Significance Statement
More than one third of hospitalized patients with coronavirus disease 2019 (COVID-19) develop AKI. The pathogenesis of AKI in this setting is poorly understood, and pathologic descriptions are limited. The authors examined kidney histopathology of 42 patients who died of COVID-19. The most significant findings included mild acute tubular injury as well as the absence of classic viral nephropathy, diffuse thrombotic microangiopathy, or acute GN. In situ hybridization could not identify definitive positivity for SARS-CoV-2. The finding of only mild acute tubular injury in the setting of severe creatinine elevation suggests a pathogenesis involving tubular injury and hemodynamic factors (such as aggressive fluid management) and potential for recovery of renal function upon resolution of infection.
Keywords: acute renal failure, pathology, acute tubular injury, COVID-19, SARS-CoV-2, acute kidney injury
Visual Abstract
Abstract
Background
AKI is common among hospitalized patients with coronavirus disease 2019 (COVID-19) and is an independent risk factor for mortality. Although there are numerous potential mechanisms underlying COVID-19–associated AKI, our current knowledge of kidney pathologic findings in COVID-19 is limited.
Methods
We examined the postmortem kidneys from 42 patients who died of COVID-19. We reviewed light microscopy findings in all autopsies and performed immunofluorescence, electron microscopy, and in situ hybridization studies for SARS-CoV-2 on a subset of samples.
Results
The cohort had a median age of 71.5 years (range, 38–97 years); 69% were men, 57% were Hispanic, and 73% had a history of hypertension. Among patients with available data, AKI developed in 31 of 33 patients (94%), including 6 with AKI stage 1, 9 with stage 2, and 16 with stage 3. The predominant finding correlating with AKI was acute tubular injury. However, the degree of acute tubular injury was often less severe than predicted for the degree of AKI, suggesting a role for hemodynamic factors, such as aggressive fluid management. Background changes of hypertensive arterionephrosclerosis and diabetic glomerulosclerosis were frequent but typically mild. We identified focal kidney fibrin thrombi in 6 of 42 (14%) autopsies. A single Black patient had collapsing FSGS. Immunofluorescence and electron microscopy were largely unrevealing, and in situ hybridization for SARS-CoV-2 showed no definitive positivity.
Conclusions
Among a cohort of 42 patients dying with COVID-19, autopsy histologic evaluation revealed acute tubular injury, which was typically mild relative to the degree of creatinine elevation. These findings suggest potential for reversibility upon resolution of SARS-CoV-2 infection.
Since its emergence in Wuhan, China, infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) leading to coronavirus disease 2019 (COVID-19) has rapidly evolved into a global pandemic. As of July 6, 2020, approximately 11.3 million cases have been reported worldwide, including 2.8 million cases from the United States, where New York City was the epicenter of the epidemic when the study was performed.1,2 COVID-19 disease is most prominently a respiratory illness, which in the most severe cases, is characterized by pneumonia and hypoxemic respiratory failure.3 Additional complications have also been reported, including AKI.
Early reports estimated AKI rates of 0.5%–28% in China and Italy.3–12 In the Chinese experience, the burden of AKI appeared to be highest in patients with severe COVID-19 disease, particularly in the intensive care unit setting, where 54%–64% of AKI was at least moderate by the 2012 Kidney Disease Improving Global Outcomes (KDIGO) criteria.5,6 Development of AKI in the setting of COVID-19 infection was also an independent risk factor for in-hospital mortality.5,6 The first United States data included 21 critically ill patients in Washington state, 19% of whom developed AKI.13 In a more recent report of over 5000 patients from a large health network in the New York metropolitan area, 37% of hospitalized patients developed AKI, 53% of whom were moderate or severe and 14% of whom required RRT.14
There are numerous potential mechanisms underlying COVID-19–associated AKI; however, our current knowledge of pathologic findings is limited. An autopsy series from China described features of acute tubular injury (ATI) in all 26 decedents, 85% of whom were graded as at least moderate, despite only 9 of 26 patients showing clinical evidence of kidney injury.15 Interstitial inflammation was confined to areas of interstitial fibrosis. Other relevant findings included congestion of glomeruli and peritubular capillaries, rare instances of glomerular fibrin thrombi, occasional pigmented casts, and ultrastructural evidence of endothelial injury. Evidence of kidney tropism, including detection of viral RNA and putative virions, has raised concern that direct infection by SAR-CoV-2 could contribute to kidney injury.15,16 Reports of COVID-19–associated collapsing FSGS in Blacks suggest that heightened cytokine release associated with COVID-19 infection may place patients with high-risk APOL1 genotypes at increased risk of severe kidney disease, as occurs in other viral-mediated forms of collapsing FSGS.17,18 Herein, we report our experience with postmortem renal pathologic findings from 42 patients in New York City, the epicenter of the United States COVID-19 pandemic.
Methods
Clinical Data
This study highlights the clinical and kidney pathology findings in 42 of the initial 44 autopsies on patients who died with COVID-19 at Columbia University Irving Medical Center; two autopsies were excluded because the patients had ESKD requiring chronic hemodialysis, and thus, the renal histologic findings were noncontributory. Consent for autopsy was obtained from the patient’s next of kin. The electronic health record was reviewed for age, sex, race, body mass index, hospitalization date, date of death, and nephrotoxin exposure during the hospitalization. Nephrotoxins were defined as medications known to be associated with ATI. Comorbidities, including hypertension (HTN), diabetes mellitus (DM), coronary artery disease (CAD), cerebrovascular accident (CVA), and any history of immunosuppression, were noted if they were present in the hospital admission note. Laboratory parameters of interest were collected, including baseline creatinine (prior to final admission), creatinine at the time of admission, peak creatinine during hospitalization, and final creatinine prior to death. Baseline GFR was calculated using the Modification of Diet in Renal Disease equation to identify patients who have CKD. On the basis of creatinine measurements, patients were classified as having AKI or no AKI. AKI was further classified into AKI stage 1, stage 2, or stage 3 on the basis of KDIGO definitions for AKI.19 When baseline serum creatinine was not available and the patient had a creatinine >2 mg/dl, the degree of tubular atrophy and interstitial fibrosis (TA/IF)was assessed, and if it involved <25% of the cortex sampled, normal baseline renal function was assumed for the purpose of classifying the degree of AKI. Urinary parameters, including proteinuria, hematuria, and glucosuria, were collected from the first available urinalysis during the admission. Inflammatory markers were also collected. If multiple values were available during the hospital course, the highest IL-6, C-reactive protein, and d-dimer levels and the lowest fibrinogen level and platelet count were recorded as these values reflect the severity of the patients’ condition.
Histopathologic Data
For each autopsy, tissue was evaluated from both kidneys, including renal cortex and medulla, with a typical sampling of >200 glomeruli. Sections were stained with hematoxylin and eosin and evaluated. When needed, additional staining with periodic acid–Schiff was performed, often to better elucidate the glomerular findings. For each autopsy, the light microscopic findings were reviewed by a minimum of two renal pathologists, and any discrepant scores were adjudicated. Immunofluorescence (IF) and/or electron microscopy (EM) were performed on ten separate autopsies as follows: frozen IF and EM on six autopsies (selected on the basis of short postmortem interval [PMI]), pronase IF and deparaffin EM on one autopsy, pronase IF only on two autopsies, and deparaffin EM only on one autopsy.
Autolysis, which refers to the postmortem autodigestion of tissue by endogenous cytoplasmic enzymes of predominantly lysosomal origin, was graded for each autopsy. Briefly, the kidney and in particular, the proximal tubule are highly susceptible to autolysis owing to the high concentration of autolytic enzymes. Histologically, autolytic changes in the proximal tubules are characterized by detachment of tubular epithelia from the tubular basement membrane (TBM); a pale, amorphous eosinophilic coloration (due to reduced staining with hematoxylin); and nuclear changes that evolve from peripheral clumping of chromatin to chromatolysis (dissolution of nuclear chromatin).20 In the setting of autolysis, proximal tubular nuclei will typically progress to chromatolysis before early autolytic changes are seen in distal tubules or glomeruli. There is no uniform, accepted system for grading autolysis in the kidney. As such, we applied the following grading system for autolysis: (1) absent to mild: changes confined to proximal tubules, typically involving <50% of proximal tubules, and limited to pale eosinophilia, only focal detachment from TBMs, and early nuclear chromatin clumping without chromatolysis; (2) severe autolysis: >75% of proximal tubules exhibiting widespread detachment from TBMs, extensive cytoplasmic pale eosinophilia, and diffuse chromatolysis usually accompanied by similar but less severe changes in distal tubules and glomeruli; and (3) moderate autolysis: changes that are more extensive than those described for mild autolysis but not meeting criteria for severe autolysis.
Findings of ATI were graded in hematoxylin and eosin–stained sections and included luminal ectasia, apical cytoplasmic blebbing, epithelial simplification, cytoplasmic vacuolization, loss of brush border, nuclear enlargement, prominent nucleoli, epithelial cell necrosis, and apoptosis. The distribution (<50% versus >50% of tubular profiles) and severity of these changes were assessed. The changes were considered to be mild when involving <50% of tubular profiles and mainly consisted of luminal ectasia, epithelial simplification, and vacuolization. When the changes involved >50% of tubules and were more prominent, they were classified into a combined category of “moderate or severe.” When scoring of ATI differed between pathologists, rereview by two or three renal pathologists was performed to reach consensus. Of note, there are no widely accepted criteria for grading of ATI.21
Viral Probes
In situ hybridization (ISH) for SARS-CoV-2 was performed in ten autopsies, which were preferentially selected on the basis of shorter PMI. ISH for SARS-CoV-2 RNA was performed on formalin-fixed paraffin-embedded tissues using the chromogenic RNAscope 2.5 HD Reagent Kit-RED (catalog no. 322350; Advanced Cell Diagnostics) and the RNAscope 2.5 HD Duplex Reagent Kit (catalog no. 322430; Advanced Cell Diagnostics) according to the manufacturer’s protocols. Two probes specific to the SARS-CoV-2 RNA encoding the spike protein were used, one in each channel: V-nCoV2019-S (catalog no. 848561) and V-nCoV2019-S-C2 (catalog no. 848561-C2). A probe for kidney injury biomarker, LCN2 (catalog no. 559441), was used in combination with V-nCoV2019-S for dual-channel detection. Tissue sections were then counterstained with hematoxylin.
Analyses
Early in the pandemic, autopsies were requested less frequently and disproportionately sought for patients who had died at or shortly after the time of hospital arrival. There was often a long PMI between patient death and performance of autopsy. Later in the pandemic, the potential for autopsy to inform understanding, guide treatment decisions, and provide valuable tissue for research was recognized. A close collaborative effort among clinical departments succeeded in increasing autopsy rates and reducing PMI. We do not have data to compare the initial 42 patients with COVID-19 who underwent autopsy with the significantly larger number of patients who died at New York–Presbyterian/Columbia University Irving Medical Center with COVID-19 during the pandemic or even the larger number of patients who recovered. Thus, we report our findings in a largely descriptive fashion.
Results
Clinical Characteristics
The demographics of the 42 patients who underwent autopsy are provided in Table 1. The cohort had a median age of 71.5 years (range, 38–97 years), and 37 of 42 (88%) were above the age of 60. There were 29 men and 13 women, and 24 of 42 self-identified as Hispanic (57%). Five additional patients self-identified as Black (12%), and three patients identified as White (7%), whereas self-identification of race was not available for the remaining eight patients (19%). The time spent in the hospital varied widely, with median of 7.7 days. Notably, 6 of the 42 patients (14%) were deceased at the time of arrival, and another 9 subjects (21%) died within 3 days. In contrast, 18 individuals (43%) were hospitalized for >10 days prior to death. Frequent and notable comorbidities included HTN in 73%, DM in 42%, CAD or CVA in 32%, and obesity in 31%. Eight of 28 patients with available baseline serum creatinine had CKD (CKD stage 3 or higher in all 8 patients). Of note, only 2 of 28 patients (7%) had no history of HTN, DM, CAD, CKD, or obesity.
Table 1.
Patient characteristics
| Patient Characteristics | No. of Patients (%) |
|---|---|
| Age, yr; median, 71.5 yr (range, 38–97 yr) | |
| <50 | 1/42 (2.4) |
| 51–60 | 4/42 (9.5) |
| 61–70 | 15/42 (35.7) |
| 71–80 | 13/42 (31.0) |
| 81–90 | 6/42 (14.3) |
| >90 | 3/42 (7.1) |
| Men | 29/42 (69.0) |
| Self-identified race | |
| Hispanic | 24/42 (57.1) |
| White | 3/42 (7.1) |
| Black | 5/42 (11.9) |
| Other | 2/42 (4.8) |
| Unknown | 8/42 (19.0) |
| Comorbidities | |
| HTNa | 30/41 (73.2) |
| DMa | 17/41 (41.5) |
| CAD or CVAa | 13/41 (31.7) |
| Obesity (BMI>30)b | 10/32 (31.3) |
| Immunosuppressedc | 2/40 (5.0) |
| CKDd | 8/28 (28.5) |
| Time spent in hospital, d; median, 7.7 d (range, 0–40 d) | |
| Deceased upon arrival | 6/42 (14.3) |
| <1 | 1/42 (2.4) |
| 1–3 | 8/42 (19.0) |
| 3–5 | 2/42 (4.8) |
| 5–10 | 7/42 (16.7) |
| 10–15 | 3/42 (7.1) |
| >15 | 15/42 (35.7) |
BMI, body mass index.
Comorbidities were unknown in one patient.
BMI was not available in ten patients.
Of the two immunosuppressed patients, one had amyotrophic lateral sclerosis on adalimumab, and one was a liver transplant recipient.
Baseline creatinine was missing in 14 patients.
The majority of patients who died with COVID-19 developed AKI (Table 2). Assessment of serum creatinine was not available for nine individuals. Among the remaining 33 patients, 31 (94%) developed AKI, including 6 with AKI stage 1 (18%), 9 with AKI stage 2 (27%), and 16 with AKI stage 3 (48%). Eight patients with AKI stage 3 received continuous RRT (CRRT). Urine dipstick assessment of proteinuria was positive in 23 of 29 subjects (79%) but yielded a urine protein concentration of ≤100 mg/dl in 76%. A urine protein-creatinine ratio was available for ten patients, among whom a single Black individual had nephrotic-range proteinuria (urine protein-creatinine ratio of 8.5 g/g), which corresponded to autopsy findings of collapsing FSGS. Hematuria was present in 19 of 29 individuals, all of whom had indwelling urinary catheters at the time of collection. Red blood cell casts were not identified in the 25 patients whose urine sediment was examined. Glucosuria (17%), hypokalemia (6%), and hypophosphatemia (17%), indicators of possible proximal tubular injury, were encountered in a minority of patients.
Table 2.
Renal parameters and inflammatory markers
| Renal Parameters and Inflammatory Markers | No. of Patients (%) | Median | Range |
|---|---|---|---|
| AKI stage | |||
| No AKI | 2/42 (4.8) | ||
| AKI stage 1 | 6/42 (14.3) | ||
| AKI stage 2 | 9/42 (21.4) | ||
| AKI stage 3 | 16/42 (38.1) | ||
| Serum creatinine not available | 9/42 (21.4) | ||
| Proteinuria (dipstick), mg/dla | |||
| <30 | 6/29 (20.7) | ||
| 30 | 6/29 (20.7) | ||
| 100 | 10/29 (34.5) | ||
| 300 | 4/29 (13.8) | ||
| >1000 | 3/29 (10.3) | ||
| No. of nephrotoxin exposures | |||
| 0 | 13/42 (31.0) | ||
| 1 | 17/42 (40.4) | ||
| 2 | 10/42 (23.8) | ||
| 3 | 1/42 (2.4) | ||
| 4 | 1/42 (2.4) | ||
| Inflammatory markers | |||
| Elevated IL-6 (reference range: ≤5 pg/ml)b | 26/26 (100) | >315 | 36 to >315 |
| Elevated high-sensitivity CRP (reference range: 0.00–10.00 mg/L)c | 30/30 (100) | 245.7 | 82.07 to >300 |
| Elevated d-dimer (reference range: ≤0.80 μg/ml FEU)d | 27/28 (96.4) | 10.2 | 0.58 to >20 |
| Abnormal fibrinogen activity (reference range: 191–430 mg/dl)e | 12/14 (92.9) | 456.5 | <60 to 855 |
CRP, C-reactive protein; FEU, fibrinogen equivalent units.
Proteinuria was unavailable in 13 patients.
IL-6 levels were unavailable in 16 patients.
CRP levels were unavailable in 12 patients.
d-dimer levels were unavailable in 14 patients.
Fibrinogen activity was unavailable in 28 patients.
Twenty-nine patients (69%) were exposed to at least one potential nephrotoxin during hospitalization, and 29% were exposed to two or more. The most frequent nephrotoxin exposure was vancomycin, which was administered to 28 patients, including 19 who received a concomitant dose of piperacillin-tazobactam (not classified as a nephrotoxin for the purpose of this study). Vancomycin levels were available for 18 patients, among whom 10 (56%) had at least one supratherapeutic trough level, defined as a trough level of >20 μg/ml. Seven patients received an aminoglycoside antibiotic, all in conjunction with vancomycin and piperacillin-tazobactam. In the three patients in whom aminoglycoside levels were checked, two had supratherapeutic levels. Four patients received intravenous contrast; no other individual nephrotoxin was administered to more than one patient (Supplemental Table 1).
Inflammatory laboratory parameters are provided in Table 2. IL-6, C-reactive protein, d-dimer levels and fibrinogen activity were significantly elevated in nearly all patients. Among the 36 patients who were alive at the time of arrival and therefore had available data, 32 had an abnormal white blood cell count (94%), and 22 had evidence of thrombocytopenia (61%) (Supplemental Table 1). Fourteen of the 36 who were alive on arrival had a code status of “do not resuscitate/do not intubate”; only 1 of 14 was started on vasopressors during the hospitalization. Among the remaining 22 patients, 21 received ventilatory support (95%), 20 received vasopressors (91%), and 8 received CRRT (36%). A few additional patients died before CRRT could be initiated. Twenty-two of the 36 patients were treated with plaquenil (61%), 22 were treated with steroids (61%), and 6 were treated with tocilizumab (17%). None of the patients received remdesivir.
Autopsy Findings
Kidney autopsy findings are highlighted in Table 3. For each patient, coronal sections of both kidneys containing >200 glomeruli were evaluated. A critical determinant in the evaluation of the kidneys at autopsy is the PMI. Autolysis increases in proportion to PMI and is also influenced by temperature (Figure 1A). Although refrigeration reduces autolysis, access to rapid refrigeration was at times limited during the height of the pandemic. Importantly, autolysis mainly obscures the ability to identify ATI but does not significantly obscure the scoring of glomerulosclerosis, TA/IF, or vascular disease; even in the setting of severe autolysis, GN, interstitial inflammation, and fibrin thrombi can be identified. The median PMI in our series was 21.8 hours (range, 2.5–186 hours), with PMI<12 hours in 15 autopsies (34%), <24 hours in 24 autopsies (55%), and <48 hours in 32 autopsies (73%). Autolysis was graded as absent to mild in 23 (55%), moderate in 5 (12%), and severe to near complete in 14 (33%).
Table 3.
Renal autopsy findings
| Renal Autopsy Findings | No. of Patients |
|---|---|
| PMI, h; median, 21.8 ha | |
| 0–6 | 13 |
| 6–12 | 2 |
| 12–24 | 9 |
| 24–48 | 8 |
| 48–72 | 2 |
| >72 | 10 |
| Degree of tissue autolysis | |
| Absent to mild | 23 |
| Moderate | 5 |
| Severe/near complete | 14 |
| Glomeruli | |
| Glomerulosclerosis | |
| 0%–25% of glomeruli | 34 |
| 26%–50% of glomeruli | 6 |
| >50% of glomeruli | 2 |
| Diagnosis of glomerular disease | |
| Diabetic glomerulosclerosis | 7b |
| Fibrin thrombi (<5% of glomeruli) | 4c |
| Collapsing FSGS | 1 |
| Idiopathic nodular GS | 1 |
| IgAN | 1 |
| No glomerular diagnosis | 29 |
| Tubules, interstitium, and blood vessels | |
| ATI | |
| Absent/minimal | 12 |
| Mild | 12 |
| Moderate or severe | 7 |
| Cannot assess due to autolysis | 11 |
| TA/IF | |
| 0%–25% of cortex | 36 |
| 26%–50% of cortex | 4 |
| >50% of cortex | 2 |
| Arteriosclerosis | |
| Mild | 7 |
| Moderate | 26 |
| Severe | 9 |
| Fibrin thrombi in arteries or arterioles | |
| Present | 3d |
GS, glomerulosclerosis.
PMI ranged from 2.5 to 186 h.
Diabetic glomerulosclerosis was accompanied by fibrin thrombi in one autopsy.
Fibrin thrombi were identified in six autopsies in glomeruli (n=3), blood vessels (n=2), and glomeruli and blood vessels (n=1).
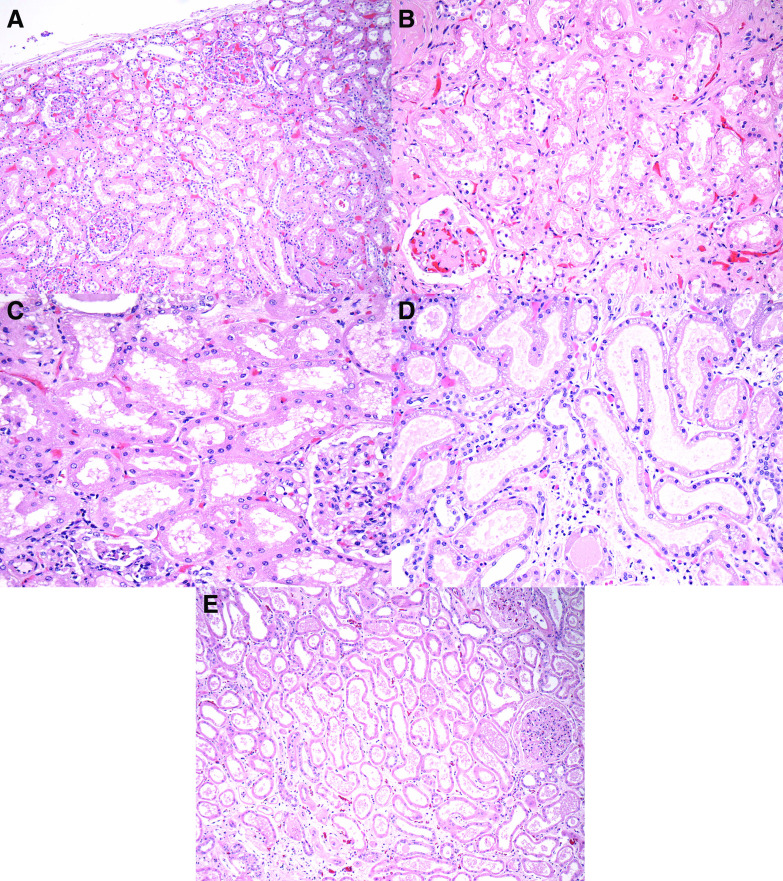
Figure 1.
Autopsy kidneys deomstarted arteriosclerosis, variable degrees of autolysis and, rarely, fibrin thrombi. (A) A low-magnification view reveals complete autolysis in an autopsy with a prolonged PMI. Tubular nuclei are not visible, and tubular injury cannot be assessed. In contrast, chronic changes of glomerulosclerosis, arteriosclerosis, and TA/IF are still visualized. (Hematoxylin and eosin, ×100.) (B) A glomerulus exhibits mild changes of NDGS. (Hematoxylin and eosin, ×400.) (C) A glomerulus displays red blood cell congestion and an intracapillary fibrin thrombus. (Hematoxylin and eosin, ×400.) (D) A fibrin thrombus is seen in the lumen of an artery. (Hematoxylin and eosin, ×400.) (E) In this area of microscopic infarction, tubules exhibit coagulative-type necrosis, and there is prominent neutrophil infiltration with neutrophilic debris. (Hematoxylin and eosin, ×200.) (F) In this patient with hypertensive arterionephrosclerosis, an artery exhibits severe intimal sclerosis, compromising >50% of the lumen. (Hematoxylin and eosin, ×400.)
Glomeruli were well preserved in the majority of kidneys. Despite a median age of 71.5 years and a high incidence of HTN (73.2%), ≤25% of glomeruli were globally sclerotic in 34 of 42 autopsies (81%). In the remaining eight patients, the renal parenchymal scarring was primarily attributed to hypertensive arterionephrosclerosis in six, nodular diabetic glomerulosclerosis (NDGS) in one (Figure 1B), and a combination of hypertensive arterionephrosclerosis and NDGS in one. Glomeruli exhibited no more than mild, nonspecific changes, such as enlargement, mild mesangial sclerosis, and focal ischemic-type wrinkling of the glomerular basement membrane, in 29 of 42 autopsies (69%). Seven autopsies exhibited findings of diabetic glomerulosclerosis (mainly NDGS), and one each displayed collapsing FSGS, idiopathic nodular glomerulosclerosis, and IgA nephropathy (IgAN; in the setting of chronic liver disease). Fibrin thrombi were identified in the kidneys in six autopsies and were distributed in glomeruli (n=3), in arteries or arterioles (n=2), or in both glomeruli and arterioles (n=1) (Figure 1, C and D). In two of six autopsies with fibrin thrombi, focal microinfarction was noted, measuring approximately 3 and 10 mm (Figure 1E). Importantly, renal fibrin thrombi were focal in all autopsies; when present, <5% of glomeruli contained thrombi. There was no macroscopic evidence of infarction on gross examination.
Similar to glomerulosclerosis, chronic changes of TA/IF involved ≤25% of the cortex sampled in 36 of 42 autopsies (86%). The remaining six autopsies had renal parenchymal scarring related to hypertensive arterionephrosclerosis or NDGS. Arteriosclerosis was the most prominent chronic finding in the majority of autopsies, was present in 100% of autopsies, and was graded as moderate or severe in 35 (83%) (Figure 1F). The majority of these patients would meet criteria for the diagnosis of hypertensive arterionephrosclerosis (alternatively referred to as arterionephrosclerosis in the setting of HTN). Interstitial inflammation was mild in all autopsies, and it was largely confined to zones of TA/IF. None of the autopsies exhibited significant tubulitis or other findings suggestive of interstitial nephritis. No viral inclusions were identified.
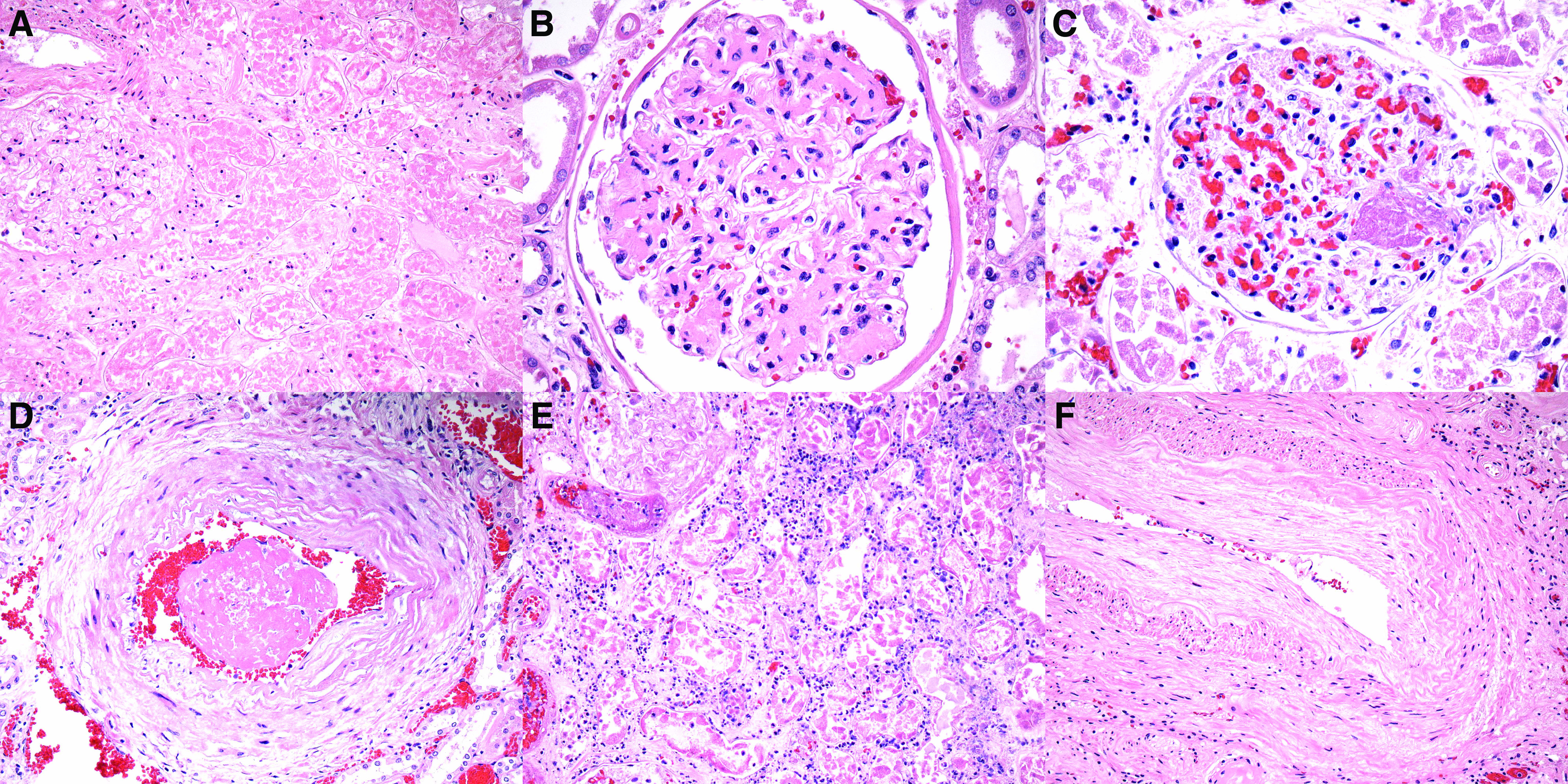
The ability to identify ATI, which was the main finding that correlated with a history of AKI, was obscured by autolysis to varying degrees. Although 11 autopsies could not be assessed for ATI due to autolysis, in the remaining 31, ATI was graded as absent or minimal in 12 (39%), mild in 12 (39%), or moderate to severe in 7 (23%) (Figure 2, A–E). Specifically, ATI was scored on the basis of the distribution (focal versus diffuse) and severity of injurious changes, which most commonly included luminal ectasia, cytoplasmic simplification, vacuolization, attenuation and loss of brush border, and nuclear degenerative and regenerative changes. Findings of frank necrosis and/or apoptosis were not encountered or were extremely focal in all cases. When scoring of ATI differed between pathologists, rereview by two or three renal pathologists was performed to reach consensus.
Figure 2.
ATI is the main finding in autopsy kidneys from patients with COVID-19 and AKI. (A) A low-magnification view reveals intact renal cortex, without evidence of ATI. (Hematoxylin and eosin, ×40.) (B) In this patient with AKI stage 1 with an increase in creatinine from 1.09 to 1.6 mg/dl, histologic evaluation revealed mild ATI characterized by mild luminal ectasia, irregular luminal contours, and vacuolization. Prominent cytoplasmic simplification and loss of brush border are not apparent. (Hematoxylin and eosin, ×100.) (C) In this patient with AKI stage 3 with an increase in creatinine from 0.94 to 6.83 mg/dl requiring CRRT, only mild ATI is noted, characterized by luminal ectasia and mild vacuolization. (Hematoxylin and eosin, ×200.) (D) In this patient with AKI stage 3 with an increase in creatinine from 0.67 to 3.48 mg/dl, the kidneys exhibit severe ATI, with more prominent luminal ectasia, cytoplasmic simplification, vacuolization, and loss of brush border. (Hematoxylin and eosin, ×200.) (E) In this patient with AKI stage 3 requiring CRRT, severe ATI is also apparent. (Hematoxylin and eosin, ×100.)
Table 4 provides the degree of ATI among the 31 patients with AKI. Histologic assessment was not obscured by autolysis in 23 autopsies with AKI and revealed ATI that ranged from absent to minimal in 7 to mild in 10 to moderate or severe in 6. Not surprisingly, ATI was less evident in AKI stage 1. Somewhat surprisingly, even in the presence of AKI stage 2 or 3, tubular injury was graded as moderate to severe in only 5 of 17 autopsies (29%), and it was largely absent in 2. As such, the most distinctive finding was relatively profound elevation in serum creatinine (AKI stage 2 or 3) associated with no more than mild ATI in the majority of cases.
Table 4.
Tubular injury in patients with AKI
| Degree of ATI | No. of Patients per AKI Stage | ||
|---|---|---|---|
| AKI 1 | AKI 2 | AKI 3 | |
| Absent/minimal | 5 | 2 | 0 |
| Mild | 0 | 4 | 6 |
| Moderate or severe | 1 | 2 | 3 |
| Cannot assess due to autolysis | 0 | 1 | 7 |
IF was performed on nine autopsies, including IF on frozen tissue on six cases that were randomly selected on the basis of short PMI and IF on pronase-digested tissue for three cases that had mild increases in glomerular cellularity. IF was unrevealing in seven of nine autopsies. In the remaining two autopsies, IF revealed positivity consistent with IgAN in a patient with chronic liver disease and staining for fibrinogen in the distribution of arterial fibrin thrombi in another.
EM was performed on eight autopsies that were randomly selected mainly on the basis of short PMI. EM revealed extensive (90%) podocyte foot process effacement in the case with collapsing FSGS, mesangial deposits consistent with IgAN in one autopsy, and mild findings of NDGS in another. In the remaining five, glomeruli appeared largely unremarkable by EM. In particular, electron dense deposits were not apparent, and only mild foot process effacement (<25%) was noted. Tubules displayed degenerative changes, which most notably included attenuation and loss of brush border, dilation of endoplasmic reticulum, and intraluminal cellular debris. No definitive virions were identified at the ultrastructural level.
ISH for SARS-CoV-2 RNA (RNAscope) was performed on ten autopsies that were randomly selected on the basis of short PMI. Although multiple autopsies exhibited rare tubular epithelial staining (<1 in 200 tubular epithelial cells) with low-intensity dot-like positivity, this staining was seen in the setting of a high background staining, appeared to be present focally in a negative autopsy kidney control, and was substantially less impressive than the positivity noted in lung specimens from autopsies with COVID-19. Despite activation of the transcription of lipocalin 2-neutrophil gelatinase-associated lipocalin in distal segments (diffuse positive in one, focally positive in eight, and absent in one), typical of intrinsic ATI, COVID-19 transcripts were difficult to confirm in autopsied kidneys using RNAscope in situ probes. As a result, we concluded that we could not identify definitive positivity for SARS-CoV-2 by ISH in autopsy kidneys.
On the basis of the autopsy findings, the cause of death in 30 of 42 autopsies was respiratory failure resulting from COVID-19 pneumonia with diffuse alveolar damage and in a few cases, superimposed bacterial pneumonia. Three additional patients died from decompensated heart failure, three died from acute pulmonary emboli, two died from intracranial hemorrhage, and one died from myocardial infarction.
Discussion
We report the largest autopsy series detailing clinical and renal pathology findings in patients dying of confirmed COVID-19 disease. Although not generalizable to the general patient population with COVID-19 at New York–Presbyterian/Columbia University Irving Medical Center, several clinical demographics echoed overall mortality data from the New York City area, where at the time of writing, 85% of fatalities were over the age of 60 and 58% were men22 The high percentage of Hispanic patients in our study in part reflects the patient population we serve in the Washington Heights section of Manhattan. The relatively low percentage of Black patients compared with overall New York City COVID-19 mortality data likely reflects decisions by next of kin to consent for autopsy rather than being indicative of overall mortality at Columbia University Irving Medical Center. Rates of comorbid conditions, including HTN, DM, CAD, CVA, and obesity, were also similar to the New York City area as a whole.22 AKI, a relatively established risk factor for in-hospital mortality,6 was present in the majority of our decedents, and was accompanied by markedly increased levels of inflammatory markers and near-universal requirement for ventilatory support and vasopressors. A similarly high rate of AKI in patients who died (78%; 694 of 888) has been reported from another large health network in the New York metropolitan area.14
The most notable observation in our autopsy cohort was that ATI was the main pathologic finding correlating with a history of AKI. There are many potential—and likely inter-related—causes of AKI in critically ill patients with COVID-19 disease. Elevated inflammatory markers were present in all patients, indicative of cytokine release syndrome, a well documented complication of severe COVID-19 infection.23 Hypercytokinemia (“cytokine storm”) may lead to AKI secondary to the effects of distributive septic shock, the most common cause of AKI in the intensive care unit setting.24 Indeed, nearly all nonpalliative patients in our cohort required vasopressor support. Other mechanisms of septic shock–related AKI include exposure of tubular epithelial cells to pathogen- or damage-associated molecular patterns, intrarenal microcirculatory dysfunction, and metabolic reprogramming.25 The need for mechanical ventilation for suspected acute respiratory distress syndrome (ARDS), a well documented complication of severe COVID-19 disease, was high in our cohort. ARDS is a known risk factor for the development of AKI in critically ill patients, and AKI in the setting of ARDS confers significant risk of mortality.26,27 Potential mechanisms include complex lung-kidney interactions, impaired gas exchange leading to tissue hypoxia, hemodynamic effects of aggressive volume removal (i.e., the use of diuretics or RRT to “dry out the lungs”), and secondary right heart failure.28 Most decedents in our cohort also had iatrogenic exposure to known nephrotoxins, raising the possibility of a component of toxic ATI. We did not find sufficient evidence of myoglobinuric AKI.
An equally distinctive finding in our series is that the degree of ATI was typically mild compared with the degree of serum creatinine elevation (AKI). Specifically, among 17 patients with stage 2 or 3 AKI, ATI was graded as absent to mild in 12 (71%). Previous studies have observed a poor correlation between histopathologic findings of ATI and clinical parameters in animal models and human studies of septic shock–related AKI.29–31 Alternatively and more likely, this observation suggests that prerenal or functional factors, such as aggressive fluid management in the settings of ARDS, are contributing to the development of AKI in the setting of COVID-19. Importantly, the sole finding of ATI that was typically mild, on a background of mostly mild chronic glomerular and tubulointerstitial changes, among patients with COVID-19–associated AKI suggests that recovery from infection has the potential to be associated with significant improvement in renal function.
Evidence of kidney tropism of SARS-CoV-2, together with reports of putative viral particles in the kidney, has raised concern for a possible “viral nephropathy.”15,16 The angiotensin-converting enzyme 2 receptor, the main cellular entry point for SARS-CoV-2, is expressed on the apical brush border of proximal tubular cells and to a lesser degree, on podocytes.32,33 In our autopsy cohort, viral inclusions were not identified at the light microscopic level, and prominent interstitial inflammation characteristic of many viral nephropathies (i.e., BK polyoma virus and adenovirus) was not seen. Furthermore, viral particles were not identified at the ultrastructural level, and we were unable to demonstrate SARS-CoV-2 by ISH. Whether rare, equivocal staining represents nonspecific staining or low viral abundance requires further study. The reported presence of virus demonstrable by RT-PCR16 leads us to conclude that SARS-CoV-2 is likely present at very low levels in the renal parenchyma. It is unclear whether this low-level renal infection may increase susceptibility to tubular insults.
The absence of many significant findings may be the most notable finding in autopsy kidneys from patients with COVID-19. Interstitial inflammation was only mild and localized in all autopsies, thus excluding acute interstitial nephritis. Similarly, there were no examples of acute GN, although one autopsy exhibited mild IgAN (in the setting of chronic liver disease). Despite multiple recent reports of collapsing FSGS in Black patients with COVID-19,17,18 collapsing FSGS was only identified in 1 of 42 autopsies (a Black patient). Importantly, hypercoagulability and risk of thromboembolic events are major concerns during the COVID-19 pandemic,34 raising the significant possibility that microangiopathy, with or without overt fibrin thrombus formation and cortical infarction, might underlie AKI in some patients with COVID-19–associated AKI. In our experience, glomerular and/or vascular fibrin thrombi were limited to 6 of 42 (14%) autopsies and when present, involved <5% of glomeruli sampled. Furthermore, well developed ultrastructural features of endothelial injury were not apparent at the ultrastructural level. None of the six patients with kidney fibrin thrombi had clinical evidence of deep vein thrombosis or pulmonary embolus or died as a result of pulmonary emboli. These findings argue strongly against thrombosis as a significant cause of AKI in the setting of COVID-19.
Our study has several limitations. First, we are unable to compare the 42 autopsied patients at New York–Presbyterian/Columbia University Irving Medical Center with the significantly larger number of patients who died or recovered at our center during the COVID-19 pandemic; thus, the generalizability of the data is unknown. Second, tissue evaluation was limited by autolysis related to prolonged PMI. In particular, the kidneys from four of eight patients who required CRRT had severe autolysis, precluding our ability to assess the degree of AKI. As such, additional cases of severe ATI may have been missed. Third, tissue evaluation was largely limited to light microscopy and hematoxylin and eosin–stained tissue sections, with periodic acid–Schiff stain, IF, and EM available as needed and on a randomly selected subset of autopsies with reduced PMI. This limitation was partially offset by the availability of abundant tissue with >200 glomeruli examined per case. Fourth, our inability to detect SARS-CoV-2 by ISH may relate to postmortem RNA degradation (although we were able to detect SARS-CoV-2 by ISH in lung from COVID-19 autopsies).
In conclusion, histologic evaluation of the kidneys from autopsies of patients dying with COVID-19 is most notable for the presence of ATI, and the degree of ATI is most commonly mild compared with the degree of AKI. These findings suggest a complex etiology that likely involves ischemia, hypoxia, sepsis-associated factors, and toxin exposure. Equally notable is the absence of findings of classic viral nephropathy, diffuse thrombotic microangiopathy, or acute GN. The relatively mild degree of ATI, even in the setting of severe AKI, suggests that with resolution of SARS-CoV-2 infection, there is potential for renal functional recovery.
Disclosures
J. Barasch reports that Columbia University owns patents, including European Patent Register 1 616 184; United States Patent Register 7,977,110; European Patent Register 1 616 184; and United States Patent Register 7,977,110, and has licensed biomarker data to Abbott and Bioporto. Bioporto provides royalties to Columbia University. All remaining authors have nothing to disclose
Funding
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020050744/-/DCSupplemental.
Supplemental Table 1. Expanded clinical data.
References
- 1.World Health Organization : WHO Coronavirus disease (COVID-19) Situation Report–119, 2020. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200518-covid-19-sitrep-119.pdf?sfvrsn=4bd9de25_4. Accessed July 6, 2020
- 2.Centers for Disease Control and Prevention : Cases, Data & Surveillance, 2020. Available at: https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/cases-in-us.html. Accessed July 6, 2020
- 3.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. : Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study [published correction appears in Lancet Respir Med 8: e26, 2020]. Lancet Respir Med 8: 475–481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. : Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study [published correction appears in BMJ 368: m1295, 2020]. BMJ 368: m1091, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. : Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. : Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fanelli V, Fiorentino M, Cantaluppi V, Gesualdo L, Stallone G, Ronco C, et al. : Acute kidney injury in SARS-CoV-2 infected patients. Crit Care 24: 155, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. ; China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. : Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. : Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395: 507–513, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. : Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395: 1054–1062, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arentz M, Yim E, Klaff L, Lokhandwala S, Riedo FX, Chong M, et al. : Characteristics and outcomes of 21 critically ill patients with COVID-19 in Washington state. JAMA 323: 1612–1614, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. ; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. : Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al.: Multiorgan and renal tropism of SARS-CoV-2 [published online ahead of print May 13, 2020]. N Engl J Med doi:10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larsen CP, Bourne TD, Wilson JD, Saqqa O, Sharshir MA: Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19). Kidney Int Rep 5: 935–939, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peleg Y, Kudose S, D’Agati V, Siddall E, Ahmad S, Kisselev S, et al. : Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep 5: 940–945, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl 2: 1–138, 2012 [Google Scholar]
- 20.Cruickshank J: The histologic appearance occuring in organs undergoing autolysis. J Pathol XVI: 167–186, 1911 [Google Scholar]
- 21.Moeckel GW: Pathologic perspectives on acute tubular injury assessment in the kidney biopsy. Semin Nephrol 38: 21–30, 2018. [DOI] [PubMed] [Google Scholar]
- 22.New York State Department of Health: NYSDOH COVID-19 Tracker—Fatalities, 2020. Available at: https://covid19tracker.health.ny.gov/views/NYS-COVID19-Tracker/NYSDOHCOVID-19Tracker-Fatalities?%3Aembed=yes&%3Atoolbar=no&%3Atabs=n. Accessed May 20, 2020
- 23.Moore JB, June CH: Cytokine release syndrome in severe COVID-19. Science 368: 473–474, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Uchino S, Kellum JA, Bellomo R, Doig GS, Morimatsu H, Morgera S, et al. ; Beginning and Ending Supportive Therapy for the Kidney (BEST Kidney) Investigators : Acute renal failure in critically ill patients: A multinational, multicenter study. JAMA 294: 813–818, 2005. [DOI] [PubMed] [Google Scholar]
- 25.Gomez H, Ince C, De Backer D, Pickkers P, Payen D, Hotchkiss J, et al. : A unified theory of sepsis-induced acute kidney injury: Inflammation, microcirculatory dysfunction, bioenergetics, and the tubular cell adaptation to injury. Shock 41: 3–11, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Darmon M, Clec’h C, Adrie C, Argaud L, Allaouchiche B, Azoulay E, et al. : Acute respiratory distress syndrome and risk of AKI among critically ill patients. Clin J Am Soc Nephrol 9: 1347–1353, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson JG, Calfee CS: ARDS subphenotypes: Understanding a heterogeneous syndrome. Crit Care 24: 102, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Joannidis M, Forni LG, Klein SJ, Honore PM, Kashani K, Ostermann M, et al. : Lung-kidney interactions in critically ill patients: Consensus report of the acute disease quality initiative (ADQI) 21 Workgroup. Intensive Care Med 46: 654–672, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosaka J, Lankadeva YR, May CN, Bellomo R: Histopathology of septic acute kidney injury: A systematic review of experimental data. Crit Care Med 44: e897–e903, 2016. [DOI] [PubMed] [Google Scholar]
- 30.Langenberg C, Bagshaw SM, May CN, Bellomo R: The histopathology of septic acute kidney injury: A systematic review. Crit Care 12: R38, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aslan A, van den Heuvel MC, Stegeman CA, Popa ER, Leliveld AM, Molema G, et al. : Kidney histopathology in lethal human sepsis. Crit Care 22: 359, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serfozo P, Wysocki J, Gulua G, Schulze A, Ye M, Liu P, et al. : Ang II (angiotensin II) conversion to angiotensin-(1-7) in the circulation is POP (prolyloligopeptidase)-dependent and ACE2 (angiotensin-converting enzyme 2)-independent. Hypertension 75: 173–182, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D: Glomerular localization and expression of angiotensin-converting enzyme 2 and angiotensin-converting enzyme: Implications for albuminuria in diabetes. J Am Soc Nephrol 17: 3067–3075, 2006. [DOI] [PubMed] [Google Scholar]
- 34.Connors JM, Levy JH: COVID-19 and its implications for thrombosis and anticoagulation. Blood 135: 2033–2040, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.