Abstract
Numerous nonmalignant diseases can be treated with radiation therapy (RT). Among them, Heterotopic Ossification (HO) is a benign condition resulting from several causes that can be successfully managed with ionizing radiation. More often seen in the hip area after major surgical procedures, HO is of major concern as it can lead to functional disorders, pain and even to joint ankylosis. We retrospectively analyzed the outcome of therapeutic irradiation for the prevention of HO in 14 patients treated in our hospital between 2005 and 2011. All patients were irradiated with a dose ranging from 7 to10 Gy in a single fraction for prevention of HO after surgery. After a median follow up of 126 months (range 96 – 156 months) none of our patients developed HO. Impaired wound healing or other post surgery complications like trochanteric nonunion were not observed. A single fraction of RT seems to be a sufficient, cost effective and safe treatment regimen. In our study we report excellent results as none of our patients developed HO.
Key words: heterotopic ossification, radiation therapy, single fraction
Introduction
From the introduction of the ionizing radiation, nearly a century ago, its therapeutic role in treating various benign diseases is well established.1 Among nonmalignant conditions, heterotopic ossification (HO) is a frequent disorder, defined as ectopic formation of mature lamellar bone in nonosseous soft tissues. HO has been given several names, including myositis ossificans, neurogenic osteoma, ossifying fibromyopathy, heterotopic calcification, but the best and more accurate definition of this entity is heterotopic ossification.2-4
Resulting from various causes like traumatic, neurogenic or genetic disorders, trauma is the most frequent cause of HO. Fractures or myoskeletal trauma, surgery to major joints, central nervous system injury and severe burns are the usual etiologic factors of HO. Several other risk factors that are responsible and strongly correlated with the progression and extend of the disease are: previous history of HO, hypertrophic osteoarthritis, diffuse idiopathic hyperostosis, male gender, severity of trauma or multiple injuries and specific surgical approaches.5-9
Following major surgical procedures to hip area, like Total Hip Arthroplasty (THA), patients may develop HO with the incidence varying in different studies between 5 to 90%. HO occurs right after surgery with radiographic signs from plain x ray films, computed tomography or bone scans usually apparent between 4–8 week after surgery.7
Even not completely understood, most investigators believe that responsible for the pathogenesis of HO is a pluripotent mesenchymal cell and its inappropriate differentiation to mature osteoblasts.10 The whole procedure from the initial irritative factor like trauma until the formation of ectopic bone tissue involves more than one parameters and requires a “friendly” environment. This process is much complex as it seems that peri implant microenvironment with its osteogenic potential, along with cytokines and nervous system stimuli responses, are associated with an increased risk of ectopic bone formation.11 Urist et al in 1965, hypothesized the existence of bone morphogenic proteins in demineralized bone matrix that stimulate the differentiation of mesenchyme cells, a procedure that begins within 16 hours after surgery with a peak at 32 hours.12 Other investigators have further analyzed the role of several growth factors originating from traumatic tissues and their role in HO.8 Summarizing, and according to a study from Chalmers et al, HO is a complex process, resulting from the inappropriate maturation of a pluripotent mesenchymal cell, the coexistence of other inducing agents all coacting with a suitable environment that permits ectopic bone formation.5
Most cases are asymptomatic and only one-third progresses to clinically significant HO. If symptomatic it presents with local pain, reduced range of motion and in severe cases it may lead to complete ankyloses of the joint. In rare cases when it approaches skin, it may present with signs and symptoms of local inflammation like erythema and edema.13 As mentioned above HO occurs usually 2-3 weeks after surgery. Signs from simple imaging tests are profound after usually 4-6 weeks and further evaluation can be performed via computed tomography scans or magnetic resonance imaging.7
The most widely accepted classification of HO is Brooker classification, which uses radiographic criteria from simple X-ray imaging. Depending on the extent of the ossification around hip joint, the disease is classified as clinically silent or insignificant (class I – II) and clinically significant (class III – IV).
Materials and Methods
We retrospectively analyzed the efficacy and toxicity outcome in 14 patients that underwent radiation therapy (RT) for prevention of HO after hip surgery between January 2005 and December 2011.
4 patients were suffering from HO class II, 9 from class III (clinically significant) while 1 patient was treated after surgery for class IV HO (joint ankyloses), according to Brooker classification. Patients’ characteristics are shown in Table 1.
All patients received a single fraction dose of 7, 8.5, 9 or 10 Gy depending on HO severity (class II, III or IV) and certain risk factors according to the literature: previous history of HO (major factor), hypertrophic osteoarthritis, diffuse idiopathic skeletal hyperostosis or ankylosing spondylitis, prior surgery, multiple injuries, fracture with dislocation and extent of surgical approach (minor factors). Major and minor risk factors are summarized in Table 2. The patient with class IV HO, with a major and two minor factors received 10 Gy. Patients with class III HO received 8.5 or 9 Gy. Those with a major and a minor factor were treated with 9 Gy, while patients with only history of HO received 8.5 Gy. Finally, patients with class II HO and only minor factors apart from male gender received a single fraction of 7 Gy.
Patients were treated with threedimensional conformal radiotherapy (3DCRT) technique, a process that employees individualized 3D anatomy data and gives the ability to develop complex plans in order to deliver highly conformed radiation doses to sites or volumes of interest, while sparing normal tissues. During the first step of the procedure, computed tomography (CT) images of 3-5mm slice thickness were acquired with the patient in the supine treatment position, covering the region of interest, usually from the level of L4/L5 interspace to diaphysis of femur. Delineation of the target volumes of interest followed: Clinical Target Volume (CTV) which included periarticular tissues with the operation bed plus a margin of 1.5 cm and Planning Target Volume (PTV), which corresponded to CTV plus extra margin in order to compensate for inter- and intra-fraction uncertainties consequent to daily setup errors and to potential internal organ motion.
Among 14 patients, 7 underwent RT within the first day after surgery, 5 were treated the second day and 2 patients within 48 to 96 hours from surgery. Treatment was delivered via a Varian 2100C linac accelerator with typically opposed anterioposterior and posterioanterior fields with 6 megavolt (6MV) photons prescribed to midplane (Figure 1).
Special instructions, physiotherapy or kinesiotherapy as well as individualized home program exercise for each patient was prescribed, according to a multidisciplinary approach from orthopedic surgeon, radiation oncologist and physiotherapist.
All patients were regularly followed with physical exam every two months and radiographic images every six months after completion of RT. Final end points were impaired joint motility or any bone formation seen on X-rays.
Figure 1.
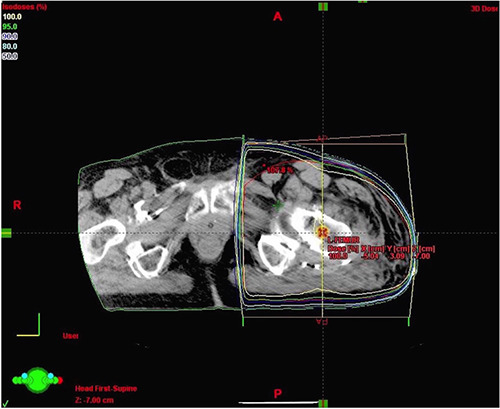
Axial image at the level of isocenter with isodose distribution. Treatment delivered via a 2100C linac accelerator with opposed anterioposterior and posterioanterior fields with 6 megavolt (6MV) photons prescribeda to midplane.
Table 1.
Patients’ characteristics.
| Risk factors | Time of RT (hours after surgery) | ||||||
|---|---|---|---|---|---|---|---|
| Age | Dose RT (Gy) | Major risk factor (HO) | Minor risk factors -N | Brooker Class. | Within 24 h | 24-48 h | 48-96 h |
| 22 | 9 | √ | 1 | III | √ | ||
| 25 | 8.5 | √ | - | III | √ | ||
| 38 | 9 | √ | 1 | III | √ | ||
| 28 | 7 | - | 1 | II | √ | ||
| 41 | 8.5 | √ | - | III | √ | ||
| 26 | 7 | - | 2 | II | √ | ||
| 34 | 8.5 | √ | - | II | √ | ||
| 38 | 8.5 | √ | - | III | √ | ||
| 33 | 10 | √ | 2 | IV | √ | ||
| 32 | 9 | √ | 1 | III | √ | ||
| 29 | 8.5 | √ | - | III | √ | ||
| 38 | 7 | - | 2 | II | √ | ||
| 32 | 8.5 | √ | - | III | √ | ||
| 24 | 9 | √ | 1 | III | √ | ||
Results
Between January 2005 and December 2011, 14 patients were treated for prevention of HO after hip surgery. All patients were at high risk for developing HO after surgery for various reasons, but typically due to previous history of ectopic bone formation.
After a median follow up of 126 months (range 96 – 156 months) none of our patients developed HO. Impaired wound healing or other post surgery complications like trochanteric nonunion were not observed. No early or late radiation induced toxicity was documented in all our patients, including infertility problems, as half of our patients were young male patients at reproductive age.
Discussion
The role of RT in preventing HO is well established and documented from numerous studies. Coventry et al early from the 70s, introduced a scheme of 20 Gy in 10 fractions with excellent results and minimal toxicity for patients treated early after surgery.14 Several other trials, reported also good results with similar RT schemes by means of total dose administered and fraction size.2
In years followed, investigators compared different treatment regimens trying to define the optimal total therapeutic dose. Anthonty et al in 1987, compared 20 Gy in 10 fractions to a 10 Gy scheme administered in 5 fractions. He reported that the 20 Gy regimen was slightly more effective in preventing HO but with higher radiation toxicity rates, 19.4% versus 7.3%.15 A year later, Sylvester et al reported results from 28 patients treated with the same treatment schemes: 20 Gy in 10 fractions or 10 Gy in 5 fractions. They concluded that the two radiation regimens were equally effective when RT administered within 4 days after surgery.16 In a study from Healy et al a 7 Gy regimen was compared to a 5.5 Gy RT scheme. Patients treated with 5.5 Gy developed clinically significant HO in 63% of cases, while only 10% of patients treated with 7 Gy developed HO.17 Comparable results came from a study from Padgett et al in which authors compared RT of 5 or 10 Gy administered postoperatively. Even not statistically significant difference between two arms, the 10 Gy regimen was slightly more efficient than the 5 Gy.18 In a very recent trial from Ruo Redda et al, investigators evaluated the prophylactic role of RT especially in high risk patients. They found that a single fraction of 7 Gy showed excellent results as 76% of patients experienced complete response.19
Many investigators also examined multiple versus single fraction radiotherapy. In a study from Blount et al a 7 Gy RT administered in a single fraction was found as efficacious as conventional 2 Gy RT schemes.20 Konski et al confirmed these results after randomizing patients to 8 Gy in one fraction or 10 Gy in 5 fractions. They reported that both schemes have equal therapeutic results.20 Several other studies from many institutions agreed that single dose irradiation is as effective as classic (2 Gy per fraction) or other hypofractioanted RT schemes (Table 3).15,16,18,20-23 Interestingly in a recently reported meta-analysis of 12 randomized control trials from Milakovic et al, multiple fractions of radiotherapy was found to be superior to single fraction for the overall progression endpoint, implying possibly that optimal dose and fractionation has yet to be defined and further research is needed.24
Another issue that investigators tried to answer, after the established effectiveness of RT, was the time interval between surgical operation or trauma and the administration of radiation as well as whether prophylactic RT should be administerd pre- or postoperatively. In an early study from Kantorowitz et al preoperative RT in HO prophylaxis was examined in rats. Investigators found that preoperative RT soon before the procedure and postoperative RT were superior to prophylactic treatment administered 2 days before operation.25 Many other reports since then agree that prolongation of time between trauma and radiotherapy significantly increases the risk of HO while there seems to be no difference if radiotherapy is administered early before surgery or postoperatively.24,26,27
Shielding of the hip prosthesis seems to have a negative impact on HO incidence after prophylactic RT, as seen from numerous studies. Soon after the employment of RT for the prevention of HO, concern has arisen regarding the possible risk of prosthesis failure due to radiation.28 Seegenschmiedt et al in 1997, found that prosthesis failure was independent to shielding during RT.29 In a retrospective analysis from Balboni et al shielding of the hip prosthesis did not reduce the incidence of prosthesis failure while also led to an increased risk of HO after prophylactic RT. Among 44 patients with hip shielding, HO occurred in 21 cases (48%) while only 8 of 40 unshielded patients (20%) developed HO.30 Another interesting issue in the relative literature is the comparison of RT to other conservative pharmaceutical therapies, mainly NSAID’s. NSAID agents are generally prescribed after the procedure with good results as documented from numerous studies.31-33Many investigators compared RT to NSAIDs with different results and no general agreement about the most effective treatment. Kolbl et al compared a 7 Gy single fraction RT postoperatively, to a 5 Gy scheme and the use of NSAIDs in HO prophylaxis after prosthetic total hip replacement in 301 patients. They reported that HO incidence was 11.1 %, 30.1% and 16% respectively. They concluded that a single 7 Gy fraction is superior to NSAIDs and represents the most effective postoperative prophylactic treatment for HO.34 Burd et al in two prospective randomized trials found that both prophylactic modalities had the same rates of HO. They also noted that patients receiving indomethacin had a significant risk of bone nonunion compared to irradiated patients.35,36 In a meta-analysis from Pakos et al, the efficacy of RT versus NSAIDs was evaluated. RT was superior in preventing clinically significant (Brooke’s III, IV) HO, as it was almost twice more effective than NSAIDs, but absolute benefit gain was small.37 In a review article from Legosz et al, the efficacy of RT and nonsteroidal antiinflammatory drugs was evaluated. Single fraction RT of 7 Gy postoperatively and indomethacin were the two most common used regimens with equal effectiveness.38 Finally, three meta-analysis of randomizes clinical trials (RCTs) have compared RT to other treatment options mainly NSAIDs. In the two meta-analysis from Vanken et al, there was not any statistically significant difference, although Grade 3 and 4 HO was less often seen after radiation therapy while NSAIDs were considerably more cost effective as a treatment modality.39,40 In the third very recent meta-analysis from Cai et al, the main strategies for prevention of HO were evaluated. After collecting data from 31 RCTs and statistical analysis of these, the investigators found that RT was the most efficient option for the prevention of HO after THA.41
Table 2.
Major and minor risk factors for Heterotopic Ossification (HO).
| Major factor | Minor factors |
|---|---|
| Previous history of HO | Hypertrophic osteoarthritis |
| Diffuse idiopathic skeletal hyperostosis | |
| Ankylosing spondylitis | |
| Prior surgery | |
| Multiple injuries | |
| Fracture with dislocation | |
| Extent of surgical approach |
Table 3.
Radiation Therapy in Heterotopic Ossification.
| Author | Year | Study | N. hips | Conclusions |
|---|---|---|---|---|
| Anthony et al.15 | 1987 | 20Gy/10fr vs 10Gy/5fr | 46 | 10 Gy as effective as 20Gy |
| Sylvester et al.16 | 1988 | 20Gy/10fr vs 10Gy/5 fr | 27 | Reduced dose effective |
| Blount et al.20 | 1990 | 10Gy/5fr vs 12/6fr vs 20/10fr vs 8Gy/4fr vs 7/single fr | 97 | 8 Gy and 7Gy same efficacy |
| Konski et al.21 | 1990 | 10Gy/5fr vs 8Gy/single fr | 37 | 8 Gy as effective as 10 Gy |
| Pellegrini et al.22 | 1992 | 8Gy/single fr vs 10Gy/5fr | 62 | Single-dose limited-field radiation is effective |
| Padgett et al.18 | 2003 | 5Gy/2fr vs 10Gy/5fr | 59 | 10 Gy more efficient |
All the above data regarding HO are confirmed by two recent meta-analysis studies from Canada. Several factors influencing HO formation in patients receiving prophylactic radiotherapy were analyzed. After statistical analyses of all 5464 treatment sites that were included in the first study, and after meta-analysis of 12 well designed controlled randomized trials in the second study, authors concluded that low dose radiation therapy administered either preoperatively or postoperatively represents an efficacious treatment regimen that can decrease the rate of HO.24, 42
Toxicity of treatment
Due to low RT doses, the toxicity from treatment is uncommon and most investigators agree that usually no side effects are expected from prophylactic RT for HO.43 The issue of carcinogenesis and second malignancies from radiation treatment is gaining more interest in latest years. In a recent report from Mourad et al. a case of radiation-induced sarcoma was reported after prophylactic RT for HO.44 Further data from literature are scarce and come often from case reports.45 Even if the risk for second malignancies is minimal, a general principal that physicians should follow is that administering prophylactic RT to young patients as prophylaxis for HO should be carefully evaluated. Fertility problems are another potential side effect from radiotherapy in young males. RT doses in the range of 70 to 100 cGy may cause oligospermia while slightly higher doses may even cause permanent azoospermia. Every attempt should be made for testis shielding in young males and patients should always be informed about the potential impact on fertility.46,47
Conclusions
Many benign diseases are managed with Radiation Therapy. Among them prevention of Heterotopic Ossification is a usual indication for RT among many centers worldwide. A single fraction of RT seems to be a sufficient, cost effective and a safe treatment regimen. In our study we report excellent results as none of our patients developed HO.
Funding Statement
Funding: Self funding.
References
- 1.Micke O, Seegenschmiedt MH: Consensus guidelines for radiation therapy of benign diseases: a multicenter approach in Germany. Int J Radiat Oncol Biol Phys 2002; 52:496-513. [DOI] [PubMed] [Google Scholar]
- 2.MacLennan I, Keys HM, McCollister Evarts C, et al. : Usefulness of postoperative hip irradiation in the prevention of heterotopic bone formation in a high risk group of patients. Int J Radiat Oncol Biol Phys 1984; 10:49-53. [DOI] [PubMed] [Google Scholar]
- 3.Balboni TA, Gobezie R, Mamon HJ: Heterotopic ossification: Pathophysiology, clinical features, and the role of radiotherapy for prophylaxis. Int J Radiat Oncol Biol Phys 2006; 65:1289-299. [DOI] [PubMed] [Google Scholar]
- 4.Ritter MA VR: Ectopic ossification after total hip arthroplasty. Predisposing factors, frequency, and effect on results. J Bone Joint Surg Am 1977;59:345-51. [PubMed] [Google Scholar]
- 5.Chalmers J GD, Rush J: Observations on the induction of bone in soft tissues. J Bone Joint Surg Br 1975;57:36-45 [PubMed] [Google Scholar]
- 6.Cohen RB HG, Tabas JA, et al. : The natural history of heterotopic ossification in patients who have fibrodysplasia ossificans progressiva. A study of forty - four patients. J Bone Joint Surg Am 1993;75:215-9. [DOI] [PubMed] [Google Scholar]
- 7.Ledermann HP SM, Morrison WB: Pelvic Heterotopic Ossification: MR Imaging Characteristics. Radiology 2002;222:189-95. [DOI] [PubMed] [Google Scholar]
- 8.Nilsson OS PP: Heterotopic bone formation after joint replacement. Curr Opin Rheumatol 1999;11:127-31. [DOI] [PubMed] [Google Scholar]
- 9.Pacifici M: Acquired and congenital forms of heterotopic ossification: new pathogenic insights and therapeutic opportunities. Curr Opin Pharmacol 2018;40:51-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naraghi FF DT, Moncim MS, et al. : Heterotopic Ossification. Orthopedics 1996;19:145-51. [DOI] [PubMed] [Google Scholar]
- 11.Henze K, Monika Herten M, Haversath M, et al. : Surgical vacuum filter-derived stromal cells are superior in proliferation to human bone marrow aspirate. Stem Cell Res Ther. 2019;10:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Urist MR: Bone: formation by autoinduction. Science 1965;150:893-899. [DOI] [PubMed] [Google Scholar]
- 13.Schafer S, Schafer LO, Anglen JO, Childers M: Heterotopic ossification in rehabilitation patients who have had internal fixation of an acetabular fracture. J Rehabil Res Dev 2000;37: 389-93. [PubMed] [Google Scholar]
- 14.Coventry MB SP: The use of radiation to discourage ectopic bone. A nine-year study in surgery about the hip. J Bone Joint Surg Am 1981;63:201-8. [PubMed] [Google Scholar]
- 15.Anthony P, Keys H, McCollister Evarts C, et al. : Prevention of heterotopic bone formation with early post operative irradiation in high risk patients undergoing total HIP arthroplasty: Comparison of 10.00 Gy VS 20.00 Gy schedules. Int J Radiat Oncol Biol Phys 1987;13:365-9. [DOI] [PubMed] [Google Scholar]
- 16.Sylvester J, Greenberg P, Selch M, et al. : The use of postoperative irradiation for the prevention of heterotopic bone formation after total hip replacement. Int J Radiat Oncol Biol Phys 1988;14:471-6. [DOI] [PubMed] [Google Scholar]
- 17.Healy W, Lo T, DeSimone A, et al. : Single-dose irradiation for the prevention of heterotopic ossification after total hip arthroplasty. A comparison of doses of five hundred and fifty and seven hundred centigray. J Bone Joint Surg Am 1995;77:590-5. [DOI] [PubMed] [Google Scholar]
- 18.Padgett DE, Holley KG, Cummings M, et al. : The efficacy of 500 centigray radiation in the prevention of heterotopic ossification after total hip arthroplasty: a prospective, randomized, pilot study. J Arthroplasty 2003;18:677-86. [DOI] [PubMed] [Google Scholar]
- 19.Ruo Redda MG, De Colle C, Bianco L, Ruggieri A, Nassisi D, Rossi A, Gino E, Airaldi C. Heterotopic ossifications: role of radiotherapy as prophylactic treatment. Radiol Med 2018; 123:463-468. [DOI] [PubMed] [Google Scholar]
- 20.Blount LH, Thomas BJ, Tran L, et al. : Postoperative irradiation for the prevention of heterotopic bone: Analysis of different dose schedules and shielding considerations. Int J Radiat Oncol Biol Phys 1990;19:577-81. [DOI] [PubMed] [Google Scholar]
- 21.Konski A, Pellegrini V, Poulter C, et al. : Randomized trial comparing single dose versus fractionated irradiation for prevention of heterotopic bone: A preliminary report. Int J Radiat Oncol Biol Phys 1990;18:1139-42. [DOI] [PubMed] [Google Scholar]
- 22.Pellegrini V, Konski A, Gastel J, et al. : Prevention of heterotopic ossification with irradiation after total hip arthroplasty. Radiation therapy with a single dose of eight hundred centigray administered to a limited field. J Bone Joint Surg Am 1992;74:186-200. [PubMed] [Google Scholar]
- 23.Lee DS, Kim Y, Cho HJ, Kim M, Whang IY. Hypofractionated Radiation Therapy for Progressive Heterotopic Ossification: The Relationship between Dose and Efficacy. Int J Radiat Oncol Biol Phys. 2020;106:993-7. [DOI] [PubMed] [Google Scholar]
- 24.Milakovic M, Popovic M, Raman S, et al. : Radiotherapy for the prophylaxis of heterotopic ossification: A systematic review and meta-analysis of randomized controlled trials. Radiother Oncol 2015;116:4-9. [DOI] [PubMed] [Google Scholar]
- 25.Kantorowitz DA, Miller GJ, A. Ferrara J, et al: Preoperative versus postoperative irradiation in the prophylaxis of heterotopic bone formation in rats. Int J Radiat Oncol Biol Phys 1990;19:1431-8. [DOI] [PubMed] [Google Scholar]
- 26.Mourad WF, Packianathan S, Shourbaji RA, et al. : A Prolonged Time Interval Between Trauma and Prophylactic Radiation Therapy Significantly Increases the Risk of Heterotopic Ossification. Int J Radiat Oncol Biol Phys 2012;82:e339-44. [DOI] [PubMed] [Google Scholar]
- 27.Morcos M., Smith K., Tanzer M. The effect of late radiotherapy on the progression of heterotopic ossification following total hip arthroplasty. Eur J Orthop Surg Traumatol 2018; 28:1125–31. [DOI] [PubMed] [Google Scholar]
- 28.Konski A, Weiss C, Rosier R, et al. : The use of postoperative irradiation for the prevention of heterotopic bone after total hip replacement with biologic fixation (porous coated) prosthesis: An animal model. Int J Radiat Oncol Biol Phys 1990;18:861-5. [DOI] [PubMed] [Google Scholar]
- 29.Seegenschmiedt MH, Keilholz L, Martus P, et al. : Prevention of heterotopic ossification about the hip: Final results of two randomized trials in 410 patients using either preoperative or postoperative radiation therapy. Int J Radiat Oncol Biol Phys 1997;39:161-71. [DOI] [PubMed] [Google Scholar]
- 30.Balboni TA, Gaccione P, Gobezie R, et al. : Shielding of the Hip Prosthesis During Radiation Therapy for Heterotopic Ossification is Associated with Increased Failure of Prophylaxis. Int J Radiat Oncol Biol Phys 2007;67:1499-505. [DOI] [PubMed] [Google Scholar]
- 31.Barthel T, Baumann B, Nöth U, et al. : Prophylaxis of heterotopic ossification after total hip arthroplasty. Acta Orthop Scand 2002;73:611-4. [DOI] [PubMed] [Google Scholar]
- 32.Hofmann S TH, Metzenroth H: General shortterm indomethacin prophylaxis to prevent heterotopic ossification in total hip arthroplasty. Orthopedics 199;22:207-11. [DOI] [PubMed] [Google Scholar]
- 33.Neal BC, Rodgers A, Clark T, et al. : A systematic survey of 13 randomized trials of non-steroidal anti-inflammatory drugs for the prevention of heterotopic bone formation after major hip surgery. Acta Orthop Scand 2000;71:122-8. [DOI] [PubMed] [Google Scholar]
- 34.Kölbl O, Knelles D, Barthel T, et al. : Randomized trial comparing early postoperative irradiation vs. the use of nonsteroidal antiinflammatory drugs for prevention of heterotopic ossification following prosthetic total hip replacement. Int J Radiat Oncol Biol Phys 1997;39:961-6. [DOI] [PubMed] [Google Scholar]
- 35.Burd TA, Hughes MS, Anglen JO: Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br 2003;85:700-5. [PubMed] [Google Scholar]
- 36.Burd TA, Lowry KJ, Anglen JO: Indomethacin Compared with Localized Irradiation for the Prevention of Heterotopic Ossification Following Surgical Treatment of Acetabular Fractures. J Bone Joint Surg Am 2001;83:1783-8. [DOI] [PubMed] [Google Scholar]
- 37.Pakos E, Ioannidis J: Radiotherapy vs. nonsteroidal anti-inflammatory drugs for the prevention of heterotopic ossification after major hip procedures: a metaanalysis of randomized trials. Int J Radiat Oncol Biol Phys 2004;60:888-95. [DOI] [PubMed] [Google Scholar]
- 38.Łęgosz P, Otworowski M, Sibilska A, Starszak K, Kotrych D, Kwapisz A, Synder M. Heterotopic Ossification: A Challenging Complication of Total Hip Arthroplasty: Risk Factors, Diagnosis, Prophylaxis, and Treatment. Biomed Res Int. 2019; doi:10.1155/2019/3860142.eCollection 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vanken Vavken P, Castellani L, Sculco TP. Prophylaxis of heterotopic ossification of the hip. Clin Orthop Relat Res. 2009;467:3283–3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vanken Vavken P, Dorotka R. Economic evaluation of NSAID and radiation to prevent heterotopic ossification after hip surgery. Arch Orthop Trauma Surg. 2011;131:1309–1315. [DOI] [PubMed] [Google Scholar]
- 41.Cai L, Wang Z, Luo X, She W, Zhang H. Optimal strategies for the prevention of heterotopic ossification after total hip arthroplasty: A network meta-analysis. Int J Surg. 2019;62:74-85. [DOI] [PubMed] [Google Scholar]
- 42.Popovic M, Agarwal A, Zhang L, et al. : Radiotherapy for the prophylaxis of heterotopic ossification: A systematic review and meta-analysis of published data. Radioth Oncol 2014;113:10-17. [DOI] [PubMed] [Google Scholar]
- 43.Kim J, Chu F, Woodard H, et al. : Radiation induced soft tissue and bone sarcoma. Radiology 1978;129:501-8. [DOI] [PubMed] [Google Scholar]
- 44.Mourad WF, Packianathan S, Shourbaji RA, et al. : Radiation-induced sarcoma following radiation prophylaxis of heterotopic ossification. Pract Radiat Oncol 2012;2:151-4. [DOI] [PubMed] [Google Scholar]
- 45.Farris M, Chowdhry V, Lemke S, et al. : Osteosarcoma following single fraction radiation prophylaxis for heterotopic ossification. Radiat Oncol 2012;7:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel H, Silverman C, Carrascosa L, et al. : Evaluation of scrotal and testicular radiation doses for heterotopic ossification prophylaxis. Am J Orthop (Belle Mead NJ) 2008;37:E163-6. [PubMed] [Google Scholar]
- 47.Schwartz CL: Survivors of Childhood and Adolescent Cancer: A Multidisciplinary Approach. Baltimore, Springer, 2005. [Google Scholar]


