Abstract
As the battle against coronavirus disease 2019 pandemic continues, an increase in workload and medical expenses have been a concern to the health care system worldwide. Developing a measure that helps to conserve the health care resource is, therefore, highly desirable, and the pooling of the specimens for testing is one of the attractive strategies. Recently, we showed that saliva could be a potential alternative specimen for the detection of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) by real‐time polymerase chain reaction (RT‐PCR). In the present study, we performed the pooling of saliva specimens for testing by SARS‐CoV‐2 RT‐PCR. We showed that the saliva pool of either 5 or 10 samples, by allowing the detection of either gene in the pool at an increased cycle threshold cutoff value, further performing individual sample testing in the positive pools did not compromise the detection of SARS‐CoV‐2.
Keywords: COVID‐19, pooling, RT‐PCR, saliva, SARS‐CoV‐2
Highlights
‐ Saliva pooling does not compromise the detection of SARS‐CoV‐2 among symptomatic patients under investigation.
‐ Saliva pooling provides advantages in the ease of specimen collection and resource conservation.
‐ Saliva pooling may be useful for mass‐screening program or sentinel surveillance in resource‐limited settings.
1. INTRODUCTION
The detection of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) in clinical specimens is one of the crucial steps of coronavirus disease 2019 (COVID‐19) diagnosis and control. Real‐time polymerase chain reaction (RT‐PCR) is an important and widely used diagnostic tool for the detection of SARS‐CoV‐2. For the detection of SARS‐CoV‐2, the preferred specimen from the upper respiratory tract includes nasopharyngeal, mid‐turbinate, or nasal swab. 1 However, the drawback of the test is the nature of the relatively invasive procedure, the need for trained personnel, swab, and personal protective equipment (PPE).
Williams et al, 2 as well as our group 3 recently showed that saliva might be an alternative specimen for the diagnosis of COVID‐19 in ambulatory patient settings. The advantages of saliva as a clinical specimen include the ease and noninvasive nature of the specimen collection, and the reduced use of the swabs and PPE. However, during the outbreak or pandemic, availability of resources, including reagents and laboratory capacity, might be in short of supply. 4
Sample pooling for RT‐PCR has been used to detect various infections, including HIV, and hepatitis B and C viruses. 5 , 6 Specimen pooling for the detection of SARS‐CoV‐2 using nasopharyngeal swab specimens was demonstrated to reduce the resources and laboratory workload in low prevalence areas. 7 However, sample pooling might decrease the sensitivity of viral detection due to specimen dilution and the influence of storage time and conditions. In this study, we investigated the potential of pooling saliva specimens for the detection of SARS‐CoV‐2.
2. MATERIALS AND METHODS
2.1. Specimens and pooling
The storage RNA specimens extracted from saliva samples of patients under investigation for COVID‐19 during the outbreak in Bangkok, Thailand, in the previous study 3 were retrieved. The extracted RNA volume of 10 μL from each patient was pooled consecutively into pools of five samples and pools of 10 samples. The 20 μL of RNA from each pool was used to perform RT‐PCR. RT‐PCR of the pools was carried out using a SARS‐CoV‐2 Nucleic Acid Diagnostic Kit (Sansure, Changsha, China), which targets the ORF1ab and N gene fragments as previously described. The kit also targets RNase P gene as a control. The pools that detected either of ORF1ab and N gene fragments before 45 cycles were retested for the detection of SARS‐CoV‐2 individually. The study protocol was reviewed and approved by the Human Research Ethics Committee, Faculty of Medicine Ramathibodi Hospital, Mahidol University.
2.2. Statistical analysis
Wilcoxon signed‐rank test was used to compare the cycle threshold (Ct) value in the pool samples and individual samples. The negative RT‐PCR of the target gene was set at the Ct value of 45.01 for the statistical analysis. A one‐tailed P < .05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism version 8 (GraphPad Software, San Diego, CA).
3. RESULTS
Two hundred RNA specimens extracted from saliva samples were pooled consecutively into the pools of five samples and the pools of 10 samples given a total of 40 pools of five samples and 20 pools of 10 samples.
3.1. The pool of five samples
Of the 40 pools of five samples, there were 27 negative pools. Eleven pools detected of both ORF1ab and N genes, and two pools detected only N gene (Table 1). RT‐PCR of the individual RNA extracted from saliva samples in each positive pool of either one gene or both of the genes was performed. Ten pools contained one positive sample, two pools contained two positive samples, and one pool contained three positive samples (Figure 1). In the pools that contained one positive specimen, the median Ct value of the ORF1ab gene in the pools of five samples was higher that of the individual specimens; the median (interquartile range; IQR) Ct values of the ORF1ab gene in the pools of five samples and the individual specimens were 37.6 (34.8‐40.7) and 35.1 (30.9‐36.5), respectively (P = .007). However, the median Ct value of the N gene in the pools of five was not significantly different from that of the individual specimens; the median Ct values of the N gene in the pools of five samples and the individual specimens were 34.9 (32.4‐35.2) and 36.7 (32.4‐37.5), respectively (P = .138). Ct values of both ORF1ab and N genes of the pools that contained two or three positive specimens were lower than those of the individual specimens (Table S1).
Table 1.
Summary of the SARS‐CoV‐2 gene detection by specimen pooling
| Total | Positive 2 genes | Positive 1 gene | Negative | |
|---|---|---|---|---|
| Pools of 5 samples | 40 | 11 | 2 | 27 |
| Positive 1 in 5 | 10 | 8 | 2 | 0 |
| Positive 2 in 5 | 2 | 2 | 0 | 0 |
| Positive 3 in 5 | 1 | 1 | 0 | 0 |
| Pools of 10 samples | 20 | 12 | 1 | 7 |
| Positive 1 in 10 | 10 | 9 | 1 | 0 |
| Positive 2 in 10 | 2 | 2 | 0 | 0 |
| Positive 3 in 10 | 1 | 1 | 0 | 0 |
Abbreviation: SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
Figure 1.
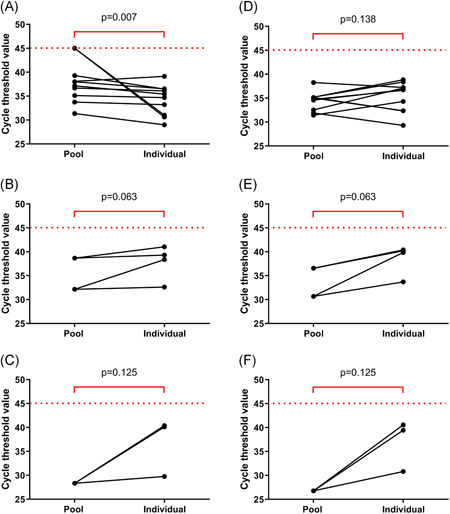
Cycle threshold values of ORF1ab (A‐C) and N genes (D‐F) in the SARS‐CoV‐2 detectable pools of five and individual samples. A and D, B and E, and C and F show the pools that contained one, two, and three positive samples, respectively. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.2. The pool of 10 samples
Of the 20 pools of 10 samples, there were seven negative pools. Twelve pools detected both ORF1ab and N genes, and one pool detected only the ORF1ab gene (Table 1). Of the total, 10 pools contained one positive sample, two pools contained two positive samples, and one pool contained three positive samples. The comparison of the Ct values between the positive pools and the individual specimens was performed (Figure 2). In the pools that contained one positive specimen, the median Ct values of the ORF1ab gene in the pools of 10 was 36.7 (33.7‐39.1), which was significantly higher than that of the individual specimens (P = .001). The median Ct value of the N gene in the pools of 10 was 34.7 (32.1‐38.9), which not significantly different from that of the individual specimens (P = .461). Ct values of both ORF1ab and N genes of the pools that contained two or three positive specimens were lower than those of the individual specimens (Table S1).
Figure 2.
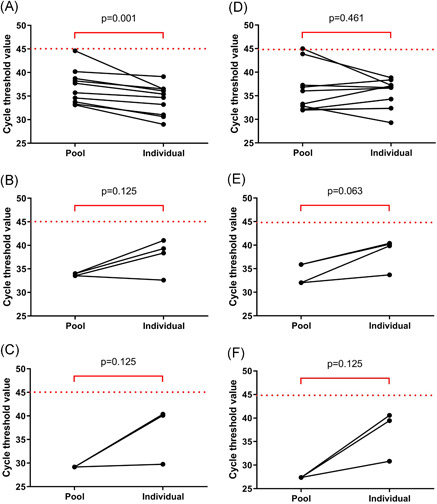
Cycle threshold values of ORF1ab (A‐C) and N genes (D‐F) in the SARS‐CoV‐2 detectable pools of 10 and individual samples. A and D, B and E, and C and F show the pools that contained one, two, and three positive samples, respectively. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
3.3. Effect of storage condition
All Ct values of ORF1ab and N genes in this study were compared with the Ct of immediate RT‐PCR testing for SARS‐CoV‐2 performed in the previous study. 3 A significant increase in the Ct values of the stored samples was observed (Figure 3). The median Ct value of ORF1ab gene in the immediate testing and the stored samples were 32.7 (28.4‐35.2) and 36.3 (32.2‐39.5), respectively (P < .001), and the median Ct values of N gene in the immediate testing and the stored samples were 31.8 (28.3‐33.9) and 37.2 (33.4‐40.0), respectively (P < .001). With the storage condition at −20°C for ∼2 months, one sample that was positive when immediate RT‐PCR testing was performed was classified as negative in the individual testing. The original Ct values of ORF1ab and N genes of the missed classified specimen were 36.16 and 33.70, respectively.
Figure 3.
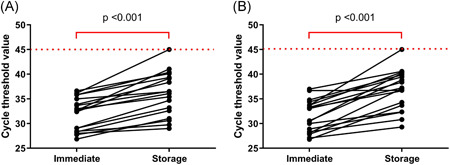
Cycle threshold values of ORF1ab (A) and N genes (B) in the SARS‐CoV‐2 positive samples comparing immediate testing and testing after the storage. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
4. DISCUSSION
This study demonstrated the feasibility of saliva pooling as a method for the detection of SARS‐CoV‐2 in symptomatic patients under investigation in a low prevalence setting. We showed that saliva pools of either 5 or 10 samples did not compromise the detection of SARS‐CoV‐2 if an increased Ct cutoff value and the detection of either gene from the pool were allowed for further individual specimen tests.
The detection of SARS‐CoV‐2 from a saliva sample is convenient. The previous studies showed that saliva might be an alternative type of specimen for the detection of SARS‐CoV‐2 in symptomatic patients in an acute respiratory infection clinic setting. 2 , 3 The pooling of saliva specimens is an appealing method for conserving testing resources in a low prevalence area. The feasibility of the pooling of nasopharyngeal swab specimens for the detection of the virus has been shown recently. 8 , 9 Similar to the nasopharyngeal swab specimen, we demonstrated the efficiency of saliva pooling for SARS‐CoV‐2 detection and also revealed an increase in Ct values of the ORF1ab gene in the pool saliva specimens that contained one positive sample. However, there was no significant difference in the Ct values of the N gene between the pool and the individual test. This might be explained by a higher genetic diversity of the SARS‐CoV‐2 genome on the N gene primer‐probe set, which might affect the efficacy of the gene detection. 10 In the pool that contained more than one positive sample, RT‐PCR testing detected the virus at lower Ct values compared to Ct values of the individual samples, which could be explained by an increase in viral concentration in the pool.
Although either the saliva pool of 5 or 10 samples could detect SARS‐CoV‐2 in the specimens, pool size should be selected according to the disease prevalence to save the test, and hence the cost, for each negative pool. 7 , 11 In our setting, the prevalence of the disease was 9.0%. The total test numbers performed in this study were 105 and 150 in the pool of five samples and the pool of 10 samples, respectively. Therefore, the pool of five samples was more appropriate than the pool of 10 samples to decrease the resource burden in our setting. This study described a proof‐of‐concept of saliva pooling for the diagnosis of COVID‐19, to conserve the available resource.
We demonstrated that the storage condition, storage time, and freeze‐thaw affected the detection of the virus, which was consistent with the recent study. 12 This presented an important implication that the nucleic acid testing of SARS‐CoV‐2 should be performed as soon as possible after specimen collection. However, in resource‐limited settings, facilities and testing capacity might not be available in those areas. Our study showed one sample that was misclassified as negative after storage and freeze‐thaw. False‐negative results are of concern because infected persons might not be isolated and can infect others. 13 However, the recent study showed that respiratory samples from patients with at least 8 days of symptoms and a SARS‐CoV‐2 E gene RT‐PCR at the Ct value of more than or equal to 24 might predict lack of infectivity. 14 For the specimen that was initially positive but became negative in the individual test after the storage and freeze‐thaw in our study, the specimen collection in this patient was performed at day 15 after the onset of symptoms, and the nasopharyngeal and throat swab of this patient was negative at the simultaneous collection. The Ct values of ORF1ab and N genes from the original saliva sample were high, reflecting the small amount of viral load in the sample. This suggested the low chance of disease transmission from the patient with missed viral detection in a saliva specimen after the storage and freeze‐thaw. Further study on saliva stabilizing solution to increase viral nucleic acid stability should be investigated.
There were some concerns about the use of pool saliva sample testing. The sensitivity of SARS‐CoV‐2 detection in saliva varies in different clinical settings. SARS‐CoV‐2 detection in saliva was found in all severe COVID‐19 patients. 15 Our previous study showed that the sensitivity of SARS‐CoV‐2 detection in saliva was 84.2% comparing to nasopharyngeal and throat swab in ambulatory patients under investigation. 3 However, a recent study demonstrated viral detection in 64% of asymptomatic SARS‐CoV‐2 infected cases from saliva specimens. 16 Of note, viral loads in saliva were lower than nasopharyngeal and throat swab. Therefore, the detection of the virus in saliva probably affected by the severity of the disease. Another limitation of the pooling strategy included the inability to evaluate the adequacy of each specimen in a pool due to the loss of ability to detect the housekeeping gene from each sample. However, with the ease of saliva collection, the saliva pooling might be an appealing method for mass‐screening program or sentinel surveillance, especially in resource‐limited settings.
The strength of this study included the nature of our study that performed consecutive pools of the saliva samples. This study design simulated the real‐life situation, which included different numbers of positive samples in the pools. We accepted the limitation that the sensitivity of the virus detection by saliva sample pooling in this study might be compromised due to RNA degradation during the storage and freeze‐thaw. Also, we used the leftover extracted viral RNA instead of the saliva sample for the pooling.
In conclusion, we demonstrated the efficiency of saliva pooling for the detection of SARS‐CoV‐2 in ambulatory patients under investigation for COVID‐19 in a low prevalence setting. Saliva pooling does not compromise the sensitivity of viral detection if an increased Ct cutoff value and the detection of either gene from the pool are allowed for further individual specimen testing. However, immediate RT‐PCR testing should be performed to minimize the effect of storage conditions that can decrease the sensitivity of the testing. Saliva pooling may facilitate the detection of the disease in suspected symptomatic patients during the disease outbreak, providing the advantages in the ease of specimen collection and resource conservation.
CONFLICT OF INTERESTS
The authors declare that there are no conflict of interests.
AUTHOR CONTRIBUTIONS
EP and AP designed the study. TW and KR performed the experiment. AP analyzed the data. BT and AP wrote the manuscript. EP, SPW, and SK edited the manuscript.
Supporting information
Supporting information
ACKNOWLEDGMENTS
This study was supported by a grant from the Faculty of Medicine Ramathibodi Hospital, Mahidol University. We would like to thank physicians and nurses at the acute respiratory infection clinic at Ramathibodi Hospital and Worramin Suksuwan for their help in collecting the samples.
Pasomsub E, Watcharananan SP, Watthanachockchai T, et al. Saliva sample pooling for the detection of SARS‐CoV‐2. J Med Virol. 2021;93:1506–1511. 10.1002/jmv.26460
DATA AVAILABILITY STATEMENT
The data that supports the findings of this study are available in the supplementary material of this article.
REFERENCES
- 1. Hanson KE, Caliendo AM, Arias CA, et al. Infectious Diseases Society of America guidelines on the diagnosis of COVID‐19 [published online ahead of print August 20, 2020]. Clin Infect Dis. 2020. 10.1093/cid/ciaa760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams E, Bond K, Zhang B, Putland M, Williamson DA. Saliva as a non‐invasive specimen for detection of SARS‐CoV‐2. J Clin Microbiol. 2020;58. 10.1128/JCM.00776-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pasomsub E, Watcharananan SP, Boonyawat K, et al. Saliva sample as a non‐invasive specimen for the diagnosis of coronavirus disease 2019: a cross‐sectional study [published online ahead of print May 15, 2020]. Clin Microbiol Infect. 2020. 10.1016/j.cmi.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McAdam AJ. Special issue on diagnostic testing for severe acute respiratory syndrome coronavirus 2 and lessons from this pandemic. J Clin Microbiol. 2020;58:58. 10.1128/JCM.01324-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Emmanuel JC, Bassett MT, Smith HJ, Jacobs JA. Pooling of sera for human immunodeficiency virus (HIV) testing: an economical method for use in developing countries. J Clin Pathol. 1988;41:582‐585. 10.1136/jcp.41.5.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mine H, Emura H, Miyamoto M, et al. High throughput screening of 16 million serologically negative blood donors for hepatitis B virus, hepatitis C virus and human immunodeficiency virus type‐1 by nucleic acid amplification testing with specific and sensitive multiplex reagent in Japan. J Virol Methods. 2003;112:145‐151. 10.1016/s0166-0934(03)00215-5 [DOI] [PubMed] [Google Scholar]
- 7. Abdalhamid B, Bilder CR, McCutchen EL, Hinrichs SH, Koepsell SA, Iwen PC. Assessment of specimen pooling to conserve SARS CoV‐2 testing resources. Am J Clin Pathol. 2020;153:715‐718. 10.1093/ajcp/aqaa064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Torres I, Albert E, Navarro D. Pooling of nasopharyngeal swab specimens for SARS‐CoV‐2 detection by RT‐PCR [published online ahead of print May 05, 2020]. J Med Virol. 2020. 10.1002/jmv.25971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wacharapluesadee S, Kaewpom T, Ampoot W, et al. Evaluating the efficiency of specimen pooling for PCR‐based detection of COVID‐19 [published online ahead of print May 13, 2020]. J Med Virol. 2020:jmv.26005. 10.1002/jmv.26005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vogels CBF, Brito AF, Wyllie AL, et al. Analytical sensitivity and efficiency comparisons of SARS‐CoV‐2 RT‐qPCR primer‐probe sets [published online ahead of print July 10, 2020]. Nat Microbiol. 2020. 10.1038/s41564-020-0761-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bilder CR, Iwen PC, Abdalhamid B. Pool size selection when testing for SARS‐CoV‐2 [published online ahead of print June 16, 2020]. Clin Infect Dis. 2020. 10.1093/cid/ciaa774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Li L, Li X, Guo Z, et al. Influence of storage conditions on SARS‐CoV‐2 nucleic acid detection in throat swabs. J Infect Dis. 2020;222:203‐205. 10.1093/infdis/jiaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Woloshin S, Patel N, Kesselheim AS. False negative tests for SARS‐CoV‐2 infection ‐ challenges and implications. N Engl J Med. 2020;383:38. 10.1056/NEJMp2015897 [DOI] [PubMed] [Google Scholar]
- 14. Bullard J, Dust K, Funk D, et al. Predicting infectious SARS‐CoV‐2 from diagnostic samples [published online ahead of print May 22, 2020]. Clin Infect Dis. 2020. 10.1093/cid/ciaa638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Azzi L, Carcano G, Gianfagna F, et al. Saliva is a reliable tool to detect SARS‐CoV‐2. J Infect. 2020;81:e45‐e50. 10.1016/j.jinf.2020.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chau NVV, Thanh Lam V, Thanh Dung N, et al. The natural history and transmission potential of asymptomatic SARS‐CoV‐2 infection [published online ahead of print June 04, 2020]. Clin Infect Dis. 2020. 10.1093/cid/ciaa711 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The data that supports the findings of this study are available in the supplementary material of this article.


