Significance Statement
HLA matching for three HLA loci (HLA-A, HLA-B, and HLA-DR) at a low-resolution antigen level has been integral to algorithms for allocating donor kidneys for transplant since the 1970s. The authors used high-resolution genotyping of the 11 HLA loci and analysis of mismatches of HLA eplets—small patches of surface-exposed amino acids of the HLA molecule—to evaluate the effect of eplet mismatches on de novo formation of donor-specific HLA antibodies (DSAs) and kidney transplant outcome. They found that eplet mismatches in the HLA-DQ locus are most important for DSA formation, rejection, decline of graft function, and graft failure. Their findings suggest that molecular HLA-DQ matching might be more helpful than antigen matching for HLA-A, HLA-B, and HLA-DR when aiming to minimize formation of DSAs and improve outcomes after transplant.
Keywords: acute allograft rejection, kidney transplantation, HLA, histocompatibility, organ allocation
Visual Abstract
Abstract
Background
In kidney transplantation, evaluating mismatches of HLA eplets—small patches of surface-exposed amino acids of the HLA molecule—instead of antigen mismatches might offer a better approach to assessing donor-recipient HLA incompatibility and improve risk assessment and prediction of transplant outcomes.
Methods
To evaluate the effect of number of eplet mismatches (mismatch load) on de novo formation of donor-specific HLA antibodies (DSAs) and transplant outcomes, we conducted a cohort study that included consecutive adult kidney recipients transplanted at a single center from March 2004 to February 2013. We performed retrospective high-resolution genotyping of HLA loci of 926 transplant pairs and used the HLAMatchmaker computer algorithm to count HLA eplet mismatches.
Results
De novo DSAs occurred in 43 (4.6%) patients. Multivariable analysis showed a significant independent association between antibody-verified eplet mismatch load and de novo DSA occurrence and graft failure, mainly explained by DQ antibody-verified eplet effects. The association with DQ antibody-verified eplet mismatches was linear, without a safe threshold at which de novo DSA did not occur. Odds for T cell– or antibody-mediated rejection increased by 5% and 12%, respectively, per antibody-verified DQ eplet mismatch.
Conclusions
Eplet mismatches in HLA-DQ confer substantial risk for de novo DSA formation, graft rejection, and graft failure after kidney transplantation. Mismatches in other loci seem to have less effect. The results suggest that antibody-verified HLA-DQ eplet mismatch load could be used to guide personalized post-transplant immunosuppression. Adoption of molecular matching for DQA1 and DQB1 alleles could also help to minimize de novo DSA formation and potentially improve transplant outcomes.
Immunologic rejection plays an important role in kidney transplant failure, both antibody-mediated rejection (ABMR) caused by donor-specific HLA antibodies (DSA) and T cell–mediated rejection (TCMR).1,2 HLA disparity between donors and recipients is the main driver of these rejection types, as reflected by the persistent association between the number of HLA antigen mismatches and transplant outcome, despite the use of combinations of powerful immunosuppressive agents to diminish rejection.3,4 Therefore, many allocation systems have integrated some degree of HLA antigen matching in their algorithms.
Advances in protein modeling make it possible to evaluate donor/recipient HLA mismatch at the molecular level. HLA immunogenicity is mediated through a limited number of mismatched polymorphic amino acid residues called epitopes.5 HLAMatchmaker is a structurally based computer algorithm that enables evaluation at the level of the “eplet”—small patches of surface-exposed amino acids of the HLA molecule.6 Eplet mismatch analysis in retrospective studies seems to explain, better than antigen-mismatch analysis, antibody reactivity patterns observed in patients who are sensitized. Because not all eplet mismatches were shown to lead to antibody formation, the HLA Epitope Registry proposes some (“antibody-verified”) eplets to be of higher immunogenicity.7,8
Eplet mismatches, especially in class 2 HLA, have been associated with risk of DSA formation and ABMR.9–12 Eplet mismatch load is also associated with increased risk of graft failure.10,13,14
Recently, eplet mismatch analysis has been proposed as a risk stratification tool to optimize immunosuppression.11,12 The success of such concepts in the Eurotransplant Acceptable Mismatch Program of patients who are highly sensitized further points to the potential of eplet mismatch analysis in risk stratification of this specific high-risk subgroup of patients waiting for kidney transplantation.15 However, it remains unclear whether such a strategy provides added value over current HLA antigen matching in the overall kidney transplant population.16
We investigated whether high-resolution genotyping could identify a locus-specific eplet mismatch associated with DSA formation, rejection, and kidney graft outcome. Such a sensitive predictor could be more helpful than testing for HLA antigen mismatches to improve graft outcomes and guide personalized post-transplant immunosuppression.
Methods
Patients
All consecutive adult recipients of a kidney transplant at the University Hospitals Leuven between March 1, 2004 and February 6, 2013 were eligible for this observational cohort study (n=1137). Recipients of combined transplantation (n=113) or kidney transplantation after another solid-organ transplantation (n=24) were excluded. All transplants were performed with negative complement-dependent cytotoxicity crossmatches. Baseline immunosuppression consisted primarily of tacrolimus, mycophenolic acid, and corticosteroids, with addition of basiliximab induction in patients who were higher risk. No desensitization therapies for HLA antibodies were used. During routine follow-up, we prospectively collected clinical data on donor/recipient sex and age, donor type (after brain/circulatory death, or living donor), transplantation rank (first versus repeat transplantation), recipient body mass index, cold ischemia time, DSA, number of HLA antigen mismatches, and eplet mismatch load for each HLA-antigen locus. Full data were required on these variables, therefore we assumed cases without missing values to be a completely random subset of the original data. To avoid selection bias, all post-transplant renal allograft biopsies performed ≤5 years after transplantation, until data extraction, were included. Clinical follow-up was updated until December 31, 2018. The study was approved by the Ethics Committee of the University Hospitals Leuven (S53364 and S61788).
HLA Genotyping and Eplet Mismatch Evaluation
Donor and recipient DNA samples were retrospectively genotyped at high resolution (four digits) for all HLA-A, -B, -C, -DRB1, -DRB3, -DRB4, -DRB5, -DQA1, -DQB1, DPA1, and DPB1 loci by next-generation sequencing (NGS). Half of the donors were genotyped using the MIA FORA NGS FLEX 11 HLA Typing Kit (Immucor, Norcross, GA) on the MiSeq sequencing instrument (Illumina, San Diego, CA); the other half and all recipients were genotyped at a high-resolution level (typing exons 2, 3, and 4 for HLA class 1; exons 2 and 3 for HLA class 2) using the HiSeq sequencing system (Illumina). The high-resolution HLA genotypes were uploaded to HLAMatchmaker (HLA-ABC version 02 and HLA-DRDQDP version 02.2; downloaded December 2018; www.epitopes.net).6 The total number of eplet mismatches (“eplet mismatch load”) and the total number of antibody-verified eplet mismatches were calculated for all HLA molecules together, as well as for each locus and each donor HLA molecule separately. HLA antigen mismatches were calculated for HLA-A, -B, -DR, and -DQ at the antigen split level. For HLA class 2 loci without serologic equivalents (HLA-DQA1, -DPA1, and -DPB1), first field (two-digit) molecular mismatches were calculated. Finally, we calculated second field high-resolution (four-digit) molecular mismatches for all HLA loci.
Detection of Circulating Anti-HLA Antibodies and Donor Specificity
Pre- and post-transplant anti-HLA antibodies were systematically monitored in one histocompatibility laboratory (Histocompatibility and Immunogenetics Laboratory, Red Cross—Flanders) at day 0, at 3 months after transplantation, yearly after transplantation, and at time of an indication biopsy. If this was not done in routine clinical practice, we retested biobanked sera for the presence of circulating DSA in the same laboratory. All sera were screened using a LIFECODES LifeScreen Deluxe (LMX) kit (Immucor) and, in case of positive screening, donor specificity was assessed using LIFECODES Single Antigen Bead (LSA) kits (Immucor). Antibodies against HLA-A, -B, -C, -DRB1, DRB345, -DQA1, -DQB1, -DPA1, and -DPB1 loci in the recipient sera were determined for DSA at the high-resolution level. A possible presence of circulating DSA was indicated by a background-corrected median fluorescence intensity value ≥500 but, for the final DSA assignment, we analyzed patients’ sera reactivity by taking account of both donor and recipient HLA-genotyping results.17
Histologic Scoring and Treatment of Rejection Episodes
One pathologist (E.L.) reviewed all biopsy specimens and scored the severity of the histologic lesions semiquantitatively according to the Banff categories, with a small deviation for C4d thresholds.18 Diagnosis of histologic phenotypes was based on Banff 2017 criteria.19 Borderline changes were diagnosed as foci of tubulitis (t>0) with minor interstitial inflammation (i1) or moderate-severe interstitial inflammation (i2 or i3) with mild (t1) tubulitis. ABMR was diagnosed by the presence of the three 2017 Banff criteria for either acute or chronic active ABMR, but did not take non-HLA antibodies or gene expression changes into account. Chronic TCMR was not considered separately, but included as borderline rejection/acute TCMR.
TCMR in protocol biopsies was treated with high-dose steroids, whereas TCMR or borderline changes in indication biopsies were treated with high-dose steroids and subsequent second-line therapy with anti-thymocyte globulin in steroid-resistant cases. Borderline changes in protocol biopsies were not treated. Only very few cases with ABMR received specific therapy, as presented previously,18 due to the retrospective rescoring of the biopsy specimens and by the lack of access to efficacious therapies.
Statistical Analyses
Characteristics of patients were described by means and SDs for continuous variables and frequencies and percentages for categoric variables. We used Kaplan–Meier curves to visualize the relationship of HLA antigen matching and eplet mismatch load with DSA formation and death-censored graft failure, and statistically evaluated these using log-rank tests and univariable/multivariable Cox models. With regard to DSA formation, patients were censored at the time of their last anti-HLA antibody measurement or, when earlier, at the experience of graft failure or at last clinical follow-up. In case of death with a functioning graft, we censored graft failure at the time of death. Patients who did not experience graft failure and had continuing clinical follow-up were administratively censored on December 31, 2018. We also considered the composite end point of graft failure or 50% eGFR decline from 3 months post-transplantation onwards. A 50% eGFR decline was defined as a persistent eGFR decline below the 50% threshold of a patient’s eGFR measured at 3 months post-transplantation, using a landmarked starting point at 3 months. To address confounding, we adjusted all multivariable models for donor and recipient sex, donor and recipient age, recipient race (white/nonwhite), recipient body mass index, donor type (living, brain death, or cardiac death), cold ischemia time (including the very short cold ischemia time in the living donor transplants), repeat transplantation, and pretransplant HLA antibodies (absence, non-DSA HLA antibodies, and DSA). We included quantitative variables linearly in the models. Determining thresholds for eplet mismatch load was done by plotting a locally estimated regression line through the Martingale residuals from the Cox models and checking its deviation of linearity. C-statistics and competing risk analyses (treating graft failure and death with a functioning graft as competing events), as proposed by Fine and Gray,20 were performed to check the predictive ability of HLA antigen and eplet mismatches for de novo DSA (dnDSA) occurrence.21 More specifically, in the Fine and Gray model, we estimated the effect of HLA antigen and eplet mismatches on the absolute risk for developing dnDSA. For the effect of total antibody-verified eplet mismatch load on dnDSA occurrence, and for the effect of antibody-verified DQ eplet mismatch load on dnDSA occurrence against DQ (all-cause censored), we also provided a receiver operating characteristic curve at 5 years post-transplantation as well as a plot of the time-dependent area under the curve, using the inverse probability of censoring weighting technique.22 We used logistic mixed models to evaluate the association of number of HLA antigen mismatches and the eplet mismatch load with post-transplant histology (ABMR present/absent, TCMR present/absent, no/any rejection). We included random intercepts and a linear fixed and random effect of post-transplant time while controlling for the same confounders as in the survival models. All tests were two-sided and P values <0.05 were considered statistically significant. We used SAS software (version 9.4; SAS Institute, Cary, NC).
Results
The donor/recipient HLA genetic mismatch was evaluated in 926 transplant pairs; for 74 donors no DNA samples were available (Supplemental Figure 1, Table 1). The median follow-up of this cohort was 7.45 years. A total of 43 (4.64%) patients developed dnDSA during follow-up, most of them (n=34; 79.1%) developed dnDSA against HLA class 2. In total, 38 different dnDSAs developed against class 2: ten against DRB1345, 25 against DQA1B1 (n=25), and 3 against DPA1B1 molecules. Five patients with pretransplant DSA developed dnDSA after transplantation, with different locus specificity and all later than 1 year post-transplant. A total of 134 grafts failed during follow-up, and 193 patients died with a functioning graft. A total of 142 (15.3%) patients did not have clinical follow-up in the last year of the study and were censored at the last clinical visit.
Table 1.
Main demographic, clinical characteristics, and follow-up of the study population (n=926)
| Cohort Characteristics | Total (n=926) |
|---|---|
| Data at time of transplantation | |
| Recipient demographics | |
| Female, n (%) | 372 (40.2) |
| Age (yr), mean±SD | 53.8±13.2 |
| Repeat transplantation, n (%) | 132 (14.3) |
| Weight (kg), mean±SD | 72.8±14.8 |
| Body mass index (kg/m2), mean±SD | 25.4±4.51 |
| White race, n (%) | 911 (98.4) |
| Pretransplant diabetes mellitus, n (%) | 163 (17.6) |
| Donor demographics | |
| Female, n (%) | 431 (46.5) |
| Age (yr), mean±SD | 47.9±14.9 |
| Living donor, n (%) | 42 (4.54) |
| Donation after brain death, n (%) | 732 (79.1) |
| Donation after cardiac death, n (%) | 152 (16.4) |
| Cold ischemia time (h), mean±SD | 14.4±5.50 |
| Transplant characteristics | |
| HLA-A/B/DR antigen mismatches (0–6), mean±SD | 2.70±1.30 |
| HLA-A/B/DR/DQ antigen mismatches (0–8), mean±SD | 3.40±1.61 |
| A antigen, mean±SD | 0.97±0.70 |
| B antigen, mean±SD | 1.02±0.64 |
| DR antigen, mean±SD | 0.71±0.56 |
| DQ antigen, mean±SD | 0.70±0.61 |
| Total eplet mismatch load, mean±SD | 36.7±18.0 |
| Antibody-verified eplet mismatch load, mean±SD | 16.0±8.7 |
| Pretransplant HLA antibodies, n (%) | 225 (24.3) |
| HLA class 1, n (%) | 76/225 (33.8) |
| HLA class 2, n (%) | 52/225 (23.1) |
| HLA class 1 and 2, n (%) | 97/225 (43.1) |
| Pretransplant DSAs, n (%) | 94 (10.2) |
| HLA class 1, n (%) | 33/94 (35.1) |
| HLA class 2, n (%) | 40/94 (42.6) |
| HLA class 1 and 2, n (%) | 21/94 (22.3) |
| Immunosuppression regimen of TAC-MPA-CS, n (%) | 808 (87.3) |
| Induction therapy, n (%) | 383 (41.4) |
| Basiliximab, n (%) | 331 (35.8) |
| Thymoglobulin, n (%) | 19 (2.1) |
| Other, n (%) | 33 (3.6) |
| Post-transplant data | |
| Overall graft survival, %a | |
| At 1 yr | 93.8 |
| At 2 yr | 91.1 |
| At 5 yr | 81.0 |
| Death-censored graft survival, %b | |
| At 1 yr | 95.6 |
| At 2 yr | 94.5 |
| At 5 yr | 89.0 |
| dnDSAs, n (%) | 43 (4.64) |
| HLA class 1, n (%) | 9/43 (20.9) |
| HLA class 2, n (%) | 32/43 (74.4) |
| HLA class 1 and 2, n (%) | 2/43 (4.7) |
TAC, tacrolimus; MPA, mycophenolic acid; CS, corticosteroids.
Overall graft survival: composite of graft failure and recipient death.
Death-censored graft survival: graft failure censored at time of recipient death with a functioning graft.
The total and antibody-verified eplet mismatch loads were 36.7±18.0 and 16.0±8.7, respectively, distributed over all HLA loci (Supplemental Table 1). Most eplet mismatches were found in HLA class 2 molecules, and more specifically in the DQ molecule. Pearson correlations between the number of total eplet mismatches and number of HLA-A/B/DR (0–6) and HLA-A/B/DR/DQ (0–8) antigen mismatches amounted to 0.63 (P<0.001) and 0.72 (P<0.001), respectively (Supplemental Figure 2). The number of antigen mismatches at the individual loci correlated highly with the number of total eplet mismatches at each HLA molecule (Supplemental Figure 3). However, the eplet mismatch approach provides more details than the actual HLA mismatch by revealing a very large heterogeneity in the eplet mismatch load that is not reflected by the antigen mismatch calculations. Patients with no DR antigen mismatches had 0.23±0.43 antigen mismatches and 2.83±4.93 eplet mismatches in the DQ molecule. In the cohort of 226 patients with zero DQB1 (four-digit)/DQA1 (two-digit) mismatches, 85.5% were completely matched for DR on the antigen level and none of these patients had two DRB1 mismatches.
The mean number of total eplet mismatches in patients with dnDSA was not significantly higher than in patients without dnDSA (41.2±15.6 versus 36.5±18.1; P=0.09), but the mean number of antibody-verified eplet mismatches did differ between groups (18.8±7.5 versus 15.8±8.7, respectively; P=0.03). Kaplan–Meier curves for dnDSA occurrence according to total number of HLA mismatches, number of HLA mismatches in the DR and DQ locus, and total and antibody-verified eplet mismatch load for DR and DQ molecules are shown in Supplemental Figure 4. Following the Cox models (Table 2), the total number of HLA antigen mismatches was associated univariably and multivariably with the occurrence of DSA.
Table 2.
Univariable and multivariable hazard ratios for de novo occurrence of DSA according to HLA antigen mismatches and eplet mismatch load of the different HLA loci (n=926)
| HLA Mismatches | Patients at Risk | Events | Univariable HR (95% CI) | P Value | Multivariable HR (95% CI)a | P Value |
|---|---|---|---|---|---|---|
| Antigen (split level) | ||||||
| A/B/DR (0–6) | 926 | 43 | 1.31 (1.03 to 1.68) | 0.03 | 1.34 (1.03 to 1.75) | 0.03 |
| A/B/DR/DQ (0–8) | 926 | 43 | 1.32 (1.08 to 1.60) | 0.006 | 1.38 (1.11 to 1.72) | 0.004 |
| A antigen | ||||||
| 0 mismatch | 243 | 11 | 1 | — | 1 | — |
| 1 mismatch | 468 | 20 | 0.99 (0.48 to 2.07) | 0.98 | 1.01 (0.479 to 2.128) | 0.98 |
| 2 mismatches | 215 | 12 | 1.44 (0.63 to 3.26) | 0.39 | 1.43 (0.62 to 3.30) | 0.40 |
| B antigen | ||||||
| 0 mismatch | 180 | 7 | 1 | — | 1 | — |
| 1 mismatch | 545 | 26 | 1.28 (0.55 to 2.94) | 0.57 | 1.26 (0.54 to 2.95) | 0.59 |
| 2 mismatches | 201 | 10 | 1.60 (0.61 to 4.20) | 0.34 | 1.53 (0.56 to 4.15) | 0.41 |
| DR antigen | ||||||
| 0 mismatch | 319 | 8 | 1 | — | 1 | — |
| 1 mismatch | 560 | 31 | 2.50 (1.15 to 5.44) | 0.02 | 2.74 (1.22 to 6.15) | 0.02 |
| 2 mismatches | 47 | 4 | 5.28 (1.58 to 17.66) | 0.007 | 8.76 (2.24 to 34.28) | 0.002 |
| DQ antigen | ||||||
| 0 mismatch | 358 | 11 | 1 | — | 1 | — |
| 1 mismatch | 491 | 25 | 1.68 (0.83 to 3.42) | 0.15 | 1.59 (0.77 to 3.27) | 0.21 |
| 2 mismatches | 77 | 7 | 3.64 (1.41 to 9.40) | 0.008 | 4.68 (1.75 to 12.48) | 0.002 |
| Eplet | ||||||
| Total eplets | 926 | 43 | 1.02 (1.00 to 1.04) | 0.03 | 1.02 (1.00 to 1.04) | 0.02 |
| Antibody-verified eplets | 926 | 43 | 1.05 (1.02 to 1.08) | 0.004 | 1.06 (1.02 to 1.10) | 0.002 |
| A molecule | 926 | 43 | 1.08 (0.99 to 1.19) | 0.08 | 1.08 (0.99 to 1.19) | 0.09 |
| B molecule | 926 | 43 | 0.94 (0.81 to 1.10) | 0.45 | 0.94 (0.80 to 1.10) | 0.41 |
| C molecule | 926 | 43 | 0.98 (0.83 to 1.15) | 0.77 | 0.98 (0.83 to 1.16) | 0.82 |
| DR molecule | 926 | 43 | 1.04 (0.96 to 1.13) | 0.30 | 1.05 (0.96 to 1.14) | 0.27 |
| DQ molecule | 926 | 43 | 1.14 (1.07 to 1.21) | <0.001 | 1.14 (1.07 to 1.22) | <0.001 |
| DP molecule | 926 | 43 | 0.97 (0.81 to 1.18) | 0.79 | 1.00 (0.82 to 1.21) | 0.98 |
HR, hazard ratio.
Multivariable models were corrected for donor and recipient sex, donor and recipient age, recipient race, recipient body mass index, donor type, cold ischemia time, repeat transplantation, and pretransplant HLA antibodies (absence, non-DSA HLA antibodies, and DSA).
Total and antibody-verified eplet mismatch loads were independently associated with overall dnDSA occurrence (Table 2). Nonetheless, at the individual HLA molecule level, only the antibody-verified eplet mismatch load on the DQ molecule increased the overall rate of dnDSA occurrence (hazard ratio [HR], 1.14; 95% CI, 1.07 to 1.22; P<0.001), which could explain the overall eplet effects observed before. There was no association between the occurrence of dnDSA and antigen/eplet mismatches in any of the HLA class 1 loci.
In the Cox models for HLA molecule–specific dnDSA occurrence (Supplemental Table 2), only for the DQ molecule were sufficient events observed to perform significance testing in the multivariable models. We concluded that the antibody-verified eplet mismatch load on the DQ molecule increased the hazard rate of HLA-DQ dnDSA occurrence (HR, 1.30; 95% CI, 1.18 to 1.44; P<0.001). Univariably, there was also a significant association of the antibody-verified eplet mismatch load on the A molecule and on the DR molecule with dnDSA occurrence on their respective loci (HR, 1.33; 95% CI, 1.04 to 1.71; P=0.03 and HR, 1.20; 95% CI, 1.04 to 1.39; P=0.02, respectively). Events were too scarce to evaluate the association between the occurrence of dnDSA and the number of antigen mismatches in any of the HLA loci.
In the multivariable model, including the DQ antibody-verified eplet mismatch load led to the best estimate of discriminative performance for overall dnDSA occurrence (C-statistic, 0.74; 95% CI, 0.69 to 0.85), compared with including HLA-A/B/DR (C-statistic, 0.71; 95% CI, 0.67 to 0.83) or HLA-A/B/DR/DQ antigen-mismatch load (C-statistic, 0.71; 95% CI, 0.66 to 0.84) (Table 3). Even adding the HLA-A/B/DR/DQ antigen mismatches to the number of antibody-verified DQ eplet mismatches did not improve the risk prediction for overall DSA formation (C-statistic, 0.74; 95% CI, 0.70 to 0.86). In the multivariable model for prediction of HLA molecule–specific dnDSA occurrence, the DQ antibody-verified eplet mismatch load again led to high discriminative performance for HLA-DQ dnDSA occurrence (C-statistic, 0.89; 95% CI, 0.87 to 0.96).
Table 3.
Harrell C-statistics, evaluating the discriminative ability of HLA mismatch calculations for dnDSA occurrence (n=926)
| HLA Mismatch Models | dnDSA Occurrence | |
|---|---|---|
| Univariable C-Statistic (95% CI)a | Multivariable C-Statistic (95% CI)a,b | |
| Overall dnDSA occurrence | ||
| HLA-A/B/DR antigens | 0.60 (0.51 to 0.69) | 0.71 (0.67 to 0.83) |
| HLA-A/B/DR/DQ antigens | 0.61 (0.52 to 0.70) | 0.71 (0.66 to 0.84) |
| Total antibody-verified eplets | 0.61 (0.53 to 0.71) | 0.72 (0.68 to 0.84) |
| DQ antibody-verified eplets | 0.65 (0.55 to 0.76) | 0.74 (0.69 to 0.85) |
| HLA-A/B/DR/DQ antigens and DQ antibody-verified eplets | 0.66 (0.56 to 0.77) | 0.74 (0.70 to 0.86) |
| HLA molecule-specific dnDSA occurrence | ||
| DQ antibody-verified eplets | 0.81 (0.73 to 0.89) | 0.89 (0.87 to 0.96) |
95% CIs are calculated based on 1000 bootstrapped samples.
Multivariable models were corrected for donor and recipient sex, donor and recipient age, recipient race, recipient body mass index, donor type, cold ischemia time, repeat transplantation, and pretransplant HLA antibodies (absence, non-DSA HLA antibodies, and DSA).
Multivariable competing risk analyses confirmed there was an effect of DQ antibody-verified eplet mismatch load on the cumulative incidence for dnDSA occurrence on the DQ molecule (subdistribution hazard ratio [SHR], 1.31; 95% CI, 1.19 to 1.43; P<0.001), an effect of total antibody-verified eplet mismatches on the cumulative incidence for overall dnDSA occurrence (SHR, 1.06; 95% CI, 1.02 to 1.09; P=0.003), and an effect of HLA-A/B/DR/DQ antigen-mismatch load on the cumulative incidence for overall dnDSA occurrence (SHR, 1.36; 95% CI, 1.05 to 1.77; P=0.02). The number of HLA-A/B/DR antigen mismatches was not significantly related to the cumulative incidence of overall dnDSA occurrence (SHR, 1.33; 95% CI, 0.99 to 1.78; P=0.06).
To determine a threshold in the DQ eplet mismatch load for risk stratification, we checked the linearity of the effect in the multivariable Cox model (Figure 1). The locally estimated regression lines, fitted through the Martingale residuals, showed no deviation from a straight line, indicating a linear effect of DQ eplet mismatches on the hazard rate of HLA-DQ dnDSA formation. Given this linearity between the DQ eplet mismatch load and hazard of HLA-DQ dnDSA formation, our analysis does not suggest any specific threshold for differentiating low and high risk of DSA formation. With regard to previously suggested single molecular thresholds for DRB1345 (<7) and DQA1B1 (<9) mismatched eplets,12 17.1% (6/35) of DSAs occurred below the threshold for the corresponding DRB1345 or DQA1B1 molecules. When we grouped patients with dnDSA according to both thresholds together, dnDSA occurred in only 9.38% (3/32) patients who were “low risk” (DRB1345<7 and DQA1B1<9 mismatched eplets). In the total population, 28.5% (264/926) of patients had an eplet mismatch load above the proposed single molecule threshold of ≥15 HLA-DQA1B1 eplet mismatches.12 Only 18/264 (6.82%) of these patients who were “high risk” developed dnDSA. The 5-year cumulative incidence estimates reached 6.79% and 3.39%, respectively. There was no safe eplet mismatch threshold at which no DSA occurs: two cases (patients #4 and #11) illustrated that just one eplet mismatch was sufficient to develop dnDSA against the mismatched eplet (Supplemental Table 3). On the other hand, the absolute risk of dnDSA formation remained low, even in the higher-risk group.
Figure 1.
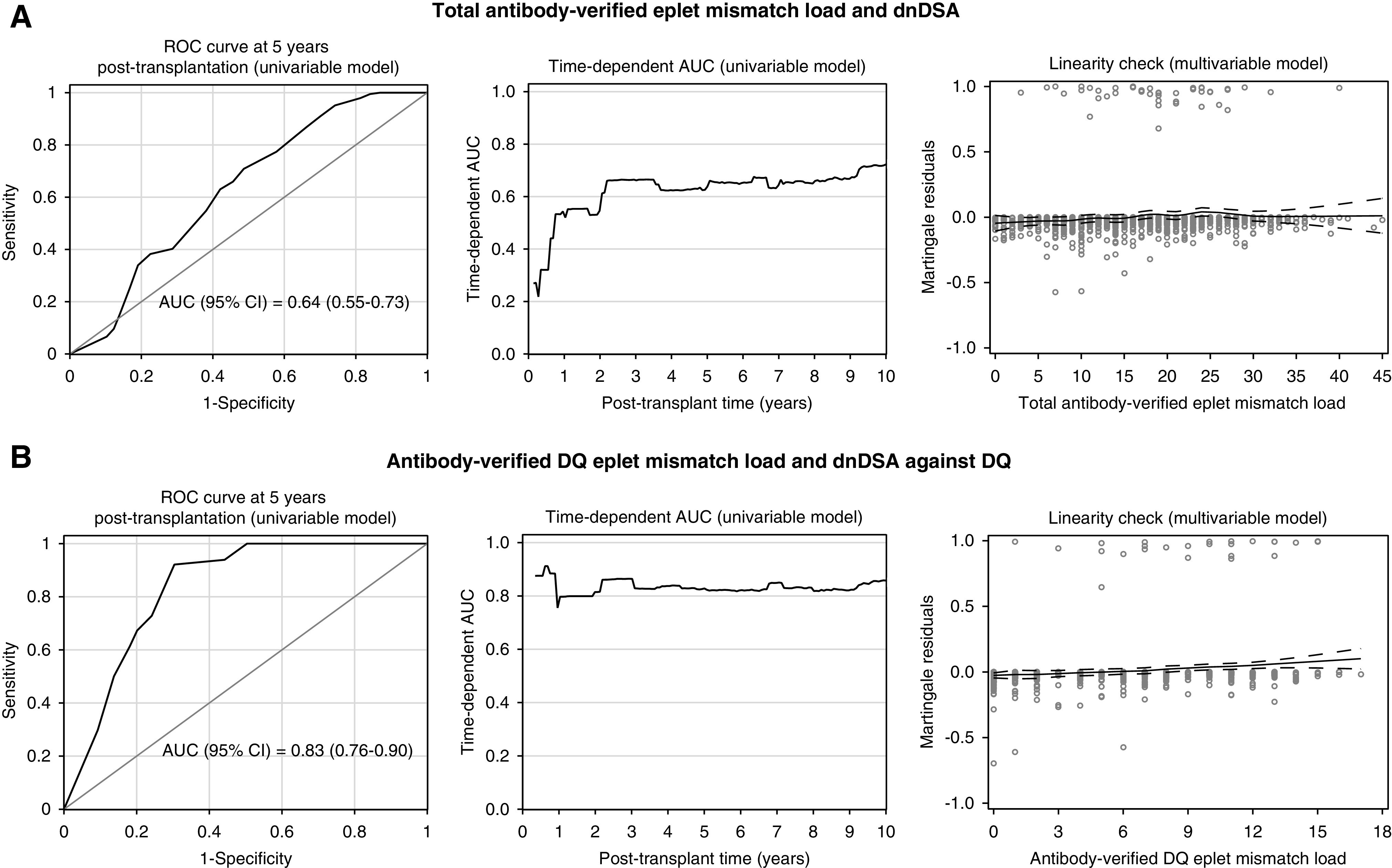
Antibody-verified eplet mismatch load associated with the hazard rate of dnDSA formation, with a linear effect (dnDSA; n=926). The receiver operator characteristic (ROC) curve at 5 years after transplantation is depicted (left panel), together with the evolution of the time-dependent area under the curve (AUC) up to 10 years after transplantation (middle panel), and a linearity check of the association between eplet mismatch load and the risk of dnDSA occurrence (right panel). (A) The association between the total antibody-verified eplet mismatch load and overall dnDSA. (B) The association between the antibody-verified DQ eplet mismatch load and dnDSA against DQ. ROC curves and AUCs were based on the univariable Cox models; the Martingale residual plots were based on the multivariable Cox models. Because AUCs of the time-dependent ROC curves change at every event time, they differ from the overall C-statistics in Table 3. The latter could be interpreted as the “integrated AUC” over all event time points. In the Martingale residual plots, fitted Loess lines indicate which functional form is appropriate for modeling the covariates. There is no substantial deviation from a straight line in both plots, thereby showing no evidence for including the covariates other than linearly in the Cox model. Hence our data suggest no specific hazard threshold for total antibody-verified eplet mismatch load or for antibody-verified DQ eplet mismatch load.
Because eplet mismatches in the DQ molecule had the predictive potential for DSA formation, we investigated which molecular mismatches in the two different DQA1 and DQB1 loci were associated with HLA class 2 DSA formation (Supplemental Tables 4 and 5). Besides one (HR, 5.07; 95% CI, 1.19 to 21.63; P=0.03) and two (HR, 8.27; 95% CI, 1.69 to 40.46; P=0.01) four-digit DQB1 molecular mismatches, one and two two-digit mismatches for DQA1 were also independently associated with class 2 dnDSA occurrence (HR, 4.62; 95% CI, 2.09 to 10.20; P<0.001 and HR, 9.73, 95% CI 1.12 to 84.65; P=0.04, respectively). This suggests the DQA1 chain has an important role in the immunogenicity of the DQ molecule. In our cohort, of the patients matched for four-digit DQB1, and two-digit DQA1 loci (n=226) 88.5% (200/226) had zeroDRB1 antigen mismatches, whereas only 11.5% (26/226) had one DRB1 mismatched antigen.
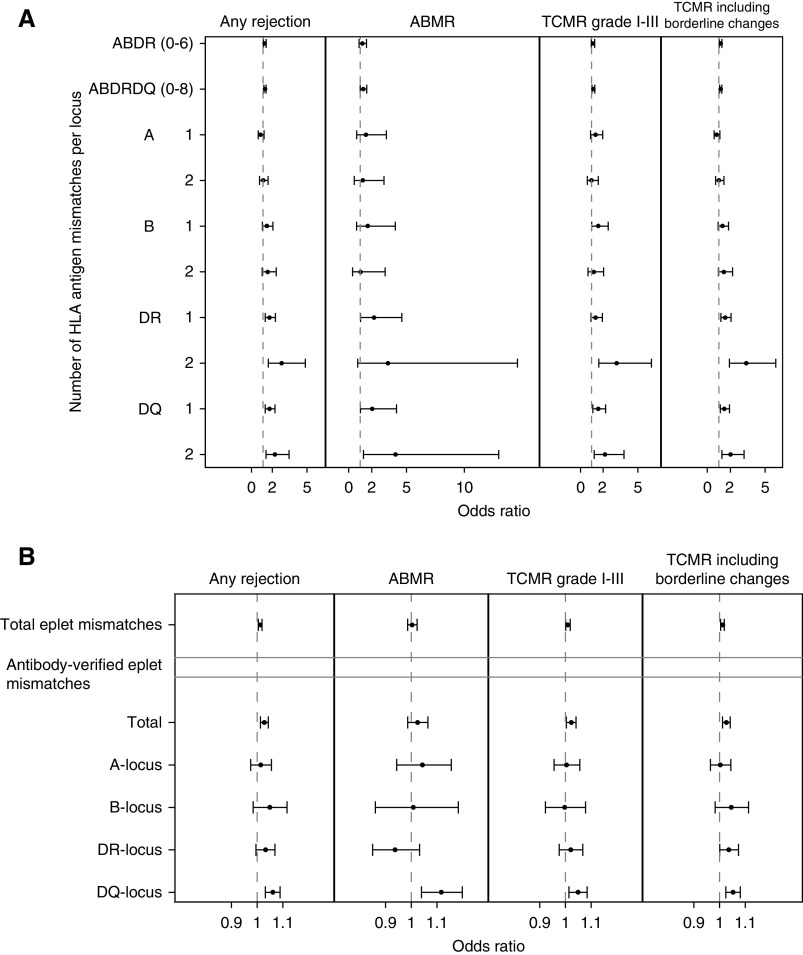
In total, 3372 post-transplant biopsies were performed in 883 transplantations (Supplemental Figure 1): 751 indication biopsies and 2621 protocol biopsies at 3 months and 1, 2, 3, 4, and 5 years. A total of 43/926 cases (4.64%) without biopsy follow-up were excluded from the histologic analyses. Supplemental Figure 5 and Supplemental Table 6 show the distribution of the different rejection types across all biopsies; 821 of the 883 patients included (93.0%) had more than one biopsy performed during follow-up. The risk for rejection (any type) associated with DR (one mismatch, odds ratio [OR], 1.57; 95% CI, 1.19 to 2.07; P=0.002; two mismatches, OR, 2.60; 95% CI, 1.46 to 4.66; P=0.001) and with DQ antigen mismatches (one mismatch, OR, 1.55; 95% CI, 1.19 to 2.03; P=0.001; two mismatches, OR, 2.02; 95% CI, 1.26 to 3.25; P=0.004). The risk for TCMR was associated with the number of both DR and DQ antigen mismatches. Also, the risk for ABMR was affected by the number of antigen mismatches in DR and DQ. There was no association between the risk for rejection and other HLA antigens than DR and DQ, except for that between one antigen mismatch on the B locus and the occurrence of TCMR (Figure 2A, Supplemental Table 7).
Figure 2.
HLA antigen mismatches and antibody-verified eplet mismatches, especially on the DQ molecule, associated with the risk for rejection, both ABMR and TCMR. Effects of the number of HLA (A) antigen and (B) antibody-verified eplet mismatches per locus on kidney allograft histology (n=3372 biopsies). The estimates and confidence bounds are based on separate logistic mixed models with random intercepts, a linear fixed and random effect of time, corrected for donor/recipient sex, donor/recipient age, recipient race, recipient body mass index, donor type, cold ischemia time, repeat transplantation, and pretransplant HLA antibodies/DSA. All post-transplant biopsy specimens (n=3372) were used for these analyses.
Log-rank tests showed that rejection-free survival associated with HLA-DR and HLA-DQ antigen mismatches, as well as with total and antibody-verified eplet mismatches, and also for DR and DQ separately (Supplemental Figures 6 and 7). In multivariable logistic mixed model analysis, adjusted for baseline confounders (Supplemental Table 7), antibody-verified eplet mismatch load was associated with rejection (OR, 1.03; 95% CI, 1.01 to 1.04; P<0.001), but this appeared to be determined primarily by the eplet mismatch load on the DQ molecule (OR, 1.06; 95% CI, 1.03 to 1.09; P<0.001). This was the case for any type of rejection (ABMR, TCMR, and TCMR including borderline changes). HLA-DR eplet mismatch load also associated with TCMR including borderline changes (OR, 1.05; 95% CI, 1.02 to 1.08; P<0.001). Each extra antibody-verified eplet mismatch on the DQ molecule corresponded to an increase in the subject-specific odds for ABMR (OR, 1.12; 95% CI, 1.04 to 1.20; P=0.002). Mismatched eplets on the other HLA molecules were not associated with the risk of any type of rejection (Figure 2B, Supplemental Table 7).
Finally, we evaluated the effect of antigen and eplet mismatches on graft failure. In both univariable and multivariable analyses, the number of HLA-A/B/DR and HLA-A/B/DR/DQ antigen mismatches was associated with increased rate of graft failure, with HRs of 1.25 (95% CI, 1.08 to 1.45; P=0.003) and 1.21 (95% CI, 1.07 to 1.36; P=0.002), respectively (Supplemental Figures 7 and 8, Supplemental Table 8, Table 4). With regard to the eplet mismatch load, an independent effect was found for antibody-verified eplet mismatches on the DQ (HR, 1.05; 95% CI, 1.01 to 1.09; P=0.01) and B (HR, 1.09; 95% CI, 1.00 to 1.18; P=0.04) molecules. Eplet mismatches in other loci were not consistently associated with graft failure (Supplemental Figure 8, Table 4). C-statistics of the association between eplet mismatch load and death-censored graft survival illustrated that the discriminative performance for graft failure was low, even for HLA-DQ eplet mismatches (Supplemental Table 9). We noted similar results in the univariable and multivariable analyses for the effect of HLA-A/B/DR/DQ antigen mismatches and antibody-verified DQ eplet mismatches on the composite end point of graft failure and graft functional decrease (Supplemental Table 10).
Table 4.
Univariable and multivariable hazard ratios for death-censored graft survival according to HLA antigen mismatches and eplet mismatches (n=926)
| HLA Mismatches | Patients at Risk | Events | Univariable HR (95% CI) | P Value | Multivariable HR (95% CI)a | P Value |
|---|---|---|---|---|---|---|
| Antigen (split level) | ||||||
| A/B/DR (0–6) | 926 | 134 | 1.24 (1.08 to 1.43) | 0.002 | 1.25 (1.08 to 1.45) | 0.003 |
| A/B/DR/DQ (0–8) | 926 | 134 | 1.19 (1.07 to 1.33) | 0.002 | 1.21 (1.07 to 1.36) | 0.002 |
| A antigen | ||||||
| 0 mismatch | 243 | 32 | 1 | — | 1 | — |
| 1 mismatch | 468 | 64 | 1.03 (0.68 to 1.58) | 0.88 | 1.08 (0.70 to 1.65) | 0.73 |
| 2 mismatches | 215 | 38 | 1.42 (0.89 to 2.27) | 0.15 | 1.50 (0.93 to 2.42) | 0.10 |
| B antigen | ||||||
| 0 mismatch | 180 | 19 | 1 | — | 1 | — |
| 1 mismatch | 545 | 75 | 1.31 (0.79 to 2.17) | 0.29 | 1.27 (0.76 to 2.10) | 0.36 |
| 2 mismatches | 201 | 40 | 2.08 (1.20 to 3.59) | 0.009 | 1.88 (1.07 to 3.31) | 0.03 |
| DRB1 antigen | ||||||
| 0 mismatch | 319 | 40 | 1 | — | 1 | — |
| 1 mismatch | 560 | 84 | 1.25 (0.86 to 1.83) | 0.24 | 1.31 (0.90 to 1.93) | 0.16 |
| 2 mismatches | 47 | 10 | 2.27 (1.13 to 4.54) | 0.02 | 2.12 (1.00 to 4.50) | 0.051 |
| DQ antigen | ||||||
| 0 mismatch | 358 | 43 | 1 | — | 1 | — |
| 1 mismatch | 491 | 78 | 1.37 (0.94 to 1.98) | 0.10 | 1.40 (0.96 to 2.04) | 0.08 |
| 2 mismatches | 77 | 13 | 1.54 (0.83 to 2.86) | 0.17 | 1.49 (0.79 to 2.80) | 0.22 |
| Eplet | ||||||
| Total eplets | 926 | 134 | 1.008 (1.00 to 1.02) | 0.08 | 1.01 (1.00 to 1.02) | 0.072 |
| Antibody-verified eplets | 926 | 134 | 1.02 (1.00 to 1.04) | 0.03 | 1.02 (1.00 to 1.04) | 0.02 |
| A molecule | 926 | 134 | 1.03 (0.98 to 1.09) | 0.23 | 1.05 (0.99 to 1.10) | 0.11 |
| B molecule | 926 | 134 | 1.11 (1.02 to 1.20) | 0.01 | 1.09 (1.00 to 1.18) | 0.04 |
| C molecule | 926 | 134 | 1.00 (0.92 to 1.10) | 0.93 | 0.98 (0.89 to 1.07) | 0.60 |
| DRB molecule | 926 | 134 | 1.00 (0.95 to 1.05) | 0.94 | 1.01 (0.96 to 1.06) | 0.85 |
| DQ molecule | 926 | 134 | 1.04 (1.01 to 1.08) | 0.03 | 1.05 (1.01 to 1.09) | 0.01 |
| DP molecule | 926 | 134 | 1.01 (0.91 to 1.12) | 0.89 | 1.00 (0.90 to 1.12) | 0.97 |
HR, hazard ratio.
Multivariable models were corrected for donor and recipient sex, donor and recipient age, recipient race, recipient body mass index, donor type, cold ischemia time, repeat transplantation, and pretransplant HLA antibodies (absence, non-DSA HLA antibodies, and DSA).
Discussion
Our study shows that DQ antibody-verified eplet mismatch load, calculated using high-resolution genotyping, is associated with an increased risk of dnDSA formation, kidney transplant rejection, decline of graft function, and graft failure. Eplet mismatches in other HLA molecules did not contribute to the risk of post-transplant rejection and the rate of dnDSA formation. Although DQ antibody-verified eplet mismatch load predicted dnDSA formation better than the number of antigen mismatches, the absolute risk for development of dnDSA was low even in patients with high DQ antibody-verified mismatch load. Importantly, there was no threshold below which the risk of dnDSA occurrence was absent. These findings are relevant for organ allocation schemes and post-transplant risk stratification.
In most allocation schemes, HLA match between donors and recipients is determined on the basis of HLA-A, -B, and -DR antigens only—not on the DQ antigens, which are deemed less relevant given the strong DR-DQ linkage disequilibrium. However, a recent study showed that 11% of the patients matched for both DR antigens were mismatched for DQ,23 with correlation to dnDSA formation and impaired graft outcome.9–12,17,24 We found that DQ eplet load has the best discriminative performance for dnDSA formation, regardless of other HLA molecule mismatches. We further demonstrated that mismatches at either DQB1 or DQA1 are independently associated with dnDSA formation. Therefore, our results clearly indicate that adding molecular DQA1/DQB1 matching to the current antigen HLA-A/B/DR algorithm will decrease rejection rates and dnDSA formation after transplantation, perhaps improving long-term graft outcomes. This can be best achieved by having two-digit and four-digit DQA1/DQB1 typing, respectively. This is especially important for young recipients, in whom repeat transplantations are anticipated and so the goal is to minimize HLA sensitization. Large-scale simulation studies are warranted to assess the potential effect of such change on wait-list times and allocation equity.
We observed that the association between mismatched eplet load and outcome was explained entirely by antibody-verified mismatched eplets. The HLAMatchmaker algorithm assumes similar antigenic or immunogenic properties to all mismatched eplets. However, it seems that some mismatched eplets carry higher immunogenicity: in some patients, a single eplet mismatch was sufficient to induce dnDSA formation. With the current knowledge, we cannot exclude the possibility that these single nonself eplet mismatches are shared between different HLA or even non-HLA molecules. We therefore question the practice of simply summing the total number of eplet mismatches to guide organ allocation.
Our study has also implications for patient risk stratification. Despite the acknowledged goal of personalized treatment, physicians remain somewhat inflexible when planning the induction and maintenance of immunosuppression,25 often following center-specific—rather than patient-informed—protocols.26,27 Being able to stratify organ transplant recipients into risk brackets will greatly assist the choice of immunosuppression approach for individual patients. Patients who are high risk could benefit from close monitoring and heavy immunosuppression, and patients who are low risk could benefit from less intensive therapy.28–30 Two recent studies suggested that HLA-DR/DQ eplet mismatch analysis can risk stratify transplant patients on the basis of eplet mismatch thresholds, allowing more tailored immunosuppression.11,12
Our finding that the association between the eplet mismatch load and the rate of dnDSA formation is linear, however, questions the universal validity of proposed thresholds for patient risk stratification.9,12 According to such thresholds, three patients who developed dnDSA in our cohort—despite standard immunosuppression—would have been considered at low immunologic risk. On the other hand, the absolute risk of dnDSA formation in our cohort was low (4.64%), although 71.5% of patients had an eplet mismatch load above the proposed single molecular thresholds for DRB1345 (≥7) and DQA1B1 (≥9) molecules. We therefore analyzed our data to determine whether a different threshold can be identified, but found no clinically safe threshold for alloimmunization. Even a single mismatched eplet can lead to DSA formation. Thus, the observed association between an increased eplet load and dnDSA might not result from the numeric quantity of mismatches, but rather from the increased likelihood that one (or more) of those mismatches is immunogenic. For risk stratification in clinical trials, thresholds can be proposed based on the study design and study end points. In clinical practice, however, there is always a risk for dnDSA formation. This supports the need for additional studies to define and assign immunogenicity scores to different donor/recipient molecular mismatches, beyond eplet mismatch load, for optimal risk stratification. Whereas eplet mismatch analysis is an attempt to define the immunogenic epitopes, eplets represent only a small part of the complete epitope and do not reflect the surrounding structure that could influence alloimmune reactivity.24
Our study has some limitations. This is a single‐center study in a white population with general and continued access to immunosuppression, representing a population at low immunologic risk, as exemplified by the low absolute risk of DSA formation, especially for HLA class 1. Also, inherent to the retrospective design of our study, we did not have reliable data on nonadherence, we have no insight in the kinetics of the median fluorescence intensity values of the DSA, and we cannot fully exclude selection and indication bias. Our results might not be generalizable to a population of different ethnicity or with less access to immunosuppressive drugs. Because our center does not systematically reduce immunosuppression, we could not assess the potential contribution of immunosuppressive drug minimization on the risk for DSA formation, rejection, and graft failure. Similarly, we systematically treated TCMR, whereas virtually none of the patients with ABMR were treated.18 Further studies are warranted in other populations and other clinical settings. In the survival analyses, we assumed missing completely at random for the 74 patients without epitope data (n=74),31 therefore adhering to complete-case analysis without using imputation. Because the missingness in the eplet data were due to the nonavailability of DNA samples for some donor-recipient pairs and the data collection on these DNA samples was done retrospectively, we assumed that the eplet missingness was not informative to any of the variables/models used in our paper. Also in the longitudinal (mixed) models for rejection, 43 patients without biopsy follow-up were excluded, assuming missing completely at random. For the remaining biopsy analyses, this assumption was relaxed to missing at random. This study relies on the current algorithm for calculating the eplet mismatches using the incomplete list of antibody-verified eplets and eplets that are shared across different HLA molecules in the HLA Epitope Registry. Further refinement of this algorithm or updates in the Epitope Registry will demand re-evaluation of our findings. Finally, the effect of non-HLA genetic donor/recipient mismatch needs to be considered.14
We conclude that a higher HLA-DQ antibody-verified eplet mismatch load, assessed using high-resolution HLA genotyping, confers an increased risk for the development of dnDSA, rejection episodes, decline in graft function, and graft failure after kidney transplantation. Mismatches in other loci seem to have a lesser effect. Although there is no threshold below which the risk of DSA formation is absent, molecular matching for the DQA1 and DQB1 alleles can be used to minimize DSA formation, improve graft outcome after transplantation, and guide personalized post-transplant immunosuppression.
Disclosures
A.R. Tambur reports advisory board membership to Astellas, Sanofi, and Viela Bio. All remaining authors have nothing to disclose.
Funding
This work was supported by Fonds Wetenschappelijk Onderzoek (FWO; Research Foundation – Flanders) and the Agentschap Innoveren en Ondernemen (VLAIO; Agency for Innovation and Entrepreneurship) by the TBM project grant IWT.150199. M. Naesens and B. Sprangers are senior clinical investigators of FWO, grants 1844019N and 1842919N, respectively. E. Van Loon and J. Callemeyn hold FWO fellowship grants, 1143919N and 1196119N, respectively. M. Naesens is also funded by KU Leaven C3 internal grant C32/17/049.
Supplementary Material
Acknowledgments
The authors thank the centers of the Leuven Collaborative Group for Kidney Transplantation, the clinicians and surgeons, the nursing staff, and the patients. Dr. Jasper Callemeyn, Dr. Maarten Coemans, Dr. Dirk Kuypers, Dr. Maarten Naesens, Dr. Aleksandar Senev, and Dr. Elisabet Van Loon were involved in clinical data collection and data quality control; Dr. Frans H.J. Claas, Dr. Maarten Coemans, Dr. Marie-Paule Emonds, Dr. Maarten Naesens, Dr. Aleksandar Senev, and Dr. Anat R. Tambur interpreted the results; Dr. Maarten Coemans did the statistical analyses and created the figures and tables, with input from Dr. Maarten Naesens, Dr. Aleksandar Senev, and Dr. Geert Verbeke; Dr. Maarten Coemans, Dr. Marie-Paule Emonds, Dr. Maarten Naesens, and Dr. Aleksandar Senev designed the study and the analysis plan; Dr. Maarten Coemans, Dr. Maarten Naesens, and Dr. Aleksandar Senev wrote the article; Dr. Evelyne Lerut performed all biopsy readings; Dr. Aleksandar Senev produced and analyzed the HLA genotyping and antibody data with support from Dr. Veerle Compernolle, Dr. Liesbeth Daniëls, Dr. Marleen Vanden Driessche, Dr. Marie-Paule Emonds, Dr. Johan Kerkhofs, and Dr. Vicky Van Sandt; and all coauthors revised and approved the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “Molecular Mismatch—the Renaissance of HLA in Kidney Transplantation,” on pages 1922–1925.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020010019/-/DCSupplemental.
Supplemental Figure 1. Flow chart inclusion criteria.
Supplemental Figure 2. Violin plots of total eplet mismatch load per number of HLA antigen mismatches for (A) locus A, B, and DR (0–6) and for (B) locus A, B, DR, and DQ (0–8) (n=926).
Supplemental Figure 3. Violin plots of total eplet mismatch load per number of HLA antigen mismatches for HLA-A, B, DR, and DQ (n=926).
Supplemental Figure 4. Cumulative incidence of overall dnDSA occurrence.
Supplemental Figure 5. Prevalence of ABMR, TCMR and mixed rejection in (A) protocol and (B) indication biopsies.
Supplemental Figure 6. Kaplan–Meier survival plots of rejection-free graft survival (n=883)
Supplemental Figure 7. Low, intermediate and high eplet mismatch risk groups versus dnDSA, rejection and graft failure.
Supplemental Figure 8. Kaplan–Meier survival plots of death-censored graft survival (n=926).
Supplemental Table 1. Number of eplet mismatches in overall cohort, per class and per locus (n=926).
Supplemental Table 2. HLA class- and molecule-specific dnDSA according to HLA antigen mismatches and eplet mismatch load (n=926).
Supplemental Table 3. Eplet mismatch load calculated per HLA locus and single HLA molecule for DRB1345, DQA1B1 and DPA1B1 dnDSA (n=38).
Supplemental Table 4. Univariable and multivariable HRs for dnDSA occurrence.
Supplemental Table 5. Univariable and multivariable HR for dnDSA class II occurrence.
Supplemental Table 6. The number of biopsies per patient and rejection subtypes (n=883).
Supplemental Table 7. Effects of number of HLA antigen and total eplet mismatches and antibody-verified eplet mismatches per locus on kidney allograft histology (n=926).
Supplemental Table 8. Univariable analysis of death-censored graft survival for all confounders included in the multivariable models (n=926).
Supplemental Table 9. Harrell’s C-statistics, evaluating the discriminative ability for death-censored graft survival.
Supplemental Table 10. Univariable and multivariable HRs for composite of graft survival and 50% eGFR decline from 3 months onwards according to HLA antigen mismatches and eplet mismatch load (n=886).
Supplemental Table 11. STROBE Statement—Checklist of items included in this cohort study.
References
- 1.Nankivell BJ, Alexander SI: Rejection of the kidney allograft. N Engl J Med 363: 1451–1462, 2010. [DOI] [PubMed] [Google Scholar]
- 2.Loupy A, Lefaucheur C: Antibody-mediated rejection of solid-organ allografts. N Engl J Med 379: 1150–1160, 2018. [DOI] [PubMed] [Google Scholar]
- 3.Williams RC, Opelz G, McGarvey CJ, Weil EJ, Chakkera HA: The risk of transplant failure with hla mismatch in first adult kidney allografts from deceased donors. Transplantation 100: 1094–1102, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Summers DM, Johnson RJ, Allen J, Fuggle SV, Collett D, Watson CJ, et al.: Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet 376: 1303–1311, 2010. [DOI] [PubMed] [Google Scholar]
- 5.Tambur AR, Claas FHJ: HLA epitopes as viewed by antibodies: what is it all about? Am J Transplant 15: 1148–1154, 2015. [DOI] [PubMed] [Google Scholar]
- 6.Duquesnoy RJ: HLA epitope based matching for transplantation. Transpl Immunol 31: 1–6, 2014. [DOI] [PubMed] [Google Scholar]
- 7.Duquesnoy RJ, Marrari M, Mulder A, Sousa LC, da Silva AS, do Monte SJH: First report on the antibody verification of HLA-ABC epitopes recorded in the website-based HLA Epitope Registry. Tissue Antigens 83: 391–400, 2014. [DOI] [PubMed] [Google Scholar]
- 8.Duquesnoy RJ, Marrari M, Tambur AR, Mulder A, Sousa LC, da Silva AS, et al.: First report on the antibody verification of HLA-DR, HLA-DQ and HLA-DP epitopes recorded in the HLA Epitope Registry. Hum Immunol 75: 1097–1103, 2014. [DOI] [PubMed] [Google Scholar]
- 9.Wiebe C, Pochinco D, Blydt-Hansen TD, Ho J, Birk PE, Karpinski M, et al.: Class II HLA epitope matching-A strategy to minimize de novo donor-specific antibody development and improve outcomes. Am J Transplant 13: 3114–3122, 2013. [DOI] [PubMed] [Google Scholar]
- 10.Lachmann N, Niemann M, Reinke P, Budde K, Schmidt D, Halleck F, et al.: Donor-recipient matching based on predicted indirectly recognizable HLA epitopes independently predicts the incidence of de novo donor-specific HLA antibodies following renal transplantation. Am J Transplant 17: 3076–3086, 2017. [DOI] [PubMed] [Google Scholar]
- 11.Snanoudj R, Kamar N, Cassuto E, Caillard S, Metzger M, Merville P, et al.: Epitope load identifies kidney transplant recipients at risk of allosensitization following minimization of immunosuppression. Kidney Int 95: 1471–1485, 2019. [DOI] [PubMed] [Google Scholar]
- 12.Wiebe C, Kosmoliaptsis V, Pochinco D, Gibson IW, Ho J, Birk PE, et al.: HLA-DR/DQ molecular mismatch: A prognostic biomarker for primary alloimmunity. Am J Transplant 19: 1708–1719, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiebe C, Rush DN, Nevins TE, Birk PE, Blydt-Hansen T, Gibson IW, et al.: Class II eplet mismatch modulates tacrolimus trough levels required to prevent donor-specific antibody development. J Am Soc Nephrol 28: 3353–3362, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reindl-Schwaighofer R, Heinzel A, Kainz A, van Setten J, Jelencsics K, Hu K, et al. iGeneTRAiN consortium : Contribution of non-HLA incompatibility between donor and recipient to kidney allograft survival: Genome-wide analysis in a prospective cohort. Lancet 393: 910–917, 2019. [DOI] [PubMed] [Google Scholar]
- 15.Heidt S, Haasnoot GW, van Rood JJ, Witvliet MD, Claas FHJ: Kidney allocation based on proven acceptable antigens results in superior graft survival in highly sensitized patients. Kidney Int 93: 491–500, 2018. [DOI] [PubMed] [Google Scholar]
- 16.Tambur AR: HLA-epitope matching or eplet risk stratification: The devil is in the details. Front Immunol 9: 2010, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Senev A, Lerut E, Van Sandt V, Coemans M, Callemeyn J, Sprangers B, et al.: Specificity, strength, and evolution of pretransplant donor-specific HLA antibodies determine outcome after kidney transplantation. Am J Transplant 19: 3100–3113, 2019. [DOI] [PubMed] [Google Scholar]
- 18.Senev A, Coemans M, Lerut E, Van Sandt V, Daniëls L, Kuypers D, et al.: Histological picture of antibody-mediated rejection without donor-specific anti-HLA antibodies: clinical presentation and implications for outcome. Am J Transplant 19: 763–780, 2019. [DOI] [PubMed] [Google Scholar]
- 19.Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al.: The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fine JP, Gray RJ: A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94: 496–509, 1999 [Google Scholar]
- 21.Harrell FE Jr., Califf RM, Pryor DB, Lee KL, Rosati RA: Evaluating the yield of medical tests. JAMA 247: 2543–2546, 1982. [PubMed] [Google Scholar]
- 22.Uno H, Cai T, Tian L, Wei LJ: Evaluating prediction rules for t-year survivors with censored regression models. J Am Stat Assoc 102: 527–537, 2007 [Google Scholar]
- 23.Lim WH, Chapman JR, Coates PT, Lewis JR, Russ GR, Watson N, et al.: HLA-DQ mismatches and rejection in kidney transplant recipients. Clin J Am Soc Nephrol 11: 875–883, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tambur AR, McDowell H, Hod-Dvorai R, Abundis MAC, Pinelli DF: The quest to decipher HLA immunogenicity: telling friend from foe. Am J Transplant 19: 2910–2925, 2019. [DOI] [PubMed] [Google Scholar]
- 25.Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Robinson A, et al.: OPTN/SRTR 2016 annual data report: Kidney. Am J Transplant 18[Suppl 1]: 18–113, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dharnidharka VR, Naik AS, Axelrod DA, Schnitzler MA, Zhang Z, Bae S, et al.: Center practice drives variation in choice of US kidney transplant induction therapy: a retrospective analysis of contemporary practice. Transpl Int 31: 198–211, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Axelrod DA, Naik AS, Schnitzler MA, Segev DL, Dharnidharka VR, Brennan DC, et al.: National variation in use of immunosuppression for kidney transplantation: A call for evidence-based regimen selection. Am J Transplant 16: 2453–2462, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hricik DE, Formica RN, Nickerson P, Rush D, Fairchild RL, Poggio ED, et al. Clinical Trials in Organ Transplantation-09 Consortium : Adverse outcomes of tacrolimus withdrawal in immune-quiescent kidney transplant recipients. J Am Soc Nephrol 26: 3114–3122, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matas AJ, Gaston RS: Moving beyond minimization trials in kidney transplantation. J Am Soc Nephrol 26: 2898–2901, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kiberd BA, Rose C, Gill JS: Cancer mortality in kidney transplantation. Am J Transplant 9: 1868–1875, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Rubin DB: Trust inference and missing data. Biometrika 63: 581–592, 1976 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.