Abstract
Recent advances in high throughput RNA sequencing have revealed that, in addition to messenger RNAs (mRNAs), long non-coding RNAs (lncRNAs) play an important role in the regulation of many cell functions and of organ development. While a number of lncRNAs have been identified in pancreatic islets, their function remains largely undetermined. Here, we identify a novel long non-coding RNA regulated by the transcription factor GLIS3, which we refer to as GLIS3 Regulated 1 (G3R1). This lncRNA was identified for its significant loss of expression in GLIS3 knockout mouse pancreatic islets. G3R1 appears to be specifically expressed in mouse pancreatic β-cells and in a β-cell line (βTC-6). ChIP-Seq analysis indicated that GLIS3 and other islet-enriched transcription factors bind near the G3R1 gene, suggesting they directly regulate G3R1 transcription. Similarly, an apparent human homolog of G3R1 displays a similar expression pattern, with additional expression seen in human brain. In order to determine the function of G3R1 in mouse pancreatic β-cells, we utilized CRISPR to develop a knockout mouse where ~80% of G3R1 sequence is deleted. Phenotypic analysis of these mice did not reveal any impairment in β-cell function or glucose regulation, indicating the complexity underlying the study of lncRNA function.
Keywords: GLIS3, lncRNA, pancreas, islets, beta-cells
Introduction
Long non-coding RNAs (lncRNAs) make up a subset of non-coding RNA of >200bp that do not code for protein. They are transcribed similar to mRNAs, with enhancers/promoters bound by transcription factors leading to recruitment of RNA polymerase II. LncRNAs can have a variety of roles within the cell, including nuclear regulation of transcription and mRNA stability, and cytoplasmic regulation of a microRNA, translation, or other functions (Chen, 2016). The expression of lncRNAs have increasingly been linked to a variety of human diseases, including both Type 1 and Type 2 Diabetes (Akerman et al., 2017, Motterle et al., 2015, Moran et al., 2012, Motterle et al., 2017). For instance, Single Nucleotide Polymorphisms (SNPs) that have been located near lncRNAs, but not to adjacent protein coding genes, have been associated with Type 2 Diabetes (Moran et al., 2012). Other studies have suggested that lncRNAs could be used as biomarkers for diabetes (Carter et al., 2015, de Gonzalo-Calvo et al., 2016). However, few lncRNA have been examined beyond expression levels during disease states, and their regulation remains poorly understood.
One method of identifying novel lncRNA with a potential role in organ development is through their regulation by tissue-specific transcription factors. Within the pancreas, the C2H2 zinc finger transcription factor GLIS3 regulates many genes involved in β-cell function (Scoville et al., 2019, Kang et al., 2009, Kim et al., 2012, ZeRuth et al., 2013). While many of these genes encode proteins with known functions, other genes regulated by GLIS3 have no known function. One of the most highly downregulated genes in pancreatic islets of GLIS3 knockout mice was B830017H08Rik (G3R1), a novel gene with unknown function. Interestingly, this gene had been annotated as a long non-coding RNA, with low probability of protein coding. We therefore examined the expression of this gene and its regulation by GLIS3. Our data demonstrate that G3R1 expression in mouse is highly specific for pancreatic β cells and indicates that its transcription is directly regulated by GLIS3. This tissue specific expression pattern could prove a useful biomarker for functional β-cells. In an attempt to identify the biological function of G3R1, a knockout mouse was generated; however, study of these mouse did not reveal any phenotypes.
Materials and Methods
RNA-seq and ChIP-seq data.
Mouse RNA-seq analysis on Glis3Δpanc mice and ChIP-seq analysis were performed previously (Scoville et al., 2019). Briefly, pancreatic islets from 4-week old Glis3Δpanc and control littermates were isolated and RNA analyzed. For ChIP-seq, raw data for mouse were obtained from the Gene Expression omnibus for GLIS3 (GSE122120), NKX2.2 (GSE79785), MAFA and NEUROD1 (GSE30298), NKX6.1 (GSE40975), ISL1 and LDB1 (GSE84759); from SRA for: PDX1 and FOXA2 (SRA008281); and from ArrayExpress for: PDX1 (E-MTAB-1143). Raw data from human were obtained from Array Express (E-MTAB-1919). For human, due to duplication of the lncRNA and surrounding region, reads were aligned against the hg38 reference assembly via Bowtie v1.2.2 with the following parameters: -a -m 3 --best --strata (Langmead et al., 2009). Duplicate reads were removed with MarkDuplicates.jar from the Picard tool suite v2.18.25, and independent experiments merged. For visualization of mapped read depth on the UCSC Genome Browser, uniquely-mapped non-duplicate reads were extended to 200nt, converted to bedGraph format using BEDTools v2.24.0 genomeCoverageBed, and converted to bigwig using bedGraphToBigWig (Quinlan and Hall, 2010).
qRT-PCR analysis of gene expression.
Mouse tissue and cell line RNA were isolated from wild type C57BL/6J or leptin receptor deficient mice (db/db, JAX #000697), and RNA extracted with either Trizol LS (Life tech, Waltham, MA) or RNeasy mini kit (Qiagen, Venlo, Netherlands), with DNAse I digestion following the manufacturers protocol. For human tissue RNA, the Human Total RNA Master Panel II (#636643, Clontech, Mountain View, CA), which contains pooled human tissues, was utilized. βTC-6 cells (ATCC CRL-11506) and EndoC-βH1 cells (Univercell Biosolutions, Toulouse, France) were obtained commercially. 100–1000 ng of RNA was reverse transcribed with a High Capacity DNA Reverse Transcription Kit (Applied Biosystems; Foster City, CA). qRT-PCR was carried out in triplicate in a StepOnePlus Real Time PCR System (Applied Biosystems; Foster City, CA). All results were normalized to 18S expression using the 2ΔΔct method. Primer sequences are listed in Supplementary Table 1.
In situ detection of RNA.
Isolated mouse pancreas was fixed with 4% paraformaldehyde in PBS overnight, and embedded in OCT for cryosectioning. RNAscope in situ hybridization (ACD, Newark, CA) was used following manufacturer’s protocol, using the multiplex fluorescence assay (Catalog # 320850). Probes for mouse G3R1 (B830017H08Rik) and Ins2 are available from ACD (Catalog # 474671 and 497811, respectively).
Cell lines and glucose treatment.
Min6 cells (Ohno et al., 1993) were grown in DMEM high glucose media supplemented with 10% Fetal Bovine Serum (FBS), 71.5 μM beta mercaptoethanol, and penicillin/streptomycin. For low or high glucose treatment, cells were pre-incubated for 1 hour in Krebs/HEPES Ringer solution (20 mM NaCl, 24 mM NaHCO3, 5 mM KCl, 1 mM MgCl2, 1 mM CaCl2, and 25 mM HEPES, pH 7.4) supplemented with 1mM glucose. Cells were then treated with Krebs/HEPES Ringer solution supplemented with either low (1 mM) or high (16.7 mM) glucose for 1 hour. Cells were lysed and RNA collected using the RNeasy kit (Qiagen # 74134) with on column DNAse I digestion, per manufacturers protocol. RNA was reverse transcribed to cDNA using the High Capacity cDNA synthesis kit (Thermo Fisher, # 4368813).
Cytoplasmic/Nuclear RNA isolation.
The Protein and RNA Isolation Kit (Thermo Fisher, #AM1921) was used following manufacturer’s protocol, with DNAse I digestion preformed during the first wash step. Equal volumes of nuclear/cytoplasmic RNA were used for reverse transcription.
Luciferase Assays.
A ~500 bp sequence containing the overlapping binding peaks for GLIS3, PDX1, NKX6.1, NKX2.2, and NEUROD1 was cloned into pGL4.27 luciferase vector (primer sequences available in Supplementary Table 1). Luciferase plasmids were co-transfected with CMV-driven β-galactosidase plasmid using Lipofectamine 2000 (Thermo Fisher # 11668027) in βTC-6 cells, and Luciferase signal (Promega #E1500) normalized to Galactosidase activity (Clontech #631712) was measured 24 hours after transfection. Experiments were performed in triplicate.
Generation of G3R1 knockout mice and Glucose Tolerance Tests (GTT).
The G3R1 knockout mice (C57BL/6-B830017H08Rik<em1Amj>) were generated via CRISPR/Cas9 targeted deletion of the second exon of G3R1 in C57BL/6J albino embryos. Mouse lines were backcrossed onto C57BL/6J for at least 2 generations. Animal studies followed guidelines outlined by the NIH Guide for the Care and Use of Laboratory Animals and protocols were approved by the Institutional Animal Care and Use Committee at the NIEHS. For GTT, 8-week old mice were fasted for 6 hours followed by intraperitoneal injection of 2 mg glucose/g body weight in sterile PBS. Blood glucose levels were monitored via tail snip blood using a Nova Max Plus glucose monitoring system at the indicated timepoints. For high fat diet, mice were placed on diabetogenic HFD (Open Source Diets D12492) for 8 weeks starting at 8–10 weeks of age.
Immunohistochemical analysis.
Mouse pancreases were fixed for 4 hours in PBS + 4% Paraformaldehyde and embedded in OCT. Cryosectioned tissue from at least 2 mice from different litters for each genotype were then used for immunofluorescence staining with the indicated antibodies (Supplementary Table 1). Alexa Fluor-conjugated secondary antibodies were from Molecular Probes (Carlsbad, CA). Nuclei were stained with DAPI Prolong Diamond from Invitrogen (Carlsbad, CA). Fluorescent images were captured using a Zeiss LSM 880 confocal microscope and ZEN software.
Statistical analysis.
Statistical significance was calculated using a two-tailed student T-test. P-values are indicated.
Results
G3R1 is a β-cell specific lncRNA.
We initially identified B830017H08Rik (which we refer to as G3R1) as a gene downregulated in RNAseq analysis of Glis3Δpanc islets (Fig 1A), indicating it is a target of GLIS3 regulation in pancreatic β-cells. There is some disagreement between databases as to the annotation of G3R1, with ENSEMBL identifying it as a potential protein coding gene, while NCBI and the NONCODE database identify it as a non-coding gene. While we cannot rule out the possibility that a small peptide is encoded within lncRNA or its intron, according to PhyloCSF, a tool used to predict coding potential of gene sequences, G3R1 encodes a lncRNA (Lin et al., 2011). It is annotated as a two exon, one intron gene totaling 1,901 base pairs (1,119 base pairs of mature transcript). Cloning of G3R1 based on its annotated sequence indicates a different intron-exon junction than has been annotated previously, producing a shorter exon 1 (90 bp vs 451 bp) and longer exon 2 (861 bp vs 668 bp), yielding a mature transcript of 951bp (Supplementary Fig 1). This sequence also correlates with RNAseq data from other investigators, including FACS mouse β-cells (ArrayExpress ID E-MTAB-3351). Additionally, we performed 3’ Rapid Amplification of cDNA Ends (RACE) experiments in mouse islets and confirmed the polyA termination of the 3’ end prior to neighboring protein coding gene, CA15 (data not shown).
Fig 1. G3R1 is an islet-enriched long non-coding RNA controlled by GLIS3.
(A) RNA-seq analysis of islets from a pancreas-specific deletion of Glis3 (Glis3Δpanc) revealed downregulation of G3R1. (B) Expression analysis of G3R1 across multiple mouse tissues, normalized to pancreas expression. Error bars indicate standard error of the mean, N=3.
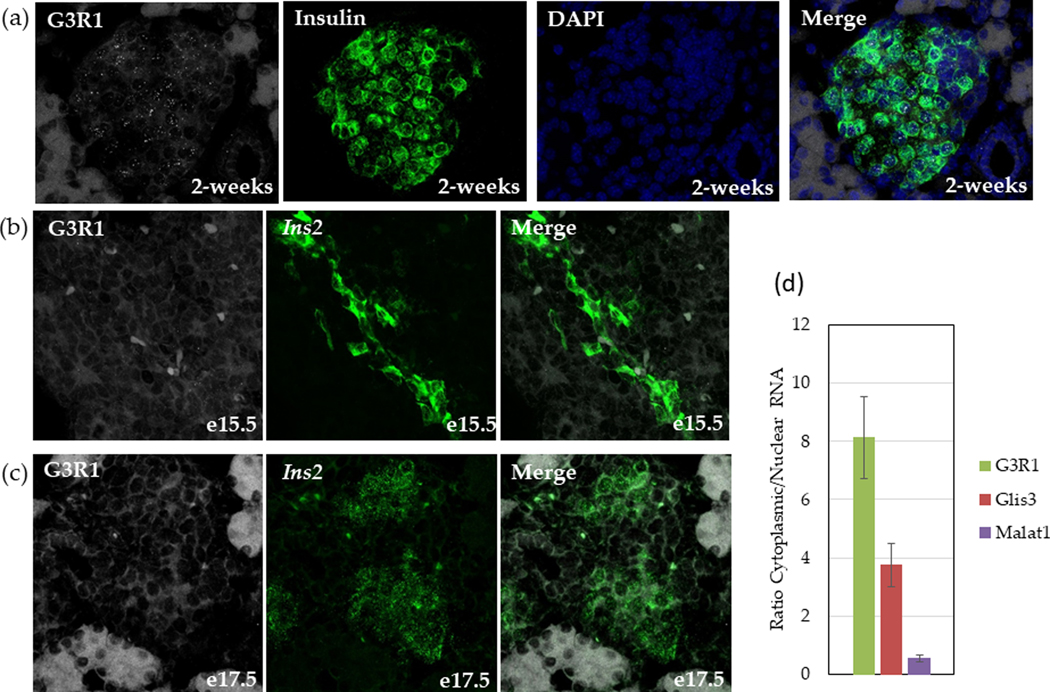
To examine its tissue-specific expression pattern, relative levels of G3R1 were analyzed in several mouse tissues by RT-qPCR (Fig 1B). G3R1 appears most highly expressed in islets and in a mouse β-cell line (βTC-6), followed by testis, with low expression in the brain. The similar expression between β-cell line and islets, as well as the low expression in whole pancreas (only ~5% of which consists of islets) indicated that G3R1 expression may be restricted to β-cells. In situ hybridization confirmed the β-cell specific expression of G3R1, as indicated by co-localization of G3R1 and Ins2 mRNA (Fig 2A). Interestingly, expression of G3R1 appears to be limited to postnatal islets, as expression was not detected at embryonic day (e)15.5 or e17.5 (Fig 2B–C). Additionally, G3R1 appears to be localized predominately in the cytoplasm. We therefore isolated cytoplasmic and nuclear RNA and determined that G3R1 is indeed predominately localized to the cytoplasm in βTC-6 cells (Fig 2D).
Fig 2. G3R1 is β-cell specific, cytoplasmic lncRNA within the islet.
In situ hybridization of 2-week old (A), e15.5 (B), and e17.5 (C) mouse pancreas for G3R1 (white) and Ins2 (green), with DAPI (blue) nuclear stain. Images shown are representative. (D) RT-qPCR analysis of nuclear and cytoplasmic RNA for G3R1, Glis3 (an mRNA), and Malat1 (a nuclear lncRNA). Cytoplasmic/Nuclear ratio was determined by calculating the relative amount of RNA in cytoplasmic and nuclear fractions of equal volume. Error bars indicate standard error of the mean, N=5.
G3R1 expression is stimulated by GLIS3, NKX2.2, and MAFA.
ChIP-seq analysis of several islet enriched transcription factors revealed binding peaks ~2.2 kB upstream of the transcriptional start site of G3R1 (Fig 3A). We therefore examined the ability of this region to promote transcription using a luciferase assay. In a mouse β-cell line, this upstream regulatory region of G3R1 (labeled G3R1-URR1) stimulated luciferase transcription ~4 fold (Fig 3B). In addition, overexpression of GLIS3, NKX2.2, or MAFA further stimulated the transcriptional activity of this region, indicating potential direct regulation by these factors. In addition to transcription factor regulation, we also examined whether G3R1 expression changed in response to glucose stimulation. However, levels of G3R1 remined constant during 1 hour high glucose exposure in a mouse β-cell line (Supplementary Fig 2A). Interestingly, leptin receptor deficient mice (db/db) had reduced islet expression of G3R1 (Supplementary Fig 2B), indicating a potential role for G3R1 in response to chronic, physiological hyperglycemia.
Fig 3. GLIS3 and other Islet transcription factors regulate G3R1 expression.
(A) ChIP-seq analysis of islet enriched transcription factors GLIS3, PDX1 (performed independently by two different labs), MAFA, NEUROD1, NKX6.1, NKX2.2, ISL1, LDB1, and FOXA2 revealed binding upstream of G3R1. Data is displayed via UCSC genome browser. (B) Luciferase assay of G3R1-URR1 in βTC-6 cells in the presence of overexpressed GLIS3, PDX1, NEUROD1, ISL1, NKX6.1, NKX2.2, PAX6, and MAFA. Error bars indicate standard deviation of a representative experiment.
Two apparent human homologs of G3R1 exist.
We next took an in silico approach to identify human homologs of G3R1. While lncRNAs are not evolutionarily well conserved (Zampetaki et al., 2018), we were able to identify two apparent human G3R1 homologs via Basic Local Alignment Search Tool (BLAST) search, LOC105372871 and LOC105377190, both annotated as lncRNA and located on chromosome 22. BLAST initially identified >70% identity over 8–12% of the sequence, however alignment of the entire sequences for G3R1 with LOC105372871 and LOC105377190 revealed ~46% identity. Interestingly, LOC105372871 and LOC105377190 only differ by the presence of an additional 40 nucleotides at the beginning of LOC105372871 (which are present in the genomic DNA, but not in the annotated RNA), and by 2 differences in nucleotide sequence over the remaining 777 nucleotides. Indeed, this >99% conservation extends both 5’ and 3’ of the lncRNA sequence (~84 kb 5’ and ~24 kb 3’ of the lncRNA sequence), suggesting an evolutionarily recent duplication event within chromosome 22.
Further examination of the sequences surrounding LOC105372871 and LOC105377190 indicated that Carbonic Anhydrase 15 (a pseudogene in humans) has 3 annotated copies, CA15P1, CA15P2, and CA15P3. While LOC105372871 and LOC105377190 are flanked by CA15P2 and CA15P3, CA15P1 is neighbored by DGCR2 (similar to mouse) and DGCR5, a predicted non-coding RNA not currently annotated in the mouse genome (Supplementary Fig 3). Interestingly, homology exists in this region similar to that of the region surrounding CA15P2 and CA15P3, including a third potential orthologue of G3R1 within the last intron of DGCR5, which is not annotated as an independent gene. Similar to the high homology between LOC105372871 and LOC105377190, this genomic sequence neighboring CA15P1 contained >98% homology with LOC105372871 and LOC105377190.
This high conservation makes detection of one lncRNA versus the others difficult, and unlikely that they are independently regulated, as conservation encompasses neighboring potential regulatory sequences. We therefore developed RT-qPCR primers that recognize LOC105372871 and LOC105377190, as well as potentially the unannotated region neighboring CA15P1. For simplicity, we subsequently refer to these three genes as hG3R1. Expression of hG3R1 followed a similar pattern to that of mouse, with enriched expression in the brain, islets, and testis (Fig 4A). Additionally, the transcription factors FOXA2, MAFB, NKX2.2, NKX6.1, and PDX1 all bound upstream of LOC105372871, LOC105377190, and the unannotated region neighboring CA15P1 in an upstream regulatory region homologous to that of mouse G3R1-URR1 (Fig 4B–D).
Fig 4. Two apparent human homologs of G3R1 display a similar tissue expression pattern.
(A) RT-qPCR expression analysis using primers that recognized both LOC105372871 and LOC105377190. Error bars indicate standard deviation of technical triplicates. ChIP-seq analysis of binding for FOXA2, MAFB, NKX2.2, NKX6.1, and PDX1 near (B) LOC105372871 and (C) LOC105377190.
G3R1 knockout in mice lacks a detectable islet phenotype.
In order to determine the function of G3R1, particularly its role in pancreatic islets, we developed a CRISPR knockout strategy for removal of Exon 2 (containing ~90% of the lncRNA sequence, Fig 5A). We obtained two mouse lines, one with a deletion of 765 nucleotides (80.4% of the total G3R1 sequence) and the other with an 821 nucleotide deletion (86.3% of G3R1, Fig 5A). We primarily examined this second line with the larger deletion, which we named G3R1-KO mice. Mice from both lines were born at expected Mendelian ratios and displayed no overt phenotypes (data not shown). While G3R1 did have some detectable expression in other tissues (as noted in Fig 1B), internal organs such as kidneys and testis appeared grossly normal, and both male and female knockout mice were fertile (data not shown). G3R1-KO mice also did not display an impairment in glucose tolerance (Fig 5B), nor were random blood glucose readings different from control littermates (Fig 5C). Correspondingly, markers for functional islet β-cells appeared unaffected as well, including INSULIN, MAFA, PDX1, and SYNAPTOPHYSIN (Fig 5D–F). In addition, G3R1-KO and control littermates were put on high fat diet (HFD), in order to determine whether diet-induced stress led to greater glucose impairment in the G3R1-KO mice. However, G3R1-KO and control littermates showed similar impairments in glucose tolerance tests on HFD (data not shown).
Fig 5. G3R1 knockout mice lack a detectable islet phenotype.
(A) CRISPR strategy for deletion of G3R1 exon 2, and genotyping indicating successful creation of a mouse lines with >80% deletion of G3R1. WT = Wild type genotyping, KO = Knockout genotyping. (B) Intraperitoneal glucose tolerance test of G3R1-KO mice, and both wild type and heterozygous littermates at 8-weeks of age. +/+ = 8, +/− = 4, −/− = 4. (C) Random blood glucose levels measured at 8-weeks of age. +/+ = 5, +/− = 5, −/− = 7. Error bars indicate standard error of the mean. Immunofluorescence staining of 8-week old pancreas from WT and G3R1-KO mice for (D) INSULIN and GLUCAGON, (E) INSULIN and MAFA, and (F) SYNAPTOPHYSIN and PDX1, as well as DAPI. Images shown are representative.
Discussion
In this study, we identified a novel lncRNA, referred to as G3R1, within the mouse pancreas as a β-cell specific, cytoplasmic lncRNA (Fig 2). That G3R1 encodes a lncRNA was supported by a combination of annotations in public databases, such as NCBI, and algorithmic approaches, such as PhyloCSF (Lin et al., 2011). However, as others have noted for other lncRNAs and despite our best predictions (Ji et al., 2015), it remains a possibility that there is a small peptide encoded within G3R1 or its intron. Additionally, the sequence that we cloned from mouse islets showed a different intron-exon junction than what had previously been annotated. RNA-seq analysis of 60 day old mouse β-cells performed by Guoqiang Gu at Vanderbilt University (ArrayExpress #E-MTAB-3351) showed the same intron-exon junction as we observed in our cloned G3R1 (Supplementary Fig 1). This highlights an important difficulty in identifying novel lncRNA from tissue databases based largely on whole organ sequencing, when many lncRNAs are expressed at relatively low levels (Zampetaki et al., 2018).
In addition to determining its tissue-specific pattern of expression, we demonstrated that G3R1 expression is repressed in pancreatic islets from Glis3Δpanc mice and identified a potential upstream regulatory element that was bound by GLIS3, suggesting that G3R1 transcription is directly regulated by GLIS3 (Fig 3A). Several additional islet-enriched transcription factors were found to bind within the same regulatory region, indicating that GLIS3 regulates G3R1 in coordination with these transcription factors (Fig 3A). This further highlights the cell-type specific and complex nature of G3R1 regulation in mice. In order to test the ability of GLIS3 and other factors to regulate G3R1 transcription through this region, we utilized a luciferase reporter assay. GLIS3, NKX2.2, and MAFA were all able to stimulate transcription (Fig 3B), although other transcription factors may have been limited by the relatively small sequence of DNA used (~500 bp), or by the lack of unknown upstream signals affecting transcriptional activity. For example, in a mouse model of postnatal deletion of Pdx1 in β-cells G3R1 was downregulated, indicating the possibility of direct or indirect regulation(Gao et al., 2014). The coordinated regulation by multiple transcription factors also indicated the evolutionary conservation of the region we observed in humans.
Indeed, in silico analysis of mouse G3R1 identified potential human homologs of G3R1 (LOC105372871, LOC105377190, and the region neighboring CA15P1) displaying a somewhat similar expression pattern to mouse G3R1, with expression in the pancreatic islets (Fig 4A). Because of the near total conservation between LOC105372871, LOC105377190, and the region neighboring CA15P1 we assume they provide redundancy for each other. Additionally, a similar pattern of islet-enriched transcription factor binding to the mouse was observed for PDX1, MAFB, NKX2.2, NKX6.1, and FOXA2 in a conserved regulatory region in human islets (Fig 3C–D). It should be noted that this region is home to a known islet enhancer (Pasquali et al., 2014), and that other islet genes, such as the lncRNA HI-LNC1255, neighbor this region (Moran et al., 2012). However, while we cannot rule out the possibility that this region regulates other genes, hG3R1 is the closest gene to this regulatory region, and in a diabetic mouse model, G3R1 expression is down (Supplementary Fig 2). Interestingly, LOC105372871, LOC105377190, and CA15P1 all reside on chromosome 22q11.21 in matching ~106KB regions that have gone through a relatively recent duplication event, as evidenced by the extremely high (>99%) conservation. This region of the chromosome is home to frequent deletions within 22q11.2, leading to DiGeorge syndrome, a genetic disorder characterized by impaired heart development, impaired immune system, cleft palate, delayed development and behavioral problems (Burnside, 2015). However, we did not observe any craniofacial defects in our G3R1 knockout mice (data not shown), indicating that human G3R1 can likely be ruled out as a potential cause of DiGeorge syndrome. Also, to our knowledge there is not a strong connection between DiGeorge syndrome and diabetes, despite the presence of islet enhancers and regulatory regions, suggesting that there could be multiple physiological roles for genes within this region of the chromosome.
We next sought to determine the function of G3R1 in vivo through CRISPR-mediated knockout. However, these mice failed to develop any detectable phenotypes, especially in regard to islet development, unlike other β-cell lncRNA knockouts (Arnes et al., 2016). There are many potential reasons why the G3R1-KO mice lacked any overt phenotypes. One potential explanation is that our knockout strategy targeted the second exon, leaving the first, shorter exon intact. LncRNA have been reported to have modular functions, with some portion of the sequence enough to recapitulate key functions, such as with Xist and HOTAIR (Chu et al., 2015, Somarowthu et al., 2015). It is therefore possible that the ~10% of G3R1 encoded in exon 1 is sufficient to promote normal β-cell development/function. A more likely possibility is that the function of G3R1 is redundant with another lncRNA. LncRNA are generally poorly conserved across evolution, which not only makes it nearly impossible to determine the function from the sequence, but also makes it difficult to identify lncRNA with similar functions based on sequence alone (Chen, 2016). Therefore, we cannot rule out the possibility that another lncRNA exists with compensatory function. Finally, other investigators recently looked at the deletion of 12 novel lncRNA in mice, and found that 11 of these 12 mice did not have a discernable phenotype, similar to what we observed with G3R1 (Han et al., 2018). The authors suggested that this lack of phenotype may be the result of lncRNA only fine-tuning the expression of neighboring genes (Morris and Mattick, 2014). It is therefore possible that G3R1 plays some regulatory function of nearby known or novel genes in a minor and temporally restricted manner.
The tightly controlled tissue-specific expression of G3R1 in both mouse and human pancreatic β-cells suggests that it likely has some role in β-cell development, function, or response to environmental challenge. G3R1 is predominately localized to the cytoplasm, making it difficult to distinguish the role of G3R1 in the cell verses the large number of potential roles. Future studies should attempt to identify which proteins or RNAs G3R1 interacts with, in order to identify its role in the pancreatic β-cell. Nevertheless, with its β-cell specific expression in the mouse and conserved regulatory regions in humans, G3R1 could prove an intriguing biomarker of β-cell fate or dysfunction.
Supplementary Material
G3R1 observed sequence differs from the annotated sequence. Sequence of G3R1, with annotated sequence marked in green and observed sequence marked in blue.
Chronic hyperglycemia, but not acute glucose treatment, regulates G3R1. (A) Min6 cells were pre-treated with low glucose (1mM) for 1 hour, then stimulated with either low (1 mM) or high (16 mM) glucose. N=3. (B) Freshly isolated pancreatic islets from wild type (C57BL/6J) and db/db mice at 8-weeks of age of both genders. N=10 for each genotype, * indicates p<0.01. Error bars indicate standard error of the mean.
Identification of hG3R1 conserved regions. Indicated regions of high homology on human chromosome 22. Adapted from the NCBI genome browser for hg38.
Acknowledgements
We would like to acknowledge Dr. Xiao-Ping Yang at NIEHS for her assistance in preliminary investigations, and Dr. Sara Grimm for her assistance in bioinformatics analysis.
Funding: This research was supported by the Intramural Research Program of the NIEHS, NIH Z01-ES-101585.
Footnotes
Declaration of Interests
We declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Akerman I, Tu Z, Beucher A, Rolando DMY, Sauty-Colace C, Benazra M, Nakic N, Yang J, Wang H, Pasquali L, et al. 2017. Human Pancreatic beta Cell lncRNAs Control Cell-Specific Regulatory Networks. Cell Metab, 25, 400–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnes L, Akerman I, Balderes DA, Ferrer J & Sussel L 2016. betalinc1 encodes a long noncoding RNA that regulates islet beta-cell formation and function. Genes Dev, 30, 502–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnside RD 2015. 22q11.21 Deletion Syndromes: A Review of Proximal, Central, and Distal Deletions and Their Associated Features. Cytogenet Genome Res, 146, 89–99. [DOI] [PubMed] [Google Scholar]
- Carter G, Miladinovic B, Patel AA, Deland L, Mastorides S & Patel NA 2015. Circulating long noncoding RNA GAS5 levels are correlated to prevalence of type 2 diabetes mellitus. BBA Clin, 4, 102–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LL 2016. Linking Long Noncoding RNA Localization and Function. Trends Biochem Sci, 41, 761–772. [DOI] [PubMed] [Google Scholar]
- Chu C, Zhang QC, Da Rocha ST, Flynn RA, Bharadwaj M, Calabrese JM, Magnuson T, Heard E & Chang HY 2015. Systematic discovery of Xist RNA binding proteins. Cell, 161, 404–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Gonzalo-Calvo D, Kenneweg F, Bang C, Toro R, Van Der Meer RW, Rijzewijk LJ, Smit JW, Lamb HJ, Llorente-Cortes V & Thum T 2016. Circulating long-non coding RNAs as biomarkers of left ventricular diastolic function and remodelling in patients with well-controlled type 2 diabetes. Sci Rep, 6, 37354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Mckenna B, Li C, Reichert M, Nguyen J, Singh T, Yang C, Pannikar A, Doliba N, Zhang T, et al. 2014. Pdx1 maintains beta cell identity and function by repressing an alpha cell program. Cell Metab, 19, 259–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Luo S, Peng G, Lu JY, Cui G, Liu L, Yan P, Yin Y, Liu W, Wang R, et al. 2018. Mouse knockout models reveal largely dispensable but context-dependent functions of lncRNAs during development. J Mol Cell Biol, 10, 175–178. [DOI] [PubMed] [Google Scholar]
- Ji Z, Song R, Regev A & Struhl K 2015. Many lncRNAs, 5’UTRs, and pseudogenes are translated and some are likely to express functional proteins. Elife, 4, e08890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang HS, Kim YS, Zeruth G, Beak JY, Gerrish K, Kilic G, Sosa-Pineda B, Jensen J, Pierreux CE, Lemaigre FP, et al. 2009. Transcription factor Glis3, a novel critical player in the regulation of pancreatic beta-cell development and insulin gene expression. Mol Cell Biol, 29, 6366–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YS, Kang HS, Takeda Y, Hom L, Song HY, Jensen J & Jetten AM 2012. Glis3 regulates neurogenin 3 expression in pancreatic beta-cells and interacts with its activator, Hnf6. Mol Cells, 34, 193–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langmead B, Trapnell C, Pop M & Salzberg SL 2009. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol, 10, R25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MF, Jungreis I & Kellis M 2011. PhyloCSF: a comparative genomics method to distinguish protein coding and non-coding regions. Bioinformatics, 27, i275–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran I, Akerman I, Van De Bunt M, Xie R, Benazra M, Nammo T, Arnes L, Nakic N, Garcia-Hurtado J, Rodriguez-Segui S, et al. 2012. Human beta cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metab, 16, 435–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris KV & Mattick JS 2014. The rise of regulatory RNA. Nat Rev Genet, 15, 423–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motterle A, Gattesco S, Caille D, Meda P & Regazzi R 2015. Involvement of long non-coding RNAs in beta cell failure at the onset of type 1 diabetes in NOD mice. Diabetologia, 58, 1827–35. [DOI] [PubMed] [Google Scholar]
- Motterle A, Gattesco S, Peyot ML, Esguerra JLS, Gomez-Ruiz A, Laybutt DR, Gilon P, Burdet F, Ibberson M, Eliasson L, et al. 2017. Identification of islet-enriched long non-coding RNAs contributing to beta-cell failure in type 2 diabetes. Mol Metab, 6, 1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T, Kato N, Ishii C, Shimizu M, Ito Y, Tomono S & Kawazu S 1993. Genistein augments cyclic adenosine 3’5’-monophosphate(cAMP) accumulation and insulin release in MIN6 cells. Endocr Res, 19, 273–85. [DOI] [PubMed] [Google Scholar]
- Pasquali L, Gaulton KJ, Rodriguez-Segui SA, Mularoni L, Miguel-Escalada I, Akerman I, Tena JJ, Moran I, Gomez-Marin C, Van De Bunt M, et al. 2014. Pancreatic islet enhancer clusters enriched in type 2 diabetes risk-associated variants. Nat Genet, 46, 136–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR & Hall IM 2010. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics, 26, 841–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville D, Lichti-Kaiser K, Grimm S & Jetten A 2019. GLIS3 binds pancreatic beta cell regulatory regions alongside other islet transcription factors. J Endocrinol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somarowthu S, Legiewicz M, Chillon I, Marcia M, Liu F & Pyle AM 2015. HOTAIR forms an intricate and modular secondary structure. Mol Cell, 58, 353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampetaki A, Albrecht A & Steinhofel K 2018. Long Non-coding RNA Structure and Function: Is There a Link? Front Physiol, 9, 1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeruth GT, Takeda Y & Jetten AM 2013. The Kruppel-like protein Gli-similar 3 (Glis3) functions as a key regulator of insulin transcription. Mol Endocrinol, 27, 1692–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
G3R1 observed sequence differs from the annotated sequence. Sequence of G3R1, with annotated sequence marked in green and observed sequence marked in blue.
Chronic hyperglycemia, but not acute glucose treatment, regulates G3R1. (A) Min6 cells were pre-treated with low glucose (1mM) for 1 hour, then stimulated with either low (1 mM) or high (16 mM) glucose. N=3. (B) Freshly isolated pancreatic islets from wild type (C57BL/6J) and db/db mice at 8-weeks of age of both genders. N=10 for each genotype, * indicates p<0.01. Error bars indicate standard error of the mean.
Identification of hG3R1 conserved regions. Indicated regions of high homology on human chromosome 22. Adapted from the NCBI genome browser for hg38.