To the Editor,
SARS‐CoV‐2 infection may induce a broad spectrum of consequences ranging from asymptomatic infection to fatal pneumonia. 1 The most severe complication is the acute respiratory distress syndrome (ARDS) which is often fatal. The so‐called “IL‐6 cytokine storm” and a disseminated intravascular cascade in the lung characterize most severe cases of COVID‐19. The coincidence of these events with the rise of the adaptive immune response suggests that the response itself may play a role. In effect, COVID‐19 patients with agammaglobulinemia recovered without lung complications. 2 Atopic status is the genetic predisposition to produce a Type 2 immune response to environmental antigens. Such response relies on some key cytokines, including interleukin (IL) 4, IL‐5, IL‐9, and IL‐13. 3 In infection, the Th2 response counteracts the microbicidal Th1 response, which could limit the tissue damage induced by Th1‐mediated inflammation 4 on one hand, but also cause a less efficient anti‐virus response, as shown in a study on experimental Coronavirus 229E infection in healthy volunteers, where atopy appeared to be associated with a more severe rhinitis score. 5 Further, atopic subjects show a reduced expression of ACE2, the SARS‐CoV‐2 receptor, which could be associated with reduced susceptibility to the virus. 6 We therefore hypothesized that atopic subjects infected by SARS‐CoV‐2 might have a milder clinical course than nonatopic subjects, and tested this hypothesis in a large cohort of hospitalized COVID‐19 patients.
We performed a retrospective study on patients with SARS‐CoV‐2‐induced pneumonia, as confirmed by the detection of viral nucleic acid in nasal and/or pharyngeal clinical specimens, hospitalized in several Italian hospitals. Doctors recorded clinical data (age, sex, smoking habits, diabetes, hypertension, coronary heart disease, and thrombosis) along with respiratory allergy and graded the severity of the respiratory disease at the end of the hospital stay as mild, severe, or very severe based on no need for respiratory assistance, need for noninvasive respiratory assistance or need for invasive respiratory assistance or death, respectively. Patients were considered “atopic” in the presence of an unequivocal history of respiratory allergy to airborne allergens confirmed by positive skin prick tests performed by a specialist allergy center and/or by elevated specific IgE. All allergy investigations had been performed before hospitalization. Patients’ data were anonymized, and the internal review board of the promoting center approved the study. The association between severity of COVID‐19 and both the atopy status and the clinical co‐factors recorded was studied in both univariate and multivariate analyses (details in the Appendix S1). Type I statistic error probability values<5% were considered significant.
Five hundred and thirty one adult individuals (214 females, 40%) aged 68 ± 14 years (range 25‐100 years) were studied. Their disease was classified as mild in 191 cases (36%) and severe or very severe in 340 cases (64%). The clinical features are summarized in Table 1. Male gender was significantly associated with severe disease (P = .001), and men were more likely to be admitted to intensive care units (P = .01). Smokers had a significantly higher prevalence of coronary heart disease (P = .023), and intensive care admission (P = .032) than nonsmokers. Male smokers showed a higher prevalence of coronary heart disease (39.7% vs 21.6%, P = .002) and thrombosis (P = .027) than female smokers (Table 1).
TABLE 1.
Demographic and clinical features of the study population
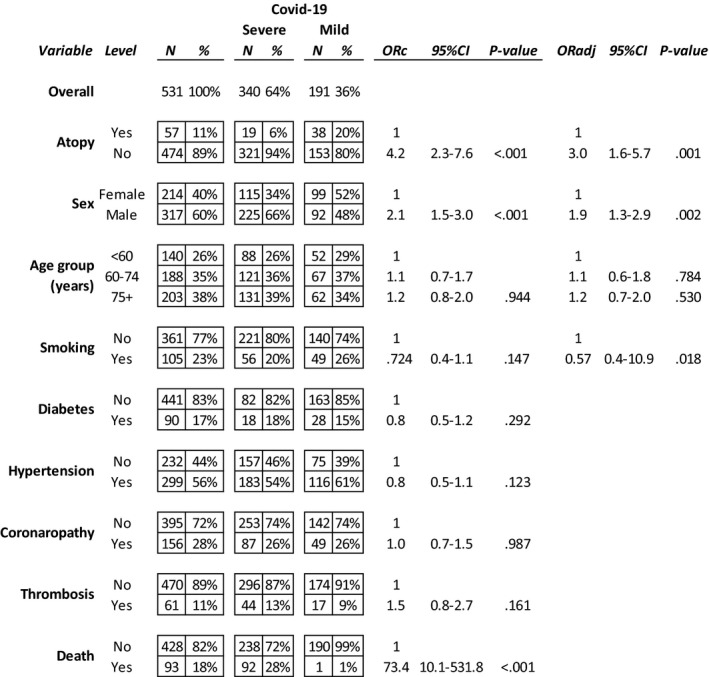
The multiple logistic regression analysis including age, sex, smoking, and all the comorbidities shows a significant association between nonatopic status and a significantly higher risk of having severe COVID‐19‐related pneumonia.
The multiple logistic regression analysis including age, sex, smoking, and all the comorbidities shows a significant association between nonatopic status and a significantly higher risk of having severe COVID‐19‐related pneumonia.
Fifty‐seven participants (10.7%) were atopic. 32/57 (56.1%) were females (mean age 62 ± 14, range 28‐93 years). Atopic subjects were younger (69 ± 14 years, P = .01) than nonatopic patients, showed a lower prevalence of coronary heart disease (P < .050)and, notably, showed a much lower occurrence of severe or very severe COVID‐19 pneumonia(33.3% vs 67.7%, P < .0001).The protective effect of atopic status against severe lung disease was evident throughout all the age subsets (P = .001; Figure 1). The multiple logistic regression analysis (details in Appendix S1) confirmed a significant association between atopic status and milder COVID‐19; nonatopic patients had a significantly higher risk of having severe COVID‐19 (ORadj 3.0, 95% CI 1.6‐5.7, P = .001) (Table 1). The unsupervised two‐way hierarchical clustering analysis identified a clear cluster including mild COVID‐19‐related pneumonia and atopy status (P < .0001; ORa = 4.523, 95% CI = 2.221‐9.221) (Figure S1).
FIGURE 1.
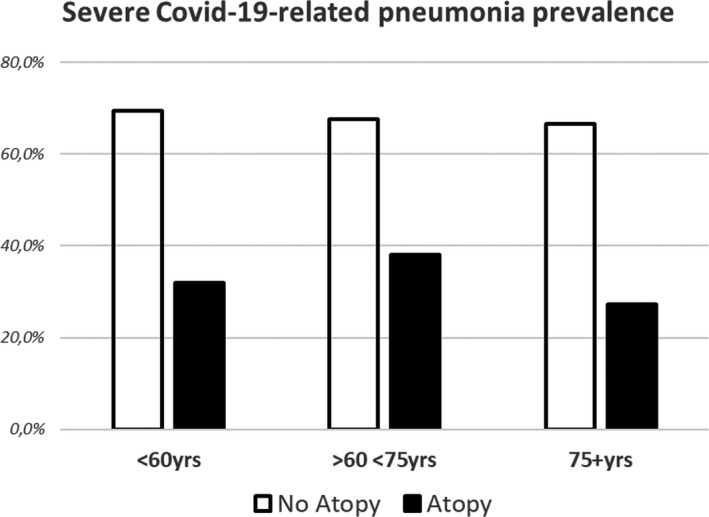
Association between atopic status and mild COVID‐19 in different age groups
In severe SARS‐CoV‐2 infection hyper‐expressed cytokines include IFN‐gamma, TNF‐alpha, and IL‐6, which cause fever, fatigue, flu‐like symptoms, vascular leakage due to endothelial dysfunction, cardiomyopathy, hypotension, lung injury, activation of the coagulation cascade, and diffuse intravascular coagulation. 7 The cytokine storm, which does not occur in patients with uncomplicated SARS‐CoV‐2 infection, 8 leads to the rapid proliferation and activation of T cells, macrophages, and natural killer cells that eventually secrete >150 inflammatory cytokines, chemokines, and chemical mediators. 9 Atopic patients are genetically predisposed to mount Th2 type immune‐mediated responses; these responses do not imply the expression of the main cytokines involved in the ARDS. We hypothesized that allergic patients might be both less prone to SARS‐CoV‐2 infection and/or might have a less severe SARS‐CoV‐2 infection than nonatopic individuals. In the impossibility to carry out a field epidemiological study, we tried to address the second hypothesis focusing on more than 500 patients with confirmed COVID‐19 infection severe enough to warrant hospital admission. Thus, our study population lacks both asymptomatic and symptomatic SARS‐CoV‐2 infected patients with very mild disease, as these patients had to spend their quarantine period at home. Despite these obvious limitations, our study demonstrates that among hospitalized patients with severe COVID‐19, atopic patients have less severe disease. Interestingly, the prevalence of atopic patients in our population (10.7%) appeared lower than that in the age‐matched general population, although recent epidemiological data on allergy in Italy are missing. On the other hand, we cannot exclude that also other of our patients were atopic, although undiagnosed, as they had not undergone allergy investigations before. Notably, the “protective” effect of atopic status did not depend on the age or sex of patients nor the presence of other co‐factors, such as cigarette smoke, coronary heart disease, diabetes, thrombosis, or hypertension. We are not in the position to ascertain whether the immunological scenario that we have hypothesized and that prompted us to perform this study is correct, as this would require an immune‐histochemical analysis of patients' sputum. Nonetheless, our clinical findings make our initial hypothesis believable suggesting that the genetic predisposition to a Th2‐oriented immune response might help to avoid the cytokine storm. Altogether, our findings indirectly confirm the observed low prevalence of asthmatics among patients admitted due to COVID‐19 pneumonia. 10 , 11 Of course, we cannot exclude that other factors such as treatment with inhaled corticosteroids might have played a role in our observation of a milder disease among atopic patients, particularly in the light of the recent finding of effective dexamethasone protection against severe pneumonia. However, all our patients, both atopic and nonatopic underwent systemic corticosteroid treatment during their hospital stay, and most atopic patients were not asthmatic and hence did not use inhaled corticosteroids before their admission.
In conclusion, atopic status seems to protect against the most severe, often fatal consequences of SARS‐CoV‐2 infection. Such finding may be of help for future studies investigating how to limit the clinical consequences of this infection.
CONFLICT OF INTEREST
No author has conflicts of interest to declare.
Supporting information
App S1
REFERENCES
- 1. Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727‐733. [e‐pub]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Soresina A, Moratto D, Chiarini M, et al. Favorable outcome of COVID19 in two patients with X‐linked agammaglobulinemia. Pediatric Allergy Immunol. 2020. 10.1111/pai.13263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15:271‐282. [DOI] [PubMed] [Google Scholar]
- 4. Berger A. Th1 and Th2 responses: what are they? BMJ. 2000;321(7258):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Callow KA, Tyrrell DA, Shaw RJ, Fitzharris P, Wardlaw AJ, Kay AB. Influence of atopy on the clinical manifestations of coronavirus infection in adult volunteers. Clin Allergy. 1988;18:119‐129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jackson JJ, Busse WW, Bacarier LB, et al. Association of respiratory allergy, asthma and expression of the SARS‐CoV‐2 receptor, ACE2. J Allergy Clin Immunol. 2020;146(1):203. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus‐infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497‐506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signalling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci USA. 2014;111(10):3799‐3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carli G, Cecchi L, Stebbing J, Parronchi P, Farsi A. Is asthma protective against COVID‐19? Allergy. 2021;76:866‐936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Heffler E, Detoraki A, Contoli M, et al. COVID‐19 in severe asthma network in Italy (SANI) patients: clinical features, impact of comorbidities and treatments. Allergy. 2020. 10.1111/all.14532 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
App S1


