. The exams were performed using an Aplio XG ultrasound machine (Toshiba Medical Systems, Tokyo, Japan), with a 7.5 to 10 MHz multifrequency linear transducer or a 3.5 to 5 MHz convex transducer in B mode. The ultrasound exams were performed in six areas of each hemithorax (two anterior, two lateral and two posterior) while the patients were seated. 6 The transducer was covered with a probe cover, and the ultrasound device was cleaned with disinfectant wipes after each use. LUS images were examined for the following signs: B‐lines >2, coalescent B‐lines, consolidations, pleural effusion and pleural thickening. To classify the lung disease in the LUS, the tests obtained from the two anterior, two lateral and two posterior areas were evaluated. In each area, a score ranging from 1 to 3 was assigned to each LUS finding (1 = B‐lines >2; 2 = coalescent B‐lines; 3 = consolidations), and the sum of all areas represented the aeration score. 12
The study was approved by the National Research Ethics Commission under number CAAE‐30135320.0.0000.5259. All of the participants signed an informed consent form.
2.1. Statistical analysis
The normal distribution of the data was assessed using the Shapiro‐Wilk test and graphical analysis of histograms; because a significant number of variables had no normal distribution, nonparametric tests were selected. Median and interquartile range or frequency (percentage) values were used to express the results. The association between the clinical variables (dichotomous data) and the number of affected lung areas or the aeration score was evaluated by the Mann‐Whitney test, while the association between the clinical variables and the number of affected lung areas or the consolidation finding were evaluated by chi‐square or Fisher's exact test. The association between age and aeration score was analyzed using Spearman's correlation coefficient. The significance level was set at 5%. Statistical analysis was carried out using SPSS Statistics 26.0 (IBM, New York).
3. RESULTS
The sample comprised 409 symptomatic healthcare professionals with positive RT‐PCR tests for COVID‐19 and abnormal LUS findings. Of these, 134 (32.8%) patients were male, and 275 (67.2%) were female. The median age was 41 (35‐51) years, and 34 of them (8.31%) were aged ≥60 years. All 409 participants had clinical symptoms on admission, with cough in 344 (84.1%), fever in 285 (69.7%), and dyspnea in 148 (36.2%) of the cases.
Regarding the LUS signs, 297 (72.6%) participants had B‐lines >2, 148 (36.2%) had coalescent B‐lines, and 33 (8.06%) had subpleural consolidations, as shown in Table 1 and Figure 2. Of all participants, 204 (49.9%) had unilateral lesions, while 205 (50.1%) had bilateral lesions. The median aeration score was 3 (2‐7), with minimum and maximum values of 1 and 33, respectively.
TABLE 1.
Distribution of the number of affected areas, consolidation, and other findings of lung ultrasound in the sample evaluated
| Lung ultrasound findings | All (n = 409) |
|---|---|
| Number of areas with B‐lines >2 | |
| Absent | 112 (27.4%) |
| One area | 141 (34.5%) |
| Two areas | 69 (16.9%) |
| ≥Three areas | 87 (21.3%) |
| Number of areas with coalescent B‐lines | |
| Absent | 261 (63.8%) |
| One area | 78 (19.1%) |
| ≥Two areas | 70 (17.1%) |
| Consolidations | |
| Absent | 376 (91.9%) |
| Present | 33 (8.07%) |
| Aeration score | 3 (2–7) |
Note: Data are expressed as medians and interquartile ranges for continuous data and number (percentage) for categorical data.
FIGURE 2.
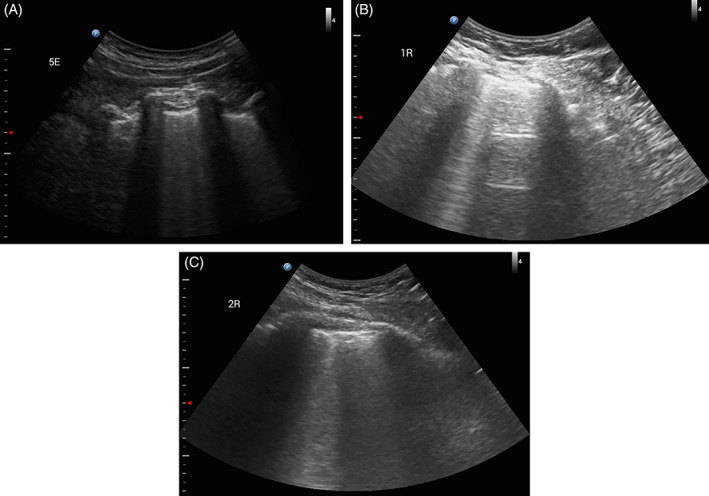
Lung ultrasound images showing B‐lines, A and B, and subpleural consolidations associated with B‐lines, C
We compared the demographic and clinical data according to the LUS aeration score. In this analysis, the sex and age of the participant (<60 years or ≥60 years) did not influence the LUS aeration score. However, there were significant differences in the aeration score regarding the presence of cough, fever, and dyspnea, as shown in Table 2 and Figure 3. Furthermore, according to Spearman's coefficient, there was no significant correlation between age and the LUS aeration score (r = .072; P = .14) in the total sample evaluated.
TABLE 2.
Aeration score on lung ultrasound according to demographic and clinical data in the total sample
| Aeration score | P value | |
|---|---|---|
| Genre | ||
| Men | 4 (2‐8) | .079 |
| Women | 3 (2‐6) | |
| Age ≥60 y | ||
| Yes | 4 (1.80‐11.3) | .46 |
| No | 3 (2‐6) | |
| Cough | ||
| Present | 3 (2‐7) | .002 |
| Absent | 2 (1‐4) | |
| Fever | ||
| Present | 4 (2‐7.50) | .001 |
| Absent | 2 (1‐5) | |
| Dyspnea | ||
| Present | 8 (4‐12) | <.0001 |
| Absent | 2 (1‐4) | |
Note: Data are expressed as medians and interquartile ranges. Bold type indicates significant P values.
FIGURE 3.
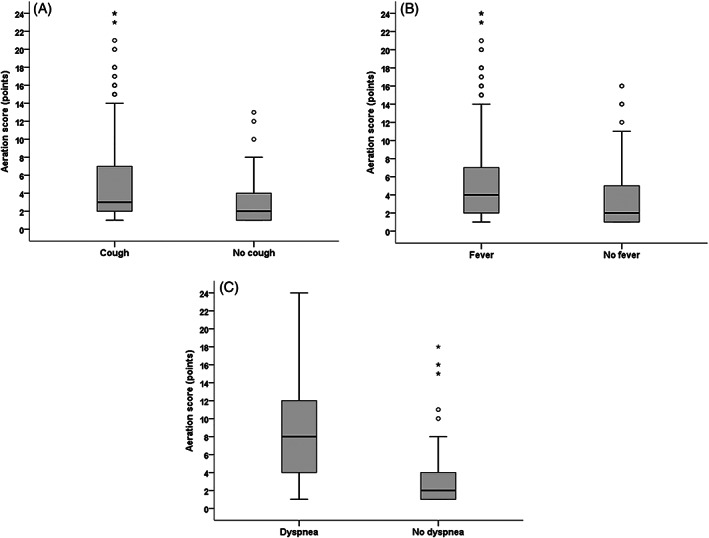
Cough, fever, and dyspnea according to the aeration score
Regarding the LUS signs, there were no significant differences for B‐lines >2 and coalescent B‐lines when these variables were compared to demographic and clinical data. However, the finding of subpleural consolidations showed significant differences between participants with or without dyspnea, as shown in Table 3 and Figure 4.
TABLE 3.
Consolidations on Lung Ultrasound According to Demographic and Clinical Data in the Total Sample
| Consolidations | No consolidations | P value | |
|---|---|---|---|
| Genre | |||
| Men | 10 (30.3%) | 124 (33%) | .75 |
| Women | 23 (69.7%) | 252 (67%) | |
| Age ≥60 y | |||
| Yes | 5 (15.2%) | 29 (7.09%) | .12 |
| No | 28 (84.8%) | 347 (92.3%) | |
| Cough | |||
| Present | 28 (84.8%) | 316 (84%) | .90 |
| Absent | 5 (15.2%) | 60 (16%) | |
| Fever | |||
| Present | 25 (75.8%) | 260 (69.1%) | .43 |
| Absent | 8 (24.2%) | 116 (30.9%) | |
| Dyspnea | |||
| Present | 27 (81.8%) | 121 (32.2%) | <.0001 |
| Absent | 6 (18.2%) | 255 (67.8%) | |
Note: Data are expressed as number (percentage). Bold type indicates significant P values.
FIGURE 4.
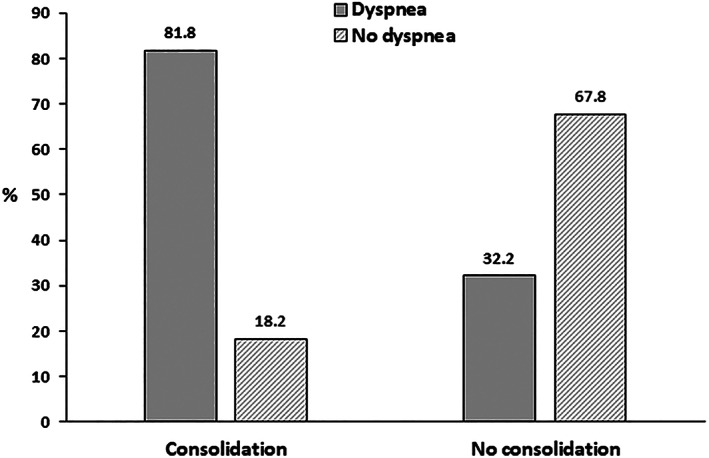
Lung ultrasound consolidations according to the presence or absence of dyspnea
4. DISCUSSION
The high rate of lung involvement caused by SARS‐CoV‐2 infection requires a rapid diagnostic tool that complements the diagnostic test by RT‐PCR and is also useful in evaluating the progression of lung lesions. Since most of these lesions are peripheral, LUS has been increasingly used in the early diagnosis of lung abnormalities without the need to use CT. 13 Thus, it is crucial to define patterns of LUS signs for COVID‐19 pneumonia to promptly diagnose viral infection in acute stages and to conduct a more accurate screening. The main findings of the present study were that in patients with COVID‐19 pneumonia, the most common abnormal ultrasound sign was B‐lines >2, while 8% of patients had subpleural consolidations, with bilateral lesions being seen in half of the cases. There was a relationship between the aeration score and the presence of cough, fever, and dyspnea. In addition, we also found that the finding of subpleural consolidations was the one that most impacted the pulmonary condition, especially in relation to the presence of dyspnea. To our knowledge, this is the first study that evaluated the association between LUS signs and clinical findings in symptomatic healthcare professionals with COVID‐19 pneumonia during the first phase of the disease.
LUS has several advantages, including the lack of radiation exposure, the possibility of multiple repetitions, the reduction of exposure of healthcare professionals to SARS‐CoV‐2 and the mitigation of the shortage of personal protective equipment. 6 Furthermore, LUS also shows prognostic abilities in acute respiratory distress syndrome (ARDS) before hypoxemia becomes evident. 5 In fact, in the present study, LUS allowed us to perform a large number of tests in tents set up in our institution after the beginning of community transmission in Brazil, with no report of infection of any member of the team conducting the testing. It is noteworthy that the median age of the population in our study was slightly above that reported for young adults and close to the average described by Li et al, 14 who evaluated only 83 patients with COVID‐19 pneumonia in the Chinese population.
LUS is highly sensitive in the detection of multiple lung pathologies, which can also be demonstrated in COVID‐19 pneumonia. However, to date, there are no pathognomonic findings related to COVID‐19 in LUS. 8 The histopathological aspect of initial COVID‐19 pneumonia is characterized by alveolar damage, which includes alveolar edema, whereas the inflammatory component is mild and irregular. 15 In the present study, we observed that half of the patients had unilateral lung damage, unlike a systematic review of thoracic CT scans in patients with COVID‐19 who observed bilateral lung involvement in 87% of cases and multilobar involvement in 78.8% of cases. 16 Although there is a high correlation between LUS and chest CT, 6 one possible explanation for these differences is that we saw patients in their first assessment, and those hospitalized or undergoing intensive care were not included.
The most common abnormal ultrasound finding in our study were B‐lines >2, which was observed in almost two‐thirds of our sample. In line with our findings, Lomoro et al 10 also observed a high frequency of this finding in their sample of 22 patients with COVID‐19 pneumonia. The presence of multiple B‐lines per ultrasound field generally corresponds to the ground‐glass opacity (GGO) pattern in CT and, in turn, to a high‐grade interstitial syndrome. 6 However, this type of abnormality may also indicate underlying parenchymal consolidation that does not come in direct contact with visceral pleura, alveolar edema, or pneumonia with predominant interstitial involvement. 13 It is important to note that despite the high frequency of B‐lines in our study, both B‐lines >2 and coalescent B‐lines were not individually associated with the demographic or clinical data.
The more advanced stages of COVID‐19 pneumonia show gravitational consolidations similar to those of ARDS, with alveolar congestion, edema, hemorrhagic necrosis, desquamation, and fibrosis. 15 In the current study, consolidations were observed in less than 10% of the LUS exams, being present at a frequency lower than that observed in the study by Lomoro et al, 10 who observed subpleural consolidations in 27.3% of their patients in LUS. It is important to note that ultrasound has a sensitivity and specificity of 89% and 94%, respectively, for the identification of parenchymal consolidation, but these lesions must be in contact with the pleura to enable visualization. 17 The presence of consolidation in LUS imaging may represent air bronchogram, fluid bronchogram, consolidated pneumonia or vascular lesions, 13 and is associated with disease progression based on previous studies on CT findings in patients with COVID‐19. 6
Because most patients with COVID‐19 develop GGO lesions with peripheral distribution that progress over time to form more consolidated changes, LUS can likely detect most symptomatic patients with COVID‐19 requiring hospitalization. 6 In this context, we observed a strong association between the presence of consolidations in LUS and the symptom of dyspnea. Soldati et al 7 showed that the progression of consolidations, especially in the gravitational position, with or without air bronchograms, and its increasing extension along the lung surface may indicate progression toward the respiratory failure phase that requires invasive ventilatory support. In that sense, we believe that future studies using LUS to follow‐up the consolidation images diagnosed during the first assessment of patients with COVID‐19 may be of interest.
In the presence of typical clinical symptoms and previous exposure to other individuals with SARS‐CoV‐2, a combination of chest imaging elements and RT‐PCR positivity may facilitate the diagnosis of COVID‐19 pneumonia. In the present study, the aeration score was associated with the clinical manifestations evaluated (including cough, fever, and dyspnea), which shows that the extent of the disease in LUS can assist in the evaluation of the prognosis of COVID‐19 pneumonia, even during the first phase of the disease. It is unclear whether there are LUS sign thresholds that can predict significant clinical deterioration in patients with good general appearance who present with lung findings. However, observations that the LUS findings may precede the clinical findings suggest that LUS can identify more severe cases even before the emergence of important symptoms, such as progressive dyspnea. 5
The strength of this study is that it includes a large number of healthcare professionals with COVID‐19 pneumonia and associates the LUS signs with clinical findings. However, similar to any other study, ours also has limitations. First, we did not evaluate the follow‐up of these patients; in fact, we included in our analysis only the imaging test performed at the initial assessment at our institution, without evaluating the imaging changes in the disease together with its temporal phases. Second, we did not evaluate the correlation of the LUS signs with CT findings because a significant portion of our sample did not undergo a CT examination. Third, our sample consisted of healthcare professionals and, therefore, is not representative of the general population of patients with COVID‐19. Future studies are needed to determine the role of LUS as a screening tool to better elucidate the associations between LUS findings and the presence of pneumonia in CT, the prognostic value of the different lesions diagnosed, and its use in monitoring patients with COVID‐19 after hospital discharge. We suggest that future research should be performed to verify the sensitivity and specificity of LUS and to evaluate the use of this method in patients with more advanced stages of SARS‐CoV‐2 infection.
5. CONCLUSION
This study showed that in healthcare professionals with COVID‐19 pneumonia, LUS plays a key role in the characterization of the disease. In these patients, B‐lines >2 was the most common abnormal ultrasound sign, followed by coalescent B‐lines and, less frequently, subpleural consolidations. There was an association between the aeration score and the presence of clinical symptoms. In addition, the ultrasound sign of subpleural consolidations is the only sign that was strongly associated with the presence of dyspnea. Since LUS has well‐established advantages in terms of portability, safety, and repeatability, its use should be further explored during the first phase of COVID‐19 to recognize lung involvement as early as possible in this emerging critical infectious disease.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
ACKNOWLEDGMENTS
The authors thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq: 302215/2019‐0) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ: #E‐26/202.679/2018 and #E‐26/010.002124/2019).
Mafort TT, Lopes AJ, da Costa CH, et al. Changes in lung ultrasound of symptomatic healthcare professionals with COVID‐19 pneumonia and their association with clinical findings. J Clin Ultrasound. 2020;48:515–521. 10.1002/jcu.22905
Funding information Conselho Nacional de Desenvolvimento Científico e Tecnológico, Grant/Award Number: 302215/2019‐0; Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro, Grant/Award Numbers: #E‐26/010.002124/2019, #E‐26/202.679/2018
REFERENCES
- 1. Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708‐1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in coronavirus disease 2019 patients: a systematic review and meta‐analysis. Int J Infect Dis. 2020;94:91‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rothan HA, Byrareddy SN. The epidemiology and pathogenesis of coronavirus disease (COVID‐19) outbreak. J Autoimmun. 2020;109:102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID‐19): a perspective from China. Radiology. 2020;296:E15‐E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liu RB, Tayal VS, Panebianco NL, et al. Ultrasound on the frontlines of COVID‐19: report from an international webinar. Acad Emerg Med. 2020;27:523‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yasukawa K, Minami T. Point‐of‐care lung ultrasound findings in patients with novel coronavirus disease (COVID‐19) pneumonia. Am J Trop Med Hyg. 2020;102:1198‐1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lung ultrasound during the COVID‐19 pandemic? J Ultrasound Med. 2020;39:1459‐1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moore S, Gardiner E. Point of care and intensive care lung ultrasound: a reference guide for practitioners during COVID‐19. Radiography. 2020. 10.1016/j.radi.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Poggiali E, Dacrema A, Bastoni D, et al. Can lung US help critical care clinicians in the early diagnosis of novel coronavirus (COVID‐19) pneumonia? Radiology. 2020;295:E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lomoro P, Verde F, Zerboni F, et al. COVID‐19 pneumonia manifestations at the admission on chest ultrasound, radiographs, and CT: single‐center study and comprehensive radiologic literature review. Eur J Radiol Open. 2020;7:100231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tierney DM, Huelster JS, Overgaard JD, et al. Comparative performance of pulmonary ultrasound, chest radiograph, and CT among patients with acute respiratory failure. Crit Care Med. 2020;48:151‐157. [DOI] [PubMed] [Google Scholar]
- 12. Volpicelli G, Lamorte A, Villén T. What's new in lung ultrasound during the COVID‐19 pandemic. Intensive Care Med. 2020;46:1445‐1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pérez Pallarés J, Flandes Aldeyturriaga J, Cases Viedma E, et al. SEPAR‐AEER consensus recommendations on the usefulness of the thoracic ultrasound in the management of the patient with suspected or confirmed infection with COVID‐19. Arch Bronconeumol. 2020;56 Suppl 2:27‐30. [Google Scholar]
- 14. Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID‐19 pneumonia. Invest Radiol. 2020;55:327‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salehi S, Abedi A, Balakrishnan S, Gholamrezhad A. Coronavirus disease 2019 (COVID‐19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020;215:87‐93. [DOI] [PubMed] [Google Scholar]
- 17. Wimalasena Y, Kocierz L, Strong D, Watterson J, Burns B. Lung ultrasound: a useful tool in the assessment of the dyspnoeic patient in the emergency department: fact or fiction? Br Med J. 2016;35:1e9. [DOI] [PubMed] [Google Scholar]


