Abstract
Metastasis is the most common cause of death, with treatments failing to provide a durable response. Aging is a key prognostic factor in many cancers. Emerging data suggest that normal age-related changes in the tumor microenvironment can contribute to metastatic progression. These changes encompass secreted factors, biophysical changes, and changes in both stromal and immune cell populations. These data also highlight the importance of conducting studies in preclinical models of appropriate age. Ultimately, therapies may also need to be tailored to reflect patient age, as markers of metastatic disease differ in young and aged populations. In this review, we will discuss some of the changes that occur during aging that increase the metastatic capacity of tumor cells.
Metastasis is the major cause of cancer-related deaths, with the majority of treatments failing to provide a durable response. Cancer is referred to as a disease of aging. Incidence rates dramatically increase as we age, and cancer is the number one cause of death in 60–79 yr old individuals (Siegel et al. 2018). Alarmingly, the probability of developing invasive cancer in patients aged 60 or older is double that of younger patients (Siegel et al. 2018). While this implicates age-related changes within the body as a potential factor in driving a metastatic tumor phenotype, few studies have directly linked tumor cell dissemination, extravasation, dormancy, metastatic outgrowth, and other metastatic progressive factors with an aged microenvironment. Given the vast improvements seen in healthcare and technology, The World Health Organization estimates a shift in the proportion of the world's population over 60 yr old from 12% to 22% by 2050, with an estimated population of over 2 billion people. This places an emphasis on more studies needed to investigate age-related contributions toward metastatic disease for effective therapeutic targeting.
Genomic pairwise analysis of patient primary tumor and distant metastases across many tumors reveal mutations common to both, but importantly, identifies mutations that are distinct to metastasis (Steeg 2016). Despite the clear contribution that certain driver mutations play in promoting metastasis, in vivo modeling within many cancer subtypes reveal no changes in mutational status. This emphasizes the importance of both the tumor microenvironment (TME) and premetastatic niche environments as key contributors toward the reprogramming of cancer cells toward states that promote metastatic progression. As we age, tissue microenvironments change dramatically, and we are now only beginning to understand how significant these changes are in driving tumor metastasis. For example, very recent studies have shown that the multistage model of carcinogenesis (tumor initiation, tumor promotion, malignant conversion, and tumor progression) requires incorporation of aging-dependent somatic selection to make it capable of generalizing cancer incidence across tissues and species (Rozhok and DeGregori 2019). This process of somatic selection has been defined as noncell-autonomous and is driven by microenvironment-imposed increases in positive selection for previously accumulated genetic/phenotypic diversity in aged tissues (Rozhok et al. 2014).
The mechanisms of cancer progression and aging underlie an accumulation of cellular damage and other genomic changes at the transcriptional and epigenetic level over time. The process of aging (decreased function and growth) versus cancer (increased growth and survival) seem diametrically opposed, however, studies show that many of the hallmarks of aging are in fact shared with cancer (Aunan et al. 2017). The stromal microenvironment within tissues is made up of many components including fibroblasts, endothelial cells, pericytes, adipocytes, extracellular matrix (ECM), immune cells, and more. Even though many of these populations appear to decrease functionally as we age, these changes appear to promote efficient metastatic progression in established TMEs and within premetastatic niches.
This chapter will focus on the role that age-related changes in tumor and tissue microenvironments play in promoting efficient metastatic progression. We will discuss the interactions between cancer cells and aged microenvironments, focusing on how aging can reprogram fibroblasts, the ECM and immune subtypes and infiltration to promote progression and the effect that this has on targeting metastatic populations therapeutically.
AGE-RELATED SECRETED CHANGES DRIVE METASTASIS
The intrinsic changes required for tumor initiation, growth, and progression are well documented. Malignant transformation often requires genetic mutations in growth pathways to drive hyperproliferation and bypass senescence. Many of the hallmarks of aging promote this. These include increased genomic instability, telomere attrition, epigenetic alteration, impaired proteostasis, and deregulation of nutrient sensing (Campisi 2013; Aunan et al. 2017; Zinger et al. 2017). Environmental factors to which we are exposed as we age, such as UV exposure, alcohol, smoking, and pollution, further contribute to the chronic accumulation of DNA damage and other events associated with cellular aging. Paradoxically, many of these factors involved in aged tissue evolution that promote malignant transformation and hyperplastic growth also contribute to growth arrest (senescence), apoptosis, and degradation of other cells and structural tissue components. There is a significant correlation between these age-related degradative features within tissues/cells and cancer cell metastasis. Studies are now finally beginning to investigate a direct link between local and systemic aging of tissue and metastatic progression.
Fibroblasts are the most common stromal component within tissues. They play an integral role in maintaining the homeostatic integrity within tissues. These cells are required for the synthesis of ECM and collagen necessary for the structural integrity of connective tissue and play a key role in wound healing and inflammation. These processes are achieved in large part through secretion of soluble factors into the microenvironment. These factors include cytokines, chemokines, growth factors, enzymes, and structural components of the ECM (Coppé et al. 2010a; Campisi 2013). Given the dynamic nature of different tissue microenvironments, there is large context specificity within different tissues which tightly regulates the soluble factors secreted by fibroblasts along with their migratory and proliferative characteristics. For example, the fibroblast renewal rate (growth and proliferation) is highly diverse throughout the body, with factors including local temperature, vascularization, mechanical stress, and hormonal response within the microenvironment contributing to this diversity (Ruchti et al. 1983). The age-specific changes that occur in fibroblasts and other stromal components during aging are also likely to differ between organ sites. One of the distinctive features of aging in fibroblasts involves them adopting a senescent phenotype. Furthermore, aged fibroblasts undergo distinct metabolic changes, and have higher levels of reactive oxygen species (ROS) and nitric oxide (NO). This allows them to create a hostile microenvironment that not only promotes tumorigenesis, but that tumor cells can co-opt to become more aggressive (Martinez-Outschoorn et al. 2010, 2011; Pavlides et al. 2010; Balliet et al. 2011).
Cellular Senescence—A Model of Aging?
Cellular senescence refers to the process of irreversible cell growth arrest. It is linked to many of the cellular processes of aging described above but can also occur in direct response to intrinsic or extrinsic oncogenic stimuli. It is a key homeostatic mechanism used by cells to inhibit malignant or uncontrolled growth of cells. Often, immune components can recognize senescent cells and clear them from the microenvironment. However, senescence induction is an example of antagonistic pleiotropy. This is defined as a singular gene trait that elicits a phenotype that can be simultaneously beneficial and detrimental to an organism. Accumulation of senescent cells is one of the key pathological features associated with aging, and is highly prevalent within fibroblasts (Campisi 2013; Faragher et al. 2017; McHugh and Gil 2018; Calcinotto et al. 2019). As we age, it is hypothesized that a reduction immune function decreases the recognition and clearance of these growth-arrested cells, which eventually results in their accumulation (Burton and Stolzing 2018). Given the accumulation of senescent fibroblasts throughout the body as we age, senescence was originally used as an artificial model to study aging of fibroblasts (Campisi and Robert 2014). However, as we will discuss below, recent studies show that while there is a great similarity between senescence and aging, not all secreted markers of senescence are preserved in aging cells, and aging cells can have an impact on tumor cells that extend beyond that of senescence (Kaur et al. 2016a; Behera et al. 2017).
Age-dependent accumulation of senescence-associated secretory phenotype (SASP) cells can contribute to metastatic progression by reprogramming primary TMEs to drive tumor cell dissemination and by reprogramming premetastatic niches microenvironments over time to a state that is growth permissive for malignant or dormant tumor cells (Fig. 1). Senescent fibroblasts and many SASP factors have been shown to induce invasion in many types of cancers in culture (Krtolica et al. 2001; Lawrenson et al. 2010; Kim et al. 2013). Furthermore, coinjection of senescent fibroblasts, but not nonsenescent fibroblasts, stimulates metastatic progression of various mouse and human tumors in immunocompromised and syngeneic mice (Krtolica et al. 2001; Liu and Hornsby 2007). Below, we discuss the role of senescence in cancer metastasis.
Figure 1.
Secreted changes in the aged TME. Fibroblasts make up the largest proportion of the stromal microenvironment in tissues. They are responsible for regulating tissue structure via ECM deposition and supporting cellular and microenvironmental homeostasis via the tightly regulated secretion of soluble factors such as cytokines, chemokines growth factors, and other key signaling proteins. A decrease in fibroblast renewal rate in the elderly, coupled with decreased senescent cell clearance by the immune system results in aged tissue microenvironments having an accumulation of SASP fibroblast cells. The SASP is composed of about 75 defined soluble factors that promote metastatic progression via a diverse range of functions. A large number of proteases (MMPs, PAI, TPA) promote ECM remodeling to reinforce an invasive phenotype within the primary tumor and enable a growth permissive ECM in the metastatic tissue. They secrete angiogenic factors such as VEGF to promote angiogenesis to allow efficient dissemination and nutrient supply, and a large number of growth factors (IGFBP, CSF) to reinforce an aggressive cancer phenotype. Cytokines and chemokines including CXCL1-2, IL6, IL10, GMCSF, and others allow them to promote an immune microenvironment that favors metastatic progression. Many of these factors also appear to have powerful paracrine effects, which can induce a SASP phenotype in surrounding stromal cells. SASP fibroblasts also promote extensive ECM remodeling to increase key signaling components involved in metastatic outgrowth along with structurally altering deposition to promote invasion of tumor cells and efficient immune cell trafficking. Finally, dramatic metabolic changes in SASP cells results in the secretion of high energy metabolites, coupled with an increase in ROS and NO production, which not only further reprograms the microenvironment but also increases cancer aggressiveness. Recent evidence from our group shows that aging within the skin reprograms the fibroblast secretome of healthy patients. Aged skin fibroblasts secrete factors such as sFRP2, while decreasing secretion of molecules such as Klotho and HAPLN1, which significantly increases tumor cell invasion, dissemination, tumor angiogenesis, ECM remodeling, and resistance to targeted therapy. It remains to be seen if aging has an effect on healthy patient fibroblast secretomes in other tissues. Such a phenomenon may promote aggressive primary tumors or premetastatic niche formation which may help explain increased cancer metastasis in aged patients.
Senescent-Associated Secretory Phenotype in Cancer Cell Metastasis
One of the key features of senescence in cells is a widespread change in epigenetic gene expression (Maegawa et al. 2010), whereby cells dramatically increase the secretion of proinflammatory cytokines, chemokines, growth factors, and proteases. This secretome is defined as the senescence-associated SASP (Coppé et al. 2010a). Typically, the SASP is thought to be made up of about 75 secreted factors which has been extensively reviewed (Coppé et al. 2010a; Campisi 2013; Campisi and Robert 2014). Many of these SASP factors have highly diverse functions that can promote efficient metastatic progression (Fig. 1). A large group of proteases including matrix metalloproteases (MMPs), plasminogen activators (PAI), and tissue plasminogen activator (TPA) (Coppé et al. 2010a) act to reinforce the invasive phenotype in many malignancies via remodeling of the ECM. These proteases also regulate the activity of other soluble factors via cleavage and induce their activation/degradation to promote growth at distant metastatic sites (Coppé et al. 2010a; Gialeli et al. 2011). The SASP can also overcome age-related decreases in systemic angiogenesis (Moriya and Minamino 2017) by secreting vascular endothelial growth factors (VEGF). Senescence induction within fibroblasts also results in a metabolic switch toward aerobic glycolysis, along with increased mitochondrial dysfunction and hydrogen peroxide production. As such, SASP cells secrete a large number of high energy metabolites including lactate, ketones, and glutamine into the microenvironment (Martinez-Outschoorn et al. 2010, 2011; Pavlides et al. 2010; Balliet et al. 2011). These metabolites have been shown to enhance cancer cell aggressiveness toward an invasive phenotype. SASP cells also secreted increased amounts of ROS and NO, which further accelerates age-related cellular damage that can further promote a permissive metabolic microenvironment for aggressive cancer development.
SASP cells also secrete high amounts of growth factors and cytokines such as CXCL1–CXCL2 (Coppé et al. 2010b; Lesina et al. 2016), IL6 (Zinger et al. 2017), insulin-like growth factor binding proteins (IGFBPs) (Coppé et al. 2008; Elzi et al. 2012; Severino et al. 2013; Sanada et al. 2018), and colony-stimulating factors (CSF) (Coppé et al. 2008). These factors are capable of modulating the TME toward an aggressive phenotype and also can reprogram premetastatic niche environments to make them growth permissive. Many of these factors including CXCL1/CXCL2 (Acosta et al. 2008), IGFBP7 (Wajapeyee et al. 2008), IL6 (Kuilman et al. 2008), and PAI-1 (Kortlever et al. 2006) appear to have powerful paracrine effects in the maintenance and induction of other senescent cells and can further increases the accumulation of senescent populations. IL1-α for example, is a key SASP factor involved in tumor initiation and progression within pancreatic cancer. Genetic knockout of the senescence inducing factor SIN3B in the pancreas in a pancreatic ductal adenocarcinoma (PDAC) mouse model protected animals from metastatic progression via a reduction in fibroblast SASP activation and reduced IL1-α secretion (Rielland et al. 2014) and uncoupling of this pathway within this PDAC model via IL1-α genetic ablation further inhibited metastatic growth (Lau et al. 2019).
The SASP has been shown to have many beneficial roles in embryonic development (Munoz-Espin et al. 2013; Storer et al. 2013), wound healing (Demaria et al. 2014) and alerting the immune system that clearance of senescent cells is required. However, age-related accumulation of SASP cells involved in a number of age-related pathologies (Baker et al. 2011; Liu et al. 2019) and as described above, plays a role in driving tumor cell invasion and progression. While senescence induction and accumulation in stroma is a key issue in tumorigenesis, senescence plays an important role in the regulation of cancer cells. Under normal circumstances, the oncogenic transformation of a normal cell initially promotes senescence induction to prevent uncontrolled growth and provides a major barrier for tumor progression. However, malignant cells often bypass this process through genetic mutation or epigenetic down-regulation of tumor suppressor associated pathways such as p53/p21 and p16INK4a/pRB pathway (Lee and Schmitt 2019). Furthermore, the microenvironmental regulation of certain signaling pathways can contribute to this. In melanocytes, for example, the BRAF oncogene induces senescence, but melanocytes can undergo malignant transformation and bypass this senescence via activation of the canonical Wnt signaling pathway (Kaur et al. 2016b). Nonmalignant senescent cells that are able to persist have also been shown to dramatically contribute toward metastatic progression in many models of cancer (Coppé et al. 2010a; Calcinotto et al. 2019; Lee and Schmitt 2019), and this may involve noncanonical Wnt signaling (Webster et al. 2015).
Secreted Changes—Beyond the SASP
While the accumulation of senescent fibroblasts and other cells is well documented with age, there is much debate as to whether these SASP related effects on tumor development can truly be attributed to the aging process (Burton and Faragher 2015; Faragher et al. 2017). Many studies have now shown that the mode of senescence initiation (oncogene induced, replicative induced, stress induced, and therapy induced) dramatically alters the SASP factors secreted by these cells and thus, not all senescence is equal, nor may it may truly be indicative of aging (Nelson et al. 2014; Salama et al. 2014; Özcan et al. 2016).
Recent studies from our group have now begun to show that nonsenescent aged fibroblasts from healthy human donors appear to promote melanoma metastasis (Kaur et al. 2016a) and have a different secretome profile compared to that of senescent fibroblasts (Fig. 1; Kaur et al. 2018). Using a syngeneic mouse model of melanoma, subcutaneous injection of Yumm1.7 cells resulted in faster-growing primary tumors in younger animals (8 wk); however aged animals (52 wk) had significantly increased vessel density and lung micrometastasis. Using an organotypic 3D human skin reconstruction, aged fibroblasts (>55 yr) taken from healthy human donors induced significantly more melanoma invasion but less proliferation then younger healthy donors (<45 yr). Proteomic analysis of conditioned media (CM) from these young versus aged dermal fibroblasts confirmed that sFRP2 (a canonical-WNT antagonist) was secreted at significantly higher levels in aged CM and was responsible for the increases in melanoma cell invasiveness. Furthermore, recombinant sFRP2 treatment in young mice significantly increased tumor angiogenesis, lung metastasis and increased resistance to targeted therapy.
While sFRP2 is secreted by aged fibroblasts, just as significant are factors that are lost in aged fibroblasts. These include molecules such as klotho, “the fountain of youth” hormone, and hyaluronan and proteoglycan link protein 1 (HAPLN1), both of which are secreted by young but not aged fibroblasts. We discuss HAPLN1 in more detail later in this chapter. Klotho knockout mice present with a plethora of age-related pathologies, including osteoporosis, atherosclerosis, skin atrophy and so on, and die within 9 mo (Masuda et al. 2005). Klotho transgenic mice are conversely more robust and live almost a year longer than their wild-type counterparts (Kurosu et al. 2005). Klotho is a circulating serum protein that modulates oxidative stress and suppresses cellular senescence. We have shown that the loss of Klotho drives melanoma metastasis (Camilli et al. 2011), and alters the metabolism of melanoma cells such that they no longer respond effectively to targeted therapy (Behera et al. 2017). In conjunction with increases in molecules such as sFRP2 that also drives metastasis and resistance to targeted therapy, secreted changes in the aged microenvironment seem to converge on a prometastatic environment.
THE MATRIX AND AGING
The Extracellular Matrix and Metastasis
Another highly dynamic microenvironmental component of the metastatic cascade that continuously evolves during the aging process is the ECM. The ECM maintains the integrity of tissue microenvironments and governs protein and cell-specific trafficking across the body. It is defined as the noncellular component of tissue and provides structural and biochemical support for cells. The ECM provides biochemical cues to cells via the expression of various proteins such as integrins that are key in regulating many of the aspects of cancer cell metastasis including cell adhesion, apoptosis, proliferation, migration, survival, and invasion. They also provide biomechanical support to cells via the cross-linking, stiffness and directionality of collagen, fibrillar proteins, scaffold proteins and other molecules that make up the ECMs structure. The ECM is largely secreted by fibroblasts and is constantly undergoing remodeling controlled by a delicate balance between degradation and deposition.
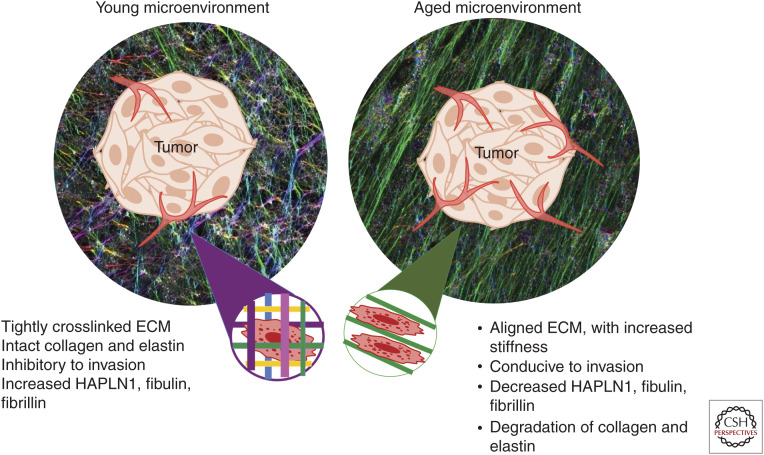
The ECM plays a critical role in cancer cell dissemination and metastatic outgrowth. Cancer cells must first navigate through the ECM scaffold and dense ECM network. Throughout this process, cancer cells will encounter various ECM proteins that can aid in altering a cell state toward invasion/migration during dissemination or may facilitate extravasation and outgrowth within distant metastatic tissue (extensively reviewed in Eble and Niland 2019). On the other hand, the ECM may also impair dissemination and outgrowth by barring the way of cancer cells and forming impermeable, dense ECM barriers or structures that inhibit growth. Such a state requires considerable ECM remodeling to facilitate progression. The loss of tissue ECM integrity is a well-defined feature of an aged microenvironment (Fig. 2), encompasses one of the hallmarks of cancer and is heavily associated with metastatic dissemination and outgrowth (Hanahan and Weinberg 2011; Pickup et al. 2014; Poltavets et al. 2018; Walker et al. 2018).
Figure 2.
ECM changes in the aged TME. The manner in which fibroblasts lay down matrix changes with age. Young dermal fibroblasts lay down matrices that are nonlinear, and in a “basketweave” pattern, and refract light at multiple different angles. This is visualized as multiple colors. Aged fibroblasts lay down matrices that are linear, and refract light in a singular direction, visualized as a single color. This change in cross-linking is in part directed by the loss of HAPLN1 during aging, which acts to stabilize the cross-links of collagen, elastin, and hyaluronic acid (HA). An aligned matrix predicts for more metastatic behavior. Further breakdown of the ECM around the vasculature can also affect the predicted route of metastasis for disseminated tumor cells.
ECM Composition Diversity Across Organs
The ECM microenvironment differs greatly due to the complex variation required for organ structure and function. While there is diversity in many of the biochemical cues in an ECM environment, stiffness and elasticity are very heterogenous throughout the body and change dramatically as we age. In healthy individuals, softer tissues such as the brain, breast, and lung require a more elastic, “looser” connective tissue environment, whereas harder tissues such as the skin and bone require stiffer structures to provide a protective barrier. Pathologically, this diversity in ligand expression and stiffness across differing microenvironments plays a dramatic role in cancer progression (Cox and Erler 2011). This diversity makes it much harder to study the general effects of ECM composition across various cancer subtypes within the TME and in the establishment of premetastatic niches. The difficulty in general modeling of the ECM within cancer was recently highlighted by the fact that cancer cell metastasis does not fit a linear model of increasing ECM stiffness but in fact fits a biphasic model, whereby too much stiffening inhibits metastasis, as cell nuclei are unable to efficiently fit through the smaller pores created in very stiffly cross-linked matrices (Ahmadzadeh et al. 2017). This ultimately inhibits tumor cell invasion/dissemination along with extravasation into metastatic tissue sites (Kaur et al. 2018). Discrete changes in fiber realignment, strain-stiffening and increased cross-linking also alter cancer cell metastasis (Levental et al. 2009; Wang et al. 2014). These overall changes also have a dramatic effect on immune infiltration within aged tumor models (Kaur et al. 2018). As such, there is a clear need to define the age-related changes in ECM structure and function across different tissue and cancer subtypes.
These site-specific differences are achieved via regulation of cross-linking of ECM components and overall collagen density. In the skin, for example, collagen and elastin are tightly bound to each other by HA in a basket weave pattern. HA alterations have been shown to increase the ability of fibroblasts to contract elastin and collagen matrices (Huang-Lee and Nimni 1994). HA's role in cancer is believed to be tissue specific; reduced HA is associated with increased tumorigenesis in normally HA-rich tissues such as skin (Karjalainen et al. 2000), and it is known that HA levels decrease dramatically with aging.
The ECM in Aging and Cancer Cell Metastasis
ECM integrity decreases substantially as we age. This process is exemplified visually during the wrinkling of the skin. Age-related changes in the physical properties of the ECM include decreased collagen density (Diridollou et al. 2001; Fisher et al. 2002; Panwar et al. 2015), ECM fiber area and thickness (Lee et al. 2011; Oh et al. 2011; Marcos-Garces et al. 2014) as well as changes in the mechanical properties of the ECM such as stiffness (Fig. 2; Panwar et al. 2015). Furthermore, natural age-related turnover of collagen cross-linking proteins such as fibulin, fibrillin, and elastin (Sephel and Davidson 1986; Roark et al. 1995) decreases cross-linking within the ECM which further damages integrity (Martin and Dean 1993).
Studies from our group provide an example of how aged microenvironments can drive efficient metastatic progression via age-specific remodeling of the ECM. Our first study found that in young versus aged healthy human patient fibroblast samples, young dermal fibroblasts secrete high levels of ECM constituents, including proteoglycans, glycoproteins, and cartilage-linking proteins when compared with aged (Kaur et al. 2018). The most abundantly secreted was HAPLN1, a hyaluronic and proteoglycan link protein. HAPLN1 is a cross-linking protein that stabilizes proteoglycan monomer aggregates with HA. It was originally thought that this protein was secreted exclusively by tumor cells during invasion (Naba et al. 2012). However, we specifically found that HAPLN1 expression was secreted by young fibroblasts and is lost in healthy aged human skin fibroblasts. These changes resulted in a decrease in collagen density and fiber cross-linking (Fig. 2). There was also a dramatic increase in fiber alignment, which promoted melanoma cell invasion and increased dissemination from the primary tumor.
A follow-up to this study from our group investigated the importance of ECM breakdown on visceral metastases (Ecker et al. 2018). Paradoxically, melanoma patients have lower rates of sentinel lymph metastases but have inferior survival and increased visceral metastases (Page et al. 2012). Our study found that lymphatic expression of HAPLN1 was prognostic of long-term survival. HAPLN1 secreted from fibroblasts reduced endothelial cell permeability via modulation of VE-cadherin junctions. Treating aged mice with recombinant HAPLN1 increased lymph node metastases while reducing visceral metastases. These findings may have considerable clinical implications for other cancers that follow a sequential model of progression, where cancer cells spread from the primary tumor, to the lymph node, and then finally to visceral sites (Leong et al. 2006). Importantly, it highlights the importance of maintaining a dense and tightly cross-linked ECM across various microenvironments to inhibit visceral metastatic outgrowth. However, as we will discuss below, it highlights context specificity in that many cancers require increased stiffness, cross-linking, and density within their microenvironment to induce a metastatic phenotype.
Site-Specific Stiffening of ECM in an Aged Microenvironment is Required Contextually for Tumor Progression
Age-related increases in senescent stromal components with a SASP phenotype can secrete soluble factors that contribute to ECM remodeling and matrix stiffening in the local microenvironment (Mavrogonatou et al. 2019). This appears to be a key factor in the TMEs in softer tissue environments which require increased cross-linking and stiffening to allow tumor dissemination. This phenomenon can account for the fact that while the body-wide breakdown of ECM architecture occurs rapidly with aging, local age-related stromal accumulation of SASP cells may foster a tumor-specific niche required for metastasis. Very few studies have directly linked these mechanisms with aging. Malignancies of the lung, breast, and other soft-tissue environments are directly correlated with age and increases in ECM stiffness, cross-linking and collagen density (Benz 2008; Burgstaller et al. 2017). Studies in the aged lung have shown that age-related accumulation of SASP cells increase collagen density and cross linking when compared with the young lung in both human and mouse samples (Calhoun et al. 2016). Similarly, aging of human breast fibroblasts via passaging in culture has been shown to significantly increase secretion of MMPs, proteases, growth factors and other ECM modifying components (Martens et al. 2003), however, an actual comparison of young versus aged human breast fibroblast secretomes was not performed. Further studies are warranted to link these age-induced changes in soft-tissue environments with ECM remodeling and cancer progression.
Overall, these findings highlight the importance of physical changes in the ECM as a mediator of tumor cell trafficking and metastasis. Unfortunately, few studies have been performed directly investigating age-related ECM remodeling on metastatic outgrowth or premetastatic niche formation. There is also a clear need for more mechanistic studies to be performed on the role of aging in immune, SASP and stromal related regulation of ECM components required for metastatic progression. Understanding these influences may allow a therapeutic avenue to alter the ECM and potentially inhibit metastasis.
THE IMMUNE MICROENVIRONMENT
Inflammaging and Metastasis
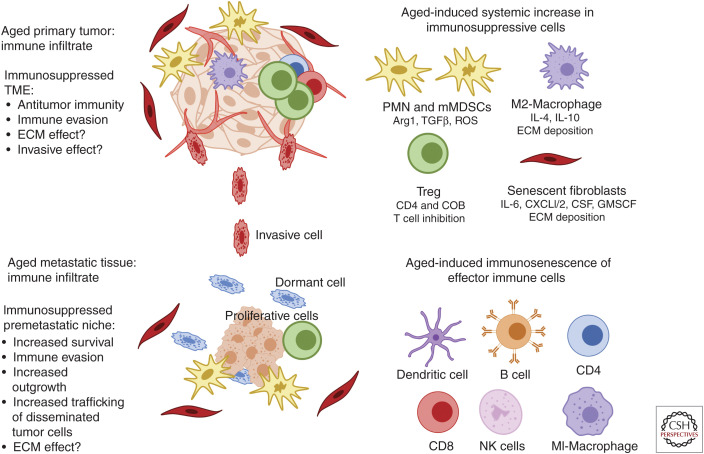
A new concept emerging within the aging field is the idea that an age-related systemic increase in low-grade chronic inflammation (inflammaging), coupled with a decrease in effector immune cell activity and cytotoxicity (immunosenescence) and an increases in systemic immunosuppressive subpopulations may contribute toward efficient metastatic dissemination and outgrowth within elderly patients (Fig. 3; Pawelec 2017; Fulop et al. 2018). One of the hallmarks of aging is an increase in systemic low-grade chronic inflammation, a process termed “inflammaging” (Lopez-Otin et al. 2013). This low-grade, persistent inflammatory response can lead to tissue degeneration, disrupt acute inflammation and is heavily associated with metastatic progression (Leonardi et al. 2018). Cellular senescence has been proposed as a key contributor in linking inflammaging to many age-related malignancies (Leonardi et al. 2018; Olivieri et al. 2018). SASP induction in stromal populations results in the persistently increased secretion of multiple inflammatory cytokines that maintains a low-grade adaptive immune response. Other age-related changes to the gut microbiota, obesity, and tissue degradation also appear to drive the inflammaging response (Zinger et al. 2017). Overall, these age-related processes appear to drive a chronic inflammatory microenvironment which aids in efficient metastatic outgrowth by increasing systemic levels of various interleukins (IL1, IL6, IL1-α, IL1-β), GM-CSF, IFN-γ, Tumor Necrosis Factor alpha (TNFa), and C reactive protein (CRP). These factors are heavily associated with multiple morbidities and mortalities in aged patients (Greene and Loeser 2015; Zinger et al. 2017), however, more direct studies are required linking this process directly with age-related metastatic progression.
Figure 3.
Changes in the aged tumor immune microenvironment. The immune system is crucial in recognizing and targeting cancer cells in both primary and metastatic sites. One of the critical factors involved in age-related pathologies is immunosenescence, a process defined by a decline in effector immune function. Subpopulations of effector immune cells including CD4/CD8 T cells, NK cells, macrophages, B-cells, and dendritic cells, all dramatically decrease in cytotoxic activity during the aging process. Studies are now finding that age-related immunosenescence seems to induce a systemic subtype switch toward more suppressive immune populations. In particular, MDSCs and Tregs are significantly increased in aged tissues and blood, and contextually contribute toward the progression of aged tumor models. These components are also critical for the establishment of premetastatic niche across many cancer subtypes; however, a direct relationship between aging and these populations in premetastatic niche formation has yet to be established. Furthermore, effector cells such as macrophages appear to switch phenotypically toward suppressive M2 states in the elderly, which has been shown to promote cancer cell aggressiveness and metastatic disease, but more direct studies of their involvement in age-related metastasis are warranted. The accumulation of SASP stromal components also results in “inflammaging,” a process defined by persistent low-grade inflammation. This process has been shown to disrupt acute inflammatory responses toward malignant tissue, induce infiltration of immunosuppressive MDSCs and promote the secretion of anti-inflammatory components. These overall changes in immune subtype and functionality appear to contribute toward immune evasion in primary and metastatic tumor sites, while also allowing an immunosuppressed premetastatic niche that promotes efficient outgrowth and increased trafficking of disseminated cancer cells toward these sites. More studies are warranted to directly relate age-related systemic increases in immunosuppressive populations and the effect it has on metastasis, specifically investigating how they may contribute to invasion and ECM remodeling.
Immunosenescence
Immunosenescence is another contributing factor to many age-related malignancies. It is defined as an age-related dysregulation of the innate immune system, whereby subpopulations of effector immune cells, and overall immune function decline (Fig. 3). This process is the result of multiple factors including thymic atrophy (Palmer 2013), decreases in naive T cells(Haynes et al. 2003), a reduction in memory T cell function (Saule et al. 2006) and decreased antigen recognition diversity by T cells (Yager et al. 2008). Inflammaging appears to play a key role in accelerating many of these processes, as chronic inflammatory signals and responses associated with this process are often inhibitory (Fulop et al. 2018). Along with T cell loss of function, NK cells, macrophages, and dendritic cells, which play an immediate role in tumor recognition and suppression, appear to also undergo phenotypic decreases in cytotoxic activity as we age (Fig. 3; Colonna-Romano et al. 2004; Gayoso et al. 2011; Linton and Thoman 2014; Linehan and Fitzgerald 2015). Age-induced immunosenescence occurs largely in effector T cells and other immune subtypes crucial for tumor immunity. It has been hypothesized by our group and others that these changes may induce a shift toward the activation and infiltration of more immunosuppressive populations in the elderly, which may be key in the establishment of a premetastatic niche or a TME that promotes dissemination (Hurez et al. 2018).
Immune Suppressive Populations in the Aging Microenvironment
Tumor-Associated Macrophages
Studies have shown that M2 tumor-associated macrophages (TAMs), which have an immunosuppressive phenotype, are significantly higher in the spleen and bone marrow of aged mice (Jackaman et al. 2013). While the binary M1–M2 classification of macrophages is heavily debated, there is a large amount of evidence that these M2-like immunosuppressive macrophages are key drivers of metastatic progression in an aging context (Aras and Zaidi 2017). TAM induction has also been shown to play a role in setting up a premetastatic niche in the liver by secreting CXCL1 and inducing the recruitment of myeloid-derived suppressor cells (MDSCs), which are necessary for efficient colorectal metastasis (Wang et al. 2017).
Myeloid-Derived Suppressor Cells
One of the key elements that appears to link inflammaging to many types of cancers involves the recruitment of immunosuppressive MDSCs. MDSCs are a heterogenous population of cells defined by their myeloid origin that are potent repressors of T cells through secretion of Arg-1, TGFB, and ROS (Veglia et al. 2018). MDSCs are also significantly linked to the formation of the premetastatic niche in a wide variety of cancers by inducing an immunosuppressive microenvironment that allows outgrowth without being targeted by the immune system (Wang et al. 2019). They also play a key role in promoting tumor-specific trafficking toward metastatic tissue sites (Wang et al. 2019). Studies using the ret transgenic spontaneous murine melanoma model show that persistent up-regulation of inflammaging factors such as IL-1β, GM-CSF and IFN-γ correlate with progression (Meyer et al. 2011). These factors induced MDSC infiltration in primary tumor sites and metastatic lymph nodes. Importantly, treatment with the MDSC inhibitor sildenafil increased survival, decreased metastatic outgrowth and decreased these inflammaging mediators systemically. MDSCs show a consistent increase during aging in human blood (Verschoor et al. 2013), bone marrow and lymphoid organs (Hurez et al. 2012) in mice (Enioutina et al. 2011). MDSCs are one of the most highly associated immune subtypes in the formation of the premetastatic niche in cancer (Wang et al. 2019). It is still unclear how they are recruited to form these premetastatic niches. It is very possible that age-related increases in systemic MDSCs (Enioutina et al. 2011) and other major age-related processes such as SASP accumulation, inflammaging, and ECM modulation described above may directly link MDSCs and aging with premetastatic niche formation and age-related cancer predisposition, however, this has yet to be investigated directly.
Regulatory T Cells (Tregs)
The effects of regulatory T cell (Tregs) contribution to an age-related decline in the immune response are contradictory (Hurez et al. 2018). Tregs play a key role in maintaining tolerance to self-antigens and suppress induction and proliferation of effector T cells. Many studies show a dramatic increase in Treg number and function in age-related pathologies, and in organs such as lymph node and spleen (Sharma et al. 2006; Rosenkranz et al. 2007; Zhao et al. 2007; Kryczek et al. 2009); however, others show no change or reduced contribution in other settings (Kozlowska et al. 2007; Thomas et al. 2007; Kugel et al. 2018), suggesting they have a context-specific role in different microenvironments. Importantly, Treg recruitment appears to be a crucial process in the establishment of the premetastatic niche in many cancer subtypes (Aguado et al. 2017), however, it has yet to be established whether the age-related increases in Tregs seen systemically in certain mouse models is directly linked with increases in age-related metastasis.
Age-Induced Proinflammatory Populations Driving Metastatic Progression
An interesting contradictory observation in certain tumors such as prostate cancer shows that age-induced reprogramming of effector T-cells and proinflammatory cytokines appear to contribute to progression. Many studies in prostate cancer highlight that CD3+, CD4+, and CD8+ T-cell infiltration are seen as protumorigenic correlate with tumor growth, and increase metastatic spread (Strasner and Karin 2015). This highlights further microenvironment contextuality required for effector versus suppressive immune infiltration and age in establishing tumor cell metastasis and premetastatic niche establishment. The bone microenvironment provides another important niche that has been shown to require age-related changes in proinflammatory signals for efficient metastatic growth. Osteoblasts are responsible for bone formation via secretion of a mineralized ECM (Ducy et al. 2000). The bone microenvironment is considered growth restrictive to almost all subtypes of cancers and often promotes dormancy following metastatic dissemination and requires significant remodeling to allow efficient outgrowth (Sosa et al. 2014; Gao et al. 2017). Importantly, studies are now showing age-related changes in stromal components of the bone metastatic niche leads to an accumulation of senescent populations that contribute toward metastatic tumor progression. The fibroblasts accelerate stromal-supported tumorigenesis (FASST) mouse model uses a stromal-specific, estrogen-responsive Cre-recombinase (Cre-ERT2) to create senescent osteoblasts in mice by inducing expression of the cell cycle inhibitor, p27Kip1 (Luo et al. 2016). This led to the discovery that IL6-driven SASP secretion led to an osteoclastogenesis specific remodeling of the bone matrix that enabled the formation of a premetastatic niche for efficient breast cancer colonization (Luo et al. 2016). Given that other cancers such as multiple myeloma are also dependent on a supportive bone microenvironment for their progression (Hameed et al. 2014), it is critical to further understand how this stromal environment ages and supports tumor progression. These studies also highlight a potential overlap in age-related immune signaling and the off-target effects they may have on ECM remodeling and fibroblast function. The interplay between all three aspects and their dramatic age-associated changes may be key in the understanding and targeting of metastasis in elderly patients.
CONCLUSIONS
Age is the predominant prognostic factor for the vast majority of cancers. Cancers arising in the elderly are often more aggressive, and harder to treat. As the population ages, understanding the role of aging in cancer becomes ever more critical. To this end, preclinical studies need to better reflect the role of the aging TME in cancer and the age of patients needs to be considered when deciding on which course of therapy to provide. Understanding the molecular and biophysical changes that occur as a result of normal aging may also provide some insight into the microenvironmental regulation of cancer metastasis. Targeting age-related changes in the stroma will likely provide avenues to overcome therapy resistance and reduce metastatic progression of the cancer cells. Further, changes that occur in the immune system that occur during aging may augment or hamper some of the novel therapies we have for the treatment of cancer, and this needs to be taken into careful consideration.
Footnotes
Editors: Jeffrey W. Pollard and Yibin Kang
Additional Perspectives on Metastasis: Mechanism to Therapy available at www.perspectivesinmedicine.org
REFERENCES
- Acosta JC, O'Loghlen A, Banito A, Guijarro MV, Augert A, Raguz S, Fumagalli M, Da Costa M, Brown C, Popov N, et al. 2008. Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133: 1006–1018. 10.1016/j.cell.2008.03.038 [DOI] [PubMed] [Google Scholar]
- Aguado BA, Bushnell GG, Rao SS, Jeruss JS, Shea LD. 2017. Engineering the pre-metastatic niche. Nat Biomed Eng 1: pii: 0077 10.1038/s41551-017-0077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadzadeh H, Webster MR, Behera R, Valencia AMJ, Wirtz D, Weeraratna AT, Shenoy VB. 2017. Modeling the two-way feedback between contractility and matrix realignment reveals a nonlinear mode of cancer cell invasion. Proc Natl Acad Sci 114: E1617–E1626. 10.1073/pnas.1617037114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aras S, Zaidi MR. 2017. TAMeless traitors: macrophages in cancer progression and metastasis. Br J Cancer 117: 1583–1591. 10.1038/bjc.2017.356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aunan JR, Cho WC, Søreide K. 2017. The biology of aging and cancer: a brief overview of shared and divergent molecular hallmarks. Aging Dis 8: 628–642. 10.14336/AD.2017.0103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, van de Sluis B, Kirkland JL, van Deursen JM. 2011. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479: 232–236. 10.1038/nature10600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balliet RM, Capparelli C, Guido C, Pestell TG, Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Chiavarina B, Pestell RG, Howell A, et al. 2011. Mitochondrial oxidative stress in cancer-associated fibroblasts drives lactate production, promoting breast cancer tumor growth: understanding the aging and cancer connection. Cell Cycle 10: 4065–4073. 10.4161/cc.10.23.18254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behera R, Kaur A, Webster MR, Kim S, Ndoye A, Kugel CH, Alicea GM, Wang J, Ghosh K, Cheng P, et al. 2017. Inhibition of age-related therapy resistance in melanoma by rosiglitazone-mediated induction of Klotho. Clin Cancer Res 23: 3181–3190. 10.1158/1078-0432.CCR-17-0201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benz CC. 2008. Impact of aging on the biology of breast cancer. Crit Rev Oncol Hematol 66: 65–74. 10.1016/j.critrevonc.2007.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgstaller G, Oehrle B, Gerckens M, White ES, Schiller HB, Eickelberg O. 2017. The instructive extracellular matrix of the lung: basic composition and alterations in chronic lung disease. Eur Respir J 50: 1601805 10.1183/13993003.01805-2016 [DOI] [PubMed] [Google Scholar]
- Burton DG, Faragher RG. 2015. Cellular senescence: from growth arrest to immunogenic conversion. Age (Dordr) 37: 27 10.1007/s11357-015-9764-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton DGA, Stolzing A. 2018. Cellular senescence: immunosurveillance and future immunotherapy. Ageing Res Rev 43: 17–25. 10.1016/j.arr.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Calcinotto A, Kohli J, Zagato E, Pellegrini L, Demaria M, Alimonti A. 2019. Cellular senescence: aging, cancer, and injury. Physiol Rev 99: 1047–1078. 10.1152/physrev.00020.2018 [DOI] [PubMed] [Google Scholar]
- Calhoun C, Shivshankar P, Saker M, Sloane LB, Livi CB, Sharp ZD, Orihuela CJ, Adnot S, White ES, Richardson A, et al. 2016. Senescent cells contribute to the physiological remodeling of aged lungs. J Gerontol A Biol Sci Med Sci 71: 153–160. 10.1093/gerona/glu241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilli TC, Xu M, O'Connell MP, Chien B, Frank BP, Subaran S, Indig FE, Morin PJ, Hewitt SM, Weeraratna AT. 2011. Loss of Klotho during melanoma progression leads to increased filamin cleavage, increased Wnt5A expression, and enhanced melanoma cell motility. Pigment Cell Melanoma Res 24: 175–186. 10.1111/j.1755-148X.2010.00792.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J. 2013. Aging, cellular senescence, and cancer. Annu Rev Physiol 75: 685–705. 10.1146/annurev-physiol-030212-183653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, Robert L. 2014. Cell senescence: role in aging and age-related diseases. Interdiscip Top Gerontol 39: 45–61. 10.1159/000358899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna-Romano G, Aquino A, Bulati M, Lio D, Candore G, Oddo G, Scialabba G, Vitello S, Caruso C. 2004. Impairment of γ/δ T lymphocytes in elderly: implications for immunosenescence. Exp Gerontol 39: 1439–1446. 10.1016/j.exger.2004.07.005 [DOI] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J. 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6: 2853–2868. 10.1371/journal.pbio.0060301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Desprez PY, Krtolica A, Campisi J. 2010a. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5: 99–118. 10.1146/annurev-pathol-121808-102144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Krtolica A, Beausejour CM, Parrinello S, Hodgson JG, Chin KE, Desprez PY, Campisi J. 2010b. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One 5: e9188 10.1371/journal.pone.0009188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Erler JT. 2011. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech 4: 165–178. 10.1242/dmm.004077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dollé MET, et al. 2014. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell 31: 722–733. 10.1016/j.devcel.2014.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diridollou S, Vabre V, Berson M, Vaillant L, Black D, Lagarde JM, Gregoire JM, Gall Y, Patat F. 2001. Skin ageing: changes of physical properties of human skin in vivo. Int J Cosmet Sci 23: 353–362. 10.1046/j.0412-5463.2001.00105.x [DOI] [PubMed] [Google Scholar]
- Ducy P, Schinke T, Karsenty G. 2000. The osteoblast: a sophisticated fibroblast under central surveillance. Science 289: 1501–1504. 10.1126/science.289.5484.1501 [DOI] [PubMed] [Google Scholar]
- Eble JA, Niland S. 2019. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastas 36: 171–198. 10.1007/s10585-019-09966-1 [DOI] [PubMed] [Google Scholar]
- Ecker BL, Kaur A, Douglass SM, Webster MR, Almeida FV, Marino G, Sinnamon AJ, Neuwirth MG, Alicea GM, Ndoye A, et al. 2018. Age-related changes in HAPLN1 increase lymphatic permeability and affect routes of melanoma metastasis. Cancer Discov 9: 82–95. 10.1158/2159-8290.CD-18-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzi DJ, Lai Y, Song M, Hakala K, Weintraub ST, Shiio Y. 2012. Plasminogen activator inhibitor 1–insulin-like growth factor binding protein 3 cascade regulates stress-induced senescence. Proc Natl Acad Sci 109: 12052–12057. 10.1073/pnas.1120437109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enioutina EY, Bareyan D, Daynes RA. 2011. A role for immature myeloid cells in immune senescence. J Immunol 186: 697–707. 10.4049/jimmunol.1002987 [DOI] [PubMed] [Google Scholar]
- Faragher RG, McArdle A, Willows A, Ostler EL. 2017. Senescence in the aging process. F1000Res 6: 1219 10.12688/f1000research.10903.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher GJ, Kang S, Varani J, Bata-Csorgo Z, Wan Y, Datta S, Voorhees JJ. 2002. Mechanisms of photoaging and chronological skin aging. Arch Dermatol 138: 1462–1470. 10.1001/archderm.138.11.1462 [DOI] [PubMed] [Google Scholar]
- Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C. 2018. Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front Immunol 8: 1960 10.3389/fimmu.2017.01960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XL, Zhang M, Tang YL, Liang XH. 2017. Cancer cell dormancy: mechanisms and implications of cancer recurrence and metastasis. Onco Targets Ther 10: 5219–5228. 10.2147/OTT.S140854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayoso I, Sanchez-Correa B, Campos C, Alonso C, Pera A, Casado JG, Morgado S, Tarazona R, Solana R. 2011. Immunosenescence of human natural killer cells. J Innate Immun 3: 337–343. 10.1159/000328005 [DOI] [PubMed] [Google Scholar]
- Gialeli C, Theocharis AD, Karamanos NK. 2011. Roles of matrix metalloproteinases in cancer progression and their pharmacological targeting. Febs J 278: 16–27. 10.1111/j.1742-4658.2010.07919.x [DOI] [PubMed] [Google Scholar]
- Greene MA, Loeser RF. 2015. Aging-related inflammation in osteoarthritis. Osteoarthritis Cartilage 23: 1966–1971. 10.1016/j.joca.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hameed A, Brady JJ, Dowling P, Clynes M, O'Gorman P. 2014. Bone disease in multiple myeloma: pathophysiology and management. Cancer Growth Metastasis 7: 33–42. 10.4137/CGM.S16817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. 2011. Hallmarks of cancer: the next generation. Cell 144: 646–674. 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- Haynes L, Eaton SM, Burns EM, Randall TD, Swain SL. 2003. CD4 T cell memory derived from young naive cells functions well into old age, but memory generated from aged naive cells functions poorly. Proc Natl Acad Sci 100: 15053–15058. 10.1073/pnas.2433717100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang-Lee LL, Nimni ME. 1994. Crosslinked CNBr-activated hyaluronan–collagen matrices: effects on fibroblast contraction. Matrix Biol 14: 147–157. 10.1016/0945-053X(94)90004-3 [DOI] [PubMed] [Google Scholar]
- Hurez V, Daniel BJ, Sun L, Liu AJ, Ludwig SM, Kious MJ, Thibodeaux SR, Pandeswara S, Murthy K, Livi CB, et al. 2012. Mitigating age-related immune dysfunction heightens the efficacy of tumor immunotherapy in aged mice. Cancer Res 72: 2089–2099. 10.1158/0008-5472.CAN-11-3019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurez V, Padrón Á, Svatek RS, Curiel TJ. 2018. Considerations for successful cancer immunotherapy in aged hosts. Exp Gerontol 107: 27–36. 10.1016/j.exger.2017.10.002 [DOI] [PubMed] [Google Scholar]
- Jackaman C, Radley-Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, Nelson DJ. 2013. Targeting macrophages rescues age-related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 12: 345–357. 10.1111/acel.12062 [DOI] [PubMed] [Google Scholar]
- Karjalainen JM, Tammi RH, Tammi MI, Eskelinen MJ, Ågren UM, Parkkinen JJ, Alhava EM, Kosma VM. 2000. Reduced level of CD44 and hyaluronan associated with unfavorable prognosis in clinical stage I cutaneous melanoma. Am J Pathol 157: 957–965. 10.1016/S0002-9440(10)64608-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Webster MR, Marchbank K, Behera R, Ndoye A, Kugel CH, Dang VM, Appleton J, O'Connell MP, Cheng P, et al. 2016a. sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance. Nature 532: 250–254. 10.1038/nature17392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Webster MR, Weeraratna AT. 2016b. In the Wnt-er of life: Wnt signalling in melanoma and ageing. Br J Cancer 115: 1273–1279. 10.1038/bjc.2016.332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur A, Ecker BL, Douglass SM, Kugel CH, Webster MR, Almeida FV, Somasundaram R, Hayden J, Ban E, Ahmadzadeh H, et al. 2018. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov 9: 64–81. 10.1158/2159-8290.CD-18-0193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Rebecca V, Fedorenko IV, Messina JL, Mathew R, Maria-Engler SS, Basanta D, Smalley KS, Anderson AR. 2013. Senescent fibroblasts in melanoma initiation and progression: an integrated theoretical, experimental, and clinical approach. Cancer Res 73: 6874–6885. 10.1158/0008-5472.CAN-13-1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R. 2006. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol 8: 877–884. 10.1038/ncb1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowska E, Biernacka M, Ciechomska M, Drela N. 2007. Age-related changes in the occurrence and characteristics of thymic CD4+ CD25+ T cells in mice. Immunology 122: 445–453. 10.1111/j.1365-2567.2007.02667.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtolica A, Parrinello S, Lockett S, Desprez PY, Campisi J. 2001. Senescent fibroblasts promote epithelial cell growth and tumorigenesis: a link between cancer and aging. Proc Natl Acad Sci 98: 12072–12077. 10.1073/pnas.211053698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczek I, Liu R, Wang G, Wu K, Shu X, Szeliga W, Vatan L, Finlayson E, Huang E, Simeone D, et al. 2009. FOXP3 defines regulatory T cells in human tumor and autoimmune disease. Cancer Res 69: 3995–4000. 10.1158/0008-5472.CAN-08-3804 [DOI] [PubMed] [Google Scholar]
- Kugel CH 3rd, Douglass SM, Webster MR, Kaur A, Liu Q, Yin X, Weiss SA, Darvishian F, Al-Rohil RN, Ndoye A, et al. 2018. Age correlates with response to anti-PD1, reflecting age-related differences in intratumoral effector and regulatory T-cell populations. Clin Cancer Res. 24: 5347–5356. 10.1158/1078-0432.CCR-18-1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS. 2008. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell 133: 1019–1031. 10.1016/j.cell.2008.03.039 [DOI] [PubMed] [Google Scholar]
- Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, et al. 2005. Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833. 10.1126/science.1112766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L, Porciuncula A, Yu A, Iwakura Y, David G. 2019. Uncoupling the senescence-associated secretory phenotype from cell cycle exit via IL-1 inactivation unveils its pro-tumorigenic role. Mol Cell Biol 39: pii: e00586-18 10.1128/MCB.00586-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrenson K, Grun B, Benjamin E, Jacobs IJ, Dafou D, Gayther SA. 2010. Senescent fibroblasts promote neoplastic transformation of partially transformed ovarian epithelial cells in a three-dimensional model of early stage ovarian cancer. Neoplasia 12: 317–325. 10.1593/neo.91948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Schmitt CA. 2019. The dynamic nature of senescence in cancer. Nat Cell Biol 21: 94–101. 10.1038/s41556-018-0249-2 [DOI] [PubMed] [Google Scholar]
- Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. 2011. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer 11; 245 10.1186/1471-2407-11-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonardi GC, Accardi G, Monastero R, Nicoletti F, Libra M. 2018. Ageing: from inflammation to cancer. Immun Ageing 15: 1 10.1186/s12979-017-0112-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong SP, Cady B, Jablons DM, Garcia-Aguilar J, Reintgen D, Jakub J, Pendas S, Duhaime L, Cassell R, Gardner M, et al. 2006. Clinical patterns of metastasis. Cancer Metastasis Rev 25: 221–232. 10.1007/s10555-006-8502-8 [DOI] [PubMed] [Google Scholar]
- Lesina M, Wörmann SM, Morton J, Diakopoulos KN, Korneeva O, Wimmer M, Einwächter H, Sperveslage J, Demir IE, Kehl T, et al. 2016. RelA regulates CXCL1/CXCR2-dependent oncogene-induced senescence in murine Kras-driven pancreatic carcinogenesis. J Clin Invest 126: 2919–2932. 10.1172/JCI86477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, et al. 2009. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell 139: 891–906. 10.1016/j.cell.2009.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linehan E, Fitzgerald DC. 2015. Ageing and the immune system: focus on macrophages. Eur J Microbiol Immunol (Bp) 5: 14–24. 10.1556/EuJMI-D-14-00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linton PJ, Thoman ML. 2014. Immunosenescence in monocytes, macrophages, and dendritic cells: lessons learned from the lung and heart. Immunol Lett 162: 290–297. 10.1016/j.imlet.2014.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Hornsby PJ. 2007. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res 67: 3117–3126. 10.1158/0008-5472.CAN-06-3452 [DOI] [PubMed] [Google Scholar]
- Liu JY, Souroullas GP, Diekman BO, Krishnamurthy J, Hall BM, Sorrentino JA, Parker JS, Sessions GA, Gudkov AV, Sharpless NE. 2019. Cells exhibiting strong p16INK4a promoter activation in vivo display features of senescence. Proc Natl Acad Sci 116: 2603–2611. 10.1073/pnas.1818313116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. 2013. The hallmarks of aging. Cell 153: 1194–1217. 10.1016/j.cell.2013.05.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X, Fu Y, Loza AJ, Murali B, Leahy KM, Ruhland MK, Gang M, Su X, Zamani A, Shi Y, et al. 2016. Stromal-initiated changes in the bone promote metastatic niche development. Cell Rep 14: 82–92. 10.1016/j.celrep.2015.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maegawa S, Hinkal G, Kim HS, Shen L, Zhang L, Zhang J, Zhang N, Liang S, Donehower LA, Issa JP. 2010. Widespread and tissue specific age-related DNA methylation changes in mice. Genome Res 20: 332–340. 10.1101/gr.096826.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcos-Garcés V, Molina Aguilar P, Bea Serrano C, García Bustos V, Benavent Seguí JB, Ferrández Izquierdo A, Ruiz-Sauri A. 2014. Age-related dermal collagen changes during development, maturation and ageing—a morphometric and comparative study. J Anat 225: 98–108. 10.1111/joa.12186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens JWM, Sieuwerts AM, Bolt-de Vries J, Bosma PT, Swiggers SJJ, Klijn JGM, Foekens JA. 2003. Aging of stromal-derived human breast fibroblasts might contribute to breast cancer progression. Thromb Haemostasis 89: 393–404. 10.1055/s-0037-1613457 [DOI] [PubMed] [Google Scholar]
- Martin H, Dean M. 1993. An N-terminal peptide from link protein is rapidly degraded by chondrocytes, monocytes and B-cells. Eur J Biochem 212: 87–94. 10.1111/j.1432-1033.1993.tb17636.x [DOI] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Trimmer C, Lin Z, Whitaker-Menezes D, Chiavarina B, Zhou J, Wang C, Pavlides S, Martinez-Cantarin MP, Capozza F, et al. 2010. Autophagy in cancer-associated fibroblasts promotes tumor cell survival: role of hypoxia, HIF1 induction and NFκB activation in the tumor stromal microenvironment. Cell Cycle 9: 3515–3533. 10.4161/cc.9.17.12928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Outschoorn UE, Pavlides S, Howell A, Pestell RG, Tanowitz HB, Sotgia F, Lisanti MP. 2011. Stromal-epithelial metabolic coupling in cancer: integrating autophagy and metabolism in the tumor microenvironment. Int J Biochem Cell B 43: 1045–1051. 10.1016/j.biocel.2011.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H, Chikuda H, Suga T, Kawaguchi H, Kuro-o M. 2005. Regulation of multiple ageing-like phenotypes by inducible klotho gene expression in klotho mutant mice. Mech Ageing Dev 126: 1274–1283. 10.1016/j.mad.2005.07.007 [DOI] [PubMed] [Google Scholar]
- Mavrogonatou E, Pratsinis H, Papadopoulou A, Karamanos NK, Kletsas D. 2019. Extracellular matrix alterations in senescent cells and their significance in tissue homeostasis. Matrix Biol 75-76: 27–42. 10.1016/j.matbio.2017.10.004 [DOI] [PubMed] [Google Scholar]
- McHugh D, Gil J. 2018. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol 217: 65–77. 10.1083/jcb.201708092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W, Borrello I, Kato M, Schadendorf D, Baniyash M, et al. 2011. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci 108: 17111–17116. 10.1073/pnas.1108121108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriya J, Minamino T. 2017. Angiogenesis, cancer, and vascular aging. Front Cardiovasc Med 4: 65 10.3389/fcvm.2017.00065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Espin D, Cañamero M, Maraver A, Gómez-López G, Contreras J, Murillo-Cuesta S, Rodríguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, et al. 2013. Programmed cell senescence during mammalian embryonic development. Cell 155: 1104–1118. 10.1016/j.cell.2013.10.019 [DOI] [PubMed] [Google Scholar]
- Naba A, Clauser KR, Hoersch S, Liu H, Carr SA, Hynes RO. 2012. The matrisome: in silico definition and in vivo characterization by proteomics of normal and tumor extracellular matrices. Mol Cell Proteomics 11: M111.014647 10.1074/mcp.M111.014647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DM, McBryan T, Jeyapalan JC, Sedivy JM, Adams PD. 2014. A comparison of oncogene-induced senescence and replicative senescence: implications for tumor suppression and aging. Age (Dordr) 36: 9637 10.1007/s11357-014-9637-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh JH, Kim YK, Jung JY, Shin JE, Kim KH, Cho KH, Eun HC, Chung JH. 2011. Intrinsic aging- and photoaging-dependent level changes of glycosaminoglycans and their correlation with water content in human skin. J Dermatol Sci 62: 192–201. 10.1016/j.jdermsci.2011.02.007 [DOI] [PubMed] [Google Scholar]
- Olivieri F, Prattichizzo F, Grillari J, Balistreri CR. 2018. Cellular senescence and inflammaging in age-related diseases. Mediators Inflamm 2018: 9076485 10.1155/2018/9076485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özcan S, Alessio N, Acar MB, Mert E, Omerli F, Peluso G, Galderisi U. 2016. Unbiased analysis of senescence associated secretory phenotype (SASP) to identify common components following different genotoxic stresses. Aging (Albany NY) 8: 1316–1329. 10.18632/aging.100971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page AJ, Li A, Hestley A, Murray D, Carlson GW, Delman KA. 2012. Increasing age is associated with worse prognostic factors and increased distant recurrences despite fewer sentinel lymph node positives in melanoma. Int J Surg Oncol 2012: 456987 10.1155/2012/456987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer DB. 2013. The effect of age on thymic function. Front Immunol 4: 316 10.3389/fimmu.2013.00316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar P, Lamour G, Mackenzie NC, Yang H, Ko F, Li H, Brömme D. 2015. Changes in structural-mechanical properties and degradability of collagen during aging-associated modifications. J Biol Chem 290: 23291–23306. 10.1074/jbc.M115.644310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides S, Tsirigos A, Migneco G, Whitaker-Menezes D, Chiavarina B, Flomenberg N, Frank PG, Casimiro MC, Wang C, Pestell RG, et al. 2010. The autophagic tumor stroma model of cancer: role of oxidative stress and ketone production in fueling tumor cell metabolism. Cell Cycle 9: 3485–3505. 10.4161/cc.9.17.12721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G. 2017. Immunosenescence and cancer. Biogerontology 18: 717–721. 10.1007/s10522-017-9682-z [DOI] [PubMed] [Google Scholar]
- Pickup MW, Mouw JK, Weaver VM. 2014. The extracellular matrix modulates the hallmarks of cancer. EMBO Rep 15: 1243–1253. 10.15252/embr.201439246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltavets V, Kochetkova M, Pitson SM, Samuel MS. 2018. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front Oncol 8: 431 10.3389/fonc.2018.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rielland M, Cantor DJ, Graveline R, Hajdu C, Mara L, Diaz Bde D, Miller G, David G. 2014. Senescence-associated SIN3B promotes inflammation and pancreatic cancer progression. J Clin Invest 124: 2125–2135. 10.1172/JCI72619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roark EF, Keene DR, Haudenschild CC, Godyna S, Little CD, Argraves WS. 1995. The association of human fibulin-1 with elastic fibers: an immunohistological, ultrastructural, and RNA study. J Histochem Cytochem 43: 401–411. 10.1177/43.4.7534784 [DOI] [PubMed] [Google Scholar]
- Rosenkranz D, Weyer S, Tolosa E, Gaenslen A, Berg D, Leyhe T, Gasser T, Stoltze L. 2007. Higher frequency of regulatory T cells in the elderly and increased suppressive activity in neurodegeneration. J Neuroimmunol 188: 117–127. 10.1016/j.jneuroim.2007.05.011 [DOI] [PubMed] [Google Scholar]
- Rozhok A, DeGregori J. 2019. A generalized theory of a age-dependent carcinogenesis. Elife 8: pii: e3995 10.7554/eLife.39950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozhok AI, Salstrom JL, DeGregori J. 2014. Stochastic modeling indicates that aging and somatic evolution in the hematopoietic system are driven by non-cell-autonomous processes. Aging (Albany NY) 6: 1033–1048. 10.18632/aging.100707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruchti C, Haller D, Nuber M, Cottier H. 1983. Regional differences in renewal rates of fibroblasts in young adult female mice. Cell Tissue Res 232: 625–636. 10.1007/BF00216434 [DOI] [PubMed] [Google Scholar]
- Salama R, Sadaie M, Hoare M, Narita M. 2014. Cellular senescence and its effector programs. Genes Dev 28: 99–114. 10.1101/gad.235184.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada F, Taniyama Y, Muratsu J, Otsu R, Shimizu H, Rakugi H, Morishita R. 2018. IGF binding protein-5 induces cell senescence. Front Endocrinol (Lausanne) 9: 53 10.3389/fendo.2018.00053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saule P, Trauet J, Dutriez V, Lekeux V, Dessaint JP, Labalette M. 2006. Accumulation of memory T cells from childhood to old age: central and effector memory cells in CD4+ versus effector memory and terminally differentiated memory cells in CD8+ compartment. Mech Ageing Dev 127: 274–281. 10.1016/j.mad.2005.11.001 [DOI] [PubMed] [Google Scholar]
- Sephel GC, Davidson JM. 1986. Elastin production in human skin fibroblast cultures and its decline with age. J Invest Dermatol 86: 279–285. 10.1111/1523-1747.ep12285424 [DOI] [PubMed] [Google Scholar]
- Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A. 2013. Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells. Cell Death Dis 4: e911 10.1038/cddis.2013.445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Dominguez AL, Lustgarten J. 2006. High accumulation of T regulatory cells prevents the activation of immune responses in aged animals. J Immunol 177: 8348–8355. 10.4049/jimmunol.177.12.8348 [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. 2018. Cancer statistics, 2018. CA Cancer J Clin 68: 7–30. 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- Sosa MS, Bragado P, Aguirre-Ghiso JA. 2014. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nature Rev Cancer 14: 611–622. 10.1038/nrc3793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeg PS. 2016. Targeting metastasis. Nat Rev Cancer 16: 201–218. 10.1038/nrc.2016.25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storer M, Mas A, Robert-Moreno A, Pecoraro M, Ortells MC, Di Giacomo V, Yosef R, Pilpel N, Krizhanovsky V, Sharpe J, et al. 2013. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155: 1119–1130. 10.1016/j.cell.2013.10.041 [DOI] [PubMed] [Google Scholar]
- Strasner A, Karin M. 2015. Immune infiltration and prostate cancer. Front Oncol 5: 128 10.3389/fonc.2015.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DC, Mellanby RJ, Phillips JM, Cooke A. 2007. An early age-related increase in the frequency of CD4+ Foxp3+ cells in BDC2·5NOD mice. Immunology 121: 565–576. 10.1111/j.1365-2567.2007.02604.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veglia F, Perego M, Gabrilovich D. 2018. Myeloid-derived suppressor cells coming of age. Nat Immunol 19: 108–119. 10.1038/s41590-017-0022-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschoor CP, Johnstone J, Millar J, Dorrington MG, Habibagahi M, Lelic A, Loeb M, Bramson JL, Bowdish DM. 2013. Blood CD33(+)HLA-DR(−) myeloid-derived suppressor cells are increased with age and a history of cancer. J Leukoc Biol 93: 633–637. 10.1189/jlb.0912461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajapeyee N, Serra RW, Zhu X, Mahalingam M, Green MR. 2008. Oncogenic BRAF induces senescence and apoptosis through pathways mediated by the secreted protein IGFBP7. Cell 132: 363–374. 10.1016/j.cell.2007.12.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C, Mojares E, Del Rio Hernandez A. 2018. Role of extracellular matrix in development and cancer progression. Int J Mol Sci 19: pii: E3028 10.3390/ijms19103028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Abhilash AS, Chen CS, Wells RG, Shenoy VB. 2014. Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys J 107: 2592–2603. 10.1016/j.bpj.2014.09.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DZ, Sun HY, DuBois RN. 2017. CXCL1 is critical for pre-metastatic niche formation and metastasis in colorectal cancer. Cancer Res 77: 3655 10.1158/0008-5472.CAN-16-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ding Y, Guo N, Wang S. 2019. MDSCs: key criminals of tumor pre-metastatic niche formation. Front Immunol 10: 172 10.3389/fimmu.2019.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MR, Xu M, Kinzler KA, Kaur A, Appleton J, O'Connell MP, Marchbank K, Valiga A, Dang VM, Perego M, et al. 2015. Wnt5A promotes an adaptive, senescent-like stress response, while continuing to drive invasion in melanoma cells. Pigment Cell Melanoma Res 28: 184–195. 10.1111/pcmr.12330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yager EJ, Ahmed M, Lanzer K, Randall TD, Woodland DL, Blackman MA. 2008. Age-associated decline in T cell repertoire diversity leads to holes in the repertoire and impaired immunity to influenza virus. J Exp Med 205: 711–723. 10.1084/jem.20071140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L, Sun L, Wang H, Ma H, Liu G, Zhao Y. 2007. Changes of CD4+CD25+Foxp3+ regulatory T cells in aged Balb/c mice. J Leukoc Biol 81: 1386–1394. 10.1189/jlb.0506364 [DOI] [PubMed] [Google Scholar]
- Zinger A, Cho WC, Ben-Yehuda A. 2017. Cancer and aging—the inflammatory connection. Aging Dis 8: 611–627. 10.14336/AD.2016.1230 [DOI] [PMC free article] [PubMed] [Google Scholar]