Abstract
Background:
MiR-103a-3p is a small non-coding RNA and has been reported to be involved in osteogenic proliferation and differentiation, but the role of miR-103a-3p in human osteoarthritis (OA) remains unclear.
Objectives:
In this study, we aimed to explore its function and molecular target in chondrocytes during OA pathogenesis.
Materials and Methods:
Total 12 experimental OA rat models, together with 12 rats without knee OA lesions were established and cartilage samples were collected. Chondrocytes were treated with LPS in vitro. MiR-103a-3p expression was detected in articular cartilage tissues and chondrocytes using quantitative real-time PCR. Knee OA chondrocytes were transfected with miR-103a-3p mimics, and siHMGB1, respectively. Then cellular proliferation, apoptosis, apoptosis related factors and inflammatory cytokines were analyzed by MTT, flow cytometry, western blot, caspase-3 activity and ELISA, respectively. Potential targets of miR-103a-3p were predicted using series of bioinformatics analysis, then confirmed by luciferase reporter assay.
Results:
We first found miR-103a-3p was significantly down-regulated in the articular cartilage tissues from experimental OA rats, as well as in chondrocytes treated with LPS in vitro. The gain-of-function assay further demonstrated that up-regulation of miR-103a-3p significantly promoted cell proliferation, inhibited apoptosis and inflammation, which was accompanied with elevated expression of PCNA, and reduced expression of caspase-3, PARP, IL-1β, IL-6, IL-10 and TNF-α. Furthermore, high mobility group box 1 (HMGB1), an important inflammatory mediator of OA, was a target of miR-103a-3p. Moreover, knockdown of HMGB1 mimicked the effects of miR-103a-3p on chondrocytes treated with LPS.
Conclusions:
Taken together, our study suggests that miR-103a-3p inhibits chondrocyte apoptosis and inflammation in OA, which appears to be an attractive approach to OA treatment.
Keywords: Apoptosis, Chondrocytes, HMGB1, Inflammation, miR-103a-3p, Osteoarthritis
1. Background
Osteoarthritis (OA) is the most common serious rheumatic disease and a leading cause of pain, disability, diminished life quality, and socioeconomic cost in the United States and globally ( 1 , 2 ). It is primarily characterized as articular cartilage degradation, bone alteration, and secondary synovial joint inflammation ( 3 ). It is considered as a complex multifactor etiology, i.e., genetic, biological and biomechanical components ( 4 , 5 ). Although some factors, including dysregulation of body composition, lipids, Vitamin D, pro- and anti-inflammatory have been shown to be correlated with the pathobiology of OA, the detailed molecular mechanisms remain largely unclear ( 4 ).
MicroRNAs (miRNAs) are small (20-to-24 nt) non-coding RNAs that function as post-transcriptional regulators on gene expression in eukaryotes though binding to the 3’UTR of their target genes ( 6 ). Until now, more than 2000 miRNAs have been identified in humans and these tiny RNAs participate in nearly all fundamental processes, including cell growth, differentiation, apoptosis, and inflammation ( 7 ). In recent years, accumulating studies revealed aberrant of miRNAs play a crucial role in various diseases, such as cancer, fibrosis, cardiovascular disease, and rheumatoid arthritis ( 8 ). Among these miRNAs, miR-103a-3p has been identified as a potential diagnostic biomarker and therapeutic target in several cancers, including gastric cancer ( 9 ), glioma ( 10 ) and bladder cancer ( 11 ). In addition, combination of miR-103a-3p and mesothelin could improve the biomarker detection sensitivity in malignant mesothelioma ( 12 ). The complex network created by miR-103a-3p and GPRC5A has greatly contributed to the investigation on pancreatic tumor development and progression ( 13 ). More recently, miR-103a-3p expression was found to be up-regulated in rheumatoid arthritis patients compared to controls ( 14 ). However, the molecular mechanisms underlying miR-103a-3p involved in OA are unknown.
HMGB1, as a chromatin component and a secreted protein, is extracellularly released as a potent pro-inflammatory cytokine in sepsis ( 15 ). It is also generated by necrotic cells, activated monocytes and macrophages, and plays in important role in mediating infection, injury and inflammation ( 16 ). Multiple recent studies support a critical role for HMGB1 on the pathogenesis of OA. For example, Sun et al. ( 17 ) found that the number of HMGB1-positive cells was significantly elevated in synovial tissue compared with control. Moreover, HMGB1 has been reported to be involved in chondrocyte apoptosis and inflammation in OA ( 18 , 19 ). But whether HMGB1 is regulated by miR-103a-3p in OA still remains unclear.
2. Objectives
In the current study, by employing molecular and biological behavioral experiments, we identified miR-103a-3p as an important regulator of OA physiology and pathophysiology. We further investigated the mRNA targets of miR-103a-3p in the context of OA, aiming to provide potential alternative strategies for OS treatment.
3. Materials and Methods
3.1. Experimental Animals and OA Rat Model
Twenty-four 8-week male Sprague–Dawley (SD) rats (250–300 g) were purchased from the Animal Experiment Center of Sichuan Province and randomly divided into normal group (n = 12) and knee OA group (n = 12). Briefly, in the knee OA group, the rats were anesthetized by 30 mg/kg pentobarbital sodium. Both knees were shaved and disinfected. Then, an incision was made at the center of the knee to expose the patellar ligament. Each rat was positioned on its back, and the leg was flexed 90° at the knee joint. The patellar ligament was palpated below the patella, and monosodium iodoacetate (MIA) was injected into the medial side of the ligament of both knees using a 29-gauge, 0.5-inch needle. Care was taken to ensure that the needle was not advanced too far into the cruciate ligaments. The normal group with no knee lesions was not treated. After the MIA injection and during the experimental period, no intervention was performed in any of the animals. All the rats were killed and cartilage tissues were harvested under sterile conditions after 4 weeks. The animal experiments accorded with the national guidelines of the care and use of laboratory animals and were approved by the Ethics Committee of Experimental Animal Center, Sichuan Province.
3.2. Cell Culture and Treatment
Chondrocytes were isolated from knee OA cartilage samples as previously described ( 20 ). Briefly, rat cartilage tissues were cut into small pieces and digested with trypsin (Sigma-Aldrich, MO, USA). After that, cell suspension was filtered through 40 μm cell filter (BD Falcon, MA), and cells were collected. Chondrocytes were then cultured in Dulbecco’s Modified Eagles Medium (DMEM; Gibco) containing with 10% FBS (Gibco), 100 U.mL-1 penicillin (Gibco), 100 mg.mL-1 streptomycin (Gibco) in incubator at 37 °C with 5% CO2. For induction of inflammation in cultured chondrocytes, adherent chondrocytes at 70–80% confluence were stimulated with100 mg.mL-1 lipopolysaccharide (LPS; Sigma, MO, USA) for 6 h to mimick OA chondrocytes.
3.3. Cell Transfection
MiR-103a-3p mimics (5’-AGCAGCAUUGUACAGGGCUAUGA-3’), negative control miRNA (miR-NC: 5’-AUAGCCCUGUACAAUGCUGCUUU-3’), small interfering RNA targeting HMGB1 (siHMGB1: 5’-CUCACCAAGUCUCCUCAAU-3’) and siRNA negative control (siNC: 5’-AUUGAGGAGACUUGGUGAG-3’) were synthesized and purified by GenePharma Co., Ltd (Shanghai, China). These oligonucleotides were cloned into the lentivirus expression vector of Hu6-MCS-CMV-EGFP (GenePharma Co., Ltd). The recombined vector was triple transfected into 80% confluent HEK293T cells with packaging vectors pHelper 1.0 and 2.0 (GeneChem Co., Ltd.) with Lipofectamine 2000 to produce corresponding viral particles. For cell transfection, chondrocytes were divided into 4 different groups, including the miR-NC group, miR-103a-3p group, siNC and siHMGB1 group according the transfected viral particles. They were transfected with scramble miRNA mimics as miRNA (miR-NC), miR-103a-3p mimics, siNC, and siHMGB1, respectively (purchased from Genepharma, Shanghai, China) using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. Forty-eight hours after incubation, the transfection efficiency was observed using a fluorescence microscope (Olympus Co., Ltd., Beijing, China) and miR-103a-3p expression level was evaluated by quantitative real-time PCR.
3.4. Quantitative Real-Time PCR (qRT-PCR)
Total RNA from articular cartilage tissues and chondrocytes was isolated with GenElute™ Total RNA Purification Kit (Sigma-Aldrich) and reverse transcribed into cDNA with miScript Reverse Transcription Kit (QIAGEN, Dusseldorf, Germany). TaqMan microRNA Assay Kit was used for miR-103a-3p and U6 expression analysis. SYBR Green Gene Expression Assay Kit (QIAGEN) was used to detect HMGB1 and β-actin expression. A quantitative real-time PCR assay was performed on the ABI7500 Instrument (Applied Biosystems, Warrington, UK). The primers used are listed as follows: miR-103a-3p (forward: 5’-ATCCAGTGCGTGTCGTG-3’; reverse: 5’-TGCTAGCAGCATTGTACAGG-3’); HMGB1 (forward: 5’-CCAACAGGCAAATGGGGTCT; reverse: 5’-TAACTGGTGGGCCAGGGATA-3’); U6 (forward: 5’-GCTTCGGCAGCACATATACTAAAAT-3’; reverse: 5’-CGCTTCACGAATTTGCGTGTCAT-3’) or β-actin (forward: 5’- CCAACCGCGAGAAGATGA-3’; reverse: 5’-CCAGAGGCGTACAGGGATAG-3’) was used as internal reference and the 2−ΔΔCt method was used to calculate the relative gene expression.
3.5. MTT Assay
The cell growth and proliferation of chondrocytes were assessed by MTT assay. In brief, treated cells were plated on a 96-well plate at a density of 1 × 105 cells/well after transfected for 24, 48, and 72 h. Subsequently, 20 μL of MTT stock solution (Sigma; 5 mg.mL-1) was added to each well and incubated for 4 h at 37 °C. After removing the medium, 200 μL dimethyl sulfoxide (Sigma) was added to each well to dissolve the formazan crystals. The absorbance at 595 nm was measured using a microplate reader (Bio-Rad Laboratories, CA, USA).
3.6. Flow Cytometry
The apoptosis of treated chondrocytes was determined by flow cytometry assay with Annexin V-APC/7-AAD Apoptosis Detection Kit (KeyGEN Biotech). Briefly, the harvested chondrocytes were washed and subjected to Annexin V-APC/7-AAD double staining. The labeled chondrocytes were then analyzed by a flow cytometer (FACSCalibur, Becton Dickinson, CA, USA).
3.7. Western Blot Analysis
Total proteins were isolated from chondrocytes using lysis buffer (Takara, Dalian, China) and then centrifuged at 8000 g for 5 min. The proteins were separated by 12 % SDS-PAGE, and transferred to PVDF membranes (Millipore, CA, USA). After blocking with 2 % skim milk at 37 °C for 1 h, the membrane was incubated with primary antibodies against PCNA (1:1000, #4578, Cell signaling Technology, MA, USA), caspase-3 (1:1000, #9661, Cell signaling Technology, MA, USA), PARP (1:1000, #9542, Cell signaling Technology, MA, USA), HMGB1 (1:500, #9213, Cell signaling Technology, MA, USA) and GAPDH (1:500000, #10494-1-AP, Proteintech Group, Inc. IL, USA) at 4 °C overnight. After washed with TBST, the PVDF membranes were incubated with HRP-conjugated secondary antibodies (1:5000, SC-2054, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at room temperature for 2 h. The protein bands were visualized by an enhanced chemiluminescence kit (Pierce, Rockford, IL, USA).
3.8. Enzyme-Linked Immunosorbent Assay (ELISA)
Inflammatory cytokines, including interleukin (IL)-1β, IL-6, IL-10 and tumor necrosis factor α (TNF-α), in cell culture supernatants were detected using ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions.
3.9. MiRNA Target Prediction and Dual-Luciferase Reporter Assay
Putative targets of miR-103a-3p were bioinformatically predicted using the TargetScan (http://www.targetscan.org/), miRanda (http://www.targetscan.org/) and PicTar (http://pictar.mdc-berlin.de/). Among putative predicted genes, HMGB1 was indicated as a potential target gene of miR-103a-3p. For the luciferase reporter assay, the wild-type HMGB1-3’-UTR (Wt HMGB1) and mutant HMGB1-3’-UTR (Mut HMGB1) containing the putative binding site of miR-103a-3p were constructed and cloned into the psiCHECK-2 dual-luciferase expression vector (Promega, Madison, WI, USA). Then the reporter vectors containing Wt HMGB1 or Mut HMGB1 and miR-103a-3p mimic or miR-NC were co-transfected into 293T cells (ATCC, Manassas, VA, USA) using Lipofectamine 2000 (Invitrogen). After transfection for 48 h, the luciferase activity was analyzed with the Dual Luciferase Reporter Assay System (Promega, Madison, USA) according to the manufacturer’s instructions.
3.10. Detection of Caspase-3 Activity
The caspase-3 activity was detected using colorimetric assay kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Briefly, the cells were lysed in lysis buffer on ice for 20 min. After centrifugation, supernatants were incubated with the supplied reaction buffer at 37 °C for 4 h for 2 h in the dark. The reactions were measured by changes at the wavelength of 405 nm using an ELISA reader.
3.11. Statistical Analysis
All statistical analyses were performed by SPSS 19.0 software (SPSS, Inc., Armonk, NY, USA). Quantitative data are presented as the mean ± standard deviation (SD). Statistical difference was determined the unpaired t-tests between two groups, or one-way ANOVA for more than two groups. A p value less than 0.05 was defined as statistically significant.
4. Results
4.1. Mir-103a-3p Expression Was Decreased in Cartilage Tissues from the OA Rat Model and Suppressed by LPS in Chondrocytes
To investigate the potential role of miR-103a-3p in OA, the OA rat model was constructed and the expression of miR-103a-3p was determined using qRT-PCR. As shown in Fig. 1A, the expression level of miR-103a-3p was significantly decreased in tissues from OA rat as compared with tissues from normal mice (p < 0.001). Moreover, the expression of miR-103a-3p was detected in the in vitro chondrocytes treated with LPS. Consistently, there was a marked decrease in miR-103a-3p expression in the LPS induced inflammation reaction (Fig. 1B, p < 0.001). These results suggest that miR-103a-3p might play an important role in the development of OA.
Fig 1.
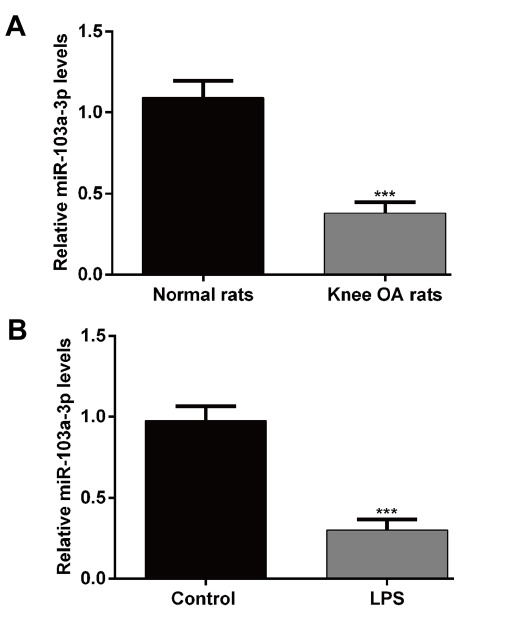
The expression of miR-103a-3p in cartilage tissues from the OA rat model and chondrocytes treated with LPS. (A) Quantitative real-time PCR was performed to detect the expression of miR-103a- 3p in cartilage tissues from the OA rat model. Normal rats without treatment were used as the control. ***p < 0.001, as compared with normal; (B) Quantitative real-time PCR was performed to detect the expression of miR-103a-3p in chondrocytes treated with LPS. PBS treatment was used as control. ***p < 0.001, as compared with co
4.2. Confirmation of Mir-103a-3p Oligonucleotide Transfection into LPS Induced Chondrocytes
To investigate the function of miR-103a-3p in OA, we transfected LPS induced chondrocytes with miR-103a-3p mimics and miR-NC oligonucleotides, respectively. At 48 h after transfection, the fluorescence microscopy image showed that the transfection efficiency of miR-103a-3p oligonucleotides was obviously higher than that of miR-NC in human chondrocytes (Fig.2A). QRT-PCR analysis further demonstrated that the miR-103a-3p level was significantly elevated after miR-103a-3p mimics transfection (Fig.2B, p < 0.001).
Fig 2.
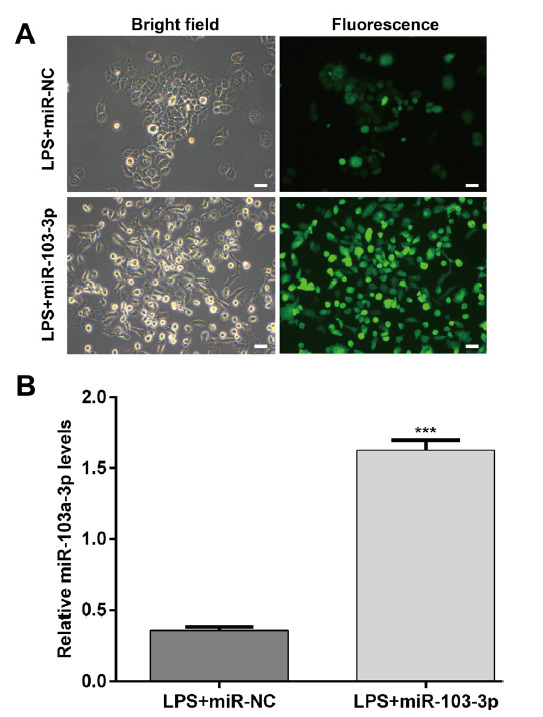
MicroRNA-103a-3p oligonucleotide transfection and miR- 103a-3p expression in LPS-induced chondrocytes. (A) Representative fluorescence images captured using fluorescence microscopy. (B) MiR- 103a-3p expression levels in transfected chondrocytes. ***p < 0.001, as compared with LPS+miR-NC
4.3. MiR-103a-3p Regulated Cell Proliferation, Apoptosis and Inflammation in Chondrocytes from LPS-Induced Injury
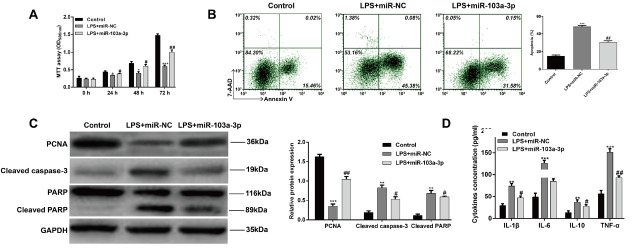
As miR-103a-3p was down-regulated in the OA rat model and LPS induced chondrocytes, we further performed gain-of-functional assays to assess the functional role of miR-103a-3p in the in vitro chondrocytes treated with LPS. As shown in Figure 3A, MTT assay showed that LPS significantly reduced cell proliferation of chondrocytes (p < 0.05, p < 0.001), whereas overexpression of miR-103a-3p by transfection of miR-103a-3p mimics significantly reversed the inhibited effect of LPS on cell proliferation (p < 0.05, p < 0.01). We further detected whether miR-103a-3p overexpression affects the percentage of apoptosis in chondrocytes treated with LPS. As shown in Figure 3B, LPS treatment significantly elevated the percentage of apoptosis in chondrocytes (p < 0.001). However, cell apoptosis induced by LPS was remarkably attenuated by miR-103a-3p overexpression (p < 0.01). The protein expression levels of related molecules associated with proliferation and apoptosis were also examined. As shown in Figure 3C, miR-103a-3p overexpression obviously reversed the inhibition of LPS treatment on PCNA expression, the enhancement of LPS treatment on the expression of caspase-3 and PARP in chondrocytes. Furthermore, the pro-inflammatory cytokines, including IL-1β, IL-6, IL-10 and TNF-α were all significantly decreased by miR-103a-3p overexpression (Fig. 3D, p < 0.05, p < 0.01, p < 0.001). These results demonstrated that miR-103a-3p overexpression could protect the chondrocytes from LPS-induced apoptosis and inflammation.
Fig 3.
Effects of miR-103a-3p overexpression in LPS-induced chondrocytes. Cells were transfected with miR-103a-3p mimics or miR-NC for 48 h and then treated with LPS for 6 h. (A) MTT assay of LPS-induced chondrocytes. (B) Cell apoptosis was detected by Flow cytometry assay. (C) The relative protein levels of PCNA, caspase-3, and PARP were detected by western blot assay. (D) The production of IL-1β, IL-6, IL-10 and TNF-α in the cell culture supernatants was detected by ELISA. *p < 0.05, **p < 0.01, ***p < 0.001, as compared with control; #p < 0.05, ##p < 0.01, as compared with LPS+miR-NC
4.4. HMGB1 Is a Direct Target of miR-103a-3p
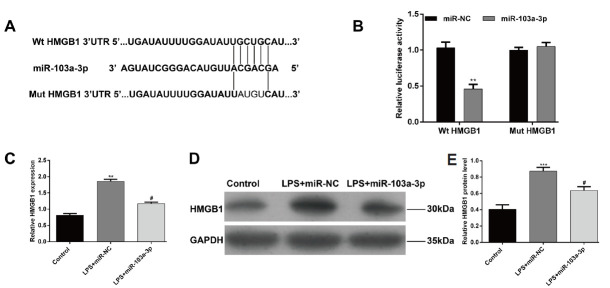
To investigate the potential molecular mechanism of miR-103a-3p, bioinformatic analysis was performed to screen its putative target genes. Among putative predicted genes, HMGB1, an important regulator of inflammation, was indicated as a potential target gene of miR-103a-3p. Perfect base pairing was observed between the seed sequence of miR-103a-3p and the 3’UTR of HMGB1 (Fig.4A). Subsequently, dual luciferase reporter assay was used to validate the direct targeting of HMGB1 by miR-103a-3p. As shown in Figure 4B, miR-103a-3p significantly decreased the luciferase activity of Wt HMGB1 3’-UTR, while it had no significant effect on the HMGB1 mutant type 3’UTR luciferase activity (p < 0.01). Both qRT-PCR and western blot assay showed overexpression of miR-103a-3p significantly inhibited the mRNA (Fig. 4C, p < 0.05, p < 0.01) and protein (Fig.4D) expression of HMGB1 in chondrocytes treated with LPS. These results indicated that HMGB1 was a direct target of miR-103a-3p.
Fig 4.
HMGB1 is a direct target of miR-142-3p. (A) Diagram of the binding site between miR-103a-3p and HMGB1 3’-UTR. (B) Dual-luciferase reporter assay of miR-103a-3p and HMGB1 3’-UTR. 293T cells were co-transfected with miR-103a-3p mimics and WT or MT reporter vector. Luciferase activity was measured after 48 h incubation. (C) Quantitative real-time PCR and (D) Western blot analysis of HMGB1 expression in chondrocytes transfected with miR-103a-3p mimics in the presence with LPS. **p < 0.01, ***p < 0.001, as compared with control; #p < 0.05, as compared with LPS+miR-NC (E) Relative HMGB1 protein level.
4.5. Knockdown of HMGB1 mimicked the protective effects of miR-103a-3p overexpression on LPS-Induced Chondrocytes
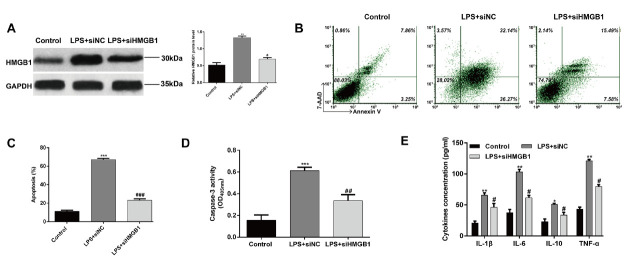
To investigate the effect of HMGB1 on chondrocytes, we examined whether the decrease of HMGB1 affects the apoptosis and inflammation of chondrocyte treated with LPS. First, siHMGB1 or siNC oligonucleotides were transfected in to LPS-induced chondrocytes. As shown in Figure 5A, western blot analysis confirmed the expression of HMGB1 protein levels were obviously elevated in chondrocytes after LPS treatment, but remarkably decreased after siHMGB1 transfection. Subsequently, flow cytometry assay showed that knockdown of HMGB1 significantly decreased the cell apoptosis induced by LPS in chondrocytes (Fig. 5B and C, p < 0.001). The caspase-3 activity of chondrocytes was significantly increased after LPS treatment (Fig.5D, p < 0.001), but knockdown of HMGB1 alleviated the cell apoptosis induced by LPS (p < 0.01). Furthermore, the pro-inflammation of LPS on chondrocytes was also abolished by HMGB1 knockdown, as confirmed by decreased IL-1β, IL-6, IL-10 and TNF-α (Fig. 5E, p < 0.05, p < 0.01). These results suggested that miR-103a-3p protected chondrocytes from LPS-induced injury might by down-regulating HMGB1.
Fig 5.
Effects of knockdown of HMGB1 on LPS-induced chondrocytes. Cells were transfected with siHMGB1 or siNC for 48 h and then treated with LPS for 6 h. (A) The expression of HMGB1 protein levels were detected using western blot analysis. (B and C) Cell apoptosis was detected by Flow cytometry assay and (D) caspase-3 activity assay. (E) The production of IL-1β, IL-6, IL-10 and TNF-α in the cell culture supernatants was detected by ELISA. *p < 0.05, **p < 0.01, ***p < 0.001, as compared with control; #p < 0.05, ##p < 0.01, ###p < 0.001, as compared with LPS+siNC
5. Discussion
OA as the most prevalent joint disease has been diagnosed among a considerable proportion of the adult human population ( 1 ). Previous findings identified some critical pathogenic factors for OA, including inflammation, chondrocytes injury, extracellular matrix degradation, and failure to remodeling articular cartilage ( 21 ). Meanwhile, miRNA levels are frequently changed in response to these pathological disorders, therefore leading to degenerative diseases, including OA.
This study identified lower miR-103a-3p expression profiles in tissue samples from rheumatoid arthritis than in the normal subject. Using a model of OA in rat, we now extend insight into the role of miR-103a-3p in OA. Quantitative RT-PCR analysis in cartilage tissues and chondrocytes treated with LPS showed down-regulation of miR-103a-3p compared with control. More importantly, forced expression of miR-103a-3p promoted proliferation, inhibited apoptosis and pro-inflammatory cytokines (IL-1β, IL-6, IL-10 and TNF-α) in LPS-treated chondrocytes. HMGB1 was found to be a direct target of miR-103a-3p and knockdown of HMGB1 can successfully imitate the phenotype of miR-103a-3p overexpression. These results suggest that miR-103a-3p has the potential to protect against LPS-induced chondrocytes injury though directly targeting HMGB1.
Chondrocytes play a crucial role in maintaining the homeostasis and function of ECM ( 22 ). Decreased cloning and increased apoptosis of chondrocytes in OA have been validated in numerous studies ( 23 - 25 ). It is clear that a number of empty lacunae and cellular debris-like material occurred at the light-microscopic level, supporting apoptosis is a central event in the development and progression of OA ( 22 ). In this study, transfection of a synthetic miR-103a-3p resulted in increased proliferation and decreased apoptosis in LPS-treated rat chondrocytes. PCNA, an endogenous marker of cell proliferation, is associated with DNA synthesis and repair (26). As a commonly activated death protease, caspase-3 is a considered to be the final step executor of apoptosis and catalyzes the cleavage of multiple key cellular proteins ( 27 ). Here, we found that upregulation of miR-103a-3p increased PCNA expression and decreased the expression levels of cleavage of caspase-3 and PARP as a major substrate of caspase-3 ( 28 ). These data suggest that PCAN, caspase-3, and PARP might play essential roles in miR-103a-3p-mediated acceleration of proliferation and inhibition of apoptosis in rat OA model chondrocytes.
Pro-inflammatory cytokines secreted by inflammatory cells play a critical role in cartilage destruction during the pathogenesis of OA can induce production of extracellular matrix degrading enzymes, in chondrocytes, thus contributing to cartilage damage ( 29 ). We found that pro-inflammatory cytokines (IL-1β, IL-6, IL-10 and TNF-α) levels were reduced in chondrocytes upon combination treatment with miR-103a-3p mimics and LPS. Therefore, miR-103a-3p appeared to exert anti-inflammatory activities in chondrocytes.
With the rapid progress on miRNAs studies, thousands of genes were found to be directly targeted by miRNAs and participate in pathological disorders ( 30 ). Here, we found HMGB1 was a direct target of miR-103a-3p. What’s more, knockdown of HMGB1 exhibited similar chondrocytes behavior to miR-103a-3p. Scaffidi et al. ( 31 ) reported that HMGB1 is passively released by necrotic or damaged cells but not apoptotic cells. A markedly reduced capacity to induce inflammation was observed in necrotic cells with HMGB1 knockdown ( 31 ). We guess that miR-103a-3p decreased the number of necrotic or damaged chondrocytes and inhibited release of HMGB1, causing reduced the concentration of pro-inflammatory cytokines. However, in MGC-803 gastric cancer cells and osteosarcoma MG-63 cells, HMGB1 depletion significantly decreased cell proliferation and sensitized cells to apoptosis ( 32 , 33 ), which is contrary to our findings. We thus hypothesize that HMGB1 may exerts different effects in different types of diseases.
In conclusion, the present studies reveal miR-103a-3p as an important regulator in the chondrocytes injury involving in the pathogenesis of OA though directly targeting HMGB1. These preliminary findings will facilitate and deepen our understanding on the development and progression of human OA.
Footnotes
Declaration of conflicting interests : The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthr Cartilage. 2014;22(3):363–388. doi: 10.1016/j.joca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Silverwood V, Blagojevic-Bucknall M, Jinks C, Jordan JL, Protheroe J, Jordan KP. Current evidence on risk factors for knee osteoarthritis in older adults: a systematic review and meta-analysis. Osteoarthr Cartilage. 2015;23(4):507–515. doi: 10.1016/j.joca.2014.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Sun Y, Pan X, Ho K, Li G. Joint distraction attenuates osteoarthritis by reducing secondary inflammation, cartilage degeneration and subchondral bone aberrant change. Osteoarthr Cartilage. 2015;23(10):1728–1735. doi: 10.1016/j.joca.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 4.Wang X, Hunter D, Xu J, Ding C. Metabolic triggered inflammation in osteoarthritis. Osteoarthr Cartilage. 2015;23(1):22–30. doi: 10.1016/j.joca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Mobasheri A, Batt M. An update on the pathophysiology of osteoarthritis. Ann Phys Rehabil Med. 2016;59(5-6):333–339. doi: 10.1016/j.rehab.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Manikandan M, Deva Magendhra Rao AK, Arunkumar G, Manickavasagam M, Rajkumar KS, Rajaraman R, et al. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol Cancer. 2016;15:28. doi: 10.1186/s12943-016-0512-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, Liu L, Hu J, Song L. MicroRNA Regulatory Network Revealing the Mechanism of Inflammation in Atrial Fibrillation. Med Sci Monit. 2015;21:3505–3513. doi: 10.12659/MSM.895982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Piletic K, Kunej T. MicroRNA epigenetic signatures in human disease. Arch Toxicol. 2016;90(10):2405–2419. doi: 10.1007/s00204-016-1815-7. [DOI] [PubMed] [Google Scholar]
- 9.Hu X, Miao J, Zhang M, Wang X, Wang Z, Han J, et al. miRNA-103a-3p Promotes Human Gastric Cancer Cell Proliferation by Targeting and Suppressing ATF7 in vitro. Mol cells. 2018;41(5):390–400. doi: 10.14348/molcells.2018.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yu M, Xue Y, Zheng J, Liu X, Yu H, Liu L, et al. Linc00152 promotes malignant progression of glioma stem cells by regulating miR-103a-3p/FEZF1/CDC25A pathway. Mol Cancer. 2017;16(1):110. doi: 10.1186/s12943-017-0677-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 11.Zhong Z, Lv M, Chen J. Screening differential circular RNA expression profiles reveals the regulatory role of circTCF25-miR-103a-3p/miR-107-CDK6 pathway in bladder carcinoma. Sci Rep. 2016;6:30919. doi: 10.1038/srep30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weber DG, Casjens S, Johnen G, Bryk O, Raiko I, Pesch B, et al. Combination of MiR-103a-3p and mesothelin improves the biomarker performance of malignant mesothelioma diagnosis. PLoS One. 2014;9(12):e114483. doi: 10.1371/journal.pone.0114483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou H, Rigoutsos I. MiR-103a-3p targets the 5' UTR of GPRC5A in pancreatic cells. RNA. 2014;20(9):1431–1439. doi: 10.1261/rna.045757.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anaparti V, Smolik I, Meng X, Mookherjee N, El-Gabalawy H. OP0295 Unique whole blood microrna biosignature for rheumatoid arthritis. Ann Rheum Dis. 2017;76(Suppl 2):178. doi: 10.1136/annrheumdis-2017-eular.1480. [DOI] [Google Scholar]
- 15.Huang W, Tang Y, Li L. HMGB1, a potent proinflammatory cytokine in sepsis. Cytokine. 2010;51(2):119–126. doi: 10.1016/j.cyto.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 16.Bonaldi T, Talamo F, Scaffidi P, Ferrera D, Porto A, Bachi A, et al. Monocytic cells hyperacetylate chromatin protein HMGB1 to redirect it towards secretion. EMBO J. 2003;22(20):5551–5560. doi: 10.1093/emboj/cdg516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun XH, Liu Y, Han Y, Wang J. Expression and Significance of High-Mobility Group Protein B1 (HMGB1) and the Receptor for Advanced Glycation End-Product (RAGE) in Knee Osteoarthritis. Med Sci Monit. 2016;22:2105–12. doi: 10.12659/MSM.895689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qin Y, Chen Y, Wang W, Wang Z, Tang G, Zhang P, et al. HMGB1-LPS complex promotes transformation of osteoarthritis synovial fibroblasts to a rheumatoid arthritis synovial fibroblast-like phenotype. Cell Death Dis. 2014;5:e1077. doi: 10.1038/cddis.2014.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X, Guo Y, Wang C, Yu H, Yu X. MicroRNA-142- 3p Inhibits Chondrocyte Apoptosis and Inflammation in Osteoarthritis by Targeting HMGB1 . Inflammation . 2016;39(5):1718–1728. doi: 10.1007/s10753-016-0406-3. [DOI] [PubMed] [Google Scholar]
- 20.Tanida S, Yoshitomi H, Nishitani K, Ishikawa M, Kitaori T, Ito H, et al. CCL20 produced in the cytokine network of rheumatoid arthritis recruits CCR6+ mononuclear cells and enhances the production of IL-6. Cytokine. 2009;47(2):112–118. doi: 10.1016/j.cyto.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 21.Karlsen TA, de Souza GA, Odegaard B, Engebretsen L, Brinchmann JE. microRNA-140 Inhibits Inflammation and Stimulates Chondrogenesis in a Model of Interleukin 1beta-induced Osteoarthritis. Mol Ther Nucleic Acids. 2016;5(10):e373. doi: 10.1038/mtna.2016.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aigner T, Hemmel M, Neureiter D, Gebhard PM, Zeiler G, Kirchner T, et al. Apoptotic cell death is not a widespread phenomenon in normal aging and osteoarthritis human articular knee cartilage: a study of proliferation, programmed cell death (apoptosis), and viability of chondrocytes in normal and osteoarthritic human knee cartilage. Arthritis Rheum. 2001;44(6):1304–1312. doi: 10.1002/1529-0131(200106)44:6<1304::AID-ART222>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 23.Bao G, Xu L, Xu X, Zhai L, Duan C, Xu D, et al. SGTB Promotes the Caspase-Dependent Apoptosis in Chondrocytes of Osteoarthritis. Inflammation. 2016;39(2):601–610. doi: 10.1007/s10753-015-0285-z. [DOI] [PubMed] [Google Scholar]
- 24.Wu XF, Zhou ZH, Zou J. MicroRNA-181 inhibits proliferation and promotes apoptosis of chondrocytes in osteoarthritis by targeting PTEN. Biochem Cell Biol. 2017;95(3):437–344. doi: 10.1139/bcb-2016-0078. [DOI] [PubMed] [Google Scholar]
- 25.Liu CL, Yan L, Cai KR, Sun K, Qi Y, Han YL, et al. Effects of soybean isoflavones on Wnt/beta-catenin and the TGF-beta1 signaling pathway in renal tissue of type 2 diabetic rats. J Biol Reg Homeos Ag. 2018;32(3):455–464. [PubMed] [Google Scholar]
- 26.Chieffi P, Franco R, Fulgione D, Staibano S. PCNA in the testis of the frog, Rana esculenta: a molecular marker of the mitotic testicular epithelium proliferation. Gen Comp Endocrinol. 2000;119(1):11–16. doi: 10.1006/gcen.2000.7500. [DOI] [PubMed] [Google Scholar]
- 27.Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6(2):99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 28.Gobeil S, Boucher CC, Nadeau D, Poirier GG. Characterization of the necrotic cleavage of poly(ADP-ribose) polymerase (PARP-1): implication of lysosomal proteases. Cell Death Differ. 2001;8(6):588–594. doi: 10.1038/sj.cdd.4400851. [DOI] [PubMed] [Google Scholar]
- 29.Schuerwegh AJ, Dombrecht EJ, Stevens WJ, Van Offel JF, Bridts CH, De Clerck LS. Influence of pro-inflammatory (IL-1 alpha, IL-6, TNF-alpha, IFN-gamma) and anti-inflammatory (IL-4) cytokines on chondrocyte function. Osteoarthritis Cartilage. 2003;11(9):681–687. doi: 10.1016/s1063-4584(03)00156-0. [DOI] [PubMed] [Google Scholar]
- 30.Wang X. miRDB: a microRNA target prediction and functional annotation database with a wiki interface. RNA. 2008;14(6):1012–1017. doi: 10.1261/rna.965408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scaffidi P, Misteli T, Bianchi ME. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 32.Song B, Song WG, Li ZJ, Xu ZF, Wang XW, Wang CX, et al. Effect of HMGB1 silencing on cell proliferation, invasion and apoptosis of MGC-803 gastric cancer cells. Cell Biochem Funct. 2012;30(1):11–17. doi: 10.1002/cbf.1811. [DOI] [PubMed] [Google Scholar]
- 33.Meng Q, Zhao J, Liu H, Zhou G, Zhang W, Xu X, et al. HMGB1 promotes cellular proliferation and invasion, suppresses cellular apoptosis in osteosarcoma. Tumour Biol. 2014;35(12):12265–12274. doi: 10.1007/s13277-014-2535-3. [DOI] [PubMed] [Google Scholar]