Abstract
Background:
Recent years have seen a rise in the diversity and use of synthetic cannabinoids. The present study evaluated the behavioral effects of the third-generation indazole-3-carboxamide-type synthetic cannabinoid, AB-FUBINACA.
Methods:
Adult male and female C57BL/6J mice were treated with AB-FUBINACA (0–3 mg/kg, i.p.) and tested repeatedly in the tetrad battery measuring catalepsy, antinociception, hypothermia, and locomotor activity. Mice treated with AB-FUBINACA (≥2 mg/kg, i.p.) displayed classic cannabinoid effects in the tetrad that were blocked by the CB1 receptor selective antagonist rimonabant. To address tolerance and withdrawal effects, a second group of mice was injected with AB-FUBINACA (3 mg/kg, s.c.) or vehicle consisting of 5% ethanol, 5% Kolliphor EL, and 90 % saline every 12 h and tested daily in modified tetrad over the course of 5 days. On the 6th day, withdrawal was precipitated using rimonabant (3 mg/kg, s.c.), and somatic signs of withdrawal (i.e., head twitches and paw tremors) were quantified.
Results:
Although mice did not develop tolerance to AB-FUBINACA or cross-tolerance to Δ9-tetrahydrocannabinol (THC; 50 mg/kg, i.p.), somatic precipitated withdrawal signs were observed. Repeated tetrad testing up to 48 h post injection indicated that AB-FUBINACA effects are relatively short-lived, as compared with THC. Brain levels of AB-FUBINACA, as quantified by UHPLC-MS/MS, were undetectable 4 h post injection.
Conclusions:
These data indicate that the cannabinoid effects of AB-FUBINACA are relatively short-lived, yet sufficient to induce dependence in mice.
Keywords: Cannabis use disorder, Substance use disorder, Synthetic marijuana, Drug dependence
1. Introduction
The term “synthetic cannabinoid” technically refers to any lab-produced compound that affects CB1 or CB2 function, and includes receptor agonists and antagonists, inhibitors of enzymes, and allosteric modulators (Hess et al., 2016). Colloquially, "synthetic cannabinoid" is used as an umbrella term that encompasses CB1 agonists found in"Spice" products. Synthetic cannabinoid receptor agonists (SCRAs) were originally synthesized for research purposes, such as receptor/ligand interaction studies, or to selectively agonize one receptor without affecting the other (Banister et al., 2015; Huffman et al., 2005). Once the chemical structures of SCRAs were published, clandestine chemists hi-jacked the compounds and produced purportedly “safe” alternatives to cannabis (Jarbe and Raghav, 2016).
Though some individuals ingest powdered forms of SCRAs, the most common route of administration is inhalation of smoked or vaporized plant material adulterated with one or more of the SCRAs (Seely et al., 2012). Solutions of one or more SCRAs, in a solvent, are often sprayed onto inert plant material, but may also be applied to plant material containing psychoactive alkaloids (Dresen et al., 2010; EMCDDA, 2009; Havenon et al., 2011; Seely et al., 2012). Sold under the broad umbrella term “Spice,” these synthetic cannabinoid preparations are labeled "not for human consumption" to avoid regulation by the Food and Drug Administration (Brents et al., 2013), despite their disguised intended use.
SCRAs are broadly categorized into 7 families, based on chemical structure: cyclohexyl-substituted phenols (e.g., CP 47,497), naphtholindoles (e.g., AM-2201, JWH-018), benzoylindoles (e.g., 6-APB), tetramethylcyclopropylindoles (e.g., UR-144, XLR-11), adamantoylindoles (e.g., AKB48), indazole carboxamides (e.g., AB-FUBINACA, AB-PINACA), and quinolinyl esters (e.g., PB-22) (Canazza et al., 2016; Ford et al., 2017; Hess et al., 2016). AB-FUBINACA is a member of the indazole carboxamide family of synthetic cannabinoids, which includes two other compounds that have been linked to deaths in the United States: AB–CHMINACA and AB-PINACA (Trecki et al., 2015). Whereas Δ9-tetrahydrocannabinol (THC) is a partial agonist of CB1 and CB2, many SCRAs act as full agonists at CB1, and, in some cases, CB2 (Fantegrossi et al., 2014; Ford et al., 2017; Hess et al., 2016), and thus SCRAs induce similar cannabimimetic effects to THC, but with increased intrinsic activity (Hess et al., 2016; Wiley et al., 2015).
Classic behavioral and physiological effects of cannabinoids in rodents include catalepsy, antinociception, and hypothermia and depressed locomotor activity, and are typically assessed using the Billy R. Martin tetrad battery (Lichtman et al., 2001; Wiley and Martin, 2003). When analyzed in tetrad, both first generation indole (e.g., JWH-018, CP 55,940) and later generation (e.g., AB-PINACA, AB–CHMINACA, AB-FUBINACA) indazole-3-carboxamide-type SCRAs induce catalepsy, antinociception, and hypothermia in mice (Canazza et al., 2017; Wiley et al., 2015). SCRAs also have inconsistent effects on locomotor activity. For instance, whereas only high doses of AB-FUBINACA induces immobility in mice (Canazza et al., 2017; Gatch and Forster, 2015; Schreiber et al., 2019), decreased locomotor activity is reported at low and high doses, in rats (Kevin et al., 2017).
In addition to changes in physiology, cannabinoids have well-established effects on emotion in people and experimental animal models. In humans, acute SCRA use can cause symptoms such as agitation, anxiety, and hallucinations (Benford and Caplan, 2011; Besli et al., 2015; Durand et al., 2015). Several deaths and serious injuries have been reported following acute SCRA use, including self-mutilation and several suicides, most of which have been attributed to hallucinations (Gay, 2010; Meijer et al., 2014; Patton et al., 2013; Thomas et al., 2014; Trecki et al., 2015). In particular, the indazole-3-carboxamide-type SCRAs, are used as street drugs and adulterants in commercial cannabinoid products. For example, MDMB-FUBINACA was identified in e-cigarette liquid (Peace et al., 2017) and AB-FUBINACA was detected in a commercial "cannabidiol oil" administered to a child (Rianprakaisang et al., 2020). Moreover, SCRAs have shown mixed effects on rodent assays used to screen anxiolytic and antidepressant drugs. For example, in mice, AB-FUBINACA increases time spent in the open arms of the elevated plus maze at low doses, but, has the opposite effect at high doses (Schreiber et al., 2019). In addition, AB-FUBINACA decreases struggling in the forced swim test at a low dose, but increases struggling at a high dose (Schreiber et al., 2019), suggesting that AB-FUBINACA produces dose-dependent locomotor effects observed in other cannabinoids including THC (Katsidoni et al., 2013; Sañudo-Peña et al., 2000).
Little is known about the effects of repeated SCRA administration. One case report indicates that chronic SCRA use may lead to relatively long-term psychosis (Durand et al., 2015). It is plausible that 5HT2A receptors mediate the psychogenic effects of SCRAs, given their upregulation in schizophrenia (Fantegrossi et al., 2018). More commonly, chronic drug use results in physical dependence. There is evidence, in both preclinical models and from case reports, that repeated use of SCRAs induces dependence, typically evidenced by the development of tolerance and withdrawal following cessation of use (Aceto et al., 2001; Nacca et al., 2013; Sampson et al., 2015; Trexler et al., 2018). The goals of the present study were to evaluate the acute cannabimimetic effects of the new generation SCRA AB-FUBINACA. To this end, we tested the acute effects of AB-FUBINACA using the classic "tetrad" battery. We also monitored the development of physical cannabinoid dependence (i.e., tolerance and withdrawal) following repeated AB-FUBINACA administration in mice, as a predictive proxy of Cannabis Use Disorder in humans. Brain levels of AB-FUBINACA were quantified at various timepoints following injection and matched to "tetrad" effects to establish a timecourse.
2. Methods
2.1. Animals
Adult male and female C57BL/6J mice (The Jackson Laboratory; Bar Harbor, ME) were group housed (4–5 per cage) in Polysulfone plastic cages with food and water available ad libitum. Mice were housed in a temperature (20–22 °C) and humidity (50±5%) controlled room, on a 12:12 h light/dark cycle in an AAALAC accredited facility. Mice were randomly assigned to each treatment group, such that each cage contained mice from at least two different treatment groups (i.e., no cage contained only AB-FUBINACA-treated mice). Male and female mice were stratified by sex before random assignment. All experiments were carried out by trained technicians who were blinded to treatment conditions. The Animal Care and Use Committee at West Virginia University approved all experimental protocols prior to the start of any experimental manipulation.
2.2. Drugs
The SCRA AB-FUBINACA, the phytocannabinoid Δ9-THC, and the selective CB1 receptor antagonist rimonabant (SR141716A), were generously provided by the National Institute on Drug Abuse (NIDA) Drug Supply Program (Bethesda, MD). All drugs were dissolved in a vehicle composed of 5% ethanol, 5% Kolliphor EL (Sigma-Aldrich, St. Louis, MO), and 90 % normal saline (Kinsey and Cole, 2013). All solutions were warmed to room temperature before administration at a volume of 10 μL/g body mass.
2.3. Behavioral assessments
2.3.1. Tetrad
The “Billy Martin tetrad” is a well-characterized battery of four assays used to evaluate the effects of cannabinoid agonists (Lichtman et al., 2001; Wiley and Martin, 2003). It consists of assessments of catalepsy, antinociception, core body temperature, and locomotor activity. Mice were acclimated to the test room for a minimum of 1 h prior to tetrad testing (i.e., in order: locomotor activity, catalepsy, tail immersion, and body temperature) (Grim et al., 2016; Schlosburg et al., 2009). Catalepsy was assessed by gently laying the forepaws of individual mice over a metal bar elevated 3 cm above the benchtop. Total latency to move one or both forepaws off the bar was recorded, with a maximum cutoff of 60 s (Long et al., 2009). Antinociception was measured via immersing the distal tip of the tail (i.e., the last 1 cm) into a 56 °C water bath (Falenski et al., 2010). Latency to remove the tail from the water was recorded, with a maximum cutoff of 10 s. Hypothermia was assessed by taking rectal temperature using a micro probe thermocouple thermometer designed for use with mice (BAT-12 with RET-3 probe, Physitemp Instruments Inc., Clifton, NJ, USA). Spontaneous locomotor activity was measured by placing individual mice into an empty, plastic test chamber (30 cm W x40 cm L x16 cm H) inside a sound-attenuating chamber outfitted with a fan, white LED lighting and an overhead video camera. Mice were recorded for 5 min and locomotor activity was scored in real time using ANY-maze video tracking software (Stoetling, Wool Dale, IL). Time immobile was determined by setting the tracking parameters to a latency of 1200 ms for 90 % of the mouse image pixels (Trexler et al., 2019). The test chamber was cleaned between subjects with a paper towel dampened with distilled water. In cases where mice were repeatedly tested, a modified tetrad that excluded locomotor testing was used, because mice quickly habituate to locomotor testing.
2.3.2. Precipitated withdrawal paradigm
Mice were weighed daily and injected subcutaneously (s.c.) with AB-FUBINACA (1 or 3 mg/kg; Canazza et al., 2017; Wiley et al., 2017) or vehicle every 12 h for 5 days, as described previously (Falenski et al., 2010; Schlosburg et al., 2009; Trexler et al., 2018). Subcutaneous (s.c.) injection was used for repeated administration in lieu of intraperitoneal (i.p.) injection to decrease risk of injection site irritation or accidental intra-intestinal administration (Das and North, 2007). On the morning of the sixth day, all mice received a final injection of AB-FUBINACA or vehicle. After 30 min, mice received an i.p. injection of rimonabant (3 mg/kg) (Lichtman et al., 2001; Trexler et al., 2018) to precipitate withdrawal.
2.3.3. Somatic withdrawal signs testing
Somatic signs of withdrawal were measured as described previously (Trexler et al., 2018). Each mouse was placed into an empty, plastic test chamber (20 cm W x20 cm L x15 cm H) inside a sound-attenuating chamber outfitted with a fan and white LED lighting. The apparatus had three clear sides and one mirrored side that faced a video camera to allow for observation of behavior when the mouse faced away from the camera.
Mice were habituated to the test apparatus for 30 min following final AB-FUBINACA or vehicle injection and were then removed and injected with rimonabant (3 mg/kg) or vehicle, as previously reported (Schlosburg et al., 2009). The boxes were cleaned between subjects using a paper towel moistened with distilled water. Each mouse was then placed back into the test chamber and video was recorded for 60 min.
Video files were deidentified and scored by a trained observer. A subset of 15 videos was scored by a second observer to ensure inter-rater reliability (r2=.97). The dependent variables were (1) incidences of paw tremors and (2) head twitches (i.e., an incidence was scored for ‘paw tremor’ when the behavior was observed, not for each individual motion). Incidences were considered separate when either (1) another behavior occurred between the incidences, or (2) there was at least 1 s between incidences (Schlosburg et al., 2009).
2.4. Pharmacokinetics: ultrahigh-performance liquid chromatograph-tandem mass spectrometer (UHPLC-MS/MS) analysis
2.4.1. Reagents
AB-FUBINACA and AB–CHMINACA were purchased from Cerilliant Corporation (Round Rock, TX). Acetonitrile, ammonium formate, formic acid, methanol, and water were purchased from Fisher Scientific (Hanover Park, IL, USA). All reagents were ACS grade or better.
2.4.2. Method
Adult male and female mice were injected with AB-FUBINACA (3 mg/kg, i.p.) at various timepoints (0.25, 0.5, 1, 4, or 12 h) prior to being euthanized via CO2 asphyxia, and brains were placed in 1 mL ice-cold distilled water, homogenized, snap frozen in LN2, and stored at −80 °C until assay. A separate group of mice was injected with vehicle 1 h before tissue collection. Brain tissue samples were homogenized with water (1:3 tissue:water). All samples and controls were kept at −20 °C until analyzed. On the day of analysis, a seven-point calibration curve at concentrations of 1, 2, 5, 10, 20 and 100 ng/g AB-FUBINACA in brain tissue along with a drug free control and a negative control without internal standard (i.e., 1 ng/g AB–CHMINACA) in drug-free serum were prepared. AB-FUBINACA was extracted from tissue using a technique modified from a previously published liquid/liquid extraction method (Poklis et al., 2011). In brief, 1 ng of AB–CHMINACA, the internal standard, was added to 400 μL of brain homogenate of each calibrator, control or specimen except the negative control. To each specimen, 1 mL of ice-cold acetonitrile was added dropwise while vortexing. The samples were then centrifuged for 5 min at 3000 rpm. After centrifuging the samples were placed in −40 °C freezer for at least 2 h to create clear separation between the aqueous and organic layers. The upper organic layer was transferred to a clean test tube and eva-porated to dryness under a gentle stream of nitrogen in a 40 °C dry bath. The samples were reconstituted with 100 μL of mobile phase and placed in auto-sampler vials for analysis.
The ultrahigh-performance liquid chromatograph-tandem mass spectrometer (UHPLC-MS/MS) analysis was performed on a Sciex 6500 + QTRAP system with an IonDrive Turbo V source for TurbolonSpray® (Sciex, Ontario, Canada) attached to a Shimadzu UPLC system (Kyoto, Japan) controlled by Analyst software (Sciex, Ontario, Canada). Chromatographic separation was performed on an Agilent Zorbax Eclipse XDB-C18 4.6 × 75 mm, 3.5 μ column (Santa Clara, CA). The mobile phase consisted of 10:90 10 mM ammonium formate and 0.1 % formic acid in water:methanol. The UHPLC flow rate was set at 1 mL/min. The mass spectrometer source temperature was set at 650 °C and had a curtain gas flow rate of 30 mL/min. The ionspray voltage was 5000 V, with the ion source gases 1 and 2 at flow rates of 40 mL/min. The acquisition mode used was multiple reaction monitoring (MRM). The retention times for AB-FUBINACA and AB–CHMINACA were 0.9 and 1.2 min, respectively. The declustering potential was set at 30 eV. The following transition ions (m/z) were monitored with their corresponding collection energies (eV) in parentheses: AB-FUBINACA, 369>352 (12) and 369>324 (19); and AB–CHMINACA, 357>340 (14) and 357>312 (24). The total run time for the analytical method was 2.0 min. A linear regression of the peak area of ratios of the quantification and the ISTDs transition ion were used to construct the calibration curves.
2.5. Statistical analyses
Data were analyzed using a between- (e.g., acute AB-FUBINACA, brain metabolism) or within-subjects (e.g., AB-FUBINACA timecourse, tolerance) analysis of variance (ANOVA), with the exception of the withdrawal experiment, which used unmatched t-tests to compare the two groups (i.e., veh vs. AB-FUBINACA 1 mg/kg), because an ANOVA of two groups is mathematically equivalent to a t-test. Main or interaction effects were followed by either Dunnett (e.g., for dose-response curves, comparing to vehicle treatment) or Bonferonni post hoc tests, as appropriate. Differences were considered statistically significant at α = 05.
2.6. Experimental design
Exp 1a. Determine the dose-response relation of the acute effects of AB-FUBINACA (0.1, 1, 2, or 3 mg/kg, i.p.; 30 min pretreatment) or vehicle in male and female mice in the classic tetrad battery, as detailed in 2.3.1 above.
Exp 1b. Challenge the effects of AB-FUBINACA in Exp 1a by using the CB1 selective antagonist/inverse agonist rimonabant (3 mg/kg, i.p.). Rimonabant was administered 60 min prior to testing, and either AB-FUBINACA (3 mg/kg, i.p.) or vehicle was administered 30 min prior to testing in modified tetrad (i.e., catalepsy, tail immersion, and body temperature) testing.
Exp 2a. Determine ability to induce AB-FUBINACA withdrawal. To assess withdrawal effects, male and female mice were injected twice daily with AB-FUBINACA (1 mg/kg, s.c.) or vehicle for 5 days. On the 6th day, mice were administered a final dose of AB-FUBINACA (or vehicle) and placed in the test chamber for 30 min. Withdrawal was precipitated with rimonabant (3 mg/kg, s.c.) immediately before mice were returned to the boxes for testing for somatic signs of withdrawal, as detailed in 2.3.2 above.
Exp 2b. Direct replication of Exp 2a, using 3 mg/kg (s.c.) dose of AB-FUBINACA.
Exp 2c. Determine drug tolerance effects of repeated administration of AB-FUBINACA. Following baseline testing, a separate group of male and female mice was administered AB-FUBINACA (3 mg/kg, s.c.) or vehicle every 12 h for 5 days and tested daily in tetrad to determine the degree to which AB-FUBINACA tolerance develops. Mice were tested daily in a modified tetrad battery, starting 30 min after morning drug administration. To probe THC cross-tolerance, approximately 24 h after the final AB-FUBINACA dose, mice were administered vehicle or THC (50 mg/kg, i.p.) and tested in the modified tetrad battery.
Exp 3a. To assess possible pharmacokinetic explanation for the relatively mild AB-FUBINACA withdrawal signs and lack of tolerance, the timecourse of AB-FUBINACA behavioral effects was compared to the well-studied phytocannabinoid, THC. After baseline tests, male and female mice were administered AB-FUBINACA (3 mg/kg, i.p.), THC (50 mg/kg, i.p.) or vehicle and tested repeatedly (0, 0.25, .5, 1, 2, 4, 8, 12, 24, and 48 h post-injection) in a modified tetrad battery. THC dose was chosen based on pilot data (Fig. S1).
Exp 3b. Determine brain levels of AB-FUBINACA. Whole brain levels of AB-FUBINACA were quantified, using a parallel group of mice injected with vehicle or AB-FUBINACA (3 mg/kg, i.p.) as detailed in 2.4.2 above. The collection timeline was based on behavioral effects of AB-FUBINACA observed in Exp 3a.
3. Results
3.1. AB-FUBINACA induces classic cannabinoid effects
In the tetrad, AB-FUBINACA (3 mg/kg) significantly increased latency in both catalepsy [F(4,32) = 6.6,p<.05; Fig. 1A] and tail immersion [F(3,35) = 6.9,p<.05; Fig. 1B] tests. Mice treated with AB-FUBINACA (≥2 mg/kg) displayed hypothermia [F(34,35) = 24.9,p<.05; Fig. 1C] and increased time immobile [F(4,35) = 11.4,p<.05; Fig. 1D]. To determine the necessity of CB1 receptor involvement, male and female mice were administered the CBi selective antagonist rimonabant (3 mg/kg, i.p.) or vehicle 30 min prior to AB-FUBINACA (3 mg/kg, i.p.) or vehicle treatment. Testing was conducted 60 min after rimonabant administration, and 30 min after AB-FUBINACA or vehicle. Again, AB-FUBINACA induced catalepsy [F(1,24) = 11.8; p<.05; Fig. 1E], antinociception [F(1,24) = 5.6; p<.05; Fig. 1F], and hypothermia [F(1,24) = 60.8; p<.05; Fig. 1G], and each of these effects was blocked by rimonabant pretreatment, consistent with a mechanism requiring CB1.
Fig. 1.
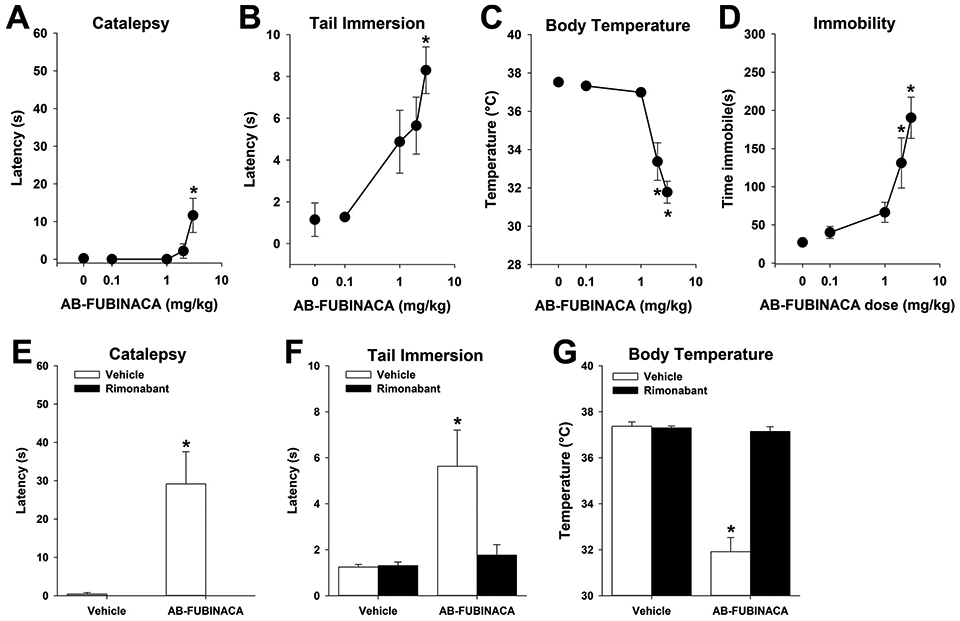
AB-FUBINACA induces classic cannabinoid effects via CB1 receptors. Male and female mice were treated with AB-FUBINACA (0-3 mg/kg, i.p.) 30 min prior to testing in the tetrad battery. AB-FUBINACA induced catalepsy (A) antinociception (B), hypothermia (C), and hypolocomotion (D), consistent with established cannabinoid effects. Pretreatment with the CB1 receptor selective antagonist rimonabant (3 mg/kg) blocked each of these effects of AB-FUBINACA (E-G). Data represent mean±SEM (n = 8[4 m/4f]/group);*p<.05 v. vehicle or baseline.
3.2. Repeated administration of AB-FUBINACA induces limited dependence
Rimonabant significantly increased paw tremors [t(18) = 3.8,p<.05; Fig. 2A] and head twitches [t(18) = 3.0,p<.05; Fig. 2B] in mice repeatedly administered AB-FUBINACA (1 mg/kg) compared to vehicle-treated mice. Similarly, rimonabant precipitated withdrawal increased both paw tremors [F(2,20) = 9.7,p<.05; Fig. 2C] and head twitches [F(2,20) = 4.3,p<.05; Fig. 2D] in the AB-FUBINACA (3 mg/kg, s.c.)-treated mice. It is noteworthy that, using these experimental procedures, mice administered AB-FUBINACA alone did not display spontaneous somatic withdrawal signs (Fig. 2C-D).
Fig. 2.
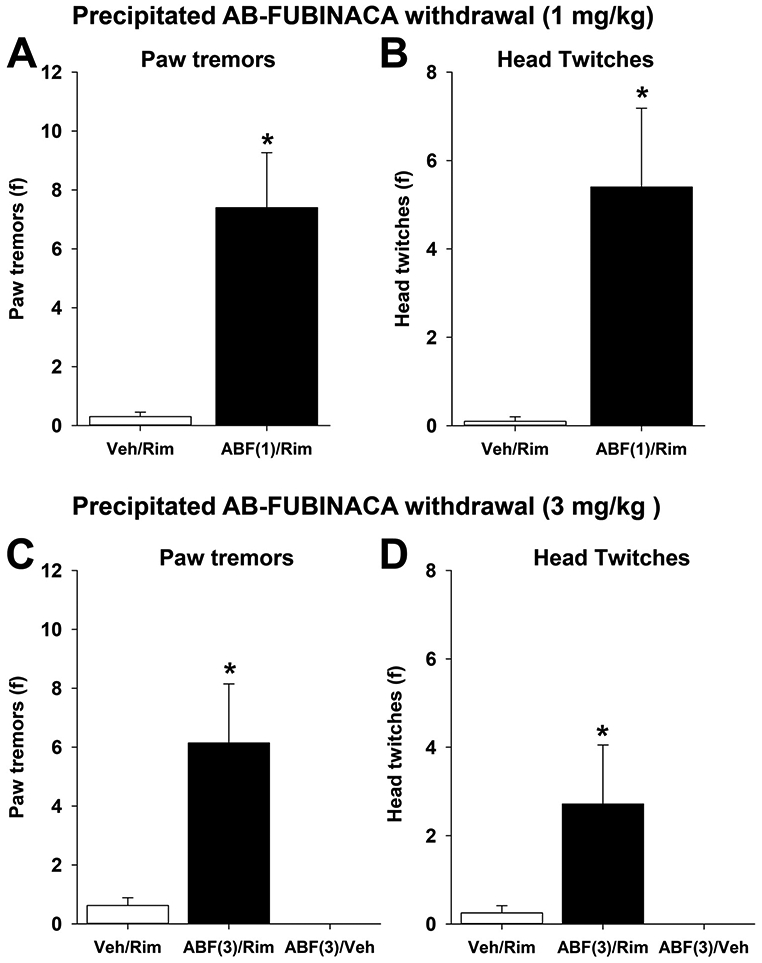
Rimonabant precipitates AB-FUBINACA withdrawal. Mice were treated with AB-FUBINACA (1 or 3 mg/kg, s.c.) twice daily for 6 days. On the 6th day, mice were injected with rimonabant (3 mg/kg, i.p following the morning injection and evaluated for somatic signs of withdrawal. AB-FUBINACA (1 or 3 mg/kg) increased paw tremors (A & C) and head twitches (B & D). Data represent mean±SEM (n = 8-10 [4-5 m & 4-5f] /group); *p<.05 v. vehicle/rim control group.
Mice repeatedly administered AB-FUBINACA maintained increased effects of catalepsy [Main effect drug F(1,70) = 31.8,p<.05; Fig. 3A], antinociception [Main effect drug F(1,70) = 15.3,p<.05; Fig. 3B], and hypothermia despite five days of treatment [Interaction F(5,70) = 8.94,p<.05; Fig. 3C] indicating that tolerance did not develop. Similarly, THC (50 mg/kg, i.p.) administered on dosing day 6 (i.e., in place of AB-FUBINACA) induced cannabimimetic effects, regardless of AB-FUBINACA treatment history, indicating a lack of cross-tolerance to THC in catalepsy [p=.48; Fig. 3D], antinociception [p=.87; Fig. 3E], or body temperature [p = .27; Fig. 3F].
Fig. 3.
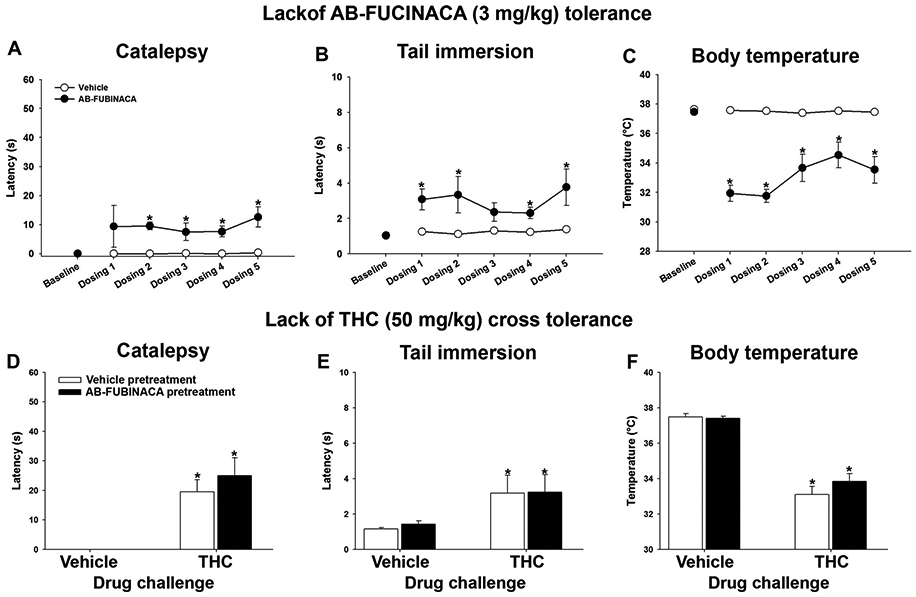
Repeated AB-FUBINACA administration does not induce tolerance. Mice were treated twice daily with AB-FUBINACA (3 mg/kg, i.p.) or vehicle for 5 days and assessed in a modified tetrad battery each day, approximately 30 min after injections. AB-FUBINACA continued to induce cataleptic (A), antinociceptive (B), or hypothermic (C) effects despite repeated administration. On the 6th day, mice were injected with vehicle or THC (50 mg/kg, i.p.) to evaluate cross-tolerance. Repeated AB-FUBINACA had no effect on THC-induced catalepsy (D), antinociception (E), or hypothermia (F). Data represent mean±SEM (n = 8-10[4-5 m/4-5f]/group); *p<.05 v. vehicle or baseline.
3.3. AB-FUBINACA is rapidly cleared from the brain
Although the onset of AB-FUBINACA and THC effects was similar, the duration of cataleptic (1 h vs 2 h for THC) [F(1,130) = 2.9; p<.05; Fig. 4A], antinociceptive (30 min vs. 12 h for THC) [F(1,130) = 5.7; p<.05; Fig. 4B], and hypothermic (2 h vs. 12 h for THC) [F(1,130) = 19.3; p<.05; Fig. 4C] effects of AB-FUBINACA were shorter than the same effects of THC (50 mg/kg).
Fig. 4.
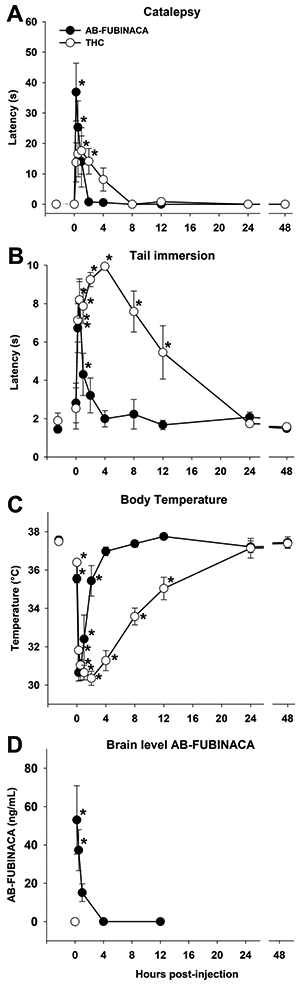
AB-FUBINACA has short-acting, in vivo cannabinoid effects. Male and female mice were injected with AB-FUBINACA (3 mg/kg, i.p.) or THC (50 mg/kg) and tested repeatedly in a modified tetrad battery, following baseline testing. The effects of AB-FUBINACA abated more quickly than the effects of THC in catalepsy (A), antinociception (B), and hypothermia (C). n = 8[4 m/4f]/group);*p<.05 vs. baseline. A second group of mice was injected with vehicle (i.e., 0 h), 0.25, 0.5, 1, 4, or 12 h prior to tissue collection (D). AB-FUBINACA levels in whole brain homogenates were quantified by UHPLC-MS/MS (n = 6 [3 m/3f]/group); *p<.05 vs. vehicle group. Data represent mean±SEM.
Given that tetrad effects are brain mediated, whole brain concentrations of AB-FUBINACA were quantified, using a parallel group of mice injected with vehicle or AB-FUBINACA (3 mg/kg, i.p.) 0.25, 0.5, 1, 4, and 12 h prior to tissue collection. The collection timeline was based on behavioral effects of AB-FUBINACA. Brain concentrations of AB-FUBINACA were detectable in whole brain homogenates [F(5,30)=6.8, p<0.05; Fig. 4D]. Post hoc analyses revealed significant levels of AB-FUBINACA detected in brain at 15 and 30 min after injection but did not differ from control by 1 h post-injection. In contrast, no drug (i.e., false positives) was detected in the brains of the vehicle control group.
4. Discussion
The current project used the tetrad assay to evaluate the acute cannabimimetic effects of the synthetic cannabinoid receptor agonist (SCRA) AB-FUBINACA. It also evaluated whether the pharmacological effects of AB-FUBINACA underwent tolerance following repeated administration or if the CB1 receptor antagonist rimonabant would precipitate withdrawal signs in mice given repeated administration of AB-FUBINACA. AB-FUBINACA produced classic cannabimimetic effects, including catalepsy, antinociception, hypothermia, and hypolocomotion.
A unique finding in this study was the short duration of action of AB-FUBINACA compared with THC as well as earlier developed SCRAs. While the onset of tetrad effects and detectable drug brain levels observed here was similar as previously reported with other SCRAs (Samano et al., 2014; Poklis et al., 2012), we found that AB-FUBINACA effects in the present study abated more quickly than those in the prior study in rats (Canazza et al., 2017). It is plausible that the differences in observed effect timecourse and magnitude are due to a species differ-ence. Regardless, the relatively short hypothermic timecourse in the present study mirrors published mouse data of similar indazole SCRAs AB-PINACA (10 mg/kg, i.p.) (Hutchison et al., 2018) and AB–CHMINACA (3 mg/kg, i.p.), which induced hypothermia that abated 3 h after injection, whereas the indole SCRA CP55,940 (3 mg/kg, i.p.) induced hypothermia persisted for at least 5 h post-injection (Lefever et al., 2017). Brain THC levels are detectable for hours after administration in mice (Bornheim et al., 1995) and for at least 1 day in rats (Hlož et al., 2017). A likely reason for the relatively quick recovery from these compounds is their rapid metabolism, as was illustrated in the analysis of brain homogenate concentrations of AB-FUBINACA (see Fig. 4). Thus, rapid metabolism likely accounts for the relatively short timecourse of injected AB-FUBINACA, which concomitantly may contribute to the apparent lack of tolerance and minimal dependence following its repeated administration.
It is noteworthy that the doses of AB-FUBINACA selected for repeated administration produced a relatively equivalent magnitude of acute effects as the acute effects of THC and other SCRAs, including WIN55,212-2 and JWH-018 (Aceto et al., 2001; Lichtman et al., 2001; Schlosburg et al., 2009; Trexler et al., 2018), which reliably produce tolerance upon repeated administration. In addition to the relatively short pharmacokinetics of AB-FUBINACA, this agonist possesses considerably higher efficacy at the CB1 receptor than THC (Hess et al., 2016; Wiley et al., 2015), which could further contribute to the lack of tolerance observed here. Specifically, as high efficacy agonists produce pharmacological effects with less receptor occupancy than partial efficacy agonists, they are resistant to decreases in CB1 receptor expression and function (Grim et al., 2016).
That said, the lack of tolerance to the single dose of AB-FUBINACA and cross-tolerance to THC were nonetheless somewhat surprising, as repeated administration of short-acting μ opioid receptor agonists show tolerance and cross-tolerance to longer acting μ opioid receptor agonists. For instance, mice treated chronically with fentanyl displayed dose-dependent cross-tolerance to morphine, a longer acting μ receptor; however, once daily fentanyl injections had no effect on morphine efficacy (Sirohi et al., 2008). Thus, it is plausible that, for tolerance to develop, mice must be exposed more frequently, or perhaps continuously, to AB-FUBINACA (e.g., via osmotic minipump). It has been further suggested that an alternate route of administration (e.g., inhalation) may be a better model of SCRA use because these compounds are generally administered in vapor (Lefever et al., 2017), which also most closely resembles intravenous administration with regard to speed of delivery to brain. Moreover, the short timecourse of indazole-3-carboxamide-type SCRAs is even further accelerated in an inhaled aerosol model (Lefever et al., 2017). Although such approaches have limited construct validity and may not reflect patterns of human cannabinoid self-administration, they may contribute to increased understanding of how duration of receptor activation affects drug tolerance. It is also plausible that THC cross-tolerance effects were masked by the use of a relatively high (i.e., 50 mg/kg) dose of THC. Future studies will probe possible ceiling effects by administering lower doses of THC in mice with a history of repeated SCRA exposure.
Although increasing the dose of AB-FUBINACA may result in more pronounced withdrawal effects, higher doses also increase probability of proconvulsant effects, as AB-FUBINACA (6 mg/kg) induces convulsions in male ICR mice (Canazza et al., 2017). In humans, seizures induced by SCRA use may occur immediately (i.e., within minutes of use) or after a delay of several hours or even days (Havenon et al., 2011; Schaefer et al., 2013). Although the physiological mechanism(s) are poorly understood, it is hypothesized that off-target effects, lack of quality control, toxicity (absence of mitigating phytocannabinoids and endocannabinoids), ligand bias, or active metabolites/degradants are likely contributing factors (Chimalakonda et al., 2012; Pertwee, 2009). Further complicating mechanistic studies, the metabolites of many SCRAs are active and bind to CB receptors at similar affinities to the parent compound (Erratico et al., 2015; Fantegrossi et al., 2014). Previous studies have demonstrated that SCRAs tend to be rapidly metabolized, and that potent metabolites remain in the organism, inter-acting with cannabinoid receptors (Brents et al., 2012). This is in contrast to THC, which has only a single known psychoactive metabolite, 11–OH-THC (Huestis, 2007; Matsuda et al., 1990).
Although no statistically significant sex differences were found in present study, it was not a goal to probe sex differences, but rather to ensure that mice of both sexes are included (and clearly reported) in each of our experiments. It is worth noting that the lack of sex differences is consistent with our previous reports of phytocannabinoid (e.g., THC) and SCRA (e.g., JWH-018) withdrawal in mice (Trexler et al., 2019, 2018).
Given the unpredictable negative effects of SCRA use in humans, it is tempting to dismiss synthetic cannabinoids as a risk to public health. Yet, it is important to keep in mind that some SCRAs elicit promising pharmacological effects. For example, nabilone (sold as Cesamet) is approved by the US Food and Drug Administration to reduce nausea, vomiting, and wasting in cancer patients. In preclinical models of cancer, JWH-133, a selective CB2 receptor agonist, and WIN55,212–2, a nonselective CB1 and CB2 agonist, both inhibit breast tumor growth and metastasis in vitro and in vivo (Olea-Herrero et al., 2009; Qamri et al., 2009). The therapeutic use of SCRAs has been severely limited, however, by the presence of side effects in preclinical models, including abuse potential and seizures (Cooper, 2016; Havenon et al., 2011; Schaefer et al., 2013). Recreational use of SCRAs has increased dramatically in recent years, with a concomitant increase in emergency department visits. Few assays currently detect SCRAs in blood or urine (Islam et al., 2018; Muehlethaler et al., 2016; Sobolevsky et al., 2010), an issue that is compounded by the ever-changing SCRAs used in spice compounds and the difficulty detecting them. Thus, it remains difficult to track exactly which SCRA(s) caused a given adverse health episode. Even determining the dose ingested can be difficult, because the adulterated plant materials often have “hot spots,” as a result of uneven distribution, and high inter- and intra-batch variability in quality and dose (Frinculescu et al., 2017; Hudson and Ramsey, 2011; Marshell et al., 2014; Van Amsterdam et al., 2015). Moreover, of the SCRA poisoning cases in which the compounds involved are known, there is often more than one compound present (Musshoff et al., 2014). Thus, the pharmacokinetic and pharmacodynamic features of SCRAs clearly differ from cannabis in their unpredictable effects in humans.
5. Conclusions
The present studies revealed that AB-FUBINACA, a third-generation SCRA, produces cannabimimetic effects that abate much more rapidly than the phytocannabinoid, THC. This rapid offset coincides with drug clearance from the brain, consistent with rapid metabolism and clearance of AB-FUBINACA. As human use of cannabinoids continues, information gained from preclinical studies remains informative for basic understanding of physiological mechanisms and treatments for SCRA use.
Supplementary Material
Acknowledgements
This work was supported by the National Institutes of Health [DA033934, DA039335, and GM132494]. The authors thank Ethan Mick and Brian Kotson for technical support and Dr. Aron Lichtman for editorial assistance.
Funding source
This project was supported by The National Institutes of Health [DA033934, DA039335, and GM132494]. Sponsors had no role in the design, collection, analysis and interpretation of data, writing of the report, or the decision to submit the article for publication.
Abbreviations:
- CB1
Cannabinoid receptor subtype 1
- CB2
Cannabinoid receptor subtype 2
- Rimonabant
SR141716A
- THC
Δ9-tetrahydrocannabinol
Footnotes
Declaration of Competing Interest
None.
Appendix A. Supplementary data
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.drugalcdep.2020.108179.
References
- Aceto MD, Scates SM, Martin BB, 2001. Spontaneous and precipitated withdrawal with a synthetic cannabinoid, WIN 55212-2. Eur. J. Pharmacol 416, 75–81. 10.1016/S0014-2999(01)00873-1. [DOI] [PubMed] [Google Scholar]
- Banister SD, Stuart J, Kevin RC, Edington A, Longworth M, Wilkinson SM, Beinat C, Buchanan AS, Hibbs DE, Glass M, Connor M, McGregor IS, Kassiou M, 2015. Effects of bioisosteric fluorine in synthetic cannabinoid designer drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135. ACS Chem. Neurosci. 6, 1445–1458. 10.1021/acschemneuro.5b00107. [DOI] [PubMed] [Google Scholar]
- Benford DM, Caplan JP, 2011. Psychiatric sequelae of spice, K2, and synthetic cannabinoid receptor agonists. Psychosomatics. 10.1016/j.psym.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Besli GE, Ikiz MA, Yildirim S, Saltik S, 2015. Synthetic cannabinoid abuse in adolescents: a case series. J. Emerg. Med 49, 644–650. 10.1016/j.jemermed.2015.06.053. [DOI] [PubMed] [Google Scholar]
- Bornheim LM, Kim KY, Li J, Perotti BYT, Benet LZ, 1995. Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain. Drug Metab. Dispos. 23, 825–831. [PubMed] [Google Scholar]
- Brents LK, Gallus-Zawada A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, Moran JH, Prather PL, 2012. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem. Pharmacol 83, 952–961. 10.1016/j.bcp.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brents LK, Zimmerman SM, Saffell AR, Prather PL, Fantegrossi WE, 2013. Differential drug-drug interactions of the synthetic cannabinoids JWH-018 and JWH-073: implications for drug abuse liability and pain therapys. J. Pharmacol. Exp. Ther 346, 350–361. 10.1124/jpet.113.206003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canazza I, Ossato A, Trapella C, Fantinati A, De Luca MA, Margiani G, Vincenzi F, Rimondo C, Di Rosa F, Gregori A, Varani K, Borea PA, Serpelloni G, Marti M, 2016. Effect of the novel synthetic cannabinoids AKB48 and 5F-AKB48 on “tetrad”, sensorimotor, neurological and neurochemical responses in mice. In vitro and in vivo pharmacological studies. Psychopharmacology (Berl.) 233, 3685–3709. 10.1007/s00213-016-4402-y. [DOI] [PubMed] [Google Scholar]
- Canazza I, Ossato A, Vincenzi F, Gregori A, Di Rosa F, Nigro F, Rimessi A, Pinton P, Varani K, Borea PA, Marti M, 2017. Pharmaco-toxicological effects of the novel third-generation fluorinate synthetic cannabinoids, 5F-ADBINACA, AB-FUBINACA, and STS-135 in mice. In vitro and in vivo studies. Hum. Psychopharmacol 32. 10.1002/hup.2601. [DOI] [PubMed] [Google Scholar]
- Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, James LP, Hollenberg PF, Prather PL, Radominska-Pandya A, Moran JH, 2012. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab. Dispos. 40, 2174–2184. 10.1124/dmd.112.047530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, 2016. Adverse effects of synthetic cannabinoids: management of acute toxicity and withdrawal. Curr. Psychiatry Rep. 10.1007/s11920-016-0694-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das RG, North D, 2007. Implications of experimental technique for analysis and interpretation of data from animal experiments: outliers and increased variability resulting from failure of intraperitoneal injection procedures. Lab. Anim 41, 312–320. 10.1258/002367707781282802. [DOI] [PubMed] [Google Scholar]
- Dresen S, Ferreirós N, Pütz M, Westphal F, Zimmermann R, Auwärter V, 2010. Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J. Mass Spectrom 45, 1186–1194. 10.1002/jms.1811. [DOI] [PubMed] [Google Scholar]
- Durand D, Delgado LL, De La Parra-Pellot DM, Nichols-Vinueza D, 2015. Psychosis and severe rhabdomyolysis associated with synthetic cannabinoid use: a case report. Clin. Schizophr. Relat. Psychoses 10.3371/CSRP.DUDE.031513. [DOI] [PubMed] [Google Scholar]
- EMCDDA, 2009. The State of the Drugs Problem in Europe. Annual Report 2009. European Monitoring Centre of Drugs and Drugs addiction. [Google Scholar]
- Erratico C, Negreira N, Norouzizadeh H, Covaci A, Neels H, Maudens K, van Nuijs ALN, 2015. In vitro and in vivo human metabolism of the synthetic cannabinoid AB-CHMINACA. Drug Test. Anal. 7, 866–876. 10.1002/dta.1796. [DOI] [PubMed] [Google Scholar]
- Falenski KW, Thorpe AJ, Schlosburg JE, Cravatt BF, Abdullah RA, Smith TH, Selley DE, Lichtman AH, Sim-Selley LJ, 2010. FAAH−/− mice display differential tolerance, dependence, and cannabinoid receptor adaptation after Δ9-Tetrahydrocannabinol and anandamide administration. Neuropsychopharmacology 35, 1775–1787. 10.1038/npp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Moran JH, Radominska-Pandya A, Prather PL, 2014. Distinct pharmacology and metabolism of K2 synthetic cannabinoids compared to Δ9-THC: mechanism underlying greater toxicity? Life Sci. 10.1016/j.lfs.2013.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantegrossi WE, Wilson CD, Berquist MD, 2018. Pro-psychotic effects of synthetic cannabinoids: interactions with central dopamine, serotonin, and glutamate systems. Drug Metab. Rev 10.1080/03602532.2018.1428343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford BM, Tai S, Fantegrossi WE, Prather PL, 2017. Synthetic pot: not your grandfather’s marijuana. Trends Pharmacol. Sci 10.1016/j.tips.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frinculescu A, Lyall CL, Ramsey J, Miserez B, 2017. Variation in commercial smoking mixtures containing third-generation synthetic cannabinoids. Drug Test. Anal 9, 327–333. 10.1002/dta.1975. [DOI] [PubMed] [Google Scholar]
- Gatch MB, Forster MJ, 2015. 9 -Tetrahydrocannabinol-Like effects of novel synthetic cannabinoids found on the gray market michael. Behav. Pharmacol 26, 460–468. 10.1097/FBP.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay M, 2010. Synthetic Marijuana Spurs State Bans. New York Times, pp. 2–5. [Google Scholar]
- Grim TW, Morales AJ, Gonek MM, Wiley JL, Thomas BF, Endres GW, Sim-Selley LJ, Selley DE, Negus SS, Lichtman AH, 2016. Stratification of cannabinoid 1 receptor (CB1R) agonist efficacy: manipulation of CB1R density through use of transgenic mice reveals congruence between in vivo and in vitro assays. J. Pharmacol. Exp. Ther 359, 329–339. 10.1124/jpet.116.233163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havenon Ade, Chin B, Thomas KC, Afra P, 2011. The secret “Spice”: an undetectable toxic cause of seizure. Neurohospitalist 1, 182–186. 10.1177/1941874411417977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess C, Schoeder CT, Pillaiyar T, Madea B, Müller CE, 2016. Pharmacological evaluation of synthetic cannabinoids identified as constituents of spice. Forensic Toxicol. 34, 329–343. 10.1007/s11419-016-0320-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hložek T, Uttl L, Kadeřábek L, Balíková M, Lhotková E, Horsley RR, Nováková P, Šichová K, Štefková K, Tylš F, Kuchař M, Páleníček T, 2017. Pharmacokinetic and behavioural profile of THC, CBD, and THC+CBD combination after pulmonary, oral, and subcutaneous administration in rats and confirmation of conversion in vivo of CBD to THC. Eur. Neuropsychopharmacol 27, 1223–1237. 10.1016/j.euroneuro.2017.10.037. [DOI] [PubMed] [Google Scholar]
- Hudson S, Ramsey J, 2011. The emergence and analysis of synthetic cannabinoids. Drug Test. Anal 3, 466–478. 10.1002/dta.268. [DOI] [PubMed] [Google Scholar]
- Huestis MA, 2007. Human cannabinoid pharmacokinetics. Chem. Biodivers, 10.1002/cbdv.200790152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, Thompson ALS, Bushell S, Tartal C, Hurst DP, Reggio PH, Selley DE, Cassidy MP, Wiley JL, Martin BR, 2005. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl) indoles at the cannabinoid CB 1 and CB 2 receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB 2 receptor agonists. Bioorg. Med. Chem. Lett 13, 89–112. 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Hutchison RD, Ford BM, Franks LN, Wilson CD, Yarbrough AL, Fujiwara R, Su MK, Fernandez D, James LP, Moran JH, Patton AL, Fantegrossi WE, Radominska-Pandya A, Prather PL, 2018. Atypical pharmacodynamic properties and metabolic profile of the abused synthetic cannabinoid AB-PINACA: potential contribution to pronounced adverse effects relative to Δ9-THC. Front. Pharmacol 9. 10.3389/fphar.2018.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam SK, Cheng YP, Birke RL, Green O, Kubic T, Lombardi JR, 2018. Rapid and sensitive detection of synthetic cannabinoids AMB-FUBINACA and α-PVP using surface enhanced Raman scattering (SERS). Chem. Phys 506, 31–35. 10.1016/j.chemphys.2018.03.028. [DOI] [Google Scholar]
- Jarbe TU, Raghav JG, 2016. Tripping with synthetic cannabinoids (“Spice”): anecdotal and experimental observations in animals and Man. Neuropharmacology of New Psychoactive Substances (NPS). pp. 263–281. 10.1007/7854. [DOI] [PubMed] [Google Scholar]
- Katsidoni V, Kastellakis A, Panagis G, 2013. Biphasic effects of Δ9-tetrahydrocannabinol on brain stimulation reward and motor activity. Int. J. Neuropsychopharmacol 2273–2284. 10.1017/S1461145713000709. [DOI] [PubMed] [Google Scholar]
- Kevin RC, Wood KE, Stuart J, Mitchell AJ, Moir M, Banister SD, Kassiou M, McGregor IS, 2017. Acute and residual effects in adolescent rats resulting from exposure to the novel synthetic cannabinoids AB-PINACA and AB-FUBINACA. J. Psychopharmacol 10.1177/0269881116684336. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Cole EC, 2013. Acute Δ9-tetrahydrocannabinol blocks gastric hemorrhages induced by the nonsteroidal anti-inflammatory drug diclofenac sodium in mice. Eur. J. Pharmacol 715. 10.1016/j.ejphar.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefever TW, Marusich JA, Thomas BF, Barrus DG, Peiper NC, Kevin RC, Wiley JL, 2017. Vaping synthetic cannabinoids: a novel preclinical model of E-cigarette use in mice. Subst. Abus. Res. Treat 11. 10.1177/1178221817701739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtman AH, Fisher J, Martin BR, 2001. Precipitated cannabinoid withdrawal is reversed by Delta(9)-tetrahydrocannabinol or clonidine. Pharmacol. Biochem. Behav 69, 181–188. [DOI] [PubMed] [Google Scholar]
- Long JZ, Li W, Booker L, Burston JJ, Kinsey SG, Schlosburg JE, Pavón FJ, Serrano AM, Selley DE, Parsons LH, Lichtman AH, Cravatt BF, 2009. Selective blockade of 2-arachidonoylglycerol hydrolysis produces cannabinoid behavioral effects. Nat. Chem. Biol 5, 37–44. 10.1038/nchembio.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshell R, Kearney-Ramos T, Brents LK, Hyatt WS, Tai S, Prather PL, Fantegrossi WE, 2014. In vivo effects of synthetic cannabinoids JWH-018 and JWH-073 and phytocannabinoid Δ9-THC in mice: inhalation versus intraperitoneal injection. Pharmacol. Biochem. Behav 124, 40–47. 10.1016/j.pbb.2014.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI, 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 436, 561–564. 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Meijer KA, Russo RR, Adhvaryu DV, 2014. Smoking synthetic marijuana leads to self-mutilation requiring bilateral amputations. Orthopedics, 10.3928/01477447-20140401-62. [DOI] [PubMed] [Google Scholar]
- Muehlethaler C, Leona M, Lombardi JR, 2016. Review of surface enhanced raman scattering applications in forensic science. Anal. Chem 10.1021/acs.analchem.5b04131. [DOI] [PubMed] [Google Scholar]
- Musshoff F, Madea B, Kern-Wighton G, Bicker W, Kneisel S, Hutter M, Auwarter V, 2014. Driving under the influence of synthetic phenethylamines: a case series. Int. J. Legal Med 128, 59–64. 10.1007/s00414-015-1150-1. [DOI] [PubMed] [Google Scholar]
- Nacca N, Vatti D, Sullivan R, Sud P, Su M, Marraffa J, 2013. The synthetic cannabinoid withdrawal syndrome. J. Addict. Med 7, 296–298. 10.1097/ADM.0b013e31828e1881. [DOI] [PubMed] [Google Scholar]
- Olea-Herrero N, Vara D, Malagarie-Cazenave S, Díaz-Laviada I, 2009. Inhibition of human tumour prostate PC-3 cell growth by cannabinoids R-Methanandamide and JWH-015: involvement of CB 2. Br. J. Cancer 101, 940–950. 10.1038/sj.bjc.6605248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton AL, Chimalakonda KC, Moran CL, Mccain KR, Radominska-Pandya A, James LP, Kokes C, Moran JH, 2013. K2 Toxicity: fatal case of psychiatric complications following AM2201 exposure. J. Forensic Sci. 58, 1676–1680. 10.1111/1556-4029.12216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peace MR, Krakowiak RI, Wolf CE, Poklis A, Poklis JL, 2017. Identification of MDMB-FUBINACA in commercially available e-liquid formulations sold for use in electronic cigarettes. Forensic Sci. Int 271, 92–97. 10.1016/j.forsciint.2016.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, 2009. Emerging strategies for exploiting cannabinoid receptor agonists as medicines. Br. J. Pharmacol 10.1111/j.1476-5381.2008.00048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis JL, Thompson CC, Long KA, Lichtman AH, Poklis A, 2011. NIH Public Access 34, 516–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poklis JL, Amira D, Wise LE, Wiebelhaus JM, Haggerty BJ, Lichtman AH, Poklis A, 2012. Determination of naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in mouse blood and tissue after inhalation exposure to “buzz” smoke by HPLC/MS/MS. Biomed. Chromatogr 26, 1393–1398. 10.1002/bmc.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamri Z, Preet A, Nasser MW, Bass CE, Leone G, Barsky SH, Ganju RK, 2009. Synthetic cannabinoid receptor agonists inhibit tumor growth and metastasis of breast cancer. Mol. Cancer Ther 8, 3117–3129. 10.1158/1535-7163.MCT-09-0448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rianprakaisang T, Gerona R, Hendrickson RG, 2020. Commercial cannabidiol oil contaminated with the synthetic cannabinoid AB-FUBINACA given to a pediatric patient. Clin. Toxicol, 10.1080/15563650.2019.1619758. [DOI] [PubMed] [Google Scholar]
- Samano Kimberly L., Poklis Justin L., Lichtman Aron H., Poklis Alphonse, 2014. Development of a high-performance liquid chromatography-tandem mass spectrometry method for the identification and quantification of CP-47,497, CP-47,497-C8 and JWH-250 in mouse brain. J. Anal. Toxicol 38. 10.1093/JAT/BKU043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson CS, Bedy SME, Carlisle T, 2015. Withdrawal seizures seen in the setting of synthetic cannabinoid abuse. Am. J. Emerg. Med 33,1712. 10.1016/j.ajem.2015.03.025.e3. [DOI] [PubMed] [Google Scholar]
- Sañudo-Peña MC, Romero J, Seale GE, Fernandez-Ruiz JJ, Walker JM, 2000. Activational role of cannabinoids on movement. Eur. J. Pharmacol 391, 269–274. 10.1016/S0014-2999(00)00044-3. [DOI] [PubMed] [Google Scholar]
- Schaefer N, Peters B, Bregel D, Kneisel S, Schmidt PH, Ewald AH, 2013. A fatal case involving several synthetic cannabinoids. Toxichem Krimtech. [Google Scholar]
- Schlosburg JE, Carlson BLA, Ramesh D, Abdullah RA, Long JZ, Cravatt BF, Lichtman AH, 2009. Inhibitors of endocannabinoid-metabolizing enzymes reduce precipitated withdrawal responses in THC-dependent mice. AAPS J. 11, 342–352. 10.1208/s12248-009-9110-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber S, Bader M, Lenchinski T, Meningher I, Rubovitch V, Katz Y, Cohen E, Gabet Y, Rotenberg M, Wolf E.(Udi), Pick CG, 2019. Functional effects of synthetic cannabinoids versus Δ 9 -THC in mice on body temperature, nociceptive threshold, anxiety, cognition, locomotor/exploratory parameters and depression. Addict. Biol 24, 414–425. 10.1111/adb.12606. [DOI] [PubMed] [Google Scholar]
- Seely KA, Lapoint J, Moran JH, Fattore L, 2012. Spice drugs are more than harmless herbal blends: a review of the pharmacology and toxicology of synthetic cannabinoids. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 10.1016/j.pnpbp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Walker EA, Yoburn BC, 2008. The analgesic efficacy of fentanyl: relationship to tolerance and μ-opioid receptor regulation. Pharmacol. Biochem. Behav 91, 115–120. 10.1016/j.pbb.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolevsky T, Prasolov I, Rodchenkov G, 2010. Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci. Int 200, 141–147. 10.1016/j.forsciint.2010.04.003. [DOI] [PubMed] [Google Scholar]
- Thomas G, Kloner RA, Rezkalla S, 2014. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: what cardiologists need to know. Am. J. Cardiol 10.1016/j.amjcard.2013.09.042. [DOI] [PubMed] [Google Scholar]
- Trecki J, Gerona RR, Schwartz MD, 2015. Synthetic cannabinoid-related illnesses and deaths. N. Engl. J. Med 373, 103–107 https://doi.org/10.1056NEJMp1505328. [DOI] [PubMed] [Google Scholar]
- Trexler KR, Nass SR, Crowe MS, Gross JD, Jones MS, McKitrick AW, Siderovski DP, Kinsey SG, 2018. Novel behavioral assays of spontaneous and precipitated THC withdrawal in mice. Drug Alcohol Depend. 191,14–24. 10.1016/j.drugalcdep.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trexler KR, Eckard ML, Kinsey SG, 2019. CB1 positive allosteric modulation attenuates Δ9-THC withdrawal and NSAID-induced gastric inflammation. Pharmacol. Biochem. Behav 177, 27–33. 10.1016/j.pbb.2018.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Amsterdam J, Brunt T, Van Den Brink W, 2015. The adverse health effects of synthetic cannabinoids with emphasis on psychosis-like effects. J. Psychopharmacol 29, 254–263. 10.1177/0269881114565142. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Martin BR, 2003. Cannabinoid pharmacological properties common to other centrally acting drugs. Eur. J. Pharmacol 471, 185–193. [DOI] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Lefever TW, Antonazzo KR, Wallgren MT, Cortes RA, Patel PR, Grabenauer M, Moore KN, Thomas BF, 2015. AB-CHMINACA, AB-PINACA, and FUBIMINA: affinity and potency of novel synthetic cannabinoids in producing 9-Tetrahydrocannabinol-like effects in mice. J. Pharmacol. Exp. Ther 354, 328–339. 10.1124/jpet.115.225326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JL, Marusich JA, Thomas BF, 2017. Combination chemistry: structure-activity relationships of novel psychoactive cannabinoids. Curr. Top. Behav. Neurosci 32, 231–248. 10.1007/7854_2016_17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


