Significance Statement
Coronavirus disease 2019 (COVID-19) is a new contagious disease. Previous studies reported AKI with varying results regarding the incidence, severity, and outcomes. This study provides detailed clinical data of 81 patients critically ill with COVID-19 and a prolonged disease course, and provides renal pathologic findings from ten deceased patients with AKI in a single intensive care unit in Wuhan, China. The incidence of AKI was 50.6%, with 41.5% of cases of AKIs were Kidney Disease Improving Global Outcomes (KDIGO) stage 3. The primary pathological findings were those of acute tubular injury. Nucleic acid tests and immunohistochemistry failed to detect the virus in kidney tissues. Older age and serum IL-6 levels were risk factors of AKI. KDIGO stage 3 AKI independently predicted death.
Keywords: renal pathology, acute renal failure, kidney disease, COVID-19, critically ill
Visual Abstract
Abstract
Background
The incidence, severity, and outcomes of AKI in COVID-19 varied in different reports. In patients critically ill with COVID-19, the clinicopathologic characteristics of AKI have not been described in detail.
Methods
This is a retrospective cohort study of 81 patients critically ill with COVID-19 in an intensive care unit. The incidence, etiologies, and outcomes of AKI were analyzed. Pathologic studies were performed in kidney tissues from ten deceased patients with AKI.
Results
A total of 41 (50.6%) patients experienced AKI in this study. The median time from illness to AKI was 21.0 (IQR, 9.5–26.0) days. The proportion of Kidney Disease Improving Global Outcomes (KDIGO) stage 1, stage 2, and stage 3 AKI were 26.8%, 31.7%, and 41.5%, respectively. The leading causes of AKI included septic shock (25 of 41, 61.0%), volume insufficiency (eight of 41, 19.5%), and adverse drug effects (five of 41, 12.2%). The risk factors for AKI included age (per 10 years) (HR, 1.83; 95% CI, 1.24 to 2.69; P=0.002) and serum IL-6 level (HR, 1.83; 95% CI, 1.23 to 2.73; P=0.003). KDIGO stage 3 AKI predicted death. Other potential risk factors for death included male sex, elevated D-dimer, serum IL-6 level, and higher Sequential Organ Failure Assessment score. The predominant pathologic finding was acute tubular injury. Nucleic acid tests and immunohistochemistry failed to detect the virus in kidney tissues.
Conclusions
AKI was a common and multifactorial complication in patients critically ill with COVID-19 at the late stage of the disease course. The predominant pathologic finding was acute tubular injury. Older age and higher serum IL-6 level were risk factors of AKI, and KDIGO stage 3 AKI independently predicted death.
Coronavirus disease 2019 (COVID-19) is a new contagious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), causing >10 million cases and 500,000 deaths worldwide.1,2 Most of patients had mild cases of the disease which primarily presented as acute respiratory illness.3–7 About 15.7%–26.0% of the COVID-19 cases were classified as severe or critically ill, with mortality as high as 60%.3,7,8 Based on the published studies, the median time from illness to death in early severe cases was 16–18.5 days.3,7,9 Early reports suggested the renal involvement in patients with COVID-19 might not be very common.7,10,11 Recent studies showed the incidence of AKI in severe cases varied from 2.9% to 50%, due to different definitions and clinical situations.7,8,10,12–14 The detection of SARS-CoV-2 in urine samples and kidney tissues of patients with COVID-19 also showed varying results (B. Diao, C. Wang, R. Wang, Z. Feng, Y. Tan, H. Wang, et al.: Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection, 2020; doi:10.1101/2020.03.04.20031120).15–19 Elevated inflammatory markers, such as high-sensitivity C-reactive protein and IL-6, in patients with COVID-19 were described.7,8 It is reasonable to raise the concern that kidney damage might be related to direct viral infection of the kidney and the following hyperinflammation process.20 So far, the limited evidence of kidney histologic changes in COVID-19 cases revealed mainly tubular injury.10,18,21 Electron microscopic (EM) evidence of virus infection in kidney was also under debate.18,22,23 In summary, it is a pressing demand to improve our understanding of the kidney involvement in COVID-19 in different clinical contexts, especially in patients who are critically ill with a relatively long disease course. In this study, we provide a detailed description of the clinical data from 81 patients who were critically ill, and renal pathologic findings from ten deceased patients with AKI in intensive care unit (ICU) settings.
Methods
Study Design and Participants
This is a single-center, retrospective study conducted in an ICU designated for patients critically ill with COVID-19 at the Sino-French New City Campus of Tongji Hospital in Wuhan, China, which is run by the medical team from Peking Union Medical College Hospital (PUMCH). All of the patients in ICU who met the criteria of having a critical case of the disease from February 5 to March 20, 2020 were included in this study. The deceased adult patients whose family member had provided oral consent for a minimally invasive autopsy received needle autopsies of the kidneys. The written consent was waived due to the visiting restrictions during the epidemic. This study was approved by the PUMCH Institutional Review Board (ZS-2328, SK-1197).
The diagnosis of COVID-19 was made according to the Guideline of Chinese National Health Commission (Fifth Trial Edition).24 The clinical diagnosis criteria were as follows: (1) fever or respiratory symptoms, (2) leukopenia or lymphopenia, and (3) computerized tomography scan showing radiographic abnormalities in lung. Those with two or more clinical diagnosis criteria and a positive result to high-throughput sequencing or RT-PCR assay of SARS-CoV-2 were diagnosed with COVID-19.
Critically ill COVID-19 cases were defined as including at least one of the following: septic shock, respiratory failure requiring mechanical ventilation, and a combination of other organ failures and admission to ICU.
Data Collection and Definitions
The electronic medical records, laboratory results, and medical order lists were carefully reviewed to extract the data. AKI was diagnosed according to the Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines.25 For those who had no baseline serum creatinine (sCr) data, the diagnosis was made by the clinical evaluations of three independent nephrologists. eGFR was calculated based on the CKD–Epidemiology Collaboration equation using sCr. Volume insufficiency–induced AKI was diagnosed based on the history of poor intake and/or volume loss and rapid recovery of AKI after volume supplementation. Fever was defined as an axillary temperature of at least 37.3°C. Sepsis and septic shock were defined according to the 2016 Third International Consensus Definitions for Sepsis and Septic Shock.26 Acute respiratory distress syndrome (ARDS) was diagnosed according to the Berlin definition.27 Cardiac injury was diagnosed if serum levels of cardiac biomarkers (e.g., high-sensitivity cardiac troponin I) were above the 99th percentile upper reference limit. Coagulopathy was defined as a 3-second extension of prothrombin time (PT) or a 5-second extension of activated partial thromboplastin time. Hypoalbuminemia was defined as serum albumin <25 g/L. The primary outcome was defined as death in hospital before March 20, 2020.
Minimally Invasive Needle Autopsy of Kidneys
The minimally invasive needle autopsies were performed by the nephrologists using the 16-guage biopsy needle (BARD MN1620) under ultrasound guidance. The autopsies were performed in the right kidneys of the deceased patients. The first slices of kidney tissues were sent for PCR tests of SARS-CoV-2. Other samples were fixed in 4% neutral formaldehyde and 2.5% glutaraldehyde for further pathologic processing.
Light Microscopic Examinations and Transmission EM Examinations
Specimens were fixed with 4% neutral formaldehyde and embedded in paraffin wax. Sections (2 μm thick) were cut; stained with hematoxylin and eosin, Periodic acid–Schiff, Periodic acid–silver methenamine, and Masson trichrome; and scanned as high-resolution digital images (Nanozoomer Digital Pathology, NDP-2.0RS; Hamamatsu Photonics, Hamamatsu, Japan).
For EM examination, tissues were fixed in 2.5% glutaraldehyde before the following procedures. After osmium tetroxide postfixation and gradient dehydration, Epon-embedded, Toluidine Blue–stained semithin sections were examined, and selected areas were chosen for thin sections. Thin sections were then cut and stained with uranyl acetate and lead citrate. EM grids were then viewed with a transmission electron microscope (HT-7800; Hitachi, Tokyo, Japan).
Immunohistochemistry Staining
Formalin-fixed, paraffin-embedded sections were heated in the pressure cooker in 100× Tris-EDTA buffer (pH 9.0) for 2 minutes to allow antigen retrieval. Standard immunohistochemistry (IHC) was performed using the EnVision Detection System (K5007; Dako) according to the manufacturer’s protocol. The primary antibodies used were as follows: anti-CD3 (clone number F7.2.38, 1:400; DAKO), anti-CD4 (clone number UMAB64, ready to use; ZSGB-BIO), anti-CD8 (clone number SP16, ready to use; ZSGB-BIO), anti-CD20 (clone number L28, 1:400; DAKO), anti-CD34 (clone number QBend-10, 1:400; DAKO), anti-CD68 (clone number KP1, 1:400; DAKO), anti-CD138 (ready to use; ZSGB-BIO), anti-granzyme B (polyclonal, ready to use; ZSGB-BIO), anti-angiotensin converting enzyme 2 (anti-ACE2; polyclonal, 1:1600; Proteintech), anti-SARS-CoV-2 spike (clone number HA14FE2402, 1:1000; Sino Biologic). Paracarcinoma normal kidney tissue from a patient with clear cell renal carcinoma was used as control for ACE2 staining. Lung tissue from an included patient was used as control for staining of SARS-CoV-2.
Nucleic Acid Tests of SARS-CoV-2 in Urine Samples and Kidney Tissues
Patients’ urine samples were extracted on a Nextractor automatic extraction system (Genolution Pharmaceuticals, Seoul, South Korea) with the manufacturer’s RNA extraction kits. Kidney tissues were extracted manually with Qiagen RNeasy Plus Universal Mini Kit (Thermo Fisher Scientific, Dreieich, Germany) following the manufacturer’s instructions. RT-PCR of SARS-CoV-2 was performed using the commercially available kit targeting the SARS-CoV-2-specific N-gene (BGI Biotechnology [Wuhan] Co., Ltd., Wuhan, China) that has been approved by the Chinese National Medical Products Administration on the ABI 7500 system (Applied Biosystems, Thermo Fisher Scientific). Any positive or suspected positive results were repeated for a second time for confirmation.
Statistical Analyses
Categoric variables were described as frequency and percentage, and continuous variables were described as using mean, median, and interquartile range (IQR) values. When the data were normally distributed, independent t tests were used to compare the means of continuous variables. Otherwise, the Mann–Whitney test was used. Although the Fisher exact test was used with limited data, the chi-squared test was used to compare the proportion of categoric variables. Cox proportional hazards models were used to identify the potential risk factors. Time trends of creatinine and eGFR for different groups of patients with AKI were calculated by a cubic polynomial regression model based on least square means fit using GraphPad Prism 6.0. All statistical analyses were performed using SPSS version 22.0 software. A P value of <0.05 is statistically significant.
Results
Clinical Characteristics of Patients Critically Ill with COVID-19
A total of 1752 patients with COVID-19 were admitted to the Sino-French New City Campus of Tongji Hospital from February 5 to March 20, 2020. Of these, 95 were transferred to our ICU. Five patients transferred for non-COVID-19-related conditions, and nine patients missing important data were excluded. Finally, 81 patients were included.
The mean age of these patients was 66.6±11.4 years. Male/female ratio was 2:1. Comorbidities were present in over half of the patients, with hypertension being the most common (Table 1). Neutrophil-predominant leukocytosis complicated with lymphocytopenia occurred in >70% of the patients. Significant elevation of D-dimer and inflammatory markers, including high-sensitivity C-reactive protein, IL-6, and ferritin, were also observed (Table 2). The median Acute Physiology and Chronic Health Evaluation II (APACHE II) and Sequential Organ Failure Assessment (SOFA) scores were 15 (IQR, 11–19) and 6 (IQR, 4–8), respectively, on ICU day 1 (Table 1). ARDS, septic shock, and coagulopathy were present in 95.1%, 63.0%, and 68.0% of the patients. A total of 58.0% of patients experienced secondary infections after hospitalization (Supplemental Figure 1). Aggressive treatment including glucocorticoids, antiviral medication, antibiotics, and intravenous Ig were deployed. Invasive mechanical ventilation and vasopressors were required in 81.5% (66/81) and 77.8% (63/81) of the patients. Five patients received extracorporeal membrane oxygenation support (Table 3).
Table 1.
Demographic and clinical characteristics of patients
| Characteristics | All Patients (n=81) | Presence of AKI | Survival | ||||
|---|---|---|---|---|---|---|---|
| AKI (n=41) | Non-AKI (n=40) | P Value | Nonsurvivors (n=60) | Survivors (n=21) | P Value | ||
| Clinical characteristics | |||||||
| Age (yr), mean±SD | 66.6±11.4 | 69.6±9.3 | 63.6±12.7 | 0.02a | 68.2±10.4 | 62.0±13.3 | 0.03a |
| Sex, n (%) | |||||||
| Female | 27 (33.3%) | 11 (26.8%) | 16 (40.0%) | 0.21 | 15 (25.0%) | 12 (57.1%) | 0.007a |
| Male | 54 (66.7%) | 30 (73.2%) | 24 (60.0%) | – | 45 (75.0%) | 9 (42.9%) | – |
| APACHE II score on ICU day 1, median (IQR) | 15 (11–19) | 16 (12–22) | 14 (10–16) | 0.02a | 16 (12–20) | 12 (10–16) | 0.01a |
| SOFA score on ICU day 1, median (IQR) | 6 (4–8) | 7 (5–10) | 6 (4–8) | 0.03a | 7 (5–10) | 4 (3–5) | <0.001a |
| Median arterial pressure (mm Hg), median (IQR) | 93 (82–103) | 93 (82–104) | 92 (82–103) | 0.79 | 90 (80–103) | 97 (89–104) | 0.16 |
| Comorbidity, n (%)b | |||||||
| Hypertension | 43 (53.1%) | 23 (56.1%) | 20 (50.0%) | 0.58 | 30 (50.0%) | 13 (61.9%) | 0.35 |
| Diabetes mellitus | 19 (23.5%) | 11 (26.8%) | 8 (20.0%) | 0.47 | 12 (20.0%) | 7 (33.3%) | 0.22 |
| Coronary heart disease | 17 (21.0%) | 8 (19.5%) | 9 (22.5%) | 0.74 | 12 (20.0%) | 5 (23.8%) | 0.71 |
| Cerebrovascular disease | 11 (13.6%) | 2 (4.9%) | 9 (22.5%) | 0.02a | 6 (10.0%) | 5 (23.8%) | 0.11 |
| CKD | 3 (3.7%) | 1 (2.4%) | 2 (5%) | 0.62 | 1 (1.6%) | 2 (9.5%) | 0.16 |
| Current smoker, n (%)c | 12 (16.0%) | 7 (18.4%) | 5 (13.5%) | 0.56 | 9 (16.7%) | 3 (14.3%) | >0.99 |
| Complications, n (%) | |||||||
| ARDS | 77 (95.1%) | 37 (90.2%) | 40 (100.0%) | – | 57 (95.0%) | 20 (95.2%) | >0.99 |
| Septic shock | 51 (63.0%) | 28 (68.3%) | 23 (57.5%) | 0.32 | 44 (73.3%) | 7 (33.3%) | 0.001a |
| Altered mental status | 39 (48.1%) | 19 (46.3%) | 20 (50.0%) | 0.74 | 30 (50.0%) | 9 (42.9%) | 0.57 |
| Cardiac injury | 61 (79.0%) | 33 (80.5%) | 31 (77.5%) | 0.74 | 51 (85.0%) | 13 (61.9%) | 0.03a |
| Arrythmias | 29 (35.8%) | 17 (41.5%) | 12 (30.0%) | 0.28 | 25 (41.7%) | 4 (19.0%) | 0.07 |
| Coagulopathy | 51 (63.0%) | 30 (73.2%) | 21 (52.5%) | 0.05 | 43 (71.7%) | 8 (38.1%) | 0.006a |
| Liver injury | 32 (39.5%) | 19 (46.3%) | 13 (32.5%) | 0.20 | 28 (46.7%) | 4 (19.0%) | 0.03a |
| Hypercapnia | 44 (54.3%) | 26 (63.4%) | 18 (45.0%) | 0.10 | 37 (61.7%) | 7 (33.3%) | 0.03a |
| Secondary infection | 47 (58.0%) | 26 (63.4%) | 21 (52.5%) | 0.32 | 34 (56.7%) | 13 (61.9%) | 0.68 |
| Pneumothorax | 7 (8.6%) | 5 (12.2%) | 2 (5.0%) | 0.43 | 7 (11.7%) | 0 (0.0%) | – |
| AKI | 41 (50.6%) | – | – | – | 34 (56.7%) | 7 (33.3%) | 0.07 |
| Initial symptoms, n (%) | |||||||
| Fever | 72 (88.9%) | 34 (82.9%) | 38 (95.0%) | 0.08 | 54 (90.0%) | 18 (85.7%) | 0.69 |
| Cough | 63 (77.8%) | 33 (80.5%) | 30 (75.0%) | 0.55 | 46 (76.7%) | 17 (81.0%) | 0.77 |
| Sputum | 33 (40.7%) | 15 (36.6%) | 18 (45.0%) | 0.44 | 22 (36.7%) | 11 (52.4%) | 0.21 |
| Fatigue | 46 (56.8%) | 21 (51.2%) | 25 (62.5%) | 0.31 | 34 (56.7%) | 12 (57.1%) | 0.97 |
| Anorexia | 26 (32.1%) | 11 (26.8%) | 15 (37.5%) | 0.30 | 22 (36.7%) | 4 (19.0%) | 0.18 |
| Vomit | 8 (9.9%) | 4 (9.8%) | 4 (10.0%) | 0.97 | 5 (8.3%) | 3 (14.3%) | 0.42 |
| Diarrhea | 20 (24.7%) | 9 (22.0%) | 11 (27.5%) | 0.56 | 17 (28.3%) | 3 (14.3%) | 0.25 |
| Headache | 9 (11.1%) | 6 (14.6%) | 3 (7.5%) | 0.48 | 8 (13.3%) | 1 (4.8%) | 0.43 |
| Myalgia | 15 (18.5%) | 6 (14.6%) | 9 (22.5%) | 0.36 | 8 (13.3%) | 7 (33.3%) | 0.04a |
| Dyspnea | 55 (67.9%) | 29 (70.7%) | 26 (65.0%) | 0.58 | 42 (70.0%) | 13 (61.9%) | 0.49 |
P<0.05.
Hypertension and diabetes histories were diagnosed based on self-reports from patients or family members. Coronary heart disease was diagnosed based the self-reports from patients or family members about the coronary angiography results or histories of coronary stenting. Cerebrovascular disease was diagnosed based on the self-reports from patients or family members about the histories of ischemic or hemorrhagic cerebrovascular incidents and positive image findings. CKD was defined as abnormalities of kidney structure or function for at least 3 months.
All patients, n=75; AKI, n=38; non-AKI, n=37; nonsurvivors, n=54; survivors, n=21.
Table 2.
The laboratory findings of patients
| Laboratory Findings | All Patients (n=81) | Presence of AKI | Survival | ||||
|---|---|---|---|---|---|---|---|
| AKI (n=41) | Non-AKI (n=40) | P Value | Nonsurvivors (n=60) | Survivors (n=21) | P Value | ||
| White blood cell count (×109/L), median (IQR) | 12.20 (9.00–17.29) | 13.11 (9.88–17.81) | 11.60 (8.66–15.30) | 0.41 | 13.05 (10.20–18.30) | 9.93 (6.40–15.13) | 0.05a |
| >10, n (%) | 56 (69.1%) | 30 (73.2%) | 26 (65.0%) | 0.43 | 46 (76.7%) | 10 (47.6%) | 0.01a |
| 4–10, n (%) | 24 (29.6%) | 11 (26.8%) | 13 (32.5%) | – | 13 (21.7%) | 11 (52.4%) | – |
| <4, n (%) | 1 (1.2%) | 0 (0.0%) | 1 (2.5%) | – | 1 (1.7%) | 0 (0.0%) | – |
| Neutrophil count (×109/L), median (IQR) | 11.13 (8.14–16.02) | 12.49 (9.14–16.43) | 10.65 (7.80–14.42) | 0.31 | 11.89 (9.33–16.87) | 9.22 (5.64–13.10) | 0.03a |
| Lymphocytes (×109/L), median (IQR) | 0.54 (0.34–0.74) | 0.50 (0.34–0.60) | 0.65 (0.34–0.88) | 0.02a | 0.53 (0.32–0.70) | 0.69 (0.42–0.94) | 0.10 |
| <0.4, n (%) | 24 (29.6%) | 13 (31.7%) | 11 (27.5%) | 0.08 | 19 (31.7%) | 5 (23.8%) | 0.59 |
| 0.4–0.8, n (%) | 39 (48.1%) | 23 (56.1%) | 16 (40.0%) | – | 30 (50.0%) | 9 (42.9%) | – |
| Hemoglobin (g/L), mean±SD | 122.1±22.8 | 124.5±25.6 | 119.8±19.5 | 0.36 | 124.8±21.5 | 114.7±25.2 | 0.08 |
| Anemia, n (%) | 29 (35.8%) | 14 (34.1%) | 15 (37.5%) | 0.75 | 19 (31.7%) | 10 (47.6%) | 0.19 |
| Platelets (×109/L), median (IQR) | 159.0 (100.0–225.5) | 145.0 (77.5–209.5) | 176.5 (122.5–268.0) | 0.03a | 153.0 (82.5–210.8) | 202.0 (138.0–271.0) | 0.03a |
| <100, n (%) | 19 (23.5%) | 12 (29.3%) | 7 (17.5%) | 0.21 | 17 (28.3%) | 2 (9.5%) | 0.13 |
| Serum albumin (g/L), median (IQR) | 28.5 (26.1–31.0) | 28.4 (25.2–31.8) | 28.7 (26.2–31.0) | 0.87 | 27.9 (24.1–30.4) | 29.0 (28.1–33.0) | 0.06 |
| <25, n (%) | 18 (22.2%) | 10 (24.4%) | 8 (20.0%) | 0.64 | 17 (28.3%) | 1 (4.8%) | 0.03a |
| ALT (U/L), median (IQR) | 29.0 (19.5–45.0) | 32.0 (19.0–52.5) | 25.0 (20.2–39.8) | 0.44 | 29.5 (20.2–45.8) | 24.0 (16.5–38.5) | 0.33 |
| AST (U/L), median (IQR) | 33.0 (20.5–63.5) | 37.0 (28.0–59.0) | 29.0 (19.0–68.0) | 0.27 | 33.5 (21.0–68.2) | 33.0 (20.0–54.5) | 0.38 |
| LDH (U/L), median (IQR) | 523.0 (373.5–700.5) | 562.0 (382.5–688.0) | 504.5 (345.2–742.5) | 0.45 | 558.0 (417.2–742.5) | 373.0 (274.5–618.5) | 0.01a |
| Total bilirubin (μmol/L), median (IQR) | 12.9 (8.6–20.6) | 12.2 (8.2–21.6) | 13.4 (8.8–20.6) | 0.81 | 14.5 (9.8–21.7) | 10.3 (7.6–17.0) | 0.05a |
| sCr (μmol/L), median (IQR) | 80.0 (58.0–113.0) | 104.0 (74.0–188.5) | 65.5 (48.0–80.0) | <0.001a | 85.5 (65.0–114.5) | 61.0 (43.5–82.0) | 0.02a |
| Elevated sCr, n (%) | 24 (29.6%) | 21 (51.2%) | 3 (7.5%) | <0.001a | 20 (33.3%) | 4 (19.0%) | 0.27 |
| Cystatin C (mg/L), median (IQR)b | 1.26 (0.99–2.01) | 1.74 (1.22–2.44) | 1.06 (0.86–1.28) | <0.001a | 1.32 (0.95–2.01) | 1.22 (1.05–1.82) | 0.96 |
| >1.55, n (%) | 23 (35.3%) | 20 (60.6%) | 3 (9.1%) | <0.001a | 17 (36.2%) | 6 (31.6%) | 0.72 |
| eGFR (ml/min per 1.73 m2), median (IQR) | 83.2 (50.1–99.6) | 53.0 (27.0–85.8) | 96.0 (79.8–107.8) | <0.001a | 78.3 (45.2–97.4) | 94.8 (64.5–115.9) | 0.06 |
| <60, n (%) | 26 (32.1%) | 22 (53.7%) | 4 (10.0%) | <0.001a | 21 (35.0%) | 5 (23.8%) | 0.34 |
| BUN (mmol/L), median (IQR) | 9.6 (6.4–15.4) | 12.5 (8.6–27.8) | 7.1 (5.5–10.6) | <0.001a | 10.2 (7.0–17.8) | 7.3 (5.2–10.9) | 0.02a |
| Serum potassium (mmol/L), median (IQR) | 4.37 (3.88–5.01) | 4.50 (4.09–5.04) | 4.19 (3.78–4.82) | 0.16 | 4.48 (3.96–5.04) | 4.16 (3.76–4.60) | 0.18 |
| >5.5, n (%) | 12 (14.8%) | 6 (14.6%) | 6 (15.0%) | 0.96 | 9 (15.0%) | 3 (14.3%) | >0.99 |
| 3.5–5.5, n (%) | 62 (76.5%) | 31 (75.6%) | 31 (77.5%) | – | 46 (76.7%) | 16 (76.2%) | – |
| <3.5, n (%) | 7 (8.6%) | 4 (9.8%) | 3 (7.5%) | – | 5 (8.3%) | 2 (9.5%) | – |
| Serum sodium (mmol/L), median (IQR) | 142.6 (138.0–146.2) | 144.4 (138.9–148.2) | 139.9 (137.5–145.1) | 0.10 | 144.2 (139.4–146.9) | 137.8 (135.2–140.0) | 0.001a |
| >145, n (%) | 28 (34.6%) | 18 (43.9%) | 10 (25.0%) | 0.07 | 25 (41.7%) | 3 (14.3%) | 0.03a |
| 135–145, n (%) | 43 (53.1%) | 18 (43.9%) | 25 (75.0%) | – | 29 (48.3%) | 14 (66.7%) | – |
| <135, n (%) | 10 (12.3%) | 5 (12.2%) | 5 (12.5%) | – | 6 (10.0%) | 4 (19.0%) | – |
| Serum calcium (mmol/L), median (IQR) | 2.10 (1.96–2.29) | 2.12 (1.98–2.26) | 2.09 (1.95–2.29) | 0.77 | 2.08 (1.96–2.22) | 2.24 (1.98–2.34) | 0.05a |
| Serum phosphorus (mmol/L), median (IQR)c | 0.98 (0.84–1.24) | 1.12 (0.84–1.48) | 0.94 (0.82–1.09) | 0.09 | 0.99 (0.86–1.32) | 0.92 (0.74–1.14) | 0.14 |
| <0.81, n (%) | 16 (21.9%) | 8 (22.2%) | 8 (21.6%) | 0.95 | 9 (17.0%) | 7 (35.0%) | 0.08 |
| Serum uric acid (μmol/L), median (IQR)d | 201.0 (137.5–327.0) | 289.5 (184.8–448.8) | 164.0 (119.2–219.2) | <0.001a | 208.0 (147.0–346.0) | 187.0 (125.0–259.0) | 0.28 |
| Proteinuria at admission to ICU, n (%)e | |||||||
| >2+ | 11 (15.5%) | 6 (18.2%) | 5 (13.2%) | 0.78 | 10 (19.2%) | 1 (5.3%) | 0.27 |
| ±1+ | 50 (70.4%) | 23 (69.7%) | 27 (71.1%) | – | 37 (71.2%) | 13 (68.4%) | – |
| Negative | 10 (14.1%) | 4 (12.1%) | 6 (15.8%) | – | 5 (9.6%) | 5 (26.3%) | – |
| Hematuria at admission to ICU, n (%)e | |||||||
| >2+ | 23 (32.4%) | 11 (33.3%) | 12 (31.6%) | 0.75 | 20 (38.5%) | 3 (15.8%) | 0.09 |
| ±1+ | 24 (33.8%) | 12 (36.4%) | 12 (31.6%) | – | 18 (34.6%) | 6 (31.6%) | – |
| Negative | 24 (33.8%) | 10 (30.3%) | 14 (36.8%) | – | 14 (26.9%) | 10 (52.6%) | – |
| Proteinuria at hospital presentation, n (%)f | |||||||
| >2+ | 5 (13.9%) | 3 (15.8%) | 2 (11.8%) | >0.99 | 5 (17.9%) | 0 (0.0%) | – |
| ±1+ | 26 (72.2%) | 14 (73.7%) | 12 (70.6%) | – | 20 (71.4%) | 6 (75%) | – |
| Negative | 5 (13.9%) | 2 (10.5%) | 3 (17.6%) | – | 3 (10.7%) | 2 (25.0%) | – |
| Hematuria at hospital presentation, n (%)f | |||||||
| >2+ | 10 (25.0%) | 6 (31.6%) | 4 (23.5%) | 0.72 | 9 (32.1%) | 1 (12.5%) | 0.40 |
| ±1+ | 17 (47.2%) | 9 (47.4%) | 8 (47.4%) | – | 14 (50.0%) | 3 (37.5%) | – |
| Negative | 9 (27.8%) | 4 (21.1%) | 5 (21.1%) | – | 5 (17.9%) | 4 (50.5%) | – |
| ESR (mm/h), median (IQR)g | 40.5 (27.8–65.5) | 47.5 (32.0–72.0) | 35.0 (22.0–61.8) | 0.10 | 38.5 (24.5–58.5) | 67.0 (31.0–91.0) | 0.02a |
| hsCRP (mg/L), median (IQR)h | 105.5 (50.8–142.2) | 109.0 (66.8–147.9) | 66.0 (36.1–140.7) | 0.10 | 108.9 (58.8–149.4) | 66.0 (29.0–122.5) | 0.06 |
| IL-6 (pg/ml), median (IQR)i | 59.0 (31.0–167.2) | 100.4 (36.4–265.6) | 36.8 (20.3–103.2) | 0.01a | 93.2 (35.5–214.9) | 31.8 (22.1–69.5) | 0.002a |
| Ferritin (μg/ml), median (IQR)j | 1386.4 (758.7–2207.8) | 1301.8 (758.7–2207.8) | 1484.8 (765.2–2229.8) | 0.62 | 1469.0 (831.3–2261.3) | 973.3 (649.7–2207.8) | 0.34 |
| PT (s), median (IQR) | 16.3 (15.3–18.1) | 17.2 (15.3–18.5) | 16.0 (15.1–17.2) | 0.04a | 17.0 (15.3–18.2) | 15.6 (14.6–16.2) | 0.006a |
| aPTT (s), median (IQR) | 41.0 (37.2–45.5) | 41.7 (37.4–46.8) | 40.9 (35.9–43.6) | 0.37 | 40.7 (37.0–45.9) | 43.0 (38.2–45.4) | 0.48 |
| Fibrinogen (g/L), median (IQR) | 3.95 (3.08–5.56) | 4.08 (2.92–5.57) | 3.92 (3.10–5.55) | 0.88 | 3.86 (2.77–5.52) | 4.60 (3.42–5.62) | 0.21 |
| INR, median (IQR) | 1.29 (1.19–1.48) | 1.40 (1.20–1.52) | 1.26 (1.18–1.36) | 0.02a | 1.36 (1.20–1.49) | 1.24 (1.08–1.28) | 0.009a |
| D-dimer (μg/ml FEU), n (%) | |||||||
| >21.0 | 36 (44.4%) | 19 (46.3%) | 17 (42.5%) | 0.73 | 32 (53.3%) | 4 (19.0%) | 0.01a |
| 5.0–21.0 | 19 (23.5%) | 10 (24.4%) | 9 (22.5%) | – | 14 (23.3%) | 5 (23.8%) | – |
| 0.5–5.0 | 26 (32.1%) | 12 (29.3%) | 14 (35.0%) | – | 14 (23.3%) | 12 (57.1%) | – |
| <0.5 | 0 | 0 | 0 | – | 0 | 0 | – |
| cTnI (pg/ml), median (IQR)k | 62.6 (15.1–520.4) | 69.8 (18.8–320.8) | 52.4 (8.3–555.8) | 0.49 | 67.1 (19.1–653.2) | 20.5 (3.9–167.4) | 0.02a |
| NT-proBNP (pg/ml), median (IQR)l | 992.0 (402.0–3589.0) | 1902.0 (617.0–5590.0) | 843.0 (320.2–2929.2) | 0.03a | 1021.5 (486.0–3634.5) | 992.0 (193.0–3808.0) | 0.74 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; LDH, lactate dehydrogenase; ESR, erythrocyte sedimentation rate; hsCRP, high-sensitivity C-reactive protein; aPTT, activated partial thromboplastin time; INR, international normalized ratio; FEU, fibrinogen equivalent units; cTnI; cardiac troponin I; NT-proBNP, N-terminal prohormone of brain natriuretic peptide.
P≤0.05.
All patients, n=66; AKI, n=33; non-AKI, n=33; nonsurvivors, n=47; survivors, n=19.
All patients, n=73; AKI, n=36; non-AKI, n=37; nonsurvivors, n=53; survivors, n=20.
All patients, n=80; AKI, n=40; non-AKI, n=40; nonsurvivors, n=59; survivors, n=21.
All patients, n=71; AKI, n=33; non-AKI, n=38; nonsurvivors, n=52; survivors, n=19.
All patients, n=36; AKI, n=19; non-AKI, n=17; nonsurvivors, n=28; survivors, n=8.
All patients, n=58; AKI, n=30; non-AKI, n=28; nonsurvivors, n=42; survivors, n=16.
All patients, n=77; AKI, n=39; non-AKI, n=38; nonsurvivors, n=56; survivors, n=21.
All patients, n=64; AKI, n=32; non-AKI, n=32; nonsurvivors, n=45; survivors, n=19.
All patients, n=63; AKI, n=31; non-AKI, n=32; nonsurvivors, n=48; survivors, n=15.
All patients, n=80; AKI, n=40; non-AKI, n=40; nonsurvivors, n=59; survivors, n=21.
All patients, n=79; AKI, n=39; non-AKI, n=40; nonsurvivors, n=58; survivors, n=21.
Table 3.
The treatment and outcome of patients during the hospitalization
| Treatment and Outcome | Total (n=81) | Presence of AKI | Survival | ||||
|---|---|---|---|---|---|---|---|
| AKI (n=41) | Non-AKI (n=40) | P Value | Nonsurvivors (n=60) | Survivors (n=21) | P Value | ||
| Therapies, n (%) | |||||||
| Glucocorticoids | 71 (87.7%) | 34 (82.9%) | 37 (92.5%) | 0.31 | 54 (90.0%) | 17 (81.0%) | 0.28 |
| Antiviral medication | 66 (81.5%) | 33 (80.5%) | 33 (82.5%) | 0.82 | 49 (81.7%) | 17 (81.0%) | 0.94 |
| Antibiotics | 75 (92.6%) | 38 (92.7%) | 37 (92.5%) | >0.99 | 57 (95.0%) | 18 (85.7%) | 0.18 |
| Intravenous Ig | 66 (81.5%) | 33 (80.5%) | 33 (82.5%) | 0.82 | 48 (80.0%) | 18 (85.7%) | 0.75 |
| Blood transfusion | 22 (27.2%) | 14 (34.1%) | 8 (20.0%) | 0.15 | 18 (30.0%) | 4 (19.0%) | 0.40 |
| Anticoagulation | 41 (50.6%) | 21 (51.2%) | 20 (50.0%) | 0.91 | 29 (48.3%) | 12 (57.1%) | 0.49 |
| Anti-inflammatory therapies | 13 (16.0%) | 8 (19.5%) | 5 (12.5%) | 0.39 | 9 (15.0%) | 4 (19.0%) | 0.73 |
| Life-sustaining treatment, n (%) | |||||||
| Invasive mechanic ventilation | 66 (81.5%) | 36 (87.8%) | 30 (75.0%) | 0.14 | 53 (88.3%) | 13 (61.9%) | 0.007 |
| ECMO | 5 (6.2%) | 2 (4.9%) | 3 (7.5%) | 0.68 | 3 (5.0%) | 2 (9.5%) | 0.60 |
| Vasopressors | 63 (77.8%) | 36 (87.8%) | 27 (67.5%) | 0.03a | 56 (93.3%) | 7 (33.3%) | <0.001a |
| CRRT | 8 (9.9%) | 8 (19.5%) | 0 (0.0%) | – | 8 (13.3%) | 0 (0.0%) | – |
| Outcome, n (%) | |||||||
| Death | 60 (74.1%) | 34 (82.9%) | 26 (65.0%) | 0.07 | – | – | – |
| Survive before this study | 21 (25.9%) | 7 (17.1%) | 14 (35.0%) | – | – | – | – |
| Time from illness to admission (d), median (IQR) | 9.0 (5.5–13.5) | 8.0 (5.0–11.0) | 10.5 (7.0–16.0) | 0.09 | 9.0 (5–11.8) | 11.0 (6.0–16.5) | 0.11 |
| Time from illness to intubation (d), median (IQR)b | 15.5 (12.0–21.0) | 14.0 (11.0–20.8) | 17.0 (13.0–23.5) | 0.13 | 16.0 (12.5–21.0) | 13.0 (10.0–22.5) | 0.65 |
| Time of mechanic ventilation (d), median (IQR)b | 9.5 (3.8–16.5) | 10.0 (3.0–18.0) | 9.0 (4.0–15.0) | 0.89 | 8 (3.0–13.5) | 27.0 (13.5–41.0) | <0.001a |
| Time from illness to AKI (d), median (IQR)c | 21.0 (9.5–26.0) | 21.0 (9.5–26.0) | – | – | 20.5 (9.0–24.2) | 21.0 (15.0–35.0) | 0.40 |
| Time from illness to death (d), median (IQR)d | 24.0 (18.0–31.8) | 24.0 (18.0–32.2) | 24.5 (18.8–31.0) | 0.96 | 24.0 (18.0–31.8) | – | – |
| Time of hospitalization (d), median (IQR) | 17.0 (10.0–26.0) | 18.0 (10.0–28.0) | 15.5 (9.5–23.8) | 0.76 | 14.0 (9.2–22.8) | 29.0 (14.5–39.0) | <0.001a |
| Disease course before this study (d), median (IQR) | 28.0 (19.0–36.0) | 26.0 (18.0–36.0) | 28.5 (19.2–38.2) | 0.42 | 24.0 (18.0–31.8) | 39.0 (29.0–52.5) | <0.001a |
ECMO, extracorporeal membrane oxygenation.
P<0.05.
All patients, n=66; AKI, n=30; non-AKI, n=36; nonsurvivors, n=53; survivors, n=13.
All patients, n=41; nonsurvivors, n=34; survivors, n=7.
All patients, n=60; AKI, n=34; non-AKI, n=26.
A total of 60 patients died in hospital before conclusion of the study. The median time from illness onset to death was 24.0 (IQR, 18.0–31.8) days. Nonsurvivors were mainly male (45/60, 75.0%). They were also of an older age, had a higher white blood cell count, prolonged PT and international normalized ratio, higher baseline sCr and serum IL-6 levels, and higher APACHE II and SOFA scores (Table 1). They also displayed higher prevalence of organ dysfunction including septic shock, liver injury, cardiac injury, and coagulopathy (Table 1). At the time of this study, 57.2% (12/21) of the survivors had been discharged, 23.8% (5/21) of them had been transferred to general wards, and 19.0% (4/21) of them were still in ICU.
Elevated sCr and eGFR <60 ml/min per 1.73 m2 were present in 29.6% and 35.3% of patients, respectively, at admission to ICU. Of the tested patients, 15.5% had proteinuria >2+, whereas 32.4% had hematuria >2+. AKI occurred in 41/81 (50.6%) of patients. Patients with AKI were of an older age, had lower lymphocyte and platelet counts, slightly prolonged PT, and higher serum IL-6 levels (Table 2).
Risk Factors of AKI and Death
The risk factors for AKI included older age (per 10 years) (hazard ratio [HR], 1.83; 95% CI, 1.24 to 2.69; P=0.002) and serum IL-6 levels (ng/ml) (HR, 1.83; 95% CI, 1.23 to 2.73; P=0.003) (Supplemental Table 1, Table 4). Univariate Cox proportional hazards models showed that older age, male sex, elevated baseline sCr, reduced eGFR, leukocytosis, hypoalbuminemia, hypernatremia, elevated D-dimer, and higher APACHE II and SOFA scores were associated with death (Supplemental Table 2). Different multivariate Cox proportional hazards models demonstrated that, besides male sex and KDIGO stage 3 AKI, serum IL-6 levels (ng/ml) (HR, 2.12; 95% CI, 1.27 to 3.53; P=0.004), higher levels of D-dimer (HR, 1.57; 95% CI, 1.13 to 2.18; P=0.008), and SOFA score (HR, 1.08; 95% CI, 1.01 to 1.15; P=0.03) predicted death (Table 5).
Table 4.
Risk factors for AKI identified by multivariate Cox proportional hazards models
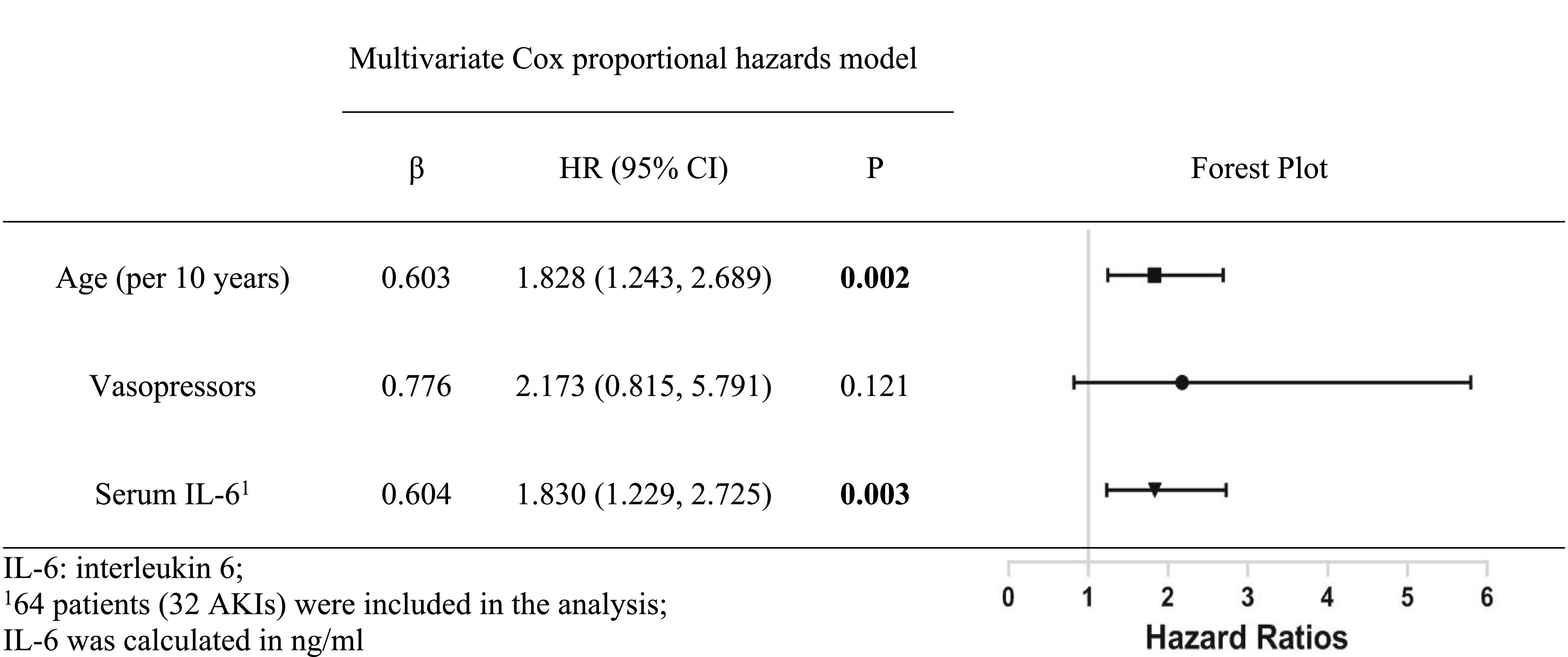 |
Table 5.
Different multivariate Cox proportional hazards models for risk factors of in-hospital death
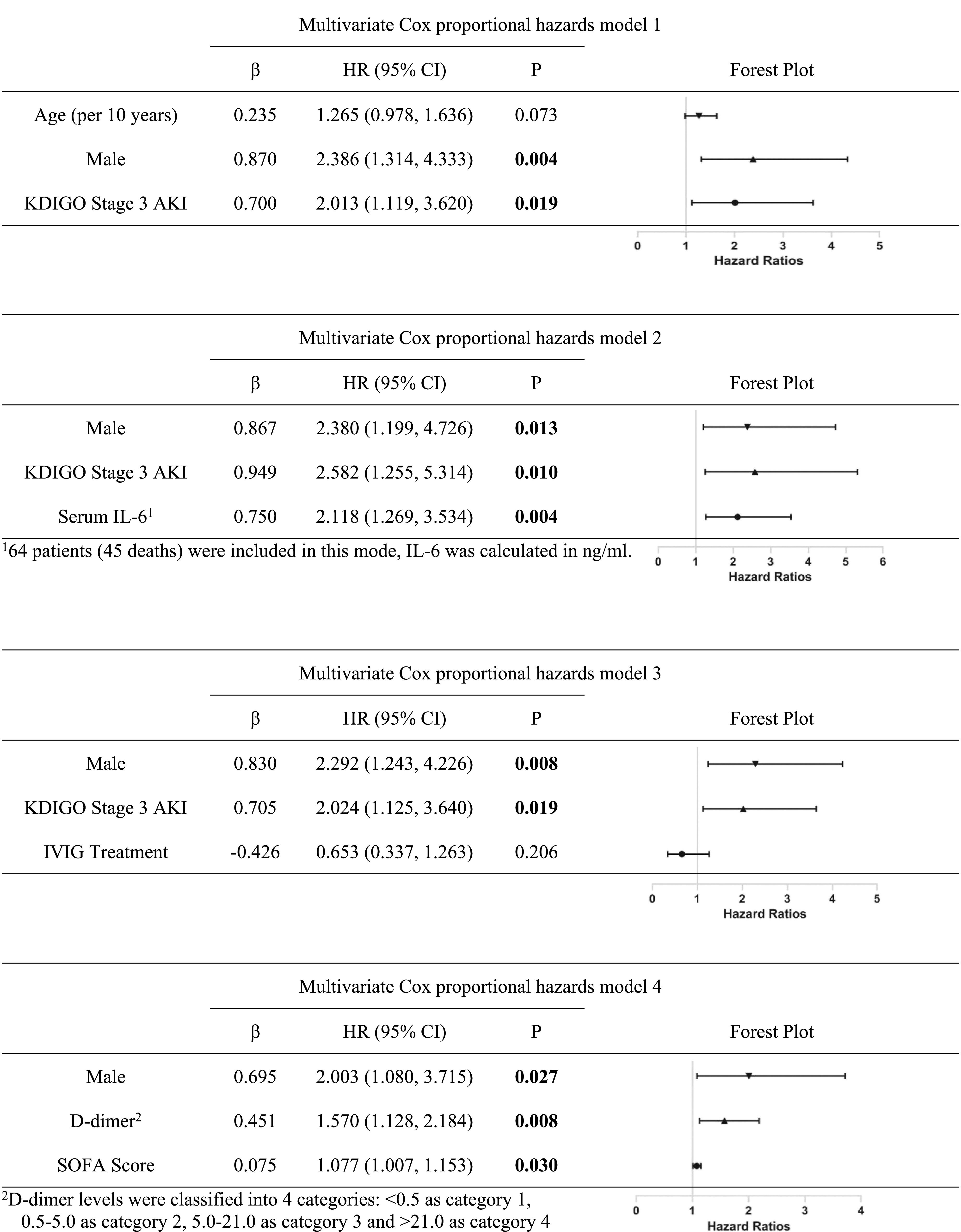 |
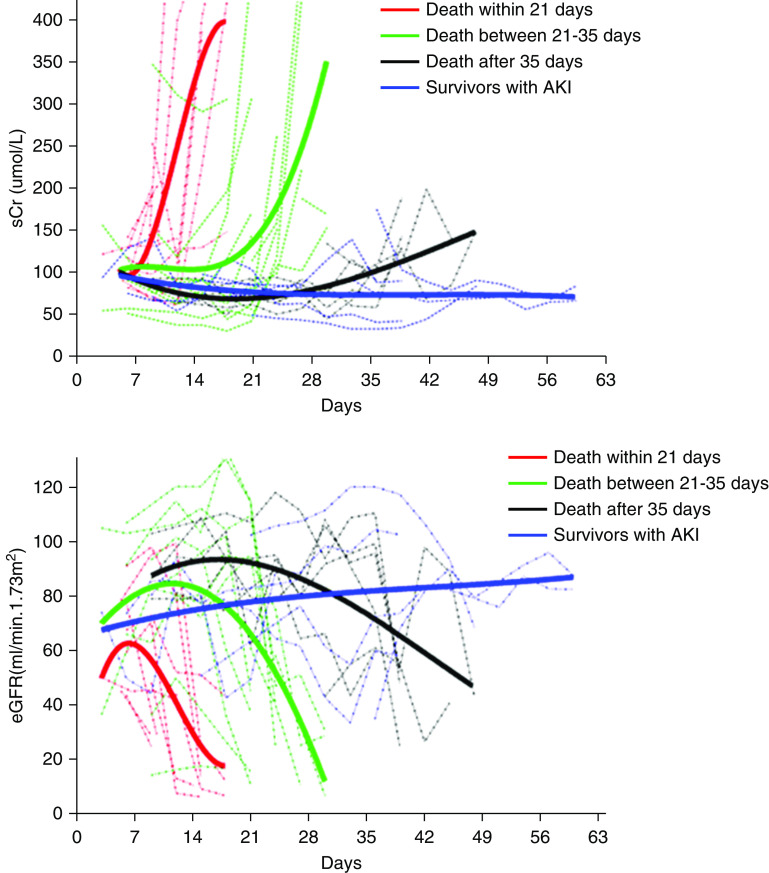
Trends of sCr, AKI Staging, and Etiologies during the Disease Course
The median time from illness to AKI was 21.0 (IQR, 9.5–26.0) days. Cubic polynomial regressions suggested the decline of renal function was faster in patients with AKI who died within shorter course of the disease (Figure 1). Seven of the 81 (8.6%) patients had AKI at hospital presentation, whereas 21.0% (17/81) of the patients had AKIs at ICU admission. The proportion of KDIGO stage 1, stage 2, and stage 3 AKI cases were 26.8%, 31.7%, and 41.5%, respectively. Eight patients with AKI required continuous RRT (CRRT). Most of the KDIGO stage 3 AKI cases occurred in the first 4 weeks (Figure 2A). Various AKI etiologies were identified: 61.0% (25/41) of the AKIs occurred either on the same day of septic shock, or within 24 hours after septic shock. Four patients with septic shock had AKI complicated with possible rhabdomyolysis (significant elevated serum myoglobin and lactate dehydrogenase). Eight patients (19.5%) had histories of poor intake or volume loss (high fever, vomit, diarrhea, or diuresis) before AKI; most of these patients (6/8) recovered from AKI quickly after volume supplementations consisting of isotonic fluids and enteral nutrition support. Five (12.2%) of the AKIs occurred after the administration of antiviral drugs or antibiotics, and no other triggers were identified. One patient experienced diabetic ketoacidosis before AKI. One patient who had received kidney transplantation 6 years previously suffered AKI possibly caused by acute rejection and cardiac failure. One patient with AKI had positive anti-phospholipid antibodies and a probable cerebrovascular incident; his renal pathology showed venous thrombosis. In general, AKI caused by septic shock and adverse drug effects occurred throughout the disease course, whereas volume insufficiency usually occurred in the early phase of the disease (Figure 2B).
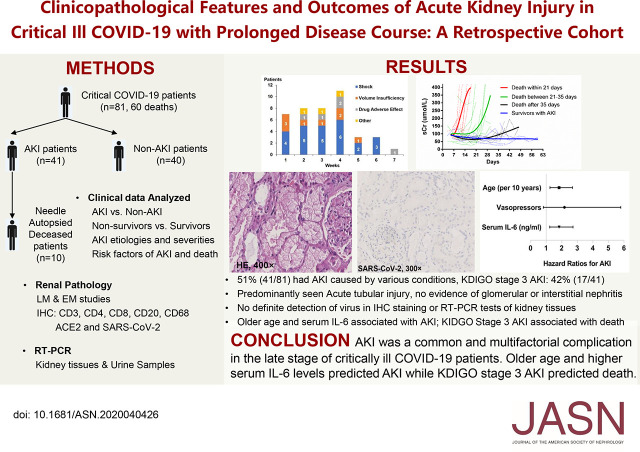
Figure 1.
The patients with AKI who had a shorter disease course before death showed faster decline of renal function. Cubic polynomial regression of (A) sCr and (B) eGFR of patients with AKI who died within 21 days (n=11), between 21 and 35 days (n=17), after 35 days (n=6), and those who survived (n=7).
Figure 2.
The compositions of AKI stages and etiologies during the disease course. (A) The proportion of KDIGO stage 1, stage 2, and stage 3 AKI were 26.8%, 31.7%, and 41.5%, respectively. Most stage 3 AKI occurred in the first 4 weeks. KDIGO stage 1: increase in sCr to 1.5–1.9 times baseline within 7 days or ≥0.3 mg/dl (≥26.5 μmol/L) increase within 48 hours, or urine output <0.5 ml/kg per hour for 6–12 hours. KDIGO stage 2: increase in sCr to 2.0–2.9 times baseline within 7 days, or urine output <0.5 ml/kg per hour for ≥12 hours. KDIGO stage 3: increase in sCr to three or more times baseline, or increase in sCr to ≥4.0 mg/dl (≥353.6 μmol/L) within 7 days, or initiation of RRT, or anuria for ≥12 hours. (B) Various AKI etiologies were identified: 61.0% (25/41) of the AKIs were considered to be related to septic shock; eight patients (19.5%) had histories of poor intake or volume loss before AKI; 12.2% (5/41) of AKIs occurred after administration of antiviral drugs or antibiotics, and no other triggers were identified. In general, AKI caused by septic shock and adverse drug effects occurred throughout the whole disease course, whereas volume insufficiency usually occurred in the early phase of the disease.
Pathologic Findings in Ten Deceased Patients with COVID-19 and AKI
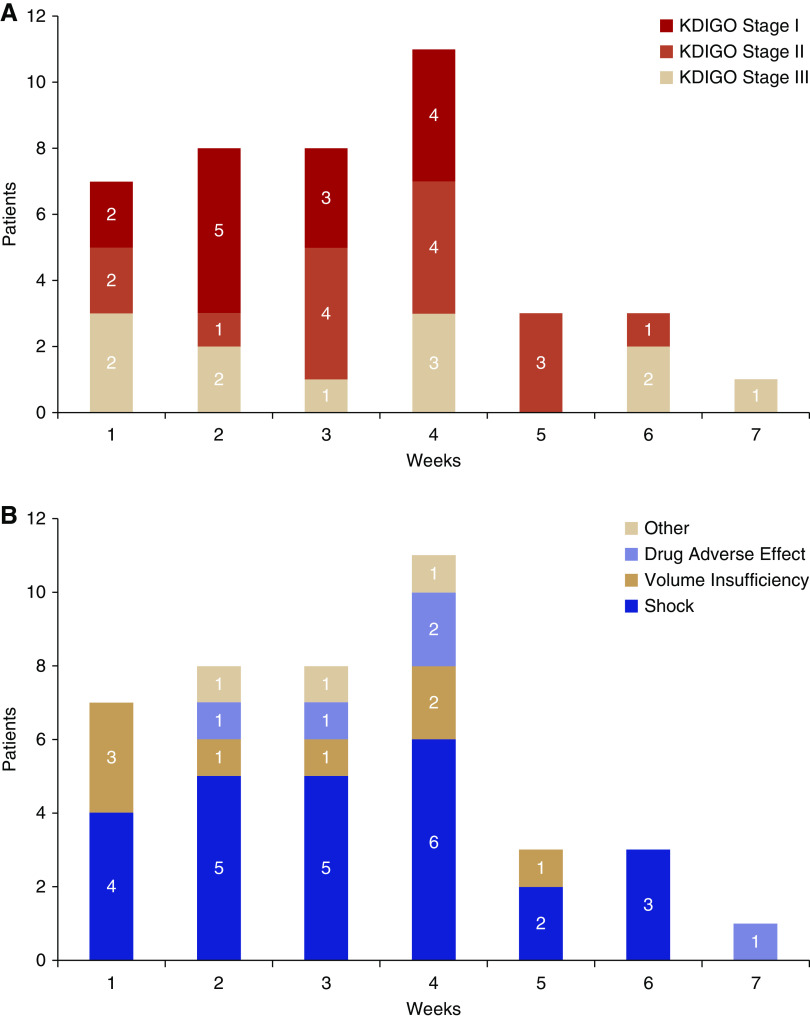
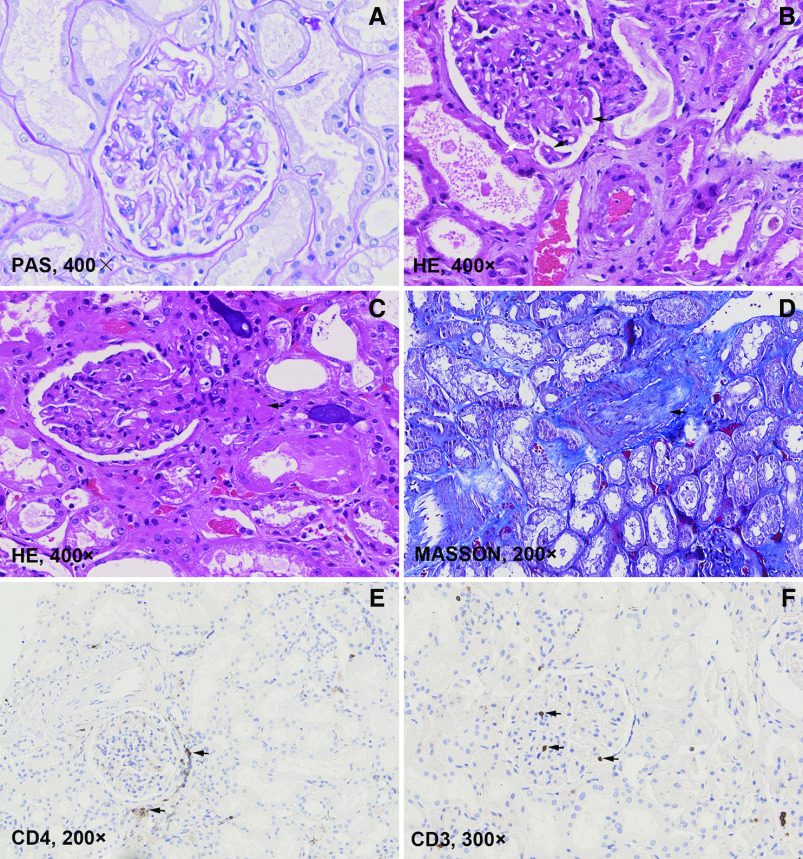
Kidney pathologic findings of ten deceased patients with AKI are summarized in Table 6. The autopsied patients with AKI had comparable demographic and clinical characteristics with other patients with AKI (Supplemental Table 3). All of the patients showed differing degrees of tubular injury and cytoplasmic vacuolization in tubular epithelial cells (Figure 3A). Patient 1 developed anuric AKI requiring CRRT after receiving antiviral medications. Crystallizations were observed in the proximal tubular cells and casts (Figure 3B), which suggested drug-induced AKI. Localized inflammatory cell infiltration was present in the interstitial area (Figure 3C). Glomerular lesions were not remarkable, except swollen endothelial cells and wrinkling of the glomerular basement membrane with double contours were occasionally observed (Figure 4, A and B). Arteriolar hyalinosis and intimal fibrosis were observed in several patients (Figure 4, C and D). Patient 5 had high titers of positive anti-phospholipid antibodies and probable cerebrovascular incidents before death. His renal pathology revealed venous thrombosis (Figure 3D).
Table 6.
Light microscopic pathologic findings of kidney tissues from ten deceased patients critically ill with COVID-19 and AKI
| Patient | Sex/Age | PMH | Baseline sCr (μmol/L) | Peak sCr (μmol/L) | Disease Course (d) | Glomerular Changes | Tubulointerstitial Changes | Vascular Changes |
|---|---|---|---|---|---|---|---|---|
| 1 | M/62 | HTN | N/A | 427 | 33 | Endothelial swollen | CV, focal TA and IF, crystallization casts, inflammatory cells infiltration | Intimal hyperplasia |
| 2 | M/82 | DM | 115 | 136 | 14 | NR | CV, focal ATI | NR |
| 3 | M/62 | – | 73 | 220 | 29 | NR | CV, focal ATI | Intimal hyperplasia |
| 4 | M/78 | HTN | 93 | 197 | 30 | Focal ischemic sclerosis | CV, focal TA and IF | Intimal hyperplasia, hyalinosis |
| 5 | M/73 | HTN, HBV | 70 | 147 | 24 | NR | CV, focal TA and IF, focal ATI | Intimal hyperplasia, venous thrombosis |
| 6 | M/59 | HTN | 48 | 139 | 39 | Hypertrophy | CV, focal ATI | Intimal hyperplasia, hyalinosis |
| 7 | M/86 | HTN, CAD | 82 | 195 | 45 | NR | CV, focal ATI | Intimal hyperplasia, hyalinosis |
| 8 | M/66 | – | 68 | 121 | 48 | Focal ischemic sclerosis | CV, focal ATI, focal TA and IF | Intimal hyperplasia, hyalinosis |
| 9 | F/57 | – | 32 | 128 | 36 | NR | CV, focal ATI | NR |
| 10 | F/66 | DM | 80 | 125 | 35 | NR | CV, focal ATI | NR |
M, male; PMH, past medical history; HTN, hypertension; DM, diabetes Mellitus; CAD, coronary artery disease; HBV, Hepatitis B virus infection; N/A, not available; CV, cytoplasmic vacuolization; TA, tubular atrophy, IF, interstitial fibrosis; NR, nonremarkable; ATI, acute tubular injuries; F, female.
Figure 3.
Pathologic findings in kidney tissues from patients critically ill with COVID-19 and AKI. (A) Diffuse tubular injury and cytoplasmic vacuolization in tubular epithelial cells (black arrow). (B) Crystallizations in casts and proximal tubular cells in a patient (black arrows). (C) Localized inflammatory cell infiltration was present in the interstitial area (black arrow). (D) Venous thrombosis in a patient with positive anti-phospholipid antibodies. (E–G) Scattered distribution of CD3-, CD20-, and CD8-positive lymphocytes in the interstitial area (black arrows). (H) Scattered distribution of CD68-positive macrophages in the interstitial area (black arrows). HE, hematoxylin and eosin.
Figure 4.
Pathologic findings in kidney tissues from patients critically ill with COVID-19 and AKI (continued). (A) Glomerulus with nonremarkable changes. (B) Glomerulus with swollen endothelial cells (white arrow), wrinkling of the glomerular basement membrane with double contours (black arrows), which was consistent with chronic glomerular ischemia. (C) Arteriolar hyalinosis (black arrow). (D) Intimal hyperplasia in interlobular artery. (E) CD4-positive cells around a glomerulus. (F) CD3 was occasionally positive within the glomerulus (black arrows). HE, hematoxylin and eosin; PAS, Periodic acid–Schiff.
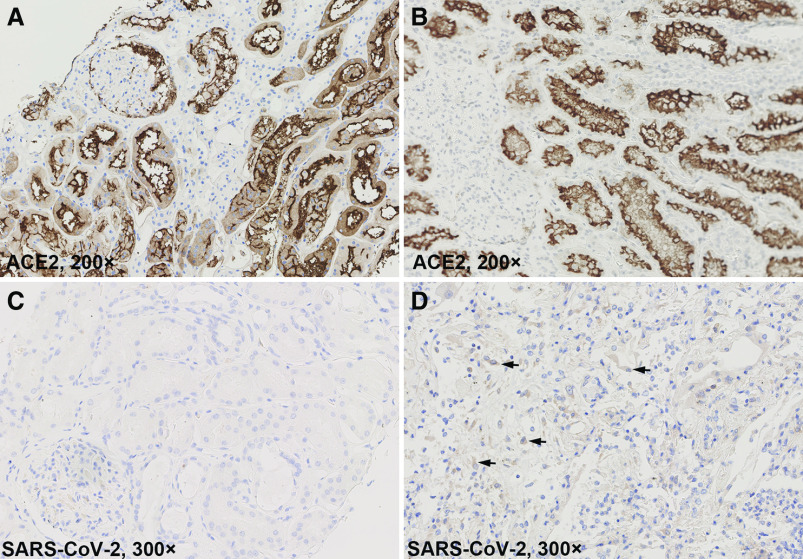
The IHC staining of CD3, CD4, CD8, CD20, and CD68 displayed scattered distributed lymphocytes and macrophages in the interstitial area (Figures 3, E–H and 4E). CD3-positive lymphocytes were also occasionally seen within the glomerulus (Figure 4F). Strong ACE2 expression was seen in both parietal epithelial cells of glomeruli and tubular epithelial cells (Figure 5A), which was not significantly different from normal kidney tissues (Figure 5B). SARS-CoV-2 was not detected by IHC in kidney tissues (Figure 5C).
Figure 5.
Immunochemistry staining of ACE2 and SARS-CoV-2. (A) ACE2 was strongly expressed in both parietal epithelial cells of glomeruli and tubular epithelial cells (brown area). (B) ACE expression in paracarcinoma normal kidney tissue from a patient with clear cell renal carcinoma. (C) IHC failed to detect SARS-CoV-2 in kidney tissues of patients with COVID-19. (D) Positive SARS-CoV-2 detection in both alveolar pneumocytes and macrophages in lung tissues (black arrows) of a patient with AKI (patient 5 in Table 6).
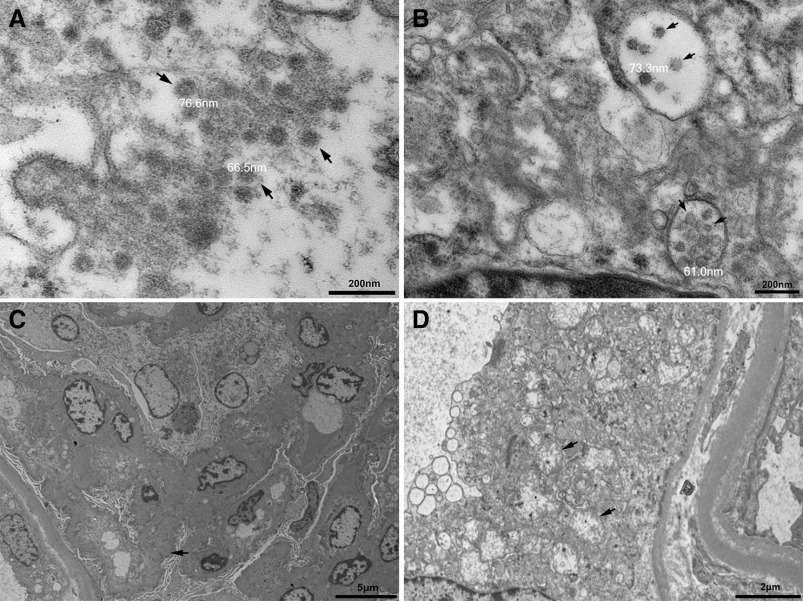
A few particles of a diameter of about 60–100 nm were observed in the cytoplasm of renal proximal tubular epithelial cells under EM (Figure 6A). Some of these particles were enclosed in vesicles (Figure 6B). Wrinkling of the glomerular basement membrane, varying degrees of foot process effacement, and subendothelial space expansion were present occasionally (Figure 6C). Cytoplasmic vacuolization and remnants of mitochondrial cristae at the periphery were observed in proximal tubular epithelial cells (Figure 6D). Peritubular capillary lumens were obstructed by abundant erythrocytes.
Figure 6.
EM findings in patients with AKI. (A) EM studies showed small particles (black arrows) in the cytoplasm in renal proximal tubular epithelium. The diameter of these particles varied from about 60 to 100 nm. (B) Particles in vesicles (black arrows) in proximal tubular epithelium. (C) Wrinkling of the glomerular basement membrane (black arrow), subendothelial space expansion, and varying degrees of foot process effacement were present occasionally. (D) Remnants of mitochondrial cristae at the periphery (black arrows) were observed in proximal tubular epithelial cells.
Nucleic Acid Tests of Urine Samples and Kidney Tissues
Kidney tissues from nine patients with AKI (except patient 8 in Table 6) were sent for nucleic acid RT-PCR tests for SARS-CoV-2, and the results were all negative. The PCR results of urine samples from another nine patients were also negative. The median time from disease onset to these tests was 29.0 (26.0–35.0) days.
Discussion
COVID-19 is currently a worldwide pandemic affecting >10 million people, causing >500,000 deaths. Most previous studies were conducted in patients with a short disease course (median time from illness to death was 16–18.5 days).3,7,9 As a multidisciplinary medical team that had been taking care of patients critically ill with COVID-19 in ICU since early February, we feel obligated to share our experience with the medical community. This study described the clinical characteristics of 81 patients who were old (mean age 66.6 years) and critically ill (rate of intubation >80%), with a relatively long disease course (median time from illness to death was 24 days). Hopefully, our study can provide helpful information to any physicians who are fighting against COVID-19 worldwide.
Our data showed that, in patients critically ill with COVID-19 in the ICU, the incidence of AKI was 50.6%, and >40% of these AKIs were KDIGO stage 3. These patients had a very high rate of invasive mechanical ventilation and multiple critical complications requiring aggressive management. Pei et al.28 also reported distinct incidences of AKI between patients noncritically ill with COVID-19 and those who were critically ill (4.0% versus 42.9%), supporting our findings. These findings were also consistent with the incidence of AKI in patients with non-COVID-19 ARDS (49.0%–68.3%).29,30
AKI is a common complication of patients in ICU settings, which can be caused by various conditions.31 Septic shock happened in >60% of our patients and was the leading cause for AKI, which was confirmed by the medical history and the pathologic findings of acute tubular injury (Figure 3, Table 6). Several patients with AKI had a history of poor intake and volume loss, and most of them recovered from AKI after fluid supplementation. Great attention should be paid to the hemodynamics and volume balance of patients with COVID-19.
A variety of antiviral drugs are being tested for their efficacy against COVID-19 worldwide.32,33 All types of antibiotics were also used to manage the secondary bacterial infections. In this study, three patients had AKI after receiving antiviral medications. One of them recovered from AKI after discontinuing the suspicious drug, and the other two patients required CRRT support. The pathologic findings of crystallizations in the proximal tubular cells and casts highly implies drug-induced AKI in one patient (Figure 3B). Another two patients experienced AKI after antibiotic treatment. One of them received vancomycin, and the other one received piperacillin-sulbactam, which are both well known renal-toxic antibiotics. These findings indicate that we should carefully select treatments and closely monitor the renal functions of patients with COVID-19.
Another concern is the possible direct attack by SARS-CoV-2 to the kidney because of the abundant expression of ACE2 in kidney tissues (Figure 5A). However, the current evidence of SARS-CoV-2 infecting the kidney are not universally agreed (B. Diao, C. Wang, R. Wang, Z. Feng, Y. Tan, H. Wang, et al.: Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection, 2020; doi:10.1101/2020.03.04.20031120).19,34–36 Our EM examinations of kidney tissues revealed particles that greatly resemble the structures found in a recent report of virus-like particles.18 However, some pathologists suspected these particles were clathrin-coated vesicles or multivesicular bodies based on the ultrastructural features and virus morphology and morphogenesis.22,23,37 Additional tools including immunogold labeling and in situ hybridization might be useful for further identification.38 Furthermore, the RT-PCR and IHC tests of kidney tissues in this study were all negative. A previous study suggested the median time of virus shedding was 20 days.3 Viral load in tissues other than the lungs were also reported to be either negative or much lower.19,36 Considering the median disease course of the ten patients who were autopsied was 34 days (IQR, 28–40), this might account for the lack of virus detection in the kidneys. Regardless, the renal pathologic changes were predominately acute tubular injury rather than glomerulopathy or interstitial nephritis. These changes were more likely caused by ischemia rather than viral infection.
The hypothesis that inflammatory cytokines might contribute to the immunologic disorders and multiorgan dysfunctions of patients with COVID-19 has been proposed.20,39 We found significantly elevated serum IL-6, lower platelet count, and prolonged international normalized ratio in patients with AKI (Tables 1 and 2). Serum IL-6 levels were also associated with both AKI and death (Tables 4 and 5). It is believed that rigorous inflammatory reactions could activate the platelets and injure the endothelial cells, leading to clots, coagulopathy, and microvascular dysfunction.32,40 Antiphospholipid syndrome was recently reported in COVID-19.41 We also found one patient with suspected antiphospholipid syndrome who presented with AKI and venous thrombosis in kidney tissues (Figure 3D). Considering potential correlations between inflammation and organ dysfunctions (Table 5), we speculated that inflammation-mediated vascular endothelial injury and a dysregulated immune response might play an important role in patients critically ill with COVID-19. Blood purification therapies targeting inflammatory molecules were delivered to several patients and their serologic response turned out to be promising.42
AKI served as a risk factor for mortality of patients with COVID-19 in Cheng et al.’s12 study. Due to the limited size of samples, different Cox proportional hazards models were tested. The results showed that KDIGO stage 3 AKI was associated with death in this group of patients (Table 5). Supporting this finding, the patients with AKI who died within a shorter disease course also had a faster decline of renal function. Other potential risk factors for death included male sex, elevated D-dimer, higher serum IL-6 levels, and higher SOFA scores, which is consistent with another report.3
Our study does have several limitations. The number of enrolled patients were limited. A small proportion of the patients had no baseline sCr value available, which might lead to misestimation of AKI, although three nephrologists from PUMCH reviewed the medical records independently to generate the consensual diagnosis. The tests of tubular functions were lacking and we failed to collect enough data from urine protein/creatinine results.
In conclusion, we reported the clinicopathologic characteristics and prognosis of AKI in patients critically ill with COVID-19 who had a long disease course. AKI was a common and multifactorial complication in the late stage of patients critically ill with COVID-19 in the ICU. Patients with AKI were of an older age, had a lower lymphocyte and platelet count, and a tendency for coagulopathy and hyperinflammation. Acute tubular injury was predominant in renal pathology. Older age and higher serum IL-6 level were associated with AKI. KDIGO stage 3 AKI independently predicted death. The early involvement of nephrologists might improve the outcome of AKI in critical patients with COVID-19.
Disclosures
All authors have nothing to disclose.
Funding
This work was supported by National Natural Sciences Foundation of China grant 81970621 (to Y. Qin); Foundation of Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology grant XXGZBDYJ010 (to G. Wang); and Fundamental Research Funds for the Central Universities grant 3332019029 (to P. Xia).
Supplementary Material
Acknowledgments
We wish to thank Ms. Cynthia S. Goldsmith, US Centers for Disease Control and Prevention for her kind help in reviewing the EM images and providing inspiring suggestions. We thank Dr. Xiaoyin Bai, Dr. Siyuan Fan, and Dr. Hua Zheng from PUMCH for their advice and assistance. We wish to thank all of the patients and their family members included in this study.
P. Xia, Y. Wen, W. Cao, G. Wang, and Y. Qin designed this study; S. Zhang, X. Yan, T. Li, B. Du, Z. Liu, X. Zhou, Y. Qin, J. Wang, J. Ma, and P. Xia cared for the included patients; P. Xia, J. Ma, Y. Zhou, G. Chen, W. Jiang, and Y. Hu extracted the clinical data; Y. Duan, S. Xu, H. Cai, H. Wu, Z. Liang, W. Cao, and G. Wang performed the light microscope and IHC studies; H. Su and C. Zhang performed the EM studies; and Y. Qin, G. Wang, L. Chen, C. Zhang, and X. Li critically reviewed the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020040426/-/DCSupplemental.
Supplemental Table 1. Risk factors for AKI tested by univariate cox proportional hazards models.
Supplemental Table 2. Risk factors predicting death tested by univariate cox proportional hazards model.
Supplemental Table 3. The autopsied patients had comparable demographic and clinical features with other patients.
Supplemental Figure 1. Compositions of secondary infections.
References
- 1.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. ; China Novel Coronavirus Investigating and Research Team : A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382: 727–733, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization: Coronavirus disease (COVID-19) pandemic. Available at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed July 31, 2020
- 3.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al.: Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study [published correction appears in Lancet 395: 1038, 2020]. Lancet 395: 1054–1062, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. : Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323: 1061–1069, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang T, Du Z, Zhu F, Cao Z, An Y, Gao Y, et al. : Comorbidities and multi-organ injuries in the treatment of COVID-19. Lancet 395: e52, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497–506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. ; China Medical Treatment Expert Group for Covid-19 : Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382: 1708–1720, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. : Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med 8: 475–481, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T, Wu D, Chen H, Yan W, Yang D, Chen G, et al. : Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 368: m1091, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Li X, Chen H, Yan S, Li D, Li Y, et al. : Coronavirus disease 19 infection does not result in acute kidney injury: An analysis of 116 hospitalized patients from Wuhan, China. Am J Nephrol 51: 343–348, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. : Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395: 507–513, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. : Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 97: 829–838, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naicker S, Yang CW, Hwang SJ, Liu BC, Chen JH, Jha V: The novel coronavirus 2019 epidemic and kidneys. Kidney Int 97: 824–828, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirsch JS, Ng JH, Ross DW, Sharma P, Shah HH, Barnett RL, et al. ; Northwell COVID-19 Research Consortium; Northwell Nephrology COVID-19 Research Consortium : Acute kidney injury in patients hospitalized with COVID-19. Kidney Int 98: 209–218, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ling Y, Xu SB, Lin YX, Tian D, Zhu ZQ, Dai FH, et al. : Persistence and clearance of viral RNA in 2019 novel coronavirus disease rehabilitation patients. Chin Med J (Engl) 133: 1039–1043, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie C, Jiang L, Huang G, Pu H, Gong B, Lin H, et al. : Comparison of different samples for 2019 novel coronavirus detection by nucleic acid amplification tests. Int J Infect Dis 93: 264–267, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, et al. ; Singapore 2019 Novel Coronavirus Outbreak Research Team : Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA 323: 1488–1494, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Su H, Yang M, Wan C, Yi LX, Tang F, Zhu HY, et al. : Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 98: 219–227, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Puelles VG, Lütgehetmann M, Lindenmeyer MT, Sperhake JP, Wong MN, Allweiss L, et al. : Multiorgan and renal tropism of SARS-CoV-2 [published online ahead of print May 13, 2020]. N Engl J Med 10.1056/NEJMc2011400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin L, Lu L, Cao W, Li T: Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect 9: 727–732, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, et al. : A pathological report of three COVID-19 cases by minimally invasive autopsies. Zhonghua Bing Li Xue Za Zhi 49: E9, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Miller SE, Brealey JK: Visualization of putative coronavirus in kidney. Kidney Int 98: 231–232, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calomeni E, Satoskar A, Ayoub I, Brodsky S, Rovin BH, Nadasdy T: Multivesicular bodies mimicking SARS-CoV-2 in patients without COVID-19. Kidney Int 98: 233–234, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Health Commission of China: Guideline of management of COVID-19 (5th trial edition). Available at: http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml
- 25.Khwaja A: KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract 120: c179–c184, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Shankar-Hari M, Phillips GS, Levy ML, Seymour CW, Liu VX, Deutschman CS, et al. ; Sepsis Definitions Task Force : Developing a new definition and assessing new clinical criteria for septic shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 315: 775–787, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fan E, Brodie D, Slutsky AS: Acute respiratory distress syndrome: Advances in diagnosis and treatment. JAMA 319: 698–710, 2018. [DOI] [PubMed] [Google Scholar]
- 28.Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C, et al. : Renal involvement and early prognosis in patients with COVID-19 pneumonia. J Am Soc Nephrol 31: 1157–1165, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panitchote A, Mehkri O, Hastings A, Hanane T, Demirjian S, Torbic H, et al. : Factors associated with acute kidney injury in acute respiratory distress syndrome. Ann Intensive Care 9: 74, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McNicholas BA, Rezoagli E, Pham T, Madotto F, Guiard E, Fanelli V, et al. ; ESICM Trials Group and the Large observational study to UNderstand the Global impact of Severe Acute respiratory FailurE (LUNG SAFE) Investigators : Impact of early acute kidney injury on management and outcome in patients with acute respiratory distress syndrome: A secondary analysis of a multicenter observational study. Crit Care Med 47: 1216–1225, 2019. [DOI] [PubMed] [Google Scholar]
- 31.Griffin BR, Liu KD, Teixeira JP: Critical care nephrology: Core curriculum 2020. Am J Kidney Dis 75: 435–452, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li T, Lu H, Zhang W: Clinical observation and management of COVID-19 patients. Emerg Microbes Infect 9: 687–690, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalil AC: Treating COVID-19–off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 323: 1897–1898, 2020 [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, et al. : Detection of SARS-CoV-2 in different types of clinical specimens. JAMA 323: 1843–1844, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q: Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis 20: 411–412, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yao XH, He ZC, Li TY, Zhang HR, Wang Y, Mou H, et al. : Pathological evidence for residual SARS-CoV-2 in pulmonary tissues of a ready-for-discharge patient. Cell Res 30: 541–543, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldsmith CS, Miller SE, Martines RB, Bullock HA, Zaki SR: Electron microscopy of SARS-CoV-2: A challenging task. Lancet 395: e99, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goldsmith CS, Tatti KM, Ksiazek TG, Rollin PE, Comer JA, Lee WW, et al. : Ultrastructural characterization of SARS coronavirus. Emerg Infect Dis 10: 320–326, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK : COVID-19: Consider cytokine storm syndromes and immunosuppression. Lancet 395: 1033–1034, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chanchal S, Mishra A, Singh MK, Ashraf MZ: Understanding inflammatory responses in the manifestation of prothrombotic phenotypes. Front Cell Dev Biol 8: 73, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Xiao M, Zhang S, Xia P, Cao W, Jiang W, et al. : Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 382: e38, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ma J, Xia P, Zhou Y, Liu Z, Zhou X, Wang J, et al. : Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol 214: 108408, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.