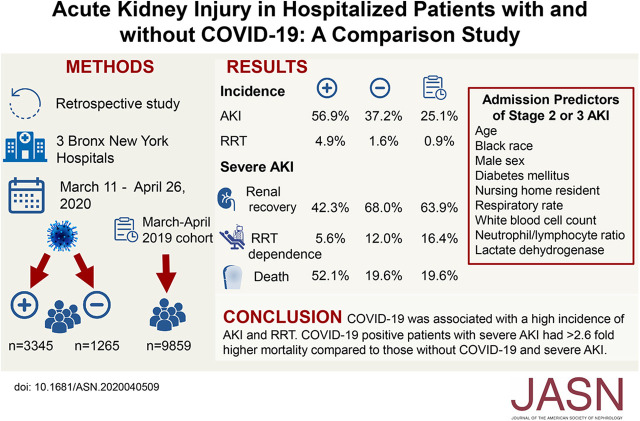
Significance Statement
Centers have reported a wide range of AKI incidence rates among patients hospitalized with coronavirus disease 2019 (COVID-19). In a retrospective observational study, the authors compared the incidence, risk factors, and outcomes of AKI in hospitalized adults with and without COVID-19 in a large New York City health system. Compared with patients without COVID-19 and with historical controls, patients with COVID-19 had a significantly higher incidence of AKI; were more likely to require RRT, intensive care unit admission, and mechanical ventilation; and were more likely to experience in-hospital death. Male sex, Black race, and older age were associated with AKI, but these associations were not unique to COVID-19. Select initial vital signs at hospital admission and inflammatory markers were predictors of severe AKI.
Keywords: AKI, COVID-19, sex, race, outcomes, risk factors
Visual Abstract
Abstract
Background
Reports from centers treating patients with coronavirus disease 2019 (COVID-19) have noted that such patients frequently develop AKI. However, there have been no direct comparisons of AKI in hospitalized patients with and without COVID-19 that would reveal whether there are aspects of AKI risk, course, and outcomes unique to this infection.
Methods
In a retrospective observational study, we evaluated AKI incidence, risk factors, and outcomes for 3345 adults with COVID-19 and 1265 without COVID-19 who were hospitalized in a large New York City health system and compared them with a historical cohort of 9859 individuals hospitalized a year earlier in the same health system. We also developed a model to identify predictors of stage 2 or 3 AKI in our COVID-19.
Results
We found higher AKI incidence among patients with COVID-19 compared with the historical cohort (56.9% versus 25.1%, respectively). Patients with AKI and COVID-19 were more likely than those without COVID-19 to require RRT and were less likely to recover kidney function. Development of AKI was significantly associated with male sex, Black race, and older age (>50 years). Male sex and age >50 years associated with the composite outcome of RRT or mortality, regardless of COVID-19 status. Factors that were predictive of stage 2 or 3 AKI included initial respiratory rate, white blood cell count, neutrophil/lymphocyte ratio, and lactate dehydrogenase level.
Conclusions
Patients hospitalized with COVID-19 had a higher incidence of severe AKI compared with controls. Vital signs at admission and laboratory data may be useful for risk stratification to predict severe AKI. Although male sex, Black race, and older age associated with development of AKI, these associations were not unique to COVID-19.
Since the detection of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of coronavirus disease 2019 (COVID-19), there have been millions of confirmed cases of COVID-19 worldwide.1,2 The first confirmed case of COVID-19 in New York was on March 1, 2020.3 In the weeks that followed, New York City became the epicenter for the COVID-19 pandemic.
The kidney may serve as a target for organ injury in COVID-19 because angiotensin-converting enzyme 2, the binding site for SARS-CoV-2, is highly expressed in proximal tubule cells and podocytes.4–6 Postmortem biopsy series have demonstrated marked renal tubular injury (B. Diao, C. Wang, R. Wang, Z. Feng, Y. Tan, H. Wang, et al.: Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection. medRxiv, 2020; DOI:10.1101/2020.03.04.20031120).7 Additionally, collapsing FSGS has been reported in Black patients with high risk APOL1 alleles.8,9 Data from the single center studies in China showed an incidence of AKI ranging from 0.5% to 29% in hospitalized patients with COVID-19.10–12 In contrast, early studies in the United States have reported a much higher incidence of AKI of between 28%–46% and in-hospital mortality of 35%–41% in those who developed AKI (L. Chan, K. Chaudhary, A. Saha, K. Chauhan, A. Vaid, M. Baweja, et al.: Acute kidney injury in hospitalized patients with COVID-19. medRxiv, 2020; doi:10.1101/2020.05.04.20090944).13,14 Although most of these reports identify risk factors for AKI, there have been no direct comparisons to patients negative for COVID-19 that would identify aspects of AKI risk, course, and outcomes that are unique to COVID-19.
The Bronx is the one of the nation’s poorest urban counties and has excess mortality rates from heart disease, stroke, and diabetes compared with national averages.15 A recent study of the five boroughs of New York City demonstrated that the Bronx had the highest rate of hospitalizations and deaths due to COVID-19 per 100,000 population.16 Due to an increased burden of comorbidities, this population is representative of a group at increased risk for adverse outcomes from COVID-19, including AKI.17,18
Furthermore, this population helps to identify risk factors that predict development of AKI as well as adverse outcomes after AKI in patients hospitalized with COVID-19. The aims of our study are the following: (1) to report the incidence, risk factors, and outcomes of COVID-19–associated AKI in a high-risk population; and (2) to determine whether there exist risk factors for AKI unique to COVID-19 infection by comparison with a cohort negative for COVID-19.
Methods
Study Population
During the surge of COVID-19 in the New York City area in mid-March 2020, Montefiore Health System (MHS) was the largest hospital system providing care to patients with COVID-19 infection in the Bronx. Using Clinical Looking Glass—an interactive software application developed at MHS to gather and integrate demographic, clinical, and administrative data sets—we performed a retrospective observational study of patients who tested positive or negative for COVID-19 upon hospitalization at any of the three main tertiary care hospitals of MHS (Moses, Wakefield, and Weiler Hospitals) in the Bronx between March 11 and April 26, 2020.19–21 We also compared patients with COVID-19 infection to a historical cohort of patients hospitalized at MHS between March 1 and April 26, 2019. The Institutional Review Board from Albert Einstein College of Medicine approved this study. Informed consent was waived and only de-identified data were analyzed.
Patients >18 years of age with a COVID-19 test performed upon hospitalization during the study period were included. A confirmed case of COVID-19 was defined by a positive result on an RT-PCR assay of a specimen collected on a nasopharyngeal or oral swab at either the Montefiore virology laboratory or a commercial laboratory used by MHS (Viracor, Lee’s Summit, MO).
Data Collection
We identified 4610 patients hospitalized during the study period after excluding those <18 years of age (n=246), patients with ESKD (n=430), patients without creatinine values (n=120), and one patient with an unknown sex assignment (Figure 1). Information on sociodemographic variables (age, sex, race/ethnicity, and socioeconomic status [SES]) was extracted. Race/ethnicity was self-reported and categorized as non-Hispanic White, non-Hispanic Black, Hispanic, or other. SES was calculated using census tract data from the Census Bureau and was reported as SD from the New York State mean.22 A “crowding” variable was defined as the percentage of households by census tract with >1.5 occupants per room and was obtained from the American Community Survey (Census Bureau).4 Data were extracted on comorbidities including obesity (body mass index), diabetes mellitus (DM), CKD, congestive heart failure (CHF), lung disease, HIV/AIDS, malignancy, and rheumatologic disease using International Classification of Diseases, Ninth (ICD-9) or Tenth Revision (ICD-10) codes within 6 months before hospitalization. These were then used to create a Charlson Comorbidity Index Score.23 In addition to ICD-9/-10 codes, hemoglobin A1c >6.5% within 2 years before hospitalization was used to define DM and eGFR <60 ml/min per 1.73 m2 (the mean of all GFRs between 7 and 365 days before hospitalization) was used to define CKD. Data were extracted on initial vital signs and laboratory data on hospitalization, the need for mechanical ventilation, or RRT, type of RRT provided, intensive care unit (ICU) admission, and death.
Figure 1.
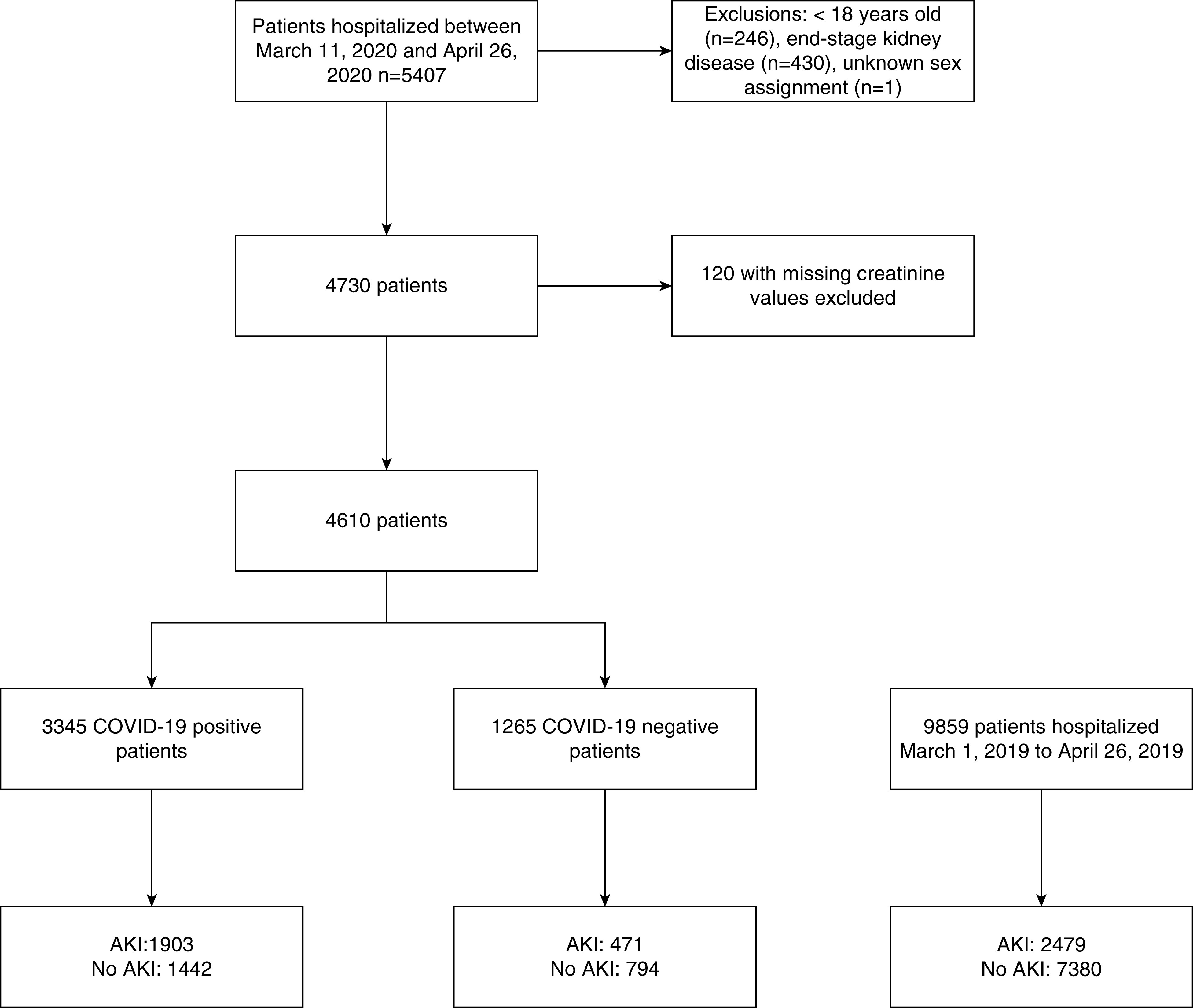
Study population.
Measurements and Variable Definitions
AKI was defined using a 0.3 mg/dl increase or >50% increase in serum creatinine from the baseline creatinine to maximum in-hospital creatinine.24 Baseline creatinine was defined as the mean creatinine value between 7 and 365 days before hospitalization (available for n=2245; 49%).25 In those without a baseline creatinine (n=2365), the minimum creatinine value during hospitalization was used as the baseline creatinine as described by Siew et al.26 AKI severity was staged according to Kidney Disease Improving Global Outcomes criteria. Those who required RRT automatically met the definition of stage 3 AKI.24 Urine output criteria to define AKI were not used because urine output was not reliably collected. A sensitivity analysis was performed that limited the definition of AKI to those with an increase in serum creatinine of 50% or greater. The eGFR was calculated using the Modification of Diet in Renal Disease equation.27 Renal recovery in patients with AKI was defined by a fall in the serum creatinine to a level 50% lower than the maximum creatinine achieved during hospitalization in the absence of an ongoing need for RRT.28
Outcomes
The primary outcome was incident AKI. The secondary outcome was the composite of need for RRT or mortality.
Statistical Analyses
Descriptive statistics were summarized as mean (SD) or median (interquartile range [IQR]) for continuous variables and number (%) for categoric variables. The proportion of sociodemographic and clinical data, initial vital signs, and laboratory values in those with AKI as compared with those without AKI were tested separately for the COVID-19–positive and COVID-19–negative groups using t test and Wilcoxon rank sum testing for parametric and nonparametric continuous data. Chi-squared analysis was used to test the association of categoric variables. We further compared the proportion of patients with AKI, stage 3 AKI, renal recovery, need for RRT, mortality, ICU admission, mechanical ventilation, and discharge status between the COVID-19–positive, COVID-19–negative, and historical cohort groups using risk ratios with 95% confidence intervals.
Logistic regression was used to test the association of the following exposure variables with AKI incidence: (1) sex, (2) race/ethnicity, and (3) age deciles in unadjusted and adjusted models. Adjustment variables were selected if bivariate associations showed significance at the α level of 0.05 or if clinically relevant. Model 1 was unadjusted. Model 2 was model 1 plus adjustment for other demographic variables and clinical comorbidities (CKD, DM, CHF, Charlson Comorbidity Index, prehospitalization use of angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, nursing home resident). Model 3 was model 2 plus adjustment for SES and crowding. To evaluate whether the same risks of AKI apply to patients who were COVID-19 positive as compared with those who were COVID-19 negative, interaction testing was used to test the association of exposure variables and COVID-19 status with respect to AKI occurrence in the logistic regression models.
To test the association between the above exposure variables and AKI incidence, we built a competing risk model of time to AKI with death as a competing variable in unadjusted and adjusted models.29 Similar adjustments were made as those described above.
To define the effect of AKI in this cohort, we evaluated the composite outcome of RRT or mortality using Cox proportional hazards modeling which derived hazard ratios of (1) sex, (2) race/ethnicity, and (3) age decile in unadjusted and adjusted models. Adjustment variables were similar to those used for logistic regression. Cumulative incidence plots were used to represent the hazard of composite outcome by group and likelihood ratio testing was used to test the difference in those outcomes by (1) sex, (2) race/ethnicity, (3) and age decile. Data were censored on April 26, 2020.
We built a predictive model using logistic regression and incorporated established sociodemographic and clinical risk factors for AKI (from our models above) and initial vital signs and laboratory data. We generated random number assignments for the observations and built a predictive model for stage 2 or 3 AKI as the outcome variable in 60% of the population. We then tested the predictive model using the remaining 40% of the population and postestimation testing derived receiving operating curves and estimated area under the precision recall curves. The predictive model was limited by the number of missing values for inflammatory markers checked within 24 hours of admission. The following variable were not used because of a large proportion with missing data points: proteinuria (n missing=1508/3345), ferritin (n missing=1281/3345), fibrinogen (n missing=1416/3345), procalcitonin (n missing=1496/3345), D-dimer (n missing=1070/3345), and C-reactive protein value (n missing=855/3345).
Stata 14.0 was used for all analyses. Statistical significance was defined at α≤0.05.
Results
Baseline Characteristics
Baseline characteristics of patients hospitalized with COVID-19 are outlined in Table 1, and comparisons with patients negative for COVID-19 are provided in the Supplemental Table 1. Between March 11 and April 26, 2020, 4610 patients with a COVID-19 test performed were hospitalized at MHS; 3345 were positive for COVID-19 and 1265 were negative for COVID-19 (Figure 1). In the COVID-19–positive cohort, mean age was 64 (SD, 16.4) years old, 8.2% were White, 35.9% were Black, and 37.3% were Hispanic. More than half were men (53.1%) and 16.4% were nursing home residents. Obesity was present in 1351 (42.9%), CKD was present in 409 (12.2%), and DM was present in 906 (27.1%) of the patients. Median SES was below the state average (−2.2 as compared with 1 representing mean SES for New York State) (Table 1). Compared with patients positive for COVID-19, those who were negative for COVID-19 were younger (mean age, 58.5 years), more were female (51.9%), fewer were obese (36.7%), and fewer were nursing home residents (11.2%) (Supplemental Table 1).
Table 1.
Baseline characteristics of hospitalized patients positive for COVID-19
| Total Cohort (n=4610) | Total Positive for COVID-19 (n=3345) | Positive for COVID-19 with AKI (n=1903; 56.9%) | Positive for COVID-19 and No AKI (n=1442; 43.1%) | P Value |
|---|---|---|---|---|
| Sociodemographic data, n (%) | ||||
| Age, yr, mean (SD) | 64.4 (16.4) | 67.1 (15.3) | 60.7 (17.0) | <0.001 |
| Age decile, yr | <0.001 | |||
| <30 | 113 (3.4) | 42 (2.2) | 71 (4.9) | |
| 31–40 | 189 (5.6) | 69 (3.6) | 120 (8.3) | |
| 41–50 | 311 (9.3) | 135 (7.1) | 176 (12.2) | |
| 51–60 | 55316.5) | 286 (15.0) | 267 (18.5) | |
| 61–70 | 779 (23.3) | 436 (22.9) | 343 (23.8) | |
| 71–80 | 771 (23.0) | 513 (27.0) | 258 (17.9) | |
| >80 | 629 (18.8) | 422 (22.2) | 207 (14.4) | |
| Sex | <0.001 | |||
| Female | 1569 (46.9) | 812 (42.7) | 757 (52.5) | |
| Male | 1776 (53.1) | 1091 (57.3) | 685 (47.5) | |
| Race/ethnicity | <0.001 | |||
| Non-Hispanic White | 275 (8.2) | 155 (8.2) | 120 (8.3) | |
| Non-Hispanic Black | 1201 (35.9) | 771 (40.5) | 430 (29.8) | |
| Hispanic | 1247 (37.3) | 638 (33.5) | 609 (42.2) | |
| Other | 622 (18.6) | 339 (17.8) | 283 (19.6) | |
| Nursing home resident | 548 (16.4) | 399 (21.0) | 149 (10.3) | <0.001 |
| SES, median (IQR) (n=3680) | −2.2 [−5.3 (−1.1)] | −2.1 [−4.9 (−1.0)] | −2.4 [−5.7–(−1.1)] | 0.03 |
| Clinical comorbidities, n (%) | ||||
| BMI, kg/m2 (n=4367) | 0.005 | |||
| <30 | 1797 (57.1) | 1069 (59.6) | 728 (53.8) | |
| 30–35 | 725 (23.0) | 388 (21.6) | 337 (24.9) | |
| >35 | 626 (19.9) | 338 (18.8) | 288 (21.3) | |
| DM | 906 (27.1) | 569 (29.9) | 337 (23.4) | <0.001 |
| CKD | 409 (12.2) | 287 (15.1) | 122 (8.5) | <0.001 |
| CHF | 129 (3.9) | 87 (4.6) | 42 (2.9) | 0.01 |
| Charlson comorbidity index | <0.001 | |||
| 0–1 | 1094 (32.7) | 495 (26.0) | 599 (41.5) | |
| 1–5 | 1993 (59.6) | 1237 (65.0) | 756 (52.4) | |
| >5 | 258 (7.7) | 171 (9.0) | 87 (6.0) | |
| Initial vital signs, mean (SD) | ||||
| Temperature, °F (n=4598) | 99.2 (1.6) | 99.3 (1.6) | 99.2 (1.5) | 0.6 |
| Respiratory rate, breaths/min (n=4599) | 21.6 (6.3) | 22.3 (6.8) | 20.6 (5.4) | <0.001 |
| Pulse oximetry, %(n=4601) | 92.8 (8.0) | 91.7 (9.4) | 94.3 (5.4) | <0.001 |
| Pulse rate, beats/min (n=4601) | 98.3 (20.4) | 99.1 (21.3) | 97.2 (19.3) | 0.01 |
| Initial laboratory data | ||||
| WBC (K/µl), mean (SD) (n=4602) | 8.7 (7.0) | 9.3 (6.9) | 7.8 (7.1) | <0.001 |
| Neutrophil (K/µl), mean (SD) (n=4602) | 6.7 (4.0) | 7.3 (4.2) | 5.8 (3.5) | <0.001 |
| Neutrophil-lymphocyte ratio, median (IQR) | 5.7 (3.5–9.3) | 6.6 (4.1–10.4) | 4.7 (3–7.7) | <0.001 |
| Procalcitonin, median (IQR) | 0.2 (0.1–0.7) | 0.4 (0.1–1.3) | 0.2 (0.1–0.2) | <0.001 |
| Fibrinogen (mg/dl), mean (SD) (n=2297) | 649.0 (200.0) | 659.1 (210.9) | 631.8 (177.8) | 0.002 |
| CRP (mg/dl), median (IQR) (n=3429) | 10.8 (4.8–19.1) | 13.2 (6.1–22.4) | 7.3 (3.3–14.3) | <0.001 |
| D-dimer (µg/ml), median (IQR) (n=2726) | 1.7 (0.9–3.8) | 2.2 (1.1–6.2) | 1.1 (0.7–2.3) | <0.001 |
| LDH (U/L), median (IQR) (n=3715) | 403 (300–546) | 458 (336–637) | 350 (270–451) | <0.001 |
| Ferritin (ng/ml), median (IQR) (n=2470) | 762.5 (384–1420) | 911 (490.5–1667.5) | 609.5 (296–1086.5) | <0.001 |
| Proteinuria, n (%) (n=2590) | <0.001 | |||
| <30 mg | 14 (0.8)a | 5 (0.4) | 7 (1.4) | |
| 30–500 mg | 1761 (95.9) | 1128 (95.0) | 633 (97.5) | |
| >500 mg | 62 (3.4) | 55 (4.6) | 9 (1.1) | |
| Hospitalization, n (%) | ||||
| ICU admission | 438 (13.1) | 382 (20.1) | 56 (3.9) | <0.001 |
| Mechanical ventilation | 624 (17.6) | 569 (29.9) | 55 (3.8) | <0.001 |
BMI, body mass index; CRP; C-reactive protein.
Percentages are of the total tested for proteinuria.
In the COVID-19–positive cohort, patients with AKI were older compared with those without AKI (mean, 67.1 [SD, 15.3] years versus 60.7 [SD, 17] years; P<0.001), a higher proportion were men (57.3% versus 42.7%; P<0.001), more were Black (40.5% versus 29.8%; P<0.001), and more were nursing home residents (20.9% versus 10.3%; P<0.001) (Table 1). Those with COVID-19 and AKI had higher Charlson Comorbidity Index Scores (P<0.001) with higher prevalence of DM (29.9% versus 23.4%; P<0.001), CKD (15.1% versus 8.5%; P<0.001), and CHF (4.6% versus 2.9%; P=0.01) compared with those without AKI (Table 1). Patients with AKI but negative for COVID-19 had similar patterns of association, demonstrating older age, a higher prevalence of comorbidities, and a higher proportion of men compared with those without AKI (Supplemental Table 1).
Incidence and Severity of AKI
AKI occurred in 56.9% of patients positive for COVID-19 compared with 37.2% of those negative for COVID-19 who were hospitalized during the pandemic (RR, 1.5; 95% CI, 1.4 to 16) and 25.1% in the historical cohort (RR, 2.3; 95% CI, 2.2 to 2.4) (Table 2). In the 1903 patients with AKI who were positive for COVID-19, 942 (49.5%) had stage 1 AKI, 387 (20.3%) had stage 2 AKI, 574 (30.2%) had stage 3 AKI, and 28.5% of patients with stage 3 AKI required RRT.
Table 2.
Clinical outcomes of hospitalized patients positive for COVID-19 compared with patients negative for COVID-19 and a historical control
| Outcomes, n (%) | Hospitalization and Positive for COVID-19 (n=3345) | Hospitalization and Negative for COVID-19 (n=1265) | Risk Ratio(95% CI) | Historical control (n=9859) | Risk Ratio (95% CI) (positive for COVID-19 versus historical control) |
|---|---|---|---|---|---|
| AKI | 1903 (56.9) | 471 (37.2) | 1.5 (1.4 to 1.6) | 2479 (25.1) | 2.3 (2.2 to 2.4) |
| AKI stage | |||||
| 1 | 942 (49.5)2 | 285 (60.5) | 1830 (73.8) | ||
| 2 | 387 (20.3) | 94 (20.0) | 369 (14.9) | ||
| 3 | 574 (30.2) | 92 (19.5) | 280 (11.3) | ||
| Need for RRT | 164 (4.9) | 20 (1.6) | 3.1 (2.0 to 4.9) | 93 (0.9) | 5.2 (4.0 to 6.7) |
| Type of RRT | |||||
| CRRT | 52 (31.7) | 5 (25) | 25 (26.9) | ||
| PIRRT | 11 (6.7) | 2 (10) | 8 (8.6) | ||
| PD | 16 (9.8) | 0 | 0 | ||
| HD | 85 (51.8) | 13 (65) | 60 (64.5) | ||
| Outcomes of the cohort with stage 3 AKI | |||||
| AKI stage 3 with renal recovery | 243 (42.3) | 63 (68.5) | 0.6 (0.5 to 0.7) | 179 (63.9) | 0.7 (0.6 to 0.8) |
| AKI stage 3 with in-hospital death | 299 (52.1) | 18 (19.6) | 2.7 (1.8 to 4.1) | 55 (19.6) | 2.6 (2.1 to 3.4) |
| AKI stage 3 with RRT dependence | 32 (5.6) | 11 (12.0) | 0.5 (0.1 to 0.8) | 46 (16.4) | 0.3 (0.2 to 0.5) |
| Outcomes in the total cohort hospitalized during the study period | |||||
| Mechanical ventilation | 624 (18.7) | 126 (10.0) | 1.9 (1.6 to 2.4) | 486 (4.9) | 3.8 (3.4 to 4.3) |
| ICU | 438 (13.1)a | 46 (3.6)b | 3.6 (2.7 to 4.8) | 323 (3.3) | 4.0 (3.5 to 4.6) |
| In-hospital death | 775 (23.2) | 92 (7.3) | 3.8 (2.6 to 3.9) | 229 (2.3) | 10.0 (8.6 to 11.5) |
| Discharged to NH | 492 (14.7) | 162 (12.8) | 1.2 (1.0 to 1.4) | 1436 (14.6) | 1.0 (0.9 to 1.1) |
| Discharged home | 1650 (49.3) | 788 (62.3) | 0.8 (0.7 to 0.8) | 8108 (82.2) | 0.6 (0.57 to 0.62) |
| Hospital length of stay (d)c | 5 (3–9) | 3 (1–6) | P<0.001 | 3 (2–6) | P<0.001 |
CRRT, continuous RRT; PIRRT, prolonged intermittent RRT; PD, peritoneal dialysis; HD, hemodialysis; NH, nursing home.
Data available for n=3341.
Data available for n=1262.
For these comparisons, Wilcoxon rank sum testing was used.
On initial hospital presentation, the COVID-19–positive cohort with AKI had higher respiratory rates (22.3 [SD, 6.8] versus 20.6 [SD, 5.4] breaths per minute; P<0.001), higher pulse rates (99.1 [SD, 21.3] versus 97.2 [SD, 19.3] beats per minute; P=0.01), and lower pulse oximetry (91.7% [SD, 9.4] versus 94.3% [SD, 5.4]; P<0.001) as compared with those without AKI (Table 1). Similar findings were observed in patients with AKI who were negative for COVID-19 (Supplemental Table 1). Initial laboratory data demonstrated those with AKI had higher markers of inflammation including white blood cell (WBC) count (9.3 [SD, 6.9] K/µl versus 7.8 [SD, 7.1] K/µl; P<0.001), neutrophil/lymphocyte ratio (6.6 [IQR, 4.1–10.4] versus 4.7 [IQR, 3.0–7.7]; P<0.001), fibrinogen (659 [SD, 211] mg/dl versus 632 [SD, 178] mg/dl; P=0.002), C-reactive protein (13.2 [IQR, 6.1–22.4] mg/dl versus 7.3 [IQR, 3.3–14.3] mg/dl; P<0.001), lactate dehydrogenase (LDH; 458 [IQR, 336–637] U/L versus 350 [IQR, 270–451] U/L; P<0.001), and D-dimer (2.2 [IQR, 1.1–6.2] µ/ml versus 1.1 [IQR, 0.7–2.3] µ/ml; P<0.001) compared with those without AKI. Heavy proteinuria of >500 mg/g by urinalysis or quantitative urine protein/creatinine ratio was present in a higher proportion of patients with AKI compared with those without AKI, irrespective of COVID-19 status (Table 1).
A significantly higher proportion of patients positive for COVID-19 had stage 3 AKI compared with patients negative for COVID-19 and with the historical cohort (17.2% versus 7.3% versus 2.8%) (Table 2). Of the patients positive for COVID-19, 4.9% required acute RRT compared with 1.6% of those negative for COVID-19 (RR, 3.1; 95% CI, 2.0 to 4.9) and 0.9% in the historical cohort (RR, 5.2; 95% CI, 4.0 to 6.7) (Table 2).
ICU Admission and Mechanical Ventilation
Significantly more patients with AKI than without required ICU admission in both the COVID-19 positive (20.1% versus 3.9%, P<0.001) and COVID-19 negative cohorts (6.4% versus 2.0%, P<0.001) (Table 1, Supplemental Table 1). Of the patients positive for COVID-19 who required ICU admission, the incidence of AKI was 87.2% as compared with 65% in patients negative for COVID-19 who required the ICU (Supplemental Figure 2). Significantly more patients with AKI required mechanical ventilation in both the COVID-19–positive (29.9% versus 3.8%; P<0.001) and COVID-19–negative cohorts (19.1% versus 4.5%; P<0.001) (Table 1).
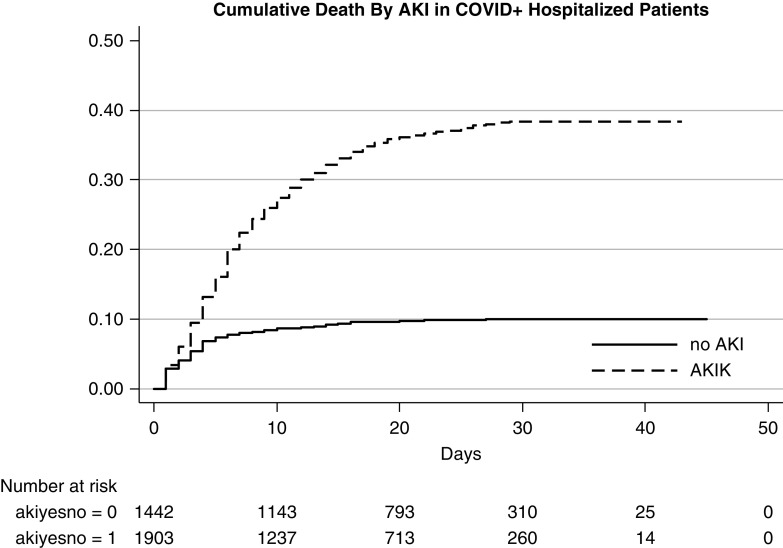
In-Hospital Death
Patients who developed AKI were at significantly higher risk of in-hospital death compared with those without AKI (Figure 2). In the COVID-19–positive cohort, in-hospital death was 33.7% in those with AKI compared with 9.3% in those without AKI. By comparison, in the patients without COVID-19, in-hospital death was 13.4% in those with AKI compared with 3.7% in those without AKI (Supplemental Table 2). In the total cohort of 4610 patients (with and without AKI), in-hospital death was significantly higher in patients positive for COVID-19 compared with those negative for COVID-19 (23.2% versus 7.3%; RR, 3.8; 95% CI, 2.6 to 3.9) and even higher compared with the historical cohort (23.2% versus 2.3%; RR, 10; 95% CI, 8.6 to 11.5) (Table 2).
Figure 2.
Higher mortality in those with AKI.
Prognosis after Stage 3 AKI
There was less renal recovery in patients with AKI who were positive for COVID-19 compared with patients with AKI who were negative for COVID-19 (42.3% versus 68.5%; RR, 0.6; 95% CI, 0.5 to 0.7) and to the historical cohort (42.3% versus 63.9%; RR, 0.7; 95% CI, 0.6 to 0.8) (Table 2). Patients with COVID-19 and stage 3 AKI were more likely to experience in-hospital death compared with patients negative for COVID-19 with stage 3 AKI (52.1% versus 19.6%; RR, 3.8; 95% CI, 2.6 to 3.9) and with the historic cohort (52.1% versus 19.6%; RR, 10; 95% CI, 8.6 to 11.5). Fewer patients with COVID-19 and stage 3 AKI requiring RRT remained RRT dependent compared with patients negative for COVID-19 or the historical cohort (5.6% versus 12% versus 16.4%), but this was likely due to the competing risk of death and high mortality observed in patients with COVID-19 and stage 3 AKI (Table 2).
Risk Factors for Development of AKI and for the Composite Outcome of RRT or Mortality
The odds of developing AKI with SARS-CoV-2 infection were higher in men in unadjusted and adjusted models (adjusted odds ratio, 1.6; 95% CI, 1.4 to 1.8) as compared with women (Table 3). Black race was associated with higher odds of developing AKI with COVID-19 in unadjusted and adjusted models (adjusted odds ratio, 1.7; 95% CI, 1.3 to 2.3). Increasing decile of age above the age of 30 years was associated with higher odds of developing AKI but did not become significant until >50 years of age. There was some attenuation of the odds of AKI in the highest decile of age, likely due to the competing risk of death. The associations of male sex, black race, and older age with the development of AKI were also observed in patients without COVID-19. Interaction between COVID-19 status and sex, race/ethnicity, and age decile were NS (P=0.9, P=0.8, and P=0.4, respectively) with respect to odds of AKI (Supplemental Table 3).
Table 3.
Odds ratio of AKI development in unadjusted and adjusted models
| Patients Positive for COVID-19 (n=3345) | Model 1 (n=3345) | Model 2 (n=3345) |
|---|---|---|
| Sex | a | |
| Female | 1 | 1 |
| Male | 1.5 (1.3 to 1.7) | 1.6 (1.4 to 1.8) |
| Race/ethnicity | b | |
| Non-Hispanic White | 1 | 1 |
| Non-Hispanic Black | 1.4 (1.1 to 1.8) | 1.7 (1.3 to 2.3) |
| Hispanic | 0.8 (0.6 to 1.0) | 1.0 (0.8 to 1.4) |
| Other | 0.9 (0.7 to 1.2) | 1.1 (0.8 to 1.5) |
| Age decile | c | |
| <30 | 1 | 1 |
| 31–40 | 1.0 (0.6 to 1.6) | 0.9 (0.6 to 1.5) |
| 41–50 | 1.3 (0.8 to 2.0) | 1.2 (0.8 to 1.8) |
| 51–60 | 1.8 (1.2 to 2.7) | 1.6 (1.1 to 2.5) |
| 61–70 | 2.1 (1.4 to 3.2) | 1.8 (1.2 to 2.8) |
| 71–80 | 3.4 (2.2 to 5.1) | 2.7 (1.7 to 4.3) |
| >80 | 3.5 (2.3 to 5.2) | 2.9 (1.8 to 4.7) |
Model 1 was unadjusted. Model 2 was model 1 plus adjustments for clinical comorbidities: DM, CKD, Charlson Comorbidity score, CHF, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker use, nursing home resident.
Model 2 adjusted for age/race.
Model 2 adjusted for age/sex.
Model 2 adjusted for sex/race.
In competing risk modeling, the odds of AKI were higher with male sex (subhazard ratio [SHR], 1.2; 95% CI, 1.1 to 1.3), black race (SHR, 1.5; 95% CI, 1.2 to 1.7), and age (SHR, 1.1 for each 10-year increase in age; 95% CI, 1.0 to 1.1) in the adjusted models (Supplemental Table 4).
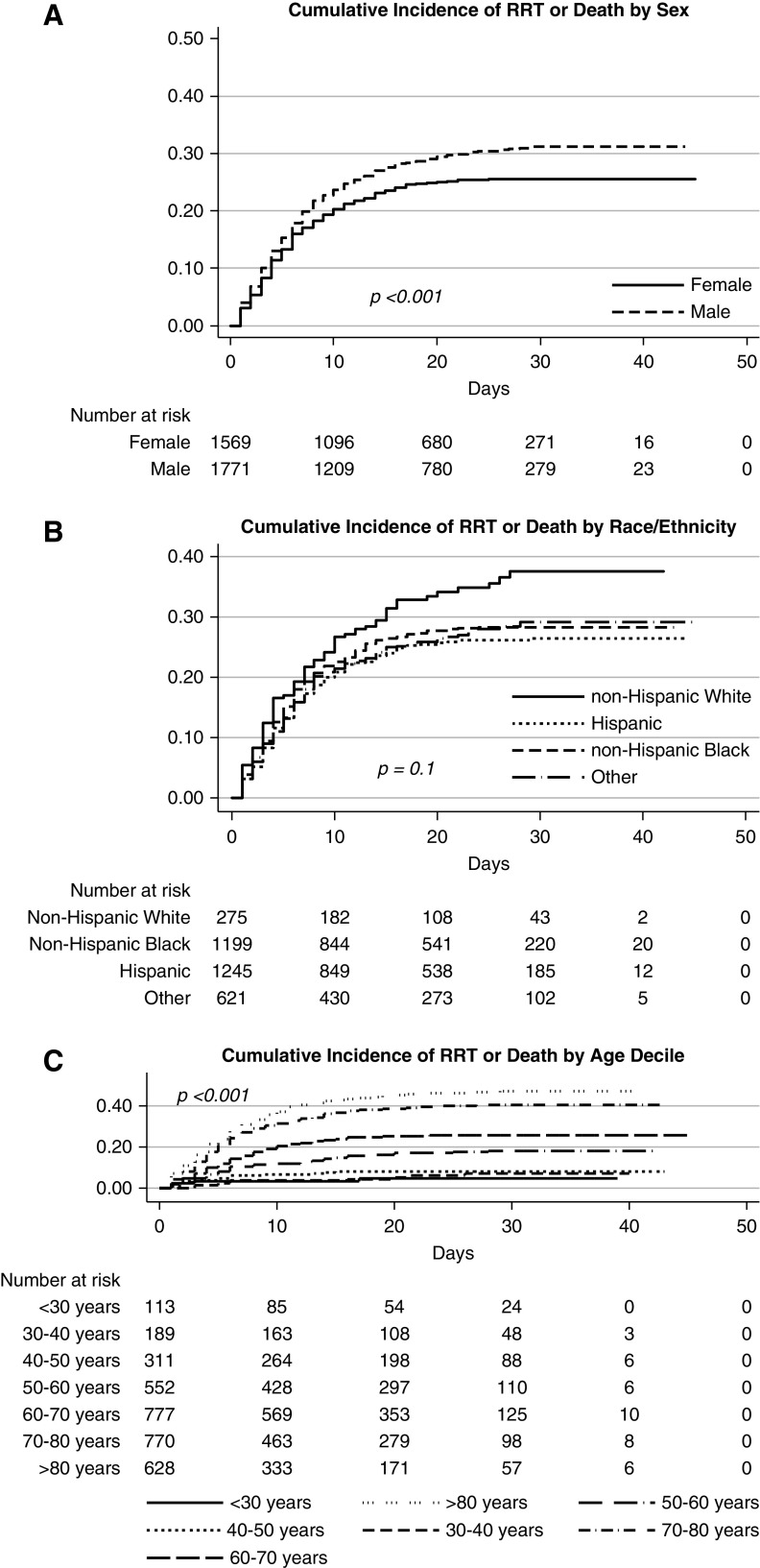
In the COVID-19–positive cohort, men were at higher risk (adjusted hazard ratio, 1.3; 95% CI, 1.2 to 1.5) of the composite outcome of RRT or mortality compared with women in the unadjusted and adjusted model (Figure 3A, Table 4). There was no association of race with the composite outcome of RRT and mortality in the unadjusted and adjusted models (Figure 3B, Table 4). Similar associations of male sex and older age with the composite outcome were observed in patients negative for COVID-19 (Figure 3, A and C, Supplemental Table 5).
Figure 3.
Male sex, Black race, and older age associated with higher incidence of RRT or death. (A) Estimates for cumulative percentage of mortality or RRT by sex. (B) Estimates for cumulative percentage of mortality or RRT by race/ethnicity (race_eth). (C) Estimates for cumulative percentage of mortality or RRT by age. age_dec, age decile.
Table 4.
Hazard ratio for composite outcome of mortality or RRT in unadjusted and adjusted models
| Patients Positive for COVID-19 (n=3345) | Unadjusted Hazard Ratio (n=3345) | Adjusted Hazard Ratioa (n=3345) |
|---|---|---|
| Sex | b | |
| Female | 1 | 1 |
| Male | 1.2 (1.1 to 1.3) | 1.3 (1.2 to 1.5) |
| Race/Ethnicity | c | |
| Non-Hispanic White | 1 | 1 |
| Non-Hispanic Black | 0.8 (0.6 to 1.0) | 0.9 (0.7 to 1.2) |
| Hispanic | 0.7 (0.6 to 0.9) | 0.9 (0.7 to 1.2) |
| Other | 0.7 (0.6 to 1.0) | 1.0 (0.8 to 1.3) |
| Age decile | d | |
| <30 | 1 | 1 |
| 31–40 | 1.2 (0.4 to 3.4) | 1.2 (0.4 to 3.4) |
| 41–50 | 1.7 (0.6 to 4.3) | 1.6 (0.6 to 4.2) |
| 51–60 | 3.5 (1.4 to 8.6) | 3.1 (1.2 to 7.5) |
| 61–70 | 5.4 (2.2 to 13.2) | 4.4 (1.8 to 10.7) |
| 71–80 | 9.2 (3.8 to 22.2) | 6.6 (2.7 to 16.3) |
| >80 | 11.2 (4.6 to 27.3) | 7.6 (3.1 to 18.9) |
Adjusted for clinical comorbidities: DM, CKD, Charlson Comorbidity Index, CHF, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, nursing home resident.
Adjusted for race/age.
Adjusted for sex/age.
Adjusted for sex/race.
Predictive Model of AKI Stage 2 or 3
The predictive model showed the following variables were independently associated with development of stage 2 or 3 AKI in COVID-19: older age, Black race, male sex, DM, nursing home resident, initial respiratory rate, WBC count, LDH (odds ratio, 2.2; 95% CI, 1.9 to 2.7), and neutrophil/lymphocyte ratio (odds ratio, 1.7; 95% CI, 1.4 to 2.1) (Supplemental Tables 5 and 6). The area under the receiving operator curve for the predictive model was 0.73 (Supplemental Figure 2).
Discussion
We examined the incidence, risk factors, and outcomes associated with AKI in a large cohort of patients with COVID-19 who were hospitalized in a single urban health system in the Bronx during the COVID-19 surge in New York City. We compared these findings with patients without COVID-19 infection who were hospitalized during the same time period and with a historical cohort. To our knowledge, this is the first study that has made such a comparison. Our goal was to identify unique features of COVID-19–associated AKI. Not only was there a much higher incidence of AKI in patients with COVID-19 compared with those without COVID-19, patients with COVID-19 also had a disproportionate burden of severe AKI, requirement for RRT, mechanical ventilation, and ICU admission compared with those without COVID-19. Additionally, patients with AKI who were positive for COVID-19 were less likely to recover kidney function and more likely to experience in-hospital death compared with patients with AKI without COVID-19. We identified male sex, Black race, and increasing age as risk factors for development of AKI, irrespective of COVID-19 status. Male sex and older age were risk factors for the composite outcome of RRT or mortality.
Recent reports from the United States demonstrate a higher incidence of AKI in COVID-19 compared with reports originating from China (L. Chan, K. Chaudhary, A. Saha, K. Chauhan, A. Vaid, M. Baweja, et al.: Acute kidney injury in hospitalized patients with COVID-19. medRxiv, 2020; doi:10.1101/2020.05.04.20090944).14–18 A study of >5400 patients with COVID-19 hospitalized in a New York hospital system reported an AKI incidence of 37% and an association of mechanical ventilation with time to AKI.17 In another study of >3200 patients hospitalized in New York City with COVID-19, the incidence of AKI was 46% and predictors of severe AKI included systolic BP, potassium level, and CKD.18 Despite differences in AKI definitions between all of these studies, the reported incidence of AKI was similar and significantly higher than earlier studies. Our study also contrasts AKI incidence and risks between patients positive for COVID-19 those negative for COVID-19 and historic controls. We were able to demonstrate a significantly higher incidence of AKI in patients positive for COVID-19 compared with those negative for COVID-19, but showed that risk factors for AKI were not exclusive to COVID-19 status. Additionally, initial respiratory rate, WBC count, LDH, and neutrophil/lymphocyte ratio were predictors of severe AKI which may be clinically relevant.
Furthermore, we were able to demonstrate the effect of COVID-19 on hospital resources including a disproportionate need for ICU, mechanical ventilation, RRT, increased hospital length of stay, and higher mortality compared with those without COVID-19. An interesting observation was worse outcomes in the COVID-19–negative group compared with the historical cohort. We attribute this difference to a high rate of false-negative tests in the COVID-19–negative group and allocation of resources away from patients without COVID-19 to those with or suspected of having COVID-19. Furthermore, there was community avoidance of the hospital setting during this time period due to concern for contracting COVID-19, thus those presenting to the hospital during the COVID-19 crisis were likely more severely ill.
Our findings are similar to other types of severe respiratory illnesses associated with AKI.30–34 In 150 patients hospitalized with Ebola virus, 50% developed AKI and nearly all cases were severe and associated with high mortality.30 Single-center studies of critically ill patients with H1N1 influenza have reported an incidence of AKI of >50% with a 17%–56% mortality.31,32 Although the reported incidence of AKI in SARS infection was only 6.7%, mortality after development of AKI was >90%.33 In contrast to SARS, AKI has been reported in up to 43% of patients with Middle East respiratory syndrome coronavirus infection.34 The clinical features we observed in patients with COVID-19 and AKI were consistent with reports of AKI in H1N1 influenza, Ebola, and other coronaviruses. We did not identify any unique risk factors distinguishing COVID-19 from these other severe respiratory viral illnesses.
Studies performed during the COVID-19 pandemic have shown that infected men are more likely to develop severe infection requiring hospitalization and therefore may be at increased risk for AKI.35,36 Consistent with other reports from the United States, we found an increased risk of AKI as well as composite outcome of RRT or mortality in men positive for COVID-19 compared with women positive for COVID-19. The Kidney Disease Improving Global Outcomes Clinical Practice Guideline for AKI concludes that female sex carries a higher risk for AKI, while at the same time recognizes that male sex predominates in AKI associated with HIV, malaria, leptospirosis, and in other community-acquired forms of AKI.24 We not only found that male sex was a risk factor for AKI associated with COVID-19 but also that male sex was associated with AKI in the COVID-19–negative and historical cohort.37 Although this observation is contrary to the consensus view that female sex is a risk factor for AKI, it is consistent with animal data and a recently published meta-analysis.38–45 Animal models have consistently demonstrated that male sex is associated with more severe kidney injury and histologic damage after ischemia-reperfusion injury.38–44 In a meta-analysis of 28 studies reporting sex-stratified data on the incidence of hospital-associated AKI in >194 million patients, men were 2.2 times more likely to develop AKI compared with women, although heterogeneity of patient population and outcome measures may limit the generalizability of this observation.45
We found black race was significantly associated with the development of AKI in the patients positive and negative for COVID-19 and in the historical cohort. Whether black race is a risk factor for non-COVID-19–associated AKI has not been clearly established as data are inconsistent.24,46–49 Several studies failed to find any relationship between race and the development of AKI.46,47 In contrast, a meta-analysis of >1.3 million individuals performed by the CKD Prognosis Consortium found that black patients had a higher risk of AKI than white patients at higher levels of eGFR and at most urinary albumin-creatinine ratios.50 Similarly, Grams et al.51 studied the incidence of AKI among >10,000 middle-aged participants in a community-based prospective cohort study and found that the adjusted risk of AKI was 26% greater in black patients compared with white patients. However, racial disparity was attenuated after adjustment for socioeconomic factors, leading to speculation that low income may be associated with reduced access to medical care, resulting in poor control of comorbidities and increased exposure to nephrotoxic agents. In our study, after adjustment for neighborhood SES and crowding, black race remained a significant risk for AKI.
Our study has several limitations. First, our cohort was limited to the New York City area. Second, white race was underrepresented in our study population so the generalizability of our findings may be limited. Third, a preadmission creatinine was only available in 49% of the patients. However, we dealt with this by using the Siew et al.25 method to estimate baseline creatinine using the minimum hospitalization creatinine value. Our estimation of stage 1 AKI may have been inflated because of lack of access to an iterative model taking into account 48-hour windows for the 0.3 mg/dL creatinine increase AKI designation throughout the hospitalization. Our sensitivity analysis reported that, after excluding the 0.3 mg/dl definition of AKI, the incidence of AKI decreased to 47.1% in the COVID-19–positive cohort, 28% in the COVID-19–negative cohort, and 15.5% in the historical cohort (Supplemental Table 7). However this phenomenon likely had very little effect on our major findings and our predictive model because they were driven and based on higher stages of AKI. Finally, the analysis of the association of AKI with mortality was limited by immortal time bias, although the competing risk model for AKI incidence did account for death as the competing risk. Despite these limitations, our study has several important strengths. This is one of the largest studies to date evaluating AKI in patients with COVID-19 and, to the best of our knowledge, this is the first detailed comparison between COVID-19–associated and non-COVID-19–associated AKI incidence, risk factors, and outcomes.
In conclusion, AKI incidence and severity was disproportionately higher and outcomes were worse in the COVID-19–positive cohort as compared with the COVID-19–negative and historical cohorts. By using a historical cohort from 1 year prior, we were able to confidently evaluate differences in the incidence, risk factors, and outcomes of AKI in patients positive and negative for COVID-19. Finally, our study highlights the burden of AKI in a large hospital system during the COVID-19 pandemic.
Disclosures
All authors have nothing to disclose.
Funding
None.
Supplementary Material
Acknowledgments
To all of the nurses, physician extenders, and fellows and faculty of the nephrology division who went above and beyond their call of duty during this pandemic.
Dr. Molly Fisher and Dr. Ladan Golestaneh were responsible for conceptualization and design; Dr. Matthew Abramowitz, Dr. Eran Bellin, Dr. Ladan Golestaneh, Dr. Tanya Johns, and Dr. Lindsay Stahl were responsible for acquisition and analysis of data; Dr. Molly Fisher, Dr. Ladan Golestaneh, and Dr. Joel Neugarten were responsible for interpretation of data and drafting of the original manuscript; Dr. Molly Fisher, Dr. Ladan Golestaneh, Dr. Rebecca Levy, Dr. Kalyan Prudhvi, and Dr. Milagros Yunes were responsible for the creation of figures and tables; Dr. Molly Fisher and Dr. Ladan Golsetaneh provided supervision; and all authors critically revised the manuscript for content and reviewed and approved the final version of the manuscript. Dr. Matthew Abramowitz reports personal fees from Tricida, Inc., outside the submitted work.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020040509/-/DCSupplemental.
Supplemental Table 1. Baseline characteristics of hospitalized COVID(+) and COVID(−) patients.
Supplemental Table 2. Odds ratio (OR) of AKI development in unadjusted and adjusted models.
Supplemental Table 3. Hazard ratio (HR) for composite outcome of mortality or RRT in unadjusted and adjusted models.
Supplemental Table 4. Incidence of AKI by ICU versus general floor admission and requirement of ICU and mechanical ventilation in COVID(+) and COVID(−) patients hospitalized during the study period.
Supplemental Table 5. Predictive model for stage 2 or 3 AKI in COVID-19 infection.
Supplemental Table 6. Sub-hazard ratios (SHR) for competing risk of death with AKI.
Supplemental Table 7. Sensitivity analysis: AKI incidence in hospitalized patients using ≥50% relative increase in creatinine.
Supplemental Figure 1. Area under the receiver operating curve for predictive model for stage 2 or 3 AKI.
Supplemental Figure 2. Flowchart of AKI Incidence in COVID(+) patients admitted to the intensive care unit or general floor.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al.; China Novel Coronavirus Investigating and Research Team: A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al.; China Medical Treatment Expert Group for Covid-19: Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.John Hopkins Coronavirus Resource Center: COVID-19 United States cases by county, 2020. Available at: https://coronavirus.jhu.edu/us-map. Accessed April 20, 2020
- 4.Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V: The novel coronavirus 2019 epidemic and kidneys. Kidney Int 97: 824–828, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pan XW, Xu D, Zhang H, Zhou W, Wang LH, Cui XG: Identification of a potential mechanism of acute kidney injury during the Covid-19 outbreak: a study based on single-cell transcriptome analysis. Intensive Care Med 46: 1114–1116, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. : Multiorgan and renal tropism of SARS-CoV-2 [published online ahead of print May 13, 2020]. N Engl J Med doi:10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al.: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA: Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19). Kidney Int Rep 5: 935–939, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Kisselev S, et al.: Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep 5: 940–945, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al.: Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al.: Clinical characteristics of 113 deceased patients with coronavirus disease 2019: retrospective study [published correction appears in BMJ 368: m1295, 2020]. BMJ 368: m1091, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al.: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395: 1054–1062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed MMB, Lukitsch I, Torres-Ortiz AE, Walker JB, Varghese V, Hernandez-Arroyo CF, et al. : Acute kidney injury associated with coronavirus disease 2019 in Urban New Orleans [published online ahead of print May 13, 2020]. Kidney360 doi:10.34067/KID.0002652020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al.; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium: Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United States Census Bureau: American community survey, 2016. Available at: https://www.census.gov/programs-surveys/acs/guidance.html. Accessed April 16, 2020
- 16.Wadhera RK, Wadhera P, Gaba P, Figueroa JF, Joynt Maddox KE, Yeh RW, et al.: Variation in COVID-19 hospitalizations and deaths across New York city boroughs. JAMA 323: 2192–2195, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Signorello LB, Schlundt DG, Cohen SS, Steinwandel MD, Buchowski MS, McLaughlin JK, et al.: Comparing diabetes prevalence between African Americans and Whites of similar socioeconomic status. Am J Public Health 97: 2260–2267, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Egede LE: Race, ethnicity, culture, and disparities in health care. J Gen Intern Med 21: 667–669, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellin E: Riddles in Accountable Healthcare: A Primer to Develop Analytic Intuition for Medical Homes and Population Health, Scotts Valley, SC, Create Space, 2015 [Google Scholar]
- 20.Bellin E: How to Ask and Answer Questions Using Electronic Medical Record Data, Scotts Valley, SC, Create Space, 2017 [Google Scholar]
- 21.Bellin E, Fletcher DD, Geberer N, Islam S, Srivastava N: Democratizing information creation from health care data for quality improvement, research, and education-the Montefiore Medical Center Experience. Acad Med 85: 1362–1368, 2010. [DOI] [PubMed] [Google Scholar]
- 22.Diez Roux AV, Merkin SS, Arnett D, Chambless L, Massing M, Nieto FJ, et al.: Neighborhood of residence and incidence of coronary heart disease. N Engl J Med 345: 99–106, 2001. [DOI] [PubMed] [Google Scholar]
- 23.Charlson ME, Pompei P, Ales KL, MacKenzie CR: A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis 40: 373–383, 1987. [DOI] [PubMed] [Google Scholar]
- 24.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group: KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 25.Siew ED, Ikizler TA, Matheny ME, Shi Y, Schildcrout JS, Danciu I, et al.: Estimating baseline kidney function in hospitalized patients with impaired kidney function. Clin J Am Soc Nephrol 7: 712–719, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siew ED, Matheny ME: Choice of reference serum creatinine in defining acute kidney injury. Nephron 131: 107–112, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, et al.; Chronic Kidney Disease Epidemiology Collaboration: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006. [DOI] [PubMed] [Google Scholar]
- 28.Xu J, Xu X, Shen B, Zhuang Y, Liu L, Wang Y, et al.: Evaluation of five different renal recovery definitions for estimation of long-term outcomes of cardiac surgery associated acute kidney injury. BMC Nephrol 20: 427, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 30.Hunt L, Knott V: Serious and common sequelae after Ebola virus infection. Lancet Infect Dis 16: 270–271, 2016. [DOI] [PubMed] [Google Scholar]
- 31.Abdulkader RC, Ho YL, de Sousa Santos S, Caires R, Arantes MF, Andrade L: Characteristics of acute kidney injury in patients infected with the 2009 influenza A (H1N1) virus. Clin J Am Soc Nephrol 5: 1916–1921, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sood MM, Rigatto C, Zarychanski R, Komenda P, Sood AR, Bueti J, et al.: Acute kidney injury in critically ill patients infected with 2009 pandemic influenza A(H1N1): report from a Canadian province. Am J Kidney Dis 55: 848–855, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chu KH, Tsang WK, Tang CS, Lam MF, Lai FM, To KF, et al.: Acute renal impairment in coronavirus-associated severe acute respiratory syndrome. Kidney Int 67: 698–705, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al.: Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis 29: 301–306, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garg S, Kim L, Whitaker M, O’Halloran A, Cummings C, Holstein R, et al.: Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 — COVID-NET, 14 states, March 1–30, 2020. MMWR Morb Mortal Wkly Rep 69: 458–464, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grasselli G, Zangrillo A, Zanella A, Antonelli M, Cabrini L, Castelli A, et al.; COVID-19 Lombardy ICU Network: Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the lombardy region, Italy. JAMA 323: 1574–1581, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ricardo AC, Yang W, Sha D, Appel LJ, Chen J, Krousel-Wood M, et al.; CRIC Investigators: Sex-related disparities in CKD progression. J Am Soc Nephrol 30: 137–146, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hutchens MP, Dunlap J, Hurn PD, Jarnberg PO: Renal ischemia: does sex matter? Anesth Analg 107: 239–249, 2008. [DOI] [PubMed] [Google Scholar]
- 39.Fekete A, Vannay A, Vér A, Rusai K, Müller V, Reusz G, et al.: Sex differences in heat shock protein 72 expression and localization in rats following renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 291: F806–F811, 2006. [DOI] [PubMed] [Google Scholar]
- 40.Fekete A, Vannay A, Vér A, Vásárhelyi B, Müller V, Ouyang N, et al.: Sex differences in the alterations of Na(+), K(+)-ATPase following ischaemia-reperfusion injury in the rat kidney. J Physiol 555: 471–480, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S: Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 303: F377–F385, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang KP, Lee JE, Lee AS, Jung YJ, Kim D, Lee S, et al.: Effect of gender differences on the regulation of renal ischemia-reperfusion-induced inflammation in mice. Mol Med Rep 9: 2061–2068, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kher A, Meldrum KK, Wang M, Tsai BM, Pitcher JM, Meldrum DR: Cellular and molecular mechanisms of sex differences in renal ischemia-reperfusion injury. Cardiovasc Res 67: 594–603, 2005. [DOI] [PubMed] [Google Scholar]
- 44.Metcalfe PD, Meldrum KK: Sex differences and the role of sex steroids in renal injury. J Urol 176: 15–21, 2006. [DOI] [PubMed] [Google Scholar]
- 45.Neugarten J, Golestaneh L: Female sex reduces the risk of hospital-associated acute kidney injury: A meta-analysis. BMC Nephrol 19: 314, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Locatelli F, Rossi F: Incidence and pathogenesis of tumor lysis syndrome. Contrib Nephrol 147: 61–68, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Thakar CV, Liangos O, Yared JP, Nelson D, Piedmonte MR, Hariachar S, et al.: ARF after open-heart surgery: influence of gender and race. Am J Kidney Dis 41: 742–751, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Grigorian A, Gabriel V, Nguyen NT, Smith BR, Schubl S, Borazjani B, et al.: Black race and body mass index are risk factors for rhabdomyolysis and acute kidney injury in trauma. J Invest Surg 33: 283–290, 2020. [DOI] [PubMed] [Google Scholar]
- 49.Mathioudakis NN, Giles M, Yeh HC, Haywood C Jr., Greer RC, Golden SH: Racial differences in acute kidney injury of hospitalized adults with diabetes. J Diabetes Complications 30: 1129–1136, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grams ME, Sang Y, Ballew SH, Gansevoort RT, Kimm H, Kovesdy CP, et al.; CKD Prognosis Consortium: A meta-analysis of the association of estimated GFR, albuminuria, age, race, and sex with acute kidney injury. Am J Kidney Dis 66: 591–601, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Grams ME, Matsushita K, Sang Y, Estrella MM, Foster MC, Tin A, et al.: Explaining the racial difference in AKI incidence. J Am Soc Nephrol 25: 1834–1841, 2014. 24722442 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.