Abstract
Background:
Nanoparticles (NPs) with unique chemical and physical properties can be used for therapeutic purposes because of their strong antimicrobial activates. Nanoparticles have been used as an antimicrobial agents to inhibit microbial growth.
Objectives:
In view of the strong antimicrobial activity of nanoparticles, the biogenic synthesis and leishmanicidal activity of rod-shaped zinc oxide (R-ZnO) nanoparticles was explored using Lilium ledebourii tuber extract
Materials and Methods:
The ensuing nanoparticles are characterized by UV-visible spectroscopy, X-ray diffraction and transmission electron microscopy and their leishmanicidal activity evaluated against the Leishmania major (L. major) by MTT assay.
Results:
The R-ZnO nanoparticles displayed excellent leishmanicidal activity against the L. major as they significantly inhibited the amastigotes. The IC50 values of R-ZnO nanoparticles being ~ 0.001 mg.mL-1. R-ZnO nanoparticles can inhibit L. major growth in a dose-dependent manner under in vitro conditions
Conclusion:
A simple, low-cost feasible and eco-friendly procedure was developed for biosynthesis of R-ZnO nanoparticles using natural bioresource that can inhibit human parasite cells growth in a dose-dependent manner under in vitro conditions
Keywords: Biosynthesis, Leishmanicidal, NPs, Rod-shaped, Zinc oxide
1. Background
Leishmaniasis is a disease caused by Leishmania species with the incidence rate of about two million cases annually ( 1 ). Currently, the increase in international travel and environmental amendments caused by war in some regions has created favorable conditions for the propagation of the parasite vectors; thus, there has been a significant increase in the incidence of leishmaniasis ( 2 ). Poverty contributes to the risk of leishmaniasis and so does the open sewerage and lack of waste management which can increase sandfly breeding sites of Leishmania vectors, as well as their access to human bodies. About 70 animal species have been found to act as natural reservoir hosts for these parasites ( 3 ). The first choice for the treatment of leishmaniasis is pentavalent antimoniate (Pa). But the Pa—because of its side effects, the emergence of resistance, and parenteral application is no longer a sufficient treatment. Therefore, it is essential to find new drugs with different mechanisms ofand greater potency ( 4 ).
Nanoparticles (NPs) can be used for therapeutic applications because of their strong antimicrobial activities ( 5 , 7 ). Such as in drug delivery, microbiology, biotechnology, and biochemistry ( 8 , 15 ). The use of metal NPs such as copper, platinum , titanium , gold, selenium, silver, zinc oxide, and palladium nanoparticles against many bacteria, viruses, and fungal pathogens has been reported ( 16 , 19 ), but the use of NPs against protozoan parasites is rather limited. Among different types of NPs, the zinc oxide (ZnO) NPs have attracted scientific attention because of their safety and high antimicrobial activity ( 20 , 21 ); they are recognized to be safe by the U.S. Food and Drug Administration (FDA). The traditional NPs synthesized by chemical methods leads to the adsorption of toxic chemical compounds onto the surface of synthesized NPs which may have adverse effects in medicine ( 22 , 23 ). Green synthesis methods using bioresources such as plants, fungi, or bacteria for the synthesis of biogenic NPs represent alternatives to conventional chemical synthesis methods ( 24 , 29 ).
The present study aims to evaluate the leishmanicidal efficiency of rod-shaped ZnO (R-ZnO) NPs on Leishmania major (L. major) cultures.
2. Objectives
The main aim of this study was to evaluate green synthesis of parasitical zinc oxide NPs using Lilium ledebourii tuber (L. ledebourii). A greener synthesis of rod shape zinc oxide nanoparticles (R-ZnO) were studied using L. ledebourii tuber as a novel bioresource and their leishmanicidal activity has been studied against L. major.
3. Materials and Methods
3.1. Materials
All the reagents and chemicals used in the experiments were purchased from Merck, Germany.
3.2. Synthesis of Rod Shaped Zinc Oxide Nanoparticles
The L. ledebourii tuber were washed thoroughly with sterile distilled water and dried, then 10 g of Lilium tuber was ground into a powder. The powder was added to 200 mL of deionized double-distilled water, heated at 80 °C, and then filtered. To conduct green synthesis of R-ZnO NPs, 20 mL of the obtained extract was added to the 100 mL of zinc acetate dehydrate solution and stirred at ~80 °C for 1 hr. The reaction mixture (extract + zinc acetate dehydrate) was incubated at 80 °C for 2 hr and calcined at 300 °C for 1 hr.
3.3. Characterization of ZnO NRs
The synthesized ZnO nanoparticles were studied using a UV-visible spectrophotometer (Analytik Jena; Germany). XRD analysis was performed to determine the formation of ZnO crystals. The resulting powder was analyzed using an X-ray diffractometer (PANalytical X'Pert PRO; The Netherlands) at 2θ. The shape, size and size distribution of nanoparticle were investigated by TEM ( 30 ).
3.4. Leishmanicidal Assay
The L. major MRHO/IR/75/ER standard strain was cultured in RPMI1640 at 25 °C supplemented with 15% heat-inactivated FBS, streptomycin (200 mg.mL-1), and penicillin (200 IUmg.mL-1). The stationary growth phase of promastigotes was added to the macrophages to generate a parasite/macrophage ratio of 10:1. It was then incubated at 37 °C in 5% CO2 for 24 hr ( 31 ). The macrophages containing antiamastigote were treated with various concentrations of 0–500 mg.mL-1 R-ZnO NPs ( 32 ).
3.5. Statistical Analysis
The differences between the groups were determined using one-way analysis of variance (ANOVA) to analyse the obtained results. A p-value of < 0.05 was considered significant.
4. Results
4.1. Biosynthesis and Characterization of ZnO NRs
The UV-Vis spectrum at 350–370 nm wavelengths (Fig. 1) shows the synthesis of R-ZnO NPs to be consistent with previous findings ( 33 ).
Fig. 1.
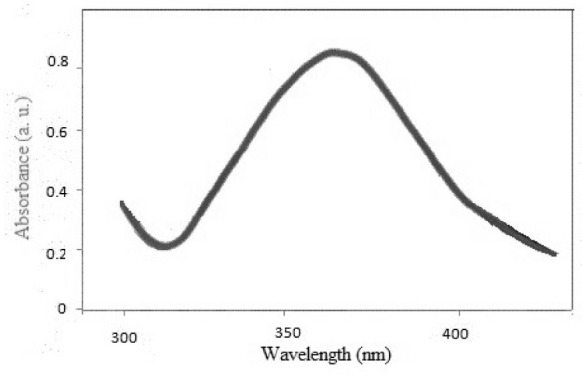
UV-visible spectroscopy of biosynthesized ZnO nanoaprticles.
The XRD pattern shows dispersion peaks at 31, 35, 37, 48, 57, 62, 66, 68, and 69, thus confirming the presence of ZnO NRs in the sample (Fig. 2). which corroborated previous with previous findings ( 33 , 34 ).
Fig. 2.
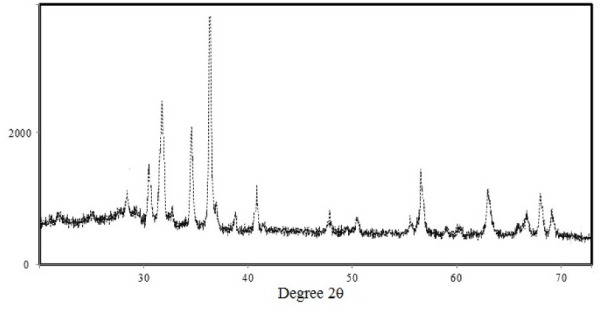
XRD pattern of biosynthesized R-ZnO nanoaprticles.
TEM images confirm that biogenic ZnO NPs have rod-shaped morphology; however, spherical NPs were observed in the TEM images. R-ZnO NPs are below 100 nm in size (Fig. 3).
Fig. 3.
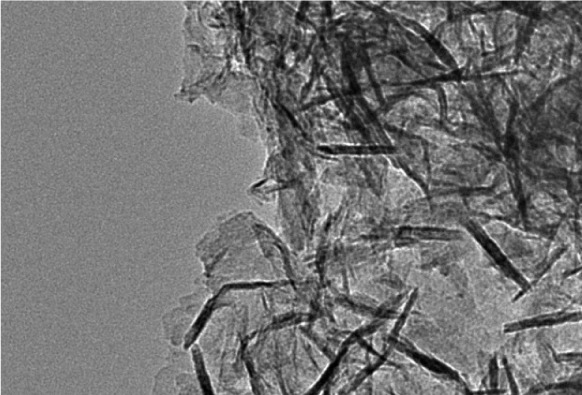
TEM image of biosynthesized R-ZnO NPs nanoaprticles.
4.2. Antiamastigote Assay
The R-ZnO NPs were found to inhibit the multiplication rate of amastigotes in a dose-dependent manner (). The IC50 values were about 10 mg.mL-1 for both R-ZnO NPs and Meglumine antimoniate (glucantime) as positive control. The concentration of 0.5 mg.mL-1 of biogenic R-ZnO NPs revealed a higher toxicity effect on L. major (amastigotes). The results disclose that by increasing the concentration of R-ZnO NPs, the viability of the tested parasites significantly decreases.
Fig. 4.
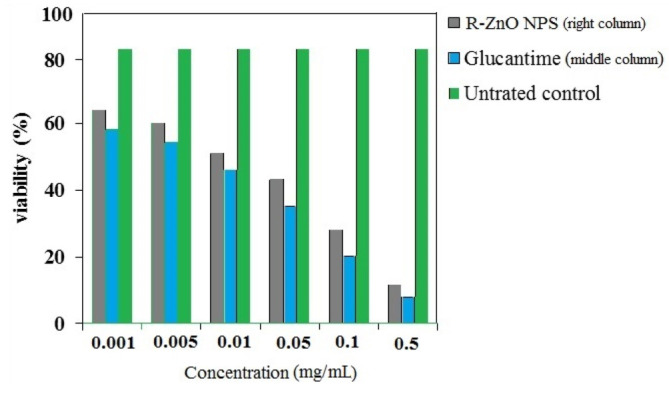
The effect of R-ZnO NPs and Glucantime on viability of Leishmania major (amastigotes).
5. Discussion
In this study, for the first time, the rod shaped ZnO NPs were biosynthesized as a safe therapeutic nanomaterial. A novel protocol for the greener production of the rod shaped ZnO NPs is presented in this study. The traditional NPs synthesized by chemical methods leads to the adsorption of toxic chemical compounds onto the surface of synthesized NPs which may have adverse effects in medicine. The nanoparticles that are produced by plant extract have lower environmental risk due to lack of harsh chemicals often in their synthesis process. Therefore, they can be applied in medical programs such as drug delivery. The salient advantages of producing plant nanoparticles via greener methods such as using bacterial or fungal extracts is the safety and high availability of medicinal plants that are more reliable and healthier than bacterial or fungal extracts mediated for the production of nanoparticles. Green synthesis methods using bioresources such as plants, fungi, or bacteria for the synthesis of biogenic NPs represent alternatives to conventional chemical synthesis methods.
The use of factors present in the plant and fungus residue not only are responsible in the synthesis of nanoparticles, but by stacking around the nanoparticles, they cause stability and prevent their agglomoration. Plants, in addition to being non-toxic, have different types of metabolites, which can be effective in the synthesis of nanoparticles which can include terpenoids, flavonoids, carbonyls, amides, and carboxylic acids, which directly contribute to the formation of nanoparticles.
The result of a study conducted against L. major showed that rod shaped ZnO NPs have good leishmanicidal activity against L. major and this outcome could help in the development of formulations as efficient means to synthesize R-ZnO NPs from natural products in our fight against the resistant microorganisms ( 35 , 36 ).
In our study, the rod shaped ZnO nanoparticles displayed strong leishmanicidal efficiency (IC50 about 0.012 mg.mL-1). There are no reports on the leishmanicidal activity of biogenic rod shaped ZnO NPs nor on the cytotoxic effect of chemically synthesized Rod shaped ZnO NPs on living cells. The study by Delavari et al. 2014 ( 37 ) have reported leishmanicidal activity of chemicaly synthesized spherial shaped ZnO NPs on L. major, the IC50 ZnO NPs being 0.0378 mg.mL-1 on promastigotes of L. major. Also Sumaira et al. ( 38 ) have reported leishmanicidal activity od spherical greener sythsized ZnO NPs, the IC50 ZnO NPs being 0.25 mg.mL-1 against Leishmania tropica. But in our present study, the biogenic greener synthesized NPs rod shaped ZnO NPs displayed much stronger leishmanicidal activity (IC50 about 0.012 mg.mL-1); they are more effective in leishmanicidal activity compared to chemically assembled spherical shape ZnO NPs or greener spherial shaped ZnO nanoarticles. Additionally, biogenic R-ZnO NPs can also be used in vivo.
6. Conclusion
These reslts show that it is possible to prepare a safe and ecofriendly synthsized NPs with leishmancidal potential. The greener synthesized rod shaped ZnO NPs, displayed stronger leishmanicidal activity (IC50 about 0.012 mg.mL-1); compared to chemically assembled spherical shape ZnO NPs and greener spherical shaped ZnO nanoparticles( 37 ).
Acknowledgement
This project was reviewed and approved by the Behbahan University of Medical Sciences.
Competing interests
The authors confirm that this article content has no competing interests.
Authors’ Contribution
All authors have participated in the manuscript preparation.
Financial Disclosure
There is no conflict of interest
References
- 1.Khatami M, Alijani H, Sharifi I, Sharifi F, Pourseyedi S, Kharazi S, et al. Leishmanicidal Activity of Biogenic Fe3O4 Nanoparticles. Sci Pharm. 2017;85(4):36. doi: 10.3390/scipharm85040036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aflatoonian MR, Sharifi I, Aflatoonian B, Bamorovat M, Heshmatkhah A, Babaei Z, et al. Associated-risk determinants for anthroponotic cutaneous leishmaniasis treated with meglumine antimoniate: A cohort study in Iran. PLoS Negl Trop Dis. 13(6):e0007423. doi: 10.1371/journal.pntd.0007423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bamorovat M, Sharifi I, Aflatoonian MR, Sadeghi B, Shafiian A, Oliaee RT, et al. Host's immune response in unresponsive and responsive patients with anthroponotic cutaneous leishmaniasis treated by meglumine antimoniate: A case-control study of Th1 and Th2 pathways. Int immunopharmacology. 2019;69:321–327. doi: 10.1016/j.intimp.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Daneshvar H, Tavakoli Kareshk A, Sharifi I, Keyhani A, Tavakoli Oliaee R, Asadi A. Host-parasite Responses Outcome Regulate the Expression of Antimicrobial Peptide Genes in the Skin of BALB/c and C57BL/6 Murine Strains Following Leishmania major MRHO/IR/75/ER Infection. Iran J Parasitol. 2018;13(4):515–523. doi: 10.1016/j.ijbiomac.2017.08.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mahmoudvand H, Fasihi Harandi M, Shakibaie M, Aflatoonian MR, ZiaAli N, Makki MS, et al. Scolicidal effects of biogenic selenium nanoparticles against protoscolices of hydatid cysts. Int J Surg. 2014;12(5):399–403. doi: 10.1016/j.ijsu.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 6.Jahani S, Khorasani-Motlagh M, Noroozifar M. DNA interaction of europium(III) complex containing 2,2′-bipyridine and its antimicrobial activity. J Biomol Struct Dynamics. 2016;34(3):612–624. doi: 10.1080/07391102.2015.1048481. [DOI] [PubMed] [Google Scholar]
- 7.Karthik K, Vijayalakshmi S, Phuruangrat A, Revathi V, Verma U. Multifunctional Applications of Microwave-Assisted Biogenic TiO2 Nanoparticles. J Clu Sci. 2019;30:1–8. doi: 10.1007/s10876-019-01556-1. [DOI] [Google Scholar]
- 8.Niroomand S, Khorasani-Motlagh M, Noroozifar M, Jahani S, Moodi A. Photochemical and DFT studies on DNA-binding ability and antibacterial activity of lanthanum(III)-phenanthroline complex. J Mol Struct. 2017;1130:940–950. doi: 10.1016/j.molstruc.2016.10.076. [DOI] [Google Scholar]
- 9.Rahi A, Sattarahmady N, Heli H. Zepto-molar electrochemical detection of Brucella genome based on gold nanoribbons covered by gold nanoblooms. Sci Rep. 2015;5:18060. doi: 10.1038/srep18060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hossein H, Masoud N. Applications of Nanoflowers in Biomedicine. Recent Pat Nanotechnol . 2018;12(1):22–33. doi: 10.2174/1872210511666170911153428. [DOI] [PubMed] [Google Scholar]
- 11.Khatami M, Iravani S, Varma R.s, Mosazade F, Darroudi M, Borhani F. Cockroach wings-promoted safe and greener synthesis of silver nanoparticles and their insecticidal activity . Bioprocess Biosyst Eng . 2019;42 doi: 10.1007/s00449-019-02193-8. [DOI] [PubMed] [Google Scholar]
- 12.Akhtartavan S, Karimi M, Karimian K, Azarpira N, Khatami M, Heli H. Evaluation of a self-nanoemulsifying docetaxel delivery system. Biomed Pharmaco. 2019;109(2019):2427–2433. doi: 10.1016/j.biopha.2018.11.110. [DOI] [PubMed] [Google Scholar]
- 13.Rajaei M, Foroughi MM, Jahani S, Shahidi Zandi M, Hassani Nadiki H. Sensitive detection of morphine in the presence of dopamine with La3+ doped fern-like CuO nanoleaves/MWCNTs modified carbon paste electrode. J Mol Liq. 2019;284:462–472. doi: 10.1016/j.molliq.2019.03.135. [DOI] [Google Scholar]
- 14.Torkzadeh-Mahani R, Foroughi MM, Jahani S, Kazemipour M, Hassani Nadiki H. The effect of ultrasonic irradiation on the morphology of NiO/Co3O4 nanocomposite and its application to the simultaneous electrochemical determination of droxidopa and carbidopa. Ultrason Sonochem. 2019;56:183–192. doi: 10.1016/j.ultsonch.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Mohammadinejad R, Moosavi MA, Tavakol S, Vardar DÖ, Hosseini A, Rahmati M, et al. Necrotic, apoptotic and autophagic cell fates triggered by nanoparticles. Autophagy. 2019;15(1):4–33. doi: 10.1016/j.ijbiomac.2017.08.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Darroudi M, Sarani M, Kazemi Oskuee R, Khorsand Zak A, Amiri MS. Nanoceria: Gum mediated synthesis and in vitro viability assay. Ceram Int. 2014;40(2):2863–2868. doi: 10.1016/j.ceramint.2013.10.026. [DOI] [Google Scholar]
- 17.Sattarahmady N, Rezaie-Yazdi M, Tondro GH, Akbari N. Bactericidal laser ablation of carbon dots: An in vitro study on wild-type and antibiotic-resistant . Staphylococcus aureus J Photochem Photobiol B. 2017;166(Supplement C):323–332. doi: 10.1016/j.jphotobiol.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Karthik K, Dhanuskodi S, Gobinath C, Prabukumar S, Sivaramakrishnan S. Fabrication of MgO nanostructures and its efficient photocatalytic, antibacterial and anticancer performance. J Photochem Photobiol B. 2019;190:8–20. doi: 10.1016/j.jphotobiol.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 19.Khatami M, Alijani H, Sharifi I. Biosynthesis of bimetallic and core shell nanoparticles: their biomedical applications: A review. IET Nanobio. 2018;12(7): 879–887. doi: 10.1049/iet-nbt.2017.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dağlıoğlu Y, Yılmaz Öztürk B. Effect of concentration and exposure time of ZnO-TiO2 nanocomposite on photosynthetic pigment contents, ROS production ability, and bioaccumulation of freshwater algae (Desmodesmus multivariabilis) Caryologia. 2018;71(1):13–23. doi: 10.1080/00087114.2017.1400262. [DOI] [Google Scholar]
- 21.Khatami M, Varma R.s, Heydari M, Peydayesh M, Sedighi A, AghaAskari H, Rohani M, Baniasadi M, Arkia S, Seyedi F, Khatami S. Copper Oxide Nanoparticles Greener Synthesis Using Tea and its Antifungal Efficiency on Fusarium solani. Geomicrobiology J . 2019;36:1. doi: 10.1080/01490451.2019.1621963. [DOI] [Google Scholar]
- 22.Karthik K, Pradeeswari K, Mohan Kumar R, Murugesan R. Microwave-assisted V2O5 nanoflowers for efficient lithium-ion battery. Mat Res Innovations. 2019;23:1–5. doi: 10.1080/14328917.2019.1618044. [DOI] [Google Scholar]
- 23.Khan AU, Yuan Q, Khan ZUH, Ahmad A, Khan FU, Tahir K, et al. An eco-benign synthesis of AgNPs using aqueous extract of Longan fruit peel: Antiproliferative response against human breast cancer cell line MCF-7, antioxidant and photocatalytic deprivation of methylene blue. J Photochem Photobiol B. 2018;183:367–373. doi: 10.1016/j.jphotobiol.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 24.Miri A, Sarani M. Biosynthesis, characterization and cytotoxic activity of CeO2 NPs. Ceram Int. 2018;44(11):12642–12647. doi: 10.1016/j.ceramint.2018.04.063. [DOI] [Google Scholar]
- 25.Miri A, Sarani M. Biological studies of synthesized silver nanoparticles using Prosopis farcta. Mol Biol Rep. 2018;45(6):1621–1626. doi: 10.1007/s11033-018-4299-0. [DOI] [PubMed] [Google Scholar]
- 26.Jamdagni P, Rana JS, Khatri P, Nehra K. Comparative account of antifungal activity of green and chemically synthesized Zinc Oxide nanoparticles in combination with agricultural fungicides. Int J Nano Dim. 2018;9(2):198–208. doi: 10.1016/j.ijbiomac.2017.08.149. [DOI] [Google Scholar]
- 27.Minhas FT, Arslan G, Gubbuk IH, Akkoz C, Ozturk BY, Asıkkutlu B, et al. Evaluation of antibacterial properties on polysulfone composite membranes using synthesized biogenic silver nanoparticles with Ulva compressa (L) and Cladophora glomerata (L) Kütz extracts Kütz . Int J Biol Macromol. 2018;107:157–165. doi: 10.1016/j.ijbiomac.2017.08.149. [DOI] [PubMed] [Google Scholar]
- 28.Tahir K, Ahmad A, Li B, Khan AU, Nazir S, Khan S, et al. Preparation, characterization and an efficient photocatalytic activity of Au/TiO2 nanocomposite prepared by green deposition method. Mat Let. 2016;178:56–59. doi: 10.1016/j.matlet.2016.04.176. [DOI] [Google Scholar]
- 29.Khan FU, Chen Y, Khan NU, Khan ZUH, Khan AU, Ahmad A, et al. Antioxidant and catalytic applications of silver nanoparticles using Dimocarpus longan seed extract as a reducing and stabilizing agent. J Photochem Photobiol B. 2016;164:344–351. doi: 10.1016/j.jphotobiol.2016.09.042. [DOI] [PubMed] [Google Scholar]
- 30.Tahir K, Nazir S, Ahmad A, Li B, Ali Shah SA, Khan AU, et al. Biodirected synthesis of palladium nanoparticles using Phoenix dactylifera leaves extract and their size dependent biomedical and catalytic applications. RSC Adv. 2016;6(89):85903–16. doi: 10.1039/C6RA11409A. [DOI] [Google Scholar]
- 31.Ahmad A, Syed F, Shah A, Khan Z, Tahir K, Khan AU, et al. Silver and gold nanoparticles from Sargentodoxa cuneata: synthesis, characterization and antileishmanial activity. RSC Adv. 2015;5(90):73793–806. doi: 10.1039/C5RA13206A. [DOI] [Google Scholar]
- 32.Khatami M, Alijani HQ, Heli H, Sharifi I. Rectangular shaped zinc oxide nanoparticles: Green synthesis by Stevia and its biomedical efficiency. Ceram Int. 2018;44(13):15596–15602. doi: 10.1016/j.ceramint.2018.05.224. [DOI] [Google Scholar]
- 33.Nagajyothi PC, Sreekanth TVM, Tettey CO, Jun YI, Mook SH. Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bioorg Med Chem Lett. 2014;24(17):4298–4303. doi: 10.1016/j.bmcl.2014.07.023. [DOI] [PubMed] [Google Scholar]
- 34.Kim K-M, Choi M-H, Lee J-K, Jeong J, Kim Y-R, Kim M-K, et al. Physicochemical properties of surface charge-modified ZnO nanoparticles with different particle sizes. Int J Nanomedicine. 2014;9(Suppl 2):41–56. doi: 10.2147/IJN.S57923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Birla S, Tiwari V, Gade A, Ingle A, Yadav A, Rai M. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol. 2009;48(2):173–179. doi: 10.1111/j.1472-765X.2008.02510.x. [DOI] [PubMed] [Google Scholar]
- 36.Rai M, Duran N. Metal nanoparticles in microbiology: . Springer Science & Business Media. 2011 doi: 10.1007/978-3-642-18312-6. [DOI] [Google Scholar]
- 37.Delavari M, Dalimi A, Ghaffarifar F, Sadraei J. In Vitro Study on Cytotoxic Effects of ZnO Nanoparticles on Promastigote and Amastigote Forms of Leishmania major (MRHO/IR/75/ER) Iran J Parasitol. 2014;9(1):6–13. [PMC free article] [PubMed] [Google Scholar]
- 38.Sumaira G, Afridi MS, Hashmi SS, Ali GS, Zia M, Abbasi BH. Comparative antileishmanial efficacy of the biosynthesised ZnO NPs from genus Verbena. IET nanobiotech. 2018;12(8):1067–1073. doi: 10.1049/iet-nbt.2018.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]


