We are concerned about the erroneous identification of coronavirus directly in tissues by authors using electron microscopy. Several recent articles have been published that purport to have identified severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly in tissue.1–4 Most describe particles that resemble, but do not have the appearance of, coronaviruses.5–7
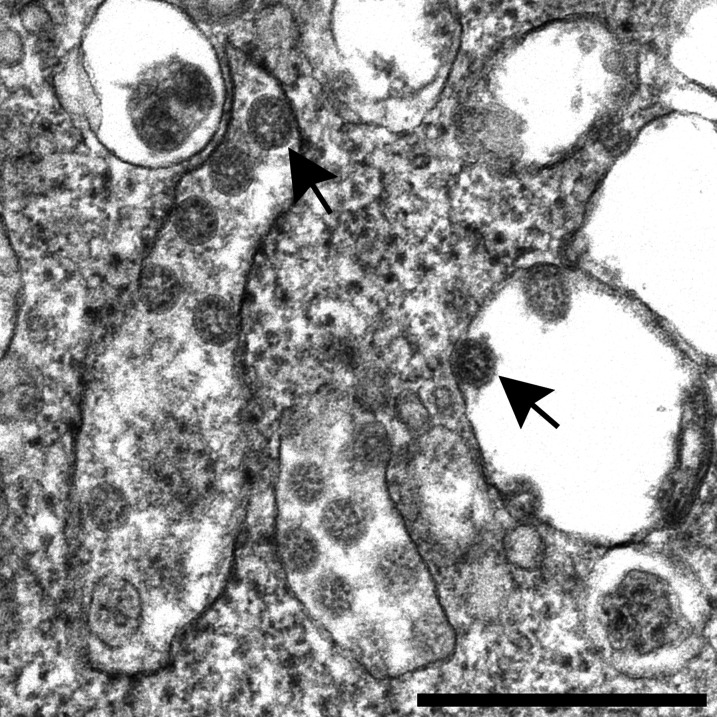
The evidence provided in the article by Farkash et al.8 in JASN likewise does not confirm the presence of SARS-CoV-2 in kidney tissue. Coronaviruses have been carefully described in electron microscopic images of thin sections.9–12 In these images of thin sections, coronaviruses appear as spherical structures containing black dots on the inside, which are cross-sections through the helical viral nucleocapsid (Figure 1). Coronaviruses receive their outer covering by budding into cellular membranes of the rough endoplasmic reticulum and Golgi complex forming a vacuole and are found in the intracisternal space. The spikes are seen with difficulty in thin sections of infected cells, but a “fuzz” is sometimes visible. However, note that these spikes face the inside of the vacuole and do not touch the cytoplasm of the cell. Complete virus particles can also be found at the cell surface, having been extruded when the vacuolar membrane fuses with the plasma membrane and exocytoses them; in this case, the spikes on the virions face the extracellular space, again, not the cytoplasm.
Figure 1.
Severe acute respiratory syndrome coronavirus 2 isolate grown in cell culture showing numerous spherical viral particles (arrows) that are in the cisternae of the rough endoplasmic reticulum/Golgi complex area of the cell. Note the black dots on the interior of the particles, which are cross-sections through the viral nucleocapsid. Scale bar: 400 nm.
In the article by Farkash et al.,8 the electron microscopic images in their Figure 3, A–C do not demonstrate coronaviruses. Rather, the structures described as virus are clathrin-coated vesicles (CCVs), normal subcellular organelles involved in intracellular transport. Figure 3A8 is a low magnification of a dying cell with nonspecific disorganized cytoplasm with an arrow pointing to an aggregation of CCVs. Panels B and C in their Figure 38 show clusters of CCVs, and the inset for Figure 3C8 shows a higher magnification. None of these spherical structures contain cross-sections through the nucleocapsid of virus particles. In addition, these CCVs are seen free in the cytoplasm, whereas coronavirus particles are found enclosed within a vacuole so that the spikes face the inside of the vacuolar contents, not the cytoplasm. Figure 3D8 contains a multivesicular body (MVB), which they have likened to double-membrane vesicles, the replication complex for coronaviruses. The structure shown in the manuscript by Farkash et al.8 does not have the two tightly opposed membranes seen in double-membrane vesicles and does not have the appearance of what is shown in the reference they cite.13 In addition, MVBs can be found in kidney tissues observed historically.6 Moreover, MVBs are formed by invaginations of endosomes and are intermediates in trafficking for lysosomes.
Additionally, Farkash et al.8 document their findings by referring to an article by Su et al.2 that purports to have identified coronavirus in kidney. Likewise, that article shows only normal cell structures that, to the non-electron microscopist virologist, may resemble coronavirus. Their interpretation has been refuted in Letters to the Editor of Kidney International.5,6
Identification of viruses is not always straight forward. Consideration should be given to the mechanism of virus production, including the location inside of cells, as well as the appearance (size, shape, internal pattern of the nucleocapsid, and surface spikes).14–16 Care should be taken to prevent mistaking cell organelles for viral particles.17,18
Disclosures
All authors have nothing to disclose.
Funding
None.
Acknowledgments
We are grateful to Dr. Roosecelis Brasil Martines and Dr. David Howell for critically reviewing the letter.
The findings and conclusions are those of the authors and do not necessarily represent the position of the US Centers for Disease Control and Prevention.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Kissling S, Rotman S, Gerber C, Halfon M, Lamoth F, Comte D, et al.: Collapsing glomerulopathy in a COVID-19 patient. Kidney Int 98: 228–231, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Su H, Yang M, Wan C, Yi L-X, Tang F, Zhu H-Y, et al.: Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al.: Endothelial cell infection and endotheliitis in COVID-19. Lancet 395: 1417–1418, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tavazzi G, Pellegrini C, Maurelli M, Belliato M, Sciutti F, Bottazzi A, et al.: Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur J Heart Fail 22: 911–915, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miller SE, Brealey JK: Visualization of putative coronavirus in kidney. Kidney Int 98: 231–232, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calomeni E, Satoskar A, Ayoub I, Brodsky S, Rovin BH, Nadasdy T: Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int 98: 233–234, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR: Electron microscopy of SARS-CoV-2: A challenging task. Lancet 395: e99, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Farkash EA, Wilson AM, Jentzen JM: Ultrastructural evidence for direct renal infection with SARS-CoV-2 [published online ahead of print May 5, 2020]. J Am Soc Nephrol doi:10.1681/ASN.2020040432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubois-Dalcq M, Holmes KV, Rentier B: Assembly of coronaviridae In: Assembly of Enveloped RNA Viruses, edited by Dubois-Dalcq M, Holmes KV, Rentier B, Vienna, Austria, Springer-Verlag, 1984, pp 100–119 [Google Scholar]
- 10.Oshiro LS, Schieble JH, Lennette EH: Electron microscopic studies of coronavirus. J Gen Virol 12: 161–168, 1971. [DOI] [PubMed] [Google Scholar]
- 11.Goldsmith CS, Tatti KM, Ksiazek TG, Rollin PE, Comer JA, Lee WW, et al.: Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis 10: 320–326, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zaki SR, Goldsmith CS: SARS coronavirus infection: Pathology and pathogenesis of an emerging virus disease In: Coronaviruses with Special Emphasis on First Insights Concerning SARS, edited by Schmidt A, Weber O, Wolff MH, Basel, Switzerland, Birkhäuser Verlag, 2005, pp 87–99 [Google Scholar]
- 13.Knoops K, Kikkert M, Worm SH, Zevenhoven-Dobbe JC, van der Meer Y, Koster AJ, et al.: SARS-coronavirus replication is supported by a reticulovesicular network of modified endoplasmic reticulum. PLoS Biol 6: e226, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dalton AJ, Haguenau F, editors: Ultrastructure of Animal Viruses and Bacteriophages, Waltham, MA, Academic Press, 1973 [Google Scholar]
- 15.Miller SE: Detection and identification of viruses by electron microscopy. J Electron Microsc Tech 4: 265–301, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller SE: Diagnosis of viral infection by electron microscopy In: Diagnostic Procedures forViral, Rickettsial and Chlamydial Infections, edited by Lennette EH, Lennette DA, Lennette ET, Washington, DC, American Public Health Association, 1995, pp 35–76 [Google Scholar]
- 17.Miller SE: Problems and pitfalls in diagnostic electron microscopy. Microsc Microanal 18[Suppl 2]: 172–173, 2012 [Google Scholar]
- 18.Haguenau F: Virus-like particles as observed with the electron microscope In: Ultrastructure of Animal Viruses and Bacteriophages, edited by Dalton AJ, Haguenau F, Waltham, MA, Academic Press, 1973, pp 391–397 [Google Scholar]