Abstract
Introduction
Recent studies have described several cardiovascular manifestations of COVID‐19 including myocardial ischemia, myocarditis, thromboembolism, and malignant arrhythmias. However, to our knowledge, syncope in COVID‐19 patients has not been systematically evaluated. We sought to characterize syncope and/or presyncope in COVID‐19.
Methods
This is a retrospective analysis of consecutive patients hospitalized with laboratory‐confirmed COVID‐19 with either syncope or presyncope. This “study” group (n = 37) was compared with an age and gender‐matched cohort of patients without syncope (“control”) (n = 40). Syncope was attributed to various categories. We compared telemetry data, treatments received, and clinical outcomes between the two groups.
Results
Among 1000 COVID‐19 patients admitted to the Mount Sinai Hospital, the incidence of syncope/presyncope was 3.7%. The median age of the entire cohort was 69 years (range 26‐89+ years) and 55% were men. Major comorbidities included hypertension, diabetes, and coronary artery disease. Syncopal episodes were categorized as (a) unspecified in 59.4% patients, (b) neurocardiogenic in 15.6% patients, (c) hypotensive in 12.5% patients, and (d) cardiopulmonary in 3.1% patients with fall versus syncope and seizure versus syncope in 2 of 32 (6.3%) and 1 of 33 (3.1%) patients, respectively. Compared with the “control” group, there were no significant differences in both admission and peak blood levels of d‐dimer, troponin‐I, and CRP in the “study” group. Additionally, there were no differences in arrhythmias or death between both groups.
Conclusions
Syncope/presyncope in patients hospitalized with COVID‐19 is uncommon and is infrequently associated with a cardiac etiology or associated with adverse outcomes compared to those who do not present with these symptoms.
Keywords: arrhythmias, coronavirus, COVID‐19, dizziness, influenza, presyncope, syncope
Abbreviations
- AV
atrioventricular
- CT
computed tomography
- EKG
electrocardiogram
- MI
myocardial infarction.
1. INTRODUCTION
The ongoing coronavirus disease 2019 (COVID‐19) pandemic has affected a total of 2.6 million people worldwide and presents itself as one of the most significant health crises ever. 1 Since the onset of the pandemic, common presenting symptoms have been well characterized and reported in several publications. 2 , 3 , 4 , 5 , 6 , 7 These reports have highlighted symptoms such as fever, cough, nasal congestion, sore throat, shortness of breath, myalgias, and headaches and have suggested these to be typical and frequent. More recently, less common but serious cardiovascular presentations of COVID‐19 such as venous thromboembolism, acute coronary syndromes, strokes, and cardiac arrhythmias have been reported. 8 , 9 , 10 , 11 Recent reports as well as our clinical experience have led to the recognition of syncope as a cardiovascular phenomenon that can occur in COVID‐19. The mechanism of syncope during severe systemic illnesses, however, can vary between benign etiologies such as orthostasis to malignant events such as atrioventricular (AV) block and ventricular arrhythmias. 12 We therefore sought to investigate syncope or presyncope in patients admitted with COVID‐19 infection by describing its incidence, characteristics, and outcomes.
2. METHODS
2.1. Study population and data collection
This single‐center retrospective cohort study included consecutive adult patients (≥18 years old) with laboratory‐confirmed COVID‐19 infection who were admitted to Mount Sinai Hospital (New York, NY). Consecutive patients were screened, and those with syncope and/or near‐syncope as a presenting symptom were enrolled in the study. The decision to admit patients was made by the emergency room physicians (with or without consultation with the admitting physician) and was based on acuity of illness and physician discretion. The choice to utilize continuous telemetry for these patients was made in a similar fashion but was also limited by the availability of beds capable of continuous telemetry. This “study” group was compared to an age and gender‐matched cohort of patients without syncope (“control” group) from within this population. Detailed review of the electronic medical records was performed to review and record patient demographics, vital statistics, clinical history, laboratory findings, chest radiographs, other imaging modalities, and electrocardiograms (EKGs) at admission. Specific laboratory tests, inpatient telemetry data, treatments received, and clinical outcomes were reviewed and recorded.
The study was approved by the institutional review board at Mount Sinai Hospital, NY. Informed consent was waived. The data were completely de‐identified with all patient identifiers removed prior to analysis.
2.2. Study definitions
Syncope was defined as an abrupt, transient, complete loss of consciousness, associated with the inability to maintain postural tone and rapid, spontaneous recovery. Presyncope was defined if the patient reported extreme lightheadedness without complete loss of consciousness. 13 The etiology of syncope was ascertained by careful and detailed review of medical records, and adjudication was confirmed by consensus between two authors (Jacob S. Koruth and Mohit K. Turagam). The etiology of syncope was categorized as (a) neurocardiogenic—accompanied by typical prodromal symptoms in the setting of a trigger with or without documentation of transient bradycardia and/or hypotension; (b) hypotensive—characterized by prominent complaints of orthostatic intolerance (e.g., dizziness) preceding the syncopal event that typically occur after adopting upright position, with or without documented low systolic blood pressure on arrival (<90 mm Hg), or demonstration of orthostatic blood pressure changes (defined as a sustained reduction of at least 20 mm Hg of systolic blood pressure [BP] or 10 mm Hg of diastolic BP after standing); (c) cardiopulmonary—sudden syncope with evidence of significant bradyarrhythmia, tachyarrhythmias, or evidence of new cardiopulmonary events such as myocardial infarction (MI) and pulmonary embolism; and (d) unspecified—this group was chosen if data did not support placement within the above three categories or other specific etiologies. Patients with syncope in whom an alternate diagnosis of fall or seizure was subsequently considered but a clear determination could not be made were placed into either of these two categories: fall versus syncope and seizure versus syncope, while clear cases of mechanical fall/seizures were excluded.
Other relevant definitions used in the study are detailed in the Supporting Information.
2.3. COVID‐19 diagnosis
Diagnostic testing for COVID‐19 was performed using the real‐time reverse transcription polymerase chain reaction (rRT‐PCR) test (Roche's cobas 6800 System, Basel, Switzerland). 14
2.4. Statistical analyses
Categorical and continuous variables were summarized as counts/percentages and median/interquartile range (IQR) or means and standard deviations, as appropriate. No imputation was made for missing data. Categorical variables between groups were compared using Fisher's exact test or χ² test. Continuous variables with normal distribution were compared using Student's t‐test, while nonnormal distributions were compared using Mann‐Whitney U test where appropriate. A P‐value ≤ .05 (two‐tailed) was considered to be statistically significant. Statistical analysis was performed using SPSS version 25.0 (IBM Corp, Armonk, NY).
3. RESULTS
3.1. Demographics and clinical characteristics
A total of 1000 consecutive patients with a diagnosis of laboratory‐confirmed COVID‐19 who were hospitalized at the Mount Sinai Hospital, NY from April 4, 2020 to April 14, 2020 were screened using the electronic medical records. Of this group, 40 patients were identified to have had either syncope or presyncope. Careful review of records led to exclusion of three of 40 patients who had been incorrectly diagnosed or had other obvious explanations for syncope (e.g., acute leukemia with severe anemia), leading to 37 patients that formed the study group. Thus, the incidence of syncope and presyncope was 3.7% in this population. From the remaining 960 patients (Figure 1), a cohort of 40 age and gender‐matched patients were identified and served as a control group.
FIGURE 1.
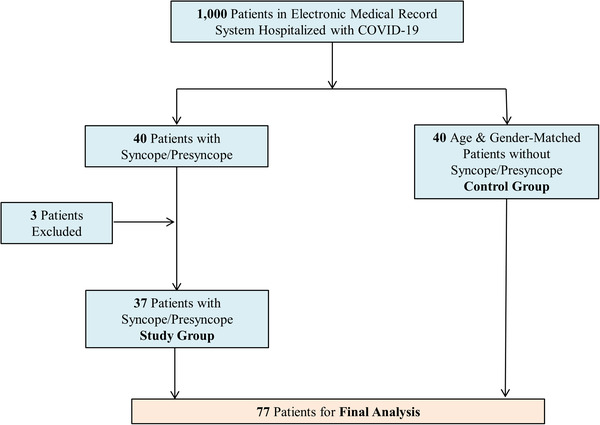
Consort diagram [Color figure can be viewed at wileyonlinelibrary.com]
The median age of the entire (study and control) cohort was 69 years (range 26‐89+ years), 42 of 77 (55%) patients were men, and 25 (33%) of the cohort were Caucasian. Hypertension was the most common comorbidity in 51 (66%) patients. Other common comorbidities included coronary artery disease, diabetes, and obesity. The distribution of comorbid states across both groups is detailed in Table 1 and was not significantly different. The study group had significantly greater use of angiotensin receptor blockers (27% vs 7%) with no significant differences in the use of other antihypertensives and oral anticoagulants between both groups. Importantly, there were no differences in usage of hydroxychloroquine and azithromycin at the time of admission between both groups. The admission vital signs including temperature, pulse rate, pulse oximetry, systolic and diastolic blood pressure were significant for systolic blood pressure, which was lower in the study group compared to control (126 [109‐138] vs 139 [120‐149] mm Hg), and pulse rate, which was lower in the study group (86 [75‐95] vs 102 [90‐109] bpm).
TABLE 1.
Clinical characteristics and laboratory data
| All patients | Patient disposition | ||||
|---|---|---|---|---|---|
| Characteristics | No. with available data | Value | Syncope(N = 37) | No syncope(N = 40) | P‐value |
| Median age (range, IQR), year | 77 | 69 (56‐73) | 69 (56.5‐73) | 68 (56‐73) | .81 |
| Male gender (%) | 77 | 42 (55) | 19 (51) | 23 (57) | .65 |
| Ethnicity (%) | 77 | ||||
| Caucasian | 25 (33) | 9 (24) | 16 (40) | .15 | |
| African‐American | 20 (25) | 9 (24) | 11 (28) | 1.00 | |
| Hispanic | 9 (12) | 1 (3) | 8 (20) | .03 | |
| Other | 23 (30) | 18 (49) | 5 (12) | .001 | |
| Body mass index, median (IQR) | 73 | 27.2 (24.7‐31.6) | 27.4 (25.6‐31.2) | 26.8 (24.1‐32.5) | .53 |
| Comorbidities—No. (%) | |||||
| Hypertension | 77 | 51 (66) | 25 (68) | 26 (65) | 1.00 |
| Insulin dependent diabetes mellitus | 77 | 9 (12) | 4 (11) | 5 (13) | 1.00 |
| Noninsulin‐dependent diabetes mellitus | 77 | 19 (25) | 8 (22) | 11(28) | .60 |
| Coronary artery disease | 77 | 15 (19) | 10 (27) | 5 (12) | .15 |
| Systolic heart failure | 77 | 5 (6) | 3 (8) | 2 (5) | .66 |
| Obesity (body mass index ≥30) | 76 | 29 (38) | 15 (42) | 14 (35) | .63 |
| Chronic kidney disease | 77 | 9 (12) | 4 (11) | 5 (13) | 1.00 |
| Chronic dialysis | 77 | 5 (6) | 3 (8) | 2 (5) | .66 |
| Chronic obstructive pulmonary disease | 77 | 9 (12) | 3 (8) | 6 (15) | .48 |
| History of atrial fibrillation | 77 | 4 (5) | 3 (8) | 1 (2) | .34 |
| History of supraventricular arrhythmias | 77 | 3 (4) | 3 (8) | 0 (0) | .11 |
| History of ventricular arrhythmias | 77 | 1 (1) | 1 (3) | 0 (0) | .48 |
| Medications—No. (%) | |||||
| Beta‐blockers | 77 | 23 (30) | 14 (38) | 9 (22) | .21 |
| Calcium‐channel blockers | 77 | 28 (36) | 11 (30) | 17 (42) | .34 |
| Angiotensin converting inhibitors | 77 | 15 (19) | 5 (13.5) | 10 (25) | .25 |
| Angiotensin receptor blockers | 77 | 13 (17) | 10 (27) | 3 (7) | .03 |
| Aldosterone antagonist | 77 | 0 (0) | 0 (0) | 0 (0) | ‐ |
| Class I/III antiarrhythmic drugs | 77 | 1 (1) | 1 (1) | 0 (0) | .50 |
| Oral anticoagulants | 77 | 5 (6) | 4 (11) | 1 (3) | .20 |
| Hydroxychloroquine | 77 | 3 (4) | 0 (0) | 3 (8) | .24 |
| Azithromycin | 77 | 10 (13) | 3 (8) | 7 (18) | .31 |
| Vital signs—on admission | |||||
| Temperature, °C | 77 | 37.1 (36.7‐38.1) | 37.2 (36.7‐38.1) | 37.1 (36.6‐38.1) | .82 |
| Pulse rate, beats/min | 77 | 93 (81‐103) | 86 (75‐95) | 102 (90‐109) | <.0001 |
| Systolic blood pressure, mm Hg | 77 | 131 (116‐143) | 126 (109‐138) | 139 (120‐149) | .01 |
| Diastolic blood pressure, mm Hg | 77 | 73 (65‐81) | 70 (61‐81) | 73 (68‐83) | .21 |
| Pulse oximetry (Spo2), % | 77 | 92. (84.0‐95.0) | 92.0 (82.5‐95.0) | 92.0 (84.2‐95.0) | .78 |
| Laboratory data | Table S1 ‐ No differences between groups | ||||
| Chest radiography—No./total No. (%) | 77 | ||||
| Bilateral pulmonary infiltrates | 60 (78) | 24 (65) | 36 (90) | 0.016 | |
| Unilateral pulmonary infiltrates | 8 (10) | 5 (13) | 3 (8) | ||
| Clear | 9 (12) | 8 (22) | 1 (2) | ||
| Baseline transthoracic echocardiography | |||||
| Left ventricular ejection fraction*, (%) | 10 | 51 ± 17 | 47 ± 19 | 60 ± 5 | 0.32 |
Values are expressed as median (IQR), unless otherwise specified. Values are on admission unless otherwise specified.
*mean ± SD; P‐values < 0.05 were bolded to highlight significance.
3.2. Study group event analysis
Of the 37 patients in this group, five patients experienced presyncope (multiple episodes in two patients) without syncope. The remaining 32 patients experienced a median of one syncopal episode (IQR_1‐2). These syncopal episodes were categorized into as (a) unspecified in 59.4% (19/32) patients, (b) neurocardiogenic in 15.6% (5/32) patients, (c) hypotensive in 12.5% (4/32) patients, and (4) cardiopulmonary in 3.1% (1/32) patients. The remaining were categorized as fall versus syncope and seizure versus syncope in 2 of 32 (6.3%) and 1 of 33 (3.1%) patients, respectively (Figure 2). Of the four patients in the hypotensive group, two were documented to have orthostatic hypotension while the other two were not tested. In the cardiac group, the one patient was unable to provide a history due to mild dementia, but presented with new onset atrial fibrillation and evidence of a recent ST‐elevation MI (peak troponin 12.6) and was presumed to have syncope of cardiac origin. Twelve of 30 (40%) patients were determined to have witnessed syncope, two of whom experienced syncope while waiting in the emergency room. Both these latter patients had episodes associated with transient bradycardia and hypotension and subsequently were adjudicated to have neurocardiogenic syncope. Furthermore, 25% (8/32) of patients were noted to have evidence of minor trauma, none requiring surgical intervention. Computed tomography (CT) imaging of the head was performed in 69% (22/32) patients, with nonsignificant findings. Only three patients underwent contrast CT scan imaging for assessment of pulmonary embolism as a potential etiology for syncope, and all were negative for emboli (but positive for typical bilateral parenchymal lung infiltrates). Only one patient had a recurrent syncopal spell after admission that was considered as a possible fall given that it was not witnessed, and the patient was unable to volunteer details of the event. Four patients had cardiac devices (one implantable loop recorder, two dual chamber pacemakers, and one biventricular defibrillator), and no arrhythmias were noted in three patients on whom data could be obtained. Finally, only three of 37 patients presented without any typical COVID‐19 symptoms of fever cough, shortness of breath, and/or myalgias. Two additional patients presented with no history of fever but had other symptoms, thus bringing the total number of patients presenting without antecedent fever to five of 37 (13.5%) within this category.
FIGURE 2.
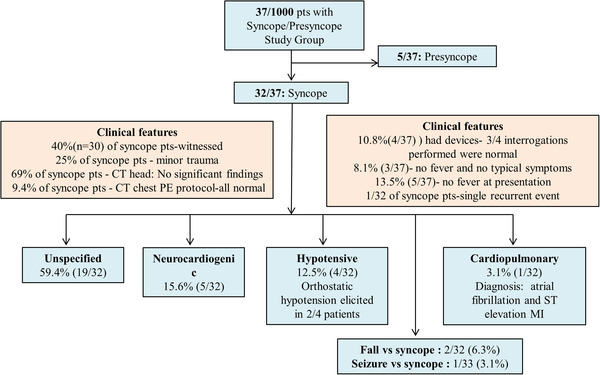
Study group details [Color figure can be viewed at wileyonlinelibrary.com]
3.3. Investigations on admission
There were no differences in laboratory data, both at the time of admission and of the peak values noted during hospitalization between both groups. Investigations included blood counts, serum sodium, aspartate aminotransferase/alanine amiontransferase (AST/ALT), creatinine, blood sugar, troponin, ferritin, lactate dehydrogenase (LDH), d‐dimer, c‐reactive protein (CRP), and brain natriuretic peptide. However, chest radiographic findings between both groups were significantly different, with bilateral pulmonary infiltrates seen in 78% of the entire cohort (Table 1 and Table S1).
The initial EKGs obtained at admission, revealed heart rates to be significantly lower in the study group compared to controls (89 [81‐97] vs 103 [93‐110]), which is consistent with the observation of slower heart rates noted during initial assessment of vital signs (Table 2). The presenting rhythm was sinus in the majority of patients (73/77 [95%]). Other presenting rhythms included atrial fibrillation and ventricular pacing, and these were not different between groups. There were only three instances of patients presenting with atrial fibrillation; two of these occurred in the study group (both patients had heart rates <100/min) and one in the control group. One of these patients (in the study group) also demonstrated evidence of a recent anterior wall infarction (mentioned above) and was managed conservatively. Occurrence of bundle branch block and repolarization abnormalities were also not different between groups, as were electrocardiographic features such as PR, QRS, and corrected QT interval (QTc) intervals. The majority of these electrocardiographic intervals were within normal limits (Table 2).
TABLE 2.
12‐lead electrocardiographic and telemetric monitoring
| Baseline EKG | All patients(N = 77) | Syncope(N = 37) | No syncope(N = 40) | P‐value |
|---|---|---|---|---|
| Heart rate/min, median (IQR) | 95 (86‐104) | 89 (81‐97) | 103 (93‐110) | <.0001 |
| Rhythm | ||||
| Sinus rhythm—No. (%) | 73 (95) | 34 (92) | 39 (98) | .35 |
| Atrial fibrillation (AF)—No. (%) | 3 (4) | 2 (5) | 1 (3) | .60 |
| Paced rhythm—No. (%) | 1 (1) | 1 (3) | 0 (0) | .50 |
| Duration of PR interval, ms | 150 (132‐160) | 150 (132‐160) | 152 (134‐162) | .71 |
| Duration of QRS interval, ms | 84 (78‐99) | 84 (78‐101) | 85 (80‐96) | .75 |
| Duration of QTC interval, ms | 444 (420‐458) | 439 (415‐464) | 448 (427‐457) | .20 |
| RBBB—No. (%) | 7 (9) | 4 (11) | 3 (8) | .70 |
| LBBB—No. (%) | 1 (1) | 0 (0) | 1 (3) | 1.00 |
| IVCD—No. (%) | 1 (1) | 0 (0) | 1 (3) | 1.00 |
| ST segment changes—No. (%) | 3 (4) | 1 (3) | 2 (5) | 1.00 |
| T wave inversion—No. (%) | 12 (16) | 7 (19) | 5 (13) | .54 |
| Last EKG prior to death or discharge | ||||
| Rhythm | ||||
| Sinus rhythm—No. (%) | 71 (92) | 35 (95) | 36 (90) | 1.00 |
| AF—No. (%) | 4 (5) | 1 (3) | 3 (8) | .61 |
| Paced rhythm—No. (%) | 1 (1) | 1 (3) | 0 (0) | .49 |
| Duration of QTC interval, ms | 454 (434‐475) | 455 (427‐486) | 454 (438‐473) | .87 |
| Change of QTC interval ≥40 ms from admission, ms | 5 (6.5) | 3 (8) | 2 (5%) | .67 |
| Telemetry | All patients (N = 39) | Syncope (N = 20) | No syncope (N = 19) | P‐value |
|---|---|---|---|---|
| Duration of continuous monitoring, days | 7.50 (3.00‐11.00) | 7.00 (2.25‐11.00) | 8.5 (3.5‐10.75) | .71 |
| Atrial arrhythmias—No. (%) | ||||
| Supraventricular tachycardia—No. (%) | 17 (44) | 11 (55) | 6 (32) | .20 |
| AF—No. (%) | 5 (13) | 1 (5) | 4 (21) | .18 |
| Atrial flutter—No. (%) | 2 (5) | 1 (5) | 1 (5) | 1.00 |
| Ventricular arrhythmias—No. (%) | ||||
| Monomorphic ventricular tachycardia—No. (%) | 0 (0) | 0 (0) | 0 (0) | ‐ |
| Ventricular fibrillation—No. (%) | 0 (0) | 0 (0) | 0 (0) | ‐ |
| Premature ventricular contractions (PVC)—No. (%) | ||||
| Occasional isolated PVCs—No. (%) | 7 (18) | 5 (25) | 2 (11) | .40 |
| PVCs >1/min—No. (%) | 4 (10) | 1 (5) | 3 (16) | .34 |
| Multiform PVCs—No. (%) | 1 (3) | 0 (0) | 1 (5) | .49 |
| Couplets—No. (%) | 1 (3) | 0 (0) | 1 (5) | .49 |
| Nonsustained ventricular tachycardia—No. (%) | 3 (8) | 1 (50) | 2 (11) | .60 |
| Bradyarrhythmias—No. (%) | 0 (0) | 0 (0) | 0 (0) | ‐ |
| Pulseless electrical activity—No. (%) | 11 (28) | 4 (20) | 7 (37) | .30 |
All values are expressed as median (IQR), unless otherwise specified.
P‐values < 0.05 were bolded to highlight significance.
Specifically, in the study group, there were two patients who had a presenting QTc >500 ms (513 and 517 ms). Both these patients had QTc intervals of <500 ms at the time of discharge. One patient was observed on continuous telemetry and was noted to have no significant ventricular arrhythmias, but developed a peak troponin of 4.4 ng/mL with evidence of T wave inversions (LV ejection fraction of 60%) leading to a diagnosis of acute MI that was conservatively managed. The second patient with the prolonged QT interval was not observed on telemetry but did not develop troponin elevations or subsequent EKG abnormalities.
The predischarge EKG and inpatient telemetry (available for 20 and 19 patients in study and control groups) failed to reveal any differences in arrhythmias. Importantly, within the study group, there were no instances of bradyarrhythmias identified either on the presenting EKGs (performed on all patients) or during continued telemetry (55% of patients). One patient in the study and three patients in the control group developed new onset atrial fibrillation. In the study group, one patient developed sudden worsening of respiratory status on the third day of admission with accompanying sinus tachycardia and an S1Q3T3 pattern that prompted CT angiography revealing pulmonary embolism (see below for further details).
3.4. Treatments, complications, and outcomes
The median duration of hospitalization for the entire cohort was 10 days (IQR 6‐15) (Table 3). The study group experienced significantly less intensive care unit (ICU) admissions (14 % vs 35%) and had lower need for mechanical ventilation (14% vs 33%, P = NS). Additional details are compared in Table 3, and it is notable that there were no significant differences. Finally, there were a total of 14 of 77 deaths and 74 of 77 discharges in this cohort with only three patients remaining hospitalized at the time of this analysis. There were no differences between groups in terms of ventricular arrhythmias and death. The initial rhythm at the time of death in the 11 of 14 patients who were on telemetry was pulseless electrical activity in all, and there were no instances of ventricular arrhythmias.
TABLE 3.
Treatments and complications
| Characteristics | All patients(N = 77) | Syncope(N = 37) | No syncope(N = 40) | P‐value |
|---|---|---|---|---|
| Treatments | ||||
| Admit to intensive care unit—No. (%) | 19 (25) | 5 (14) | 14 (35) | .03 |
| Invasive mechanical ventilation—No. (%) | 18 (23) | 5 (14) | 13 (33) | .06 |
| Bi‐level positive airway pressure—No. (%) | 16 (21) | 8 (22) | 8 (20) | 1.00 |
| Nonrebreather mask—No. (%) | 36 (47) | 18 (49) | 18 (45) | .82 |
| High‐flow nasal cannula—No. (%) | 13 (17) | 7 (19) | 6 (15) | .74 |
| Hydroxychloroquine—No. (%) | 67 (87) | 30 (81) | 37 (93) | .20 |
| Azithromycin—No. (%) | 46 (60) | 19 (51) | 27 (68) | .18 |
| Immunomodulators—No. (%) | ||||
| Sirolumab—No. (%) | 2 (3) | 1 (3) | 1 (3) | 1.00 |
| Tocilizumab—No. (%) | 3 (4) | 2 (5) | 1 (3) | .60 |
| Remdesivir—No. (%) | 3 (4) | 0 | 3 (7.5) | .24 |
| Glucocorticoids—No. (%) | 23 (30) | 9 (24) | 14 (35) | .3 |
| Therapeutic anticoagulation—No. (%) | 38 (49) | 19 (51) | 19 (48) | .82 |
| Complications | ||||
| Myocardial injury—No. (%) | 35 (44) | 14 (38) | 21 (53) | .25 |
| Acute MI | 4 (5) | 4 (11) | 0 (0) | .05 |
| Acute kidney injury—No. (%) | 24 (32) | 11 (30) | 13 (33) | 1.00 |
| Acute kidney injury requiring renal replacement therapy—No. (%) | 7 (9) | 1 (3) | 6 (15) | .11 |
| Shock, requiring pressors—No. (%) | 16 (21) | 4 (11) | 12 (30) | .08 |
| Acute respiratory distress syndrome—No. (%) | 34 (44) | 15 (41) | 19 (48) | .65 |
| Ischemic stroke—No. (%) | 1 (2) | 1 (3) | 0 (0) | .50 |
| Pulmonary embolism—No. (%) | 5 (7) | 2 (6) | 3 (8) | 1.00 |
| Deep vein thrombosis—No. (%) | 3 (4) | 1 (3) | 2 (5) | 1.00 |
| Death—No. (%) (N = 60) | 14 (23) | 5 (18) | 9 (28) | .38 |
| Palliative care—No. (%) | 7 (50) | 3 (60) | 4 (44) | 1.00 |
| ICU length of stay, mean ± SD | 3.05 ± 5.5 | 1.4 ± 3.6 | 4.1 ± 6.2 | .09 |
| Hospital length of stay, median (IQR)—No. (%) | 10 (6‐15) | 10 (7‐17) | 10 (6‐15) | .8 |
All values are expressed as median (IQR), unless otherwise specified.
P‐values < 0.05 were bolded to highlight significance.
4. DISCUSSION
In this report, we identify and report on 37 patients with laboratory‐confirmed COVID‐19 infection who presented with syncope and/or presyncope within a cohort of 1000 consecutive patients admitted over a 2‐week period during the peak of the COVID‐19 pandemic. The main findings in this report are as follows:
Syncope and presyncope is infrequent in COVID‐19 among patients who were hospitalized, with an incidence of 3.7%.
Syncopal events were categorized as unspecified in more than half (59.4%) of the patients with the remaining being attributed mainly to neurocardiogenic, hypotensive etiologies.
Only one of 32 patient had cardiac syncope related to new onset atrial fibrillation and anterior wall ST‐elevation MI.
No evidence of bradyarrhythmias either at the time of admission or during the course of their hospitalization were noted.
Compared to an age and gender‐matched control group, the only significant differences noted in the study group were a lower heart rate at admission, a lower systolic blood pressure at admission, and a lower need for escalation of care to the ICU level. There were no significant differences in all the other parameters including need for assisted ventilation or incremental oxygen requirements, and there was no difference in mortality.
During the ongoing COVID‐19 pandemic, clinicians have begun to report on less common presenting symptoms and complications outside of those related to the pulmonary involvement. These are important to recognize, both from the point of view of improving early identification, risk stratification, and treatment. Of note, several COVID‐19‐associated cardiovascular complications have received significant attention, such as myocardial ischemia, myocarditis, pseudo‐infarctions, repolarization abnormalities, left ventricular dysfunction, thromboembolism, and malignant arrhythmias. 8 , 9 , 10 , 11 While these issues are obviously important, our report is restricted to patients presenting with syncope and presyncope. Thus far, syncope has only been reported in the COVID‐19 literature as two isolated case reports and a case series of five patients. 12 , 15 , 16 The case reports describe two elderly patients; one presenting with syncope followed by altered mental status and the other with antecedent influenza like symptoms and syncope after a bowel movement. Both these reports documented orthostatic hypotension. The case series, on the other hand, described five patients who presented with syncope preceding the onset of other typical COVID‐19 symptoms. All five patients had cardiovascular implantable devices, at the time of syncope which upon interrogation did not reveal any arrhythmias leading to the conclusion that syncope was noncardiac. Two other COVID‐19 reports on hospitalized patients noted a 17‐20% incidence of dizziness. The frequent occurrence of dizziness as indicated in these reports could be viewed as supportive of the occurrence of presyncope/syncope in COVID‐19. 17 , 18 All these reports are consistent with our findings.
The majority of patients were determined to have syncope of unspecified etiology. These episodes were often ascribed to be related to “severe illness with dehydration” and ascribed to reduced peroral intake of fluids, gastrointestinal intolerance, etc., and were treated with initial intravenous fluid resuscitation and other usual supportive measures. The study cohort had lower heart rate and systolic blood pressures at admission and lower intensive care unit requirement, which suggest that syncope was likely not associated with severe COVID‐19 infection. Of note, the study cohort had a significantly greater use of angiotensin receptor blocking agents; the greater blood pressure lowering effects of these agents may have played an additional role in the occurrence of syncope in this group. Some patients reported exertional syncope and the occurrence of exertional hypoxia in this population raises the possibility that hypoxia maybe mechanistically related to syncope. The lack of any bradyarrhythmia and of recurrences is of interest as there has been concern for potential COVID‐19‐related AV block. In fact, the evidence of symptomatic bradyarrhythmias in COVID‐19 is limited to only a single case report of transient complete hear block that occurred a day after intubation and was not associated with recurrence. 19 While the authors of this manuscript have witnessed cases of complete heart block during the pandemic, coincidentally none of these presented during the 2‐week recruitment period for this study. We feel that unless AV block related to COVID‐19 is further explored and defined, it remains important for clinicians to recognize that bradyarrhythmias as well as other more serious etiologies of syncope (MI, pericardial tamponade, pulmonary embolism) should not be ignored based on this report. 20 , 21 , 22 , 23
For perspective, it is relevant to note that a prior report on a cohort of 651 patients with influenza, noted the incidence of syncope to be 2.2% (14 patients). 24 They described a significantly younger population (mean age 48 ± 20 years), but like our report, they did not note any serious arrhythmias. Orthostatic blood pressures changes were also demonstrable in only three of the 14 patients. Thus, the 3.7% incidence of syncope seen in our report is comparable to that of what was seen with influenza. This similarity raises the question if syncope is indeed more reflective of an ongoing severe debilitating illness rather than of severe end‐organ involvement.
5. LIMITATIONS
This was a retrospective analysis of only hospitalized patients based on review of electronic records. Patients were not interviewed or examined. Orthostatic hypotension, serial EKGs, echocardiography, and telemetry were not assessed on the entire cohort. Patients in this study were enrolled during the “surge” that New York City experienced where patients willingness to seek urgent care as well as the ability to pursue investigations such as CT angiography (work up of syncope) may have been reduced. Finally, during this period, patients with troponin elevations and electrocardiographic changes were classified as MIs; we are unable to distinguish these changes from myocarditis given the limited imaging options, and this limitation must be recognized.
5.1. Conclusions
Syncope and presyncope in patients hospitalized with COVID‐19 was noted to be an uncommon presentation with a total incidence of 3.7% in this retrospective analysis of 1000 patients. This presentation is infrequently associated with a cardiac etiology and was not associated with adverse outcomes compared to patients with other common presentations.
CONFLICT OF INTEREST
The authors have no relevant conflict to disclose. A complete list of all disclosures for authors Vivek Y. Reddy and Jacob S. Koruth is provided in the Supporting Information.
Supporting information
Supplementary Material
Oates CP, Turagam MK, Musikantow D, et al. Syncope and presyncope in patients with COVID‐19. Pacing Clin Electrophysiol. 2020;43:1139–1148. 10.1111/pace.14047
REFERENCES
- 1. Johns Hopkins University of Medicine Coronavirus Resource Center . COVID‐19 dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). 2020. https://coronavirus.jhu.edu/. Accessed August 26, 2020.
- 2. Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727‐733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323:1061‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhou F, Yu T, Du R, et al. Clinical course and risk factor for mortality of adult inpatients with COVID‐19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054‐1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Guo T, Fan Y, Chan M, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID‐19 in Wuhan, China. JAMA Cardiol. 2020;5:802‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS‐CoV‐2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574‐1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiong T‐Y, Redwood S, Prendergast B, Chen M. Coronaviruses and the cardiovascular system: acute and long‐term implications. Eur Heart J. 2020;41:1798‐1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ebrille E, Lucciola MT, Amellone C, Ballocca F, Orlando F, Giammaria M. Syncope as the presenting symptom of COVID‐19 infection. HeartRhythm Case Rep. 2020;6:363‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Goldberger ZD, Petek BJ, Brignole M, et al. ACC/AHA/HRS versus ESC guidelines for the diagnosis and management of syncope: JACC guideline comparison. J Am Coll Cardiol. 2019;74:2410‐2423. [DOI] [PubMed] [Google Scholar]
- 14. Corman VM, Landt O, Kaiser M, et al. Detection of 2019 novel coronavirus (2019‐nCoV) by real‐time RT‐PCR. Euro Surveill. 2020;25:2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tapé C, Byrd KM, Aung S, et al. COVID‐19 in a patient presenting with syncope and a normal chest X‐ray. R I Med J (2013). 2020;103:50‐51. [PMC free article] [PubMed] [Google Scholar]
- 16. Singhania N, Bansal S, Singhania G. An atypical presentation of novel coronavirus disease 2019 (COVID‐19). Am J Med. 2020;7:e365‐e366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Q, Zheng Z, Zhang C, et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID‐19) in Taizhou, Zhejiang, China. Infection. 2020;48:543‐551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77:683‐690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Azarkish M, Laleh Far V, Eslami M, Mollazadeh R. Transient complete heart block in a patient with critical COVID‐19. Eur Heart J. 2020;41:213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hua A, O'Gallagher K, Sado D, Byrne J. Life‐threatening cardiac tamponade complicating myo‐pericarditis in COVID‐19. Eur Heart J. 2020;41:2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Danzi GB, Loffi M, Galeazzi G, Gherbesi E. Acute pulmonary embolism and COVID‐19 pneumonia: a random association?. Eur Heart J. 2020;41:1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Xie Y, Wang X, Yang P, Zhang S. COVID‐19 complicated by acute pulmonary embolism. Radiol Cardiothorac Imaging. 2020;2:e200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Xiao M, Zhang S, et al. Coagulopathy and antiphospholipid antibodies in patients with COVID‐19. N Engl J Med. 2020;382:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Noh SM, Kang HG, Kim BJ. Syncope after influenza virus infection. J Korean Med Sci. 2020;35:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


