Abstract
Alzheimer’s disease (AD) is the most prevalent neurodegenerative disorder related to age, characterized by the cerebral deposition of fibrils, which are made from the amyloid-β (Aβ), a peptide of 40–42 amino acids. The conversion of Aβ into neurotoxic oligomeric, fibrillar, and protofibrillar assemblies is supposed to be the main pathological event in AD. After Aβ accumulation, the clinical symptoms fall out predominantly due to the deficient brain clearance of the peptide. For several years, researchers have attempted to decline the Aβ monomer, oligomer, and aggregate levels, as well as plaques, employing agents that facilitate the reduction of Aβ and antagonize Aβ aggregation, or raise Aβ clearance from brain. Unluckily, broad clinical trials with mild to moderate AD participants have shown that these approaches were unsuccessful. Several clinical trials are running involving patients whose disease is at an early stage, but the preliminary outcomes are not clinically impressive. Many studies have been conducted against oligomers of Aβ which are the utmost neurotoxic molecular species. Trials with monoclonal antibodies directed against Aβ oligomers have exhibited exciting findings. Nevertheless, Aβ oligomers maintain equivalent states in both monomeric and aggregation forms; so, previously administered drugs that precisely decrease Aβ monomer or Aβ plaques ought to have displayed valuable clinical benefits. In this article, Aβ-based therapeutic strategies are discussed and several promising new ways to fight against AD are appraised.
Keywords: Aβ, tau, Alzheimer’s disease, amyloid precursor protein, aducanumab, BAN2401
1. Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease, and globally, over 46 million people are affected by this devastating disease [1,2]. AD causes irreversible mental and cognitive deficiency including memory loss, personality disorder, and intellectual abnormality in patients older than 65 years [3,4]. Central sensory systems including the visual system are also affected during the advanced stages of the disease [5]. Collectively, complications of AD diminish the lifespan, hamper the quality of life, and cause physical impairment [6], which finally appears as a terrible difficulty in normal life activities [7]. To decrease the social and economic costs and the burden of the disease on patients and their families, recently, several attempts have been made to identify disease diagnostic markers [8]. Neuroimaging methods, such as magnetic resonance imaging and positron emission tomography, have been developed enabling researchers to diagnose AD at the early stages of the disease. Moreover, distinct biomarkers, which are essential to figure out the pathological characteristics of AD, have been observed in the cerebrospinal fluid (CSF) [9,10]. The advancement of AD is associated with three cardinal neuropathological features such as extracellular deposition of amyloid-β (Aβ) to produce neuritic plaques, intracellular neurofibrillary tangles (NFTs) and synaptic degeneration [11,12,13]. These pathological alterations arise in the neocortex, hippocampus, and other subcortical regions that are crucial for cognitive functions [14].
AD has been diagnosed on the basis of medical history, mental status tests, clinical findings, and brain imaging. Indeed, AD can exactly be identified only after death via relating clinical measures with an investigation of brain tissues upon autopsy. Currently, available therapies are mainly symptomatic and ineffective against the development of the disease [15]. Now, cholinesterase inhibitors [16] and the N-methyl-d-aspartate (NMDA) receptor antagonist memantine [17] merely represent the receivable options. Regardless of the enormous number of studies in AD pathogenesis, varieties of drug candidates entered into clinical trials, but no novel drug has been approved since memantine in 2003 [17]. Several arguments have been proposed to illustrate this failing, including an irrational selection of patients, various disease progression rates, minimal dosing, drug exposure, target engagement, improper time of intervention, inaccurate result measurements, and less effectiveness of clinical scales. Besides, imperfect perception regarding AD pathophysiology may facilitate the choice of vague targets.
Aβ has been considered as the foremost risk factor that playing a vital role in the initiation and progression of AD [18,19,20,21]. Aβ is generated into the typical individual, but in some instances, this peptide leads to aggregation, the starting point of disease progression. Many findings elucidate that Aβ oligomers might play a central role in neuronal dysfunction and AD [22,23]. Bennett et al. [24] reported that Aβ can increase tau pathology via elevating the generation of tau species that have the capacity to seed new aggregates. It was also observed that heterotypic seeded tau via pre-aggregated Aβ provides efficient seeds for prion-like initiation and propagation of tau pathology in vivo [25]. In tau-transgenic mouse models, NFTs formation was found to be increased due to the intrahippocampal infusion of synthetic Aβ fibrils, Aβ-rich extracts or pre-aggregated Aβ [25,26,27], which are similar to the activities observed in double-transgenic mouse models presenting both tau and Aβ pathology [28,29]. Human derived-tau injections in mouse models with a great Aβ plaque load boosted tau pathology, which is further indicating that Aβ plaques may induce tau propagation [30]. In this article, we have critically reviewed the recent studies targeting Aβ in Alzheimer’s pathogenesis.
2. Biomarkers for Alzheimer’s Disease
Decreased Aβ1-42 levels in the CSF can take place due to decreased Aβ clearance from the brain to the blood/CSF, along with increased deposition and aggregation of plaques in the brain. Alterations in Aβ levels in CSF vary in relation to the nature of the disease [31,32,33]. For instance, reduced levels of Aβ1-37 correlate with Lewy body dementia and Aβ1-38 levels with frontotemporal lobar degeneration [34]. In case of AD, levels of shorter Aβ1-40 forms remain unaffected or increased. Thus, evaluating the ratio of Aβ1-42/Aβ1-40 can improve AD diagnosis, but others have not observed such alterations [35,36]. Novel identification processes allow the measurement of Aβ oligomers, which may ameliorate the specificity of the diagnostic. For example, surface-enhanced laser desorption/ionization-time-of-flight-mass spectrometry has appeared as a valuable approach for the simultaneous identification and quantitation of various products generated through Aβ cleavage [37].
NFTs are another AD hallmark composed of highly phosphorylated forms of the microtubule-associated proteins tau [38,39]. Total tau levels in CSF elevate with age [40] such as < 300 pg/mL (i.e., 21–50 years), < 450 pg/mL (i.e., 51–70 years), and < 500 pg/mL (i.e., > 70 years) in healthy controls. Levels of total tau are considerably increased in AD individuals in comparison with the age-matched control participants with a cut off of > 600 pg/mL. Levels of total tau are also significantly increased in Creutzfeldt–Jakob disease (>3000 pg/mL). Levels of tau may also be a prognostic marker with a decent predictive validity for conversion from mild cognitive impairment (MCI) to AD since a high level of tau in CSF has been observed in 90% of MCI cases that later advance to AD, but not in stable MCI cases [41]. Study of other phosphorylated tau forms (i.e., phospho-tau-199, -231, -235, -396 and -404) may also provide substantial enhancements in early AD diagnosis [42].
Mo et al. [43] revealed that CSF levels of Aβ1-42 were decreased in AD as compared to non-AD dementia or controls. Nevertheless, the sole determination of the CSF levels of Aβ1-42 is not sufficient for reliable differential AD diagnosis. More studies are required based on the usage of biomarker combinations, such as Aβ1-42 levels in combination with other markers (e.g., tau, phosphorylated tau, Aβ1-40, total Aβ) to develop CSF biochemical measurements allowing reliable diagnosis of AD versus other non-AD cognitive deficits. In fact, total tau and phosphorylated tau levels rise in the CSF of AD subjects in comparison with the aged-matched subjects having normal cognition [44]. Positron-emission tomography (PET) studies revealed that accumulation of tau follows Aβ deposition in young individuals with autosomal dominant AD. In their study, Quiroz et al. [45] revealed that increased levels of tau were observed in medial temporal lobe areas in carriers of unimpaired PSEN1 E280A mutation in their late 30s, and that noticeable formation of tau tangle in neocortical areas was seen in one cognitively unaffected carrier as well as in individuals with mild cognitive impairment. In addition, these results also indicated that PET imaging of tau might be beneficial as a biomarker to differentiate people at high risk to develop clinical AD symptoms and to monitor the progression of the disease [45].
Aβ levels of blood plasma are elevated in Down syndrome and familial AD (FAD), though findings are inconsistent with sporadic AD [34]. It has also been revealed that concentrations of Aβ1-40 and Aβ1-42 can be decreased, increased or even unaltered in AD as compared to control individuals [31,34]. There was a noticeable rise in the levels of Aβ1-42 in plasma in females with MCI, but not in males, in comparison with age-matched, cognitively normal subjects [46]. It has been found by longitudinal studies that high concentrations of Aβ1-42 are also a risk factor for the development of AD, being this factor however not specific and sensitive for early AD diagnosis [47]. In AD, reduced levels of serum Aβ1-42 autoantibodies have also been observed [48], though no correlation has still been observed between Aβ levels in plasma and CSF. Antigen-spotted microarrays might be useful to validate and detect AD-selective biomarker autoantibodies in CSF and blood. Such a rapid method has also been developed for detecting differences between dissociated sera and the corresponding non-dissociated sera [49].
3. Amyloid Cascade Hypothesis
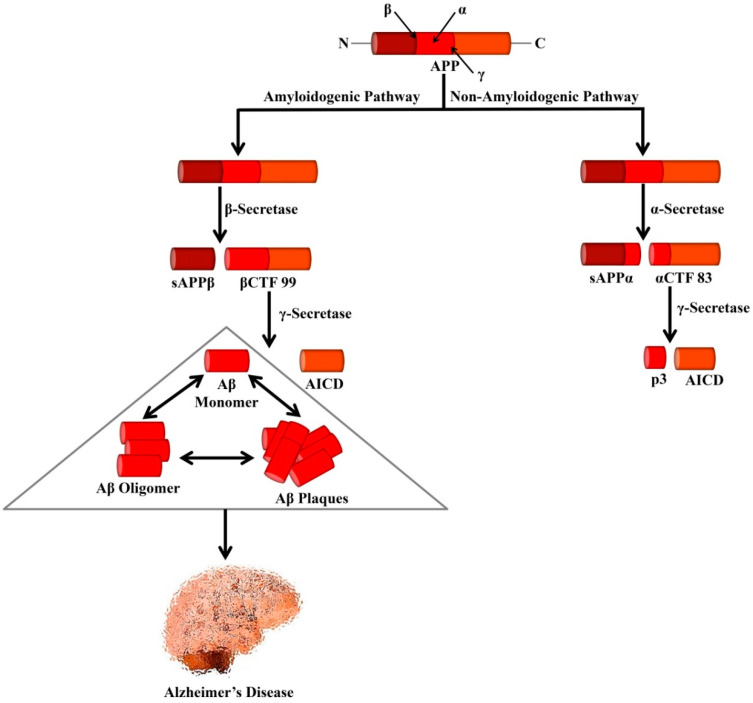
The amyloid precursor protein (APP) is a type I transmembrane glycoprotein containing 695–770 amino acids [50]. It is regarded that abnormal proteolytic processing of APP leads to the generation of Aβ [51]. In the non-amyloidogenic pathways, APP is cleaved by α- and γ-secretases. It is known that α-secretase is a metalloprotease (ADAM) which is localized to the Golgi complex and the cell membrane. This α-secretase can cleave APP at residue L688, located in the middle of the Aβ domain [52]. α-secretase causes APP cleavage leading to the formation of soluble APP alpha (sAPPα) and a cell-membrane-bound C-terminal fragment 83 (CTF83). The generated CTF83 is cleaved by γ-secretase to produce AICD and a small p3 fragment.
In the amyloidogenic pathways, APP is cleaved by β- and γ-secretases [50]. β-secretase (BACE1) belongs to the aspartic protease family and is around 500 residues in length containing 2 active sites situated at the lumenal side of the membrane [52]. The β-secretase enzyme possesses high sequence specificity [53,54], a feature that matches with BACE1, which can cleave APP at the N-terminal of the Aβ domain, either at residues Glu682 or Asp672 [52]. BACE1 also causes APP cleavage and generates soluble APP β (sAPPβ) and a cell-membrane-bound C-terminal fragment 99 (CTF99).
On the other hand, γ-secretase is an enzymatic complex of four proteins including presenilin enhancer 2, presenilin (PSEN), anterior pharynx defective 1, and nicastrin. Indeed, each of the subunits is considered as an effective therapeutic for elevating Aβ clearance or controlling Aβ generation. Within the membrane, γ-secretase causes cleavage of βCTF99 that leads to the formation of the APP intracellular domain (AICD) and Aβ peptides. Various isoforms of Aβ isoforms are available that contain a variable length of amino acid residues including Aβ1–40 (i.e., the most abundant) and Aβ1–42 (i.e., the less soluble isoform). Aggregation of Aβ triggers the generation of protofibrils, fibrils, and oligomers, which eventually can lead to the formation of plaques, which are well-known as one of the major hallmarks of AD pathology as shown in Figure 1.
Figure 1.
Processing of APP by secretases that leads to the formation of Alzheimer’s-associated Aβ peptides. In the amyloidogenic pathway, APP is cleaved by β- and γ-secretases leading to the formation of Aβ peptides and AICD. Formation of the neurotoxic Aβ is the major cause of AD. In the non-amyloidogenic pathway, APP is cleaved by α- and γ-secretases leading to the genesis of p3 and AICD. APP, Amyloid precursor protein; sAPPα, Soluble APP alpha; sAPPβ, Soluble APP beta; αCTF 83, Alpha C-terminal fragment 83; βCTF 99, Beta C-terminal fragment 99; AICD, APP intracellular domain.
It is believed that in the AD process, Aβ accumulation in the brain is one of the events that take place initially [55]. Accumulation of Aβ also initiates in the entorhinal cortex and hippocampus. Furthermore, in NFTs, intracellular hyperphosphorylated tau deposition can cause disruption in axonal transport and progressive alterations in the cytoskeleton. In 1991, several independent groups initially proposed the theory that Aβ accumulation is the major characteristic of AD pathogenesis [56,57,58]. After one year, Hardy and Higgins [59] formally proposed the ‘amyloid cascade hypothesis’. As per the hypothesis, the deposition of Aβ in the brain can trigger several events including cognitive deficit, neuronal death, synaptic loss, formation of NFTs, and phosphorylation of tau.
The amyloid cascade hypothesis was further strengthened by the fact that AD may take place due to the autosomal dominant mutations in the APP gene and these mutations in PSEN1 and PSEN2 can elevate the Aβ generation and ultimately mediate the generation of Aβ aggregates and deposits [60]. Transgenic mouse models that express forms of PSEN proteins or APP containing mutations linked with human FAD progressively show the development of memory impairments and Aβ plaques in the brain, which further strengthens the hypothesis that buildup of Aβ can trigger AD [61]. Mutations in PSEN seem to be the major cause of FAD with over 150 causative mutations that have been mapped to the genes (PSEN1 and PSEN2) encoding the PSEN proteins [62,63]. Most of these mutations seem to elevate the generation of Aβ1–42 over Aβ1–40 by mediating cleavage at residue 639 of APP over residue 637 [64]. Increased activity of BACE1 [65] and defective clearance [66] are found to contribute to the buildup of Aβ in the brain in late-onset sporadic AD (SAD). The buildup of Aβ is also found to be associated with the apolipoprotein E ε4 (APOE ε4) allele, which is regarded as the strongest genetic risk factor for late-onset SAD.
It has been revealed by post-mortem analyses that healthy elderly individuals can possess extensive amyloid pathology [67]. Moreover, brain imaging analyses showed that Aβ pathology was observed in up to 44% of cognitively healthy older people [68]. As compared to the individuals without amyloid pathology, a faster deficit in brain glucose metabolism, brain volume, and cognitive performance was experienced by individuals with amyloid deposits at baseline [69,70,71,72,73]. By 15–20 years, it has been estimated that the deposition of Aβ will precede the clinical AD symptoms [70].
4. Proteolytic Fragments of Amyloid Precursor Protein—CTF99 and sAPPα
Various studies have revealed that CTF99 generally accumulates within lysosomal, endosomal, and autophagic structures [74,75], which also correspond to the main intracellular sites for amyloidogenic APP processing [76]. In the 3xTgAD mouse model, accumulation of CTF99 did not take place due to elevated β-secretase or decreased γ-secretase cleavages, but rather because of an early lysosomal deficit [74]. Accumulation of CTF99 was also found to be similar in 3xTgAD and 2xTgAD (APPswe, TauP301L) mouse models, though the latter showed little if any, Aβ as estimated from the absence of mutated PSEN1 [74]. In line with the lysosomal dysfunction in these two mouse strains, CTF99 accumulated within aberrantly large lamp- and cathepsins-positive structures, which number being also raised in CTF99-positive neurons [74]. Findings from mouse models revealed that the accumulation of CTF99 may play a role in this pathology. Pharmacological suppression of γ-secretase in young animal models not only resulted in elevated levels of CTF99 but also worsened lysosomal dysfunction [75].
In CTF99-expressing mouse models, a study has studied the hippocampal long-term potentiation (LTP) to analyze the effect of CTF99 in synaptic alterations [75]. Interestingly, as compared to control mice infected with control virus, hippocampal LTP was found to be considerably decreased in young CTF99-expressing mouse models. Suppression of γ-secretase did not rescue LTP alterations, which is suggesting that CTF99 instead of Aβ, induced these activities [75].
Indeed, sAPPα shows a remarkable neuroprotective effect. Numerous activities of sAPPα have been highlighted through in vitro studies. Long-term survival of cultured cortical neurons can be enhanced by sAPPα and it is assumed that it has a significant contribution in protecting cultured neuroblastoma cells against glutamate toxicity [77], since it can protect cultured neuronal cells against metabolic, excitotoxic, and oxidative damages [78,79]. Findings of in vivo studies are in line with those of in vitro studies. In addition, sAPPα can also induce cortical synaptogenesis and neurite outgrowth [78,79,80]. In the subventricular zone of the lateral ventricle in adult mouse models, sAPPα functions together with epidermal growth factors to play a role as a growth factor for neuronal progenitor cells [81], which is further indicating the activity of sAPPα in adult neurogenesis as these cells hold the ability to generate new neurons during adulthood.
In a transgenic AD mouse model and cell culture, BACE1 modulation through sAPPα led to a decreased level of Aβ generation, and plaques [82]. Furthermore, it was also demonstrated that sAPPα can play a role as an endogenous inhibitor of BACE1. It was also confirmed that sAPPα reduces the BACE1 activity via binding with its allosteric site [83]. Moreover, sAPPα suppressed the activity of the glycogen synthase kinase 3beta (GSK3β) and BACE1 by acting through unknown receptors, which eventually resulted in decreased tau phosphorylation [84].
5. Crosstalk of Aβ and Tau
Molecular, genetic, and neuropathological data indicate that AD pathology can be mediated by the tau protein. Pathology of tau is associated with AD severity and duration [85,86,87] and also with the neuronal loss [88,89]. Furthermore, tau pathology also facilitates the association between the occurrence of AD and load of brain Aβ [86], which is evident in the entorhinal cortex in individuals with subjective memory complaints [90]. Without the presence of Aβ, deposition of tau in the hippocampus may be ineffective in inducing the neurodegenerative mechanisms which can cause AD [91]. In individuals with SAD [92] or FAD [93], longitudinal analyses have revealed that the levels of tau in CSF increase in the early stages of the disease but fall once the symptoms appear. Outcomes of a study involving stable isotope labeling kinetics analysis concluded that the pathology of Aβ can increase tau production [94]. However, these results contradict the hypothesis that increased CSF levels of tau in individuals with AD can take place initially from dying and dead neurons. A certain type of tau aggregate formation can be induced by the neuritic Aβ plaques, which can lead to the generation and distribution of neuropil threads and NFTs [30]. Increased tau levels and decreased Aβ1–42 levels are linked with the development of AD in individuals with MCI [95].
Post-mortem examinations based on confirmed AD cases have revealed what are the main cell types and functional systems influenced by the disease, though the primary sites of the pathology remain still not clear. As per the modified and original Braak staging protocols [96,97], primary sites of tau pathology are located within the transentorhinal and entorhinal cortex (stage I), propagated into the hippocampus (stage II), temporal cortex (stage III) and ultimately into other cerebral cortex areas (stage IV), eventually reaching the visual association cortex region (stage V) and primary visual cortex area (stage VI). In contrast, the order of Aβ plaque deposition [98] initiates within neocortical areas, especially within the temporal lobe region (phase 1), and reaches allocortical areas including amygdala and hippocampus (phase 2), and after that into subcortical areas (phase 3), brain stem (phase 4) and eventually cerebellum (phase 5) [99].
Tau interacts through its amino-terminal projection domain with the Fyn kinase (Fynomers) [100]. Fyn can phosphorylate the NMDA receptor subunit 2 to mediate the interaction of the NR complex with the postsynaptic density protein 95 (PSD-95) [101,102,103], connecting NRs to synaptic excitotoxic downstream signaling [104]. Without affecting synaptic NMDA currents, disturbance of the NR/PSD-95 interaction averted excitotoxic injury in a rat model of stroke and cultured neurons [105]. In APP transgenic mouse models, Fyn reduction prevented Aβ toxicity, whereas its overexpression enhanced toxicity [106,107].
In their study, Ittner et al. [12] generated transgenic mice (Δtau74) that only express the amino-terminal projection domain of tau and crossed them with Aβ-forming APP23 and tau−/− mice to identify how tau can cause Aβ toxicity. It was observed that tau possesses a dendritic activity in postsynaptic targeting of the Src kinase Fyn, a substrate of which is the NMDA receptor. Tau missorting in transgenic mouse models expressing truncated tau (Δtau) and absence of tau in tau−/− mouse models both disrupted postsynaptic Fyn targeting, which further uncoupled NMDA receptor-induced excitotoxicity and henceforth mitigated Aβ toxicity. Δtau expression as well as tau deficiency prevent memory impairments and ameliorates survival in Aβ-forming APP23 mouse models. Furthermore, these impairments were also completely rescued with a peptide that uncouples the Fyn-induced in vivo interaction of PSD-95 and NMDA receptors. These results indicate that the dendritic activity of tau confers Aβ toxicity at the postsynapse level with direct involvement in AD pathogenesis and treatment [12].
Kim et al. [108] have confirmed that CTFs such as C31 and AICD [C57, C59] induce neurotoxicity on rat primary cortical neurons and differentiated PC12 cells via the stimulation of the expression of GSK3β, generating a ternary complex with CP2/LSF/LBP1 and Fe65 in the nucleus, while a point mutant and deletion mutants with Y682G of the YENPTY domain, a Fe65 binding domain, do not. Besides, expression of APP770 and the Swedish mutant form of APP elevated CTFs in neuronal cells and also stimulated GSK3β up-regulation at both the protein and mRNA levels. Furthermore, the CP2/LSF/LBP1 binding site in human GSK3β promoter region is important for the stimulation of gene transcription by CTFs. The neurotoxicity mediated by CTFs (i.e., AICD and C31) was accompanied by a rise in the active form of GSK3β, and trough the stimulation of tau phosphorylation as well as a decrease in the levels of nuclear beta-catenin, resulting in apoptosis [108].
6. Therapeutic Targeting of Aβ in Alzheimer’s Pathogenesis
6.1. Decreasing Aβ Production
6.1.1. α-Secretase Activators
Since α-secretase processing of APP includes cleavage within the Aβ peptide sequence, preventing the formation of Aβ, activation of APP α-secretase cleavage is regarded as an effective AD treatment [109]. Multiple drugs are currently in use for AD treatment that can elevate the effect of α-secretases by activating related signaling cascades and this has been regarded as the best therapeutic technique, so far [110,111,112]. On the other hand, selegiline (i.e., a selective inhibitor of monoamine oxidase) was utilized to slow the progression of AD, which has been found to elevate the activity of α-secretase through a protein trafficking-related process [113,114]. Following the observation that the chronic use of statin might be protective, atorvastatin was employed for AD treatment, inducing α-secretase activation [115,116]. Feldman et al. [117] reported in a large-scale randomized controlled trial of 640 mild to moderate AD patients, that atorvastatin was not associated with a significant clinical benefit over 72 weeks.
Numerous drugs anticipated as indirect activators of α-secretase have moved to the clinical trial stage for AD. Among these drugs, etazolate (EHT 0202, a modulator of GABA receptor) was found to be the most prominent one and has reached phase II clinical trials based on the observation that it stimulated sAPPα generation and provided protection against Aβ mediated toxicity in rat cortical neurons [118,119]. Later EHT 0202 failed to show any significant cognitive or clinical benefit in an over 3-month, double-blind, placebo-controlled study in 159 mild to moderate AD patients [118]. It was reported in 2008 that PRX-03140 (i.e., an agonist of 5-HT4) can induce α-secretase and may exhibit positive outcomes in phase II clinical trial (NCT00693004) with an enhancement of the cognitive functions in AD individuals, though these experiments were not followed by additional studies [120]. Epigallocatechin-gallate (EGCG), which is a polyphenolic compound extracted from green tea, induces α-secretase activity via the protein kinase C (PKC) signaling pathway and decreases cerebral amyloid deposition in AD mouse models [121,122,123]. The benefits of this compound in AD are being studied in phase II/III trials (NCT00951834) [123]. Bryostatin (i.e., a strong modulator of PKC) can elevate the release of the α-secretase product, sAPPα. However, in a phase II study, bryostatin failed to show any significant cognitive or clinical benefit in a 12-week, double-blind, placebo-controlled study in 150 advanced AD patients [124].
6.1.2. β-Secretase Inhibitors
As stated earlier, the protease BACE1 is accountable for the primary cleavage of APP, which increases the production of Aβ [125,126]. The relationship between Aβ decline and BACE1 inhibition was reported in numerous studies with BACE1 knock-out mice [127,128]. Furthermore, it was observed that inhibition of BACE1 rescued Aβ-driven cholinergic dysfunction and improved memory deficits in APP transgenic mice [129,130].
Several BACE1 inhibitors containing properties including regulation of insulin metabolism are derived from approved drugs that are used in type 2 diabetes. Elevation of ubiquitination and inhibition of β-secretase to degrade the amyloid load can be achieved via peroxisome proliferator-activated receptor gamma (PPAR-γ) activation by thiazolidinediones [131]. Reduction of the peripheral insulin resistance can be achieved by PPARγ agonists, including thiazolidinedione derivatives (i.e., pioglitazone and rosiglitazone) [132], which can mediate aggravation of neuropathology of AD. This weakening of insulin sensitivity can help in proteolysis of Aβ. Nonetheless, pioglitazone failed to show cognitive or clinical benefits in a large prevention trial (NCT01931566) owing to the lack of efficacy of the drug and no safety concern [133]. On the other hand, in 2 double-blind, placebo-controlled trials, rosiglitazone failed to show any clinically significant efficacy in cognition or global function in mild to moderate AD patients for 48 weeks [134].
There is an ongoing investigation of several novel drugs. CTS-21166, which displays measurable brain penetration properties, lessened the plasma levels of Aβ and showed good tolerance in healthy individuals [135,136,137]. Findings from the phase I trial indicated that CTS-21166 is safe when injected intravenously in AD individuals at doses as high as 225 mg. Findings revealed a dose-dependent decrease of Aβ concentrations in plasma for a prolonged time [138]. A marked decrease in the Aβ levels in plasma lasted over 72 h [139]. Similar observations were also achieved from a second phase I clinical trial on participants receiving an oral liquid solution of 200 mg CTS-21166 [139]. Despite these initial encouraging results, CTS-21166 has not moved further in clinical trials and the 6-year collaboration between Astellas Pharma and CoMentis for developing and commercializing CTS-21166 was terminated in 2014 [140].
BI 1181181 (i.e., an orally active BACE1 inhibitor) is the first generation of BACE1 inhibitors that failed in phase I clinical trials due to their low blood-brain barrier penetration and low oral bioavailability. Subsequently, the second generation of BACE1 inhibitors including LY2886721, LY2811376, and RG7129 also failed in clinical studies owing to liver toxicity [141]. On the other hand, the third-generation of BACE1 inhibitors including JNJ-54861911, CNP520, and AZD3293 was discontinued because of the worsening of cognitive performances compared to placebo. Even though verubecestat (MK-8931) decreased Aβ levels in the central nervous system (CNS) of animal models and AD individuals [142] upon a 104-week, randomized, double-blind, placebo-controlled trial, this compound failed to improve clinical ratings of dementia among patients with prodromal AD [143].
6.1.3. γ-Secretase Inhibitors
Like BACE1, Aβ generation is a result of APP cleavage, which is mediated by γ-secretase. Henceforth, γ-secretase is regarded as a principal target in AD therapy [144,145]. This enzyme complex is involved in various physiological processes [146]. In addition to APP, there are copious substrates in the human body that γ-secretase can react with; many of them are neuronal substrates [147]. On the other hand, cleavage of Notch 1 is also done by γ-secretase, which can ultimately lead to Notch intracellular domain release and can consequently translocate to the nucleus to cause gene regulation associated with cell survival, determination of cell fate, and cell development [148]. Henceforth, to avoid the drawbacks related to abnormalities of Notch-signaling, γ-secretase inhibitors should be carefully designed. Frequently observed adverse effects of γ-secretase inhibition include changes in hair color [149], skin reactions [150,151], gastrointestinal [152], and hematological [153] toxicity. Some inhibitors of the γ-secretase have been tested during clinical studies and most of them have been found to decrease Aβ production in CSF or plasma. Nonetheless, few of them effectively avoided the side-effects induced by Notch.
A decrease in the levels of Aβ in plasma and downregulation of Aβ production in the CNS have been achieved with semagacestat (LY450139), [154] a γ-secretase inhibitor which has been forwarded to phase III clinical trials. In phase I trials, a dose-dependent reduction in the synthesis of Aβ was observed in CSF [154]. Conversely, in phase II clinical trials, side effects related to the skin were observed. However, there was a significant decrease in the plasma Aβ level; a similar trend was not noticed in CSF and no momentous activities on function and cognition were reported. Two essential phase III clinical trials were hesitantly commenced, and subsequently discontinued as a result of a lack of efficacy, increased risk of the skin infection, and cancer [155]. Fall of semagacestat and recurrent unsatisfactory results with other γ-secretase inhibitors suggested the idea that an interaction between the four subunits of the γ-secretase and their substrates is needed.
While showing target specificity, different γ-secretase inhibitors have exhibited suitable interactions with the γ-secretase subunits. Sulfonamide and MRK-560 based γ-secretase inhibitors predominantly inhibit PSEN1 rather than PSEN2, whereas L-685458 and DAPT displayed the lowest selectivity [156,157]. Even though the possibility of drug design still appears challenging, heterogeneity of Aph1 is important for the survival of individuals, which further recommends that targeting the Aph1b γ-secretase particularly would be better tolerated [158]. Therefore, selective inhibition of specific sites via the next-generation inhibitors of Notch-sparing γ-secretase came into focus and NIC5-15, begacestat (GSI-953), and avagacestat (BMS-708163) represent such inhibitors under clinical study. Moreover, it was found that, avagacestat possesses 137 times more selectivity over Notch for APP in cell cultures. In rats and dogs, avagacestat strongly decreases the levels of Aβ in CSF without producing any Notch-related toxicity [159]. Avagacestat was primarily supposed to play a role as an inhibitor of Notch-sparing γ-secretase. Nonetheless, further experiments indicated this to be false [160]. In 2010, due to liver toxicity, a clinical trial for the Notch-sparing inhibitor ELND006 (Elan Corporation) was terminated [161]. Tarenflurbil (i.e., a γ-secretase modulator) exerted positive effects on cognitive functions in phase II clinical trial but was stopped following phase III clinical trials because of poor outcomes [162,163,164]. CHF-5074 (i.e., Chiesi’s γ-secretase modulator) reached phase II clinical trials, but experiments were terminated for unrevealed causes [165]. All drugs, including Notch-sparing compounds, were discontinued for lack of efficacy and poor tolerability.
A decrease in the concentration of Aβ in the plasma, but not in CSF was noticed with a second Notch-sparing inhibitor, begacestat [148,166,167]. A phase I clinical trial in association with donepezil has also been carried out but additional data regarding this trial are not available [167]. On the other hand, NIC5-15 or pinitol, a naturally-occurring cyclic sugar alcohol displays good safety and tolerance and is presently under a phase II clinical trial [168,169].
6.2. Blocking Aβ Aggregation
6.2.1. Anti-Amyloid Aggregators
Tramiprosate is a small and orally-administered aminosulfonate compound that has the ability to bind with Lys28, Lys16, and Asp23 of Aβ42 [170]. Interestingly, this binding can lead to Aβ42 monomer stabilization, further decreasing fibrillar (plaque) and oligomeric amyloid aggregation. It was reported that suppression of oligomer generation and elongation can exert neuroprotective effects against Aβ-mediated subsequent deposition [171,172,173,174]. In a transgenic AD mouse model, it was seen that when cyclohexanehexol stereoisomers were administered orally, Aβ aggregation into high-molecular-weight oligomers was suppressed in the brain and improvement of various AD-like phenotypes in the mouse models were observed [175]. Based on good safety and tolerance profile, scyllo-inositol (i.e., a cyclohexanehexol stereoisomer) is being studied in phase II clinical trials with mild-to-moderate AD individuals [176]. On the other hand, EGCG was also found to induce the generation of nontoxic, unstructured Aβ aggregates by suppressing its structural alteration from a random-coil to a β-sheet structure [177]. The generated small Aβ oligomers in the presence of EGCG were harmless and unable to seed further fibril growth [178]. Curcumin which is a diphenol extracted from the rhizomes of Curcuma longa (turmeric), reduced the concentrations of soluble and insoluble Aβ and plaque burden in Tg2576 transgenic mouse models [179]. Curcumin can bind with both Aβ fibrils and oligomers [180]. Furthermore, it is believed that curcumin can also suppress the oligomerization of Aβ, mediate the nontoxic Aβ fibrils deposition, and reduce the neurotoxicity of Aβ aggregates [181,182]. Therefore, it might be sufficient enough to accelerate or change the pathway from toxic Aβ oligomers to nontoxic Aβ forms. Moreover, curcumin also induces the conversion of Aβ fibrils by decreasing the prefibrillar Aβ species and thus alleviates toxicity of Aβ in the transgenic Drosophila model [183]. However, curcumin in a 24-week, double-blind, placebo-controlled trial in mild to moderate AD patients failed to demonstrate any biological or clinical effects [184].
Resveratrol is a non-flavonoid polyphenol that is abundantly found in red wine and many plants [185]. Multiple studies have suggested that the neuroprotective activity of resveratrol on AD is credited to several factors including proteasome-mediated intracellular Aβ degradation [186], reduction in secreted and intracellular Aβ through inhibition of BACE1 [187], and reduction of Aβ-induced toxicity [188]. Furthermore, resveratrol can decrease the accumulation of extracellular Aβ by elevating the activity of the AMP-activated protein kinase (AMPK) to induce autophagy and lysosomal degradation of Aβ [189]. In a 52-week, double-blind, placebo-controlled study in 119 mild-to-moderate AD patients, the brain volume loss was increased by a resveratrol treatment compared to placebo [190]. The clinical effect trajectories of resveratrol remain uncertain in another 12-month study in mild-to-moderate AD [191]. Finally, flavonoids including quercetin and myricetin showed neuroprotective activities in AD via several mechanisms including inhibition of BACE1 activity [192], activation of AMPK [193], and antioxidative effects [194,195]. Quercetin has a strong capacity to suppress the formation of Aβ fibrils and protect neuronal cells from Aβ-mediated toxicity [196]. On the other hand, myricetin prevented conformational alteration of Aβ from a random-coil to a β-sheet-rich structure, and its suppressive activity against aggregation of Aβ was directly proportional to the number of hydroxyl groups on the B-ring [197].
6.2.2. Metal Chelators
Aβ-metal adducts and metal ions can produce toxic radical species that have the ability to modify biomolecules, which can further lead to neuronal death [198]. Increased concentrations of redox-active metal ions such as copper (Cu)/iron (Fe) might lead to mitochondrial dysfunction, DNA damage, and reactive oxygen species (ROS) generation [199]. Furthermore, these metal ions can cause neuronal cell damages via mediating the generation of ROS or nitrogenated species (RNS) containing unpaired electrons, primarily characterized by peroxynitrite, nitric oxide, lipid peroxyl and alkoxyl radicals, hydroxyl, and superoxide anion radical [200,201,202]. In their study, House et al. [203] revealed that Aβ1-42 alone or in the presence of Fe(III) or Al(III) generated β-pleated sheets of plaque-like amyloids which were dissolved following incubation with either ethylenediaminetetraacetic acid (EDTA) or desferrioxamine (DFO). Cu(II) prevented while Zn(II) suppressed the generation of β-pleated sheets of Aβ1-42. However, neither of these effects was influenced by incubation of the aged peptide aggregates with either EDTA or DFO. Existence of marked levels of Fe(III) and Al(III) as contaminants of Aβ1-42 preparation indicated that these two metals were associated with either inducing the generation or stabilizing the structure of β-pleated amyloid [203]. Khan et al. [204] evaluated the effect of Aβ1-42 aggregation in mediating the redox cycling of Fe(II) and the associated activities of companion metals, zinc (Zn), Cu, and aluminum (Al). It was mentioned that the main effects of Aβ1-42 in the redox cycling of iron were perhaps in binding Fe(III) and thus slowing its precipitation as redox-inactive Fe(OH)3(s). Additional aggregation state-specific binding of both Fe(III) and Fe(II) determined critical equilibrium associated with H2O2 generation via the superoxide anion (O2−) in favor of keeping Fe(II) in solution. Introduction of physiologically important levels of either Zn(II) or Cu(II) decreased the activity of Aβ1-42 in the Fe(II)/Fe(III) redox cycle, while a pathophysiologically important Al(III) level induced the redox cycle in favor of Fe(II) whether or not Zn(II) or Cu(II) were additionally present. Collectively, these findings suggest the idea that oxidative stress in the vicinity of senile plaques might take place due to Fenton chemistry catalyzed by the co-deposition of Aβ1-42 with various metals including Al(III) and Fe(II)/Fe(III) [204].
Henceforth, metal chelators that can impede Aβ burden, can be envisaged as a therapeutic approach. Instead, clioquinol (i.e., metal-induced Aβ inhibitors) can increase Aβ degradation via the redistribution of metal ions to neurons thus stimulating expression of metalloproteinases [205]. Evidence of the decrease in the concentration of Aβ1-42 in CSF and enhancement of behavioral and cognitive performances were noticed in phase II clinical trials of copper/zinc ionophore PBT2 [206]. Levels of Fe, Zn, and Cu are found to be high around and in amyloid plaques in AD brain [202,207,208,209,210]. Higher concentrations of Fe [211] and Zn [212] were also identified in the amyloid plaques of the Tg2576 (APPsw) mouse model for AD. Interestingly, Aβ shows high selectivity and low-affinity Cu2+- and Zn2+-binding sites that induce its aggregation via interactions with Zn2+, Cu2+, and to a lesser extent with Fe3+ in vitro [213,214,215]. Nuclear magnetic resonance and electron paramagnetic resonance studies suggested a model of monomer Aβ binding to a Cu ion via 3 histidine and tyrosine residues or via a bridging histidine for aggregated Aβ [216]. Raman spectroscopy of senile plaque cores confirmed that Zn and Cu ions are coordinated through histidine residues [210], which are situated at the N-terminal end of the Aβ sequence. Indeed, the affinity of Aβ variants for Cu2+ is highest for Aβ1-42 > Aβ1-40 > > rat Aβ, in association with toxicity and redox activity [214,215,217].
A significant association between amyloid formation and Cu levels has been reported by 2 complementary and independent studies using 2 different transgenic APP mouse models. Both of these studies stated reduced constitutive brain Cu levels [218,219], which is in line with earlier results [220]. Increased levels of Cu in the brain, either by the introduction of a mutant allele of the CuATPase7b Cu transporter [219] or by dietary Cu supplementation [218], caused a marked decrease in Aβ and amyloid plaque load and ameliorated mice survival. These observations indicate that increased Cu levels might trigger non-amyloidogenic APP processing, as confirmed previously in vitro [221].
6.3. Increasing Aβ Clearance
6.3.1. Aβ Vaccination
Aβ vaccination has been tested and found to be effective against cognitive impairment, cellular changes, and Aβ pathology in animal models of AD [222,223]. In PDAPP transgenic mice (i.e., which overexpress mutant human APP), most of the neuropathological AD hallmarks develop progressively in a brain-region- and age-dependent manner. It was noticed that a transgenic mouse following Aβ vaccination was found to overexpress a mutant form of the human APP, which is protected against the formation of amyloid plaque, neuritic dystrophy, and astrogliosis [224]. Besides, apart from protecting the aggregation of Aβ, it also helps in clearing the amyloids from the brains of adult mouse models [225].
Active Immunization
Active immunotherapies have been developed to activate an immune response in the human body of specific antibodies against Aβ [226]. Furthermore, active immunotherapies might be utilized to mediate anti-Aβ antibody responses in individuals with early stages of AD. Considering the potentially hazardous autoimmune responses because of newly activated Aβ1-42 specific inflammatory T cells, new peptide vaccines were developed in which the parts accountable for the activation of T cells were removed and only the parts required for Aβ production-specific antibodies persisted [227]. Currently, 3 of these peptide vaccines for active immunization including affitope, ACC001, and CAD106 are in phase II clinical trials [227]. In case of all 3 approaches, phase I trials exhibited positive antibody responses with no signs for adverse autoimmune inflammation and clinical trials of these active immunization therapies are being continued [227]. CAD106 has been developed to exert a powerful antibody response while escaping the activation of inflammatory T cells [228]. Interestingly, CAD106 can combine with multiple Aβ1-6 copies derived from the N-terminal B cell epitope of Aβ, coupled to a Qβ virus-like particle. CAD106 stimulated Aβ-antibody titers without activating the Aβ-reactive T cells in animal models. CAD106 administration resulted in a decrease of amyloid accumulation in the brain of APP-transgenic mouse models [229]. In patients with mild AD, CAD106 [230] has finished the phase II clinical trial and did not cause meningoencephalitis [231].
A different active immunization method was also established that mimics the N-terminus of Aβ. Affitope, AD01, and AD02 are keyhole limpet hemocyanin vaccines with a synthetic peptide of 6 amino acids that represent the natural Aβ sequence. In animal models, AD01 as well as AD02, mimic the N-terminus of Aβ [232]. Furthermore, the controlled selectivity averts cross-reactivity with APP, and the small size prevents autoreactive T-cell activation [233]. To examine tolerability and clinical/immunological activity, AD02 has been selected for development in phase II trial in early AD individuals [234]. It was found that AD02 could not meet its primary endpoints in early AD. IMM-AD04 (2 mg) vaccination alone (utilized in one of the 2 control arms) provided statistically significant slowing of global, functional, and cognitive decline as revealed by primary and secondary outcomes over 18 months [235]. ACC-001 is a conjugate of several short Aβ fragments associated with a carrier composed of inactivated diphtheria toxins. It was developed to avoid the safety issues linked with the prior AN1792 active vaccine against full-length Aβ1-42 [236]. However, ACC-001 was discontinued in phase II trials because of a strong autoimmune response [237,238,239].
Passive Immunization
Direct administration of antibodies is another approach to evade the immune response. Passive immunization is effective for the removal of amyloid plaques and rescuing glial and neuritic pathology [222]. Moreover, the decrease in early cytopathology [240] and hyperphosphorylation of tau [241], as well as the reversal of abnormal hippocampal synaptic plasticity [242] can also be achieved with the passive immunization. Rescue of synapse loss in brains of APP transgenic mouse models and Aβ plaques removal can be mediated by bapineuzumab (i.e., a humanized monoclonal antibody) [222]. In AD individuals, bapineuzumab was first used as passive immunotherapy. In 2 phase III clinical trials of bapineuzumab in mild-to-moderate AD individuals, Salloway et al. [243] stated that bapineuzumab did not ameliorate clinical outcomes in AD individuals, notwithstanding treatment differences in biomarkers seen in carriers of APOE ε4. Solanezumab is a humanized antibody as bapineuzumab. Nonetheless, solanezumab is targeted to an internal epitope of Aβ13-28. Furthermore, solanezumab exhibited superior binding to soluble Aβ rather than fibrillar Aβ. Early clinical trials exhibited minor enhancement in cognitive functions in individuals with moderate AD [244]. However, individuals with mild AD exhibited a 33% decrease in a rate of decline so a phase III clinical trial was started to analyze the treatment of mild AD individuals with solanezumab [245]. Moreover, early clinical trials revealed that solanezumab possesses similar efficacy in individuals without or with the APOE4 allele [246] as compared to the bapineuzumab trial. Solanezumab was discontinued for the treatment of sporadic AD.
Gantenerumab (RG1450, RO4909832) is the only fully developed human antibody. The most probable mechanism of the effects of this antibody seems to be the binding to small plaques and stimulation of a phagocytic response by microglia [247]. In clinical trials, when gantenerumab was administered to moderate AD individuals, it decreased the load of brain amyloid by up to 30% as estimated via a positron emission tomography scan, but 2 individuals experienced vasogenic cerebral edema in the high dose group [248]. In a phase II/III clinical trial (NCT01760005), Hoffmann La-Roche is working together with Eli Lilly run by Washington University School of Medicine. A randomized clinical trial (DIAN-TU-001) aims to evaluate gantenerumab and Lilly’s solanezumab in dominantly inherited AD patients (a rare, early-onset type of AD) [249,250,251]. This clinical study is estimated to be finished in March 2021. Crenezumab (a humanized antibody) is used to target Aβ with a modified human immunoglobulin G (IgG) isotype IgG4 to decrease the affinity for Fc receptor binding and decrease the risk of immune cell stimulation permitting higher dosing of the antibody as compared to that allowed with previous immunotherapy methods. It has been observed that crenezumab is likely to bind with various forms of Aβ including fibrillar, oligomeric, and monomeric forms [252]. Phase I clinical trials revealed better safety data and currently, crenezumab is entering into phase II clinical trial. Unlike other tested antibodies, ponezumab (another humanized IgG antibody) targets Aβ1-40 without targeting the full-length APP protein [253,254]. Ponezumab is now being analyzed as a treatment for cerebral amyloid angiopathy [245].
Aducanumab (BIIB037) is a human monoclonal antibody (mAb) that has the capacity to bind with various aggregated forms of Aβ such as, insoluble fibrils and soluble oligomers. However, it has been reported that this mAb does not bind with Aβ monomers. In an ongoing PRIME study (NCT01677572, phase Ib trial), aducanumab was shown to reduce Aβ plaques and slow the decline in clinical measures in prodromal or mild AD patients, with acceptable tolerability and safety [255]. Nevertheless, a side effect called amyloid-related imaging abnormalities (ARIA) was also observed along with Aβ removal in a dose-dependent manner and was mainly reported in APOE4 carriers as compared to the non-carriers. Based on the findings of PRIME and observations from previous clinical trials in AD individuals, current phase III trials of aducanumab in early AD individuals including EMERGE (NCT02484547) and ENGAGE (NCT02477800) have been designed [256,257]. Features of those study designs involve selection of participants with confirmed Aβ pathology, in order to confirm adequate target engagement, and carrying out clinical trials in participants at early symptomatic AD stages. It has been reported that the EMERGE study met its primary endpoint exhibiting a marked decrease in clinical decline in comparison with the placebo. Furthermore, Biogen (an American biotechnology company, Cambridge, MA, USA) also revealed findings from a subset of individuals in the ENGAGE study, who were randomized to the high dose of aducanumab (10 mg/kg), that is, similar with the EMERGE results [258]. As an AD treatment, Biogen declared the completion of the submission of Biologics License Application to the FDA for aducanumab on 8 July 2020. Following its approval, aducanumab will become the first therapeutic agent to decrease the AD-related clinical decline. In addition, this mAb will also become the first therapeutic agent to confirm that Aβ clearance can lead to better clinical outcomes [259].
BAN2401 is a humanized IgG1 version of the mouse monoclonal antibody mAb158 that has the capacity to bind with soluble and large Aβ protofibrils [260]. It was observed that mAB158 can decrease Aβ protofibrils in CSF and brain of Tg-ArcSwe mouse models [261]. Following experiments in mouse neuron-glial, co-cultures revealed that mAb158 might protect neurons (decrease toxicity of Aβ protofibrils) via resisting the pathological buildup of these protofibrils in astrocytes [262]. In March 2014, this mAb was licensed to Eisai and collaboration was established with Biogen to develop BAN2401. In a different phase III trial (NCT03887455), the efficacy of BAN2401 is being evaluated in early AD patients [263]. This phase III trial is projected to be completed in July 2024.
6.3.2. Receptor-Mediated Clearance
The receptor for advanced lipoprotein receptor-related protein (LRP) and glycation end products (RAGE) are both considered as multi-ligand receptors binding with copious ligands [264], which mediate the transport of Aβ out of the brain [265,266]. LRP1 is found to bind to many structurally different ligands, such as APP, Aβ, lactoferrin, and ApoE [267]. A low level of LRP1 expression or LRP1 antagonists can increase the load of Aβ [268,269].
Soluble LRP can be produced due to the cleavage of the extracellular LRP domain by β-secretase. Besides, β-secretase can also bind to free Aβ in the plasma to decrease the extracellular Aβ concentration [270]. On the other hand, RAGE can mediate the Aβ reentry into the brain through BBB. Furthermore, at a nanomolar concentration, RAGE can bind with soluble Aβ [271], and this type of interaction is suggested in inflammatory, injuries, and AD brains [272,273]. Moreover, P-glycoprotein [274,275], megalin (i.e., gp330) and reticulon 4 receptor (RTN4R) [276,277] can play a role in Aβ trafficking, with their unknown contribution in Aβ transformation via BBB.
6.4. Altering the Interplay of APP, Aβ, and Tauopathy
In addition to the amyloid burden, as stated earlier, AD is closely associated with the accumulation of CTFs of the APP. Furthermore, AD is triggered by impaired APP metabolism and advances via tau pathology. Henceforth, blocking the impairment of APP metabolism is an effective way to reduce AD pathogenesis [278]. In an AD mouse model, it was observed that the production of BACE1 and Aβ, abnormal processing of APP can be reduced by mitochondria-targeted catalase [279]. Thus, to treat patients with AD, mitochondria-targeted molecules might be an effective therapeutic technique.
6.5. Altering the Interplay of Aβ and Neuroinflammation
Inflammation has a significant role in the pathology of AD [280]. Accumulation of Aβ and activation of PKR (i.e., a pro-apoptotic kinase) can take place due to the systemic inflammation. Henceforth, brain changes triggered by lipopolysaccharides are found to be largely mediated by PKR and might be a valid target to control the production of Aβ and neuroinflammation [281]. Moreover, in an APP/PS1/tau mouse model of AD, a current study has suggested that lixisenatide (i.e., glucagon-like peptide 1 receptor agonists) can reduce neuroinflammation, NFTs, and amyloid plaques [282]. As a result, lixisenatide might be developed as an effective novel treatment for AD [282].
7. Aβ Targeting Drugs
Over 50% of drug candidates in phase III clinical trials targeted Aβ as per the updated AD drug development pipeline in 2018. From 2017 to 2018, there was a sharp decline by 40% in anti-Aβ therapies in phase I and II clinical trials, which showed alteration in AD studies after the repetitive failures of anti-Aβ therapies [283] as shown in Table 1. Common mechanisms of anti-Aβ therapies include suppression of the Aβ plaque aggregation, decrease of Aβ1-42 production, or elevation of the clearance rate of Aβ from the brain and CSF [284]. Table 2 represents various ongoing clinical studies of anti-Aβ therapies for AD.
Table 1.
Major failed clinical trials of anti-Aβ therapeutics to treat Alzheimer’s disease.
| Therapeutic Agent | Drug Class | Disease State of Participants | Clinical Trial Design | Reason of Failure | References |
|---|---|---|---|---|---|
| Avagacestat | γ-secretase inhibitor | Prodromal AD | Randomized, placebo-controlled phase II | No clinical efficacy; Adverse effects: Glycosuria, Weight loss | [285] |
| Atabecestat | BACE1 inhibitor | Early AD | Randomized, phase II/III | Adverse effects: Elevation of liver enzymes | [286,287,288] |
| Lanabecestat | BACE1 inhibitor | Mild-to-moderate AD | Randomized, phase III | Not likely to meet primary endpoint; terminated for futility | [289,290,291] |
| Verubecestat | BACE1 inhibitor | Mild-to-moderate AD | Randomized, placebo-controlled, double-blind phase III | No clinical efficacy; Adverse effects: rash, alterations in hair color, more chances of falls and injuries, weight loss, sleep disturbance, suicidal thoughts | [142,292] |
| Semagacestat | γ-secretase inhibitor | Probable AD | Double-blind, placebo-controlled phase III | No clinical efficacy, exacerbates cognitive functions at higher doses, increased incidence of skin cancer and infections | [142,155] |
| Tramiprosate | Aβ aggregation inhibitor | Mild-to-moderate AD | Phase III | No clinical efficacy | [173,293,294] |
| Gantenerumab | IgG1 humanized anti-Aβ mAbs | Prodromal AD | Randomized, placebo-controlled, double-blind phase III | Terminated owing to futility, no important differences were seen in primary and secondary endpoint | [295] |
| Crenezumab | IgG1 humanized anti-Aβ mAbs | Mild-to-moderate AD | Randomized, phase II | No clinical efficacy, Did not achieve primary and secondary endpoints. | [296] |
| Bapineuzumab | IgG1 humanized anti-Aβ mAbs | Mild-to-moderate AD | Placebo-controlled, double-blind phase III | No clinical efficacy | [297,298] |
Table 2.
Ongoing clinical studies of anti-Aβ therapeutics to treat Alzheimer’s disease.
| Therapeutic Agent | Drug Class | Number of Participants | Disease State of Participants | Study Design | Clinical Trial Phase | Current Status | References |
|---|---|---|---|---|---|---|---|
| Aducanumab | IgG1 humanized anti-Aβ mAbs | 2400 | AD patients who had participated in previous studies 221AD103, 221AD301, 221AD302 and 221AD205 | Open-label, multicenter trial | Phase III | Continuing | [299] |
| Gantenerumab and solanezumab | Monoclonal antibody | 149 | Early-onset AD caused by a genetic mutation | Randomized, placebo-controlled, double-blind trial | Phase II/III | Completed | [249] |
| BAN2401 | Monoclonal antibody | 1566 | Early AD | Placebo-controlled, double-blind, parallel-group study | Phase III | Continuing | [263] |
| CAD106 CNP520 | BACE inhibitor | 480 | Asymptomatic AD but carriers of homozygous APOE*ε4 | Randomized, placebo-controlled, double-blind trial | Phase II/III | Continuing | [300] |
| Gantenerumab | Monoclonal antibody | 1016 | Early-onset AD | Multicenter, randomized, placebo-controlled, double-blind trial | Phase III | Continuing | [301,302] |
| Solanezumab | Monoclonal antibody | 1150 | Asymptomatic AD | Randomized trial | Phase III | Continuing | [303] |
| Sodium oligomannurarate (GV-971) | Aβ aggregation inhibitor | 818 | Mild to moderate AD | Randomized trial | Phase III | Completed | [304] |
| Gantenerumab | Monoclonal antibody | 389 | Mild AD | Randomized, placebo-controlled, double-blind trial | Phase III | Continuing | [305] |
| Albumin and Immunoglobulin | Polyclonal antibodies | 347 | Mild to moderate AD | Multicenter, randomized, controlled trial | Phase II/III | Completed | [306] |
Indeed, the complex AD pathogenesis is still not clearly understood, which might include several biological mechanisms and many other proteins in addition to Aβ [307]. Perhaps this multifactorial nature of AD pathogenesis is the major cause for repetitive failures of anti-Aβ drugs since single target-based therapies might not be capable enough to tackle all the neurodegenerative event-related altered mechanisms [308].
8. Promising New Opportunities for Alzheimer’s Disease
Addressing the modifiable risk factors that play a role in AD development can be an alternative approach for therapeutics of AD [309]. Type 2 diabetes is one of such modifiable risk factors [310,311]. Neurons of the brain rely on the neurotrophic activities of insulin [312,313]. In the brain, resistance against the insulin’s neurotrophic properties can render neurons more susceptible to stress and also decrease the ability of the brain to repair the damages that accumulate over time, which can eventually cause deficits in metabolic, synaptic, and immune response activities [310]. Even in people without diabetes, insulin may lose its efficacy as a growth factor in the aged brain [314,315,316]. Thus, the use of antidiabetic agents may be considered as a useful technique in AD treatment [317]. In animal models of AD, liraglutide (i.e., an agonist of the glucagon-like peptide 1 receptor) has generated outstanding results [318]. Intranasal insulin also exerted impressive actions on cognition in individuals with AD or MCI in a small placebo-controlled study [319]. Multiple large controlled trials are also ongoing.
Neuroinflammation is another vital target for intervention. Multiple studies have revealed the link between AD and the immune system of the brain [320], where microglia plays a vital role in the association between neurodegeneration and inflammation [321]. On the other hand, epidemiological studies have revealed that a high lifetime burden of infectious diseases may elevate the risk of dementia or cognitive deficit in later life [322,323]. Peripheral inflammation and/or gut microbiota may cause activation of microglia and chronic activation may trigger harmful effects in certain situations. On the contrary, an effective adaptive immune system may avert the pathogenesis of AD via modulation of microglial activity [324]. However, further studies are required to understand microglia biology to design immune-based AD treatments.
Anti-tau therapies are also under investigation. Pathology of tau better correlates with cognitive deficits as compared to Aβ lesions. Therefore, once the clinical symptoms are apparent, targeting tau is likely to be more beneficial as compared to the clearance of Aβ. However, most of the early tau-targeting therapies were either based on the stabilization of microtubules or based on suppression of tau aggregation or kinases. Most of these therapies were discontinued due to their lack of efficacy and/or toxicity [325]. In recent times, most of the tau-based treatments in clinical studies are immunotherapies. Several preclinical studies have found them as promising [325].
There is a probability that unknown harmful neuronal stimuli can trigger compensatory changes during the early stage of the AD pathological process, which can eventually lead to decreased clearance or elevated generation of Aβ as a secondary result of the initial damage. One can draw parallels with sepsis is a life-threatening condition and can occur due to the dysregulation in the immune response. Sepsis initiates from an infectious agent, however, leukocytosis is a usual primary laboratory finding. Therapies are mainly aiming at the elimination of the main cause of the infection, rather than aiming at decreasing the numbers of white blood cells. Accumulation of Aβ may be regarded as a reaction of the brain in response to the neuronal damage. Therefore, treatments ought to have effects against the neuronal damage rather than the immune response of the host. Since initial leukocytosis in sepsis can trigger dysfunction in biological activities, thus the progressive and prolonged accumulation of Aβ in AD may have further toxic effects.
9. Conclusions
The amyloid cascade hypothesis was formulated based on strong histopathological, biochemical, and genetic evidence. Clinical, cognitive, and biomarker studies then further reinforced this hypothesis. Nevertheless, various classes of anti-Aβ agents that influence the production, aggregation, and clearance of Aβ have failed in clinical studies. Cognitive impairment was found to be accelerated by some agents that suppress the generation of nascent Aβ, including inhibitors of the γ-secretase and the BACE1. This aforesaid impairment may take place because of their off-target activities. Indeed, Aβ plays roles in memory consolidation and neuronal function. Increased generation of Aβ may take place in CNS under various clinical conditions, including cardiac arrest, general anesthesia, and sleep deprivation. Besides, Aβ generation can also be increased in response to neuronal stress. However, it is still not clear whether the accumulation of Aβ takes place due to the AD process or whether this accumulation is causing the disease. Current studies in asymptomatic or preclinical stages of AD and cognitively healthy people who are at risk of AD should provide some answers.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Author Contributions
M.S.U. conceived of the original idea and designed the outlines of the study. M.S.U., M.T.K., and M.S.R. wrote the draft of the manuscript. M.S.U. prepared the figures for the manuscript. P.J. edited the whole manuscript and improved the draft. T.B., G.M.A., A.N., M.N.B.-J., H.R.E.-S., and M.M.A.-D. performed the literature review and aided in revising the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Koudinov A.R., Berezov T.T. Alzheimer’s amyloid-beta (A beta) is an essential synaptic protein, not neurotoxic junk. Acta Neurobiol. Exp. (Wars) 2004;64:71–79. doi: 10.55782/ane-2004-1492. [DOI] [PubMed] [Google Scholar]
- 2.Sharma P., Sharma A., Fayaz F., Wakode S., Pottoo F.H. Biological Signatures of Alzheimer’s Disease. Curr. Top. Med. Chem. 2020;20:770–781. doi: 10.2174/1568026620666200228095553. [DOI] [PubMed] [Google Scholar]
- 3.Uddin M.S., Al Mamun A., Asaduzzaman M., Hosn F., Abu Sufian M., Takeda S., Herrera-Calderon O., Abdel-Daim M.M., Uddin G.M.S., Noor M.A.A., et al. Spectrum of Disease and Prescription Pattern for Outpatients with Neurological Disorders: An Empirical Pilot Study in Bangladesh. Ann. Neurosci. 2018;25:25–37. doi: 10.1159/000481812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khorrami A., Ghanbarzadeh S., Mahmoudi J., Nayebi A.M., Maleki-Dizaji N., Garjani A. Investigation of the memory impairment in rats fed with oxidized-cholesterol-rich diet employing passive avoidance test. Drug Res. (Stuttg) 2015;65:231–237. doi: 10.1055/s-0034-1370950. [DOI] [PubMed] [Google Scholar]
- 5.Grienberger C., Rochefort N.L., Adelsberger H., Henning H.A., Hill D.N., Reichwald J., Staufenbiel M., Konnerth A. Staged decline of neuronal function in vivo in an animal model of Alzheimer’s disease. Nat. Commun. 2012;3:774. doi: 10.1038/ncomms1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu C., Kivipelto M., von Strauss E. Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci. 2009;11:111–128. doi: 10.31887/DCNS.2009.11.2/cqiu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Götz J., Ittner L.M. Animal models of Alzheimer’s disease and frontotemporal dementia. Nat. Rev. Neurosci. 2008;9:532–544. doi: 10.1038/nrn2420. [DOI] [PubMed] [Google Scholar]
- 8.Nordberg A. Amyloid plaque imaging in vivo: Current achievement and future prospects. Eur. J. Nucl. Med. Mol. Imaging. 2008;35:46–50. doi: 10.1007/s00259-007-0700-2. [DOI] [PubMed] [Google Scholar]
- 9.Dubois B., Feldman H.H., Jacova C., DeKosky S.T., Barberger-Gateau P., Cummings J., Delacourte A., Galasko D., Gauthier S., Jicha G., et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 10.Uddin M.S., Kabir M.T., Jakaria M., Sobarzo-Sánchez E., Barreto G.E., Perveen A., Hafeez A., Bin-Jumah M.N., Abdel-Daim M.M., Ashraf G.M. Exploring the potential of neuroproteomics in Alzheimer’s disease. Curr. Top. Med. Chem. 2020;20 doi: 10.2174/1568026620666200603112030. [DOI] [PubMed] [Google Scholar]
- 11.Butterfield D.A., Boyd-Kimball D. Amyloid β-Peptide(1-42) Contributes to the Oxidative Stress and Neurodegeneration Found in Alzheimer Disease Brain. Brain Pathol. 2006;14:426–432. doi: 10.1111/j.1750-3639.2004.tb00087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ittner L.M., Ke Y.D., Delerue F., Bi M., Gladbach A., van Eersel J., Wölfing H., Chieng B.C., Christie M.J., Napier I.A., et al. Dendritic function of tau mediates amyloid-β toxicity in alzheimer’s disease mouse models. Cell. 2010;142:387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 13.Uddin M.S., Al Mamun A., Rahman M.A., Behl T., Perveen A., Hafeez A., Bin-Jumah M.N., Abdel-Daim M.M., Ashraf G.M. Emerging proof of protein misfolding and interactions in multifactorial Alzheimer’s disease. Curr. Top. Med. Chem. 2020;20 doi: 10.2174/1568026620666200601161703. [DOI] [PubMed] [Google Scholar]
- 14.Moreira P.I., Carvalho C., Zhu X., Smith M.A., Perry G. Mitochondrial dysfunction is a trigger of Alzheimer’s disease pathophysiology. Biochim. Biophys. Acta Mol. Basis Dis. 2010;1802:2–10. doi: 10.1016/j.bbadis.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 15.Kabir M.T., Uddin M.S., Mamun A.A., Jeandet P., Aleya L., Mansouri R.A., Ashraf G.M., Mathew B., Bin-Jumah M.N., Abdel-Daim M.M. Combination Drug Therapy for the Management of Alzheimer’s Disease. Int. J. Mol. Sci. 2020;21:3272. doi: 10.3390/ijms21093272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kabir M.T., Uddin M.S., Begum M.M., Thangapandiyan S., Rahman M.S., Aleya L., Mathew B., Ahmed M., Ashraf G.M., Barreto G.E. Cholinesterase Inhibitors for Alzheimer’s Disease: Multitargeting Strategy based on Anti-Alzheimer’s Drugs Repositioning. Curr. Pharm. Des. 2019;25:3519–3535. doi: 10.2174/1381612825666191008103141. [DOI] [PubMed] [Google Scholar]
- 17.Kabir M.T., Abu Sufian M., Uddin M.S., Begum M.M., Akhter S., Islam A., Mathew B., Islam M.S., Amran M.S., Md Ashraf G. NMDA Receptor Antagonists: Repositioning of Memantine as Multitargeting Agent for Alzheimer’s Therapy. Curr. Pharm. Des. 2019;25:3506–3518. doi: 10.2174/1381612825666191011102444. [DOI] [PubMed] [Google Scholar]
- 18.Butterfield D.A. Amyloid beta-peptide (1-42)-induced oxidative stress and neurotoxicity: Implications for neurodegeneration in Alzheimer’s disease brain. A review. Free Radic. Res. 2002;36:1307–1313. doi: 10.1080/1071576021000049890. [DOI] [PubMed] [Google Scholar]
- 19.Rahman M.A., Rahman M.R., Zaman T., Uddin M.S., Islam R., Abdel-Daim M.M., Rhim H. Emerging Potential of Naturally Occurring Autophagy Modulator against Neurodegeneration. Curr. Pharm. Des. 2020;26:772–779. doi: 10.2174/1381612826666200107142541. [DOI] [PubMed] [Google Scholar]
- 20.Uddin M.S., Al Mamun A., Jakaria M., Thangapandiyan S., Ahmad J., Rahman M.A., Mathew B., Abdel-Daim M.M., Aleya L. Emerging promise of sulforaphane-mediated Nrf2 signaling cascade against neurological disorders. Sci. Total Environ. 2020;707:135624. doi: 10.1016/j.scitotenv.2019.135624. [DOI] [PubMed] [Google Scholar]
- 21.Ibrahim A.M., Pottoo F.H., Dahiya E.S., Khan F.A., Kumar J.S. Neuron-Glia interaction: Molecular basis of Alzheimer’s Disease and Applications of Neuroproteomics. Eur. J. Neurosci. 2020;52:2931–2943. doi: 10.1111/ejn.14838. [DOI] [PubMed] [Google Scholar]
- 22.Bao F., Wicklund L., Lacor P.N., Klein W.L., Nordberg A., Marutle A. Different β-amyloid oligomer assemblies in Alzheimer brains correlate with age of disease onset and impaired cholinergic activity. Neurobiol. Aging. 2012;33:825.e1–825.e13. doi: 10.1016/j.neurobiolaging.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Uddin M.S., Kabir M.T., Tewari D., Al Mamun A., Mathew B., Aleya L., Barreto G.E., Bin-Jumah M.N., Abdel-Daim M.M., Ashraf G.M. Revisiting the role of brain and peripheral Aβ in the pathogenesis of Alzheimer’s disease. J. Neurol. Sci. 2020;416:116974. doi: 10.1016/j.jns.2020.116974. [DOI] [PubMed] [Google Scholar]
- 24.Bennett R.E., DeVos S.L., Dujardin S., Corjuc B., Gor R., Gonzalez J., Roe A.D., Frosch M.P., Pitstick R., Carlson G.A., et al. Enhanced Tau Aggregation in the Presence of Amyloid β. Am. J. Pathol. 2017;187:1601–1612. doi: 10.1016/j.ajpath.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasconcelos B., Stancu I.C., Buist A., Bird M., Wang P., Vanoosthuyse A., Van Kolen K., Verheyen A., Kienlen-Campard P., Octave J.N., et al. Heterotypic seeding of Tau fibrillization by pre-aggregated Abeta provides potent seeds for prion-like seeding and propagation of Tau-pathology in vivo. Acta Neuropathol. 2016;131:549–569. doi: 10.1007/s00401-015-1525-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Götz J., Chen F., Van Dorpe J., Nitsch R.M. Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ42 fibrils. Science. 2001;293:1491–1495. doi: 10.1126/science.1062097. [DOI] [PubMed] [Google Scholar]
- 27.Bolmont T., Clavaguera F., Meyer-Luehmann M., Herzig M.C., Radde R., Staufenbiel M., Lewis J., Hutton M., Tolnay M., Jucker M. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP x tau transgenic mice. Am. J. Pathol. 2007;171:2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pooler A.M., Polydoro M., Maury E.A., Nicholls S.B., Reddy S.M., Wegmann S., William C., Saqran L., Cagsal-Getkin O., Pitstick R., et al. Amyloid accelerates tau propagation and toxicity in a model of early Alzheimer’s disease. Acta Neuropathol. Commun. 2015;3:14. doi: 10.1186/s40478-015-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lewis J., Dickson D.W., Lin W.L., Chisholm L., Corral A., Jones G., Yen S.H., Sahara N., Skipper L., Yager D., et al. Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science. 2001;293:1487–1491. doi: 10.1126/science.1058189. [DOI] [PubMed] [Google Scholar]
- 30.He Z., Guo J.L., McBride J.D., Narasimhan S., Kim H., Changolkar L., Zhang B., Gathagan R.J., Yue C., Dengler C., et al. Amyloid-β plaques enhance Alzheimer’s brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nat. Med. 2018;24:29–38. doi: 10.1038/nm.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zetterberg H., Blennow K., Hanse E. Amyloid β and APP as biomarkers for Alzheimer’s disease. Exp. Gerontol. 2010;45:23–29. doi: 10.1016/j.exger.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Stefani A., Bernardini S., Panella M., Pierantozzi M., Nuccetelli M., Koch G., Urbani A., Giordano A., Martorana A., Orlacchio A., et al. AD with subcortical white matter lesions and vascular dementia: CSF markers for differential diagnosis. J. Neurol. Sci. 2005;237:83–88. doi: 10.1016/j.jns.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 33.Noguchi M., Yoshita M., Matsumoto Y., Ono K., Iwasa K., Yamada M. Decreased β-amyloid peptide42 in cerebrospinal fluid of patients with progressive supranuclear palsy and corticobasal degeneration. J. Neurol. Sci. 2005;237:61–65. doi: 10.1016/j.jns.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Cedazo-Minguez A., Winblad B. Biomarkers for Alzheimer’s disease and other forms of dementia: Clinical needs, limitations and future aspects. Exp. Gerontol. 2010;45:5–14. doi: 10.1016/j.exger.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Sunderland T., Mirza N., Putnam K.T., Linker G., Bhupali D., Durham R., Soares H., Kimmel L., Friedman D., Bergeson J., et al. Cerebrospinal fluid β-amyloid 1-42 and tau in control subjects at risk for Alzheimer’s disease: The effect of APOE ε4 allele. Biol. Psychiatry. 2004;56:670–676. doi: 10.1016/j.biopsych.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 36.Schoonenboom N.S., Mulder C., Van Kamp G.J., Mehta S.P., Scheltens P., Blankenstein M.A., Mehta P.D. Amyloid β 38, 40, and 42 species in cerebrospinal fluid: More of the same? Ann. Neurol. 2005;58:139–142. doi: 10.1002/ana.20508. [DOI] [PubMed] [Google Scholar]
- 37.Xiao Z., Prieto D., Conrads T.P., Veenstra T.D., Issaq H.J. Proteomic patterns: Their potential for disease diagnosis. Mol. Cell. Endocrinol. 2005;230:95–106. doi: 10.1016/j.mce.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 38.Bloom G.S. Amyloid-β and Tau. JAMA Neurol. 2014;71:505. doi: 10.1001/jamaneurol.2013.5847. [DOI] [PubMed] [Google Scholar]
- 39.Al Mamun A., Uddin M.S., Kabir M.T., Khanum S., Sarwar M.S., Mathew B., Rauf A., Ahmed M., Ashraf G.M. Exploring the Promise of Targeting Ubiquitin-Proteasome System to Combat Alzheimer’s Disease. Neurotox. Res. 2020;38:8–17. doi: 10.1007/s12640-020-00185-1. [DOI] [PubMed] [Google Scholar]
- 40.Sjögren M., Vanderstichele H., Agren H., Zachrisson O., Edsbagge M., Wikkelsø C., Skoog I., Wallin A., Wahlund L.O., Marcusson J., et al. Tau and Abeta42 in Cerebrospinal Fluid From Healthy Adults 21–93 Years of Age: Establishment of Reference Values. Clin. Chem. 2001;47:1776–1781. [PubMed] [Google Scholar]
- 41.Blennow K. CSF biomarkers for mild cognitive impairment. J. Intern. Med. 2004;256:224–234. doi: 10.1111/j.1365-2796.2004.01368.x. [DOI] [PubMed] [Google Scholar]
- 42.Blennow K. CSF biomarkers for Alzheimer’s disease: Use in early diagnosis and evaluation of drug treatment. Expert Rev. Mol. Diagn. 2005;5:661–672. doi: 10.1586/14737159.5.5.661. [DOI] [PubMed] [Google Scholar]
- 43.Mo J.-A., Lim J.-H., Sul A.-R., Lee M., Youn Y.C., Kim H.-J. Cerebrospinal Fluid β-Amyloid1–42 Levels in the Differential Diagnosis of Alzheimer’s Disease—Systematic Review and Meta-Analysis. PLoS ONE. 2015;10:e0116802. doi: 10.1371/journal.pone.0116802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hu W.T., Watts K.D., Shaw L.M., Howell J.C., Trojanowski J.Q., Basra S., Glass J.D., Lah J.J., Levey A.I. CSF beta-amyloid 1-42—what are we measuring in Alzheimer’s disease? Ann. Clin. Transl. Neurol. 2015;2:131–139. doi: 10.1002/acn3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quiroz Y.T., Sperling R.A., Norton D.J., Baena A., Arboleda-Velasquez J.F., Cosio D., Schultz A., Lapoint M., Guzman-Velez E., Miller J.B., et al. Association between amyloid and tau accumulation in young adults with autosomal dominant Alzheimer disease. JAMA Neurol. 2018;75:548–556. doi: 10.1001/jamaneurol.2017.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Assini A., Cammarata S., Vitali A., Colucci M., Giliberto L., Borghi R., Inglese M.L., Volpe S., Ratto S., Dagna-Bricarelli F., et al. Plasma levels of amyloid β-protein 42 are increased in women with mild cognitive impairment. Neurology. 2004;63:828–831. doi: 10.1212/01.WNL.0000137040.64252.ED. [DOI] [PubMed] [Google Scholar]
- 47.Borroni B., Di Luca M., Padovani A. Predicting Alzheimer dementia in mild cognitive impairment patients. Are biomarkers useful? Eur. J. Pharmacol. 2006;545:73–80. doi: 10.1016/j.ejphar.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 48.Brettschneider S., Morgenthaler N.G., Teipel S.J., Fischer-Schulz C., Bürger K., Dodel R., Du Y., Möller H.J., Bergmann A., Hampel H. Decreased serum amyloid β1-42 autoantibody levels in Alzheimer’s disease, determined by a newly developed immuno-precipitation assay with radiolabeled amyloid β1-42 peptide. Biol. Psychiatry. 2005;57:813–816. doi: 10.1016/j.biopsych.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 49.Gustaw-Rothenberg K.A., Siedlak S.L., Bonda D.J., Lerner A., Tabaton M., Perry G., Smith M.A. Dissociated amyloid-β antibody levels as a serum biomarker for the progression of Alzheimer’s disease: A population-based study. Exp. Gerontol. 2010;45:47–52. doi: 10.1016/j.exger.2009.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uddin M.S., Kabir M.T., Jeandet P., Mathew B., Ashraf G.M., Perveen A., Bin-Jumah M.N., Mousa S.A., Abdel-Daim M.M. Novel Anti-Alzheimer’s Therapeutic Molecules Targeting Amyloid Precursor Protein Processing. Oxid. Med. Cell. Longev. 2020;2020:1–19. doi: 10.1155/2020/7039138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Weggen S., Beher D. Molecular consequences of amyloid precursor protein and presenilin mutations causing autosomal-dominant Alzheimer’s disease. Alzheimer’s Res. Ther. 2012;4:1–14. doi: 10.1186/alzrt107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacLeod R., Hillert E.-K., Cameron R.T., Baillie G.S. The role and therapeutic targeting of α-, β- and γ-secretase in Alzheimer’s disease. Futur. Sci. OA. 2015;1:FSO11. doi: 10.4155/fso.15.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Citron M., Teplow D.B., Selkoe D.J. Generation of amyloid β protein from its precursor is sequence specific. Neuron. 1995;14:661–670. doi: 10.1016/0896-6273(95)90323-2. [DOI] [PubMed] [Google Scholar]
- 54.Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A.Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D.J. Mutation of the β-amyloid precursor protein in familial Alzheimer’s disease increases β-protein production. Nature. 1992;360:672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- 55.Uddin M.S., Kabir M.T., Rahman M.M., Mathew B., Shah M.A., Ashraf G.M. TV 3326 for Alzheimer’s dementia: A novel multimodal ChE and MAO inhibitors to mitigate Alzheimer’s-like neuropathology. J. Pharm. Pharmacol. 2020;72:1001–1012. doi: 10.1111/jphp.13244. [DOI] [PubMed] [Google Scholar]
- 56.Beyreuther K., Masters C.L. Amyloid precursor protein (APP) and beta A4 amyloid in the etiology of Alzheimer’s disease: Precursor-product relationships in the derangement of neuronal function. Brain Pathol. 1991;1:241–251. doi: 10.1111/j.1750-3639.1991.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 57.Hardy J., Allsop D. Amyloid deposition as the central event in the aetiology of Alzheimer’s disease. Trends Pharmacol. Sci. 1991;12:383–388. doi: 10.1016/0165-6147(91)90609-V. [DOI] [PubMed] [Google Scholar]
- 58.Selkoe D.J. The molecular pathology of Alzheimer’s disease. Neuron. 1991;6:487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- 59.Hardy J.A., Higgins G.A. Alzheimer’s disease: The amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 60.Kabir M.T., Uddin M.S., Setu J.R., Ashraf G.M., Bin-Jumah M.N., Abdel-Daim M.M. Exploring the Role of PSEN mutations in the pathogenesis of Alzheimer’s disease. Neurotox. Res. 2020 doi: 10.1007/s12640-020-00232-x. [DOI] [PubMed] [Google Scholar]
- 61.Karran E., Mercken M., Strooper B. De The amyloid cascade hypothesis for Alzheimer’s disease: An appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10:698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 62.Borchelt D.R., Thinakaran G., Eckman C.B., Lee M.K., Davenport F., Ratovitsky T., Prada C.M., Kim G., Seekins S., Yager D., et al. Familial Alzheimer’s disease-linked presenilin I variants elevate aβ1-42/1-40 ratio in vitro and in vivo. Neuron. 1996;17:1005–1013. doi: 10.1016/S0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- 63.Ertekin-Taner N. Genetics of Alzheimer’s Disease: A Centennial Review. Neurol. Clin. 2007;25:611–667. doi: 10.1016/j.ncl.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Radde R., Bolmont T., Kaeser S.A., Coomaraswamy J., Lindau D., Stoltze L., Calhoun M.E., Jäggi F., Wolburg H., Gengler S., et al. Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Rep. 2006;7:940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L.B., Lindholm K., Yan R., Citron M., Xia W., Yang X.L., Beach T., Sue L., Wong P., Price D., et al. Elevated β-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat. Med. 2003;9:3–4. doi: 10.1038/nm0103-3. [DOI] [PubMed] [Google Scholar]
- 66.Mawuenyega K.G., Sigurdson W., Ovod V., Munsell L., Kasten T., Morris J.C., Yarasheski K.E., Bateman R.J. Decreased clearance of CNS β-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bennett D.A., Schneider J.A., Arvanitakis Z., Kelly J.F., Aggarwal N.T., Shah R.C., Wilson R.S. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 68.Jansen W.J., Ossenkoppele R., Knol D.L., Tijms B.M., Scheltens P., Verhey F.R.J., Visser P.J., Aalten P., Aarsland D., Alcolea D., et al. Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA J. Am. Med. Assoc. 2015;313:1924–1938. doi: 10.1001/jama.2015.4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vos S.J.B., Xiong C., Visser P.J., Jasielec M.S., Hassenstab J., Grant E.A., Cairns N.J., Morris J.C., Holtzman D.M., Fagan A.M. Preclinical Alzheimer’s disease and its outcome: A longitudinal cohort study. Lancet Neurol. 2013;12:957–965. doi: 10.1016/S1474-4422(13)70194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Villemagne V.L., Burnham S., Bourgeat P., Brown B., Ellis K.A., Salvado O., Szoeke C., Macaulay S.L., Martins R., Maruff P., et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: A prospective cohort study. Lancet Neurol. 2013;12:357–367. doi: 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- 71.Burnham S.C., Bourgeat P., Doré V., Savage G., Brown B., Laws S., Maruff P., Salvado O., Ames D., Martins R.N., et al. Clinical and cognitive trajectories in cognitively healthy elderly individuals with suspected non-Alzheimer’s disease pathophysiology (SNAP) or Alzheimer’s disease pathology: A longitudinal study. Lancet Neurol. 2016;15:1044–1053. doi: 10.1016/S1474-4422(16)30125-9. [DOI] [PubMed] [Google Scholar]
- 72.Petersen R.C., Wiste H.J., Weigand S.D., Rocca W.A., Roberts R.O., Mielke M.M., Lowe V.J., Knopman D.S., Pankratz V.S., Machulda M.M., et al. Association of elevated amyloid levels with cognition and biomarkers in cognitively normal people from the community. JAMA Neurol. 2016;73:85–92. doi: 10.1001/jamaneurol.2015.3098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Donohue M.C., Sperling R.A., Petersen R., Sun C.K., Weiner M., Aisen P.S. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA J. Am. Med. Assoc. 2017;317:2305–2316. doi: 10.1001/jama.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lauritzen I., Pardossi-Piquard R., Bauer C., Brigham E., Abraham J.D., Ranaldi S., Fraser P., St-George-Hyslop P., Le Thuc O., Espin V., et al. The β-secretase-derived C-terminal fragment of βAPP, C99, but not Aβ, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J. Neurosci. 2012;32:16243–16255. doi: 10.1523/JNEUROSCI.2775-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lauritzen I., Pardossi-Piquard R., Bourgeois A., Pagnotta S., Biferi M.G., Barkats M., Lacor P., Klein W., Bauer C., Checler F. Intraneuronal aggregation of the β-CTF fragment of APP (C99) induces Aβ-independent lysosomal-autophagic pathology. Acta Neuropathol. 2016;132:257–276. doi: 10.1007/s00401-016-1577-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pasternak S.H., Callahan J.W., Mahuran D.J. The role of the endosomal/lysosomal system in amyloid-beta production and the pathophysiology of Alzheimer’s disease: Reexamining the spatial paradox from a lysosomal perspective. J. Alzheimer’s Dis. 2004;6:53–65. doi: 10.3233/JAD-2004-6107. [DOI] [PubMed] [Google Scholar]
- 77.Schubert D., Behl C. The expression of amyloid beta protein precursor protects nerve cells from β-amyloid and glutamate toxicity and alters their interaction with the extracellular matrix. Brain Res. 1993;629:275–282. doi: 10.1016/0006-8993(93)91331-L. [DOI] [PubMed] [Google Scholar]
- 78.Furukawa K., Mattson M.P. Secreted amyloid precursor protein a selectively suppresses N-methyl-D- aspartate currents in hippocampal neurons: Involvement of cyclic GMP. Neuroscience. 1998;83:429–438. doi: 10.1016/S0306-4522(97)00398-9. [DOI] [PubMed] [Google Scholar]
- 79.Bell K.F.S., Zheng L., Fahrenholz F., Cuello A.C. ADAM-10 over-expression increases cortical synaptogenesis. Neurobiol. Aging. 2008;29:554–565. doi: 10.1016/j.neurobiolaging.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 80.Mattson M.P. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol. Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 81.Caillé I., Allinquant B., Dupont E., Bouillot C., Langer A., Müller U., Prochiantz A. Soluble form of amyloid precursor protein regulates proliferation of progenitors in the adult subventricular zone. Development. 2004;131:2173–2181. doi: 10.1242/dev.01103. [DOI] [PubMed] [Google Scholar]
- 82.Obregon D., Hou H., Deng J., Giunta B., Tian J., Darlington D., Shahaduzzaman M., Zhu Y., Mori T., Mattson M.P., et al. Soluble amyloid precursor protein-α modulates β-secretase activity and amyloid-β 2 generation. Nat. Commun. 2012;3:777. doi: 10.1038/ncomms1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peters-Libeu C., Campagna J., Mitsumori M., Poksay K.S., Spilman P., Sabogal A., Bredesen D.E., John V. SAβPPα is a Potent Endogenous Inhibitor of BACE1. J. Alzheimer’s Dis. 2015;47:545–555. doi: 10.3233/JAD-150282. [DOI] [PubMed] [Google Scholar]
- 84.Deng J., Habib A., Obregon D.F., Barger S.W., Giunta B., Wang Y.J., Hou H., Sawmiller D., Tan J. Soluble amyloid precursor protein alpha inhibits tau phosphorylation through modulation of GSK3β signaling pathway. J. Neurochem. 2015;135:630–637. doi: 10.1111/jnc.13351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arriagada P.V., Growdon J.H., Hedley-Whyte E.T., Hyman B.T. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42:631–639. doi: 10.1212/WNL.42.3.631. [DOI] [PubMed] [Google Scholar]
- 86.Bierer L.M., Hof P.R., Purohit D.P., Carlin L., Schmeidler J., Davis K.L., Perl D.P. Neocortical neurofibrillary tangles correlate with dementia severity in Alzheimer’s disease. Arch. Neurol. 1995;52:81–88. doi: 10.1001/archneur.1995.00540250089017. [DOI] [PubMed] [Google Scholar]
- 87.Mamun A.A., Uddin M.S., Mathew B., Ashraf G.M. Toxic Tau: Structural Origins of Tau Aggregation in Alzheimer’s Disease. Neural Regen. Res. 2020;15:1417–1420. doi: 10.4103/1673-5374.274329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gómez-Isla T., Hollister R., West H., Mui S., Growdon J.H., Petersen R.C., Parisi J.E., Hyman B.T. Neuronal loss correlates with but exceeds neurofibrillary tangles in Alzheimer’s disease. Ann. Neurol. 1997;41:17–24. doi: 10.1002/ana.410410106. [DOI] [PubMed] [Google Scholar]
- 89.Kabir M.T., Uddin M.S., Abdeen A., Ashraf G.M., Perveen A., Hafeez A., Bin-Jumah M.N., Abdel-Daim M.M. Evidence linking protein misfolding to quality control in progressive neurodegenerative diseases. Curr. Top. Med. Chem. 2020;20 doi: 10.2174/1568026620666200618114924. [DOI] [PubMed] [Google Scholar]
- 90.Bennett D.A., Schneider J.A., Wilson R.S., Bienias J.L., Arnold S.E. Neurofibrillary Tangles Mediate the Association of Amyloid Load with Clinical Alzheimer Disease and Level of Cognitive Function. Arch. Neurol. 2004;61:378–384. doi: 10.1001/archneur.61.3.378. [DOI] [PubMed] [Google Scholar]
- 91.Wang L., Benzinger T.L., Su Y., Christensen J., Friedrichsen K., Aldea P., McConathy J., Cairns N.J., Fagan A.M., Morris J.C., et al. Evaluation of Tau imaging in staging Alzheimer disease and revealing interactions between β-Amyloid and tauopathy. JAMA Neurol. 2016;73:1070–1077. doi: 10.1001/jamaneurol.2016.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sutphen C.L., McCue L., Herries E.M., Xiong C., Ladenson J.H., Holtzman D.M., Fagan A.M. Longitudinal decreases in multiple cerebrospinal fluid biomarkers of neuronal injury in symptomatic late onset Alzheimer’s disease. Alzheimer’s Dement. 2018;14:869–879. doi: 10.1016/j.jalz.2018.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McDade E., Wang G., Gordon B.A., Hassenstab J., Benzinger T.L.S., Buckles V., Fagan A.M., Holtzman D.M., Cairns N.J., Goate A.M., et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology. 2018;91:E1295–E1306. doi: 10.1212/WNL.0000000000006277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sato C., Barthélemy N.R., Mawuenyega K.G., Patterson B.W., Gordon B.A., Jockel-Balsarotti J., Sullivan M., Crisp M.J., Kasten T., Kirmess K.M., et al. Tau Kinetics in Neurons and the Human Central Nervous System. Neuron. 2018;97:1284–1298. doi: 10.1016/j.neuron.2018.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hansson O., Zetterberg H., Buchhave P., Londos E., Blennow K., Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: A follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 96.Braak H., Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 97.Braak H., Alafuzoff I., Arzberger T., Kretzschmar H., Tredici K. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 2006;112:389–404. doi: 10.1007/s00401-006-0127-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thal D.R., Rüb U., Orantes M., Braak H. Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–1800. doi: 10.1212/WNL.58.12.1791. [DOI] [PubMed] [Google Scholar]
- 99.Davidson Y.S., Robinson A., Prasher V.P., Mann D.M.A. The age of onset and evolution of Braak tangle stage and Thal amyloid pathology of Alzheimer’s disease in individuals with Down syndrome. Acta Neuropathol. Commun. 2018;6:56. doi: 10.1186/s40478-018-0559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee G., Newman S.T., Gard D.L., Band H., Panchamoorthy G. Tau interacts with src-family non-receptor tyrosine kinases. J. Cell Sci. 1998;111:3167–3177. doi: 10.1242/jcs.111.21.3167. [DOI] [PubMed] [Google Scholar]
- 101.Nakazawa T., Komai S., Tezuka T., Hisatsune C., Umemori H., Semba K., Mishina M., Manabe T., Yamamoto T. Characterization of Fyn-mediated tyrosine phosphorylation sites on GluRε2 (NR2B) subunit of the N-methyl-D-aspartate receptor. J. Biol. Chem. 2001;276:693–699. doi: 10.1074/jbc.M008085200. [DOI] [PubMed] [Google Scholar]
- 102.Rong Y., Lu X., Bernard A., Khrestchatisky M., Baudry M. Tyrosine phosphorylation of ionotropic glutamate receptors by Fyn or Src differentially modulates their susceptibility to calpain and enhances their binding to spectrin and PSD-95. J. Neurochem. 2001;79:382–390. doi: 10.1046/j.1471-4159.2001.00565.x. [DOI] [PubMed] [Google Scholar]
- 103.Tezuka T., Umemori H., Akiyama T., Nakanishi S., Yamamoto T. PSD-95 promotes Fyn-mediated tyrosine phosphorylation of the N-methyl-D-aspartate receptor subunit NR2A. Proc. Natl. Acad. Sci. USA. 1999;96:435–440. doi: 10.1073/pnas.96.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salter M.W., Kalia L.V. SRC kinases: A hub for NMDA receptor regulation. Nat. Rev. Neurosci. 2004;5:317–328. doi: 10.1038/nrn1368. [DOI] [PubMed] [Google Scholar]
- 105.Aarts M., Liu Y., Liu L., Besshoh S., Arundine M., Gurd J.W., Wang Y.T., Salter M.W., Tymianski M. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 106.Chin J., Palop J.J., Puoliväli J., Massaro C., Bien-Ly N., Gerstein H., Scearce-Levie K., Masliah E., Mucke L. Fyn kinase induces synaptic and cognitive impairments in a transgenic mouse model of Alzheimer’s disease. J. Neurosci. 2005;25:9694–9703. doi: 10.1523/JNEUROSCI.2980-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chin J., Palop J.J., Yu G.Q., Kojima N., Masliah E., Mucke L. Fyn kinase modulates synaptotoxicity, but not aberrant sprouting, in human amyloid precursor protein transgenic mice. J. Neurosci. 2004;24:4692–4697. doi: 10.1523/JNEUROSCI.0277-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kim H.-S., Kim E.-M., Lee J.-P., Park C.H., Kim S., Seo J.-H., Chang K.-A., Yu E., Jeong S.-J., Chong Y.H., et al. C-terminal fragments of amyloid precursor protein exert neurotoxicity by inducing glycogen synthase kinase-3β expression. FASEB J. 2003;17:1–28. doi: 10.1096/fj.03-0106fje. [DOI] [PubMed] [Google Scholar]
- 109.Kuhn P.-H., Wang H., Dislich B., Colombo A., Zeitschel U., Ellwart J.W., Kremmer E., Roßner S., Lichtenthaler S.F. ADAM10 is the physiologically relevant, constitutive α-secretase of the amyloid precursor protein in primary neurons. EMBO J. 2010;29:3020–3032. doi: 10.1038/emboj.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.De Strooper B., Vassar R., Golde T. The secretases: Enzymes with therapeutic potential in Alzheimer disease. Nat. Rev. Neurol. 2010;6:99–107. doi: 10.1038/nrneurol.2009.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bandyopadhyay S., Goldstein L., Lahiri D., Rogers J. Role of the APP Non-Amyloidogenic Signaling Pathway and Targeting α-Secretase as an Alternative Drug Target for Treatment of Alzheimers Disease. Curr. Med. Chem. 2007;14:2848–2864. doi: 10.2174/092986707782360060. [DOI] [PubMed] [Google Scholar]
- 112.Hong-Qi Y., Zhi-Kun S., Sheng-Di C. Current advances in the treatment of Alzheimer’s disease: Focused on considerations targeting Aβ and tau. Transl. Neurodegener. 2012;1:21. doi: 10.1186/2047-9158-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yang H.Q., Sun Z.K., Ba M.W., Xu J., Xing Y. Involvement of protein trafficking in deprenyl-induced α-secretase activity regulation in PC12 cells. Eur. J. Pharmacol. 2009;610:37–41. doi: 10.1016/j.ejphar.2009.03.047. [DOI] [PubMed] [Google Scholar]
- 114.Filip V., Kolibás E. Selegiline in the treatment of Alzheimer’s disease: A long-term randomized placebo-controlled trial. Czech and Slovak Senile Dementia of Alzheimer Type Study Group. J. Psychiatry Neurosci. 1999;24:234–243. [PMC free article] [PubMed] [Google Scholar]
- 115.Zamrini E., McGwin G., Roseman J.M. Association between Statin Use and Alzheimer’s Disease. Neuroepidemiology. 2004;23:94–98. doi: 10.1159/000073981. [DOI] [PubMed] [Google Scholar]
- 116.Parvathy S., Ehrlich M., Pedrini S., Diaz N., Refolo L., Buxbaum J.D., Bogush A., Petanceska S., Gandy S. Atorvastatin-induced activation of Alzheimer’s alpha secretase is resistant to standard inhibitors of protein phosphorylation-regulated ectodomain shedding. J. Neurochem. 2004;90:1005–1010. doi: 10.1111/j.1471-4159.2004.02521.x. [DOI] [PubMed] [Google Scholar]
- 117.Feldman H.H., Doody R.S., Kivipelto M., Sparks D.L., Waters D.D., Jones R.W., Schwam E., Schindler R., Hey-Hadavi J., Demicco D.A., et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology. 2010;74:956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 118.Vellas B., Sol O., Snyder P.J., Ousset P.-J., Haddad R., Maurin M., Lemarie J.-C., Desire L., Pando M.P. EHT0202 in Alzheimers Disease: A 3-Month, Randomized, Placebo- Controlled, Double-Blind Study. Curr. Alzheimer Res. 2012;8:203–212. doi: 10.2174/156720511795256053. [DOI] [PubMed] [Google Scholar]
- 119.Marcade M., Bourdin J., Loiseau N., Peillon H., Rayer A., Drouin D., Schweighoffer F., Désiré L. Etazolate, a neuroprotective drug linking GABAA receptor pharmacology to amyloid precursor protein processing. J. Neurochem. 2008;106:392–404. doi: 10.1111/j.1471-4159.2008.05396.x. [DOI] [PubMed] [Google Scholar]
- 120.ClinicalTrials.gov Study of PRX-03140 Monotherapy in Subjects with Alzheimer’s Sisease. [(accessed on 18 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00693004.
- 121.Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., Ehrhart J., Townsend K., Zeng J., Morgan D., et al. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Obregon D.F., Rezai-Zadeh K., Bai Y., Sun N., Hou H., Ehrhart J., Zeng J., Mori T., Arendash G.W., Shytle D., et al. ADAM10 activation is required for green tea (-)-epigallocatechin-3-gallate-induced α-secretase cleavage of amyloid precursor protein. J. Biol. Chem. 2006;281:16419–16427. doi: 10.1074/jbc.M600617200. [DOI] [PubMed] [Google Scholar]
- 123.ClinicalTrials.gov Sunphenon EGCg (Epigallocatechin-Gallate) in the Early Stage of Alzheimer’s Disease. [(accessed on 18 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00951834.
- 124.Farlow M.R., Thompson R.E., Wei L.J., Tuchman A.J., Grenier E., Crockford D., Wilke S., Benison J., Alkon D.L., Moreira P. A randomized, double-blind, placebo-controlled, phase II study assessing safety, tolerability, and efficacy of bryostatin in the treatment of moderately severe to severe Alzheimer’s disease. J. Alzheimer’s Dis. 2019;67:555–570. doi: 10.3233/JAD-180759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vassar R., Bennett B.D., Babu-Khan S., Kahn S., Mendiaz E.A., Denis P., Teplow D.B., Ross S., Amarante P., Loeloff R., et al. Beta-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science. 1999;286:735–741. doi: 10.1126/science.286.5440.735. [DOI] [PubMed] [Google Scholar]
- 126.Yan R., Bienkowski M.J., Shuck M.E., Miao H., Tory M.C., Pauley A.M., Brashler J.R., Stratman N.C., Mathews W.R., Buhl A.E., et al. Membrane-anchored aspartyl protease with Alzheimer’s disease β-secretase activity. Nature. 1999;402:533–537. doi: 10.1038/990107. [DOI] [PubMed] [Google Scholar]
- 127.Roberds S.L., Anderson J., Basi G., Bienkowski M.J., Branstetter D.G., Chen K.S., Freedman S.B., Frigon N.L., Games D., Hu K., et al. BACE knockout mice are healthy despite lacking the primary beta-secretase activity in brain: Implications for Alzheimer’s disease therapeutics. Hum. Mol. Genet. 2001;10:1317–1324. doi: 10.1093/hmg/10.12.1317. [DOI] [PubMed] [Google Scholar]
- 128.Luo Y., Bolon B., Kahn S., Bennett B.D., Babu-Khan S., Denis P., Fan W., Kha H., Zhang J., Gong Y., et al. Mice deficient in BACE1, the Alzheimer’s β-secretase, have normal phenotype and abolished β-amyloid generation. Nat. Neurosci. 2001;4:231–232. doi: 10.1038/85059. [DOI] [PubMed] [Google Scholar]
- 129.Ohno M., Chang L., Tseng W., Oakley H., Citron M., Klein W.L., Vassar R., Disterhoft J.F. Temporal memory deficits in Alzheimer’s mouse models: Rescue by genetic deletion of BACE1. Eur. J. Neurosci. 2006;23:251–260. doi: 10.1111/j.1460-9568.2005.04551.x. [DOI] [PubMed] [Google Scholar]
- 130.Ohno M., Sametsky E.A., Younkin L.H., Oakley H., Younkin S.G., Citron M., Vassar R., Disterhoft J.F. BACE1 Deficiency Rescues Memory Deficits and Cholinergic Dysfunction in a Mouse Model of Alzheimer’s Disease. Neuron. 2004;41:27–33. doi: 10.1016/S0896-6273(03)00810-9. [DOI] [PubMed] [Google Scholar]
- 131.Landreth G., Jiang Q., Mandrekar S., Heneka M. PPARγ agonists as therapeutics for the treatment of Alzheimer’s disease. Neurotherapeutics. 2008;5:481–489. doi: 10.1016/j.nurt.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Craft S. The Role of Metabolic Disorders in Alzheimer Disease and Vascular Dementia. Arch. Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.ClinicalTrials.gov Biomarker Qualification for Risk of Mild Cognitive Impairment (MCI) Due to Alzheimer’s disease (AD) and Safety and Efficacy Evaluation of Pioglitazone in Delaying Its Onset. [(accessed on 18 June 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT01931566.
- 134.Harrington C., Sawchak S., Chiang C., Davies J., Donovan C., Saunders A.M., Irizarry M., Jeter B., Zvartau-Hind M., van Dyck C.H., et al. Rosiglitazone Does Not Improve Cognition or Global Function when Used as Adjunctive Therapy to AChE Inhibitors in Mild-to-Moderate Alzheimers Disease: Two Phase 3 Studies. Curr. Alzheimer Res. 2011;8:592–606. doi: 10.2174/156720511796391935. [DOI] [PubMed] [Google Scholar]
- 135.Tang J.J.N. Beta-secretase as target for amyloid-reduction therapy. Alzheimer’s Dement. 2009;5:74. doi: 10.1016/j.jalz.2009.05.177. [DOI] [Google Scholar]
- 136.Chang W.-P., Koelsch G., Wong S., Downs D., Da H., Weerasena V., Gordon B., Devasamudram T., Bilcer G., Ghosh A.K., et al. In vivo inhibition of Aβ production by memapsin 2 (β-secretase) inhibitors. J. Neurochem. 2004;89:1409–1416. doi: 10.1111/j.1471-4159.2004.02452.x. [DOI] [PubMed] [Google Scholar]
- 137.Ghosh A.K., Kumaragurubaran N., Hong L., Koelsh G., Tang J. Memapsin 2 (beta-secretase) inhibitors: Drug development. Curr. Alzheimer Res. 2008;5:121–131. doi: 10.2174/156720508783954730. [DOI] [PubMed] [Google Scholar]
- 138.Alzforum Keystone Drug News: CoMentis BACE Inhibitor Debuts. [(accessed on 3 August 2020)]; Available online: https://www.alzforum.org/news/conference-coverage/keystone-drug-news-comentis-bace-inhibitor-debuts.
- 139.Ghosh A.K., Brindisi M., Tang J. Developing β-secretase inhibitors for treatment of Alzheimer’s disease. J. Neurochem. 2012;120:71–83. doi: 10.1111/j.1471-4159.2011.07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yan R. Stepping closer to treating Alzheimer’s disease patients with BACE1 inhibitor drugs. Transl. Neurodegener. 2016;5 doi: 10.1186/s40035-016-0061-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alzforum Lilly Halts Phase 2 Trial of BACE Inhibitor Due to Liver Toxicity. [(accessed on 3 August 2020)]; Available online: https://www.alzforum.org/news/research-news/lilly-halts-phase-2-trial-bace-inhibitor-due-liver-toxicity.
- 142.Kennedy M.E., Stamford A.W., Chen X., Cox K., Cumming J.N., Dockendorf M.F., Egan M., Ereshefsky L., Hodgson R.A., Hyde L.A., et al. The BACE1 inhibitor verubecestat (MK-8931) reduces CNS b-Amyloid in animal models and in Alzheimer’s disease patients. Sci. Transl. Med. 2016;8:363ra150. doi: 10.1126/scitranslmed.aad9704. [DOI] [PubMed] [Google Scholar]
- 143.Egan M.F., Kost J., Voss T., Mukai Y., Aisen P.S., Cummings J.L., Tariot P.N., Vellas B., Van Dyck C.H., Boada M., et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med. 2019;380:1408–1420. doi: 10.1056/NEJMoa1812840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Shoji M., Golde T.E., Ghiso J., Cheung T.T., Estus S., Shaffer L.M., Cai X.D., McKay D.M., Tintner R., Frangione B. Production of the Alzheimer amyloid beta protein by normal proteolytic processing. Science. 1992;258:126–129. doi: 10.1126/science.1439760. [DOI] [PubMed] [Google Scholar]
- 145.Haass C., Schlossmacher M.G., Hung A.Y., Vigo-Pelfrey C., Mellon A., Ostaszewski B.L., Lieberburg I., Koo E.H., Schenk D., Teplow D.B., et al. Amyloid β-peptide is produced by cultured cells during normal metabolism. Nature. 1992;359:322–325. doi: 10.1038/359322a0. [DOI] [PubMed] [Google Scholar]
- 146.De Strooper B., Annaert W., Cupers P., Saftig P., Craessaerts K., Mumm J.S., Schroeter E.H., Schrijvers V., Wolfe M.S., Ray W.J., et al. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 147.Henley D.B., May P.C., Dean R.A., Siemers E.R. Development of semagacestat (LY450139), a functional γ-secretase inhibitor, for the treatment of Alzheimer’s disease. Expert Opin. Pharmacother. 2009;10:1657–1664. doi: 10.1517/14656560903044982. [DOI] [PubMed] [Google Scholar]
- 148.Imbimbo B.P. Therapeutic potential of gamma-secretase inhibitors and modulators. Curr. Top. Med. Chem. 2008;8:54–61. doi: 10.2174/156802608783334015. [DOI] [PubMed] [Google Scholar]
- 149.Fleisher A.S., Raman R., Siemers E.R., Becerra L., Clark C.M., Dean R.A., Farlow M.R., Galvin J.E., Peskind E.R., Quinn J.F., et al. Phase 2 Safety Trial Targeting Amyloid β Production With a γ-Secretase Inhibitor in Alzheimer Disease. Arch. Neurol. 2008;65:1031–1038. doi: 10.1001/archneur.65.8.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Xia X., Qian S., Soriano S., Wu Y., Fletcher A.M., Wang X.J., Koo E.H., Wu X., Zheng H. Loss of presenilin 1 is associated with enhanced beta-catenin signaling and skin tumorigenesis. Proc. Natl. Acad. Sci. USA. 2001;98:10863–10868. doi: 10.1073/pnas.191284198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nicolas M., Wolfer A., Raj K., Kummer J.A., Mill P., van Noort M., Hui C., Clevers H., Dotto G.P., Radtke F. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 152.Stanger B.Z., Datar R., Murtaugh L.C., Melton D.A. Direct regulation of intestinal fate by Notch. Proc. Natl. Acad. Sci. USA. 2005;102:12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Maillard I., Adler S.H., Pear W.S. Notch and the immune system. Immunity. 2003;19:781–791. doi: 10.1016/S1074-7613(03)00325-X. [DOI] [PubMed] [Google Scholar]
- 154.Bateman R.J., Siemers E.R., Mawuenyega K.G., Wen G., Browning K.R., Sigurdson W.C., Yarasheski K.E., Friedrich S.W., DeMattos R.B., May P.C., et al. A γ-secretase inhibitor decreases amyloid-β production in the central nervous system. Ann. Neurol. 2009;66:48–54. doi: 10.1002/ana.21623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Doody R.S., Raman R., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., He F., Sun X., Thomas R.G., et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013;369:341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 156.Borgegard T., Gustavsson S., Nilsson C., Parpal S., Klintenberg R., Berg A.-L., Rosqvist S., Serneels L., Svensson S., Olsson F., et al. Alzheimer’s Disease: Presenilin 2-Sparing-Secretase Inhibition Is a Tolerable A Peptide-Lowering Strategy. J. Neurosci. 2012;32:17297–17305. doi: 10.1523/JNEUROSCI.1451-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zhao B., Yu M., Neitzel M., Marugg J., Jagodzinski J., Lee M., Hu K., Schenk D., Yednock T., Basi G. Identification of γ-Secretase Inhibitor Potency Determinants on Presenilin. J. Biol. Chem. 2008;283:2927–2938. doi: 10.1074/jbc.M708870200. [DOI] [PubMed] [Google Scholar]
- 158.Serneels L., Van Biervliet J., Craessaerts K., Dejaegere T., Horre K., Van Houtvin T., Esselmann H., Paul S., Schafer M.K., Berezovska O., et al. gamma-Secretase Heterogeneity in the Aph1 Subunit: Relevance for Alzheimer’s Disease. Science. 2009;324:639–642. doi: 10.1126/science.1171176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Gillman K.W., Starrett J.E., Parker M.F., Xie K., Bronson J.J., Marcin L.R., McElhone K.E., Bergstrom C.P., Mate R.A., Williams R., et al. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable γ-secretase inhibitor. ACS Med. Chem. Lett. 2010;1:120–124. doi: 10.1021/ml1000239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Crump C.J., Castro S.V., Wang F., Pozdnyakov N., Ballard T.E., Sisodia S.S., Bales K.R., Johnson D.S., Li Y.-M. BMS-708,163 Targets Presenilin and Lacks Notch-Sparing Activity. Biochemistry. 2012;51:7209–7211. doi: 10.1021/bi301137h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Hopkins C.R. ACS Chemical Neuroscience Molecule Spotlight on ELND006: Another γ-Secretase Inhibitor Fails in the Clinic. ACS Chem. Neurosci. 2011;2:279–280. doi: 10.1021/cn2000469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.ClinicalTrials.gov Global Efficacy Study of MPC-7869 to Treat Patients with Alzheimer’s. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00322036.
- 163.Green R.C., Schneider L.S., Amato D.A., Beelen A.P., Wilcock G., Swabb E.A., Zavitz K.H. Effect of tarenflurbil on cognitive decline and activities of daily living in patients with mild Alzheimer disease: A randomized controlled trial. JAMA J. Am. Med. Assoc. 2009;302:2557–2564. doi: 10.1001/jama.2009.1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.ClinicalTrials.gov Open-label Treatment with MPC-7869 for Patients with Alzheimer’s Who Previously Participated in an MPC-7869 Protocol. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00380276.
- 165.ClinicalTrials.gov Evaluation of Safety & Tolerability of Multiple Dose Regimens of CHF 5074 (CT04) [(accessed on 3 August 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT01303744.
- 166.Ereshefsky L., Jhee S., Yen M., Moran S., Pretorius S., Adams J. Cerebrospinal fluid β-amyloid and dynabridging in Alzheimer’s disease drug development. Biomark. Med. 2009;3:711–721. doi: 10.2217/bmm.09.39. [DOI] [PubMed] [Google Scholar]
- 167.ClinicalTrials.gov Study Evaluating the Coadministration of Begacestat and Donepezil. [(accessed on 3 June 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT00959881.
- 168.ClinicalTrials.gov Development of NIC5-15 in the Treatment of Alzheimer’s Disease. [(accessed on 3 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT00470418.
- 169.ClinicalTrials.gov A single Site, Randomized, Double-Blind, Placebo Controlled Trial of NIC5-15 in Subjects with Alzheimer’s Disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01928420.
- 170.Manzano S., Agüera L., Aguilar M., Olazarán J. A Review on Tramiprosate (Homotaurine) in Alzheimer’s Disease and Other Neurocognitive Disorders. Front. Neurol. 2020;11:614. doi: 10.3389/fneur.2020.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gervais F., Paquette J., Morissette C., Krzywkowski P., Yu M., Azzi M., Lacombe D., Kong X., Aman A., Laurin J., et al. Targeting soluble Aβ peptide with Tramiprosate for the treatment of brain amyloidosis. Neurobiol. Aging. 2007;28:537–547. doi: 10.1016/j.neurobiolaging.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 172.Abushakra S., Porsteinsson A., Vellas B., Cummings J., Gauthier S., Hey J.A., Power A., Hendrix S., Wang P., Shen L., et al. Clinical Benefits of Tramiprosate in Alzheimer’s Disease Are Associated with Higher Number of APOE4 Alleles: The “APOE4 Gene-Dose Effect”. J. Prev. Alzheimer’s Dis. 2016;3:219–228. doi: 10.14283/jpad.2016.115. [DOI] [PubMed] [Google Scholar]
- 173.Kocis P., Tolar M., Yu J., Sinko W., Ray S., Blennow K., Fillit H., Hey J.A. Elucidating the Aβ42 Anti-Aggregation Mechanism of Action of Tramiprosate in Alzheimer’s Disease: Integrating Molecular Analytical Methods, Pharmacokinetic and Clinical Data. CNS Drugs. 2017;31:495–509. doi: 10.1007/s40263-017-0434-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Tolar M., Abushakra S., Sabbagh M. The path forward in Alzheimer’s disease therapeutics: Reevaluating the amyloid cascade hypothesis. Alzheimer’s Dement. 2020 doi: 10.1016/j.jalz.2019.09.075. [DOI] [PubMed] [Google Scholar]
- 175.McLaurin J.A., Kierstead M.E., Brown M.E., Hawkes C.A., Lambermon M.H.L., Phinney A.L., Darabie A.A., Cousins J.E., French J.E., Lan M.F., et al. Cyclohexanehexol inhibitors of Aβ aggregation prevent and reverse Alzheimer phenotype in a mouse model. Nat. Med. 2006;12:801–808. doi: 10.1038/nm1423. [DOI] [PubMed] [Google Scholar]
- 176.ClinicalTrials.gov ELND005 in Patients with Mild to Moderate Alzheimer’s Disease. [(accessed on 18 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/study/NCT00568776.
- 177.Ehrnhoefer D.E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A., Wanker E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 178.Lopez Del Amo J.M., Fink U., Dasari M., Grelle G., Wanker E.E., Bieschke J., Reif B. Structural properties of EGCG-induced, nontoxic Alzheimer’s disease Aβ oligomers. J. Mol. Biol. 2012;421:517–524. doi: 10.1016/j.jmb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 179.Lim G.P., Chu T., Yang F., Beech W., Frautschy S.A., Cole G.M. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci. 2001;21:8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Yanagisawa D., Taguchi H., Yamamoto A., Shirai N., Hirao K., Tooyama I. Curcuminoid binds to amyloid-β1-42 oligomer and fibril. J. Alzheimer’s Dis. 2011;24:33–42. doi: 10.3233/JAD-2011-102100. [DOI] [PubMed] [Google Scholar]
- 181.Liu K.N., Lai C.M., Lee Y.T., Wang S.N., Chen R.P.Y., Jan J.S., Liu H.S., Wang S.S.S. Curcumin’s pre-incubation temperature affects its inhibitory potency toward amyloid fibrillation and fibril-induced cytotoxicity of lysozyme. Biochim. Biophys. Acta Gen. Subj. 2012;1820:1774–1786. doi: 10.1016/j.bbagen.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 182.Hamaguchi T., Ono K., Murase A., Yamada M. Phenolic compounds prevent Alzheimer’s pathology through different effects on the amyloid-β aggregation pathway. Am. J. Pathol. 2009;175:2557–2565. doi: 10.2353/ajpath.2009.090417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Caesar I., Jonson M., Nilsson K.P.R., Thor S., Hammarström P. Curcumin Promotes A-beta Fibrillation and Reduces Neurotoxicity in Transgenic Drosophila. PLoS ONE. 2012;7:e31424. doi: 10.1371/journal.pone.0031424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ringman J.M., Frautschy S.A., Teng E., Begum A.N., Bardens J., Beigi M., Gylys K.H., Badmaev V., Heath D.D., Apostolova L.G., et al. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012;4:43. doi: 10.1186/alzrt146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Uddin M.S., Al Mamun A., Kabir M.T., Ahmad J., Jeandet P., Sarwar M.S., Ashraf G.M., Aleya L. Neuroprotective role of polyphenols against oxidative stress-mediated neurodegeneration. Eur. J. Pharmacol. 2020:173412. doi: 10.1016/j.ejphar.2020.173412. [DOI] [PubMed] [Google Scholar]
- 186.Marambaud P., Zhao H., Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. J. Biol. Chem. 2005;280:37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 187.Jeon S.Y., Kwon S.H., Seong Y.H., Bae K., Hur J.M., Lee Y.Y., Suh D.Y., Song K.S. β-secretase (BACE1)-inhibiting stilbenoids from Smilax Rhizoma. Phytomedicine. 2007;14:403–408. doi: 10.1016/j.phymed.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 188.Huang T.C., Lu K.T., Wo Y.Y.P., Wu Y.J., Yang Y.L. Resveratrol protects rats from Aβ-induced neurotoxicity by the reduction of iNOS expression and lipid peroxidation. PLoS ONE. 2011;6:e29102. doi: 10.1371/journal.pone.0029102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Vingtdeux V., Giliberto L., Zhao H., Chandakkar P., Wu Q., Simon J.E., Janle E.M., Lobo J., Ferruzzi M.G., Davies P., et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-β peptide metabolism. J. Biol. Chem. 2010;285:9100–9113. doi: 10.1074/jbc.M109.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Turner R.S., Thomas R.G., Craft S., Van Dyck C.H., Mintzer J., Reynolds B.A., Brewer J.B., Rissman R.A., Raman R., Aisen P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology. 2015;85:1383–1391. doi: 10.1212/WNL.0000000000002035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zhu C.W., Grossman H., Neugroschl J., Parker S., Burden A., Luo X., Sano M. A randomized, double-blind, placebo-controlled trial of resveratrol with glucose and malate (RGM) to slow the progression of Alzheimer’s disease: A pilot study. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018;4:609–616. doi: 10.1016/j.trci.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shimmyo Y., Kihara T., Akaike A., Niidome T., Sugimoto H. Flavonols and flavones as BACE-1 inhibitors: Structure-activity relationship in cell-free, cell-based and in silico studies reveal novel pharmacophore features. Biochim. Biophys. Acta Gen. Subj. 2008;1780:819–825. doi: 10.1016/j.bbagen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- 193.Lu J., Wu D.M., Zheng Y.L., Hu B., Zhang Z.F., Shan Q., Zheng Z.H., Liu C.M., Wang Y.J. Quercetin activates AMP-activated protein kinase by reducing PP2C expression protecting old mouse brain against high cholesterol-induced neurotoxicity. J. Pathol. 2010;222:199–212. doi: 10.1002/path.2754. [DOI] [PubMed] [Google Scholar]
- 194.Ansari M.A., Abdul H.M., Joshi G., Opii W.O., Butterfield D.A. Protective effect of quercetin in primary neurons against Aβ(1-42): Relevance to Alzheimer’s disease. J. Nutr. Biochem. 2009;20:269–275. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Pocernich C.P., Lange M.L.B., Sultana R., Butterfield D.A. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 2011;8:452–469. doi: 10.2174/156720511796391908. [DOI] [PubMed] [Google Scholar]
- 196.Kim H., Park B.S., Lee K.G., Cheol Y.C., Sung S.J., Kim Y.H., Lee S.E. Effects of naturally occurring compounds on fibril formation and oxidative stress of β-amyloid. J. Agric. Food Chem. 2005;53:8537–8541. doi: 10.1021/jf051985c. [DOI] [PubMed] [Google Scholar]
- 197.Shimmyo Y., Kihara T., Akaike A., Niidome T., Sugimoto H. Multifunction of myricetin on Aβ: Neuroprotection via a conformational change of Aβ and reduction of Aβ via the interference of secretases. J. Neurosci. Res. 2008;86:368–377. doi: 10.1002/jnr.21476. [DOI] [PubMed] [Google Scholar]
- 198.Uddin M.S., Kabir M.T. Biological, Diagnostic and Therapeutic Advances in Alzheimer’s Disease. Springer; Singapore: 2019. Oxidative Stress in Alzheimer’s Disease: Molecular Hallmarks of Underlying Vulnerability; pp. 91–115. [Google Scholar]
- 199.Desai V., Kaler S.G. Role of copper in human neurological disorders. Am. J. Clin. Nutr. 2008;88:855S–858S. doi: 10.1093/ajcn/88.3.855S. [DOI] [PubMed] [Google Scholar]
- 200.Bush A.I. Drug development based on the metals hypothesis of Alzheimer’s disease. J. Alzheimer’s Dis. 2008;15:223–240. doi: 10.3233/JAD-2008-15208. [DOI] [PubMed] [Google Scholar]
- 201.Faller P., Hureau C., Berthoumieu O. Role of metal ions in the self-assembly of the Alzheimer’s amyloid-β peptide. Inorg. Chem. 2013;52:12193–12206. doi: 10.1021/ic4003059. [DOI] [PubMed] [Google Scholar]
- 202.Lovell M.A., Robertson J.D., Teesdale W.J., Campbell J.L., Markesbery W.R. Copper, iron and zinc in Alzheimer’s disease senile plaques. J. Neurol. Sci. 1998;158:47–52. doi: 10.1016/S0022-510X(98)00092-6. [DOI] [PubMed] [Google Scholar]
- 203.House E., Collingwood J., Khan A., Korchazkina O., Berthon G., Exley C. Aluminium, iron, zinc and copper Influence the in vitro formation of amyloid fibrils of Aβ42 in a manner which may have consequences for metal chelation therapy in Alzheimer’s disease. J. Alzheimer’s Dis. 2004;6:291–301. doi: 10.3233/JAD-2004-6310. [DOI] [PubMed] [Google Scholar]
- 204.Khan A., Dobson J.P., Exley C. Redox cycling of iron by Aβ42. Free Radic. Biol. Med. 2006;40:557–569. doi: 10.1016/j.freeradbiomed.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 205.Adlard P.A., Cherny R.A., Finkelstein D.I., Gautier E., Robb E., Cortes M., Volitakis I., Liu X., Smith J.P., Perez K., et al. Rapid Restoration of Cognition in Alzheimer’s Transgenic Mice with 8-Hydroxy Quinoline Analogs is Associated with Decreased Interstitial Aβ. Neuron. 2008;59:43–55. doi: 10.1016/j.neuron.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 206.Faux N.G., Ritchie C.W., Gunn A., Rembach A., Tsatsanis A., Bedo J., Harrison J., Lannfelt L., Blennow K., Zetterberg H., et al. PBT2 rapidly improves cognition in alzheimer’s disease: Additional phase II analyses. J. Alzheimer’s Dis. 2010;20:509–516. doi: 10.3233/JAD-2010-1390. [DOI] [PubMed] [Google Scholar]
- 207.Smith M.A., Harris P.L.R., Sayre L.M., Perry G. Iron accumulation in Alzheimer disease is a source of redox-generated free radicals. Proc. Natl. Acad. Sci. USA. 1997;94:9866–9868. doi: 10.1073/pnas.94.18.9866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Sayre L.M., Perry G., Harris P.L.R., Liu Y., Schubert K.A., Smith M.A. In situ oxidative catalysis by neurofibrillary tangles and senile plaques in Alzheimer’s disease: A central role for bound transition metals. J. Neurochem. 2000;74:270–279. doi: 10.1046/j.1471-4159.2000.0740270.x. [DOI] [PubMed] [Google Scholar]
- 209.Suh S.W., Jensen K.B., Jensen M.S., Silva D.S., Kesslak P.J., Danscher G., Frederickson C.J. Histochemically-reactive zinc in amyloid plaques, angiopathy, and degenerating neurons of Alzheimer’s diseased brains. Brain Res. 2000;852:274–278. doi: 10.1016/S0006-8993(99)02096-X. [DOI] [PubMed] [Google Scholar]
- 210.Dong J., Atwood C.S., Anderson V.E., Siedlak S.L., Smith M.A., Perry G., Carey P.R. Metal binding and oxidation of amyloid-β within isolated senile plaque cores: Raman microscopic evidence. Biochemistry. 2003;42:2768–2773. doi: 10.1021/bi0272151. [DOI] [PubMed] [Google Scholar]
- 211.Smith M.A., Hirai K., Hsiao K., Pappolla M.A., Harris P.L.R., Siedlak S.L., Tabaton M., Perry G. Amyloid-β Deposition in Alzheimer Transgenic Mice Is Associated with Oxidative Stress. J. Neurochem. 2002;70:2212–2215. doi: 10.1046/j.1471-4159.1998.70052212.x. [DOI] [PubMed] [Google Scholar]
- 212.Lee J.Y., Mook-Jung I., Koh J.Y. Histochemically reactive zinc in plaques of the Swedish mutant beta-amyloid precursor protein transgenic mice. J. Neurosci. 1999;19:RC10. doi: 10.1523/JNEUROSCI.19-11-j0002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Bush A.I., Pettingell W.H., Multhaup G., Paradis M.D., Vonsattel J.P., Gusella J.F., Beyreuther K., Masters C.L., Tanzi R.E. Rapid induction of Alzheimer Aβ amyloid formation by zinc. Science. 1994;265:1464–1467. doi: 10.1126/science.8073293. [DOI] [PubMed] [Google Scholar]
- 214.Atwood C.S., Moir R.D., Huang X., Scarpa R.C., Bacarra N.M.E., Romano D.M., Hartshorn M.A., Tanzi R.E., Bush A.I. Dramatic aggregation of alzheimer by Cu(II) is induced by conditions representing physiological acidosis. J. Biol. Chem. 1998;273:12817–12826. doi: 10.1074/jbc.273.21.12817. [DOI] [PubMed] [Google Scholar]
- 215.Atwood C.S., Scarpa R.C., Huang X., Moir R.D., Jones W.D., Fairlie D.P., Tanzi R.E., Bush A.I. Characterization of copper interactions with Alzheimer amyloid β peptides: Identification of an attomolar-affinity copper binding site on amyloid β1-42. J. Neurochem. 2000;75:1219–1233. doi: 10.1046/j.1471-4159.2000.0751219.x. [DOI] [PubMed] [Google Scholar]
- 216.Curtain C.C., Ali F., Volitakis I., Cherny R.A., Norton R.S., Beyreuther K., Barrow C.J., Masters C.L., Bush A.I., Barnham K.J. Alzheimer’s Disease Amyloid-β Binds Copper and Zinc to Generate an Allosterically Ordered Membrane-penetrating Structure Containing Superoxide Dismutase-like Subunits. J. Biol. Chem. 2001;276:20466–20473. doi: 10.1074/jbc.M100175200. [DOI] [PubMed] [Google Scholar]
- 217.Huang X., Cuajungco M.P., Atwood C.S., Hartshorn M.A., Tyndall J.D.A., Hanson G.R., Stokes K.C., Leopold M., Multhaup G., Goldstein L.E., et al. Cu(II) potentiation of Alzheimer aβ neurotoxicity. Correlation with cell-free hydrogen peroxide production and metal reduction. J. Biol. Chem. 1999;274:37111–37116. doi: 10.1074/jbc.274.52.37111. [DOI] [PubMed] [Google Scholar]
- 218.Bayer T.A., Schäfer S., Simons A., Kemmling A., Kamer T., Tepest R., Eckert A., Schüssel K., Eikenberg O., Sturchler-Pierrat C., et al. Dietary Cu stabilizes brain superoxide dismutase 1 activity and reduces amyloid Aβ production in APP23 transgenic mice. Proc. Natl. Acad. Sci. USA. 2003;100:14187–14192. doi: 10.1073/pnas.2332818100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Phinney A.L., Drisaldi B., Schmidt S.D., Lugowski S., Coronado V., Liang Y., Horne P., Yang J., Sekoulidis J., Coomaraswamy J., et al. In vivo reduction of amyloid-β by a mutant copper transporter. Proc. Natl. Acad. Sci. USA. 2003;100:14193–14198. doi: 10.1073/pnas.2332851100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Maynard C.J., Cappai R., Volitakis I., Cherny R.A., White A.R., Beyreuther K., Masters C.L., Bush A.I., Li Q.X. Overexpression of Alzheimer’s disease amyloid-β opposes the age-dependent elevations of brain copper and iron. J. Biol. Chem. 2002;277:44670–44676. doi: 10.1074/jbc.M204379200. [DOI] [PubMed] [Google Scholar]
- 221.Borchardt T., Camakaris J., Cappai R., Masters C.L., Beyreuther K., Multhaup G. Copper inhibits beta-amyloid production and stimulates the non-amyloidogenic pathway of amyloid-precursor-protein secretion. Biochem. J. 1999;344 Pt2:461–467. doi: 10.1042/bj3440461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bard F., Cannon C., Barbour R., Burke R.-L., Games D., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6:916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 223.Vasilevko V., Xu F., Previti M.L., Van Nostrand W.E., Cribbs D.H. Experimental Investigation of Antibody-Mediated Clearance Mechanisms of Amyloid- in CNS of Tg-SwDI Transgenic Mice. J. Neurosci. 2007;27:13376–13383. doi: 10.1523/JNEUROSCI.2788-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schenk D., Barbour R., Dunn W., Gordon G., Grajeda H., Guido T., Hu K., Huang J., Johnson-Wood K., Khan K., et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 225.Weiner H.L., Lemere C.A., Maron R., Spooner E.T., Grenfell T.J., Mori C., Issazadeh S., Hancock W.W., Selkoe D.J. Nasal administration of amyloid-beta peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann. Neurol. 2000;48:567–579. doi: 10.1002/1531-8249(200010)48:4<567::AID-ANA3>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 226.Kabir M.T., Uddin M.S., Mathew B., Das P.K., Perveen A., Ashraf G.M. Emerging Promise of Immunotherapy for Alzheimer’s Disease: A New Hope for the Development of Alzheimer’s Vaccine. Curr. Top. Med. Chem. 2020;20:1214–1234. doi: 10.2174/1568026620666200422105156. [DOI] [PubMed] [Google Scholar]
- 227.Lambracht-Washington D., Rosenberg R.N. Advances in the development of vaccines for alzheimer’s disease. Discov. Med. 2013;15:319–326. [PMC free article] [PubMed] [Google Scholar]
- 228.Lemere C.A., Masliah E. Can Alzheimer disease be prevented by amyloid-Β immunotherapy? Nat. Rev. Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wiessner C., Wiederhold K.H., Tissot A.C., Frey P., Danner S., Jacobson L.H., Jennings G.T., Lüönd R., Ortmann R., Reichwald J., et al. The second-generation active Aβ immunotherapy CAD106 reduces amyloid accumulation in APP transgenic mice while minimizing potential side effects. J. Neurosci. 2011;31:9323–9331. doi: 10.1523/JNEUROSCI.0293-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Winblad B., Andreasen N., Minthon L., Floesser A., Imbert G., Dumortier T., Maguire R.P., Blennow K., Lundmark J., Staufenbiel M., et al. Safety, tolerability, and antibody response of active Aβ immunotherapy with CAD106 in patients with Alzheimer’s disease: Randomised, double-blind, placebo-controlled, first-in-human study. Lancet Neurol. 2012;11:597–604. doi: 10.1016/S1474-4422(12)70140-0. [DOI] [PubMed] [Google Scholar]
- 231.Winblad B., Minthon L., Floesser A. Results of the first-in-man study with the active Aβimmunotherapy CAD106 in Alzheimer patients. Alzheimer’s Dement. 2009;5:P113–P114. doi: 10.1016/j.jalz.2009.05.356. [DOI] [Google Scholar]
- 232.Schneeberger A., Mandler M., Otawa O., Zauner W., Mattner F., Schmidt W. Development of AFFITOPE vaccines for Alzheimer’s disease (AD)—From concept to clinical testing. J. Nutr. Health Aging. 2009;13:264–267. doi: 10.1007/s12603-009-0070-5. [DOI] [PubMed] [Google Scholar]
- 233.Khandelwal P.J., Herman A.M., Moussa C.E.-H. Inflammation in the early stages of neurodegenerative pathology. J. Neuroimmunol. 2011;238:1–11. doi: 10.1016/j.jneuroim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Winblad B., Graf A., Riviere M.-E., Andreasen N., Ryan J. Active immunotherapy options for Alzheimer’s disease. Alzheimers. Res. Ther. 2014;6:7. doi: 10.1186/alzrt237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Schneeberger A., Hendrix S., Mandler M., Ellison N., Bürger V., Brunner M., Frölich L., Mimica N., Hort J., Rainer M., et al. Results from a Phase II Study to Assess the Clinical and Immunological Activity of AFFITOPE® AD02 in Patients with Early Alzheimer’s Disease. J. Prev. Alzheimer’s Dis. 2015;2:103–114. doi: 10.14283/jpad.2015.63. [DOI] [PubMed] [Google Scholar]
- 236.Alzforum Vanutide Cridificar. [(accessed on 3 August 2020)]; Available online: https://www.alzforum.org/therapeutics/vanutide-cridificar.
- 237.ClinicalTrials.gov Long Term Extension Study Evaluating Safety, Tolerability and Immunogenicity of ACC-001 in Japanese Subjects with Mild to Moderate Alzheimer’s Disease. [(accessed on 18 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01238991.
- 238.ClinicalTrials.gov Study Evaluating Safety, Tolerability, and Immunogenicity of ACC-001 in Subjects with Mild to Moderate Alzheimer’s Disease. [(accessed on 18 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00479557.
- 239.ClinicalTrials.gov Study Evaluating ACC-001 in Subjects with Mild to Moderate Alzheimer’s Disease. [(accessed on 18 June 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT00498602.
- 240.Wilcock D.M., Gharkholonarehe N., Van Nostrand W.E., Davis J., Vitek M.P., Colton C.A. Amyloid Reduction by Amyloid- Vaccination Also Reduces Mouse Tau Pathology and Protects from Neuron Loss in Two Mouse Models of Alzheimer’s Disease. J. Neurosci. 2009;29:7957–7965. doi: 10.1523/JNEUROSCI.1339-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Oddo S., Billings L., Kesslak J.P., Cribbs D.H., LaFerla F.M. Aβ Immunotherapy Leads to Clearance of Early, but Not Late, Hyperphosphorylated Tau Aggregates via the Proteasome. Neuron. 2004;43:321–332. doi: 10.1016/j.neuron.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 242.Klyubin I., Walsh D.M., Lemere C.A., Cullen W.K., Shankar G.M., Betts V., Spooner E.T., Jiang L., Anwyl R., Selkoe D.J., et al. Amyloid β protein immunotherapy neutralizes Aβ oligomers that disrupt synaptic plasticity in vivo. Nat. Med. 2005;11:556–561. doi: 10.1038/nm1234. [DOI] [PubMed] [Google Scholar]
- 243.Salloway S., Sperling R., Fox N.C., Blennow K., Klunk W., Raskind M., Sabbagh M., Honig L.S., Porsteinsson A.P., Ferris S., et al. Two phase 3 trials of Bapineuzumab in mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014;370:322–333. doi: 10.1056/NEJMoa1304839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Doody R.S., Thomas R.G., Farlow M., Iwatsubo T., Vellas B., Joffe S., Kieburtz K., Raman R., Sun X., Aisen P.S., et al. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014;370:311–321. doi: 10.1056/NEJMoa1312889. [DOI] [PubMed] [Google Scholar]
- 245.ClinicalTrials.gov Study Evaluating the Safety, Tolerability and Efficacy of PF-04360365 in Adults with Probable Cerebral Amyloid Angiopathy. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01821118.
- 246.Samadi H., Sultzer D. Solanezumab for Alzheimer’s disease. Expert Opin. Biol. Ther. 2011;11:787–798. doi: 10.1517/14712598.2011.578573. [DOI] [PubMed] [Google Scholar]
- 247.Bohrmann B., Baumann K., Benz J., Gerber F., Huber W., Knoflach F., Messer J., Oroszlan K., Rauchenberger R., Richter W.F., et al. Gantenerumab: A novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J. Alzheimer’s Dis. 2012;28:49–69. doi: 10.3233/JAD-2011-110977. [DOI] [PubMed] [Google Scholar]
- 248.Ostrowitzki S., Deptula D., Thurfjell L., Barkhof F., Bohrmann B., Brooks D.J., Klunk W.E., Ashford E., Yoo K., Xu Z.X., et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch. Neurol. 2012;69:198–207. doi: 10.1001/archneurol.2011.1538. [DOI] [PubMed] [Google Scholar]
- 249.ClinicalTrials.gov Dominantly Inherited Alzheimer Network Trial: An Opportunity to Prevent Dementia. A Study of Potential Disease Modifying Treatments in Individuals at Risk for or with a Type of Early Onset Alzheimer’s Disease Caused by a Genetic Mutation. [(accessed on 3 August 2020)]; Available online: https://www.clinicaltrials.gov/ct2/show/NCT01760005.
- 250.Washington University School of Medicine The Dominantly Inherited Alzheimer Network: Clinical Trial. [(accessed on 3 August 2020)]; Available online: https://dian.wustl.edu/our-research/clinical-trial/
- 251.Alzheimer’s News Today Solanezumab. [(accessed on 3 August 2020)]; Available online: https://alzheimersnewstoday.com/solanezumab/
- 252.Adolfsson O., Pihlgren M., Toni N., Varisco Y., Buccarello A.L., Antoniello K., Lohmann S., Piorkowska K., Gafner V., Atwal J.K., et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J. Neurosci. 2012;32:9677–9689. doi: 10.1523/JNEUROSCI.4742-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Freeman G.B., Brown T.P., Wallace K., Bales K.R. Chronic administration of an aglycosylated murine antibody of ponezumab does not worsen microhemorrhages in Aged Tg2576 mice. Curr. Alzheimer Res. 2012;9:1059–1068. doi: 10.2174/156720512803569064. [DOI] [PubMed] [Google Scholar]
- 254.La Porte S.L., Bollini S.S., Lanz T.A., Abdiche Y.N., Rusnak A.S., Ho W.H., Kobayashi D., Harrabi O., Pappas D., Mina E.W., et al. Structural basis of C-terminal β-amyloid peptide binding by the antibody ponezumab for the treatment of Alzheimer’s disease. J. Mol. Biol. 2012;421:525–536. doi: 10.1016/j.jmb.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 255.ClinicalTrials.gov Multiple Dose Study of Aducanumab (BIIB037) (Recombinant, Fully Human Anti-Aβ IgG1 mAb) in Participants with Prodromal or Mild Alzheimer’s Disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01677572.
- 256.ClinicalTrials.gov 221AD301 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02477800.
- 257.ClinicalTrials.gov 221AD302 Phase 3 Study of Aducanumab (BIIB037) in Early Alzheimer’s Disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/results/NCT02484547.
- 258.Biogen About Aducanumab. [(accessed on 3 August 2020)]; Available online: https://biogenalzheimers.com/about-aducanumab/
- 259.Biogen Biogen Completes Submission of Biologics License Application to FDA for Aducanumab as a Treatment for Alzheimer’s Disease. [(accessed on 3 August 2020)]; Available online: https://investors.biogen.com/news-releases/news-release-details/biogen-completes-submission-biologics-license-application-fda.
- 260.Logovinsky V., Satlin A., Lai R., Swanson C., Kaplow J., Osswald G., Basun H., Lannfelt L. Safety and tolerability of BAN2401—A clinical study in Alzheimer’s disease with a protofibril selective Aβ antibody. Alzheimers. Res. Ther. 2016;8:14. doi: 10.1186/s13195-016-0181-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Tucker S., Möller C., Tegerstedt K., Lord A., Laudon H., Sjödahl J., Söderberg L., Spens E., Sahlin C., Waara E.R., et al. The murine Version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe Mice. J. Alzheimer’s Dis. 2015;43:575–588. doi: 10.3233/JAD-140741. [DOI] [PubMed] [Google Scholar]
- 262.Söllvander S., Nikitidou E., Gallasch L., Zyśk M., Söderberg L., Sehlin D., Lannfelt L., Erlandsson A. The Aβ protofibril selective antibody mAb158 prevents accumulation of Aβ in astrocytes and rescues neurons from Aβ-induced cell death. J. Neuroinflammation. 2018;15:98. doi: 10.1186/s12974-018-1134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.ClinicalTrials.gov A Study to Confirm Safety and Efficacy of BAN2401 in Participants with Early Alzheimer’s Disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03887455.
- 264.Tanzi R.E., Moir R.D., Wagner S.L. Clearance of Alzheimer’s Aβ Peptide. Neuron. 2004;43:605–608. doi: 10.1016/j.neuron.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 265.Deane R., Wu Z., Zlokovic B. V RAGE (Yin) Versus LRP (Yang) Balance Regulates Alzheimer Amyloid -Peptide Clearance through Transport across the Blood-Brain Barrier. Stroke. 2004;35:2628–2631. doi: 10.1161/01.STR.0000143452.85382.d1. [DOI] [PubMed] [Google Scholar]
- 266.Zlokovic B.V. New therapeutic targets in the neurovascular pathway in Alzheimer’s disease. Neurotherapeutics. 2008;5:409–414. doi: 10.1016/j.nurt.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Deane R., Zlokovic B. V Role of the blood-brain barrier in the pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2007;4:191–197. doi: 10.2174/156720507780362245. [DOI] [PubMed] [Google Scholar]
- 268.Deane R., Sagare A., Zlokovic B. V The role of the cell surface LRP and soluble LRP in blood-brain barrier Abeta clearance in Alzheimer’s disease. Curr. Pharm. Des. 2008;14:1601–1605. doi: 10.2174/138161208784705487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Marques M.A., Kulstad J.J., Savard C.E., Green P.S., Lee S.P., Craft S., Watson G.S., Cook D.G. Peripheral Amyloid-β Levels Regulate Amyloid-β Clearance from the Central Nervous System. J. Alzheimer’s Dis. 2009;16:325–329. doi: 10.3233/JAD-2009-0964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Sagare A., Deane R., Bell R.D., Johnson B., Hamm K., Pendu R., Marky A., Lenting P.J., Wu Z., Zarcone T., et al. Clearance of amyloid-β by circulating lipoprotein receptors. Nat. Med. 2007;13:1029–1031. doi: 10.1038/nm1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Chaney M.O., Stine W.B., Kokjohn T.A., Kuo Y.-M., Esh C., Rahman A., Luehrs D.C., Schmidt A.M., Stern D., Du Yan S., et al. RAGE and amyloid beta interactions: Atomic force microscopy and molecular modeling. Biochim. Biophys. Acta Mol. Basis Dis. 2005;1741:199–205. doi: 10.1016/j.bbadis.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 272.Du Yan S., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J., et al. RAGE and amyloid-β peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382:685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 273.Stern D.M., Du Yan S., Yan S.F., Schmidt A.M. Receptor for advanced glycation endproducts (RAGE) and the complications of diabetes. Ageing Res. Rev. 2002;1:1–15. doi: 10.1016/S0047-6374(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 274.Zlokovic B.V. Cerebrovascular transport of Alzheimer’s amyloid beta and apolipoproteins J and E: Possible anti-amyloidogenic role of the blood-brain barrier. Life Sci. 1996;59:1483–1497. doi: 10.1016/0024-3205(96)00310-4. [DOI] [PubMed] [Google Scholar]
- 275.Wang W., Bodles-Brakhop A.M., Barger S.W. A role for P-glycoprotein in clearance of Alzheimer amyloid β-peptide from the brain. Curr. Alzheimer Res. 2016;13:615–620. doi: 10.2174/1567205013666160314151012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Park J.H., Strittmatter S.M. Nogo receptor interacts with brain APP and Abeta to reduce pathologic changes in Alzheimer’s transgenic mice. Curr. Alzheimer Res. 2007;4:568–570. doi: 10.2174/156720507783018235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Tang B.L., Liou Y.C. Novel modulators of amyloid-β precursor protein processing. J. Neurochem. 2007;100:314–323. doi: 10.1111/j.1471-4159.2006.04215.x. [DOI] [PubMed] [Google Scholar]
- 278.Yamazaki Y., Kanekiyo T. Blood-brain barrier dysfunction and the pathogenesis of Alzheimer’s disease. Int. J. Mol. Sci. 2017;18:1965. doi: 10.3390/ijms18091965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Mao P., Manczak M., Calkins M.J., Truong Q., Reddy T.P., Reddy A.P., Shirendeb U., Lo H.-H., Rabinovitch P.S., Reddy P.H. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid production and BACE1 in a mouse model of Alzheimer’s disease: Implications for neuroprotection and lifespan extension. Hum. Mol. Genet. 2012;21:2973–2990. doi: 10.1093/hmg/dds128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Uddin M.S., Kabir M.T., Al Mamun A., Barreto G.E., Rashid M., Perveen A., Ashraf G.M. Pharmacological approaches to mitigate neuroinflammation in Alzheimer’s disease. Int. Immunopharmacol. 2020;84:106479. doi: 10.1016/j.intimp.2020.106479. [DOI] [PubMed] [Google Scholar]
- 281.Carret-Rebillat A.-S., Pace C., Gourmaud S., Ravasi L., Montagne-Stora S., Longueville S., Tible M., Sudol E., Chang R.C.-C., Paquet C., et al. Neuroinflammation and Aβ accumulation linked to systemic inflammation are decreased by genetic PKR down-regulation. Sci. Rep. 2015;5:8489. doi: 10.1038/srep08489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Cai H.-Y., Yang J.-T., Wang Z.-J., Zhang J., Yang W., Wu M.-N., Qi J.-S. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2018;495:1034–1040. doi: 10.1016/j.bbrc.2017.11.114. [DOI] [PubMed] [Google Scholar]
- 283.Mullane K., Williams M. Alzheimer’s disease (AD) therapeutics – 1: Repeated clinical failures continue to question the amyloid hypothesis of AD and the current understanding of AD causality. Biochem. Pharmacol. 2018;158:359–375. doi: 10.1016/j.bcp.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 284.Scheltens P., Blennow K., Breteler M.M.B., de Strooper B., Frisoni G.B., Salloway S., Van der Flier W.M. Alzheimer’s disease. Lancet. 2016;388:505–517. doi: 10.1016/S0140-6736(15)01124-1. [DOI] [PubMed] [Google Scholar]
- 285.Coric V., Salloway S., Van Dyck C.H., Dubois B., Andreasen N., Brody M., Curtis C., Soininen H., Thein S., Shiovitz T., et al. Targeting prodromal Alzheimer disease with avagacestat: A randomized clinical trial. J. Am. Med Assoc. Neurol. 2015;72:1324–1333. doi: 10.1001/jamaneurol.2015.0607. [DOI] [PubMed] [Google Scholar]
- 286.Timmers M., Streffer J.R., Russu A., Tominaga Y., Shimizu H., Shiraishi A., Tatikola K., Smekens P., Börjesson-Hanson A., Andreasen N., et al. Pharmacodynamics of atabecestat (JNJ-54861911), an oral BACE1 inhibitor in patients with early Alzheimer’s disease: Randomized, double-blind, placebo-controlled study. Alzheimer’s Res. Ther. 2018;10:85. doi: 10.1186/s13195-018-0415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.FierceBiotech Janssen Drops the BACE as Alzheimer’s Candidate Joins Fail List. [(accessed on 3 August 2020)]; Available online: https://www.fiercebiotech.com/biotech/janssen-drops-bace-as-alzheimer-s-candidate-joins-fail-list.
- 288.Janssen Update on Janssen’s BACE Inhibitor Program Regarding the Dominantly Inherited Alzheimer’s Network Trial (DIAN-TU) [(accessed on 3 August 2020)]; Available online: https://www.janssen.com/neuroscience/update-janssens-bace-inhibitor-program-regarding-DIAN-TU.
- 289.ClinicalTrials.gov A Study of Lanabecestat (LY3314814) in Early Alzheimer’s Disease Dementia. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02972658.
- 290.Cebers G., Alexander R.C., Haeberlein S.B., Han D., Goldwater R., Ereshefsky L., Olsson T., Ye N., Rosen L., Russell M., et al. AZD3293: Pharmacokinetic and Pharmacodynamic Effects in Healthy Subjects and Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;55:1039–1053. doi: 10.3233/JAD-160701. [DOI] [PubMed] [Google Scholar]
- 291.Eketjäll S., Janson J., Kaspersson K., Bogstedt A., Jeppsson F., Fälting J., Haeberlein S.B., Kugler A.R., Alexander R.C., Cebers G. AZD3293: A Novel, Orally Active BACE1 Inhibitor with High Potency and Permeability and Markedly Slow Off-Rate Kinetics. J. Alzheimer’s Dis. 2016;50:1109–1123. doi: 10.3233/JAD-150834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Egan M.F., Kost J., Tariot P.N., Aisen P.S., Cummings J.L., Vellas B., Sur C., Mukai Y., Voss T., Furtek C., et al. Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2018;378:1691–1703. doi: 10.1056/NEJMoa1706441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Selkoe D.J. Resolving controversies on the path to Alzheimer’s therapeutics. Nat. Med. 2011;17:1060–1065. doi: 10.1038/nm.2460. [DOI] [PubMed] [Google Scholar]
- 294.Sabbagh M.N. Clinical Effects of Oral Tramiprosate in APOE4/4 Homozygous Patients with Mild Alzheimer’s Disease Suggest Disease Modification. J. Prev. Alzheimer’s Dis. 2017;4:136–137. doi: 10.14283/jpad.2017.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ostrowitzki S., Lasser R.A., Dorflinger E., Scheltens P., Barkhof F., Nikolcheva T., Ashford E., Retout S., Hofmann C., Delmar P., et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2017;9:95. doi: 10.1186/s13195-017-0318-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Cummings J.L., Cohen S., Van Dyck C.H., Brody M., Curtis C., Cho W., Ward M., Friesenhahn M., Brunstein F., Quartino A., et al. A phase 2 randomized trial of crenezumab in mild to moderate Alzheimer disease. Neurology. 2018;90:E1889–E1897. doi: 10.1212/WNL.0000000000005550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Salloway S.P., Sperling R., Fox N.C., Sabbagh M.N., Honig L.S., Porsteinsson A.P., Rofael H., Ketter N., Wang D., Liu E., et al. Long-term follow up of patients with mild-to-moderate Alzheimer’s disease treated with bapineuzumab in a Phase III, open-label, extension study. J. Alzheimer’s Dis. 2018;64:689–707. doi: 10.3233/JAD-171157. [DOI] [PubMed] [Google Scholar]
- 298.Ketter N., Brashear H.R., Bogert J., Di J., Miaux Y., Gass A., Purcell D.D., Barkhof F., Arrighi H.M. Central Review of Amyloid-Related Imaging Abnormalities in Two Phase III Clinical Trials of Bapineuzumab in Mild-To-Moderate Alzheimer’s Disease Patients. J. Alzheimers. Dis. 2017;57:557–573. doi: 10.3233/JAD-160216. [DOI] [PubMed] [Google Scholar]
- 299.ClinicalTrials.gov A Study to Evaluate Safety and Tolerability of Aducanumab in Participants with Alzheimer’s Disease Who Had Previously Participated in the Aducanumab Studies 221AD103, 221AD301, 221AD302 and 221AD205. [(accessed on 30 July 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT04241068.
- 300.ClinicalTrials.gov A Study of CAD106 and CNP520 Versus Placebo in Participants at Risk for the Onset of Clinical Symptoms of Alzheimer’s disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02565511.
- 301.ClinicalTrials.gov Safety and Efficacy Study of Gantenerumab in Participants with Early Alzheimer’s Disease (AD) [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03443973.
- 302.ClinicalTrials.gov Efficacy and Safety Study of Gantenerumab in Participants with Early Alzheimer’s Disease (AD) [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT03444870.
- 303.ClinicalTrials.gov Clinical Trial of Solanezumab for Older Individuals Who May Be at Risk for Memory Loss. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02008357.
- 304.ClinicalTrials.gov An Efficacy and Safety Study of Sodium Oligo-Mannurarate (GV-971) Capsule for the Treatment of Alzheimer’s Disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02293915.
- 305.ClinicalTrials.gov A Study of Gantenerumab in Participants with Mild Alzheimer Disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT02051608.
- 306.ClinicalTrials.gov A Study to Evaluate Albumin and Immunoglobulin in Alzheimer’s Disease. [(accessed on 3 August 2020)]; Available online: https://clinicaltrials.gov/ct2/show/NCT01561053.
- 307.Doig A.J., Del Castillo-Frias M.P., Berthoumieu O., Tarus B., Nasica-Labouze J., Sterpone F., Nguyen P.H., Hooper N.M., Faller P., Derreumaux P. Why Is Research on Amyloid-β Failing to Give New Drugs for Alzheimer’s Disease? ACS Chem. Neurosci. 2017;8:1435–1437. doi: 10.1021/acschemneuro.7b00188. [DOI] [PubMed] [Google Scholar]
- 308.Selkoe D.J. Alzheimer disease and aducanumab: Adjusting our approach. Nat. Rev. Neurol. 2019;15:365–366. doi: 10.1038/s41582-019-0205-1. [DOI] [PubMed] [Google Scholar]
- 309.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 310.Butterfield D.A., Di Domenico F., Barone E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys. Acta Mol. Basis Dis. 2014;1842:1693–1706. doi: 10.1016/j.bbadis.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Arnold S.E., Arvanitakis Z., Macauley-Rambach S.L., Koenig A.M., Wang H.Y., Ahima R.S., Craft S., Gandy S., Buettner C., Stoeckel L.E., et al. Brain insulin resistance in type 2 diabetes and Alzheimer disease: Concepts and conundrums. Nat. Rev. Neurol. 2018;14:168–181. doi: 10.1038/nrneurol.2017.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Mielke J.G., Wang Y.T. Progress in Molecular Biology and Translational Science. Vol. 98. Academic Press; Cambridge, MA, USA: 2011. Insulin, synaptic function, and opportunities for neuroprotection; pp. 133–186. [DOI] [PubMed] [Google Scholar]
- 313.Chiu S.L., Chen C.M., Cline H.T. Insulin Receptor Signaling Regulates Synapse Number, Dendritic Plasticity, and Circuit Function In Vivo. Neuron. 2008;58:708–719. doi: 10.1016/j.neuron.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.De Felice F.G., Ferreira S.T. Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer Disease. Diabetes. 2014;63:2262–2272. doi: 10.2337/db13-1954. [DOI] [PubMed] [Google Scholar]
- 315.Yarchoan M., Arnold S.E. Repurposing diabetes drugs for brain insulin resistance in Alzheimer disease. Diabetes. 2014;63:2253–2261. doi: 10.2337/db14-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Bruehl H., Sweat V., Hassenstab J., Polyakov V., Convit A. Cognitive impairment in nondiabetic middle-aged and older adults is associated with insulin resistance. J. Clin. Exp. Neuropsychol. 2010;32:487–493. doi: 10.1080/13803390903224928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Benedict C., Grillo C.A. Insulin resistance as a therapeutic target in the treatment of Alzheimer’s disease: A state-of-the-art review. Front. Neurosci. 2018;12:215. doi: 10.3389/fnins.2018.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Batista A.F., Forny-Germano L., Clarke J.R., Lyra e Silva N.M., Brito-Moreira J., Boehnke S.E., Winterborn A., Coe B.C., Lablans A., Vital J.F., et al. The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer’s disease. J. Pathol. 2018;245:85–100. doi: 10.1002/path.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Craft S., Claxton A., Baker L.D., Hanson A.J., Cholerton B., Trittschuh E.H., Dahl D., Caulder E., Neth B., Montine T.J., et al. Effects of Regular and Long-Acting Insulin on Cognition and Alzheimer’s Disease Biomarkers: A Pilot Clinical Trial. J. Alzheimer’s Dis. 2017;57:1325–1334. doi: 10.3233/JAD-161256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Abbott A. Is “friendly fire” in the brain provoking Alzheimer’s disease? Nature. 2018;556:426–428. doi: 10.1038/d41586-018-04930-7. [DOI] [PubMed] [Google Scholar]
- 321.Salter M.W., Stevens B. Microglia emerge as central players in brain disease. Nat. Med. 2017;23:1018–1027. doi: 10.1038/nm.4397. [DOI] [PubMed] [Google Scholar]
- 322.Bu X.L., Yao X.Q., Jiao S.S., Zeng F., Liu Y.H., Xiang Y., Liang C.R., Wang Q.H., Wang X., Cao H.Y., et al. A study on the association between infectious burden and Alzheimer’s disease. Eur. J. Neurol. 2015;22:1519–1525. doi: 10.1111/ene.12477. [DOI] [PubMed] [Google Scholar]
- 323.Fani L., Wolters F.J., Ikram M.K., Bruno M.J., Hofman A., Koudstaal P.J., Darwish Murad S., Ikram M.A. Helicobacter pylori and the risk of dementia: A population-based study. Alzheimer’s Dement. 2018;14:1377–1382. doi: 10.1016/j.jalz.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 324.Marsh S.E., Abud E.M., Lakatos A., Karimzadeh A., Yeung S.T., Davtyan H., Fote G.M., Lau L., Weinger J.G., Lane T.E., et al. The adaptive immune system restrains Alzheimer’s disease pathogenesis by modulating microglial function. Proc. Natl. Acad. Sci. USA. 2016;113:E1316–E1325. doi: 10.1073/pnas.1525466113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Congdon E.E., Sigurdsson E.M. Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2018;14:399–415. doi: 10.1038/s41582-018-0013-z. [DOI] [PMC free article] [PubMed] [Google Scholar]