INTRODUCTION
The mitral valve is a dynamic, three-dimensional (3D) structure consisting of the mitral annulus, leaflets, tendinous chordae, and papillary muscles. During systole, increased left ventricular (LV) pressure displaces the mitral valve leaflets towards the left atrium while tethering forces from the chordae tendineae pull the leaflets in the direction of the left ventricle. The saddle-shape morphology of the mitral valve allows for the balance of these counteracting forces by optimizing leaflet curvature, thus minimizing mitral leaflet stress (1).
Mitral regurgitation (MR) is defined as an abnormal reversal of blood flow from the left ventricle to the left atrium, which occurs most commonly during ventricular systole. Complete coaptation and correct apposition of the mitral valve leaflets is essential in order to prevent the occurrence of MR. The etiologies of MR can be divided into primary abnormalities of the mitral valve apparatus (including the annulus, leaflets, chordae tendineae, and/or papillary muscles) and secondary causes that do not directly involve the mitral valve. Recognition of MR is clinically important, as it represents an increasingly prevalent valvular disease associated with significant morbidity and mortality.
In this manuscript, we will first review normal mitral valve anatomy, highlighting the individual structure and function of each component of the mitral valve apparatus. We will then discuss the approach to classification of mitral valve disease, including the classic Carpentier approach as well as recent proposed modifications to this classification schema. Thereafter, we will highlight several novel mechanisms of MR that have recently been described in the literature including mitral valve clefts, mitral annular disjunction, atrial fibrillation-related MR, and MR in hypertrophic cardiomyopathy (HCM). Finally, we will discuss the integral role of 3D echocardiography in the diagnosis and management of MR.
DISCUSSION
Anatomy
A. Annulus
The mitral valve annulus refers to the junctional zone separating the left atrium from the left ventricle. It is not a rigid fibrous ring, but rather a pliable structure which exhibits dynamic alterations in shape throughout the cardiac cycle. The annulus, which serves as the attachment point for the mitral valve leaflets and thus delineates the leaflet hinge line, is oval shaped, with the commissural diameter being larger than the anteroposterior (AP) diameter (2).
The anterior portion of the mitral valve annulus shares a fibrous region of continuity between the aortic root and the anterior mitral leaflet, where it is bordered by the right and left fibrous trigones. These are separated by a rigid span of fibrous tissue known as the inter-trigonal region, also called the aortic mitral-curtain (Figure 1). Because the anterior portion of the mitral annulus is fibrous, it is less prone to dilatation. The remaining two-thirds of the annulus is predominantly muscular. The posterior annulus receives some muscular fiber from the proximal aspect of the posterior leaflet, which may contribute to annular flexibility, and is separated from the left ventricle by collagenous elements (3). In patients with significant MR, the posterior annulus often is dilated, and is also more prone to calcification (2).
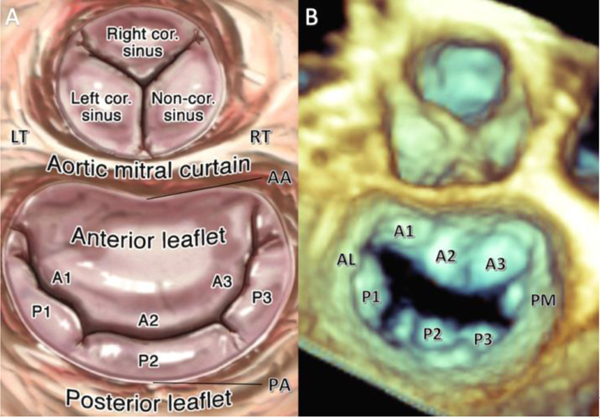
Figure 1:
(A) Normal mitral valve anatomy showing anterior and posterior leaflets and annuluses. The anterior annulus is separated from the aortic root by the fibrous intertrigonal region, also known as the aortic-mitral curtain, which is surrounded by the right and left fibrous trigones. (B) 3D TEE image from Surgeon’s View of normal mitral valve anatomy as described above. LT = left trigone, RT = right trigone, AA = anterior annulus, PA = posterior annulus, AL = anterolateral commissure, PM = posteromedial commissure. Reproduced from Lang RM, Tsang W, Weinert L, Mor-Avi V, Chandra S. J Am Coll Cardiol 2011 November 1;5 8(19):1933–1944.
In order to function properly, the mitral valve annulus requires 3D deformation in its circumference, curvature, excursion, shape, and size. All of these features are also vulnerable to alterations in left ventricular remodeling (4). As an example, in patients with dilated cardiomyopathy, mitral annular area and circumference are significantly enlarged (5,6). This is accompanied by mitral annular flattening together with a reduction in mitral annular area contraction throughout the cardiac cycle (7,8). These geometric changes result in unfavorable mitral valve leaflet remodeling, augmented leaflet stress, and increased severity of MR.
B. Leaflets
The mitral valve consists of the anterior and posterior leaflets, also known as the aortic and mural leaflets, respectively. The posterior leaflet is the narrower of the two, and spans two-thirds of the area around the left atrioventricular junction within the inlet portion of the left ventricle. In adults, the posterior leaflet commonly has two indentations resulting in three independently mobile scallops identified as P1, P2, and P3 (lateral to medial, respectively). These indentations typically do not reach the annulus. If they do, this finding is frequently associated with MR (2).
The anterior leaflet is much broader, comprising one-third of the annular circumference. The distinguishing feature of the anterior leaflet is its fibrous continuity with the left and non-coronary cusps of the aortic valve and with the inter-trigonal region located between the aortic cusps that abuts onto the membranous septum. The anterior leaflet is divided arbitrarily into three regions referred to as A1, A2, and A3 (lateral to medial), corresponding to the adjacent regions of the posterior leaflet (2). The line of closure between the anterior and posterior leaflets ends at the junctions of both mitral leaflets, forming the posteromedial and anterolateral commissures (3).
The mitral valve leaflets are described as having basal, clear, and rough zones. The basal zone is described as the area where the leaflet connects to the annulus. The thin central portion of the leaflet is the clear zone. The free edge of the leaflet represents the thick rough zone, which is the region of leaflet coaptation and apposition as well as the area of chordal attachment. The atrial surface of the leaflets is free of attachments, contrasting with the ventricular leaflet surface where the chordae tendinae connect the leaflets to the papillary muscles. As mentioned previously, the region of coaptation serves an important role, as MR can develop if the leaflets do not sufficiently cover the mitral annular orifice throughout left ventricular systole (2).
C. Chordae Tendinae
The chordae tendineae play an important role in withstanding the tensile stress exerted by the papillary muscles. Normally, the mitral valve leaflets have an array of fan-shaped chordae tendineae extending from the papillary muscles which insert into the ventricular surface of the leaflets. There are three types of chordae tendinae, identified by the region where they attach to the leaflets.
Primary chordae attach to the free edge of both leaflets (the rough zone), and are responsible for leaflet apposition. Secondary chordae attach to the body of the leaflets and contribute toward maintenance of normal ventricular geometry. Among the secondary chordae of the anterior leaflet, the two largest and thickest, called the strut cords, arise from the tip of each papillary muscle and are considered to be the strongest. Tertiary chordae are found in the posterior leaflet and attach the basal zone of the posterior leaflet directly to the ventricular wall (2,3).
D. Papillary muscles
The anterolateral (AL) and posteromedial (PM) papillary muscles are located at the mid to apical segment of the left ventricle. The AL papillary muscle chordae attach the lateral half of the mitral valve leaflets (i.e. anterolateral commissure, A1, P1, and half of P2 and A2) to the border of the anterolateral and inferolateral ventricular wall. The PM papillary muscle attaches the medial half of both leaflets (i.e. posteromedial commissure, P3, A3, and half of P2 and A2) to the inferior wall of the left ventricle. Of note, there is typically one AL papillary muscle head, compared with the multi-head PM papillary muscle, with considerable variation described in the size and number of these muscles. In some cases, the papillary muscles are indistinguishable, having been replaced with multiple small muscle bundles attaching to the ventricular wall (2).
The role of papillary muscle function is highlighted by the schematic of classic ischemic MR, which was thought to occur secondary to PM papillary muscle dysfunction. The mechanism for this was explained by the PM muscle’s dependence on a single blood supply from the posterior descending coronary artery, as opposed to the dual blood supply seen with the AL papillary muscle (9). However, over the past decade three-dimensional echocardiography (3DE) has shown that papillary muscle dysfunction is not solely responsible for ischemic MR, but rather by a wide range of geometric distortions secondary to left ventricular remodeling following myocardial infarction (MI) (10).
Categorization of Mitral Valve Dysfunction: Carpentier Classification and Beyond
In 1983, Carpentier proposed a classification scheme for the different types of mitral valve dysfunction based upon the type of leaflet motion, in an effort to inform the appropriate surgical strategy (11). Type I dysfunction refers to valve dysfunction due to mitral annular dilatation or leaflet perforation, whereas leaflet motion is normal. Type II dysfunction refers to leaflet prolapse and flail (excessive motion of the leaflet edge above the plane of the annulus) due to excessive and redundant leaflet tissue or chordal rupture, respectively. Type IIIa dysfunction is defined as leaflet restriction during both systole and diastole due to fusion of various components of the mitral valve apparatus (as is the case in rheumatic valve disease), whereas Type IIIb dysfunction occurs because of restricted leaflet motion in diastole alone, due to leaflet tethering (Figure 2).
Figure 2:
Carpentier classification of mitral valve dysfunction is based on the motion of the free margin of the affected leaflet in relation to the annular plane. Type I dysfunction is characterized by normal leaflet motion, Type II by increased leaflet motion, Type IIIa by restricted leaflet motion during systole and diastole, and Type IIIb by restricted leaflet motion during systole.
Source: Stone GW, Vahanian AS, Adams DH, et al. Clinical Trial Design Principles and Endpoint Definitions for Transcatheter Mitral Valve Repair and Replacement: Part 1: Clinical Trial Design Principles. JACC 2015; 66:278–307. Reproduced with permission.
I. Type I Mitral Valve Dysfunction
Type I dysfunction is a less frequently encountered type of mitral valve dysfunction, with typical examples arising from mitral valve endocarditis causing anterior or posterior MV leaflet perforation by localized tissue destruction, long-standing atrial fibrillation causing left atrial enlargement and subsequent annular dilatation, or the presence of a cleft mitral valve. Aortic valve endocarditis can also directly extend to involve the anterior MV leaflet via the shared fibrous trigone (12).
Diastolic MR is another example of Type I dysfunction, and can be seen in patients with atrioventricular block, when atrial contraction is asynchronous with LV contraction. MR results when ventricular pressure is higher than atrial pressure during atrial relaxation while there is incomplete mitral valve closure. Another common etiology of diastolic MR is significant elevation of the LV end-diastolic pressure (LVEDP) that may occur in the presence of significant LV dysfunction or severe aortic regurgitation (13).
We will provide an in-depth discussion of the more recently recognized Type I mechanisms in the section: Novel Mechanisms of Mitral Regurgitation.
II. Type II Mitral Valve Dysfunction
Type II mitral valve dysfunction, also known as degenerative mitral valve disease or mitral valve prolapse (MVP), is caused by disproportionate excursion of one or both mitral valve leaflets into the left atrium during systole (defined as ≥2 mm beyond the annulus). Mitral valve prolapse is the most frequent cause of MR in developed countries (14).
The two most common types of primary mitral valve disease that cause mitral valve prolapse (MVP) are fibroelastic deficiency (FED) and diffuse myxomatous disease (DMD), better known by the eponym of Barlow’s disease.
Fibroelastic deficiency is characterized by myxomatous degeneration localized to one mitral valve segment with leaflet redundancy and thickening of the flail segment, while the remainder of the valve is thin and translucent (15). The valves of these patients are often deficient in collagen, elastin, and proteoglycans. There is typically an acute loss of mechanical integrity which results in a localized, single-segment prolapse due to elongated chordae or flail leaflet due to ruptured chordae (Figure 3). Patients with fibroelastic deficiency most commonly present around the sixth decade of life with a more acute presentation of MR (16). Fibroelastic deficiency is the most common form of primary mitral valve disease for which mitral valve repair surgery is required.
Figure 3:
(A) 3D parametric image of mitral valve demonstrating flail P2 segment of posterior leaflet of mitral valve, a form of fibroelastic deficiency (B) 3D TEE image in Surgeon’s View demonstrating flail P2 segment of posterior leaflet (C) 3D TEE with transillumination (Philips) in the same patient demonstrating flail P2 leaflet with visible ruptured chord.
In contrast, Barlow’s disease is typified by diffuse thickening and redundancy of both mitral valve leaflets secondary to abnormal accumulation of mucopolysaccharides in multiple scallops as well as the chordae tendineae (16). This myxoid infiltration results in thick, redundant, billowing leaflets and elongated chordae, causing bileaflet, multi-segmental prolapse (Figure 4). Barlow’s disease is typically diagnosed in young adulthood, with indications for surgery often not met until the fourth or fifth decade of life.
Figure 4:
(A) Mid-esophageal bi-commissural (60 degree) 2D view on TEE showing bi-leaflet, multi-segmental billowing of the anterior and posterior mitral valve leaflets in a patient with Barlow’s disease (B) Mid-esophageal (0 degree) color Doppler TEE image of the same patient demonstrating mild MR as a result of bi-leaflet billowing (C) In Barlow’s disease, there is systolic protrusion of the body of the mitral valve leaflets above the annular plane (black dashed line) while the free leaflet edges remain at or below the annular plane.
A subgroup of patients with Type II dysfunction demonstrate a flail or partial flail mitral leaflet. In contrast to mitral valve prolapse, where the leaflet tip remains attached to the papillary muscle and points towards the left ventricular apex, flail leaflet is defined as the loss of the normal leaflet attachment to the left ventricular myocardium such that the leaflet tip points toward the roof of the left atrium. The leaflet is characterized as flail when most of the anterior or posterior leaflet is detached from the papillary muscle, while partial flail leaflets involve only one mitral valve scallop or a smaller segment of the leaflet. Etiologies of flail leaflet include mitral valve prolapse, endocarditis, trauma, and papillary muscle rupture in the setting of acute MI.
Type II mitral valve dysfunction has also been associated with inherited connective tissue disorders, in particular with collagen mutations (i.e. Ehlers-Danlos, Osteogenesis Imperfecta, Marfan Syndrome) (12).
III. Type IIIa Mitral Valve Dysfunction
Most commonly caused by rheumatic heart disease, Type IIIa mitral valve dysfunction is characterized by commissural fusion, leaflet thickening, chordal fusion, and leaflet restriction during both systole and diastole. Rheumatic heart disease is less commonly seen in developed nations but continues to be a significant cause of mitral valve disease worldwide (17). The mitral regurgitant jet may be either centrally or eccentrically directed, and can be present with concurrent mitral stenosis (MS). On transthoracic echocardiography (TTE), rheumatic mitral valve disease has the appearance of a “hockey stick.” An example of rheumatic heart disease leading to Type III dysfunction and subsequent MR is demonstrated in Figure 5. Another type of Type IIIa mitral valve dysfunction is caused by radiation-induced valvular heart disease, which is characterized by diffuse valvular fibrosis and focal calcification that typically involves the aortic-mitral curtain. Valvular dysfunction is more commonly seen as the time interval from radiotherapy increases. Both MR and MS can be seen with radiation due to the variable degree of valve thickening and calcification that may occur. Unlike rheumatic heart disease, commissural fusion is not typically seen with radiation-induced valvular heart disease (18).
Figure 5:
(A) Parasternal long-axis TTE view showing doming of anterior MV leaflet and restricted motion of posterior MV leaflet secondary to rheumatic mitral valve disease (RMVD) leading to mitral stenosis and MR due to leaflet malcoaptation (B) Apical two-chamber TTE view demonstrating diffuse thickening of MV leaflet as well as sub-valvular apparatus (white arrow) (C) 3D TEE view of MV from left atrium (Surgeon’s View) demonstrating reduced mitral valve area and bicommissural fusion in patient with RMVD (D) RMVD visualized from ventricular perspective using 3D TEE, demonstrating diffuse leaflet calcification and thickening leading to malcoaptation and MR. AL = anterior MV leaflet, PL = posterior MV leaflet.
IV. Type IIIb Mitral Valve Dysfunction
Type IIIb mitral valve dysfunction is characterized by leaflet restriction during diastole, and can be caused by myocardial infarction or non-ischemic dilated cardiomyopathy.
A common cause of type IIIb dysfunction is ischemic MR following MI, which occurs in approximately 20–25% of MI patients even following revascularization. In these patients, MR portends a significantly worse prognosis, regardless of the severity (14). Ischemic MR is caused by left ventricular remodeling after myocardial infarction. Most commonly the mechanism involves apical and lateral displacement of papillary muscles that leads to tethering of the mitral valve leaflets. This results in apical tenting of the leaflets and prevents excursion of the free margin to the plane of the annulus, which leads to malcoaptation and subsequently MR (19) (Figure 6).
Figure 6:
A) TTE image in parasternal long-axis view at end-diastole demonstrating inferolateral wall thinning (white arrow) secondary to myocardial infarction of right coronary artery (B) Mid-esophageal four-chamber TEE view at 0 degrees at end-diastole showing posterior leaflet retraction causing malcoaptation and leading to (C) severe MR as visualized on color Doppler at the mid-esophageal four-chamber TEE view at 0 degrees (D) 3D TEE in Surgeon’s view demonstrating asymmetric coaptation of the mitral valve leaflets due to ischemic MV disease leading to a discrete area of malcoaptation in the A3-P3 region (black arrow). AL = anterior MV leaflet, PL = posterior MV leaflet.
V. Modified Echocardiography-Based Classification of Mitral Regurgitation Mechanisms
In recent years, Shah and Raney proposed a modified echocardiographic classification of MR mechanisms as visualized by 3DE. The modified classification schema expands upon the types of mitral valve dysfunction. Type IVa describes systolic anterior motion (SAM) of the mitral valve, a phenomenon which is characteristic of HCM. Type IVb describes pathologic SAM following surgical mitral valve repair. Type IVc describes hemodynamically-induced mitral valve dysfunction (i.e. hypovolemia, inotrope-induced). Type V mitral valve dysfunction encompasses hybrid conditions, such as the combination of rheumatic valvular disease with an endocarditis-induced perforation or lesion (20).
Two-Dimensional Echocardiography
As described in the 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation, the identification of MR mechanism and etiology is most commonly obtained using TTE. MV morphology should be carefully assessed in multiple windows using B-mode imaging to evaluate structure and leaflet motion and color flow Doppler should be used to localize the origin of the MR jet. If image quality is insufficient with TTE, TEE may often be needed for better visualization of different culprit lesions such as flail segments and for more precise diagnosis of mitral valve pathology (21).
The two-dimensional (2D) appearance of the normal mitral valve may vary depending upon the imaging plane from which it is visualized. In the parasternal long axis and apical views, the mitral valve resembles clapping hands, with the anterior “hand” longer and more mobile than the posterior. In the parasternal short axis plane, the valve appears as an ovoid orifice. The papillary muscles can be seen in the parasternal short axis at approximately the four o’clock (AL) and eight o’clock (PM) orientations (Figure 7A). Other anatomic features that can be appreciated on 2D echocardiography include the chordae tendineae and the mitral annulus.
Figure 7:
(A) Parasternal short-axis view on TTE showing anterolateral (AL) and posteromedial (PL) papillary muscles at approximately the four o’clock and eight o’clock positions, respectively. (B) Transgastric TEE short-axis view at the level of the mitral valve leaflets showing the six scallops of the anterior (A1-A3) and posterior (P1-P3) mitral valve leaflets.
Mitral leaflet anatomy can be localized on standard two-dimensional imaging views. In the parasternal long axis on 2D TTE, the A2 and P2 segments are seen. In the short-axis view of the mitral valve, the A1 and P1 segments are adjacent to the left atrial appendage (which is located on the right side of the image), and it is thus possible to visualize all three anterior and posterior scallops (Figure 7B).
On 2D transesophageal echocardiography (TEE), in the four-chamber view at 0 degrees, with the anterior mitral valve leaflet located to the left of the posterior leaflet, it is possible to localize lesions to one (or both) of the two leaflets. In the mitral commissural view at approximately 60 degrees, with P3 located on the left side of the image and P1 located adjacent to the left atrial appendage, it is possible to identify which scallop is involved by different pathologies. Using these criteria, it is possible to localize the pathology to the appropriate scallop of the anterior or posterior mitral valve leaflet (Figure 8). With the recent adoption of 3DE, the diagnosis of mitral valve prolapse by conventional 2D echocardiography has been less accurate because of the discrepancy between the non-planar leaflet-annular relationship in intersecting 2D views (3).
Figure 8:
(A) Four-chamber view on TEE (0 degrees) showing A3, A2, P2, and P1 scallops (from left to right) of mitral valve (B) Mitral bi-commissural view on TEE (50–70 degrees) showing the mitral valve scallops from medial to lateral position (left to right: P3, A3, A2, A1, P1). Using the combination of these two TEE views, it is possible to localize mitral valve pathology in the majority of cases.
Three-Dimensional Echocardiography
3DE has become an invaluable tool in enhancing the understanding of mitral annular geometry and dynamics. By highlighting the role of annular nonplanarity, 3DE plays an important role in assessing the suitability of mitral valve prostheses as well as other repair strategies aimed at restoring or maintaining the saddle shape of the annulus. 3DE has also helped to clarify the mechanisms of MR in degenerative as well as ischemic MR and provides a dynamic 3D road map during surgical or transcatheter edge-to-edge repair (3,4).
The anatomic features of the mitral valve leaflets, including the quadrangular and semicircular shapes of the posterior and anterior leaflets are best visualized using 3DE. The surgeon’s view (or “en-face” view) is the recommended view to display the mitral valve on 3DE when viewed from the left atrium, with the aortic valve in the 12-o’clock position. The best window to obtain this view is in the parasternal long axis or apical 4-chamber view on TTE and in the 0–120 degree midesophageal views on TEE. This common view of the mitral valve allows the structural imager to accurately communicate with the interventionalist during structural procedures (3).
In degenerative mitral valve disease, 3DE has the ability to differentiate between Barlow’s disease and fibroelastic deficiency. When 3DE quantitative parameters were used to distinguish patients with and without degenerative mitral valve disease, billowing height and volume were the strongest predictors for the presence of degenerative mitral valve disease (4). 3DE also demonstrated that although curvature across most of the mitral valve surface is fairly constant, there is a single focus of regional curvature heterogeneity within the P1 region and two large foci in P2, which may account for the increased likelihood of these two segments to prolapse or flail in degenerative mitral valve disease (22).
Consistent with historical precedent, recent 3DE studies have confirmed the presence of two distinct phenotypes of degenerative mitral disease, diffuse myxomatous disease (DMD) and fibroelastic deficiency (FED), with considerable mechanistic differences between the two despite the common presence of prolapsing leaflets. In particular, annular dysfunction is more frequent in DMD, which can worsen the degree of MR via progressive annular enlargement. However, due to significant leaflet redundancy and tissue reserve in DMD, systolic unfurling, or unfolding of the mitral valve leaflets, occurs to compensate the leaflet prolapse and reduces the degree of regurgitation. Conversely, in FED, tissue paucity due to a lack of collagen, elastin, and proteoglycans can leave some valvular areas uncovered, thus resulting in MR (15).
The en-face views of the mitral valve on 3DE also permit visualization of leaflet prolapse and flail without rotational artifacts. In 3DE volume-rendered images, a prolapsing scallop or segment is identified as a bulge into the left atrium, and can be color-coded to aid in differentiation from normal adjacent segments. 3DE is a highly accurate and reproducible method of localizing prolapsing segments (particularly commissural or A1 involvement), when compared with the gold standard of surgical inspection (4). Concurrent use of 3D color Doppler allows for accurate localization of the mitral regurgitant jet.
Additionally, 3DE allows for reproducible volumetric measurements of annular area, circumference, and planarity index, allowing for more detailed assessment of disease complexity, planning of repair strategies, and prediction of post-surgical success (4,23). By quantifying the extent of excess leaflet length, surface area, and billowing volume, 3DE helps to identify patients at risk of SAM following MV repair. Some predictors associated with a higher probability of this entity include the degree of mitro-aortic angle, presence of excess tissue, and displacement of the mitral coaptation line, all of which can be easily characterized using 3DE (24).
3DE has also shown that conformational changes of the mitral valve annulus contribute to the development of ischemic MR. Multiple studies have demonstrated that the annulus dilates and flattens, becoming more immobile throughout the cardiac cycle. 3DE has revealed more subtle anatomic changes such as greater dilation and flattening in anterior compared with inferior infarcts (25–27). 3DE studies in animals demonstrate resolution of MR after plication of the infarcted region, supporting the idea that valvular pathology at times is linked directly to the remodeled post-ischemic myocardium (28).
In patients with dilated, non-ischemic cardiomyopathy and MR, 3DE has shown that symmetric papillary muscle displacement with simultaneous enlargement of the mitral annulus leads to progressive chordal tethering and leaflet tenting, resulting in predominantly central MR as a result of decreased leaflet coaptation. These changes are associated with a relatively non-pulsatile mitral annulus with minimal displacement towards the apex during systole (29–32).
Real-time 3D TEE can also be used for perioperative planning of mitral valve surgery as well as guidance of percutaneous interventions, including edge-to-edge repair. 3DE is able to obtain high-quality views using a combination of real-time acquisition, maximization of frame rate, narrow and wide angle acquisition, 3D zoom, cropping, and post-processing. Given the ease and speed of real-time data acquisition using 3DE, it will remain an essential tool in daily clinical practice as well as in the perioperative setting for the foreseeable future.
Novel Mechanisms of Mitral Regurgitation
A. Mitral Valve Clefts
Cleft mitral valves, an example of Carpentier Type I mitral valve dysfunction, are a rare cause of MR which are associated with congenital heart disease, most commonly ostium primum atrial septal defect (ASD). Embryologically, the normal anterior MV leaflet is formed when the superior and inferior atrioventricular (endocardial) cushions fuse together. Failure of this fusion results in cleft anterior MV, often associated with ostium primum ASD. Anterior clefts are most often located off the “midline”of A2, suggesting the possibility of asymmetric endocardial fusion (33) (Figure 9).
Figure 9:
(A) Short-axis view of the mitral valve on TTE in a patient with a large anterior MV cleft (white arrow) (B) 3D TEE from Surgeon’s view in the same patient clearly showing large A2 cleft (white arrow).
Historically, isolated cleft mitral valves in the absence of associated congenital heart disease were rarely diagnosed with 2D TTE. In recent years, with the advent of 3DE and its improved ability to discern mechanisms of MR, isolated cleft mitral valves have been identified with greater frequency. In a recent study of 1,092 patients with otherwise unexplained significant MR, isolated cleft mitral valves were found to be more prevalent than previously reported (prevalence = 3.3%). The majority of isolated cleft mitral valves were seen on the anterior mitral valve leaflet, with most clefts located in the mid-A1 or mid-A3 positions. Posterior clefts were less commonly observed and occurred at the site of the natural scallop divisions (between P1 and P2 and between P2 and P3), with those clefts that reached the mitral annulus boundary referred to as “profound indentations” due to their significantly increased depth compared to naturally occurring indentations. The mechanism for these profound posterior indentations is unknown, including whether they represent pathologic findings or a normal variant. There was a suggestion of more significant MR severity in patients with anterior clefts (33). Examples of both an anterior cleft and a profound posterior indentation can be seen in Figure 10.
Figure 10:
Three-dimensional transesophageal echocardiographic view of a normal MV (A) with the annular boundary noted in red and the division between anterior and posterior leaflets shown in yellow. (B) Anterior cleft (blue arrow) and (C) profound posterior MV indentation that extends to the annular boundary (blue arrow). The posterior scallops are labeled P1-P3.
Of note, a considerably higher prevalence of cleft mitral valve was noted using 3DE compared to 2DE, likely due to incomplete imaging of the leaflets in relation to the annulus and echo dropout on 2DE. Using 3DE, the anterior leaflet can be readily inspected for abnormal clefts, while on the posterior leaflet, one can identify the natural scallop indentations in order to understand whether pathologic indentations are present. With the use of 3D color Doppler, the MR jet can also be confirmed to originate from within the cleft (33).
B. Mitral Annular Disjunction
Another recently described entity of mitral valve dysfunction is mitral annular disjunction (MAD), which is thought to represent a more malignant type of Carpentier Type II mitral valve dysfunction. MAD is defined as detachment of the roots of the mitral annulus from the ventricular myocardium with atrial displacement of the leaflet hinge point, often localized to the base of the posterior leaflet (34) (Figures 11A and 11C). Both transthoracic and transesophageal echocardiography can recognize MAD with excellent surgical correlation (35).
Figure 11:
(A) Mitral annular disjunction (MAD) is characterized by detachment of the roots of the mitral annulus from the ventricular myocardium with atrial displacement of the posterior mitral valve leaflet. In this example, the posterior mitral valve leaflet is displaced from the ventricular myocardium and is displaced into the left atrium, as seen on 2D TTE apical four-chamber view. The MAD distance, or width, is displayed by the white dotted arrow. (B) Tissue Doppler echocardiography showing peak systolic lateral mitral annular velocity > 16 cm/s, known as the “Pickelhaube sign” (white arrow), a novel echocardiographic marker that has recently been associated with a more malignant phenotype of MVP (34). (C) Cardiac MRI three-chamber view demonstrating gap (black dotted arrow) between the posterior mitral valve leaflet insertion point and the ventricular myocardium as well as bileaflet MV prolapse.
Recognition of this entity is important due to an association with malignant arrhythmias. A recent Norwegian study noted ventricular arrhythmias were frequently observed in patients with MAD. MAD was found to be independently associated with ventricular arrhythmias, in contrast to MVP alone. MAD was detected around a large part of the mitral annulus circumference and was intermixed with normal tissue on cardiac magnetic resonance imaging (CMR) (36). Other recent studies have demonstrated an association among MAD, ventricular arrhythmia, and papillary muscle fibrosis (37–39).
MAD has also been reported to be a component feature of arrhythmic MVP and LV fibrosis. This may occur due to the excessive leaflet mobility caused by posterior systolic curling in MAD, which results in mechanical stretch of the inferobasal wall and papillary muscles, thus leading to scarring which can be detected on CMR. Of note, MAD distance was significantly increased in patients with LGE on CMR compared to those without LGE (39).
Other high-risk features associated with sudden cardiac death (SCD) and malignant arrhythmias in patients with MVP include inferior T-wave inversions (in leads II, III, and aVF) and complex ventricular ectopy on electrocardiography (including pleiomorphic PVCs, couplets, and triplets), peak systolic lateral mitral annular velocities of ≥ 16 cm/s on Tissue Doppler echocardiography (a phenomenon known as the “Pickelhaube sign”) (Figure 11B), and LV papillary muscle or inferobasal myocardial LGE, as noted above (34). The presence of the above findings can help clinicians identify which patients with MVP are at higher risk for arrhythmia and SCD.
Recent literature using dynamic 3DE details that MAD patients display paradoxical systolic expansion and flattening of the mitral valve annulus, compared to normal patients in whom the MV annulus contracts and increases in saddle shape during systole. In another study, the 3D extent of MAD correlated significantly with abnormal annular dynamics and larger effective regurgitant orifice area (35). This was the first study in humans to demonstrate the decoupling of annular and ventricular function in relation to MAD, and explains the likely mechanism of MR in this patient population.
C. Atrial Fibrillation-Associated Mitral Regurgitation
In MR caused by isolated atrial fibrillation (AF), mitral annular dilatation is the primary mechanism for mitral leaflet malcoaptation, also known as atrial functional MR (40). A recent study showed that patients with AF and MR had larger mitral annular dimensions compared to patients with AF without MR (41). Follow-up work has demonstrated that mitral annulus dimension is the most important determinant of MR in AF patients, and that patients in whom sinus rhythm was restored had greater reductions in left atrial size and annular dimension, as well as lower rates of significant MR compared with patients in whom sinus rhythm was not restored (42).
Silbiger and colleagues have proposed that the underlying mechanism of atrial functional MR involves left atrial enlargement displacing the posterior mitral annulus onto the crest of the LV inlet causing: 1) reduction of the posterior leaflet area available for coaptation and 2) tethering of the posterior leaflet due to increased annulo-papillary muscle distance. Additionally, the displacement of the posterior mitral annulus causes a counterclockwise torque of the anterior mitral annulus across the inter-trigonal axis, thereby increasing tethering of the papillary muscles and causing tenting of the anterior mitral leaflet (43).
An additional mechanism for MR in atrial fibrillation was recently proposed by Kagiyama et al, in which insufficient mitral leaflet remodeling relative to the increase in mitral annular dimension may lead to MR (44). This was demonstrated in a recent 3DE study by Chaput et al in which patients with LV dysfunction showed a 35% increase in mitral leaflet area, however in the subset of patients with significant MR, the ratio of mitral leaflet to annular area was significantly reduced as compared with patients without MR (7). In the study by Kagiyama et al, 3DE demonstrated that patients with AF and significant MR showed a significantly smaller total leaflet area relative to mitral annulus area ratio (TLA/AA) compared with AF patients without MR as well as normal controls. Each 1% decrease in the TLA/AA ratio was independently associated with significant MR, highlighting the importance of insufficient mitral leaflet remodeling in the pathophysiology of atrial functional MR (44).
D. Mitral Regurgitation in Obstructive Hypertrophic Cardiomyopathy (HCM)
In patients with obstructive HCM, functional and structural abnormalities of the MV apparatus are recognized as a phenotypic entity independent of left ventricular hypertrophy. Pathologic elongation and thickening of the anterior and posterior mitral leaflets is commonly seen, in addition to anterior displacement of the papillary muscles and abnormal attachment of the chordae tendineae (45).
The elongated mitral valve leaflets in obstructive HCM extend into the LV cavity above the plane of the mitral annulus, with the tip of the mitral anterior leaflet extending past the point of coaptation and causing SAM and consequently LV outflow tract (LVOT) obstruction. The most common cause of MR in HCM occurs when the posterior leaflet is not sufficiently long or mobile enough to move anteriorly with the anterior leaflet, resulting in leaflet malcoaptation (Type IVa dysfunction based on the Modified criteria) (19). The MR jet in this situation is typically posteriorly and laterally directed (Figure 12). In these scenarios, the MR is reduced or eliminated by removing the SAM, either medically or surgically (46). Resolution of the SAM improves leaflet coaptation and thus the degree of MR.
Figure 12:
(A) Apical three-chamber TTE view of patient with hypertrophic cardiomyopathy (HCM) and SAM (white arrow) leading to (B) posteriorly and laterally directed severe MR jet as seen with color Doppler. In HCM patients, the posterior leaflet is frequently not sufficiently long or mobile enough to move anteriorly with the anterior leaflet, resulting in leaflet malcoaptation and subsequent MR (Type IVa mitral valve dysfunction based on the Modified criteria).
Occasionally, calcification or fibrosis of the MV leaflets occur in patients with SAM causing resultant MR. A clue that this is the case is an anteriorly or centrally directed MR jet, as opposed to the classically posteriorly and laterally directed MR jet caused by SAM. A recent study by Hang et al showed that in 709 patients with obstructive HCM and significant MR who underwent septal myectomy, an isolated posteriorly directed jet of MR correlated highly with SAM as the underlying pathophysiologic mechanism (positive predictive value of 95%), but demonstrated a low negative predictive value (17%). Thus, while excellent at identifying SAM-mediated MR, jet direction should not be used exclusively to guide the decision to perform concomitant MV surgery during septal myectomy for HCM (47).
CONCLUSION
The mitral valve is a complex structure that requires 3D deformation in its circumference, curvature, excursion, shape, and size for proper functioning. Characterization of the mitral valve apparatus using 3DE has shed new light on the pathophysiology of both primary MR due to degenerative disease as well as functional MR due to both ischemic and non-ischemic cardiomyopathy. Given the complexity of the anatomy and pathophysiology of MR as well as the expanding indications for percutaneous mitral valve repair, it will be essential for structural imagers to continue to work closely with interventional and surgical colleagues in order to provide optimal, individualized care to this growing patient population.
Footnotes
Disclosure statement:
Dr. Lang has received a research grant from Philips Healthcare for other unrelated studies.
The remaining authors have nothing to disclose.
References
- 1.Salgo IS, Gorman JH 3rd, Gorman RC et al. Effect of annular shape on leaflet curvature in reducing mitral leaflet stress. Circulation 2002;106:711–7. [DOI] [PubMed] [Google Scholar]
- 2.McCarthy KP, Ring L, Rana BS. Anatomy of the mitral valve: understanding the mitral valve complex in mitral regurgitation. Eur J Echocardiogr 2010;11:i3–9. [DOI] [PubMed] [Google Scholar]
- 3.Lang RM, Tsang W, Weinert L, Mor-Avi V, Chandra S. Valvular heart disease. The value of 3-dimensional echocardiography. J Am Coll Cardiol 2011;58:1933–44. [DOI] [PubMed] [Google Scholar]
- 4.Chandra S, Salgo IS, Sugeng L et al. Characterization of degenerative mitral valve disease using morphologic analysis of real-time three-dimensional echocardiographic images: objective insight into complexity and planning of mitral valve repair. Circ Cardiovasc Imaging 2011;4:24–32. [DOI] [PubMed] [Google Scholar]
- 5.Alkadhi H, Desbiolles L, Stolzmann P et al. Mitral annular shape, size, and motion in normals and in patients with cardiomyopathy: evaluation with computed tomography. Invest Radiol 2009;44:218–25. [DOI] [PubMed] [Google Scholar]
- 6.Bulkley BH, Roberts WC. Dilatation of the mitral anulus. A rare cause of mitral regurgitation. Am J Med 1975;59:457–63. [DOI] [PubMed] [Google Scholar]
- 7.Chaput M, Handschumacher MD, Tournoux F et al. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008;118:845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flachskampf FA, Chandra S, Gaddipatti A et al. Analysis of shape and motion of the mitral annulus in subjects with and without cardiomyopathy by echocardiographic 3-dimensional reconstruction. J Am Soc Echocardiogr 2000;13:277–87. [DOI] [PubMed] [Google Scholar]
- 9.Voci P, Bilotta F, Caretta Q, Mercanti C, Marino B. Papillary muscle perfusion pattern. A hypothesis for ischemic papillary muscle dysfunction. Circulation 1995;91:1714–8. [DOI] [PubMed] [Google Scholar]
- 10.Lang RM, Goldstein SA, Kronzon I, Khandheria BK, Mor-Avi V. ASE’s comprehensive echocardiography. 2016. [Google Scholar]
- 11.Carpentier A. Cardiac valve surgery--the “French correction”. J Thorac Cardiovasc Surg 1983;86:323–37. [PubMed] [Google Scholar]
- 12.Dal-Bianco JP, Beaudoin J, Handschumacher MD, Levine RA. Basic mechanisms of mitral regurgitation. Can J Cardiol 2014;30:971–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agmon Y, Freeman WK, Oh JK, Seward JB. Diastolic mitral regurgitation. Circulation 1999;99:e13. [DOI] [PubMed] [Google Scholar]
- 14.Otto CM, Bonow RO, Company WBS. Valvular heart disease : a companion to Braunwald’s heart disease. Philadelphia: Elsevier/Saunders, 2014. [Google Scholar]
- 15.Antoine C, Mantovani F, Benfari G et al. Pathophysiology of Degenerative Mitral Regurgitation: New 3-Dimensional Imaging Insights. Circ Cardiovasc Imaging 2018;11:e005971. [DOI] [PubMed] [Google Scholar]
- 16.Anyanwu AC, Adams DH. Etiologic classification of degenerative mitral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg 2007;19:90–6. [DOI] [PubMed] [Google Scholar]
- 17.Ntusi NB, Mayosi BM. Epidemiology of heart failure in sub-Saharan Africa. Expert Rev Cardiovasc Ther 2009;7:169–80. [DOI] [PubMed] [Google Scholar]
- 18.Gujral DM, Lloyd G, Bhattacharyya S. Radiation-induced valvular heart disease. Heart 2016;102:269–76. [DOI] [PubMed] [Google Scholar]
- 19.Filsoufi F, Rahmanian PB, Anyanwu A, Adams DH. Physiologic basis for the surgical treatment of ischemic mitral regurgitation. Am Heart Hosp J 2006;4:261–8. [DOI] [PubMed] [Google Scholar]
- 20.Shah PM, Raney AA. New echocardiography-based classification of mitral valve pathology: relevance to surgical valve repair. J Heart Valve Dis 2012;21:37–40. [PubMed] [Google Scholar]
- 21.O’Gara PT, Grayburn PA, Badhwar V et al. 2017 ACC Expert Consensus Decision Pathway on the Management of Mitral Regurgitation: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2017;70:2421–2449. [DOI] [PubMed] [Google Scholar]
- 22.Ryan LP, Jackson BM, Eperjesi TJ et al. A methodology for assessing human mitral leaflet curvature using real-time 3-dimensional echocardiography. J Thorac Cardiovasc Surg 2008;136:726–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maffessanti F, Marsan NA, Tamborini G et al. Quantitative analysis of mitral valve apparatus in mitral valve prolapse before and after annuloplasty: a three-dimensional intraoperative transesophageal study. J Am Soc Echocardiogr 2011;24:405–13. [DOI] [PubMed] [Google Scholar]
- 24.Kim DH, Handschumacher MD, Levine RA et al. In vivo measurement of mitral leaflet surface area and subvalvular geometry in patients with asymmetrical septal hypertrophy: insights into the mechanism of outflow tract obstruction. Circulation 2010;122:1298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Otsuji Y, Levine RA, Takeuchi M, Sakata R, Tei C. Mechanism of ischemic mitral regurgitation. J Cardiol 2008;51:145–56. [DOI] [PubMed] [Google Scholar]
- 26.Vergnat M, Jassar AS, Jackson BM et al. Ischemic mitral regurgitation: a quantitative three-dimensional echocardiographic analysis. Ann Thorac Surg 2011;91:157–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watanabe N, Ogasawara Y, Yamaura Y et al. Mitral annulus flattens in ischemic mitral regurgitation: geometric differences between inferior and anterior myocardial infarction: a real-time 3-dimensional echocardiographic study. Circulation 2005;112:I458–62. [DOI] [PubMed] [Google Scholar]
- 28.Liel-Cohen N, Guerrero JL, Otsuji Y et al. Design of a new surgical approach for ventricular remodeling to relieve ischemic mitral regurgitation: insights from 3-dimensional echocardiography. Circulation 2000;101:2756–63. [DOI] [PubMed] [Google Scholar]
- 29.Daimon M, Saracino G, Gillinov AM et al. Local dysfunction and asymmetrical deformation of mitral annular geometry in ischemic mitral regurgitation: a novel computerized 3D echocardiographic analysis. Echocardiography 2008;25:414–23. [DOI] [PubMed] [Google Scholar]
- 30.Song JM, Fukuda S, Kihara T et al. Value of mitral valve tenting volume determined by real-time three-dimensional echocardiography in patients with functional mitral regurgitation. Am J Cardiol 2006;98:1088–93. [DOI] [PubMed] [Google Scholar]
- 31.Tsukiji M, Watanabe N, Yamaura Y et al. Three-dimensional quantitation of mitral valve coaptation by a novel software system with transthoracic real-time three-dimensional echocardiography. J Am Soc Echocardiogr 2008;21:43–6. [DOI] [PubMed] [Google Scholar]
- 32.Veronesi F, Corsi C, Sugeng L et al. Quantification of mitral apparatus dynamics in functional and ischemic mitral regurgitation using real-time 3-dimensional echocardiography. J Am Soc Echocardiogr 2008;21:347–54. [DOI] [PubMed] [Google Scholar]
- 33.Narang A, Addetia K, Weinert L et al. Diagnosis of Isolated Cleft Mitral Valve Using Three-Dimensional Echocardiography. J Am Soc Echocardiogr 2018;31:1161–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller MA, Dukkipati SR, Turagam M, Liao SL, Adams DH, Reddy VY. Arrhythmic Mitral Valve Prolapse: JACC Review Topic of the Week. J Am Coll Cardiol 2018;72:2904–2914. [DOI] [PubMed] [Google Scholar]
- 35.Lee AP, Jin CN, Fan Y, Wong RHL, Underwood MJ, Wan S. Functional Implication of Mitral Annular Disjunction in Mitral Valve Prolapse: A Quantitative Dynamic 3D Echocardiographic Study. JACC Cardiovasc Imaging 2017;10:1424–1433. [DOI] [PubMed] [Google Scholar]
- 36.Dejgaard LA, Skjolsvik ET, Lie OH et al. The Mitral Annulus Disjunction Arrhythmic Syndrome. J Am Coll Cardiol 2018;72:1600–1609. [DOI] [PubMed] [Google Scholar]
- 37.Carmo P, Andrade MJ, Aguiar C, Rodrigues R, Gouveia R, Silva JA. Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound 2010;8:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Konda T, Tani T, Suganuma N et al. The analysis of mitral annular disjunction detected by echocardiography and comparison with previously reported pathological data. J Echocardiogr 2017;15:176–185. [DOI] [PubMed] [Google Scholar]
- 39.Perazzolo Marra M, Basso C, De Lazzari M et al. Morphofunctional Abnormalities of Mitral Annulus and Arrhythmic Mitral Valve Prolapse. Circ Cardiovasc Imaging 2016;9:e005030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delgado V, Bax JJ. Atrial Functional Mitral Regurgitation: From Mitral Annulus Dilatation to Insufficient Leaflet Remodeling. Circ Cardiovasc Imaging 2017;10. [DOI] [PubMed] [Google Scholar]
- 41.Kihara T, Gillinov AM, Takasaki K et al. Mitral regurgitation associated with mitral annular dilation in patients with lone atrial fibrillation: an echocardiographic study. Echocardiography 2009;26:885–9. [DOI] [PubMed] [Google Scholar]
- 42.Gertz ZM, Raina A, Saghy L et al. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol 2011;58:1474–81. [DOI] [PubMed] [Google Scholar]
- 43.Silbiger JJ. Does left atrial enlargement contribute to mitral leaflet tethering in patients with functional mitral regurgitation? Proposed role of atriogenic leaflet tethering. Echocardiography 2014;31:1310–1. [DOI] [PubMed] [Google Scholar]
- 44.Kagiyama N, Hayashida A, Toki M et al. Insufficient Leaflet Remodeling in Patients With Atrial Fibrillation: Association With the Severity of Mitral Regurgitation. Circ Cardiovasc Imaging 2017;10. [DOI] [PubMed] [Google Scholar]
- 45.Maron MS, Olivotto I, Harrigan C et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 2011;124:40–7. [DOI] [PubMed] [Google Scholar]
- 46.Sherrid MV, Balaram S, Kim B, Axel L, Swistel DG. The Mitral Valve in Obstructive Hypertrophic Cardiomyopathy: A Test in Context. J Am Coll Cardiol 2016;67:1846–1858. [DOI] [PubMed] [Google Scholar]
- 47.Hang D, Schaff HV, Nishimura RA et al. Accuracy of Jet Direction on Doppler Echocardiography in Identifying the Etiology of Mitral Regurgitation in Obstructive Hypertrophic Cardiomyopathy. J Am Soc Echocardiogr 2019;32:333–340. [DOI] [PubMed] [Google Scholar]