Abstract
Objective
To assess the effects of protocolized recombinant human erythropoietin (r-HuEPO) therapy and standardized high dose iron supplementation on hematological and iron status measures in a cohort of extremely low gestational age newborns (ELGANs).
Study design
Charts of ELGANs admitted from 2006 to 2016 and who had received r-HuEPO per NICU protocol were reviewed. r-HuEPO was started at a dose of 900 IU/kg per week after 7 days of age and continued until 35 weeks postmenstrual age. Oral iron supplementation at 6 −12 mg/kg per day was used to maintain transferrin saturation >20% during r-HuEPO treatment. Data on demographic features, hematological and iron panel indices, RBC transfusions, and clinical outcomes were collected. Quartile groups were created based on serum ferritin levels at the conclusion of r-HuEPO treatment and the quartiles were compared.
Results
The cohort included 116 infants with mean (SD) gestational age 25.8 (1.5) wk and birth weight 793 (174.1) g. r-HuEPO promoted erythropoiesis as indicated by increasing hemoglobin, hematocrit and reticulocyte count. Serum ferritin decreased over time and was ≤75 ng/mL in 60.2% of infants at the conclusion of r-HuEPO therapy; 87% received packed RBC transfusions. Transfusion volume, total iron intake, total iron binding capacity and transferrin concentration differed among infants in the different serum ferritin quartiles (P < .05).
Conclusions
r-HuEPO therapy promoted erythropoiesis in ELGANs. Despite a biomarker-based standardized high dose iron supplementation, the majority of infants had evidence of iron deficiency to a degree that is associated with affect reduced brain function.
Keywords: Erythropoietin, extremely low gestational age neonates, iron, iron deficiency, serum ferritin, transferrin saturation
Infants born preterm develop a decrease in hemoglobin (Hgb) that is most pronounced in those born before 28 weeks of gestation (extremely low gestational age newborns, ELGANs).1, 2 This anemia of prematurity is the result of several factors, among which lower production of erythropoietin,1, 2 frequent blood sampling and decreased availability of nutrients that support erythropoiesis, including iron, folate and protein, are the major ones.3 Most ELGANs require multiple red blood cell (RBC) transfusions, iron supplementation, and in some situations, recombinant human erythropoietin (r-HuEPO) therapy to maintain hemoglobin levels.
Although RBC transfusions can improve anemia and hypoxia, there are notable risks including infection, electrolyte imbalance, circulatory overload, abnormally high serum ferritin concentrations and mortality.4, 5, 6 Associations between high serum ferritin concentrations and retinopathy of prematurity (ROP)7 and bronchopulmonary dysplasia (BPD)6 have been reported. r-HuEPO treatment mitigates the need for RBC transfusions and stabilizes or increases Hgb concentrations in preterm neonates.8–14 r-HuEPO may also have a beneficial effect on neurodevelopment.15, 16 However, adequate iron supplementation is essential for sustaining erythropoiesis during r-HuEPO treatment.17 The amount of iron necessary to support erythropoiesis, while not depleting iron stores and risking tissue iron deficiency, is not known. Inadequate iron supplementation may predispose to tissue iron deficiency, including brain iron deficiency; due to prioritization of iron to RBCs over other organs during development.18 The aim of our study was to examine the effects of r-HuEPO therapy combined with a biomarker-based standardized iron dosing strategy on hematological measures and the iron status of ELGANs.
Methods:
This is a retrospective chart review of ELGANs born at Hennepin County Medical Center (HCMC) between 2006 and 2016 following institution of a standardized r-HuEPO and iron supplementation protocol. All infants who were less than 28 weeks of gestation at birth and received r-HuEPO per protocol were included. Exclusion criteria were transfer to an outside facility, death, and failure to complete r-HuEPO treatment per protocol. The study was approved by the Institutional Review Board at HCMC.
Per protocol, r-HuEPO therapy was initiated after 7 days of life (DOL) at a dose of 300 U/kg, three times a week (Monday, Wednesday and Friday) and continued until a postmenstrual age (PMA) of 34 weeks and 6 days. Baseline iron studies were obtained before starting r-HuEPO therapy. Dose of r-HuEPO was adjusted weekly for infant’s weight. Iron supplementation was started one week after the initiation of r-HuEPO by adding iron dextran at a dose of 0.5–1mg/kg per day to total parenteral nutrition after a test dose.19 Oral iron supplementation was started using ferrous sulfate when full volume feeding was achieved. The starting oral iron supplementation dose was 6 mg/kg per day, and the maximum dose was 12 mg/kg per day. The iron dose was adjusted to maintain iron saturation (transferrin saturation) >20%. The iron dose was decreased to maintenance dose (2–4 mg/kg per day) one week after r-HuEPO was discontinued. In addition to r-HuEPO and iron supplementation, infants received 50 μg/d of folate and 25 IU/d vitamin E supplementation.20 r-HuEPO was terminated prior to 35 weeks PMA in some infants by the attending neonatologist due to clinical concerns of infection, hypertension or severe ROP. These infants were excluded from the analysis.
Hematological indices, including Hgb (g/dL), hematocrit (Hct, %), mean corpuscular volume (MCV, fL), red cell distribution width (RDW, %), reticulocyte count (%) and platelet count (109/L), and iron status panel, including serum iron (μg/dL), serum ferritin (ng/mL), total iron binding capacity (TIBC, μg/dL), transferrin saturation/iron saturation (%) and serum transferrin (mg/dL) were measured weekly for the first 4 weeks and then every other week until r-HuEPO was completed. Transferrin saturation (%) was calculated using the formula, serum iron/TIBC ×100.
Demographic, clinical, hematological and iron indices data from each infant were extracted from the electronic charts. Growth rate (g/day) was calculated using the birth and discharge weights. RBC transfusion data extracted included number of transfusions and the volume of packed RBC received by the infant. Total amount of iron (parenteral + oral) received during the r-HuEPO therapy was calculated. Clinical outcomes during hospitalization were extracted, including the occurrence of ROP, BPD, intraventricular hemorrhage, patent ductus arteriosus and necrotizing enterocolitis, and days of mechanical ventilation
Statistical Analyses:
Weight, length, and head circumference Z-scores were calculated using the Fenton 2013, growth charts for preterm infants.21 Two-sided paired t-tests were used to assess changes in the hematological and iron indices. Quantile groups were created based on the SF values at 35 weeks PMA as follows: <40 ng/mL (lowest quartile), 40 to 89 ng/mL (middle two quartiles), and ≥90 ng/mL (highest quartile). These cutoffs were determined by finding the 25th and 75th quantiles and rounding to the nearest multiple of 5. ANOVA and chi-squared tests were used to assess differences among serum ferritin quartile groups. Significant covariates underwent a post-hoc two-sample t-test with unequal variance to further compare the highest and lowest quartiles. A multivariate logistic regression model was fit and summarized to assess the overall effect of serum ferritin quartile on BPD risk. To examine the effect of serum ferritin quartile on ROP, a multivariate ordered logistic regression model was used. Cubic splines with 2 knots for iron and hematological factors were fitted to graphically display the trend of each index. Cubic splines were fitted with all data, but truncated at 38 and 42 weeks PMA for iron and hematological indices, respectively, due to insufficient data to observe meaningful trends beyond those periods. All analyses and graphing were performed with R version 3.4.0. A P-value < .05 was considered statistically significant.
Results:
Medical charts of a total of 209 infants were reviewed, and 93 infants were excluded from final analysis. Ten infants were transferred to another facility before completion of r-HuEPO treatment, thirty three infants died, thirty one infants did not receive r-HuEPO and r-HuEPO was stopped early in 15 infants (three due to positive blood culture, eight due to concern for infection, one due to high serum ferritin, one due to stage 3 ROP, one due to hypertension, and in one infant for unclear cause). In addition, data from four infants were not included (in one infant r-HuEPO and iron were started on DOL 14, in one infant r-HuEPO was started at 31 week, and in two infants transfusion data were not available). Thus, the final analysis included 116 infants (Figure 1; available at www.jpeds.com). Demographic features of the infants are shown in Table 1. The mean duration of total parenteral nutrition was 27.6±13.2 days. Enteral feedings were started on DOL 5.96±4.7; 73.3% were started on maternal breastmilk, 4.3% on donor breastmilk, 9.5% received a combination of maternal and donor breast milk and 13% received formula. Enteral feedings were fortified on DOL 26.5±14.6. At discharge 15.5% were exclusively on maternal breast milk, 14.6% received a combination of maternal breast milk and formula, 69.9% received only formula; 96% (n=111) of infants needed mechanical ventilation for a mean duration of 32.0±32.9 days. The incidence of intraventricular hemorrhage was 33.0% (n=38), severe intraventricular hemorrhage (grades 3 and 4) was 8.6 % (n=10), necrotizing enterocolitis was present in 6.0 % (n=7), and patent ductus arteriosus was present in 66.4% (n=77). The incidence of infants needing surgery, including patent ductus arteriosus ligation and gastrostomy tube placement was 40.5% (n=47). The incidence of ROP (all stages) was 77.6% (n=90), and stage 3 ROP was present in 7.8 % (n=9). BPD was present in 70.7% (n=82).
Figure 1.
Consort flow diagram (Online only)
Table 1.
Demographics of all study participants (N=116)
| Birth | Discharge | |
|---|---|---|
| Male | 55 (47.4%) | -- |
| Race/Ethnicity: | ||
| -African American | 48 (41.4%) | -- |
| -African | 8 (6.9%) | -- |
| -Caucasian | 15 (12.9%) | -- |
| -Hispanic | 32 (27.6%) | -- |
| -Other | 13 (11.2%) | -- |
| Cesarean-section delivery | 78 (67.2%) | -- |
| Antenatal steroids (%) | 100 (86.2%) | -- |
| 5min Apgar score | 7.1 (1.6) | -- |
| Weight (g) | 793 (174.1) | 3242 (621.1) |
| Weight Z-score | 0.02 (0.95) | −0.66 (0.87) |
| Length (cm) | 33.0 (3.01) | 48.2 (3.07) |
| Length Z-score | 0.08 (1.05) | −1.2 (1.23) |
| Head Circumference (cm) | 23.0 (1.85) | 34.3 (1.95) |
| Head Circumference Z-score | −0.12 (0.94) | −0.44 (0.96) |
| PMA (wk) | 25.8 (1.45) | 40.4 (3.38) |
Mean (SD) for continuous variables, or N (%) for categorical variables
r-HuEPO therapy was initiated on DOL 9.7± 2.8. Parenteral iron supplementation was started on DOL 13.6 ± 9.0, and oral iron supplementation was started on DOL 28.0±13.1; 87% (n=101) of the infants received RBC transfusions, with 78 infants receiving a transfusion during the first week after birth. The first transfusion was on DOL 5.4±5.2, and the last transfusion was on DOL 26.2±22.5. Mean number of RBC transfusions per infant was 3.3±2.7. Average volume of blood received per infant was 46.9±46.6 mL (51.0±44.8 mL/kg). Late RBC transfusions, defined as transfusions after 3 weeks of age were given to 40.5% (n=47) of the infants. Only 12.9% (n=15) of infants did not receive any transfusions during hospital stay.
Hematological indices were measured, on average, 10.8±2.3 times in each infant. Hematological indices at admission and at the end of r-HuEPO therapy and at discharge are shown in Table 2 and Table 3 (available at www.jpeds.com), respectively. Changes in the hematologic measures during hospitalization are shown in Figure 2. Infants experienced an initial drop in Hgb and Hct after birth, followed by a slight increase in both measures during r-HuEPO therapy. MCV decreased steadily from birth and was not altered during r-HuEPO therapy. Reticulocyte count and RDW increased after the beginning of r-HuEPO therapy, reached a peak at 30–32 week PMA, before steadily decreasing thereafter. Platelet count started low and steadily increased during r-HuEPO therapy.
Table 2.
Hematological and Iron Indices before r-HuEPO and at PMA 35±1 wk
| N | Before EPO and High Iron Supplementation | PMA of 35 weeks | Mean Change (95% CI) | P-value | |
|---|---|---|---|---|---|
| Hematological Indices: | |||||
| Hemoglobin (gm/dL) | 104 | 14.1 ± 0.2 | 12.1 ± 0.1 | −2 (−2.5, −1.5) | <0.001 |
| Hematocrit (%) | 104 | 42.2 ± 0.5 | 38.4 ± 0.4 | −3.7 (−5.1, −2.4) | <0.001 |
| MCV (fL) | 104 | 113.6 ± 0.8 | 92.7 ± 0.5 | −20.8 (−22.3, −19.3) | <0.001 |
| Red Cell Width (RDW %) | 103 | 16.4 ± 0.2 | 23.6 ± 0.3 | 7.1 (6.5, 7.7) | <0.001 |
| Platelet Count (10^9/L) | 98 | 208.1 ± 6.3 | 278.4 ± 11.3 | 73.2 (50.2, 96.1) | <0.001 |
| Iron Indices: | |||||
| Serum Iron (mcg/dL) | 83 | 58.1 ± 2.7 | 87.9 ± 4.3 | 28.7 (17, 40.4) | <0.001 |
| Serum Ferritin (ng/mL) | 83 | 283.8 ± 15 | 107 ± 16.1 | −161.8 (−202.6, - | <0.001 |
| TIBC (mcg/dL) | 83 | 285.2 ± 4.8 | 333.2 ± 7.6 | 48.1 (28.2, 68) | <0.001 |
| Transferrin Saturation (%) | 83 | 20.7 ± 1 | 27.1 ± 1.3 | 6 (2.1, 9.8) | 0.003 |
| Transferrin (mg/dL) | 83 | 190.8 ± 3.2 | 223.6 ± 5.1 | 33.1 (19.8, 46.4) | <0.001 |
Mean ± SE, N at PMA 35wk
Table 3.
Hematological and Iron Indices before r-HuEPO and before discharge
| N | Before EPO and High Iron Supplementation | Last Measurement Before Discharge | Mean Change (95% CI) | P-value | |
|---|---|---|---|---|---|
| Hematological Indices: | |||||
| Hemoglobin (gm/dL) | 116 | 14.1 ± 0.2 | 11.5 ± 0.1 | −2.6 (−3, −2.2) | <0.001 |
| Hematocrit (%) | 116 | 42.2 ± 0.5 | 34.9 ± 0.4 | −7.2 (−8.4, −6) | <0.001 |
| MCV (fL) | 116 | 113.6 ± 0.8 | 86.8 ± 0.7 | −26.9 (−28.6, - | <0.001 |
| Red Cell Width (RDW | 116 | 16.4 ± 0.2 | 18.9 ± 0.3 | 2.4 (1.8, 3.1) | <0.001 |
| Platelet Count (10^9/L) | 110 | 208.1 ± 6.3 | 318.9 ± 9.3 | 112.7 (92.7, | <0.001 |
| Iron Indices: | |||||
| Serum Iron (mcg/dL) | 116 | 58.1 ± 2.7 | 95.3 ± 3 | 37.2 (28.8, 45.5) | <0.001 |
| Serum Ferritin (ng/mL) | 116 | 283.8 ± 15 | 96.8 ± 10.2 | −187 (−221.8, - | <0.001 |
| TIBC (mcg/dL) | 116 | 285.2 ± 4.8 | 326.1 ± 6.1 | 41 (25, 56.9) | <0.001 |
| Transferrin Saturation | 116 | 20.7 ± 1 | 29.9 ± 0.9 | 9.1 (6.5, 11.8) | <0.001 |
| Transferrin (mg/dL) | 116 | 190.8 ± 3.2 | 218.8 ± 4.1 | 28 (17.2, 38.7) | <0.001 |
Mean ± SE
Figure 2.
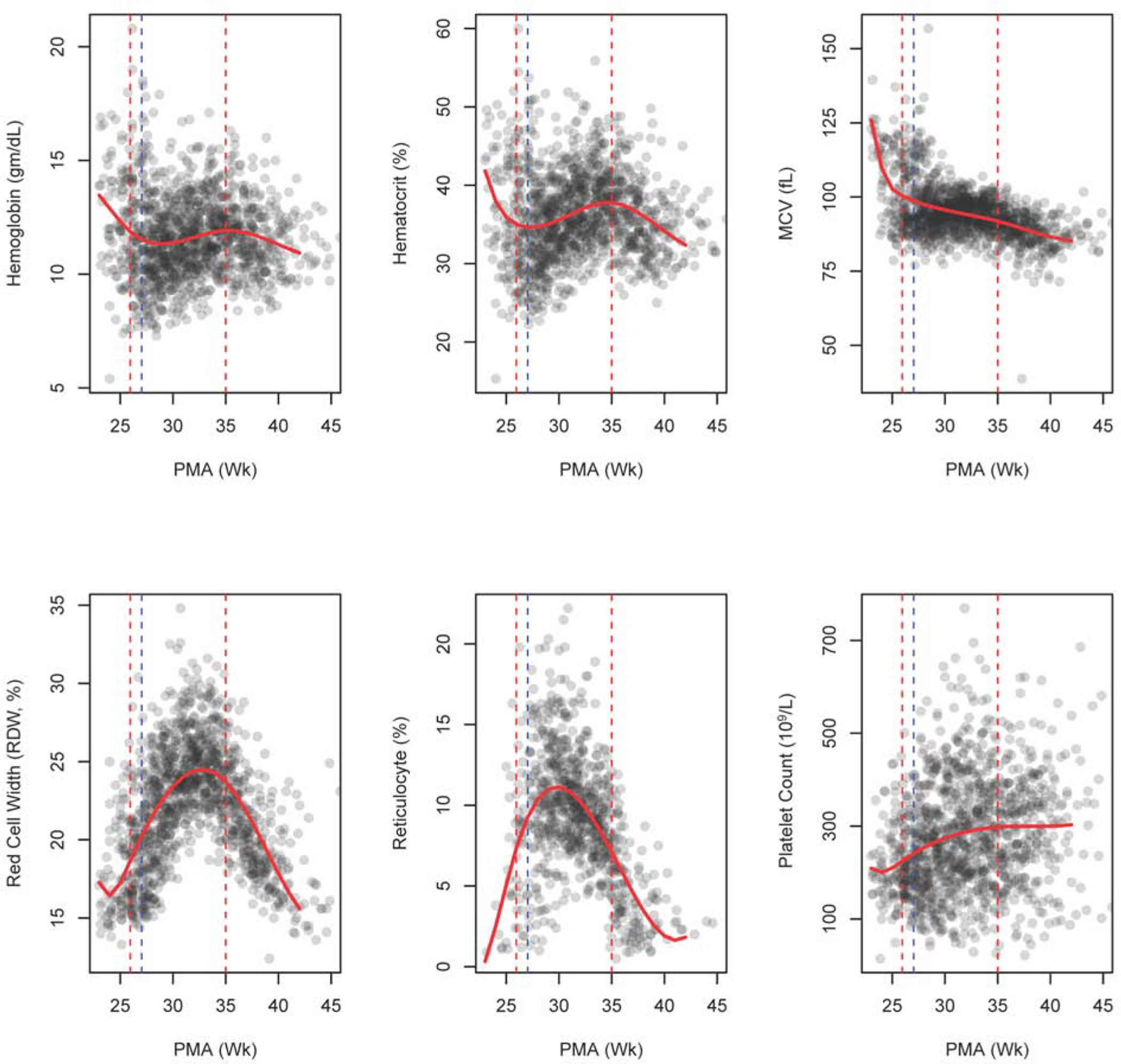
Scatter plot with non-parametric trend line for hematological indices. The red dashed vertical lines show the start and end of r-HuEPO therapy. The blue dashed vertical line represents start of iron supplementation. (PMA, postmenstrual age)
The first set of iron studies were drawn before r-HuEPO was started, and the majority of infants had been transfused before the first set of iron studies. On average, each infant had 6.5±1.5 sets of iron measurements. Iron indices before beginning r-HuEPO and iron supplementation and at the conclusion of high iron supplementation and the last values obtained during r-HuEPO therapy are given in Table 2 and Table 3, respectively. Trends in iron indices during r-HuEPO therapy are shown in Figure 3. Serum iron was low at the initiation of iron supplementation and steadily increased thereafter, whereas serum ferritin had an initial increase prior to the start of r-HuEPO and steadily decreased over time during r-HuEPO therapy. Transferrin saturation showed an initial decrease, followed by steady increase during r-HuEPO therapy. Similarly, both transferrin and TIBC showed an initial decrease, followed by an increase, before plateauing around 30 wk PMA, followed by an increase after the conclusion of r-HuEPO, whereas serum iron, serum ferritin and transferrin saturation exhibited their greatest rate of change around this time. Serum ferritin was <75 ng/mL in 60.2% of the infants, 76–400 ng/mL in 33.7% and >400 ng/mL in 6.1% at the conclusion of r-HuEPO therapy at a PMA of 35±1 weeks.
Figure 3.
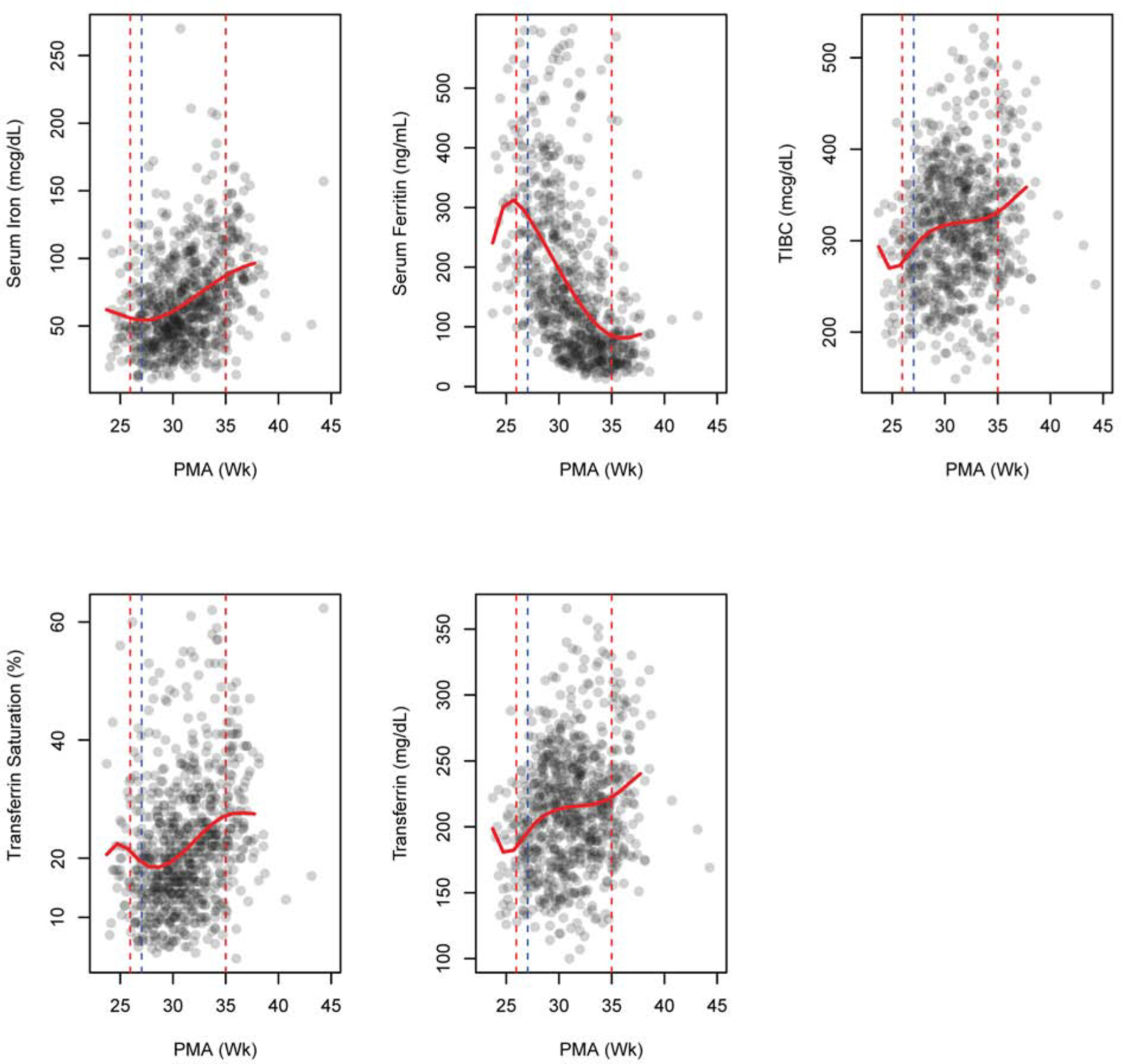
Scatter plot with non-parametric trend line for iron indices. The red dashed vertical lines show the start and end of r-HuEPO therapy. The blue dashed vertical line represents start of iron supplementation. (PMA, postmenstrual age)
In an effort to establish the factors influencing serum ferritin levels, quartiles based on serum ferritin at the conclusion of r-HuEPO therapy were determined. Thirty three infants did not have iron measurements at a PMA of 35±1 weeks, and were excluded from all serum ferritin quartile analyses. Characteristics of infants with serum ferritin in the lowest quartile (i.e., those at risk for tissue iron deficiency), middle two quartiles and the highest quartile (i.e., those at risk for iron overload) were compared (Table 4). Total iron intake, total amount of packed RBC transfused, TIBC, and transferrin concentration were statistically different between the quartile groups. A post hoc analysis confirmed that the lowest serum ferritin quartile had statistically higher amounts of total iron intake, TIBC, and transferrin, and lower volume of packed RBC transfused compared with the highest serum ferritin quartile (all P ≤ .01, Table 4). Repeating the analysis using physiological threshold of ≤ 75 ng/mL and >75 ng/mL yielded similar results to quartile-based analysis with the exception of total iron intake, which was no longer different between the groups (Table 5; available at www.jpeds.com). Results from testing the overall effects of serum ferritin quartile groups on BPD are provided in Table 6 (available at www.jpeds.com). After adjusting for sex, GA, birth weight, growth rate and total iron intake, serum ferritin quartile failed to reach significance. Similarly, no significant association was detected between serum ferritin quartile and ROP after adjustments (Table 7; available at www.jpeds.com).
Table 4:
Characteristics by Serum Ferritin Quartiles
| Lowest Serum Ferritin Quartile | 2 Median Serum Ferritin Quartiles | Highest Serum Ferritin Quartile | P-value | |
|---|---|---|---|---|
| Covariate | (N=20) | (N=40) | (N=23) | |
| Male | 12 (60.0%) | 16 (40.0%) | 13 (56.5%) | 0.249 |
| Gestational Age (wks) | 25.5 (1.4) | 25.8 (1.47) | 25.9 (1.47) | 0.707 |
| Birth weight (g) | 748 (152.3) | 792 (169.9) | 798 (198.3) | 0.579 |
| Growth Rate (g/day) | 23.8 (3.07) | 24.4 (3.19) | 24.0 (4.6) | 0.82 |
| Total Iron Intake (mg) | 693 (266.4) | 660 (253.0) | 449 (267.2)* | 0.003 |
| Total Amount of Blood (mL) | 32.0 (33.0) | 45.5 (43.6) | 79.9 (63.8)* | 0.004 |
| Total ml/kg of Blood | 39.2 (35.6) | 55.3 (47.7) | 61.7 (46.5) | 0.257 |
| Hemoglobin (gm/dL) | 11.9 (1.35) | 12.4 (1.68) | 12.4 (1.44) | 0.592 |
| Hematocrit (%) | 37.5 (3.58) | 38.9 (4.48) | 39.5 (4.78) | 0.363 |
| Serum Iron (mcg/dL) | 91.0 (43.2) | 87.4 (42.1) | 86.0 (29.3) | 0.909 |
| TIBC (mcg/dL) | 353 (68.2) | 346 (59.9) | 295 (72.9)* | 0.005 |
| Iron Saturation (%) | 25.6 (11.2) | 26.4 (12.6) | 29.7 (10.6) | 0.466 |
| Transferrin (mg/dL) | 237 (45.8) | 232 (40.2) | 198 (48.9)* | 0.006 |
| Serum Ferritin (ng/mL) | 30.4 (6.94) | 61.3 (15.3) | 253 (220.3) | <0.001 |
Mean (SD) for continuous variables, or N (%) for categorical variables Hematological and iron values at a PMA of 35±1 week).
P ≤ .01 compared to lowest serum ferritin quartile
Table 5 :
Study Sample Characteristics by Serum Ferritin Quartiles based on physiological threshold
| ≤ 75 SF at 35 PMA | >75 SF at 35 PMA | P-value | |
|---|---|---|---|
| Covariate | (N=50) | (N=28) | |
| Male | 23 (46.0%) | 15 (53.6%) | 0.685 |
| Gestational Age (wks) | 25.7 (1.43) | 25.8 (1.52) | 0.925 |
| Birth weight (g) | 778 (160.2) | 787 (208.6) | 0.825 |
| Growth Rate (g/day) | 24.0 (3.12) | 24.5 (4.44) | 0.617 |
| Total Iron Intake (mg) | 654 (237.9) | 602 (297.3) | 0.398 |
| Total Amount of Blood (mL) | 40.1 (39.6) | 62.2 (50.7) | 0.036 |
| Total ml/kg of Blood | 52.4 (47.9) | 54.1 (40.0) | 0.874 |
| Hemoglobin (gm/dL) | 12.3 (1.6) | 12.2 (1.54) | 0.887 |
| Hematocrit (%) | 38.6 (4.34) | 39.2 (4.87) | 0.617 |
| Serum Iron (mcg/dL) | 86.3 (40.3) | 90.8 (38.1) | 0.635 |
| TIBC (mcg/dL) | 349 (63.2) | 316 (75.9) | 0.047 |
| Iron Saturation (%) | 25.4 (11.6) | 29.3 (12.1) | 0.167 |
| Transferrin (mg/dL) | 234 (42.5) | 212 (50.9) | 0.047 |
| Serum Ferritin (ng/mL) | 44.8 (14.9) | 125 (57.8) | <0.001 |
Mean (SD) for continuous variables, or N (%) for categorical variables Hematological and iron values at a PMA of 35 weeks (± 1 week). 5 patients with SF > 400 at 35 PMA were removed from this analysis
Table 6:
Effect of Serum Ferritin Quartile on Risk of BPD
| Covariate | df | Deviance of Residuals | P-value |
|---|---|---|---|
| Serum Ferritin Quartile | 2 | 4.46 | 0.108 |
| Sex | 1 | 0.06 | 0.814 |
| GA (wk) | 1 | 18.09 | <0.001 |
| Birth Weight (g) | 1 | 3.84 | 0.050 |
| Growth Rate (g/day) | 1 | 0.29 | 0.592 |
| Total iron intake (mg) | 1 | 0.05 | 0.819 |
Table 7:
Effect of Serum Ferritin Quartile on ROP
| Covariate | Odds ratio (95% CI) | P-value |
|---|---|---|
| 2 Median Serum Ferritin Quartiles (vs. Lowest Quartile) | 0.869 (0.484, 1.559) | 0.637 |
| Highest Serum Ferritin Quartile (vs. Lowest Quartile) | 0.982 (0.559, 1.726) | 0.95 |
| Male Sex (vs. Female) | 1.178 (0.804, 1.727) | 0.401 |
| GA (wk) | 0.702 (0.618, 0.798) | <0.001 |
| Birth Weight (g) | 0.995 (0.992, 0.998) | <0.001 |
| Growth Rate (g/day) | 0.926 (0.83, 1.033) | 0.169 |
| Total iron intake (mg) | 0.999 (0.998, 1.001) | 0.288 |
Discussion:
In our study, despite multiple packed RBC transfusions and standardized high dose iron supplementation, the majority of ELGANs on r-HuEPO were found to be iron deficient to a degree where the developing brain is placed at risk as indicated by a serum ferritin <75 ng/mL.22, 23, 24 A parallel increase in hemoglobin and reticulocyte count indicates that prioritization of supplemented and stored iron towards erythropoiesis was likely responsible.19, 25, Inadequate absorption and/or utilization of orally supplemented iron also may have contributed as iron stores are better preserved with intravenous iron supplementation than oral iron supplementation during r-HuEPO therapy in preterm infants.26 A serum ferritin <75 ng/mL in the neonatal period is associated with abnormal short- and long-term neurodevelopment.22, 23, 24, 27 Thus, the beneficial effect of r-HuEPO on hematology may be offset by its potential to cause brain iron deficiency and adverse neurological effects.
The discharge hematocrit in our study of 34.9±0.4% is comparable with previous studies of ELGANs treated with EPO.19,28, 29 The changes in hemoglobin, hematocrit and reticulocyte count during r-HuEPO treatment in our study are also similar to the study by Ohls et al, where an increase in all three hematological measures was observed.19 Despite a positive effect on erythropoiesis, we did not see a reduction in the number of packed RBC transfusions. This suggests that transfusions were potentially given for reasons other than anemia (e.g., for correcting hypotension). Our unit does not have transfusion guidelines and infants are transfused at treating physician’s discretion. This might have contributed to higher transfusion rate in our cohort. Moreover, most transfusions were given during first week before r-HuEPO was started; 87% of preterm neonates receive RBC transfusions during their hospital stay.18, 30, 31, 32 Our results are consistent with this. Likewise, the number of transfusions per infant in our study (3.3±2.7) is comparable to previous studies (2.1 to 4.3).8, 9, 15 However, late transfusion (after 3 weeks) rate in our cohort (40.5%) is lower than the transfusion rates in ELGANs not receiving r-HuEPO therapy.33 These results suggest that r-HuEPO therapy is effective in decreasing late RBC transfusions in ELGANs. However, without a non-r-HuEPO comparison group, this possibility remains conjectural. The lower rate of late transfusions also could be reflective of an overall clinical improvement by this age, leading to a lesser need for transfusions for nonhematological reasons.
Monitoring of the iron status and iron dose adjustment were rigorously adhered to during r-HuEPO treatment. Transferrin saturation was maintained in normal range during r-HuEPO therapy, indicating that we were successful in achieving the a priori goal of therapy. Transferrin saturation in the normal range indicates adequacy of iron for utilization. Transferrin saturation is an important iron status marker as it indicates current iron availability for utilization whereas serum ferritin is an indicator of stored iron. In our study, iron status markers indicated that supplemented and stored iron were actively utilized for erythropoiesis, yet 60.2% had evidence of iron deficiency with serum ferritin below 75 ng/mL during r-HuEPO therapy. A serum ferritin <76 ng/mL in cord blood sample of infants born at 38.2 ± 2.5 wk was associated with increased risk of long-term neurodevelopmental impairments.22 The r-HuEPO dose used in the current study has been previously shown to lead to decreased serum ferritin, despite high dose iron (8–16 mg/kg per day) supplementation.28, 29 In a previous study of <32 week preterm infants by Amin et al, a serum ferritin<75 ng/mL was seen in 23% of the infants at 35 weeks PMA,14 a much lower value than the 60% in the present study. However, unlike our study, r-HuEPO was not administered to these infants. On the other hand, more infants in that study had serum ferritin>400 ng/mL than in the present study (19% vs. 6%). Serum ferritin >400 ng/mL is typically considered evidence of iron overload.14 In one study, among infants who had received >3 transfusions, 50% had serum ferritin >400 ng/mL.14 Thus, r-HuEPO administration potentially lowers the risk of excess iron storage in ELGANs by diverting iron towards erythropoiesis.
Previous studies have reported iron supplementation at a dose ranging from 1 to 16 mg/kg/d during r-HuEPO treatment.28, 29,34 Infants in our study were supplemented with up to 12 mg/kg/d of iron, yet 60% had evidence of iron deficiency, suggesting that even higher doses may be necessary while on r-HuEPO therapy. However, a randomized trial had demonstrated that oral iron supplementation at a dose of 16 mg/kg per day is not superior to a dose of 8 mg/kg per day for maintaining hematocrit and serum ferritin during r-HuEPO therapy at 900 U/kg/week (i.e., the dose used in our study).29Additional studies are necessary to determine the optimal iron dose during r-HuEPO therapy. We did not measure biomarkers of iron toxicity in our study, but iron supplementation at a dose as high as 18 mg/kg per day have been used without evidence of iron toxicity.35
Comparison of infants in different quartiles of serum ferritin at the conclusion of r-HuEPO therapy showed that total iron intake, total amount of packed RBC, TIBC and transferrin were different among the groups, an effect likely mediated by higher packed RBC volume and lower TIBC and transferrin concentration in the highest serum ferritin quartile group. In combination, these results may suggest the possibility of presence of inflammation in this group. Without other laboratory measures (e.g., C-reactive protein), this possibility remains conjectural, however. Moreover, lack of an association between serum ferritin and BPD and ROP, conditions associated with inflammation, also makes this possibility less likely. Of note, oral iron intake was not responsible for this effect as total iron intake was lower in the highest serum ferritin group, compared with the lowest serum ferritin group.
Studies have shown iron deficiency during the fetal and neonatal periods impairs short-term and long-term neurodevelopment with abnormalities in hippocampal mediated memory function, delayed maturation of auditory brainstem response and abnormal behavior, despite early recognition and treatment.36, 37, 38 Conversely, some studies have reported an association between high serum ferritin levels and BPD39 and ROP40, although there are no definitive studies confirming causation. We also failed to find an association between higher serum ferritin and BPD and ROP in our study. Our data suggest that monitoring for iron deficiency during r-HuEPO treatment is more crucial than monitoring for iron overload. Monitoring serial changes in serum ferritin is useful for this purpose as low serum ferritin is not seen in any conditions, other than iron deficiency. However, serum ferritin could be elevated in certain conditions (e.g., due to inflammation) and cannot be used as a biomarker of iron status in those conditions. Transferrin saturation, a measure of iron bound to transferrin, and TIBC, the capacity of plasma proteins to bind iron, may be better markers of iron deficiency in these situations. A low transferrin saturation and increased TIBC are indicative of iron deficiency.41, 42 Similarly, increased RDW and decreased reticulocyte hemoglobin are early indicators of iron deficient erythropoiesis.42,43 A recent study in neonatal rats has demonstrated that low reticulocyte hemoglobin is also an early biomarker of brain iron deficiency during phlebotomy-induced anemia.44 A combination of iron markers may be useful for monitoring for iron deficiency and assess adequacy of iron supplementation for erythropoiesis during r-HuEPO treatment.
The study assessed the effects of r-HuEPO only on hematological and iron measures. Effects on neurodevelopment were not determined. Given that iron plays a critical role in brain development, the finding of lower serum ferritin in r-HuEPO treated infants is of concern. Conversely, prior studies have demonstrated that r-HuEPO treatment at a dose comparable with the one used in our study has beneficial effects on neurodevelopment.16 Further studies assessing neurodevelopment in these infants would be appropriate. Other limitations of our study include lack of a non-r-HuEPO treated control group and the retrospective design. We were also unable to provide information on acute kidney injury as a clinical outcome in our cohort. Another limitation is that we were unable to compare hematological and iron measures separately during parenteral and enteral iron administration periods. Finally, our protocol and monitoring strategy may not be generalizable, particularly when r-HuEPO is used for a shorter duration or is administered to infants with documented anemia.
In conclusion, our results demonstrate that r-HuEPO increases the risk of iron deficiency in ELGANs despite a biomarker-based iron dosing strategy. Preventive measures, such as minimizing phlebotomy losses may be beneficial for preventing or decreasing the severity of anemia of prematurity in this group.
Supplementary Material
Acknowledgments:
We thank Raul F. Cifuentes, MD who developed and instituted the r-HuEPO and iron supplementation protocol at Hennepin County Medical Center, Minneapolis, MN, and Constance A. Adkisson, MD, Director of Neonatology Division at Hennepin County Medical Center, Minneapolis, MN for critical review of the manuscript.
Abbreviations:
- BPD
bronchopulmonary dysplasia
- r-HuEPO
recombinant human erythropoietin
- DOL
days of life
- ELGAN
extremely low gestational age newborn
- Hgb
hemoglobin
- HCT
hematocrit
- HCMC
Hennepin County Medical Center
- MCV
mean corpuscular volume
- PMA
postmenstrual age
- RBC
red blood cell
- RDW
red cell distribution width
- ROP
retinopathy of prematurity
- TIBC
total iron binding capacity
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Portions of this study were presented at the Pediatric Academic Societies annual meeting, May 5–8, 2018, Toronto, Canada; at the Midwest Society for Pediatric Research annual meeting, October12, 2017, Chicago, Illinois; and at the American Academy of Pediatrics annual meeting, September 15–19, 2017, Chicago, Illinois.
References
- 1.Stockman JA 3rd. Anemia of prematurity. Current concepts in the issue of when to transfuse. Pediatric Clinics of North America 1986; 33:111–28. [DOI] [PubMed] [Google Scholar]
- 2.Strauss RG. Anemia of prematurity: Pathophysiology and treatment. Blood Reviews 2010; 24:221–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Widness JA. Pathophysiology of anemia during the neonatal period, including anemia of prematurity. Neoreviews 2008; 9: e520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ibrahim R, Mohamed D, Abdelghaffar S, Mansi Y. Red blood transfusion in preterm infants: changes in glucose, electrolytes and acid base balance. Asian Journal of Transfusion Science 2012; 6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stockman III JA, Graeber JE, Clark DA, McClellan K, Garcia JF, Kavey RE. Anemia of prematurity: determinants of the erythropoietin response. J Pediatr 1984; 105:786–92. [DOI] [PubMed] [Google Scholar]
- 6.Keir A, Pal S, Trivella M, Lieberman L, Callum J, Shehata N, et al. Adverse effects of small-volume red blood cell transfusions in the neonatal population. Systematic Reviews 2014; 3:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hesse L, Eberl W, Schlaud M, Poets CF. Blood transfusion. Iron load and retinopathy of prematurity. European Journal of Pediatrics 1997; 156:465–70. [DOI] [PubMed] [Google Scholar]
- 8.Donato H, Vain N, Rendo P, Vivas N, Prudent L, Larguia M, et al. Effect of early versus late administration of human recombinant erythropoietin on transfusion requirements in premature infants: Results of a randomized, placebo-controlled, multicenter trial. Pediatrics 2000; 105:1066–72. [DOI] [PubMed] [Google Scholar]
- 9.Yeo C, Choo S, Ho L. Effect of recombinant human erythropoietin on transfusion needs in preterm infants. Journal of Paediatrics and Child Health 2001; 37:352–8. [DOI] [PubMed] [Google Scholar]
- 10.Meyer MP, Sharma E, Carsons M. Recombinant erythropoietin and blood transfusion in selected preterm infants. Arch Dis Child Fetal Neonatal Ed 2003; 88: F41–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maier RE, Obladen M, Müller-Hansen I, Kattner E, Merz U, Arlettaz R, et al. Early treatment with erythropoietin b ameliorates anemia and reduces transfusion requirements in infants with birth weights below 1000 g. J Pediatr 2002; 141:8–15. [DOI] [PubMed] [Google Scholar]
- 12.Avent M, Cory BJ, Galpin J, Ballot DE, Cooper PA, Sherman G, et al. A comparison of high versus low dose recombinant human erythropoietin versus blood transfusion in the management of anemia of prematurity in a developing country. J Trop Pediatr 2002; 48:227–33. [DOI] [PubMed] [Google Scholar]
- 13.Franz AR, Pohlandt F. Red blood cell transfusions in very and extremely low birthweight infants under restrictive transfusion guidelines: is exogenous erythropoietin necessary? Arch Dis Child Fetal Neonatal Ed 2001; 84:F96–F100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin Sanjiv B., Scholer Lori & Srivastava Manisha. Pre-discharge iron status and its determinants in premature infants. The Journal of Maternal-Fetal and Neonatal Medicine 2012; 25(11): 2265–2269 [DOI] [PubMed] [Google Scholar]
- 15.Ohls RK, Harcum J, Schibler KR, Christensen RD. The effect of erythropoietin on the transfusion requirements of preterm infants weighing 750 grams or less: a randomized, double blind, placebo-controlled study. Journal of Pediatrics 1997; 131:661–5. [DOI] [PubMed] [Google Scholar]
- 16.Fischer HS, Reibel NJ, Bührer C, Dame C. Prophylactic early erythropoietin for neuroprotection in preterm infants: A meta-analysis. Pediatrics 2017; 139:e20164317. [DOI] [PubMed] [Google Scholar]
- 17.Fujiu T, Maruyama K, Koizumi T. Oral iron supplementation in preterm infants treated with erythropoietin. Pediatr Int. 2004; 46: 635–9. [DOI] [PubMed] [Google Scholar]
- 18.Georgieff MK. Iron assessment to protect the developing brain. Am J Clin Nutr, 106(S): 1588S–1593S, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohls RK, Ehrenkranz RA, Wright LL, Lemons JA, Korones SB, Stoll BJ, et al. Effects of early erythropoietin therapy on the transfusion requirements of preterm infants below 1250 grams birth weight: a multicenter, randomized, controlled trial. Pediatrics 2001;108: 934–42. [DOI] [PubMed] [Google Scholar]
- 20.Aher SM, Ohlsson A. Late erythropoietin for preventing red blood cell transfusion in preterm and/or low birth weight infants. Cochrane Database of Systematic Reviews 2014, Issue 4 Art. No.: CD004868. DOI: 10.1002/14651858.CD004868.pub4. [DOI] [PubMed] [Google Scholar]
- 21.Fenton TR, Kim JH A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr 2013; 13:59 10.1186/1471-2431-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr 2002; 140:165–70 [DOI] [PubMed] [Google Scholar]
- 23.Armony-Sivan R, Eidelman AI, Lanir A, Sredni D, Yehuda S. Iron status and neurobehavioral development of premature infants. Journal of Perinatology 2004; 24:757–762 [DOI] [PubMed] [Google Scholar]
- 24.Amin SB, Orlando M, Wang H. Latent iron deficiency in utero is associated with abnormal auditory neural myelination in >= 35 weeks gestational age infants. Journal of Pediatrics 2013; 163:1267–1271. [DOI] [PubMed] [Google Scholar]
- 25.Finch CA. Erythropoiesis, erythropoietin and iron. Blood 1982; 60:1241–6. [PubMed] [Google Scholar]
- 26.Carinelli VP, Riol RD, Montini G. Iron supplementation enhances response to high doses of recombinant human erythropoietin in preterm infants. Arch Dis Child Fetal Neonatal Ed 1998; 79:F44–F48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petry CD, Eaton MA, Wobken JD, Mills MM, Johnson DE, Georgieff MK. Iron deficiency of liver, heart, and brain in newborn infants of diabetic mothers. J Pediatr. 1992; 121:109–114 [DOI] [PubMed] [Google Scholar]
- 28.Bader D, Blondheim O, Jonas R, et al. Decreased ferritin level, despite iron supplementation, during erythropoietin therapy in anaemia of prematurity. Acta Paediatr 1996; 85:496–501. [DOI] [PubMed] [Google Scholar]
- 29.Bader D,Kugelman A, Maor-Rogin N, Weinger-Abend M, Hershkowitz S, Tamir A, Lanir A, Attias D and Barak M. The role of high dose oral iron supplementation during erythropoietin therapy for anemia of prematurity. Journal of perinatology 2001; 21:215–220. [DOI] [PubMed] [Google Scholar]
- 30.Juul SE. Erythropoietin in the neonate. Current Problems in Pediatrics 1999; 29:133–49. [DOI] [PubMed] [Google Scholar]
- 31.Zipursky A Erythropoietin therapy for premature infants: cost without benefit? Pediatric Research 2000; 48:136. [DOI] [PubMed] [Google Scholar]
- 32.Widness JA, Seward VJ, Kromer IJ, Burmeiser LF, Bell EF, Strauss RG. Changing patterns of red blood cell transfusion in very low birth weight infants. Journal of Pediatrics 1996; 129:680–7. [DOI] [PubMed] [Google Scholar]
- 33.Garcia MG, Hutson AD, Christensen RD. Effect of recombinant erythropoietin on “late” transfusions in the neonatal intensive care unit: a meta-analysis. Journal of Perinatology 2002; 22:108–11. [DOI] [PubMed] [Google Scholar]
- 34.Kotto-Kome AC,Garcia MG, Calhoun DA, Christensen RD. Effect of beginning recombinant erythropoietin treatment within first week of life, among low birth weight neonates, on early and late erythrocyte transfusions:metaanalysis. Journal of Perinatology 2004;24: 24–9. [DOI] [PubMed] [Google Scholar]
- 35.Braekke K, Bechensteen AG, Halvorsen BL, Blomhoff R, Haaland K, Staff AC. Oxidative stress markers and antioxidant status after oral iron supplementation to very low birth weight infants. J Pediatr. 2007. July; 151:23–28. [DOI] [PubMed] [Google Scholar]
- 36.Geng F, Mai X, Zhan J, Xu L, Zhao Z, Georgieff MK, Shao J and Lozoff B. Impact of fetal-neonatal iron deficiency on recognition memory at two months of age. J Pediatr 2015. 167(6): 1226–1232. doi: 10.1016/j.jpeds.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgieff MK. Long-term brain and behavioral consequences of early iron deficiency. Nutrition Reviews 2011; 69:S43–S48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier R-A. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res 2004; 55:1034–41. [DOI] [PubMed] [Google Scholar]
- 39.Cooke RWI, Drury JA, Yoxall CW C. Blood transfusion and chronic lung disease in preterm infants. Eur J Pediatr 1997; 156: 47–50. [DOI] [PubMed] [Google Scholar]
- 40.Inder TE, Clemett RS, Austin NC, Graham P, Darlow BA. High iron status in very low birth weight infants is associated with an increased risk of retinopathy of prematurity. J Pediatr 1997;131(4):541–544. [DOI] [PubMed] [Google Scholar]
- 41.Szoke D, Panteghini M. Diagnostic value of transferrin. Clinica Chimica Acta 2012;413: 1184–1189. [DOI] [PubMed] [Google Scholar]
- 42.Beard J, deRegnier RA, Shaw M, Rao R and Georgieff M. Diagnosis of iron deficiency in infants. Labmedicine 2007;38 (2):103–108. [Google Scholar]
- 43.Amin K, Bansal M, Varley N, Wang H and Amin S. Reticulocyte hemoglobin content as a function of ion stores at 35–36 weeks post menstrual age in very premature infants. The Journal of Maternal-Fetal & Neonatal Medicine, DOI: 10.1080/14767058.2019.1680631 [DOI] [PubMed] [Google Scholar]
- 44.Ennis KM, Dahl LV, Rao RB, Georgieff MK. Reticulocyte hemoglobin content as an early predictive biomarker of brain iron deficiency. Pediatr Res. 2018. November; 84 (5):765–769. doi: 10.1038/s41390-018-0178-6. Epub 2018 Sep 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


