Abstract
Objective:
Alcohol Use Disorder (AUD) is a complex, dynamic condition that waxes and wanes with unhealthy drinking episodes and varies in drinking patterns and effects on brain structure and function with age. Its excessive use renders chronically heavy drinkers vulnerable to direct alcohol toxicity and a variety of comorbidities attributable to nonalcohol drug misuse, viral infections, and accelerated or premature aging. AUD affects widespread brain systems, commonly, frontolimbic, frontostriatal, and frontocerebellar networks.
Method and Results:
Multimodal assessment using selective neuropsychological testing and whole-brain neuroimaging provides evidence for AUD-related specific brain structure-function relations established with double dissociations. Longitudinal study using noninvasive imaging provides evidence for brain structural and functional improvement with sustained sobriety and further decline with relapse. Functional imaging suggests the possibility that some alcoholics in recovery can compensate for impairment by invoking brain systems typically not used for a target task but that can enable normal-level performance.
Conclusions:
Evidence for AUD-aging interactions, indicative of accelerated aging, together with increasing alcohol consumption in middle-age and older adults, put aging drinkers at special risk for developing cognitive decline and possibly dementia.
Keywords: neuropsychology, alcohol, alcoholism, alcoholic, alcohol use disorder, MRI, brain
Introduction
Alcohol is pervasive in American life and culture and in countries where it is not banned (Alcohol & Drug Use, 2018). Alcoholism, now diagnosed as Alcohol Use Disorder (AUD) (American Psychiatric Association, 2013), is a complex, dynamic disorder that waxes and wanes with unhealthy drinking episodes and varies in drinking patterns with age. Its excessive use renders chronically heavy drinkers vulnerable to direct alcohol toxicity, nutritional deficiency, and a variety of comorbidities attributable to nonalcohol drug misuse, viral infections, and accelerated or premature aging.
Young drinkers through college age commonly engage in heavy to binge (4 or more drinks for women and 5 or more drinks for men in 2 hours) to extreme binge (15 or more drinks in one session) drinking on weekends (Tapert & Courtney, 2019). Fast and vast consumption leads to risky behaviors and accidents (e.g., Grant et al., 2017; Thompson, Eaton, Hu, Grant, & Hasin, 2014) [www.cdc.gov/motorvehiclesafety/impaired_driving/impaired-drv_factsheet.html] and vulnerability to comorbidities from other mind-altering injectable drugs often resulting in hepatitis C virus (HCV) infection and events like unprotected sex resulting in human immunodeficiency viral (HIV) infection. Although adolescents and young adults who emerge without injury from these drinking episodes appear to recover cognitively and motorically, it remains to be tested whether such transient drinking bouts leave an “impairment scar” rendering older adults with irresponsible youthful drinking bouts especially vulnerable to the throes of aging. Until recently, middle to older age drinkers were more likely to engage in drinking throughout the week, within the NIAAA guidelines [www.niaaa.nih.gov] of no more than 2 drinks per occasion and no more than 14 drinks per week for men and half those amounts for women. New epidemiological data, however, indicate an increase in alcohol consumption in middle age to older adults with growing incidence in drinking per se and also in binge drinking especially in women (Breslow, Castle, Chen, & Graubard, 2017; Grant et al., 2017), putting older drinkers at risk for developing cognitive decline and even dementia.
There is little doubt that excessive, chronic drinking affects brain structure and function. Indeed, numerous neuroimaging and neuropathological studies have identified alcoholism-related dysfunction and dysmorphology, leading to the assumption that alcohol is a “neurotoxin.” Remarkably, although mechanisms of destruction and disruption are known for peripheral systems, including liver, lung, heart, and gut, even animal models of alcoholism have yet to reveal neural mechanisms of alcohol’s neurotoxicity. In fact, some studies suggest that alcohol itself is not severely neurotoxic but expresses neurodisruption when it interacts with nutritional deficiency, specifically, of thiamine (Vedder, Hall, Jabrouin, & Savage, 2015; Zahr et al., 2016).
In short, the signature profile of neuropsychological deficits in uncomplicated AUD involves executive dysfunction, selective to processes of working and episodic memory, visuospatial abilities, and postural stability (Oscar-Berman et al., 2014; Stavro, Pelletier, & Potvin, 2013). Brain substrates of AUD cognitive and motor deficits have been linked to MRI-derived tissue volume deficits in prefrontal (Cardenas, Studholme, Gazdzinski, Durazzo, & Meyerhoff, 2007), parietal (Cui et al., 2015), cerebellar (Sullivan, Rose, & Pfefferbaum, 2006), and thalamic (Pitel, Segobin, Ritz, Eustache, & Beaunieux, 2015) structures. These functions show varying extents and temporal courses of recovery with abstinence. An exhaustive review of the alcohol literature with reference to brain structural and functional findings requires a volume as published recently (Sullivan & Pfefferbaum, 2014); other recent reviews on selective relevant topics will also be noted. Rather, the focus of the current selective review is guided by current epidemiology of AUD including the aging of America and addresses the following: 1) which brain systems and functional correlates are affected and what ones recover; 2) use of neuroimaging modalities to seek mechanisms of cognitive and motor impairment; 3) how common comorbidities alter the course of AUD; and 4) question whether AUD has a role in the development of dementia.
Component processes of cognitive and motor functions affected in AUD
Assessment of component processes comprising dissociable functions enables identification of patterns of sparing and impairment characterizing diagnostic groups, in this case, AUD with and without comorbidities, such as non-alcohol drug use, psychiatric disorder, and head injury. Study of assessment variability provides quantitative data on performance by individuals. Some functions and their supporting brain structures may be more amenable than others to repair with sustained sobriety (e.g., Petit et al., 2017); thus, knowledge of components of impairment profiles tracked over the dynamic course of alcoholism can potentially direct therapeutic efforts toward processes with potential for recovery (cf., Cui et al., 2015).
Acute intoxication is marked by reversible cognitive, sensory, and motor impairments, including poor judgment, aggression, staggering of gait, slurring of speech, and slowed reaction time. Chronic, heavy drinking can result in AUD, which is marked by deficits in domains of executive functioning, episodic memory, visuospatial abilities, and gait and balance (for extensive reviews, Le Berre, Laniepce, Segobin, Pitel, & Sullivan, 2019; Oscar-Berman et al., 2014; Sullivan, 2017). Each of these domains comprises multiple component processes that have the potential of being spared or impaired at varying levels and at different lags between sobriety and testing. Thus, heavy drinking has short-term and long-term effects on function, some of which can be partly or fully ameliorated with abstinence or reduced drinking, whereas other impairments are enduring.
Executive function includes problem solving, working memory, sequencing, fluency, inhibitory control, selective attention, decision-making, strategic memory retrieval, and interpretation of social emotion and interaction (Brion et al., 2015; Le Berre et al., 2014; Naim-Feil, Fitzgerald, Bradshaw, Lubman, & Sheppard, 2014). These functions are difficult to detect clinically, require objective documentation (Wollenweber et al., 2014), and are usually associated with disruption of frontal-systems that can extend beyond frontal-lobe borders (Zahr, Pfefferbaum, & Sullivan, 2017).
Alcoholics as a group exhibit cognitive inflexibility and use habit learning more than controls when faced with tasks that would be better solved with more adaptive, goal-directed solutions to over-ride habitual decision making (Sebold et al., 2017; Sjoerds et al., 2013) and to switch from a previously relevant behavior to one that suits new demands (Trick, Kempton, Williams, & Duka, 2014). Such behavior emerged as impaired adaptation and inflexibility in decision-making contexts that could be influenced by manipulating reinforcement contingencies (monetary reward amount and timing) (Beylergil et al., 2017). Along with number of prior relapses, poor control of response inhibition is a good predictor of relapse (Czapla et al., 2016).
A meta-analysis tabulated data from 77 published studies spanning half a century to address which component processes of executive functions were affected in adult AUD (Stephan et al., 2017). Most affected were planning, problem solving, and inhibitory skills resulting in impulsivity, assessed by the Wisconsin Card Sorting and the Iowa Gambling Tests and less affected were fluency measures.
Studies using functional MRI (fMRI) provide evidence that alcoholics can overcome certain deficits of executive function, including verbal and spatial working memory and abstract reasoning, if they recruit brain regions beyond those activated by controls while engaged in the task (Bagga, Singh, et al., 2014; Chanraud, Pitel, Muller-Oehring, Pfefferbaum, & Sullivan, 2013; Charlet, Schlagenhauf, et al., 2014). Compensatory strategies were unsuccessful, however, under conditions of high risk and great uncertainty (Jung et al., 2014).
Episodic (explicit or declarative) memory occurs with conscious awareness and is assessed with tests of recognition or recall of newly encoded information. Episodic memory (Pitel et al., 2007) is subserved by limbic circuitry (Papez circuit), which comprises the hippocampus, amygdala, fornix, thalamus, and mammillary bodies (Le Berre et al., 2014; Pitel et al., 2015). Confabulation is also documented in AUD, can occur both in uncomplicated AUD and in KS, and was found to be more associated with impairment in episodic memory than in executive function as a means to “fill in blanks” of unconsolidated personal episodes (Rensen et al., 2015).
Procedural (or implicit) memory can be accomplished without conscious awareness or intent and is typically defined by motor learning (Fama, Rosenbloom, Sassoon, Pfefferbaum, & Sullivan, 2012; McGlinchey, Fortier, Capozzi, & Disterhoft, 2005), such as required for learning how to ride a bicycle. Procedural learning substrates are exclusive of limbic circuitry and involve frontal, basal ganglian, and cerebellar regions (Ritz et al., 2014) and may be amenable to training in recovering alcoholics (Loijen et al., 2018).
Metamemory umbrellas episodic and procedural memory and encompasses personal knowledge about one’s own ability to remember or recall information. Formal testing revealed that in guessing how well they would perform a memory test of learning a long list of random words, alcoholics over-estimated their future episodic memory performance, which correlated with smaller insular volumes, but were able to judge how well they actually recalled the word list, which correlated with frontolimbic structural volumes (Le Berre et al., 2016). Unrealistic assessment of abilities by the alcoholic can be considered a mild form of anosognosia (poor insight in knowing what you do not know) that could be misinterpreted as psychological denial (Le Berre & Sullivan, 2016). An in-depth review of metamemory and social cognition appears in the paper by Le Berre (Le Berre, 2019).
Alcoholic blackout is a transient form of a memory disorder that occurs with clear consciousness resulting from extreme alcohol consumption. Two forms are described: en bloc and fragmentary. En bloc refers to complete amnesia for a defined time period. Fragmentary blackouts are more prevalent than en bloc with the incidence of both being dose dependent; 10% (en bloc) and >30% (fragmentary) are likely to ensue when blood alcohol levels exceed 250 mg%. As few as 5 to 6 drinks in one session can precipitate transient memory impairment (Labhart, Livingston, Engels, & Kuntsche, 2018). A history of blackouts is a strong predictor of other alcohol-related problems (Hingson, Zha, Simons-Morton, & White, 2016).
Visuospatial processing includes the ability to tell where objects, including one’s self, are in space. These abilities are commonly impaired and can be resilient to improvement even with abstinence (Wollenweber et al., 2014). In light of the multi-faceted ocular dysfunctions associated with AUD and withdrawal, it may not be surprising that cognitive processes dependent on the visual system are disadvantaged. Although visuospatial abilities involve construction and memory, a direct relation between basic oculomotor dysfunction and higher-order visuospatial abilities has not been reported. Nonetheless, DTI (Bagga, Sharma, et al., 2014) and MR spectroscopy (Bagga, Khushu, et al., 2014) studies provide correlational evidence for an occipital substrate of an AUD-related impairment in visual processing skills, although selectivity of the relations was not tested (cf., Fama & Sullivan, 2014).
Emotion and Social Cognition is the ability to discern emotional valence and make judgments about facial expression, can be disrupted with cerebellar pathology and can be a facet of the Cerebellar Cognitive Affective Syndrome (Hoche, Guell, Sherman, Vangel, & Schmahmann, 2016). Judgments about emotion happen quickly and often implicitly and can be altered depending on the prevailing emotional load (positive or negative) and one’s ability to engage in perspective from another person’s view. These abilities have been documented as impaired in alcoholics. There is also evidence for enhanced midbrain activation to alcohol-related cues and for negative emotional processing in alcoholics (Muller-Oehring et al., 2013). Cross-sectional evidence indicates relations between better scores on tests of emotional functions and longer duration of alcohol abstinence (Charlet, Beck, et al., 2014; Fitzpatrick & Crowe, 2013) especially in women in recovery (Valmas, Mosher Ruiz, Gansler, Sawyer, & Oscar-Berman, 2014), and raises the need for awareness about cognitive readiness of alcoholics to engage in therapy (Le Berre et al., 2013). Correlations of social cognition measures with executive function and ataxia support a frontocerebellar substrate for the impairments. Poor self-awareness in AUD is related to lower cognitive scores (Walvoort, van der Heijden, Wester, Kessels, & Egger, 2016); similarly, the ability to recognize emotional facial expressions can be modulated by episodic memory and cognitive flexibility (Quaglino, De Wever, & Maurage, 2015), functions that can be compromised early in recovery.
Gait and balance disturbances are some of the most robust and enduring deficits detectable in abstinent alcoholics (Fein & Greenstein, 2013; Rosenbloom, Pfefferbaum, & Sullivan, 2004; T. P. Schmidt, Pennington, Durazzo, & Meyerhoff, 2014). Excessive sway can be quelled with touch, visual, and stance aids and with abstinence from alcohol (Sullivan et al., 2006). Postural stability should be assessed clinically, even with simple roadside-sobriety-type tests to assess liability for falling, especially in older alcoholics. Substrates of postural instability include the anterior superior vermis, whereas 5–7 Hz truncal tremor elicited by engaging in a challenging cognitive task while standing quietly, has been related to internal capsule-motor cortex circuitry integrity (Sullivan, Zahr, Rohlfing, & Pfefferbaum, 2015).
Neural Mechanisms of Cognitive and Motor Correlates: Evidence from MR Imaging
Structural and functional integrity of the living human brain is revealed by a variety of magnetic resonance imaging (MRI) methods. Conventional structural MRI enables quantification of regional volume, cortical thickness, surface area, tissue type and quality (e.g., Fischl, 2012). MR diffusion tensor imaging (DTI) provides measures of microstructural integrity, usually of white matter fiber systems (Jones, Knosche, & Turner, 2013; Mueller, Lim, Hemmy, & Camchong, 2015). Functional MRI (fMRI) measures differences in local vascular oxygenated-to-deoxygenated blood volume that occurs while performing a task or while at rest (Chen & Glover, 2015a, 2015b). MR arterial spin labeling (ASL) can measure local blood perfusion in gray matter tissue (Pfefferbaum et al., 2010). These and other MR modalities have been used to identify local and global differences in alcoholics compared with controls and to track the progress of recovery on brain regional integrity with sobriety or further decline with continued heavy drinking. It is beyond the scope of this paper to provide an exhaustive review of results from all of these modalities. Rather, a sampling of each is given along with references to other papers that have provided useful didactic reviews with relevance to neuropsychologists (Bigler, 2015, 2017) and others that have selectively reviewed results using these neuroimaging modalities in the context of alcoholism (e.g., Dupuy & Chanraud, 2016; Le Berre et al., 2019; Zahr, 2014; Zahr & Pfefferbaum, 2017).
Structural MRI: Patterns and Functional Correlates.
Since the advent of non-invasive neuroimaging, hundreds of papers have described structural dysmorphology associated with AUD. Early CT studies reported enlarged lateral ventricles and widened cortical sulci (e.g., Carlen, Wortzman, Holgate, Wilkinson, & Rankin, 1978; Pfefferbaum, Rosenbloom, Crusan, & Jernigan, 1988) representing non-localized abnormalities. The higher resolution of MRI along with its ability to distinguish tissue type as gray matter, white matter, and cerebrospinal fluid (CSF) has revealed localized structural differences from controls. In general, cross-sectional MRI studies report volume deficits in cortical gray matter and white matter, insula, hippocampus, and anterior cerebellum and have been reviewed previously (Zahr, 2014). Earlier longitudinal studies provided evidence for increasing volume with abstinence and continued decline with relapse (Cardenas et al., 2007; Mann, Gunther, Stetter, & Ackermann, 1999; Pfefferbaum et al., 1995). Recent, large-scale longitudinal MRI studies found volume deficits in frontal, temporal, parietal, cingulate, and insular cortices with evidence for accelerated aging in volumes of precentral and superior frontal cortices (Sullivan et al., 2018b). Volume deficits were also noted in the thalamus and hippocampus (Pfefferbaum, Zahr, et al., 2018), consistent with other cross-sectional reports (e.g., Durazzo et al., 2011; Fein et al., 2013; Makris et al., 2008). Using high-resolution MRI to quantify hippocampal subfields, follow-up study identified recovery of CA2/3 volumes in alcoholics who remained abstinent for a month (Kuhn et al., 2014). Another cross-sectional study found volume deficits in subiculum, CA1, CA4, fimbria, a composite of dentate gyrus regions, and the hippocampus-amygdala-transition-area in alcoholics compared with controls and an age-alcohol interaction in CA2/3, indicating that older alcoholics had greater volume deficits than would be expected for their age (Zahr, Pohl, Saranathan, Sullivan, & Pfefferbaum, 2019).
The most common volume deficits reported with whole-brain imaging involve selective regions of frontal cortex and implicate their dissociable, spatially disparate extra-frontal networks. To accommodate these affected systems, we recently proposed a model of three frontofugal networks implicated in AUD: frontolimbic, frontostriatal, and frontocerebellar (Zahr et al., 2017). The consistency of frontally-based functional and structural abnormalities identified in vivo comport with neuropathological findings indicating significant neuronal loss localized to the superior frontal cortex of “uncomplicated” alcoholic cases (Harper, Kril, & Daly, 1987; Kril & Halliday, 1999). These networks underlie the selective cognitive and motor functions commonly disrupted in AUD, notably, cognitive judgment, cognitive control, decision making, inhibition, working memory, affect and reward processing, visuospatial abilities, processing speed, and stability of gait and static posture (e.g., Chanraud & Sullivan, 2014; Galandra, Basso, Cappa, & Canessa, 2018; Le Berre et al., 2019). Despite the imputed relations, direct correlations between performance and markers of regional brain integrity are not always forthcoming.
Identification of these networks arose from many neuroimaging studies of uncomplicated alcoholism in comparison with alcoholism complicated by dramatic conditions that can accompany alcoholism, exacerbate its deficits, and contribute their own dysmorphological signature. The most well described AUD concomitant is the Wernicke-Korsakoff syndrome (WKS), which is caused by depletion of thiamine, an essential vitamin (Thomson, Guerrini, & Marshall, 2012). WKS is marked by lesions identified neuropathologically (Harper et al., 1998; Torvik, 1991; Victor, Adams, & Collins, 1989) and radiologically (Sullivan & Pfefferbaum, 2009) in frontolimbic sites, including the mammillary bodies, hippocampus, and thalamus, and in frontocerebellar sites, including the anterior vermis of the cerebellum (Le Berre et al., 2014). Additional diagnostic concomitants of AUD are Marchiafava-Bignami disease, marked by lesions of the corpus callosum and symptoms of a disconnection syndrome (Victor et al., 1989); alcoholic cerebellar degeneration, marked by lesions and hypometabolism of the anterior superior vermis (Gilman et al., 1990; Lhermitte, 1934); hepatic encephalopathy, marked by hyperintense MRI signal of insular cortex (Butterworth, 2014; Campagna et al., 2015; Nabi et al., 2014); central pontine myelinolysis, seen as a bat-wing shaped, edematous lesion in central pons (de la Monte & Kril, 2014; Haynes, Gallagher, Cordaro, Likeman, & Love, 2018) and when severe associated with quadriplegia (Mascarenhas & Jude, 2014); and Alcohol-Related Dementia (Cheng et al., 2017), associated with notable cortical atrophy and discussed later in this review.
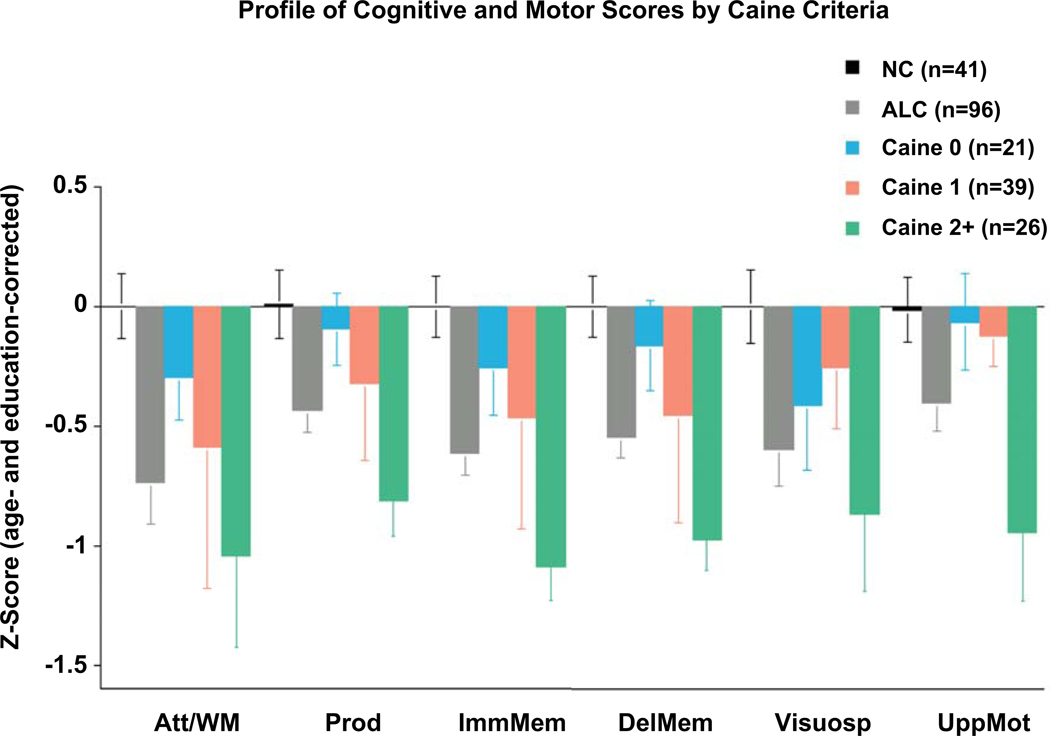
The selective lesions characterizing these AUD-associated syndromes provided leads to seeking circumscribed dysmorphology in uncomplicated AUD. Toward this end, a series of studies found graded effects in brain regions affected by these different conditions, where uncomplicated AUD showed volume deficits compared with healthy controls, and complicated AUD showed greater volume deficits than uncomplicated AUD (Le Berre et al., 2014; Sullivan & Pfefferbaum, 2009). These graded effects led to another concept about uncomplicated AUD, suggesting that such individuals carry a history, or “scar,” from subclinical bouts of any of these conditions. This hypothesis was also supported with reference to neuropsychological performance in studies, which categorized AUD individuals by Caine et al. criteria for determining history of preclinical Wernicke encephalopathy (WE) (cf., Ambrose, Bowden, & Whelan, 2001). Chart review of postmortem cases revealed that apparently uncomplicated AUD who had two of four signs of WE (dietary deficiency, ophthalmoplegia, cognitive compromise, ataxia) also had lesions consistent with WE (Caine, Halliday, Kril, & Harper, 1997). These criteria were operationalized for in vivo use to sort AUD individuals by number of criteria met. Alcoholics meeting no criteria performed at normal levels on a large neuropsychological test battery; those meeting one criterion performed at impaired levels on a few of the test composites; those meeting two or more criteria were impaired on all test composites (Fama et al., 2017; Pitel et al., 2011) (Figure 1). Thus, although these AUD individuals had no history of clinically diagnosed WE, performance impairment level conformed to the “dose effect” of a WE burden, further suggesting a nutritional substrate for explaining heterogeneity in test performance.
Figure 1.
Bar graphs depicting the alcohol Caine-categorized subgroups in comparison with the alcoholic group as a whole and the normal group. Att/WM - Attention/Working Memory; Prod - Production; ImmMem – Immediate Memory; DelMem – Delayed Memory; Visuosp –Visuospatial Construction; UppMot – Upper Limb Motor. From Fama et al. (Fama et al., 2017), Figure 2.
Quantitative MRI data indicate that the brains of individuals with AUD do not have demonstrable, frank lesions but rather are characterized by regional volume deficits and in some cases by cortical thinning or gray and white matter volume deficits (for review, Zahr & Pfefferbaum, 2017). Further, degradation of regional brain tissue can have far reaching effects based on networks, making it difficult (at best) to identify one-to-one correspondences between a selective cognitive or motor deficits and a single source brain substrate (cf., Sullivan & Pfefferbaum, 2005). Nonetheless, a number of brain structure-function relations have been reported, with the most convincing ones based on evidence for double dissociation (cf., Fama & Sullivan, 2014). The few examples that follow provide brain structural substrates, in terms of systems rather than single locations, of cognitive or motor skills commonly impaired in AUD.
Regression analysis revealed that the rostral middle frontal and cerebellar cortical volume were significant selective predictors of performance on an executive functions battery and critically not of a test of general cognitive status (Nakamura-Palacios et al., 2014). Relative to controls, alcoholics scored lower on three sets of composite scores and had smaller frontal, cingulate, insular, parietal, and hippocampal volumes; within the alcoholics, executive function scores correlated with frontal, temporal, and caudate volumes; explicit memory scores correlated with frontal volume, notably orbitofrontal cortex; and balance scores correlated with frontal, caudate, and pontine volumes (Fama et al. 2019). A visuospatial processing deficit in long-term abstinent alcoholics was related selectively to parietal volume shrinkage but not to other cortical volume deficits; greater parietal volume shrinkage occurred with greater amount of alcohol consumed over a lifetime (Fein, Shimotsu, Chu, & Barakos, 2009). An early study of alcoholics identified a series of selective, dissociable relations, where anterior vermian but not prefrontal or parietal volume was a unique predictor of balance scores; vermian and thalamic but not prefrontal cortical volumes were predictors of Wisconsin Card Sorting Test scores; and cerebellar hemispheric white matter but not parietal cortical volume was a predictor of visuospatial ability (Sullivan, 2003).
DTI: Patterns and Functional Correlates.
In addition to degraded network nodes, a network can suffer dysfunction from “fraying” of white matter fiber connections, the microstructural integrity of which can be assessed with DTI. Quantitative studies have shown evidence for anterior and superior white matter systems as being more vulnerable to alcoholism than posterior and inferior systems (Pfefferbaum, Rosenbloom, Rohlfing, & Sullivan, 2009). These in vivo findings comport with neuropathological evidence for degradation of white matter fibers observed as “swelling and fragmentation of myelin, irregular loss of axons, and degeneration of oligodentrocytes” (page 81) (de la Monte & Kril, 2014) together with loss of synaptic connections. White matter damage was especially notable in prefrontal regions, the extent of which was related to maximum daily alcohol consumption (Kril, Halliday, Svoboda, & Cartwright, 1997). Additional in vivo longitudinal data suggest a dose-like response for recovery, such that alcoholics who refrained from drinking between DTI scans showed a return toward normal levels, whereas those who relapsed showed accelerated aging (Pfefferbaum et al., 2014). Albeit cross-sectional by definition, there is neuropathological evidence for remyelination of damaged white matter fibers, for example, in a case of chronic alcoholism with central pontine myelinolysis (Haynes et al., 2018), that has the potential of supporting at least partial recovery of function.
Several studies reported functional correlates of disrupted fiber integrity, whether the DTI metric was based on abnormally low fractional anisotropy, suggestive of disruption of the linear organization of fibers, or higher than normal diffusivity, an index of excessive free water presence or edematous tissue. For example, DTI measures of the genu but not splenium of the corpus callosum correlated with working memory but not visuospatial performance, whereas the splenium but not genu diffusivity correlated with visuospatial but not working memory performance (Pfefferbaum et al., 2009). Integrity of the corpus callosum was also implicated in a task requiring interhemispheric transfer of visual information (Schulte, Sullivan, Muller-Oehring, Adalsteinsson, & Pfefferbaum, 2005). DTI results provide further support for the concept that alcoholism affects widespread regions that reflect organized and selective neural networks.
ASL: Patterns and Functional Correlates.
Only a few studies have employed this MR imaging method, which provides quantification of regional cerebral blood perfusion and ideally should be used to control for perfusion deficits that could account for abnormal BOLD responses in fMRI experiments. A longitudinal study of AUD participants who either maintained abstinence or relapsed during a 35-day span after having been sober for 7 days revealed that relapsers had significantly lower frontal and parietal gray matter perfusion than abstainers and non-smoking controls who neither differed from each other nor evidenced perfusion change over the 4-week test interval (Durazzo, Gazdzinski, Mon, & Meyerhoff, 2010). Although lobar perfusion did not correlate with either drinking severity or cognitive performance, lower frontal gray matter perfusion correlated with more cigarettes smoked per day (Gazdzinski et al., 2006; Mon, Durazzo, Gazdzinski, & Meyerhoff, 2009). Using 3-dimensional ASL protocol and controlling for smoking, alcoholics were seen to have normal perfusion and normal response while performing a spatial working memory task in all regions quantified except the insula, a hub of the salience network (Sullivan et al., 2013). The insular perfusion deficit was interpreted as having the potential to impair ability to switch from cognitive states of interoceptive cravings to cognitive control for curbing internal urge and perhaps serving as an addiction site.
PET.
Functional neuroimaging methods have enabled direct tests of brain function. Early studies used 18F-2-fluoro-deoxyglucose (FDG) positron emission tomography (PET) to measure glucose utilization in selective regional gray matter structures after performing a task (Adams et al., 1993). PET studies, however, are invasive, in that they involve infusion with a radioactive isotope but can yield robust, physiological information about absolute regional brain function if the protocol uses isotope decay timing from arterial blood collection; alternatively, rate of glucose metabolism is estimated by calculating a ratio between a target region and a referent, typically the cerebellum. An early PET study provided evidence for disruption of thalamocortical interactions in KS relative to uncomplicated alcoholics in mediating impairment in memory storage (Paller et al., 1997). When coupled with structural MRI to correct for the underlying gray matter volume, which is commonly deficient compared with age- and sex-matched controls, this method has revealed patterns of impaired glucose use differentiating uncomplicated alcoholics from alcoholics with WKS (Aupee et al., 2001). Specifically, compared with controls, uncomplicated alcoholics exhibited two major patterns of gray matter volume shrinkage and hypometabolism: nodes of Papez (limbic) circuit involving the cerebellum cingulate cortex, thalamus, and hippocampus and hippocampal gyrus showed greater volume shrinkage than hypometabolism, whereas selective cortical sites, notably the dorsolateral, premotor, and parietal cortices showed greater glucose metabolism deficits than underlying volume deficits (Ritz et al., 2014). These results suggest that neural networks have metabolic differences at rest, during cognitive task engagement, and to disease-related insult, speculations that were confirmed by a recent multimodal PET/rsfMRI study of controls and AUD participants (Shokri-Kojori, Tomasi, Alipahahi, Wiers, Wang, & Volkow, 2019).
fMRI.
For the past 20 years, functional imaging has relied on non-invasive methods using MR technology. Accordingly, compared with the two dozen PET studies published since 1993 (search for “brain PET and glucose and alcoholic” limited to human studies), approximately 1650 fMRI studies appeared on a PubMed search for “functional brain imaging and alcoholic.” Two different approaches are used in fMRI research (for review, Mumford, Poldrack, & Nichols, 2011). 1) Task-activated paradigms are designed to discover selective brain regions that are invoked when performing a task. The result is usually determined by subtracting from the signal of the regional vasculature while performing a target (experimental) task, the signal while performing a similar (control) task or no task at all. The local brain activation signal difference, which can be as small as 1–4%, indicates the contribution from the experimental condition. 2) Resting-state paradigms are presumed to reflect the brain’s activity and inter-regional functional connectivity determined by correlating the fMRI signal at every voxel in the brain with voxels in a specific regional “seed” or every other voxel while the participant is “at rest” and without an experimental task. The outcome of this synchronous activity between regionally distant voxels is assumed to identify brain regions that are intrinsically and functionally connected. Reliably identified connectivity patterns are deemed intrinsic functional networks. Given the large number of published studies and the availability of other recent targeted reviews (e.g., Dupuy & Chanraud, 2016; Galandra et al., 2018), the following will provide only some highlights.
Task-activated fMRI studies.
A number of studies have used fMRI in search of brain regions and structures that indicate disrupted neural substrates that contribute to specific deficits typically detected using cognitive testing. Paradoxically, a requirement of these studies is to devise a task that is either easy enough for an alcoholic to perform, or amenable to practice so that the alcoholic can perform at control levels. Equivalence in performance allows the investigator to ask whether the brain regions invoked by the alcoholic group are the same as or different from those invoked by the control group. Like PET studies, the underlying gray matter volume must be taken into account in analysis because the source of the fMRI response (the blood oxygen-level dependent [BOLD] signal) is the capillary bed of gray matter. Group differences in regions, spatial extents, and levels of activation can provide insight into potential compensatory mechanisms supporting a selective function (Chanraud et al., 2013; Chanraud & Sullivan, 2014).
Task-activated fMRI studies are difficult to devise, conduct, and analyze but can reveal untold mechanisms of differences that could be useful in retraining and identify brain systems that may be less able to recover and undergo restructuring. As with all aspects of alcohol research, age and timing are critical to consider: age at onset, age at test, length of sobriety, and amount drunk. Indeed, alcohol cueing experiments have been sensitive in predicting relapse and in identifying brain regions, including fronto-limbic and striatal networks (Haber & Knutson, 2010).
Initial studies focusing on working memory found that with practice alcoholics who were able to perform a verbal task activated lateral frontal and cerebellar (lobule VI/Crus I) regions by contrast, controls could perform this task by activating the frontal region only (Desmond et al., 2003). While performing a spatial working memory task, alcoholics activated the frontotemporal regions, representing the “ventral stream” of processing typically used for remembering verbal material (Ungerleider, Courtney, & Haxby, 1998), unlike the controls, who activated frontoparietal regions, representing the “dorsal stream” typically used for processing spatial information (Pfefferbaum et al., 2001). Alcoholism-related differences in activation of frontostriatal systems and sites have been detected with reward (Alba-Ferrara, Muller-Oehring, Sullivan, Pfefferbaum, & Schulte, 2016) and response inhibition (Claus, Feldstein Ewing, Filbey, & Hutchison, 2013; Courtney, Ghahremani, & Ray, 2013; Hommer, Bjork, & Gilman, 2011; Hu, Ide, Zhang, Sinha, & Li, 2015; Schuckit et al., 2012; Schulte et al., 2017).
A paradigm that used a task requiring alcoholics and controls to make decisions based on uncertainty revealed that controls showed functional synchrony between the dorsal anterior cingulate cortex and the cerebellum, whereas alcoholics showed synchronous activity between the cingulate site and the premotor cortex during decision-making (Jung et al., 2014). A potential negative outcome of the non-normal synchrony was inflexibility in response strategy to accommodate ambiguity in choices.
Functional activation of ventral tegmental area and gray matter volume of orbitofrontal and medial prefrontal cortices were better predictors of drinking relapse 3 months later than were clinical indices (Seo et al., 2015). Other functional brain systems predictive of early relapse involved enhanced connectivity between the ventral medial prefrontal cortex and the anterior cingulate cortex in neutral-relaxing cueing conditions and indices of greater craving in stress- and alcohol-related cueing conditions (Seo et al., 2013). Removal of an addictive substance causes a heightened negative response including physiological and psychological stress responses (Blaine, Seo, & Sinha, 2017), considered negative reinforcement and depicted as “the dark side of addiction” (Koob, 2015). Thus, identification of resilience factors could be a critical step in developing successful therapies, whether founded on pharmacological or psychological approaches. Accordingly, recovering alcoholics who remained sober for the next half year showed greater activation than their relapsing counterparts in prefrontal (BA10, 45, 47) and greater than controls in medial premotor (BA6,8) cortices while performing a high-load working memory task, providing evidence for a “resilience factor” as contributing to ability to sustain abstinence (Charlet, Rosenthal, Lohoff, Heinz, & Beck, 2018). Cingulate-based connectivity in processing alcohol-related vs. neutral cues has also predicted likelihood of abstinence (Zakiniaeiz, Scheinost, Seo, Sinha, & Constable, 2017). Adding to these findings is a multi-modal brain imaging study using EEG coherence and a cue-reactivity task with fMRI showing desynchronization in the BOLD response among striatolimbic regions and hypersynchronization among the same areas in the theta frequency band of the EEG, interpreted as a “central craving network” integrating components of alcohol addiction into a “unified percept” (page 923) (Huang, Mohan, De Ridder, Sunaert, & Vanneste, 2018).
Resting-state fMRI (rs-fMRI) studies.
An hypothesis-driven analysis tested differences between alcoholics and controls on connectivity patterns and strength of five intrinsic functional networks: default mode, integrative executive control, salience, attention, and primary somatosensory/auditory/visual input. Connectivity patterns were similar in the two groups but, in general, the networks were weaker and more expansive in the alcoholics than the controls, suggesting compensation through expansion for diminished network connectivity (Muller-Oehring, Jung, Pfefferbaum, Sullivan, & Schulte, 2015). Another study reported that greater severity of AUD correlated with smaller average cluster coefficient, and that longer AUD duration correlated with a global decrease in connectivity efficiency (Sjoerds et al., 2017).
Integrating findings from EEG coherence and rs-fMRI synchrony in long-term abstinent alcoholics revealed a coordinated network of heightened theta and alpha activity and greater nucleus accumbens/dorsolateral prefrontal cortical synchrony suggesting robust top-down executive control together with lower gamma activity and lower connectivity with the thalamus suggesting dampened appetitive drive (Cardenas, Price, & Fein, 2018). As with task-activated fMRI using cueing paradigms, rs-fMRI has successfully predicted relapse, where poorer synchronization within the reward, executive control, and visual networks was predictive of earlier relapse (Camchong, Stenger, & Fein, 2013b). Conversely, long-term abstinence in alcoholics was characterized by attenuated synchrony between limbic reward sites, enhanced synchrony in executive function sites, and correlation of these activation differences with performance on an intra/extradimensional set shifting task (Camchong, Stenger, & Fein, 2013c, 2013d). The rs-fMRI synchrony among nodes of the appetitive drive network (nucleus accumbens, caudate, nucleus, thalamus) was not dampened, however, in alcoholics comorbid for major depressive disorder (Fein, Camchong, Cardenas, & Stenger, 2017). Consideration of non-alcohol drug use comorbidity indicated attenuation of functional connectivity in the appetitive drive network involving the nucleus accumbens and subgenual anterior cingulate cortex in long-term abstinent alcoholics without drug comorbidity but not in those with drug comorbidity (Camchong, Stenger, & Fein, 2013a).
Compensation.
Taken together, neuroimaging studies of alcoholism have revealed widespread damage. Yet, some regions are more affected than others thereby presenting a pattern of spared and affected tissue and the potential for testing and establishing brain structure-function relations to provide neural mechanisms of neuropsychologically-detected functions. Whole-brain imaging has enabled identification of disrupted brain regions suspected from neurological and neuropsychological examination and from neuropathological reports but has gone beyond to detect brain lesions or affected tissue that is outside of traditionally sites presumed to underlie a selective functional impairment. To the extent that certain functions can be shown to be selectively associated with an unexpected region, the combination of neuroimaging and neuropsychology has advanced a fuller understanding of how brain systems work together and that wide-reaching brain systems can form networks that subserve simple to complex functions.
The recognition that complex functions are subserved by multiple brain regions forming systems or networks indicates avenues for recovery of function through compensation (Chanraud & Sullivan, 2014; Dupuy & Chanraud, 2016; Oscar-Berman et al., 2014). Evidence for overlap or apparent redundancy of functions may provide avenues for overcoming or compensating for deficits. Caution needs to be applied, however, when declaring what is compensation. For example, when in a task-activated fMRI paradigm, a patient group activates a different brain region from that activated by controls does not necessarily indicate that the different brain region is compensatory. Two conditions must be met before drawing that conclusion: 1) In a patient group, abnormally increased activity while performing a task in a region potentially considered compensatory must also be related to abnormally low activation in other brain regions associated with performing the task by unaffected controls; and 2) the abnormal activation pattern must be associated with performance at normal levels (Chanraud et al., 2013). Indeed, identification of brain regions and their associated functions that appear to be amenable to recovery can provide hope to the recovering alcoholic and a pathway for rehabilitation efforts (Bates, Buckman, & Nguyen, 2013).
Common Comorbidities of AUD: Contribution to Impairment
Epidemiology.
Comorbidities of AUD are either a result of chronic, excessive drinking or are diseases or disorders that commonly occur with AUD. An estimated 60 acute or chronic diseases result from or are exacerbated by alcohol. Leading resultants are tuberculosis, automobile accidents, suicide, hepatic cirrhosis, and a variety of carcinomas, the prevalence of which varies with the socioeconomic level of a country (Alcohol & Drug Use, 2018; Global Burden of Disease, 2018).
Leading comorbidities are selective psychiatric diagnoses, notably major depressive disorders, schizophrenia, post-traumatic stress disorder (PTSD), and anxiety disorder. A meta-analysis using data from international studies found that individuals with AUD carried a 4.1 times greater risk of bipolar disorder than those without AUD (Hunt, Malhi, Cleary, Lai, & Sitharthan, 2016). A population-based survey in France yielded similarly high incidence of AUD accompanying bipolar disorder, suicide ideation, PTSD, panic disorder, sleep disorders, and poor concentration (Carton et al., 2018). Mood disorder symptoms can change with alcohol reduction therapy, and in women an exacerbation of mood disorder can impair alcohol reduction goals (Crum et al., 2018).
Non-alcohol Substance Use Disorders (SUD) commonly co-occur with AUD, the most frequent being tobacco use (World Health Organization, 2018). AUD and smoking are considered by some to be complementary; for example, many drinkers smoke and many smokers drink. Current efforts at drinking reduction include smoking reduction or cessation in recovery programs using behavioral or pharmacological therapies (Falk, Castle, Ryan, Fertig, & Litten, 2015). Other frequently used drugs accompanying alcohol are cocaine, cannabis, benzodiazepines, and opioids, and use varies by country (World Health Organization, 2018). In the U.S., data from the National Epidemiologic Survey on Alcohol and Related Conditions-III (NESARCIII) study (Grant et al., 2017) of non-institutionalized adults revealed that alcohol and non-alcohol drugs are used concurrently in nearly half of those with AUD, and that use is especially high in individuals with psychopathology (Saha et al., 2018).
AUD is also prevalent among individuals with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) infection. For a scholarly review of AUD-HIV-HCV comorbidity with psychiatry, medication, and aging also considered, see Zahr (Zahr, 2018a). The U.S. Veterans Aging Cohort study found that the AUD prevalence rate of 32,699 people living with HIV ranged from 11.8% in rural areas to 14.4% in urban settings (Bensley et al., 2018). The comorbidities of AUD, HIV, and HCV take a toll on brain structure (Gullett et al., 2018; Pfefferbaum, Kwon, et al., 2018) and function (Fama, Sullivan, Sassoon, Pfefferbaum, & Zahr, 2016; Gongvatana et al., 2014) possibly through a combination of inflammatory mechanisms stemming especially from HCV (Zahr, 2018b), interference of anti-retroviral action from alcohol (Simon et al., 2018), or risk of non-adherence associated with drinking (Paolillo, Gongvatana, Umlauf, Letendre, & Moore, 2017) or drug use whether licit or illicit (Malbergier, Amaral, & Cardoso, 2015; but see Nolan et al., 2017). Indeed, any study focused on differentiating individuals with from those without AUD must account for past—even remote—AUD, history of which has the potential of modulating its interactions with the disorder or disease under investigation (Crane et al., 2017; Wyczechowska et al., 2017).
Alcohol use is a risk factor for mortality and morbidity for a variety of somatic diseases. Although some studies indicate a salutary or protective effect of moderate drinking (Richard et al., 2017), a community-based study on 550, non-alcohol dependent men and women in the United Kingdom found that even one alcoholic drink daily is associated with lexical fluency decline and hippocampal atrophy (Topiwala et al., 2017). A large-scale analysis on nearly 600,000 individual drinkers from 83 prospective studies, conducted in 19 high-income countries sought risk thresholds of safe or beneficial drinking (Wood et al., 2018). In contrast with lower risk of myocardial infarction, higher risk of coronary artery disease and shorter life expectancy occurred with alcohol consumption of 100g per week or higher; the minimum mortality risk occurred ≤100g per week, that is, about 7 drinks per week (Wood et al., 2018). A recent meta-analysis concluded that there is overall no beneficial quantity of alcohol consumption with a clear dose response of alcohol’s contribution to the global burden of disease (Global Burden of Disease, 2018).
Comorbidity in Neuropsychology and Neuroimaging Studies.
The neuropsychological ramifications of comorbidities are complex. Studies are commonly launched to determine whether co-occurring diagnoses contribute to selective impairments individually or synergistically and whether multiple diagnoses simply contribute to impairment severity. Ideally, study groups should include comparison groups with the separate diagnoses in addition to controls and the target group with multiple diagnoses. Such groups that are matched on critical variables such as age, sex, SES, and education, are difficult to recruit, typically resulting in small samples and restricting generalizability. Determining the primary diagnosis and whether order of onset or remoteness of drug comorbidity is influential are further considerations even before medications or other treatments are noted. With these limitations in mind, the following presents only a sampling of the burgeoning attempts to identify patterns of brain functions and structures affected and spared in disorders with high prevalence of AUD.
AUD and Tobacco.
The most commonly abused drug by alcoholics is tobacco, usually cigarettes (www.samhsa.gov). Smoked cigarettes avail the user to 100s of chemicals; the most notorious and addictive one is nicotine (Swan & Lessov-Schlaggar, 2007). Although cigarettes have an apparent beneficial effect in addicted individuals of focusing attention and psychomotor speed (Canamar & London, 2012), alcoholics who are smokers either lag their non-smoking sober alcoholic counterparts in recovery during early stages of abstinence or do not recover to the same extent (Durazzo & Meyerhoff, 2007). Component processes most stubborn to recover are gait and balance (Schmidt et al., 2014). Brain structure is also adversely affected in smokers with AUD and lag in recovery despite sobriety (Durazzo, Mon, Gazdzinski, & Meyerhoff, 2017).
Functional imaging resting-state studies posit that the effects of alcohol and nicotine dependence can be dissociated. Smokers showed poor connectivity between thalamus and putamen with high connectivity between angular gyrus and precuneus; by contrast, non-smoking alcoholics showed low connectivity among the precuneus, postcentral gyrus, insula and visual cortex, interpreted as impaired interoceptive awareness with poor connectivity between the postcentral gyrus and fusiform and lingual gyri that was associated with drinking severity (Vergara, Liu, Claus, Hutchison, & Calhoun, 2017). Although these in vivo studies indicate an untoward role for cigarette smoking on function, a recent postmortem study provided no evidence for a compounded or interactive effect of smoking and chronic alcoholism on regional lobar volume deficits (McCorkindale, Sizemova, Sheedy, Kril, & Sutherland, 2019); nevertheless, functional deficits may be detected in the absence of structural deficits.
AUD and Illicit Substance Use Disorder (SUD).
Co-occurring abuse and dependence of alcohol and illicit drug use is highly prevalent. Thus, to study the effects of one agent without consideration of others belies reality and limits generality of findings. A comparison of AUD, SUD with cocaine, and AUD+cocaine revealed impairment on tests of executive function and memory; curiously, the AUD+cocaine group did not show a compounded deficit and if anything performed better than the AUD without SUD (Blanco-Presas et al., 2018). By contrast, a neuropsychological study comparing individuals with AUD versus polysubstance use disorder (PSU) reported that the PSU group performed significantly worse than the AUD group on verbal memory and a measure of impulsivity; after 4 months of sobriety, the PSU group improved significantly on a number of cognitive measures including inhibitory control (Schmidt, Pennington, Cardoos, Durazzo, & Meyerhoff, 2017).
Quantitative MRI has been useful in discerning the effects of AUD+SUD comorbidity on regional brain structures. An attempt to parse contributions to gray matter volume deficits from alcohol, tobacco, cocaine, and cannabis each and in combination identified greater volume deficits in medial and ventral prefrontal cortex as related to more substances used (Kaag et al., 2018). A meta-analysis indicated that cocaine and methamphetamine dependencies were each associated with volume deficits notable in the inferior frontal, superior temporal, and insular cortices; further, methamphetamine had a greater effect than cocaine on superior frontal and insular volumes, whereas cocaine’s effect was greater on the superior temporal and inferior parietal volumes (Hall et al., 2015). The effect of cannabis dependence on the cortex appears to target medial orbitofrontal volumes, (Chye et al., 2017) although other studies report focal effects on medial temporal structures (Koenders et al., 2017; Lorenzetti et al., 2015; Schacht, Hutchison, & Filbey, 2012). Similarly, polysubstance abusers were found to have orbitofrontal volume deficits relative to controls without difference from non-drug-misusing alcoholic participants (Pennington et al., 2015; Tanabe et al., 2009). Recently, we examined the independent and combined effects of AUD and SUD on regional brain volumes and found the following patterns: the alcohol plus cocaine and alcohol plus opiate groups had smaller frontal volumes than the drug-dependence-free alcoholism group; critically, deficits in precentral, supplementary motor, and medial frontal volumes endured in drug-dependence-free participants with alcoholism compared with controls (Sullivan et al., 2018b). Further, although drug dependence and HCV infection compounded deleterious effects of alcohol dependence on frontal cortical volumes, HCV infection could not account for the frontally distributed volume deficits in the drug-free participants with alcoholism
AUD and HIV infection.
A principal goal of these studies is identification of salient deficits describing each diagnosis and testing whether and how comorbidity compounds impairment (for reviewZahr, 2018a). For example, studies focused on cognitive performance in HIV-infected women reported that women with HIV+AUD comorbidity made fewer optimal choices in a Game of Dice Task than did their HIV-only counterparts (Martin et al., 2016). With the success of anti-viral medication, people living with HIV have a near-normal life expectancy (Lear et al.), yet affording responders continued opportunity to engage in risky behavior. An example of this problem was reported in a study of men age 50 years and older who have sex with men showing that the strongest predictor of unsafe sex was drinking alcohol to intoxication (Kupprat, Krause, Ompad, & Halkitis, 2017).
Use of a large battery of tests examining six functional domains yielded a graded effect of impairment on tests of executive function and episodic memory: compared with unaffected controls, single-diagnosis HIV and AUD groups were similarly impaired and performed better than the group with HIV+AUD. In the comorbid group, older men and women performed worse on memory tests than younger participants. Diagnostic differences were also observed in performance levels, where the AUD group was impaired in planning and free recall of visuospatial material, whereas the HIV-only group was impaired on psychomotor speed, sequencing, narrative free recall, and pattern recognition (Fama et al., 2016). This pattern was replicated (Heinz, Fogler, Newcomb, Trafton, & Bonn-Miller, 2014) and extended (Woods et al., 2016), where poorer cognitive performance by HIV+AUD study participants was more strongly related to lifetime AUD and older age than to HIV-related disease markers of viral load and current or nadir CD4 count or even to past opiate or cocaine use (Cohen et al., 2018). Another significant factor in predicting compromised performance on tests of verbal learning and processing speed by HIV infected men with heavy alcohol use was cigarette smoking, which is highly prevalent in this group and should be a target for cessation treatment (Littleton, Barron, Prendergast, & Nixon, 2007; Monnig et al., 2016).
Mechanisms to explain the interactions of AUD and HIV infection include biochemistry of gut-brain interactions. Pro-inflammatory processes have been invoked to explain cognitive and motor deficits especially in individuals comorbid for AUD and HIV (e.g., Monnig, 2017). Study comparing AUD, HIV, and HIV+AUD also accounted for coinfection with HCV and found evidence from peripheral circulating cytokine levels for elevated tumor necrosis factor α (TNFα) and interferon gamma-induced protein 10 (IP-10) in HIV irrespective of AUD and in AUD with (but not without) HCV infection (Zahr, 2018a, 2018b). The triplet of HIV+AUD+HCV was associated with especially compromised performance on a test of remote semantic memory for public figures (Fama et al., 2011).
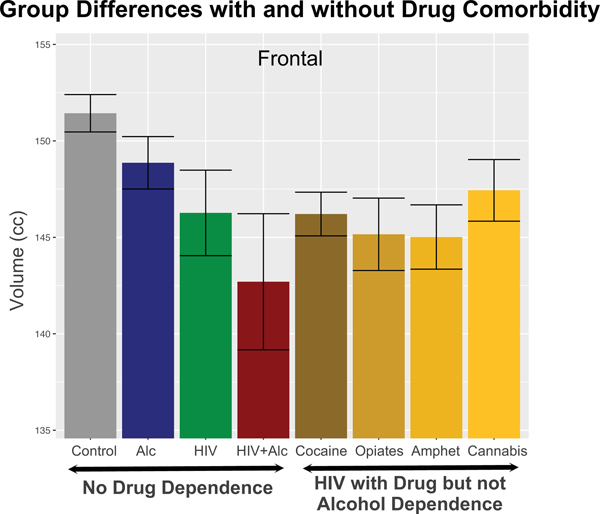
Regarding brain structural integrity, a combined HIV and HIV+AUD group showed frontal volume deficits, regardless of drug or alcohol history, relative to control subjects. In addition, the HIV-only drug-dependent group had volume deficits in parietal and temporal cortices, and the HIV+ALC drug-dependent group had volume deficits in the insular, cingulate, and parietal cortices. Critically, the frontal volume deficit endured in the HIV group without a history of drug or alcohol dependence (Figure 2). Similarly, the frontal and parietal volume deficits endured in the HIV+ALC group without drug dependence (Pfefferbaum, Zahr, et al., 2018).
Figure 2.
Color-coded brain regions and scatterplots of significant age-diagnosis interactions in the Alc group only indicating age-related declines in excess of those detected in the controls (gray regression lines). From Pfefferbaum et al. (Pfefferbaum, Zahr, et al., 2018), Supplemental Figure 2.
AUD in an Aging Population: Implications for the Development of Dementia
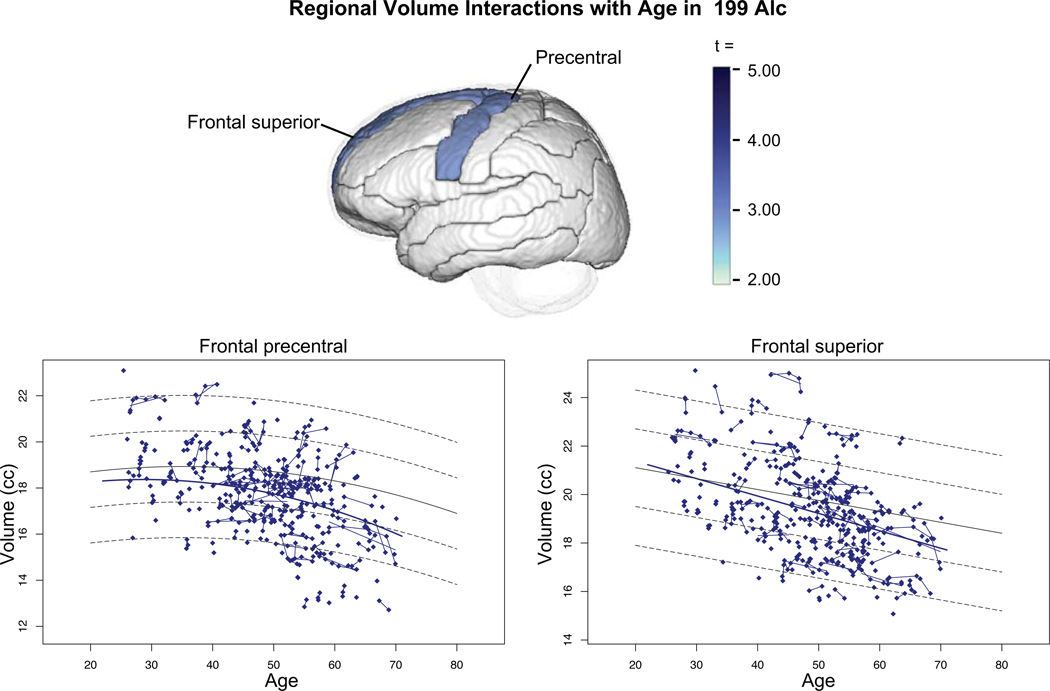
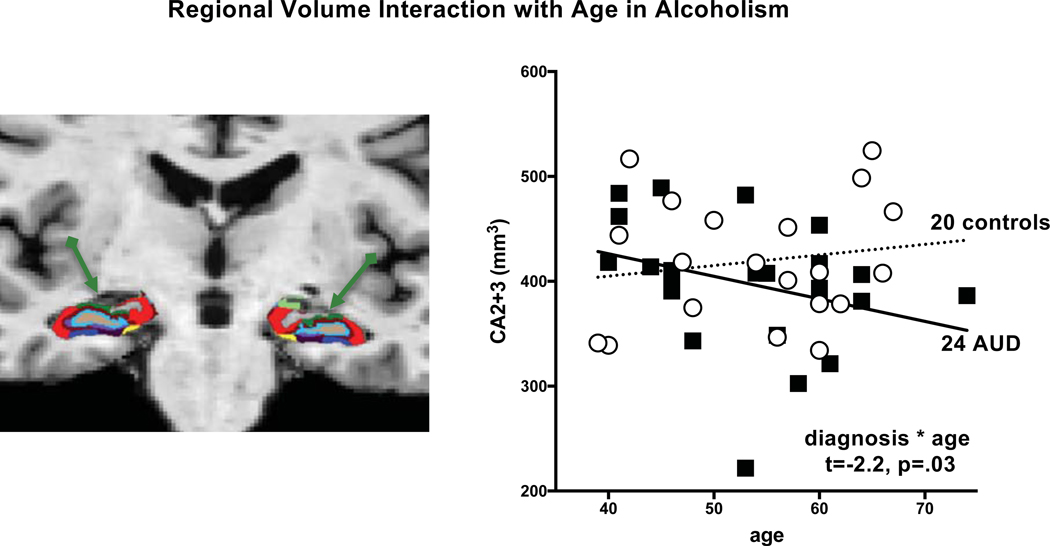
Development of cognitive decline can be considered as another form of comorbidity in AUD. Our recent longitudinal findings of accelerated aging of selective brain structures in alcohol-dependent men and women together with signs of premature aging of frontal (Pfefferbaum, Zahr, et al., 2018; Sullivan et al., 2018a) (Figure 3) and hippocampal (Zahr et al., 2019) (Figure 4) volumes lead to the speculation that that the incidence of accelerated cognitive and mnemonic decline will ensue. A controversy that remains unresolved, however, centers on whether non-WKS alcohol dependence results in a classic, irreversible dementia and whether alcohol-related cognitive and brain structural declines are precursors to Alzheimer’s Disease (AD) per se or form part of an AD-Related Dementia (ADRD) spectrum. It is timely to address these controversies given the aging of the population, increased drinking in the elderly, and high incidence of tau-based dementias.
Figure 3.
Mean ± 95% CI of the controls and each diagnostic group with and without alcohol or drug dependence in regions showing significant volume deficits. Also shown are volumes by drug-dependent comorbidity: cocaine (Coc’n), opiates, amphetamines (Amph), and cannabis (Cann). From Pfefferbaum et al. (Pfefferbaum, Zahr, et al., 2018), Supplemental Figure 3
Figure 4.
Left panel: Coronal slice through the hippocampus demonstrating parcellation of a fully processed set of images from a 65 year-old control man. Subfields are color-coded. Right panel: Age x group interaction indicating that older alcoholics had a greater CA2/3 (subfield marked in green) volume deficit for their age than did younger ones. Modified Figures 1 and 4 from Zahr et al. (Zahr et al., 2019).
A 2018 estimate noted that 10% of people 65 years and older in the U.S. have AD; remarkably, 1 in 3 older people dies with AD or ADRD (www.alz.org/facts/). Aging itself is the strongest predictor of mild cognitive impairment (MCI), which can herald unrelenting tau- and non-tau-based dementias (Jack et al., 2018; Pontecorvo et al., 2017; van de Nes, Nafe, & Schlote, 2008). With an approximate lifetime cost of care at $341,840 per dementia patient, factors, including AUD, that could be identified to ameliorate disease progress and reduce the financial and personal burden of AD to the family and society would be welcome.
Accelerated vs. premature aging in AUD.
The United States census estimated that 35% of the population as of 2016 was 50 years and older, with 15.2% aged 65+ years old (https://censusreporter.org/profiles/01000US-united-states/). The National Epidemiological Survey on Alcohol and Related Conditions (NESARC) (Grant et al., 2017) revealed substantial increases in the incidence of AUD in the U.S. Of particular relevance to problems of cognitive health facing the aging population is that the older people are increasing alcohol consumption, with women exceeding the trajectory increase of men both in regular to heavy drinking and in binge drinking (Breslow et al., 2017).
A large study of men and women, age 55–65 years at baseline, were followed after 1, 4, 10, and 20 years found that two factors--high average level of drinking and heavy episodic drinking--were better predictors than average consumption to predict risk for later drinking problems (Holahan et al., 2017). A cross-sectional study of alcoholics of a common age and representing three bands of age of onset (<25, 25–44, and ≥45 years) addressed whether late-onset drinkers with lower lifetime consumption had less severe cognitive impairment than young-onset drinkers. Results indicated that the three groups of alcoholics had a similar level of cognitive deficits despite their being of similar age at testing and proportionately different numbers of drinking years (Kist, Sandjojo, Kok, & van den Berg, 2014).
Alcoholic Related Dementia (ARD).
A 25-year review of dementia prevalence posited that ARD represents 10% of early-onset dementia but little more than 1% of late-onset dementia (Cheng et al., 2017; Schwarzinger et al., 2018). Although not everyone agrees that ARD exists or is a classical degenerative condition, age-alcoholism interactions (Durazzo et al., 2014; Pfefferbaum, Sullivan, Mathalon, & Lim, 1997; Sullivan et al., 2018b) provide correlational support for accelerated declines in some but not all brain regions, most consistently frontal cortex (Pfefferbaum, Zahr, et al., 2018; Sullivan et al., 2018b). A large longitudinal study of AD reported for the first time that heavy drinking, defined as 8 or more drinks/week, interacted with AD to accelerate cognitive decline, measured biannually with the modified Mini-Mental State Exam (MMSE) (Heymann et al., 2016).
Hippocampal Markers of AUD and AD.
Hippocampal volume loss is a robust marker of AD (e.g., Parker et al., 2018) and also occurs in AUD. Early volumetric studies found that hippocampal volume deficits were equivalent in alcoholics with WKS and individuals with AD (Sullivan & Marsh, 2003). Indeed, it has been shown that hippocampal volume deficits (greater in anterior than posterior hippocampal regions) were graded with non-WKS alcoholics showing volume loss relative to controls that were even greater in WKS alcoholics (Sullivan, Deshmukh, Desmond, Lim, & Pfefferbaum, 2000; Sullivan & Pfefferbaum, 2009). Hippocampal volume deficits relative to controls in non-WKS alcoholic (AUD) men and women have now been reported by a number of neuroimaging groups (e.g., Beresford et al., 2006; De Bellis et al., 2000; Durazzo et al., 2011; Le Berre et al., 2014; Makris et al., 2008) and include (as noted above) selective subfields also implicated in AD (Kuhn et al., 2014; Lee et al., 2016; Zahr et al., 2019). Unknown, however, is whether hippocampal subfields deficits follow the same pattern of loss as MCI and AD/ADRD and whether regional hippocampal volume shrinkage in AUD is permanent, progressive, or functionally meaningful.
Patterns of Neuropsychological Impairment in MCI/AD/ADRD and AUD/ARD.
The most salient cognitive feature of AD and amnesic MCI is impairment in memory consolidation (Alber, Della Sala, & Dewar, 2014; Dewar, Pesallaccia, Cowan, Provinciali, & Della Sala, 2012; Ribeiro, Guerreiro, & De Mendonca, 2007). The deficits ultimately affect verbal and nonverbal recognition and recall of newly presented material and are attributed to bilateral hippocampal volume loss (Di Paola et al., 2007; Kramer et al., 2004; Ostby, Tamnes, Fjell, & Walhovd, 2012). Subfield analysis has revealed some evidence for selective mnemonic declines as associated with volume shrinkage of CA1 and memory consolidation (Mueller, Chao, Berman, & Weiner, 2011), delayed recall from the California Verbal Learning Test (CVLT) in a mixed group of controls, MCI, and AD participants (Kerchner et al., 2014), and performance on the Montreal Cognitive Assessment (MoCA) with volumes of the anterolateral entorhinal cortex in undiagnosed community dwelling elderly (Olsen et al., 2017).
The neuropsychological profile of ARD implicates both cortical and subcortical involvement, with confrontation naming more impaired in AD than ARD (Schmidt et al., 2005); however, lower scores on the MMSE by the AD relative to the ARD group (Folstein, 2007) could indicate severity rather than selectivity as underlying the deficit patterns. Another study reported that even non-demented AUD patients can perform at AD deficit levels on verbal fluency, working, memory, and other front executive functions despite only mild impairment on declarative memory (Liappas et al., 2007).
Role of AUD-related Cognitive Decline in AD/ADRD.
In the U.S., alcohol related dementia (ARD) is typically diagnosed with DSM-IV or DSM-5 criteria with the primary criterion to find evidence of significant cognitive decline from a previous level of performance. Determination of ARD, as with other dementias, entails extensive interviews, neuropsychological testing, clinical examination, and hematological assays for liver and nutritional status (e.g., Ridley, Draper, & With all, 2013; Sachdeva, Chandra, Choudhary, Dayal, & Anand, 2016). DSM-5 lists dementing disorders including MCI/AD/ADRD, ARD, and traumatic brain injury. Such inclusions raise the question as to whether disorders that are reversible or preventable and not necessarily age-related should be considered a dementing disorder.
Whether and how ARD and AD/ADRD are linked remains controversial (e.g., Anstey, Mack, & Cherbuin, 2009; Gupta & Warner, 2008; Ridley et al., 2013). For example, a neuropathological study comparing three common neurodegenerative disorders – AD, Lewy body dementia, and vascular cognitive impairment – found no relation between alcohol consumption patterns and neuropathological markers even when hepatic pathology was considered (Aho, Karkola, Juusela, & Alafuzoff, 2009). A rodent in vitro study, however, showed changes in phagocytosis-related mRNAs and a reduction in the uptake of amyloid beta in microglial cells (prepared from rat mixed glial cultures) exposed to ethanol (Kalinin et al., 2018), suggesting that ethanol effects on phagocytosis could contribute to the development of AD. Other mechanisms whereby alcohol may add to the risk for development of AD include activation of toll like receptors (which might modulate amyloid beta deposition) and the downstream release of proinflammatory cytokines and chemokines (Venkataraman, Kalk, Sewell, Ritchie, & Lingford-Hughes, 2017), thereby increasing neuroinflammatory burden.
A generation ago, clinical criteria for ARD were proposed (Oslin, Atkinson, Smith, & Hendrie, 1998). Recognizing that Victor et al. (Victor et al., 1989) was an opponent of the concept of ARD, contending that ARD is not a diagnostic entity, Oslin and colleagues astutely noted, “...the elaboration of more detailed criteria may falsely tend to validate a disease that may not exist” (page 210). Rather, they proffered, alcohol may contribute to dementia but not constitute the cause of a classical dementing disease.
With the extended longevity of the U.S. population comes the looming increased numbers of individuals who will express MCI/AD (Alzheimer’s Association, 2018) and develop AUD (Wood et al., 2018). Even if mechanistic support for alcohol misuse as a cause of AD is not supported, discovery of an association between MCI/AD and AUD cognitive decline would indicate an underlying factor in hazardous alcohol consumption to cognitive decline that can be reversed or at least ameliorated with reduction in or abstinence from alcohol consumption.
Summary and Future Directions
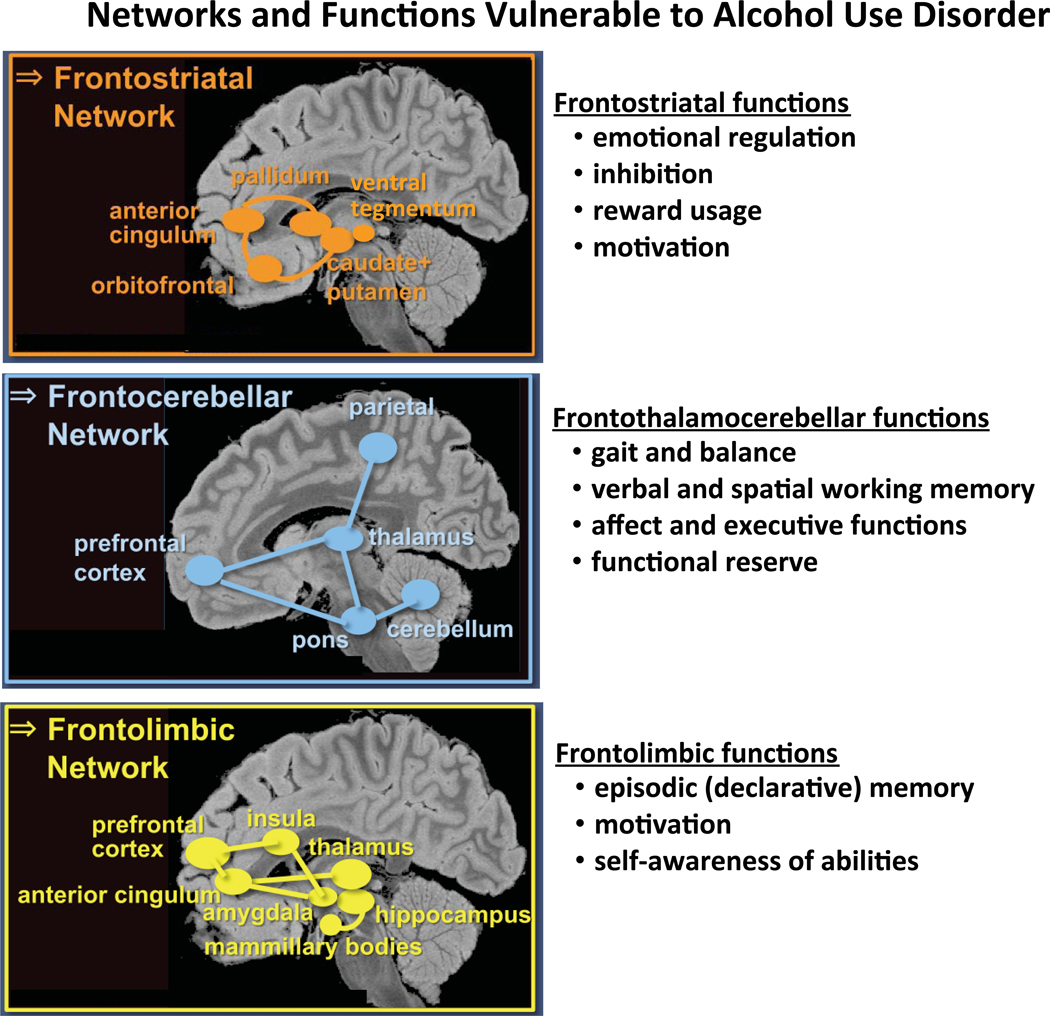
Networks antecedent and resultant in the development of AUD involve the fronto-fugal systems (Zahr et al., 2017). The fronto-limbic and fronto-striatal systems likely participate in the development of addiction through processing pleasure, stress, and memory, that evolve to become a cause for perpetuating desire for the substance of addiction. The fronto-cerebellar systems suffer the throes of alcoholism-related degeneration and may contribute to habits associated with the addiction in addition to becoming a substrate of components of executive function deficits. These frontally-based systems are far-reaching in the brain yet selective in their contributions to the neuropsychological deficits defining the dynamic development, maintenance, and resolution of alcohol dependence (Figure 5).
Figure 5.
Brain networks and associated functions found to be vulnerable to Alcohol Use Disorder: Frontostriatal nodes and network enable functions of emotional regulation, inhibition, reward usage, motivation; frontocerebellar nodes and network enable functions of gait and balance, verbal and spatial working memory, affect and executive functions, and provides functional reserve; and frontolimbic nodes and network enables functions of episodic memory to consolidate new information, motivation, self-awareness of abilities and limitations (Zahr, Pfefferbaum, & Sullivan, 2017). We speculate that knowing what is compromised and what is spared might help in therapeutic efforts to redirect neural recruitment from the usual but disrupted paths and networks to functional alternatives, possibly through behavioral therapy or pharmacological efforts for maintaining abstinence and promoting recovery.
The next generation of studies might follow two divergent paths: 1) devise highly refined tests of component cognitive, motor, and sensory processes to match the evolving high-resolution imaging methods in search of neural mechanisms of selective functional disruption and 2) plan longitudinal study to consider the myriad factors that define the heterogeneity and course of AUD and promote identification of mechanisms of functional decline and opportunities for recovery.
Public Significance Statement.
Alcohol Use Disorder (AUD) is a dynamic condition that waxes and wanes with unhealthy drinking episodes and varies with age in drinking patterns and effects on brain structure and function. Its excessive use renders chronically heavy drinkers vulnerable to direct alcohol toxicity and a variety of comorbidities attributable to nonalcohol drug misuse, viral infections, and accelerated or premature aging. Older alcoholics show accelerated aging, together with increasing alcohol consumption in middle-age and older adults, putting them at heightened risk for developing cognitive decline and possibly dementia.
Acknowledgments
Funding: Grants from the U.S. National Institute on Alcohol Abuse and Alcoholism (AA005965; AA010723; AA017347, AA017923) and the Moldow Women’s Health and Hope Fund. The content of the review is solely the responsibility of the authors and does not necessarily represent the official views of the supporting institutions.
References
- Adams KM, Gilman S, Koeppe RA, Kluin KJ, Brunberg JA, Dede D, . . . Kroll PD (1993). Neuropsychological deficits are correlated with frontal hypometabolism in positron emission tomography studies of older alcoholic patients. Alcohol Clin Exp Res, 17(2), 205–210. [DOI] [PubMed] [Google Scholar]
- Aho L, Karkola K, Juusela J, & Alafuzoff I (2009). Heavy alcohol consumption and neuropathological lesions: a post-mortem human study. J Neurosci Res, 87(12), 27862792. doi: 10.1002/jnr.22091 [DOI] [PubMed] [Google Scholar]
- Alba-Ferrara L, Muller-Oehring EM, Sullivan EV, Pfefferbaum A, & Schulte T (2016). Brain responses to emotional salience and reward in alcohol use disorder. Brain Imaging Behav, 10(1), 136–146. doi: 10.1007/s11682-015-9374-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alber J, Della Sala S, & Dewar M (2014). Minimizing interference with early consolidation boosts 7-day retention in amnesic patients. Neuropsychology, 28(5), 667–675. doi: 10.1037/neu0000091 [DOI] [PubMed] [Google Scholar]
- Alcohol GBD, & Drug Use C (2018). The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. doi: 10.1016/S2215-0366(18)30337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. (2018). Alzheimer’s Disease Facts and Figures. [Google Scholar]
- Ambrose ML, Bowden SC, & Whelan G (2001). Thiamin treatment and working memory function of alcohol-dependent people: preliminary findings. Alcohol Clin Exp Res, 25(1), 112–116. [PubMed] [Google Scholar]
- AmericanPsychiatricAssociation. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Anstey KJ, Mack HA, & Cherbuin N (2009). Alcohol consumption as a risk factor for dementia and cognitive decline: meta-analysis of prospective studies. Am J Geriatr Psychiatry, 17(7), 542–555. doi: 10.1097/JGP.0b013e3181a2fd07 [DOI] [PubMed] [Google Scholar]
- Aupee AM, Desgranges B, Eustache F, Lalevee C, de la Sayette V, Viader F, & Baron JC (2001). Voxel-based mapping of brain hypometabolism in permanent amnesia with PET. Neuroimage, 13(6 Pt 1), 1164–1173. doi: 10.1006/nimg.2001.0762 [DOI] [PubMed] [Google Scholar]
- Bagga D, Khushu S, Modi S, Kaur P, Bhattacharya D, Garg ML, & Singh N (2014). Impaired visual information processing in alcohol-dependent subjects: a proton magnetic resonance spectroscopy study of the primary visual cortex. J Stud Alcohol Drugs, 75(5), 817–826. [DOI] [PubMed] [Google Scholar]
- Bagga D, Sharma A, Kumari A, Kaur P, Bhattacharya D, Garg ML, . . . Singh N (2014). Decreased white matter integrity in fronto-occipital fasciculus bundles: relation to visual information processing in alcohol-dependent subjects. Alcohol, 48(1), 43–53. doi: 10.1016/j.alcohol.2013.10.009 [DOI] [PubMed] [Google Scholar]
- Bagga D, Singh N, Singh S, Modi S, Kumar P, Bhattacharya D, . . . Khushu S (2014). Assessment of abstract reasoning abilities in alcohol-dependent subjects: an fMRI study. Neuroradiology, 56(1), 69–77. doi: 10.1007/s00234-013-1281-3 [DOI] [PubMed] [Google Scholar]
- Bates ME, Buckman JF, & Nguyen TT (2013). A role for cognitive rehabilitation in increasing the effectiveness of treatment for alcohol use disorders. Neuropsychol Rev, 23(1), 27–47. doi: 10.1007/s11065-013-9228-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensley KM, McGinnis KA, Fortney J, Chan KCG, Dombrowski JC, Ornelas I, . . . Williams EC (2018). Patterns of Alcohol Use Among Patients Living With HIV in Urban, Large Rural, and Small Rural Areas. J Rural Health, Epub ahead of print Oct 19. doi: 10.1111/jrh.12326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beresford TP, Arciniegas DB, Alfers J, Clapp L, Martin B, Du Y, . . . Davatzikos C (2006). Hippocampus volume loss due to chronic heavy drinking. Alcohol Clin Exp Res, 30(11), 1866–1870. doi: 10.1111/j.1530-0277.2006.00223.x [DOI] [PubMed] [Google Scholar]
- Beylergil SB, Beck A, Deserno L, Lorenz RC, Rapp MA, Schlagenhauf F, . . . Obermayer K (2017). Dorsolateral prefrontal cortex contributes to the impaired behavioral adaptation in alcohol dependence. Neuroimage Clin, 15, 80–94. doi: 10.1016/j.nicl.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigler ED (2015). Structural Image Analysis of the Brain in Neuropsychology Using Magnetic Resonance Imaging (MRI) Techniques. Neuropsychol Rev, 25(3), 224–249. doi: 10.1007/s11065-015-9290-0 [DOI] [PubMed] [Google Scholar]
- Bigler ED (2017). Structural neuroimaging in neuropsychology: History and contemporary applications. Neuropsychology, 31(8), 934–953. doi: 10.1037/neu0000418 [DOI] [PubMed] [Google Scholar]
- Blaine SK, Seo D, & Sinha R (2017). Peripheral and prefrontal stress system markers and risk of relapse in alcoholism. Addict Biol, 22(2), 468–478. doi: 10.1111/adb.12320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Presas L, Moreno-Alcazar A, Alonso-Lana S, Salvador R, Pomarol-Clotet E, & McKenna P (2018). Cognitive impairment associated with cocaine use: The role of coexistent alcohol abuse/dependence. Drug Alcohol Depend, 189, 70–75. doi: 10.1016/j.drugalcdep.2018.03.054 [DOI] [PubMed] [Google Scholar]
- Breslow RA, Castle IP, Chen CM, & Graubard BI (2017). Trends in Alcohol Consumption Among Older Americans: National Health Interview Surveys, 1997 to 2014. Alcohol Clin Exp Res, 41(5), 976–986. doi: 10.1111/acer.13365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brion M, de Timary P, Vander Stappen C, Guettat L, Lecomte B, Rombaux P, & Maurage P (2015). Chemosensory Dysfunction in Alcohol-Related Disorders: A Joint Exploration of Olfaction and Taste. Chem Senses, 40(9), 605–608. doi: 10.1093/chemse/bjv047 [DOI] [PubMed] [Google Scholar]
- Butterworth RF (2014). Hepatic encephalopathy in alcoholic cirrhosis. Handb Clin Neurol, 125, 589–602. doi: 10.1016/B978-0-444-62619-6.00034-3 [DOI] [PubMed] [Google Scholar]
- Caine D, Halliday GM, Kril JJ, & Harper CG (1997). Operational criteria for the classification of chronic alcoholics: identification of Wernicke’s encephalopathy. J Neurol Neurosurg Psychiatry, 62(1), 51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, & Fein G (2013a). Resting state synchrony in long-term abstinent alcoholics with versus without comorbid drug dependence. Drug Alcohol Depend, 131(12), 56–65. doi: 10.1016/j.drugalcdep.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, & Fein G (2013b). Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex, 23(9), 2086–2099. doi: 10.1093/cercor/bhs190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, & Fein G (2013c). Resting-state synchrony in long-term abstinent alcoholics. Alcohol Clin Exp Res, 37(1), 75–85. doi: 10.1111/j.1530-0277.2012.01859.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger VA, & Fein G (2013d). Resting-state synchrony in short-term versus long-term abstinent alcoholics. Alcohol Clin Exp Res, 37(5), 794–803. doi: 10.1111/acer.12037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna F, Montagnese S, Schiff S, Ruzzoli M, Biancardi A, Iannizzi P, . . . Amodio P (2015). Confounders in the detection of minimal hepatic encephalopathy: a neuropsychological and quantified EEG study. Liver Int, 35(5), 1524–1532. doi: 10.1111/liv.12635 [DOI] [PubMed] [Google Scholar]
- Canamar CP, & London E (2012). Acute cigarette smoking reduces latencies on a Smoking Stroop test. Addict Behav, 37(5), 627–631. doi: 10.1016/j.addbeh.2012.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Price M, & Fein G (2018). EEG coherence related to fMRI resting state synchrony in long-term abstinent alcoholics. Neuroimage Clin, 17, 481–490. doi: 10.1016/j.nicl.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas VA, Studholme C, Gazdzinski S, Durazzo TC, & Meyerhoff DJ (2007). Deformation-based morphometry of brain changes in alcohol dependence and abstinence. Neuroimage, 34(3), 879–887. doi: 10.1016/j.neuroimage.2006.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen PL, Wortzman G, Holgate RC, Wilkinson DA, & Rankin JC (1978). Reversible cerebral atrophy in recently abstinent chronic alcoholics measured by computed tomography scans. Science, 200(4345), 1076–1078. [DOI] [PubMed] [Google Scholar]
- Carton L, Pignon B, Baguet A, Benradia I, Roelandt JL, Vaiva G, . . . Rolland B (2018). Influence of comorbid alcohol use disorders on the clinical patterns of major depressive disorder: A general population-based study. Drug Alcohol Depend, 187, 40–47. doi: 10.1016/j.drugalcdep.2018.02.009 [DOI] [PubMed] [Google Scholar]
- Chanraud S, Pitel AL, Muller-Oehring EM, Pfefferbaum A, & Sullivan EV (2013). Remapping the brain to compensate for impairment in recovering alcoholics. Cereb Cortex, 23(1), 97–104. doi: 10.1093/cercor/bhr381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, & Sullivan EV (2014). Compensatory recruitment of neural resources in chronic alcoholism. Handb Clin Neurol, 125, 369–380. doi: 10.1016/B978-0-444-62619-6.00022-7 [DOI] [PubMed] [Google Scholar]
- Charlet K, Beck A, Jorde A, Wimmer L, Vollstadt-Klein S, Gallinat J, . . . Heinz A (2014). Increased neural activity during high working memory load predicts low relapse risk in alcohol dependence. Addict Biol, 19(3), 402–414. doi: 10.1111/adb.12103 [DOI] [PubMed] [Google Scholar]
- Charlet K, Rosenthal A, Lohoff FW, Heinz A, & Beck A (2018). Imaging resilience and recovery in alcohol dependence. Addiction. doi: 10.1111/add.14259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlet K, Schlagenhauf F, Richter A, Naundorf K, Dornhof L, Weinfurtner CE, . . . Heinz A (2014). Neural activation during processing of aversive faces predicts treatment outcome in alcoholism. Addict Biol, 19(3), 439–451. doi: 10.1111/adb.12045 [DOI] [PubMed] [Google Scholar]
- Chen JE, & Glover GH (2015a). Erratum to: Functional Magnetic Resonance Imaging Methods. Neuropsychol Rev, 25(3), 314. doi: 10.1007/s11065-015-9298-5 [DOI] [PubMed] [Google Scholar]
- Chen JE, & Glover GH (2015b). Functional Magnetic Resonance Imaging Methods. Neuropsychol Rev, 25(3), 289–313. doi: 10.1007/s11065-015-9294-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng C, Huang CL, Tsai CJ, Chou PH, Lin CC, & Chang CK (2017). Alcohol-Related Dementia: A Systemic Review of Epidemiological Studies. Psychosomatics, 58(4), 331342. doi: 10.1016/j.psym.2017.02.012 [DOI] [PubMed] [Google Scholar]
- Chye Y, Solowij N, Suo C, Batalla A, Cousijn J, Goudriaan AE, . . . Yucel M (2017). Orbitofrontal and caudate volumes in cannabis users: a multi-site mega-analysis comparing dependent versus non-dependent users. Psychopharmacology (Berl), 234(13), 1985–1995. doi: 10.1007/s00213-017-4606-9 [DOI] [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, & Hutchison KE (2013). Behavioral control in alcohol use disorders: relationships with severity. J Stud Alcohol Drugs, 74(1), 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Gullett JM, Porges EC, Woods AJ, Lamb DG, Bryant VE, . . . Monti PM (2018). Heavy Alcohol Use and Age Effects on HIV-Associated Neurocognitive Function. Alcohol Clin Exp Res. doi: 10.1111/acer.13915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, Ghahremani DG, & Ray LA (2013). Fronto-striatal functional connectivity during response inhibition in alcohol dependence. Addict Biol, 18(3), 593–604. doi: 10.1111/adb.12013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane HM, McCaul ME, Chander G, Hutton H, Nance RM, Delaney JAC, . . . Kitahata MM (2017). Prevalence and Factors Associated with Hazardous Alcohol Use Among Persons Living with HIV Across the US in the Current Era of Antiretroviral Treatment. AIDS Behav, 21(7), 1914–1925. doi: 10.1007/s10461-017-1740-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Green KM, Stuart EA, La Flair LN, Kealhofer M, Young AS, . . . Reboussin BA (2018). Transitions through stages of alcohol involvement: The potential role of mood disorders. Drug Alcohol Depend, 189, 116–124. doi: 10.1016/j.drugalcdep.2018.02.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui C, Noronha A, Warren KR, Koob GF, Sinha R, Thakkar M, . . . Sullivan EV (2015). Brain pathways to recovery from alcohol dependence. Alcohol, 49(5), 435–452. doi: 10.1016/j.alcohol.2015.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czapla M, Simon JJ, Richter B, Kluge M, Friederich HC, Herpertz S, . . . Loeber S (2016). The impact of cognitive impairment and impulsivity on relapse of alcohol-dependent patients: implications for psychotherapeutic treatment. Addict Biol, 21(4), 873–884. doi: 10.1111/adb.12229 [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Clark DB, Beers SR, Soloff PH, Boring AM, Hall J, . . . Keshavan MS (2000). Hippocampal volume in adolescent-onset alcohol use disorders. Am J Psychiatry, 157(5), 737–744. doi: 10.1176/appi.ajp.157.5.737 [DOI] [PubMed] [Google Scholar]
- de la Monte SM, & Kril JJ (2014). Human alcohol-related neuropathology. Acta Neuropathol, 127(1), 71–90. doi: 10.1007/s00401-013-1233-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmond JE, Chen SH, DeRosa E, Pryor MR, Pfefferbaum A, & Sullivan EV (2003). Increased frontocerebellar activation in alcoholics during verbal working memory: an fMRI study. Neuroimage, 19(4), 1510–1520. [DOI] [PubMed] [Google Scholar]
- Dewar M, Pesallaccia M, Cowan N, Provinciali L, & Della Sala S (2012). Insights into spared memory capacity in amnestic MCI and Alzheimer’s Disease via minimal interference. Brain Cogn, 78(3), 189–199. doi: 10.1016/j.bandc.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Di Paola M, Macaluso E, Carlesimo GA, Tomaiuolo F, Worsley KJ, Fadda L, & Caltagirone C (2007). Episodic memory impairment in patients with Alzheimer’s disease is correlated with entorhinal cortex atrophy. A voxel-based morphometry study. J Neurol, 254(6), 774–781. doi: 10.1007/s00415-006-0435-1 [DOI] [PubMed] [Google Scholar]
- Dupuy M, & Chanraud S (2016). Imaging the Addicted Brain: Alcohol. Int Rev Neurobiol, 129, 1–31. doi: 10.1016/bs.irn.2016.04.003 [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Gazdzinski S, Mon A, & Meyerhoff DJ (2010). Cortical perfusion in alcohol-dependent individuals during short-term abstinence: relationships to resumption of hazardous drinking after treatment. Alcohol, 44(3), 201–210. doi: 10.1016/j.alcohol.2010.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, & Meyerhoff DJ (2007). Neurobiological and neurocognitive effects of chronic cigarette smoking and alcoholism. Front Biosci, 12, 4079–4100. [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Gazdzinski S, & Meyerhoff DJ (2017). Regional brain volume changes in alcohol-dependent individuals during early abstinence: associations with relapse following treatment. Addict Biol, 22(5), 1416–1425. doi: 10.1111/adb.12420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Mon A, Pennington D, Abe C, Gazdzinski S, & Meyerhoff DJ (2014). Interactive effects of chronic cigarette smoking and age on brain volumes in controls and alcohol-dependent individuals in early abstinence. Addict Biol, 19(1), 132–143. doi: 10.1111/j.1369-1600.2012.00492.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo TC, Tosun D, Buckley S, Gazdzinski S, Mon A, Fryer SL, & Meyerhoff DJ (2011). Cortical thickness, surface area, and volume of the brain reward system in alcohol dependence: relationships to relapse and extended abstinence. Alcohol Clin Exp Res, 35(6), 1187–1200. doi: 10.1111/j.1530-0277.2011.01452.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk DE, Castle IJ, Ryan M, Fertig J, & Litten RZ (2015). Moderators of Varenicline Treatment Effects in a Double-Blind, Placebo-Controlled Trial for Alcohol Dependence: An Exploratory Analysis. J Addict Med, 9(4), 296–303. doi: 10.1097/ADM.0000000000000133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Le Berre AP, Hardcastle C, Sassoon SA, Pfefferbaum A, Sullivan EV, & Zahr NM (2017). Neurological, nutritional and alcohol consumption factors underlie cognitive and motor deficits in chronic alcoholism. Addict Biol, Epub Dec 15. doi: 10.1111/adb.12584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Le Berre AP, Sassoon SA, Zahr NM, Pohl KM, Pfefferbaum A, & Sullivan EV (2019). Dissociable relations between cognitive and motor deficits and regional brain volumes in alcoholism. Brain Structure and Function, in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Sassoon SA, Pfefferbaum A, & Sullivan EV (2012). Differential effect of alcoholism and HIV infection on visuomotor procedural learning and retention. Alcohol Clin Exp Res, 36(10), 1738–1747. doi: 10.1111/j.15300277.2012.01790.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Rosenbloom MJ, Sassoon SA, Thompson MA, Pfefferbaum A, & Sullivan EV (2011). Remote semantic memory for public figures in HIV infection, alcoholism, and their comorbidity. Alcohol Clin Exp Res, 35(2), 265–276. doi: 10.1111/j.15300277.2010.01342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, & Sullivan EV (2014). Methods of association and dissociation for establishing selective brain-behavior relations. Handb Clin Neurol, 125, 175–181. doi: 10.1016/B9780-444-62619-6.00011-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fama R, Sullivan EV, Sassoon SA, Pfefferbaum A, & Zahr NM (2016). Impairments in Component Processes of Executive Function and Episodic Memory in Alcoholism, HIV Infection, and HIV Infection with Alcoholism Comorbidity. Alcohol Clin Exp Res, 40(12), 2656–2666. doi: 10.1111/acer.13250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Camchong J, Cardenas VA, & Stenger A (2017). Resting state synchrony in longterm abstinent alcoholics: Effects of a current major depressive disorder diagnosis. Alcohol, 59, 17–25. doi: 10.1016/j.alcohol.2016.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, & Greenstein D (2013). Gait and balance deficits in chronic alcoholics: no improvement from 10 weeks through 1 year abstinence. Alcohol Clin Exp Res, 37(1), 86–95. doi: 10.1111/j.1530-0277.2012.01851.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Greenstein D, Cardenas VA, Cuzen NL, Fouche JP, Ferrett H, . . . Stein DJ (2013). Cortical and subcortical volumes in adolescents with alcohol dependence but without substance or psychiatric comorbidities. Psychiatry Res, 214(1), 1–8. doi: 10.1016/j.pscychresns.2013.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein G, Shimotsu R, Chu R, & Barakos J (2009). Parietal gray matter volume loss is related to spatial processing deficits in long-term abstinent alcoholic men. Alcohol Clin Exp Res, 33(10), 1806–1814. doi: 10.1111/j.1530-0277.2009.01019.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B (2012). FreeSurfer. Neuroimage, 62(2), 774–781. doi: 10.1016/j.neuroimage.2012.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick LE, & Crowe SF (2013). Cognitive and emotional deficits in chronic alcoholics: a role for the cerebellum? Cerebellum, 12(4), 520–533. doi: 10.1007/s12311-013-0461-3 [DOI] [PubMed] [Google Scholar]
- Folstein M (2007). Improving dementia assessment by reducing sample heterogeneity. Int Psychogeriatr, 19(3), 383–389. doi: 10.1017/S1041610207005169 [DOI] [PubMed] [Google Scholar]
- Galandra C, Basso G, Cappa S, & Canessa N (2018). The alcoholic brain: neural bases of impaired reward-based decision-making in alcohol use disorders. Neurol Sci, 39(3), 423435. doi: 10.1007/s10072-017-3205-1 [DOI] [PubMed] [Google Scholar]
- Gazdzinski S, Durazzo T, Jahng GH, Ezekiel F, Banys P, & Meyerhoff D (2006). Effects of chronic alcohol dependence and chronic cigarette smoking on cerebral perfusion: a preliminary magnetic resonance study. Alcohol Clin Exp Res, 30(6), 947–958. doi: 10.1111/j.1530-0277.2006.00108.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Adams K, Koeppe RA, Berent S, Kluin KJ, Modell JG, . . . Brunberg JA (1990). Cerebellar and frontal hypometabolism in alcoholic cerebellar degeneration studied with positron emission tomography. Ann Neurol, 28(6), 775–785. doi: 10.1002/ana.410280608 [DOI] [PubMed] [Google Scholar]
- Global Burden of Disease. (2018). The global burden of disease attributable to alcohol and drug use in 195 countries and territories, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Psychiatry. doi: 10.1016/S2215-0366(18)30337-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongvatana A, Morgan EE, Iudicello JE, Letendre SL, Grant I, Woods SP, & Group HIVNRP (2014). A history of alcohol dependence augments HIV-associated neurocognitive deficits in persons aged 60 and older. J Neurovirol, 20(5), 505–513. doi: 10.1007/s13365-014-0277-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, . . . Hasin DS (2017). Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry, 74(9), 911923. doi: 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullett JM, Lamb DG, Porges E, Woods AJ, Rieke J, Thompson P, . . . Cohen RA (2018). The Impact of Alcohol Use on Frontal White Matter in HIV. Alcohol Clin Exp Res, 42(9), 1640–1649. doi: 10.1111/acer.13823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, & Warner J (2008). Alcohol-related dementia: a 21st-century silent epidemic? Br J Psychiatry, 193(5), 351–353. doi: 10.1192/bjp.bp.108.051425 [DOI] [PubMed] [Google Scholar]
- Haber SN, & Knutson B (2010). The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology, 35(1), 4–26. doi: 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MG, Alhassoon OM, Stern MJ, Wollman SC, Kimmel CL, Perez-Figueroa A, & Radua J (2015). Gray matter abnormalities in cocaine versus methamphetamine-dependent patients: a neuroimaging meta-analysis. Am J Drug Alcohol Abuse, 41(4), 290–299. doi: 10.3109/00952990.2015.1044607 [DOI] [PubMed] [Google Scholar]
- Harper C, Kril J, & Daly J (1987). Are we drinking our neurones away? Br Med J (Clin Res Ed), 294(6571), 534–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper CG, Sheedy DL, Lara AI, Garrick TM, Hilton JM, & Raisanen J (1998). Prevalence of Wernicke-Korsakoff syndrome in Australia: has thiamine fortification made a difference? Med J Aust, 168(11), 542–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes HR, Gallagher PJ, Cordaro A, Likeman M, & Love S (2018). A case of chronic asymptomatic central pontine myelinolysis with histological evidence of remyelination. Forensic Sci Med Pathol, 14(1), 106–108. doi: 10.1007/s12024-017-9933-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz AJ, Fogler KA, Newcomb ME, Trafton JA, & Bonn-Miller MO (2014). Problematic alcohol use among individuals with HIV: relations with everyday memory functioning and HIV symptom severity. AIDS Behav, 18(7), 1302–1314. doi: 10.1007/s10461-013-0602-1 [DOI] [PubMed] [Google Scholar]
- Heymann D, Stern Y, Cosentino S, Tatarina-Nulman O, Dorrejo JN, & Gu Y (2016). The Association Between Alcohol Use and the Progression of Alzheimer’s Disease. Curr Alzheimer Res, 13(12), 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson R, Zha W, Simons-Morton B, & White A (2016). Alcohol-Induced Blackouts as Predictors of Other Drinking Related Harms Among Emerging Young Adults. Alcohol Clin Exp Res, 40(4), 776–784. doi: 10.1111/acer.13010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoche F, Guell X, Sherman JC, Vangel MG, & Schmahmann JD (2016). Cerebellar Contribution to Social Cognition. Cerebellum, 15(6), 732–743. doi: 10.1007/s12311-0150746-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holahan CJ, Brennan PL, Schutte KK, Holahan CK, Hixon JG, & Moos RH (2017). Late-Life Drinking Problems: The Predictive Roles of Drinking Level vs. Drinking Pattern. J Stud Alcohol Drugs, 78(3), 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer DW, Bjork JM, & Gilman JM (2011). Imaging brain response to reward in addictive disorders. Ann N Y Acad Sci, 1216, 50–61. doi: 10.1111/j.17496632.2010.05898.x [DOI] [PubMed] [Google Scholar]
- Hu S, Ide JS, Zhang S, Sinha R, & Li CS (2015). Conflict anticipation in alcohol dependence - A model-based fMRI study of stop signal task. Neuroimage Clin, 8, 39–50. doi: 10.1016/j.nicl.2015.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mohan A, De Ridder D, Sunaert S, & Vanneste S (2018). The neural correlates of the unified percept of alcohol-related craving: a fMRI and EEG study. Sci Rep, 8(1), 923. doi: 10.1038/s41598-017-18471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt GE, Malhi GS, Cleary M, Lai HM, & Sitharthan T (2016). Comorbidity of bipolar and substance use disorders in national surveys of general populations, 1990–2015: Systematic review and meta-analysis. J Affect Disord, 206, 321–330. doi: 10.1016/j.jad.2016.06.051 [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, . . . Contributors. (2018). NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement, 14(4), 535–562. doi: 10.1016/j.jalz.2018.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knosche TR, & Turner R (2013). White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage, 73, 239–254. doi: 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Jung YC, Schulte T, Muller-Oehring EM, Namkoong K, Pfefferbaum A, & Sullivan EV (2014). Compromised frontocerebellar circuitry contributes to nonplanning impulsivity in recovering alcoholics. Psychopharmacology (Berl), 231(23), 4443–4453. doi: 10.1007/s00213-014-3594-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaag AM, Schulte MHJ, Jansen JM, van Wingen G, Homberg J, van den Brink W, . . . Reneman L (2018). The relation between gray matter volume and the use of alcohol, tobacco, cocaine and cannabis in male polysubstance users. Drug Alcohol Depend, 187, 186–194. doi: 10.1016/j.drugalcdep.2018.03.010 [DOI] [PubMed] [Google Scholar]
- Kalinin S, Gonzalez-Prieto M, Scheiblich H, Lisi L, Kusumo H, Heneka MT, . . . Feinstein DL (2018). Transcriptome analysis of alcohol-treated microglia reveals downregulation of beta amyloid phagocytosis. J Neuroinflammation, 15(1), 141. doi: 10.1186/s12974018-1184-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerchner GA, Berdnik D, Shen JC, Bernstein JD, Fenesy MC, Deutsch GK, . . . Rutt BK (2014). APOE epsilon4 worsens hippocampal CA1 apical neuropil atrophy and episodic memory. Neurology, 82(8), 691–697. doi: 10.1212/WNL.0000000000000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kist N, Sandjojo J, Kok RM, & van den Berg JF (2014). Cognitive functioning in older adults with early, late, and very late onset alcohol dependence. Int Psychogeriatr, 26(11), 1863–1869. doi: 10.1017/S1041610214000878 [DOI] [PubMed] [Google Scholar]
- Koenders L, Lorenzetti V, de Haan L, Suo C, Vingerhoets W, van den Brink W, . . . Cousijn J (2017). Longitudinal study of hippocampal volumes in heavy cannabis users. J Psychopharmacol, 31(8), 1027–1034. doi: 10.1177/0269881117718380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF (2015). The dark side of emotion: the addiction perspective. Eur J Pharmacol, 753, 73–87. doi: 10.1016/j.ejphar.2014.11.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer JH, Schuff N, Reed BR, Mungas D, Du AT, Rosen HJ, . . . Chui HC (2004). Hippocampal volume and retention in Alzheimer’s disease. J Int Neuropsychol Soc, 10(4), 639–643. doi: 10.1017/S1355617704104050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kril JJ, & Halliday GM (1999). Brain shrinkage in alcoholics: a decade on and what have we learned? Prog Neurobiol, 58(4), 381–387. [DOI] [PubMed] [Google Scholar]
- Kril JJ, Halliday GM, Svoboda MD, & Cartwright H (1997). The cerebral cortex is damaged in chronic alcoholics. Neuroscience, 79(4), 983–998. [DOI] [PubMed] [Google Scholar]
- Kuhn S, Charlet K, Schubert F, Kiefer F, Zimmermann P, Heinz A, & Gallinat J (2014). Plasticity of hippocampal subfield volume cornu ammonis 2+3 over the course of withdrawal in patients with alcohol dependence. JAMA Psychiatry, 71(7), 806–811. doi: 10.1001/jamapsychiatry.2014.352 [DOI] [PubMed] [Google Scholar]
- Kupprat SA, Krause KD, Ompad DC, & Halkitis PN (2017). Substance Use and Cognitive Function as Drivers of Condomless Anal Sex Among HIV-Positive Gay, Bisexual, and Other Men Who Have Sex with Men Aged 50 and Older: The Gold Studies. LGBT Health, 4(6), 434–441. doi: 10.1089/lgbt.2016.0163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labhart F, Livingston M, Engels R, & Kuntsche E (2018). After how many drinks does someone experience acute consequences-determining thresholds for binge drinking based on two event-level studies. Addiction. doi: 10.1111/add.14370 [DOI] [PubMed] [Google Scholar]
- Le Berre AP (2019). Social and emotional deficits in individuals with Alcohol Use Disorder. Neuropsychology, in revision. [Google Scholar]
- Le Berre AP, Laniepce A, Segobin S, Pitel AL, & Sullivan EV (2019). Alcohol Use Disorder: Permanent and transient effects on the brain and neuropsychological functions In Stern RA & Alosco M (Eds.), The Oxford Handbook of Adult Cognitive Disorders. New York: Oxford University Press. [Google Scholar]
- Le Berre AP, Muller-Oehring EM, Kwon D, Serventi MR, Pfefferbaum A, & Sullivan EV (2016). Differential compromise of prospective and retrospective metamemory monitoring and their dissociable structural brain correlates. Cortex, 81, 192–202. doi: 10.1016/j.cortex.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Pitel AL, Chanraud S, Beaunieux H, Eustache F, Martinot JL, . . . Pfefferbaum A (2014). Chronic alcohol consumption and its effect on nodes of frontocerebellar and limbic circuitry: comparison of effects in France and the United States. Hum Brain Mapp, 35(9), 4635–4653. doi: 10.1002/hbm.22500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Berre AP, Rauchs G, La Joie R, Segobin S, Mezenge F, Boudehent C, . . . Beaunieux H (2013). Readiness to change and brain damage in patients with chronic alcoholism. Psychiatry Res, 213(3), 202–209. doi: 10.1016/j.pscychresns.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Le Berre AP, & Sullivan EV (2016). Anosognosia for Memory Impairment in Addiction: Insights from Neuroimaging and Neuropsychological Assessment of Metamemory. Neuropsychol Rev, 26(4), 420–431. doi: 10.1007/s11065-016-9323-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lear JT, Migden MR, Lewis KD, Chang ALS, Guminski A, Gutzmer R, . . . Dummer R (2018). Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J Eur Acad Dermatol Venereol, 32(3), 372–381. doi: 10.1111/jdv.14542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Im SJ, Lee SG, Stadlin A, Son JW, Shin CJ, . . . Kim S (2016). Volume of hippocampal subfields in patients with alcohol dependence. Psychiatry Res Neuroimaging, 258, 16–22. doi: 10.1016/j.pscychresns.2016.10.009 [DOI] [PubMed] [Google Scholar]
- Lhermitte J (1934). Cortical cerebellar degeneration. Proceedings of the Royal Society of Medicine, 28, 379–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liappas I, Theotoka I, Kapaki E, Ilias I, Paraskevas GP, & Soldatos CR (2007). Neuropsychological assessment of cognitive function in chronic alcohol-dependent patients and patients with Alzheimer’s disease. In Vivo, 21(6), 1115–1118. [PubMed] [Google Scholar]
- Littleton J, Barron S, Prendergast M, & Nixon SJ (2007). Smoking kills (alcoholics)! shouldn’t we do something about it? Alcohol Alcohol, 42(3), 167–173. doi: 10.1093/alcalc/agm019 [DOI] [PubMed] [Google Scholar]
- Loijen A, Rinck M, Walvoort SJW, Kessels RPC, Becker ES, & Egger JIM (2018). Modification of Automatic Alcohol-Approach Tendencies in Alcohol-Dependent Patients with Mild or Major Neurocognitive Disorder. Alcohol Clin Exp Res, 42(1), 153–161. doi: 10.1111/acer.13529 [DOI] [PubMed] [Google Scholar]
- Lorenzetti V, Solowij N, Whittle S, Fornito A, Lubman DI, Pantelis C, & Yucel M (2015). Gross morphological brain changes with chronic, heavy cannabis use. Br J Psychiatry, 206(1), 77–78. doi: 10.1192/bjp.bp.114.151407 [DOI] [PubMed] [Google Scholar]
- Makris N, Oscar-Berman M, Jaffin SK, Hodge SM, Kennedy DN, Caviness VS, . . . Harris GJ (2008). Decreased volume of the brain reward system in alcoholism. Biol Psychiatry, 64(3), 192–202. doi: 10.1016/j.biopsych.2008.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malbergier A, Amaral RA, & Cardoso LD (2015). Alcohol dependence and CD4 cell count: is there a relationship? AIDS Care, 27(1), 54–58. doi: 10.1080/09540121.2014.947235 [DOI] [PubMed] [Google Scholar]
- Mann K, Gunther A, Stetter F, & Ackermann K (1999). Rapid recovery from cognitive deficits in abstinent alcoholics: a controlled test-retest study. Alcohol Alcohol, 34(4), 567–574. [DOI] [PubMed] [Google Scholar]
- Martin E, Gonzalez R, Vassileva J, Maki PM, Bechara A, & Brand M (2016). Sex and HIV serostatus differences in decision making under risk among substance-dependent individuals. J Clin Exp Neuropsychol, 38(4), 404–415. doi: 10.1080/13803395.2015.1119806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascarenhas JV, & Jude EB (2014). Central pontine myelinolysis: electrolytes and beyond. BMJ Case Rep, 2014. doi: 10.1136/bcr-2013-203516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCorkindale AN, Sizemova A, Sheedy D, Kril JJ, & Sutherland GT (2019). Reinvestigating the effects of chronic smoking on the pathology of alcohol-related human brain damage. Alcohol, 76, 11–14. doi: 10.1016/j.alcohol.2018.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlinchey RE, Fortier CB, Capozzi SM, & Disterhoft JF (2005). Trace eyeblink conditioning in abstinent alcoholic individuals: effects of complex task demands and prior conditioning. Neuropsychology, 19(2), 159–170. doi: 10.1037/0894-4105.19.2.159 [DOI] [PubMed] [Google Scholar]
- Mon A, Durazzo TC, Gazdzinski S, & Meyerhoff DJ (2009). The impact of chronic cigarette smoking on recovery from cortical gray matter perfusion deficits in alcohol dependence: longitudinal arterial spin labeling MRI. Alcohol Clin Exp Res, 33(8), 1314–1321. doi: 10.1111/j.1530-0277.2009.00960.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA (2017). Immune activation and neuroinflammation in alcohol use and HIV infection: evidence for shared mechanisms. Am J Drug Alcohol Abuse, 43(1), 7–23. doi: 10.1080/00952990.2016.1211667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnig MA, Kahler CW, Lee H, Pantalone DW, Mayer KH, Cohen RA, & Monti PM (2016). Effects of smoking and alcohol use on neurocognitive functioning in heavy drinking, HIV-positive men who have sex with men. AIDS Care, 28(3), 300–305. doi: 10.1080/09540121.2015.1093595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BA, Lim KO, Hemmy L, & Camchong J (2015). Diffusion MRI and its Role in Neuropsychology. Neuropsychol Rev, 25(3), 250–271. doi: 10.1007/s11065-015-9291-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Chao LL, Berman B, & Weiner MW (2011). Evidence for functional specialization of hippocampal subfields detected by MR subfield volumetry on high resolution images at 4 T. Neuroimage, 56(3), 851–857. doi: 10.1016/j.neuroimage.2011.03.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Pfefferbaum A, Sullivan EV, & Schulte T (2015). The Resting Brain of Alcoholics. Cereb Cortex, 25(11), 4155–4168. doi: 10.1093/cercor/bhu134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Oehring EM, Jung YC, Sullivan EV, Hawkes WC, Pfefferbaum A, & Schulte T (2013). Midbrain-driven emotion and reward processing in alcoholism. Neuropsychopharmacology, 38(10), 1844–1853. doi: 10.1038/npp.2013.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumford JA, Poldrack R, & Nichols TE (2011). Handbook of Functional MRI Data Analysis. Cambridge: Cambridge University Press. [Google Scholar]
- Nabi E, Thacker LR, Wade JB, Sterling RK, Stravitz RT, Fuchs M, . . . Bajaj JS (2014). Diagnosis of covert hepatic encephalopathy without specialized tests. Clin Gastroenterol Hepatol, 12(8), 1384–1389 e1382. doi: 10.1016/j.cgh.2013.12.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naim-Feil J, Fitzgerald PB, Bradshaw JL, Lubman DI, & Sheppard D (2014). Neurocognitive deficits, craving, and abstinence among alcohol-dependent individuals following detoxification. Arch Clin Neuropsychol, 29(1), 26–37. doi: 10.1093/arclin/act090 [DOI] [PubMed] [Google Scholar]
- Nakamura-Palacios EM, Souza RS, Zago-Gomes MP, de Melo AM, Braga FS, Kubo TT, & Gasparetto EL (2014). Gray matter volume in left rostral middle frontal and left cerebellar cortices predicts frontal executive performance in alcoholic subjects. Alcohol Clin Exp Res, 38(4), 1126–1133. doi: 10.1111/acer.12308 [DOI] [PubMed] [Google Scholar]
- Nolan S, Walley AY, Heeren TC, Patts GJ, Ventura AS, Sullivan MM, . . . Saitz R (2017). HIV-infected individuals who use alcohol and other drugs, and virologic suppression. AIDS Care, 29(9), 1129–1136. doi: 10.1080/09540121.2017.1327646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RK, Yeung LK, Noly-Gandon A, D’Angelo MC, Kacollja A, Smith VM, . . . Barense MD (2017). Human anterolateral entorhinal cortex volumes are associated with cognitive decline in aging prior to clinical diagnosis. Neurobiol Aging, 57, 195–205. doi: 10.1016/j.neurobiolaging.2017.04.025 [DOI] [PubMed] [Google Scholar]
- Oscar-Berman M, Valmas MM, Sawyer KS, Ruiz SM, Luhar RB, & Gravitz ZR (2014). Profiles of impaired, spared, and recovered neuropsychologic processes in alcoholism. Handb Clin Neurol, 125, 183–210. doi: 10.1016/B978-0-444-62619-6.00012-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin D, Atkinson RM, Smith DM, & Hendrie H (1998). Alcohol related dementia: proposed clinical criteria. Int J Geriatr Psychiatry, 13(4), 203–212. [DOI] [PubMed] [Google Scholar]
- Ostby Y, Tamnes CK, Fjell AM, & Walhovd KB (2012). Dissociating memory processes in the developing brain: the role of hippocampal volume and cortical thickness in recall after minutes versus days. Cereb Cortex, 22(2), 381–390. doi: 10.1093/cercor/bhr116 [DOI] [PubMed] [Google Scholar]
- Paller KA, Acharya A, Richardson BC, Plaisant O, Shimamura AP, Reed BR, & Jagust WJ (1997). Functional Neuroimaging of Cortical Dysfunction in Alcoholic Korsakoff’s Syndrome. J Cogn Neurosci, 9(2), 277–293. doi: 10.1162/jocn.1997.9.2.277 [DOI] [PubMed] [Google Scholar]
- Paolillo EW, Gongvatana A, Umlauf A, Letendre SL, & Moore DJ (2017). At-Risk Alcohol Use is Associated with Antiretroviral Treatment Nonadherence Among Adults Living with HIV/AIDS. Alcohol Clin Exp Res, 41(8), 1518–1525. doi: 10.1111/acer.13433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker TD, Slattery CF, Yong KXX, Nicholas JM, Paterson RW, Foulkes AJM, . . . Schott JM (2018). Differences in hippocampal subfield volume are seen in phenotypic variants of early onset Alzheimer’s disease. Neuroimage Clin, 101632. doi: 10.1016/j.nicl.2018.101632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennington DL, Durazzo TC, Schmidt TP, Abe C, Mon A, & Meyerhoff DJ (2015). Alcohol use disorder with and without stimulant use: brain morphometry and its associations with cigarette smoking, cognition, and inhibitory control. PLoS One, 10(3), e0122505. doi: 10.1371/journal.pone.0122505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit G, Luminet O, Cordovil de Sousa Uva M, Zorbas A, Maurage P, & de Timary P (2017). Differential spontaneous recovery across cognitive abilities during detoxification period in alcohol-dependence. PLoS One, 12(8), e0176638. doi: 10.1371/journal.pone.0176638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Chanraud S, Pitel AL, Shankaranarayanan A, Alsop DC, Rohlfing T, & Sullivan EV (2010). Volumetric cerebral perfusion imaging in healthy adults: regional distribution, laterality, and repeatability of pulsed continuous arterial spin labeling (PCASL). Psychiatry Res, 182(3), 266–273. doi: 10.1016/j.pscychresns.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, & Sullivan EV (2001). Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. Neuroimage, 14(1 Pt 1), 7–20. doi: 10.1006/nimg.2001.0785 [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Kwon D, Brumback T, Thompson WK, Cummins K, Tapert SF, . . . Sullivan EV (2018). Altered Brain Developmental Trajectories in Adolescents After Initiating Drinking. Am J Psychiatry, 175(4), 370–380. doi: 10.1176/appi.ajp.2017.17040469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Crusan K, & Jernigan TL (1988). Brain CT changes in alcoholics: effects of age and alcohol consumption. Alcohol Clin Exp Res, 12(1), 81–87. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom M, Rohlfing T, & Sullivan EV (2009). Degradation of association and projection white matter systems in alcoholism detected with quantitative fiber tracking. Biol Psychiatry, 65(8), 680–690. doi: 10.1016/j.biopsych.2008.10.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Rosenbloom MJ, Chu W, Sassoon SA, Rohlfing T, Pohl KM, . . . Sullivan EV (2014). White matter microstructural recovery with abstinence and decline with relapse in alcohol dependence interacts with normal ageing: a controlled longitudinal DTI study. Lancet Psychiatry, 1(3), 202–212. doi: 10.1016/S22150366(14)70301-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, & Lim KO (1997). Frontal lobe volume loss observed with magnetic resonance imaging in older chronic alcoholics. Alcohol Clin Exp Res, 21(3), 521–529. doi: 10.1111/j.1530-0277.1997.tb03798.x [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Mathalon DH, Shear PK, Rosenbloom MJ, & Lim KO (1995). Longitudinal changes in magnetic resonance imaging brain volumes in abstinent and relapsed alcoholics. Alcohol Clin Exp Res, 19(5), 1177–1191. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Zahr NM, Sassoon SA, Kwon D, Pohl KM, & Sullivan EV (2018). Accelerating and Premature Aging Characterizing Regional Cortical Volume Loss in Human Immunodeficiency Virus Infection: Contributions from Alcohol, Substance Use, and Hepatitis C Co-Infection. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, Epub 13 August 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Beaunieux H, Witkowski T, Vabret F, Guillery-Girard B, Quinette P, . . . Eustache F (2007). Genuine episodic memory deficits and executive dysfunctions in alcoholic subjects early in abstinence. Alcohol Clin Exp Res, 31(7), 1169–1178. doi: 10.1111/j.1530-0277.2007.00418.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitel AL, Segobin SH, Ritz L, Eustache F, & Beaunieux H (2015). Thalamic abnormalities are a cardinal feature of alcohol-related brain dysfunction. Neurosci Biobehav Rev, 54, 38–45. doi: 10.1016/j.neubiorev.2014.07.023 [DOI] [PubMed] [Google Scholar]
- Pitel AL, Zahr NM, Jackson K, Sassoon SA, Rosenbloom MJ, Pfefferbaum A, & Sullivan EV (2011). Signs of preclinical Wernicke’s encephalopathy and thiamine levels as predictors of neuropsychological deficits in alcoholism without Korsakoff’s syndrome. Neuropsychopharmacology, 36(3), 580–588. doi: 10.1038/npp.2010.189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontecorvo MJ, Devous MD Sr., Navitsky M, Lu M, Salloway S, Schaerf FW, . . . investigators, F. A.-A. (2017). Relationships between flortaucipir PET tau binding and amyloid burden, clinical diagnosis, age and cognition. Brain, 140(3), 748–763. doi: 10.1093/brain/aww334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaglino V, De Wever E, & Maurage P (2015). Relations Between Cognitive Abilities, Drinking Characteristics, and Emotional Recognition in Alcohol Dependence: A Preliminary Exploration. Alcohol Clin Exp Res, 39(10), 2032–2038. doi: 10.1111/acer.12841 [DOI] [PubMed] [Google Scholar]
- Rensen YC, Oosterman JM, van Damme JE, Griekspoor SI, Wester AJ, Kopelman MD, & Kessels RP (2015). Assessment of Confabulation in Patients with Alcohol-Related Cognitive Disorders: The Nijmegen-Venray Confabulation List (NVCL-20). Clin Neuropsychol, 29(6), 804–823. doi: 10.1080/13854046.2015.1084377 [DOI] [PubMed] [Google Scholar]
- Ribeiro F, Guerreiro M, & De Mendonca A (2007). Verbal learning and memory deficits in Mild Cognitive Impairment. J Clin Exp Neuropsychol, 29(2), 187–197. doi: 10.1080/13803390600629775 [DOI] [PubMed] [Google Scholar]
- Richard EL, Kritz-Silverstein D, Laughlin GA, Fung TT, Barrett-Connor E, & McEvoy LK (2017). Alcohol Intake and Cognitively Healthy Longevity in Community-Dwelling Adults: The Rancho Bernardo Study. J Alzheimers Dis, 59(3), 803–814. doi: 10.3233/JAD-161153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley NJ, Draper B, & Withall A (2013). Alcohol-related dementia: an update of the evidence. Alzheimers Res Ther, 5(1), 3. doi: 10.1186/alzrt157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz L, Segobin S, Le Berre AP, Lannuzel C, Boudehent C, Vabret F, . . . Beaunieux H (2014). Brain structural substrates of cognitive procedural learning in alcoholic patients early in abstinence. Alcohol Clin Exp Res, 38(8), 2208–2216. doi: 10.1111/acer.12486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbloom MJ, Pfefferbaum A, & Sullivan EV (2004). Recovery of short-term memory and psychomotor speed but not postural stability with long-term sobriety in alcoholic women. Neuropsychology, 18(3), 589–597. doi: 10.1037/0894-4105.18.3.589 [DOI] [PubMed] [Google Scholar]
- Sachdeva A, Chandra M, Choudhary M, Dayal P, & Anand KS (2016). Alcohol-Related Dementia and Neurocognitive Impairment: A Review Study. Int J High Risk Behav Addict, 5(3), e27976. doi: 10.5812/ijhrba.27976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha TD, Grant BF, Chou SP, Kerridge BT, Pickering RP, & Ruan WJ (2018). Concurrent use of alcohol with other drugs and DSM-5 alcohol use disorder comorbid with other drug use disorders: Sociodemographic characteristics, severity, and psychopathology. Drug Alcohol Depend, 187, 261–269. doi: 10.1016/j.drugalcdep.2018.03.006 [DOI] [PubMed] [Google Scholar]
- Schacht JP, Hutchison KE, & Filbey FM (2012). Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology, 37(11), 2368–2376. doi: 10.1038/npp.2012.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt KS, Gallo JL, Ferri C, Giovannetti T, Sestito N, Libon DJ, & Schmidt PS (2005). The neuropsychological profile of alcohol-related dementia suggests cortical and subcortical pathology. Dement Geriatr Cogn Disord, 20(5), 286–291. doi: 10.1159/000088306 [DOI] [PubMed] [Google Scholar]
- Schmidt TP, Pennington DL, Cardoos SL, Durazzo TC, & Meyerhoff DJ (2017). Neurocognition and inhibitory control in polysubstance use disorders: Comparison with alcohol use disorders and changes with abstinence. J Clin Exp Neuropsychol, 39(1), 22–34. doi: 10.1080/13803395.2016.1196165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt TP, Pennington DL, Durazzo TC, & Meyerhoff DJ (2014). Postural stability in cigarette smokers and during abstinence from alcohol. Alcohol Clin Exp Res, 38(6), 17531760. doi: 10.1111/acer.12409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Tapert S, Matthews SC, Paulus MP, Tolentino NJ, Smith TL, . . . Simmons A (2012). fMRI differences between subjects with low and high responses to alcohol during a stop signal task. Alcohol Clin Exp Res, 36(1), 130–140. doi: 10.1111/j.1530-0277.2011.01590.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Jung YC, Sullivan EV, Pfefferbaum A, Serventi M, & Muller-Oehring EM (2017). The neural correlates of priming emotion and reward systems for conflict processing in alcoholics. Brain Imaging Behav, 11(6), 1751–1768. doi: 10.1007/s11682016-9651-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte T, Sullivan EV, Muller-Oehring EM, Adalsteinsson E, & Pfefferbaum A (2005). Corpus callosal microstructural integrity influences interhemispheric processing: a diffusion tensor imaging study. Cereb Cortex, 15(9), 1384–1392. doi: 10.1093/cercor/bhi020 [DOI] [PubMed] [Google Scholar]
- Schwarzinger M, Pollock BG, Hasan OSM, Dufouil C, Rehm J, & QalyDays Study G (2018). Contribution of alcohol use disorders to the burden of dementia in France 200813: a nationwide retrospective cohort study. Lancet Public Health, 3(3), e124–e132. doi: 10.1016/S2468-2667(18)30022-7 [DOI] [PubMed] [Google Scholar]
- Sebold M, Nebe S, Garbusow M, Guggenmos M, Schad DJ, Beck A, . . . Heinz A (2017). When Habits Are Dangerous: Alcohol Expectancies and Habitual Decision Making Predict Relapse in Alcohol Dependence. Biol Psychiatry, 82(11), 847–856. doi: 10.1016/j.biopsych.2017.04.019 [DOI] [PubMed] [Google Scholar]
- Seo D, Lacadie CM, Tuit K, Hong KI, Constable RT, & Sinha R (2013). Disrupted ventromedial prefrontal function, alcohol craving, and subsequent relapse risk. JAMA Psychiatry, 70(7), 727–739. doi: 10.1001/jamapsychiatry.2013.762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Mohr J, Beck A, Wustenberg T, Heinz A, & Obermayer K (2015). Predicting the future relapse of alcohol-dependent patients from structural and functional brain images. Addict Biol, 20(6), 1042–1055. doi: 10.1111/adb.12302 [DOI] [PubMed] [Google Scholar]
- Shokri-Kojori E, Tomasi D, Alipahahi B, Wiers CE, Wang G-J, & Volkow N (2019). Correspondence between cerebral glucose metabolism and BOLD reveals relative power and cost in human brain. Nature Communications, 10(1), 690. doi: 10.1038/s41467-01908546-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon L, Siggins R, Winsauer P, Brashear M, Ferguson T, Mercante D, . . . Molina PE (2018). Simian Immunodeficiency Virus Infection Increases Blood Ethanol Concentration Duration After Both Acute and Chronic Administration. AIDS Res Hum Retroviruses, 34(2), 178–184. doi: 10.1089/AID.2017.0195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, de Wit S, van den Brink W, Robbins TW, Beekman AT, Penninx BW, & Veltman DJ (2013). Behavioral and neuroimaging evidence for overreliance on habit learning in alcohol-dependent patients. Transl Psychiatry, 3, e337. doi: 10.1038/tp.2013.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjoerds Z, Stufflebeam SM, Veltman DJ, Van den Brink W, Penninx BW, & Douw L (2017). Loss of brain graph network efficiency in alcohol dependence. Addict Biol, 22(2), 523–534. doi: 10.1111/adb.12346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stavro K, Pelletier J, & Potvin S (2013). Widespread and sustained cognitive deficits in alcoholism: a meta-analysis. Addict Biol, 18(2), 203–213. doi: 10.1111/j.13691600.2011.00418.x [DOI] [PubMed] [Google Scholar]
- Stephan RA, Alhassoon OM, Allen KE, Wollman SC, Hall M, Thomas WJ, . . . Grant I (2017). Meta-analyses of clinical neuropsychological tests of executive dysfunction and impulsivity in alcohol use disorder. Am J Drug Alcohol Abuse, 43(1), 24–43. doi: 10.1080/00952990.2016.1206113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV (2003). Compromised pontocerebellar and cerebellothalamocortical systems: speculations on their contributions to cognitive and motor impairment in nonamnesic alcoholism. Alcohol Clin Exp Res, 27(9), 1409–1419. doi: 10.1097/01.ALC.0000085586.91726.46 [DOI] [PubMed] [Google Scholar]
- Sullivan EV (2017). Contributions to Understanding the Neuropsychology of Alcoholism: An INS Legacy. J Int Neuropsychol Soc, 23(9–10), 843–859. doi: 10.1017/S1355617717000674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Deshmukh A, Desmond JE, Lim KO, & Pfefferbaum A (2000). Cerebellar volume decline in normal aging, alcoholism, and Korsakoff’s syndrome: relation to ataxia. Neuropsychology, 14(3), 341–352. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, & Marsh L (2003). Hippocampal volume deficits in alcoholic Korsakoff’s syndrome. Neurology, 61(12), 1716–1719. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Muller-Oehring E, Pitel AL, Chanraud S, Shankaranarayanan A, Alsop DC, . . . Pfefferbaum A (2013). A selective insular perfusion deficit contributes to compromised salience network connectivity in recovering alcoholic men. Biol Psychiatry, 74(7), 547–555. doi: 10.1016/j.biopsych.2013.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2005). Neurocircuitry in alcoholism: a substrate of disruption and repair. Psychopharmacology (Berl), 180(4), 583–594. doi: 10.1007/s00213-005-22676 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (2009). Neuroimaging of the Wernicke-Korsakoff syndrome. Alcohol Alcohol, 44(2), 155–165. doi: 10.1093/alcalc/agn103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, & Pfefferbaum A (Eds.). (2014). Alcohol and the Nervous System (Vol. 125). New York: Elsevier. [Google Scholar]
- Sullivan EV, Rose J, & Pfefferbaum A (2006). Effect of vision, touch and stance on cerebellar vermian-related sway and tremor: a quantitative physiological and MRI study. Cereb Cortex, 16(8), 1077–1086. doi: 10.1093/cercor/bhj048 [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Rohlfing T, & Pfefferbaum A (2015). Cognitive demands during quiet standing elicit truncal tremor in two frequency bands: differential relations to tissue integrity of corticospinal tracts and cortical targets. Front Hum Neurosci, 9, 175. doi: 10.3389/fnhum.2015.00175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, & Pfefferbaum A (2018a). Enduring Cortical Compromise in Alcoholism: Accelerated by Aging and Compounded by Drug Dependence and Hepatitis C Comorbidity. Journal of the American Medical Association, in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Zahr NM, Sassoon SA, Thompson WK, Kwon D, Pohl KM, & Pfefferbaum A (2018b). The Role of Aging, Drug Dependence, and Hepatitis C Comorbidity in Alcoholism Cortical Compromise. JAMA Psychiatry, 75(5), 474–483. doi: 10.1001/jamapsychiatry.2018.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan GE, & Lessov-Schlaggar CN (2007). The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol Rev, 17(3), 259–273. doi: 10.1007/s11065-0079035-9 [DOI] [PubMed] [Google Scholar]
- Tanabe J, Tregellas JR, Dalwani M, Thompson L, Owens E, Crowley T, & Banich M (2009). Medial orbitofrontal cortex gray matter is reduced in abstinent substance-dependent individuals. Biol Psychiatry, 65(2), 160–164. doi: 10.1016/j.biopsych.2008.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, & Courtney K (2019). Cognitive and emotional deficits associated with alcohol use in adolescence and young adults. Neuropsychology, in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RG Jr., Eaton NR, Hu MC, Grant BF, & Hasin DS (2014). Regularly drinking alcohol before sex in the United States: effects of relationship status and alcohol use disorders. Drug Alcohol Depend, 141, 167–170. doi: 10.1016/j.drugalcdep.2014.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AD, Guerrini I, & Marshall EJ (2012). The evolution and treatment of Korsakoff’s syndrome: out of sight, out of mind? Neuropsychol Rev, 22(2), 81–92. doi: 10.1007/s11065-012-9196-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topiwala A, Allan CL, Valkanova V, Zsoldos E, Filippini N, Sexton C, . . . Ebmeier KP (2017). Moderate alcohol consumption as risk factor for adverse brain outcomes and cognitive decline: longitudinal cohort study. BMJ, 357, j2353. doi: 10.1136/bmj.j2353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torvik A (1991). Wernicke’s encephalopathy--prevalence and clinical spectrum. Alcohol Alcohol Suppl, 1, 381–384. [PubMed] [Google Scholar]
- Trick L, Kempton MJ, Williams SC, & Duka T (2014). Impaired fear recognition and attentional set-shifting is associated with brain structural changes in alcoholic patients. Addict Biol, 19(6), 1041–1054. doi: 10.1111/adb.12175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungerleider LG, Courtney SM, & Haxby JV (1998). A neural system for human visual working memory. Proc Natl Acad Sci U S A, 95(3), 883–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valmas MM, Mosher Ruiz S, Gansler DA, Sawyer KS, & Oscar-Berman M (2014). Social cognition deficits and associations with drinking history in alcoholic men and women. Alcohol Clin Exp Res, 38(12), 2998–3007. doi: 10.1111/acer.12566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Nes JA, Nafe R, & Schlote W (2008). Non-tau based neuronal degeneration in Alzheimer’s disease -- an immunocytochemical and quantitative study in the supragranular layers of the middle temporal neocortex. Brain Res, 1213, 152–165. doi: 10.1016/j.brainres.2008.03.043 [DOI] [PubMed] [Google Scholar]
- Vedder LC, Hall JM, Jabrouin KR, & Savage LM (2015). Interactions between chronic ethanol consumption and thiamine deficiency on neural plasticity, spatial memory, and cognitive flexibility. Alcohol Clin Exp Res, 39(11), 2143–2153. doi: 10.1111/acer.12859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkataraman A, Kalk N, Sewell G, Ritchie CW, & Lingford-Hughes A (2017). Alcohol and Alzheimer’s Disease-Does Alcohol Dependence Contribute to Beta-Amyloid Deposition, Neuroinflammation and Neurodegeneration in Alzheimer’s Disease? Alcohol Alcohol, 52(2), 151–158. doi: 10.1093/alcalc/agw092 [DOI] [PubMed] [Google Scholar]
- Vergara VM, Liu J, Claus ED, Hutchison K, & Calhoun V (2017). Alterations of resting state functional network connectivity in the brain of nicotine and alcohol users. Neuroimage, 151, 45–54. doi: 10.1016/j.neuroimage.2016.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor M, Adams RD, & Collins GH (1989). The Wernicke-Korsakoff Syndrome and Related Neurological Disorders Due to Alcoholism and Malnutrition (2nd ed.). Philadelphia: F.A. Davis Company. [Google Scholar]
- Walvoort SJ, van der Heijden PT, Wester AJ, Kessels RP, & Egger JI (2016). Self-awareness of cognitive dysfunction: Self-reported complaints and cognitive performance in patients with alcohol-induced mild or major neurocognitive disorder. Psychiatry Res, 245, 291–296. doi: 10.1016/j.psychres.2016.08.007 [DOI] [PubMed] [Google Scholar]
- Wollenweber FA, Halfter S, Brugmann E, Weinberg C, Cieslik EC, Muller VI, . . . Eickhoff SB (2014). Subtle cognitive deficits in severe alcohol addicts--do they show a specific profile? J Neuropsychol, 8(1), 147–153. doi: 10.1111/jnp.12001 [DOI] [PubMed] [Google Scholar]
- Wood AM, Kaptoge S, Butterworth AS, Willeit P, Warnakula S, Bolton T, . . . Emerging Risk Factors Collaboration, E.-C. V. D. U. K. B. A. S. G. (2018). Risk thresholds for alcohol consumption: combined analysis of individual-participant data for 599 912 current drinkers in 83 prospective studies. Lancet, 391(10129), 1513–1523. doi: 10.1016/S01406736(18)30134-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods AJ, Porges EC, Bryant VE, Seider T, Gongvatana A, Kahler CW, . . . Cohen RA (2016). Current Heavy Alcohol Consumption is Associated with Greater Cognitive Impairment in Older Adults. Alcohol Clin Exp Res, 40(11), 2435–2444. doi: 10.1111/acer.13211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2018). Global status report on alcohol and health 2018. (ISBN 97892–4-156563–9). Geneva: World Health Organization. [Google Scholar]
- Wyczechowska D, Lin HY, LaPlante A, Jeansonne D, Lassak A, Parsons CH, . . . Peruzzi F (2017). A miRNA Signature for Cognitive Deficits and Alcohol Use Disorder in Persons Living with HIV/AIDS. Front Mol Neurosci, 10, 385. doi: 10.3389/fnmol.2017.00385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM (2014). Structural and microstructral imaging of the brain in alcohol use disorders. Handb Clin Neurol, 125, 275–290. doi: 10.1016/B978-0-444-62619-6.00017-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM (2018a). The Aging Brain With HIV Infection: Effects of Alcoholism or Hepatitis C Comorbidity. Front Aging Neurosci, 10, 56. doi: 10.3389/fnagi.2018.00056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM (2018b). Peripheral TNF-alpha elevations in abstinent alcoholics are associated with hepatitis C infection. PLoS One, 13(2), e0191586. doi: 10.1371/journal.pone.0191586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, & Pfefferbaum A (2017). Alcohol’s Effects on the Brain: Neuroimaging Results in Humans and Animal Models. Alcohol Res, 38(2), 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pfefferbaum A, & Sullivan EV (2017). Perspectives on fronto-fugal circuitry from human imaging of alcohol use disorders. Neuropharmacology, 122, 189–200. doi: 10.1016/j.neuropharm.2017.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Pohl KM, Saranathan M, Sullivan EV, & Pfefferbaum A (2019). Hippocampal subfield CA2+3 exhibits accelerated aging in AUD. NeuroImage: Clinical, in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahr NM, Sullivan EV, Rohlfing T, Mayer D, Collins AM, Luong R, & Pfefferbaum A (2016). Concomitants of alcoholism: differential effects of thiamine deficiency, liver damage, and food deprivation on the rat brain in vivo. Psychopharmacology (Berl), 233(14), 2675–2686. doi: 10.1007/s00213-016-4313-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, & Constable RT (2017). Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. Neuroimage Clin, 13, 181–187. doi: 10.1016/j.nicl.2016.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]