Abstract
A subset of individuals with autism spectrum disorder (ASD) and macrocephaly carry mutations in the gene PTEN. Animal models, particularly mice, have been helpful in establishing a causal role for Pten mutations in autism-relevant behavioral deficits. These models are a useful tool for investigating neurobiological mechanisms of these behavioral phenotypes and developing potential therapeutic interventions. Here we provide an overview of various genetic mouse models that have been used to characterize behavioral phenotypes caused by perturbation of Pten. We discuss convergent and divergent phenotypes across models with the aim of highlighting a set of behavioral domains that are sensitive to the effects of Pten mutation and that may provide useful readouts for translational and basic neuroscience research.
Autism spectrum disorder (ASD) is a neurodevelopmental disorder occurring in 1 in 59 children in the United States (Baio et al. 2018), and is highly heritable (83%; Sandin et al. 2017). The diagnostic criteria for ASD are behavioral—consisting primarily of deficits in social behavior and communication, and restricted, repetitive, and stereotyped patterns of behavior (DSM-V, American Psychiatric Association 2013). Additionally, several disorders show frequent comorbidity with ASD, including mood disorders (∼60%; Skokauskas and Gallagher 2010), anxiety disorders (∼80%; Skokauskas and Gallagher 2010), and intellectual disability (∼60%; Matson and Shoemaker 2009).
Although autism is defined and diagnosed based on behavioral criteria, a subset of individuals display alterations in the normal trajectory of head and brain growth (Courchesne et al. 2007). Macrocephaly—consisting of a head circumference more than two standard deviations above normal—is present in ∼15%–20% of individuals with ASD (Lainhart et al. 2006; Sacco et al. 2015; Albores-Gallo et al. 2017). Of these, ∼10%–25% (Butler et al. 2005; Buxbaum et al. 2007; Varga et al. 2009; McBride et al. 2010; Klein et al. 2013; Hobert et al. 2014; Yeung et al. 2017) also have mutations in Phosphatase and tensin homolog (PTEN), which is causative of macrocephaly/autism syndrome (MIM #605309).
In humans, PTEN mutations lead to a highly penetrant neurodevelopmental phenotype, resulting in an abnormal brain structure, ASD, and/or intellectual disabilities (Mester et al. 2011; Busch et al. 2013; Hobert et al. 2014). Germline heterozygous PTEN mutations are associated with macrocephaly/autism syndrome (MIM #605309), as well as Cowden 1 (MIM #158350; ∼80% have PTEN mutations; Blumenthal and Dennis 2008), Bannayan–Riley–Ruvalcaba (MIM #153480), and Lhermitte–Duclos syndromes (MIM #158350), collectively known as PTEN hamartoma tumor syndromes (PHTS; see Blumenthal and Dennis 2008 for a review). The primary behavioral phenotype for individuals with PTEN mutations, in addition to ASD, include lower verbal and nonverbal IQ (Herman et al. 2007; Conti et al. 2012; Rosti et al. 2014; Frazier et al. 2015; Stessman et al. 2017), decreased processing speed (Frazier et al. 2015), deficits in memory recall, particularly in working memory (Busch et al. 2013; Frazier et al. 2015), and short attention spans (Goffin et al. 2001; Butler et al. 2005; Boccone et al. 2006; Herman et al. 2007), as well as impaired motor and fine motor skills (Goffin et al. 2001; Butler et al. 2005; Herman et al. 2007; Orrico et al. 2009; Conti et al. 2012; Busch et al. 2013; Frazier et al. 2015).
Given the genetic heterogeneity present in the human clinical population, a key question arising from early clinical reports of individuals with PTEN mutations and ASD was whether PTEN mutations are sufficient to induce ASD-related behavioral symptoms. Animal models proved useful in addressing this question, and it was found that conditional homozygous loss-of-function (Kwon et al. 2006), germline heterozygous loss-of-function (Page et al. 2009), and germline homozygous cytoplasm-predominant knockin (Tilot et al. 2014) mutations in Pten all result in ASD-relevant behavioral deficits, along with brain overgrowth, in mice. These and numerous other mouse models of Pten mutations have been applied to understanding the neurobiological substrates of behavioral abnormalities caused by Pten mutations and to testing potential mechanisms and therapeutic interventions. In this review, we examine similarities and differences in behavioral phenotypes across Pten mouse models (see Table 1 for model descriptions), with a focus on those directly related to ASD (social behavior, repetitive behavior) and those modeling ASD comorbidities (mood and anxiety disorders, intellectual disability, sleep and circadian rhythms, motor behavior, and sensory sensitivity; see Table 2). With this scope in mind, we will limit our discussion to studies that help define a “profile” of behavioral phenotypes caused by Pten mutations, and we will not discuss here the numerous important studies that are elucidating potential molecular, cellular, neuroanatomical, and circuit-level substrates for these effects.
Table 1.
List of mouse models with Pten mutations included in this review
| Type | Pten allele | Pten perturbation | Site of Pten perturbation | Common name | References | MGI link |
|---|---|---|---|---|---|---|
| Germline | Pten+/− [Ptentm1Rps] | Loss-of-function (haploinsufficiency) | Ubiquitous | Pten+/− | Page et al. 2009; Clipperton-Allen and Page 2014, 2015; Séjourné et al. 2015; Huang et al. 2016 | www.informatics.jax.org/allele/MGI:2151804 |
| Germline | Ptenm3m4 [Ptentm1Engc] | ↓ Nuclear: cytoplasm ratio | Ubiquitous | Ptenm3m4 | Mester et al. 2011; Tilot et al. 2014 | www.informatics.jax.org/allele/reference/J:210487 |
| Germline | Ptenαmu | Deletion of PTENα isoform | Ubiquitous | Ptenαmu | Wang et al. 2017 | n/a |
| Germline | PtenTg [Tg(Pten)1Srn] | Overexpression | Ubiquitous | PtenTg | Sanchez-Puelles et al. 2019 | www.informatics.jax.org/allele/MGI:5645732 |
| Germline | Pten ΔPDZ [Ptentm1.1Jaes] | Deletion of PDZ-binding domain | Ubiquitous | PtenΔPDZ | Sanchez-Puelles et al. 2019 | www.informatics.jax.org/allele/key/868490 |
| Conditional (PtenloxP) | Ptentm2Mak | Loss-of-function | CB and DG granule cells, some CTX neurons [Tg(Gfap-cre)1Sbk] | NS-Cre; PtenloxP | Backman et al. 2001; Kwon et al. 2001, 2003; Ljungberg et al. 2009; Lugo et al. 2014, 2017; Nguyen et al. 2015; Smith et al. 2016; Binder and Lugo 2017; Hodges et al. 2018 |
Tg(Gfap-cre)1Sbk: www.informatics.jax.org/allele/MGI:2448664 Ptentm2Mak: www.informatics.jax.org/allele/MGI:2182005 |
| Conditional (PtenloxP) |
Ptentm2Mak Ptentm1Hwu |
Loss-of-function | Mature CTX and HIPP neurons [Tg(Eno2-cre)39Jme, Tg(Eno2-cre)2Lfp] | Nse-Cre; PtenloxP | Kwon et al. 2006; Ogawa et al. 2007; Zhou et al. 2009; Napoli et al. 2012; Nolan et al. 2019 |
Tg(Eno2-cre)39Jme: www.informatics.jax.org/allele/MGI:2177175 Tg(Eno2-cre)2Lfp: www.informatics.jax.org/allele/MGI:3621475 Ptentm2Mak: www.informatics.jax.org/allele/MGI:2182005 Ptentm1Hwu: www.informatics.jax.org/allele/MGI:2156086 |
| Conditional (PtenloxP) | Ptentm2Mak | Loss-of-function | Inducible; postnatal NSCs in SGZ, SVZ [Tg(Nes-cre/ERT2)73Lfp] | Nestin-Cre; PtenloxP | Amiri et al. 2012 |
Tg(Nes-cre/ERT2)73Lfp: www.informatics.jax.org/allele/MGI:3840184 Ptentm2Mak: www.informatics.jax.org/allele/MGI:2182005 |
| Conditional (PtenloxP) | Ptentm1Hwu | Loss-of-function | NSCs [Tg(Gfap-cre)77.6Mvs] | NSC-Cre; PtenloxP | Gregorian et al. 2009 |
Tg(Gfap-cre)77.6Mvs: www.informatics.jax.org/allele/MGI:3838840 Ptentm1Hwu: www.informatics.jax.org/allele/MGI:2156086 |
| Conditional (PtenloxP) | Ptentm1.1Mwst | Loss-of-function | Mesenchymal cells and WM astrocytes [Tg(S100a4-cre)1Gle] | Fsp1-Cre; PtenloxP | Borniger et al. 2016 |
Tg(S100a4-cre)1Gle: www.informatics.jax.org/allele/MGI:3775823 Ptentm1.1Mwst: www.informatics.jax.org/allele/MGI:4366755 |
| Conditional (PtenloxP) | Ptentm1Hwu | Loss-of-function | NeoCTX and HIPP neurons, subset of glia [Emx1tm1(cre)Krj] | Emx1-Cre; PtenloxP | Huang et al. 2016 |
Emx1tm1(cre)Krj: www.informatics.jax.org/allele/MGI:2684610 Ptentm1Hwu: www.informatics.jax.org/allele/MGI:2156086 |
| Conditional (PtenloxP) | Ptentm1Hwu | Loss-of-function | Oxytocinergic neurons [Oxttm1.1(cre)Dolsn] | Oxt-Cre; PtenloxP | Clipperton-Allen et al. 2016 |
Oxttm1.1(cre)Dolsn: www.informatics.jax.org/allele/MGI:5523143 Ptentm1Hwu: www.informatics.jax.org/allele/MGI:2156086 |
| Conditional (PtenloxP) | Ptentm1Hwu | Loss-of-function | Purkinje cells [Tg(Pcp2-cre)2Mpin] | L7-Cre; PtenloxP | Cupolillo et al. 2016 |
Tg(Pcp2-cre)2Mpin: www.informatics.jax.org/allele/MGI:2137515 Ptentm1Hwu: www.informatics.jax.org/allele/MGI:2156086 |
| Conditional (PtenloxP) | Ptentm1Hwu | Loss-of-function | Dopaminergic neurons [Slc6a3tm1.1(cre)Bkmn] | DAT-Cre; PtenloxP | Diaz-Ruiz et al. 2009; Clipperton-Allen and Page 2014 |
Slc6a3tm1.1(cre)Bkmn: www.informatics.jax.org/allele/MGI:3689434 Ptentm1Hwu: www.informatics.jax.org/allele/MGI:2156086 |
| Conditional (PtenloxP) | Ptentm2Mak | Loss-of-function | Subsets of DG, HAN, AMYG neurons [Tg(Pomc-cre)1Lowl] | Pomc-Cre; PtenloxP | Matsushita et al. 2016 |
Tg(Pomc-cre)1Lowl: www.informatics.jax.org/allele/MGI:4362028 Ptentm2Mak: www.informatics.jax.org/allele/MGI:2182005 |
| Conditional (PtenloxP) | Ptentm2Mak | Loss-of-function | Excitatory neurons [Tg(Camk2a-cre)T29-1Stl] | CaMKIIa-Cre; PtenloxP | Sperow et al. 2012 |
Tg(Camk2a-cre)T29-1Stl: www.informatics.jax.org/allele/MGI:2177650 Ptentm2Mak: www.informatics.jax.org/allele/MGI:2182005 |
| Conditional (PtenloxP) | Ptentm2Mak | Loss-of-function | MGE and MPOA progenitors [Tg(Nkx2-1-cre)2Sand] | Nkx2.1-Cre; PtenloxP | Vogt et al. 2015 |
Tg(Nkx2-1-cre)2Sand: www.informatics.jax.org/allele/MGI:3773076 Ptentm2Mak: www.informatics.jax.org/allele/MGI:2182005 |
| Conditional (PtenloxP) | Ptentm1Hwu | Loss-of-function | AAV-Cre in somatosensory CTX at P1 | n/a | Gutilla et al. 2016 | Ptentm1Hwu: www.informatics.jax.org/allele/MGI:2156086 |
| shRNA targeting Pten | n/a | shRNA knockdown | BLA, LA | n/a | Haws et al. 2014 | n/a |
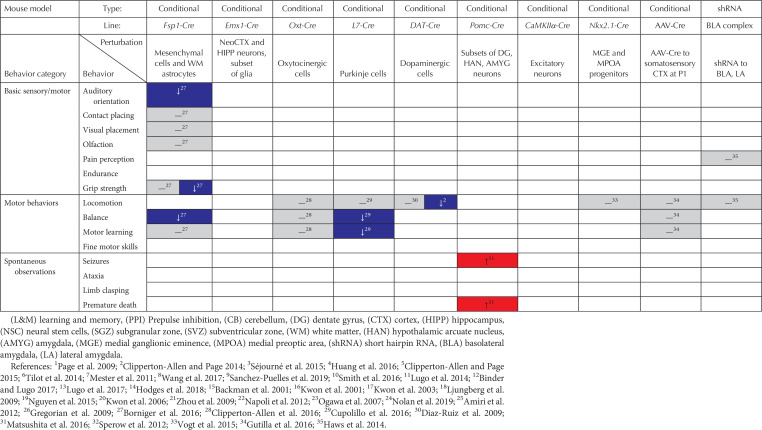
(CB) cerebellum, (DG) dentate gyrus, (CTX) cortex, (HIPP) hippocampus, (NSC) neural stem cells, (SGZ) subgranular zone, (SVZ) subventricular zone, (WM) white matter, (HAN) hypothalamic arcuate nucleus, (AMYG) amygdala, (MGE) medial ganglionic eminence, (MPOA) medial preoptic area, (shRNA) short hairpin RNA, (BLA) basolateral amygdala, (LA) lateral amygdala.
Table 2.
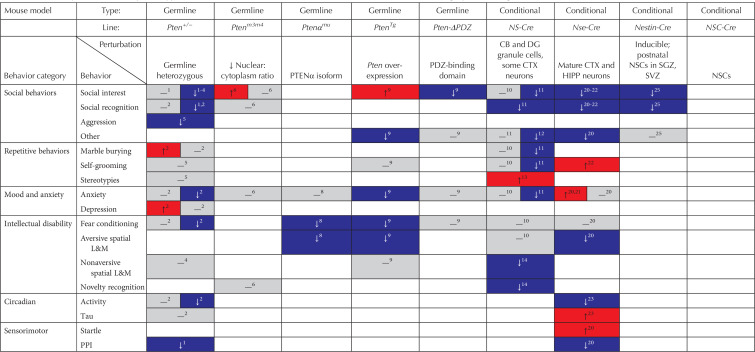
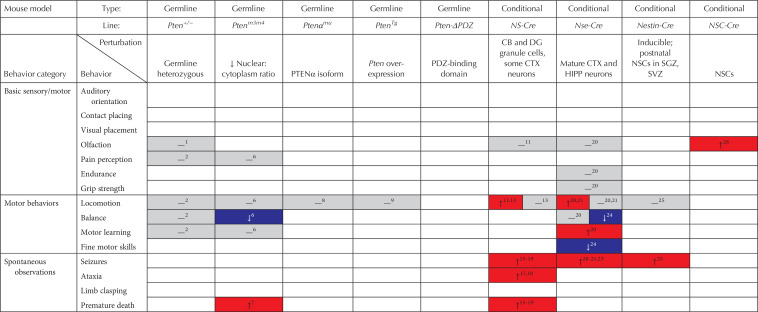
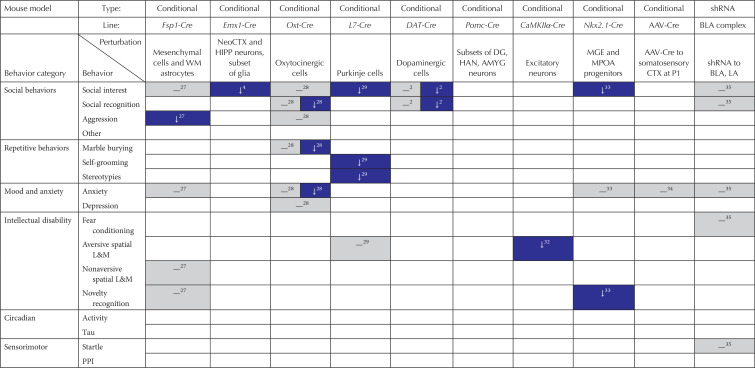
Summary of behavioral phenotypes in mouse models of Pten mutations (red, increase in behavior; gray, no change in behavior; blue, decrease in behavior)
ASD-RELEVANT BEHAVIORS
Social Behavior
As impaired social behavior and communication is one of the key diagnostic criteria for ASD (American Psychiatric Association 2013), it is not surprising that many studies of mouse models harboring Pten mutations have assessed this behavioral domain. However, “social behavior” is a very broad category that encompasses many different motivations, skills, and neural circuits. Thus, we have subdivided this category into social interest, social recognition, and other social behaviors.
Social Interest
Social interest is one of the most widely tested types of behavior across mouse models of ASD, and represents one of the most fundamental aspects of social behavior. Face validity for social impairments in ASD is a common rationale for testing social interest in mouse models; however, it is worth noting that the translational value of mouse sociability as a model for human social deficits in ASD remains to be validated. This same caveat applies to the other ASD-relevant behaviors discussed below.
There are two main ways in which social interest is analyzed in animal models. The first is by simply measuring the amount of time spent investigating a novel conspecific (adult or juvenile), either freely moving or contained within a tube (thus requiring the experimental animal to initial social contact). The second uses the three-chamber social approach test. In this assay, the experimental animal is habituated to an arena containing three chambers of equal size. A novel conspecific in a tube or cage is placed in one chamber, and a tube or cage (empty or containing a novel object) is placed in another, with the center chamber left empty. The amount of time spent either in the chambers, in the area around the tubes, or actively investigating the tubes is then calculated, and a significant difference between the social and nonsocial investigation indicates social interest or a social preference.
The majority of Pten mutant models tested have shown a decrease in or lack of social preference (see Table 2; Kwon et al. 2006; Page et al. 2009; Zhou et al. 2009; Amiri et al. 2012; Napoli et al. 2012; Clipperton-Allen and Page 2014; Lugo et al. 2014; Séjourné et al. 2015; Vogt et al. 2015; Cupolillo et al. 2016; Huang et al. 2016; Sanchez-Puelles et al. 2019). Only two models showed increased social interest: Pten overexpression mice (PtenTg; Sanchez-Puelles et al. 2019) and cytoplasm-predominant Pten mice (Ptenm3m4; Tilot et al. 2014). However, a few models showed normal social approach behavior (see Table 2; Haws et al. 2014; Borniger et al. 2016; Clipperton-Allen et al. 2016). Whereas most studies used only one sex, combined sexes, or did not state the sex of the animals tested, those that did analyze males and females separately found that the phenotype displayed sexual dimorphism and was generally only present in one sex (see Table 3; Page et al. 2009; Clipperton-Allen and Page 2014; Tilot et al. 2014; Smith et al. 2016). The exception to this pattern was a line in which Pten was conditionally deleted in oxytocinergic neurons (Oxt-Cre; PtenloxP), which displayed no deficits in either sex (Clipperton-Allen et al. 2016). This highlights the importance of including both sexes and analyzing them separately, particularly in models of a disorder as highly sexually dimorphic as ASD. Interestingly, of the models that did identify sex differences, the deficits were in females (Pten+/− [Page et al. 2009], DAT-Cre; PtenloxP [Clipperton-Allen and Page 2014], NS-Cre; PtenloxP [Lugo et al. 2014; Smith et al. 2016]), whereas the increased social interest observed in the Ptenm3m4 mice was only present in males (Tilot et al. 2014). This raises the intriguing possibility that gonadal hormones may interact with Pten mutations to influence sociability phenotypes.
Table 3.
Behavioral phenotyping in studies of mouse models that compared sexes (black, males; white, females; red, increase in behavior in males; gray, no change in behavior; blue, decrease in behavior in males [dark] or females [light])
Social Recognition
Social recognition, in its simplest form, is the ability to distinguish between a novel conspecific and one that has been encountered before. There is some evidence that this process may be disrupted in ASD (Weigelt et al. 2012; Ewbank et al. 2017). Social recognition can be tested in several ways using mouse models. Three-chamber social novelty follows from the three-chamber social approach test, with a novel social stimulus being placed into the tube that previously was empty or held an object; the test animal should show a preference for the novel social chamber, tube, or stimulus. Alternatively, social recognition can be assessed using habituation (with or without dishabituation). This takes advantage of the fact that mice will spend decreasing amounts of time investigating a conspecific with repeated exposures (habituation). In some cases, this is followed by a dishabituation trial in which a novel stimulus is introduced, and the test animal should show an increase in investigation, indicating that it has habituated to the individual stimulus and not the testing situation.
Although fewer models have been tested for social recognition than social interest, most lines do show deficits (see Table 2; Kwon et al. 2006; Zhou et al. 2009; Amiri et al. 2012; Napoli et al. 2012; Clipperton-Allen and Page 2014; Lugo et al. 2014; Clipperton-Allen et al. 2016), although others show no alterations in social recognition (Haws et al. 2014; Tilot et al. 2014). As with social interest, the majority of studies did not look for sexual dimorphism, but of those that did, with the exception of the Ptenm3m4 line, the deficit was restricted to the male subjects (see Table 3; Page et al. 2009; Clipperton-Allen and Page 2014; Clipperton-Allen et al. 2016).
Other Social Behaviors
Increased aggression has been reported in up to 70% of individuals with ASD (McClintock et al. 2003; Holden and Gitlesen 2006; Hartley et al. 2008; Kanne and Mazurek 2011; Maskey et al. 2013). When a novel male is introduced to the cage of a male mouse, they will often engage in agonistic behavior to establish a dominance hierarchy. The effect of Pten mutations on these interactions has only been assessed in males the germline heterozygous Pten+/− line and the conditional mutants Fsp1-Cre; PtenloxP and Oxt-Cre; PtenloxP, with the latter showed no major differences (Clipperton-Allen et al. 2016). Interestingly, agonistic dominance behavior, but not overt attacks, were decreased in both Pten+/− and Fsp1-Cre; PtenloxP males (Clipperton-Allen and Page 2015; Borniger et al. 2016). Although this is the opposite of what the ASD phenotype would predict, it suggests that the circuitry controlling aggression may be abnormal when Pten is perturbed.
A few other social behavior assays have also been tested in Pten mutant models. Nest building was decreased in Nse-Cre; PtenloxP mice (Kwon et al. 2006), and L7-Cre; PtenloxP males showed decreased social interaction, including decreased sexual contact, with a novel female in a free social interaction (Cupolillo et al. 2016). Social olfaction was found to be normal in Nestin-Cre; PtenloxP males (Amiri et al. 2012).
Although it is challenging to assess communication deficits in mice, measurements of ultrasonic vocalizations (USVs), typically assessed during brief maternal separation in mice <2 weeks of age, has proven to be a useful proxy. In the few lines tested for USV abnormalities, no differences were found in the Pten-ΔPDZ line (Sanchez-Puelles et al. 2019), but deficits were observed in PtenTg overexpression mice (Sanchez-Puelles et al. 2019). Interestingly, one study found USV deficits in both male and female NS-Cre; PtenloxP juveniles (Binder and Lugo 2017), but another study in mice of the same line found no differences in juveniles of unidentified sex(es) (Lugo et al. 2014).
Taken together, these data clearly indicate that Pten mutations can disrupt social behavior in mouse models, although the nature of the disruption may depend on the type and site of Pten perturbation. Further research on the less common social behaviors across models, especially social recognition, aggression, and USVs, may help to elucidate the circuitry by which social behavior is altered. Additionally, the sexual dimorphism observed in the few studies that compared males and females highlights the importance of analyzing both sexes separately, particularly in light of the sex disparity in humans with ASD.
Repetitive Behaviors
In addition to abnormal social behavior and communication, restricted, repetitive behaviors and interests is the other core symptom of ASDs (American Psychiatric Association 2013). This has been assessed in mice in three main ways. The marble burying test assesses repetitive digging by placing a mouse in a cage with clean bedding and a number of marbles; more marbles buried indicates increased repetitive behavior (Hoeffer et al. 2008; Thomas et al. 2009; Silverman et al. 2010). The observation of spontaneous stereotypies, including flips, spins, circling the cage, etc., is another indicator of repetitive behavior. Finally, self-grooming is often considered a repetitive behavior, although as this is also a normal cleaning behavior, it is preferable to only use either excessive self-grooming resulting in hair loss, or abnormal or interrupted sequences of grooming as an indication of repetitive behaviors.
Altered repetitive behavior is seen in several models of Pten mutations, often with sexually dimorphic phenotypes in those studies that assessed sex differences (see Tables 2 and 3). Marble burying was increased in male, but not female Pten+/− mice (Clipperton-Allen and Page 2014). Female, but not male Oxt-Cre; PtenloxP mice (Clipperton-Allen et al. 2016), and likely female, but not male NS-Cre; PtenloxP mice, showed decreased marble burying (Lugo et al. 2014; Smith et al. 2016). NS-Cre; PtenloxP mice showed variable results in terms of other repetitive behaviors: in unspecified sex(es), repetitive behavior on the hole-board test, another assay of repetitive behavior, and self-grooming in the open field were decreased in one study (Lugo et al. 2014), although increased stereotypy was also shown in 6-week-old males (Lugo et al. 2017), and normal self-grooming was also observed (Smith et al. 2016). No differences in this behavior were found in PtenTg mice (Sanchez-Puelles et al. 2019); decreased self-grooming and stereotypes were also found in L7-Cre; PtenloxP males (Cupolillo et al. 2016).
Because of the paucity of repetitive behavior testing in Pten mutant mice, it is difficult to draw meaningful conclusions; thus, more testing of models of both sexes is needed. However, the data do suggest that repetitive behavior may be increased in males with decreased Pten, and possibly decreased in females; Pten overexpression does not appear to affect this behavior.
COMORBID AND OTHER BEHAVIORS
Mood and Anxiety
Anxiety
ASD is frequently comorbid with anxiety disorders (Skokauskas and Gallagher 2010). Anxiety-like behavior is commonly measured using three assays: the open field test ([OFT]; an empty box lit with white light), the elevated plus maze test ([EPM]; a plus-shaped maze with two open and two enclosed arms), and the dark–light assay (a box with one dark, covered chamber and one light, brightly lit chamber). These paradigms all take advantage of the tendency of mice to avoid bright open spaces, which make them more vulnerable to predation, especially from above; as such, less time spent in the center of the open field, the open arms of the EPM, or the light chamber of the dark–light assay all indicate more anxiety-like behavior (Mozhui et al. 2010).
Although the majority of models tested for anxiety-like behavior show no phenotypes (see Table 2; Haws et al. 2014; Tilot et al. 2014; Vogt et al. 2015; Borniger et al. 2016; Gutilla et al. 2016; Wang et al. 2017; Sanchez-Puelles et al. 2019), increased anxiety-like behavior was observed in Nse-Cre; PtenloxP mice on the OFT and dark–light tests, but not the EPM (Kwon et al. 2006; Zhou et al. 2009). Interestingly, all other models showing phenotypes showed decreased anxiety-like behavior (see Table 2; Clipperton-Allen and Page 2014; Lugo et al. 2014; Clipperton-Allen et al. 2016; Sanchez-Puelles et al. 2019). As in other assays, sexual dimorphism was observed in those studies comparing males and females (see Table 3): decreased anxiety was observed in Pten+/− and Oxt-Cre; PtenloxP males but not females (Clipperton-Allen and Page 2014; Clipperton-Allen et al. 2016), and as well as being shown by NS-Cre; PtenloxP mice of indeterminate sex (Lugo et al. 2014) but not by males (Smith et al. 2016), suggesting that the observed phenotype could be present in females.
Depression
Depression and other mood disorders are frequently comorbid with ASD (Skokauskas and Gallagher 2010). Depressive-like behavior in mice is tested by measuring immobility in the forced swim test ([FST]; the mouse is put into a small pool of water and the time floating (immobile) and swimming or climbing is measured) or the tail suspension test ([TST]; the mouse is suspended by the tail and the time spent immobile or struggling is measured). Both the FST and the TST are responsive to antidepressants.
Only two Pten mutant models were tested for depressive-like behavior (see Table 2; Clipperton-Allen and Page 2014; Clipperton-Allen et al. 2016), and of these, only male Pten+/− mice showed any phenotype, showing increased depressive-like behavior. These data suggest that Pten mutations may leave mood and anxiety phenotypes largely unaffected, although further testing is warranted. Notably, anxiety or depression have not been commonly reported in patients with ASD and PTEN mutations to date.
Intellectual Disability
Intellectual disability is another common comorbidity with ASD (Matson and Shoemaker 2009). No Pten mutants showed substantially improved learning and memory when tested on aversive or appetitive tasks, both spatial and nonspatial. However, approximately half of the lines tested on each task showed deficits, whereas the remainder showed no impairment (see Table 2).
Aversive Learning and Memory Tasks
When tested in fear conditioning, which involves learning to associate a context or cue with a foot shock and thus is aversively motivated, NS-Cre; PtenloxP mice, as well as Nse-Cre; PtenloxP, Pten-ΔPDZ, and mice with shRNA in the lateral amygdala, showed no impairments (see Table 2; Kwon et al. 2006; Haws et al. 2014; Smith et al. 2016; Sanchez-Puelles et al. 2019). However, female, but not male, Pten+/− mice did show deficits, as did Ptenαmu males and PtenTg mice of combined sexes (Clipperton-Allen and Page 2014; Wang et al. 2017; Sanchez-Puelles et al. 2019).
Several lines were also tested on a variety of aversively motivated spatial learning and memory tasks. The most common aversive spatial learning task is the Morris water maze (MWM), which involves a mouse learning the location of a hidden platform in a pool of water; shorter trials or distances traveled indicate improved learning. Additionally, probe trials (with no platform present) measure the amount of time or number of crossings of the area where the platform should be. A similar task is the Barnes maze, which relies on bright light and sound to motivate mice to escape a platform by learning which hole in the round maze leads to a “safe” cage. Thus, these aversively-motivated tasks test spatial learning (acquiring the location of the safe area, shown by shorter times and distances); the probe trials in the MWM also assess memory for where the escape platform should be located. Spatial learning was normal in NS-Cre; PtenloxP, L7-Cre; PtenloxP, and CaMKIIα-Cre; PtenloxP mice (see Table 2; Sperow et al. 2012; Cupolillo et al. 2016; Smith et al. 2016), but impaired in Ptenαmu, Nse-Cre; PtenloxP, and PtenTg mutants (Kwon et al. 2006; Wang et al. 2017; Sanchez-Puelles et al. 2019). Spatial memory was also impaired in Ptenαmu and Nse-Cre; PtenloxP mice, as well as in CaMKIIα-Cre; PtenloxP mutants (Kwon et al. 2006; Sperow et al. 2012; Wang et al. 2017).
Nonaversive Learning and Memory Tasks
In novel object recognition or investigation (mice should show increased investigation of the novel object, indicating that they recognize the familiar object), NS-Cre; PtenloxP and Nkx2.1-Cre; PtenloxP mice showed impairments, whereas Fsp1-Cre; PtenloxP and Ptenm3m4 mice did not (see Table 2; Tilot et al. 2014; Vogt et al. 2015; Borniger et al. 2016; Hodges et al. 2018).
Normal spatial ability was seen in the three models that used the spontaneous alternation and novel object location assays (see Table 2), which both use the tendency of mice to prefer to explore a novel location or an object that has been moved to a new location (Borniger et al. 2016; Huang et al. 2016; Sanchez-Puelles et al. 2019). The only appetitive assay to show a deficit was the Lashley maze, in which mice are required to learn a path from the start area to the goal box; NS-Cre; PtenloxP mice made more errors and took longer to complete this task (Hodges et al. 2018).
Sleep and Circadian Rhythms
Parental reports estimate 50%–80% of children with ASD have difficulty with sleep, compared with 9%–50% of age-matched control children (Polimeni et al. 2005; Allik et al. 2006; Doo and Wing 2006; Giannotti et al. 2008; Richdale and Schreck 2009). These difficulties include problems with sleep initiation and maintenance, less time in slow-wave, REM, and overall sleep, as well as unstable sleep and irregular sleep–wake patterns (Schenck et al. 1987; Miano et al. 2007; Malow and McGrew 2008; Stores 2008; Maes et al. 2011; Vriend et al. 2011; Kotagal and Broomall 2012).
Sleep disturbances and circadian rhythm have been lightly studied in mouse models of Pten mutations. Nse-Cre; PtenloxP males show a longer free-running circadian rhythm (tau) when in constant dark (Ogawa et al. 2007), but neither male nor female Pten+/− mice showed an abnormal tau (Clipperton-Allen and Page 2014). However, both lines showed abnormal activity (as measured by wheel running) during a normal 12:12 h light/dark cycle, with Pten+/− females and Nse-Cre; PtenloxP males showing decreased activity during the dark phase (Ogawa et al. 2007; Clipperton-Allen and Page 2014). More research is needed to form conclusions about the effect of Pten mutations on sleep and circadian rhythms.
Sensorimotor and Basic Sensory and Motor Assessments
Acoustic Startle and Prepulse Inhibition
A few models of Pten mutations were assessed for their startle response to an acoustic stimulus, and/or their ability to inhibit this response following a lower decibel tone preceding the startle stimulus (prepulse inhibition [PPI]). Nse-Cre; PtenloxP mice showed an increased response to the 120 dB white noise stimulus during initial trials, but this response normalized with repeated presentations (Kwon et al. 2006). Additionally, both Nse-Cre; PtenloxP and both sexes of Pten+/− mice showed impaired PPI (Kwon et al. 2006; Page et al. 2009).
Basic Sensory and Motor Abilities
No deficits were found in any Pten mutant models for olfaction or pain perception, with deletion of Pten in neural stem cells actually improving olfactory abilities (see Table 2; Kwon et al. 2006; Gregorian et al. 2009; Page et al. 2009; Clipperton-Allen and Page 2014; Haws et al. 2014; Lugo et al. 2014; Tilot et al. 2014; Borniger et al. 2016). The only sensory deficit observed was in auditory orientation in the Fsp1-Cre; PtenloxP model (Borniger et al. 2016). Thus, Pten mutations appear to leave basic sensory skills largely intact. Similarly, although few studies have investigated basic motor abilities, Nse-Cre; PtenloxP mice show normal endurance and grip strength (Kwon et al. 2006), although the grip strength of Fsp1-Cre; PtenloxP males, but not females, was impaired (Borniger et al. 2016).
Given the growing evidence of sensory abnormalities, more investigation, particularly of tactile and auditory sensory perception, is necessary.
Motor Behaviors
One of the most common phenotypes reported in humans with PTEN mutations, outside of the core ASD domains, is motor behavior impairments.
Locomotion
Very few Pten mutant mice showed abnormal locomotion, which is typically measured by the distance traveled in an open field, or during the three-chamber assay, both as an assessment of basic activity and as a control for assays requiring locomotion (e.g., social approach, social novelty, light–dark, etc.). Only a small subset of conditional knockout models showed altered locomotor behavior, and even in these the results were inconsistent. Both the NS-Cre; PtenloxP and Nse-Cre; PtenloxP lines showed increases in distance traveled, although only when older than 6 weeks of age, and in the case of Nse-Cre; PtenloxP mice, only under stressful conditions (Kwon et al. 2006; Zhou et al. 2009; Lugo et al. 2014, 2017). DAT-Cre; PtenloxP mice of unknown sex(es) showed normal open field activity, but males traveled a shorter distance than controls in the three-chamber test (Diaz-Ruiz et al. 2009; Clipperton-Allen and Page 2014). Given these results, and the normal locomotion observed in the vast majority of models tested (see Table 2; Amiri et al. 2012; Clipperton-Allen and Page 2014; Haws et al. 2014; Tilot et al. 2014; Vogt et al. 2015; Clipperton-Allen et al. 2016; Cupolillo et al. 2016; Gutilla et al. 2016; Wang et al. 2017; Sanchez-Puelles et al. 2019), it is highly likely that effects of a Pten mutation on locomotor activity are minimal at most.
Balance, Motor Learning, and Fine Motor Skills
Rotarod testing is a standard assay used to assess balance and motor learning. This simple test involves placing the animal on a rotating bar that typically accelerates from 4 to 40 rpm over a period of 2–3 min. Balance is measured by the latency to fall, whereas motor learning is assessed by looking at increases in fall latency across several trials or days. Fall latency was increased in half of the Pten mutant models tested, specifically the Ptenm3m4, Fsp1-Cre; PtenloxP, and 5- to 6-month-old L7-Cre; PtenloxP mutants (see Table 2; Clipperton-Allen and Page 2014; Tilot et al. 2014; Borniger et al. 2016; Clipperton-Allen et al. 2016; Cupolillo et al. 2016; Gutilla et al. 2016). Additionally, Nse-Cre; PtenloxP mice were impaired in one of the studies that tested them on this assay (Nolan et al. 2019) but not the other (Kwon et al. 2006). Interestingly, only the L7-Cre; PtenloxP mice showed less improvement across trials than controls (Cupolillo et al. 2016), whereas acquisition of the task was actually improved in Nse-Cre; PtenloxP mutants (Kwon et al. 2006). No other tested line showed alterations on rotarod motor learning (Clipperton-Allen and Page 2014; Tilot et al. 2014; Borniger et al. 2016; Clipperton-Allen et al. 2016; Gutilla et al. 2016).
Despite the increasingly well-established impairment in fine motor skills in humans with PTEN mutations and ASD, only one study assessed this behavior in mice. This study used the sticker removal task, where a small adhesive sticker is placed on the nose of the animal, and the time to attempt and/or completely remove the sticker is measured. Although normal on the first trial, Nse-Cre; PtenloxP mice showed impairment on the second and third trials (Nolan et al. 2019). Clearly, more investigation of fine motor skills in mouse models of Pten mutations is a crucial need.
Spontaneous Observations
Spontaneous seizures, ataxia, and/or premature death were observed in a subset of the conditional knockout models (see Table 2). Seizures were noted in NS-Cre; PtenloxP, Nse-Cre; PtenloxP, Nestin-Cre; PtenloxP, and Pomc-Cre; PtenloxP mutants (Backman et al. 2001; Kwon et al. 2001, 2003, 2006; Ogawa et al. 2007; Ljungberg et al. 2009; Zhou et al. 2009; Amiri et al. 2012; Nguyen et al. 2015; Matsushita et al. 2016), with the NS-Cre; PtenloxP line also showing ataxia and premature death (Backman et al. 2001; Kwon et al. 2001, 2003; Ljungberg et al. 2009; Nguyen et al. 2015). No such phenotypes were noted in germline or conditional heterozygous Pten mutant lines, with the only exception being premature death in Ptenm3m4 mice and in Pten+/− mice also lacking the serotonin receptor 2C (Page et al. 2009; Mester et al. 2011; Napoli et al. 2012; Clipperton-Allen and Page 2014, 2015; Tilot et al. 2014; Séjourné et al. 2015; Huang et al. 2016; Wang et al. 2017; Sanchez-Puelles et al. 2019). Thus, although conditional knockout Pten models are highly valuable for understanding the neurobiology of Pten, it seems that more disease-relevant heterozygous mutations have a much more subtle effect on seizure susceptibility in mice.
CONCLUDING THOUGHTS AND FUTURE DIRECTIONS
The aim of this review has been to define a “profile” of behaviors that are sensitive to the effects of Pten mutations based on mouse model studies to date. Although we have highlighted some areas in which deeper phenotyping is needed, some of the most reproduced behavioral deficits across models thus far are in the domains of social interest, social recognition, and repetitive behavior. Considering the broad expression of Pten in the developing brain and the substantial changes in brain and neuronal growth, structural connectivity, intrinsic properties, and synaptic connectivity/plasticity caused by loss of Pten function, the relatively selective effects on behavior are striking (see Table 2). Identifying brain areas and circuits that are vulnerable to reduced Pten function will be a critical area of future research and will be informative for developing targeted therapeutic strategies to offset the consequences of PTEN haploinsufficiency. Conditional genetic approaches to manipulating Pten have already shown the importance of forebrain glutamatergic and GABAergic neurons, midbrain dopaminergic neurons, and cerebellar Purkinje cells. Next steps will involve narrowing down these broad categories of cell types into specific circuits that may underlie abnormal social information processing and behavior in a Pten mutant background. Likewise, it is clear that, across Pten models in which both males and females have been tested, sexual dimorphism in behavioral phenotypes appears to be more the rule than the exception (see Table 3). Thus, research into neurobiological mechanisms by which gonadal hormones may interact with Pten mutations to influence ASD-related behaviors is an important area for the future.
ACKNOWLEDGMENTS
PTEN and autism research in the Page laboratory is supported by funds provided by Ms. Nancy Lurie Marks and the National Institutes of Health (NIH) (R01MH105610 and R01MH108519).
Footnotes
Editors: Charis Eng, Joanne Ngeow, and Vuk Stambolic
Additional Perspectives on The PTEN Family available at www.perspectivesinmedicine.org
REFERENCES
- Albores-Gallo L, Fritsche-García L, Miranda-Aguirre AP, Avila-Acosta M. 2017. Brief report: macrocephaly phenotype and psychiatric comorbidity in a clinical sample of Mexican children and adolescents with autism spectrum disorders. J Autism Dev Disord 47: 2911–2917. 10.1007/s10803-017-3175-4 [DOI] [PubMed] [Google Scholar]
- Allik H, Larsson J-O, Smedje H. 2006. Sleep patterns of school-age children with Asperger syndrome or high-functioning autism. J Autism Dev Disord 36: 585–595. 10.1007/s10803-006-0099-9 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association, Arlington, VA. [Google Scholar]
- Amiri A, Cho W, Zhou J, Birnbaum SG, Sinton CM, McKay RM, Parada LF. 2012. Pten deletion in adult hippocampal neural stem/progenitor cells causes cellular abnormalities and alters neurogenesis. J Neurosci 32: 5880–5890. 10.1523/jneurosci.5462-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman SA, Stambolic V, Suzuki A, Haight J, Elia A, Pretorius J, Tsao MS, Shannon P, Bolon B, Ivy GO, et al. 2001. Deletion of Pten in mouse brain causes seizures, ataxia and defects in soma size resembling Lhermitte–Duclos disease. Nat Genet 29: 396–403. 10.1038/ng782 [DOI] [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, Kurzius-Spencer M, Zahorodny W, Robinson Rosenberg C, White T, et al. 2018. Prevalence of autism spectrum disorder among children aged 8 years—Autism and Developmental Disabilities Monitoring Network, 11 sites, United States, 2014. MMWR Surveill Summ 67: 1–23. 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder MS, Lugo JN. 2017. NS-Pten knockout mice show sex- and age-specific differences in ultrasonic vocalizations. Brain Behav 7: e00857 10.1002/brb3.857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenthal GM, Dennis PA. 2008. PTEN hamartoma tumor syndromes. Eur J Hum Genet 16: 1289–1300. 10.1038/ejhg.2008.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccone L, Dessì V, Zappu A, Piga S, Piludu MB, Rais M, Massidda C, De Virgiliis S, Cao A, Loudianos G. 2006. Bannayan–Riley–Ruvalcaba syndrome with reactive nodular lymphoid hyperplasia and autism and a PTEN mutation. Am J Med Genet A 140A: 1965–1969. 10.1002/ajmg.a.31396 [DOI] [PubMed] [Google Scholar]
- Borniger JC, Cissé YM, Cantemir-Stone CZ, Bolon B, Nelson RJ, Marsh CB. 2016. Behavioral abnormalities in mice lacking mesenchyme-specific Pten. Behav Brain Res 304: 80–85. 10.1016/j.bbr.2016.02.016 [DOI] [PubMed] [Google Scholar]
- Busch RM, Chapin JS, Mester J, Ferguson L, Haut JS, Frazier TW, Eng C. 2013. Cognitive characteristics of PTEN hamartoma tumor syndromes. Genet Med 15: 548–553. 10.1038/gim.2013.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M, Dasouki M, Zhou X-P, Talebizadeh Z, Brown M, Takahashi T, Miles J, Wang C, Stratton R, Pilarski R, et al. 2005. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J Med Genet 42: 318–321. 10.1136/jmg.2004.024646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum JD, Cai G, Chaste P, Nygren G, Goldsmith J, Reichert J, Anckarsäter H, Rastam M, Smith CJ, Silverman JM, et al. 2007. Mutation screening of the PTEN gene in patients with autism spectrum disorders and macrocephaly. Am J Med Genet B Neuropsychiatr Genet 144B: 484–491. 10.1002/ajmg.b.30493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipperton-Allen AE, Page DT. 2014. Pten haploinsufficient mice show broad brain overgrowth but selective impairments in autism-relevant behavioral tests. Hum Mol Genet 23: 3490–3505. 10.1093/hmg/ddu057 [DOI] [PubMed] [Google Scholar]
- Clipperton-Allen AE, Page DT. 2015. Decreased aggression and increased repetitive behavior in Pten haploinsufficient mice. Genes Brain Behav 14: 145–157. 10.1111/gbb.12192 [DOI] [PubMed] [Google Scholar]
- Clipperton-Allen AE, Chen Y, Page DT. 2016. Autism-relevant behaviors are minimally impacted by conditional deletion of Pten in oxytocinergic neurons. Autism Res 9: 1248–1262. 10.1002/aur.1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti S, Condò M, Posar A, Mari F, Resta N, Renieri A, Neri I, Patrizi A, Parmeggiani A. 2012. Phosphatase and tensin homolog (PTEN) gene mutations and autism: literature review and a case report of a patient with Cowden syndrome, autistic disorder, and epilepsy. J Child Neurol 27: 392–397. 10.1177/0883073811420296 [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, Schumann CM, Redcay E, Buckwalter JA, Kennedy DP, Morgan J. 2007. Mapping early brain development in autism. Neuron 56: 399–413. 10.1016/j.neuron.2007.10.016 [DOI] [PubMed] [Google Scholar]
- Cupolillo D, Hoxha E, Faralli A, De Luca A, Rossi F, Tempia F, Carulli D. 2016. Autistic-like traits and cerebellar dysfunction in purkinje cell PTEN knock-out mice. Neuropsychopharmacology 41: 1457–1466. 10.1038/npp.2015.339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Ruiz O, Zapata A, Shan L, Zhang Y, Tomac AC, Malik N, de la Cruz F, Bäckman CM. 2009. Selective deletion of PTEN in dopamine neurons leads to trophic effects and adaptation of striatal medium spiny projecting neurons. PLoS ONE 4: e7027 10.1371/journal.pone.0007027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doo S, Wing YK. 2006. Sleep problems of children with pervasive developmental disorders: correlation with parental stress. Dev Med Child Neurol 48: 650–655. 10.1017/S001216220600137X [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Pell PJ, Powell TE, von dem Hagen EAH, Baron-Cohen S, Calder AJ. 2017. Repetition suppression and memory for faces is reduced in adults with autism spectrum conditions. Cereb Cortex 27: 92–103. 10.1093/cercor/bhw373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazier TW, Embacher R, Tilot AK, Koenig K, Mester J, Eng C. 2015. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol Psychiatry 20: 1132–1138. 10.1038/mp.2014.125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannotti F, Cortesi F, Cerquiglini A, Miraglia D, Vagnoni C, Sebastiani T, Bernabei P. 2008. An investigation of sleep characteristics, EEG abnormalities and epilepsy in developmentally regressed and non-regressed children with autism. J Autism Dev Disord 38: 1888–1897. 10.1007/s10803-008-0584-4 [DOI] [PubMed] [Google Scholar]
- Goffin A, Hoefsloot LH, Bosgoed E, Swillen A, Fryns JP. 2001. PTEN mutation in a family with Cowden syndrome and autism. Am J Med Genet 105: 521–524. 10.1002/ajmg.1477 [DOI] [PubMed] [Google Scholar]
- Gregorian C, Nakashima J, Le Belle J, Ohab J, Kim R, Liu A, Smith KB, Groszer M, Garcia AD, Sofroniew MV, et al. 2009. Pten deletion in adult neural stem/progenitor cells enhances constitutive neurogenesis. J Neurosci 29: 1874–1886. 10.1523/jneurosci.3095-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutilla EA, Buyukozturk MM, Steward O. 2016. Long-term consequences of conditional genetic deletion of PTEN in the sensorimotor cortex of neonatal mice. Exp Neurol 279: 27–39. 10.1016/j.expneurol.2016.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Sikora DM, McCoy R. 2008. Prevalence and risk factors of maladaptive behaviour in young children with Autistic Disorder. J Intellect Disabil Res 52: 819–829. 10.1111/j.1365-2788.2008.01065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haws ME, Jaramillo TC, Espinosa F, Widman AJ, Stuber GD, Sparta DR, Tye KM, Russo SJ, Parada LF, Stavarache M, et al. 2014. PTEN knockdown alters dendritic spine/protrusion morphology, not density. J Comp Neurol 522: 1171–1190. 10.1002/cne.23488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman GE, Butter E, Enrile B, Pastore M, Prior TW, Sommer A. 2007. Increasing knowledge of PTEN germline mutations: two additional patients with autism and macrocephaly. Am J Med Genet A 143A: 589–593. 10.1002/ajmg.a.31619 [DOI] [PubMed] [Google Scholar]
- Hobert JA, Embacher R, Mester JL, Frazier TW II, Eng C. 2014. Biochemical screening and PTEN mutation analysis in individuals with autism spectrum disorders and macrocephaly. Eur J Hum Genet 22: 273–276. 10.1038/ejhg.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges SL, Reynolds CD, Smith GD, Jefferson TS, Gao N, Morrison JB, White J, Nolan SO, Lugo JN. 2018. Neuronal subset-specific deletion of Pten results in aberrant Wnt signaling and memory impairments. Brain Res 1699: 100–106. 10.1016/j.brainres.2018.08.007 [DOI] [PubMed] [Google Scholar]
- Hoeffer CA, Tang W, Wong H, Santillan A, Patterson RJ, Martinez LA, Tejada-Simon MV, Paylor R, Hamilton SL, Klann E. 2008. Removal of FKBP12 enhances mTOR–Raptor interactions, LTP, memory, and perseverative/repetitive behavior. Neuron 60: 832–845. 10.1016/j.neuron.2008.09.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holden B, Gitlesen JP. 2006. A total population study of challenging behaviour in the county of Hedmark, Norway: prevalence, and risk markers. Res Dev Disabil 27: 456–465. 10.1016/j.ridd.2005.06.001 [DOI] [PubMed] [Google Scholar]
- Huang WC, Chen Y, Page DT. 2016. Hyperconnectivity of prefrontal cortex to amygdala projections in a mouse model of macrocephaly/autism syndrome. Nat Commun 7: 13421 10.1038/ncomms13421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanne SM, Mazurek MO. 2011. Aggression in children and adolescents with ASD: prevalence and risk factors. J Autism Dev Disord 41: 926–937. 10.1007/s10803-010-1118-4 [DOI] [PubMed] [Google Scholar]
- Klein S, Sharifi-Hannauer P, Martinez-Agosto JA. 2013. Macrocephaly as a clinical indicator of genetic subtypes in autism. Autism Res 6: 51–56. 10.1002/aur.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotagal S, Broomall E. 2012. Sleep in children with autism spectrum disorder. Pediatr Neurol 47: 242–251. 10.1016/j.pediatrneurol.2012.05.007 [DOI] [PubMed] [Google Scholar]
- Kwon C-H, Zhu X, Zhang J, Knoop LL, Tharp R, Smeyne RJ, Eberhart CG, Burger PC, Baker SJ. 2001. Pten regulates neuronal soma size: a mouse model of Lhermitte–Duclos disease. Nat Genet 29: 404–411. 10.1038/ng781 [DOI] [PubMed] [Google Scholar]
- Kwon CH, Zhu X, Zhang J, Baker SJ. 2003. mTor is required for hypertrophy of Pten-deficient neuronal soma in vivo. Proc Natl Acad Sci 100: 12923–12928. 10.1073/pnas.2132711100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon C-H, Luikart BW, Powell CM, Zhou J, Matheny SA, Zhang W, Li Y, Baker SJ, Parada LF. 2006. Pten regulates neuronal arborization and social interaction in mice. Neuron 50: 377–388. 10.1016/j.neuron.2006.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainhart JE, Bigler ED, Bocian M, Coon H, Dinh E, Dawson G, Deutsch CK, Dunn M, Estes A, Tager-Flusberg H, et al. 2006. Head circumference and height in autism: a study by the Collaborative Program of Excellence in Autism. Am J Med Genet A 140: 2257–2274. 10.1002/ajmg.a.31465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungberg MC, Sunnen CN, Lugo JN, Anderson AE, D'Arcangelo G. 2009. Rapamycin suppresses seizures and neuronal hypertrophy in a mouse model of cortical dysplasia. Dis Model Mech 2: 389–398. 10.1242/dmm.002386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Smith GD, Arbuckle EP, White J, Holley AJ, Floruta CM, Ahmed N, Gomez MC, Okonkwo O. 2014. Deletion of PTEN produces autism-like behavioral deficits and alterations in synaptic proteins. Front Mol Neurosci 7: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugo JN, Thompson MH, Huber P, Smith G, Kwon RY. 2017. Neuron subset-specific Pten deletion induces abnormal skeletal activity in mice. Exp Neurol 291: 98–105. 10.1016/j.expneurol.2017.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes JH, Eling PA, Wezenberg E, Vissers CT, Kan CC. 2011. Attentional set shifting in autism spectrum disorder: differentiating between the role of perseveration, learned irrelevance, and novelty processing. J Clin Exp Neuropsychol 33: 210–217. 10.1080/13803395.2010.501327 [DOI] [PubMed] [Google Scholar]
- Malow B, McGrew S. 2008. Sleep disturbances and autism. Sleep Med Clin 3: 479–488. 10.1016/j.jsmc.2008.04.004 [DOI] [Google Scholar]
- Maskey M, Warnell F, Parr JR, Le Couteur A, McConachie H. 2013. Emotional and behavioural problems in children with autism spectrum disorder. J Autism Dev Disord 43: 851–859. 10.1007/s10803-012-1622-9 [DOI] [PubMed] [Google Scholar]
- Matson JL, Shoemaker M. 2009. Intellectual disability and its relationship to autism spectrum disorders. Res Dev Disabil 30: 1107–1114. 10.1016/j.ridd.2009.06.003 [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Sakai Y, Shimmura M, Shigeto H, Nishio M, Akamine S, Sanefuji M, Ishizaki Y, Torisu H, Nakabeppu Y, et al. 2016. Hyperactive mTOR signals in the proopiomelanocortin-expressing hippocampal neurons cause age-dependent epilepsy and premature death in mice. Sci Rep 6: 2299 10.1038/srep22991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride KL, Varga EA, Pastore MT, Prior TW, Manickam K, Atkin JF, Herman GE. 2010. Confirmation study of PTEN mutations among individuals with autism or developmental delays/mental retardation and macrocephaly. Autism Res 3: 137–141. 10.1002/aur.132 [DOI] [PubMed] [Google Scholar]
- McClintock K, Hall S, Oliver C. 2003. Risk markers associated with challenging behaviours in people with intellectual disabilities: a meta-analytic study. J Intellect Disabil Res 47: 405–416. 10.1046/j.1365-2788.2003.00517.x [DOI] [PubMed] [Google Scholar]
- Mester JL, Tilot AK, Rybicki LA, Frazier TWI, Eng C. 2011. Analysis of prevalence and degree of macrocephaly in patients with germline PTEN mutations and of brain weight in Pten knock-in murine model. Eur J Hum Genet 19: 763–768. 10.1038/ejhg.2011.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miano S, Bruni O, Elia M, Trovato A, Smerieri A, Verrillo E, Roccella M, Terzano MG, Ferri R. 2007. Sleep in children with autistic spectrum disorder: a questionnaire and polysomnographic study. Sleep Med 9: 64–70. 10.1016/j.sleep.2007.01.014 [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson R-M, Kash TL, Ihne J, Norcross M, Patel S, Farrell MR, Hill EE, Graybeal C, Martin KP, et al. 2010. Strain differences in stress responsivity are associated with divergent amygdala gene expression and glutamate-mediated neuronal excitability. J Neurosci 30: 5357–5367. 10.1523/jneurosci.5017-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli E, Ross-Inta C, Wong S, Hung C, Fujisawa Y, Sakaguchi D, Angelastro J, Omanska-Klusek A, Schoenfeld R, Giulivi C. 2012. Mitochondrial dysfunction in Pten haplo-insufficient mice with social deficits and repetitive behavior: interplay between Pten and p53. PLoS ONE 7: e42504 10.1371/journal.pone.0042504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LH, Brewster AL, Clark ME, Regnier-Golanov A, Sunnen CN, Patil VV, D'Arcangelo G, Anderson AE. 2015. mTOR inhibition suppresses established epilepsy in a mouse model of cortical dysplasia. Epilepsia 56: 636–646. 10.1111/epi.12946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan SO, Jefferson TS, Reynolds CD, Smith GD, Holley AJ, Hodges SL, Lugo JN. 2019. Neuronal deletion of phosphatase and tensin homolog results in cerebellar motor learning dysfunction and alterations in intracellular signaling. Neuroreport 30: 556–561. 10.1097/WNR.0000000000001241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa S, Kwon C-H, Zhou J, Koovakkattu D, Parada LF, Sinton CM. 2007. A seizure-prone phenotype is associated with altered free-running rhythm in Pten mutant mice. Brain Res 1168: 112–123. 10.1016/j.brainres.2007.06.074 [DOI] [PubMed] [Google Scholar]
- Orrico A, Galli L, Buoni S, Orsi A, Vonella G, Sorrentino V. 2009. Novel PTEN mutations in neurodevelopmental disorders and macrocephaly. Clin Genet 75: 195–198. 10.1111/j.1399-0004.2008.01074.x [DOI] [PubMed] [Google Scholar]
- Page DT, Kuti OJ, Prestia C, Sur M. 2009. Haploinsufficiency for Pten and Serotonin transporter cooperatively influences brain size and social behavior. Proc Natl Acad Sci 106: 1989–1994. 10.1073/pnas.0804428106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polimeni MA, Richdale AL, Francis AJ. 2005. A survey of sleep problems in autism, Asperger's disorder and typically developing children. J Intellect Disabil Res 49: 260–268. 10.1111/j.1365-2788.2005.00642.x [DOI] [PubMed] [Google Scholar]
- Richdale AL, Schreck KA. 2009. Sleep problems in autism spectrum disorders: prevalence, nature, and possible biopsychosocial aetiologies. Sleep Med Rev 13: 403–411. 10.1016/j.smrv.2009.02.003 [DOI] [PubMed] [Google Scholar]
- Rosti RO, Sadek AA, Vaux KK, Gleeson JG. 2014. The genetic landscape of autism spectrum disorders. Dev Med Child Neurol 56: 12–18. 10.1111/dmcn.12278 [DOI] [PubMed] [Google Scholar]
- Sacco R, Gabriele S, Persico AM. 2015. Head circumference and brain size in autism spectrum disorder: a systematic review and meta-analysis. Psychiatry Res 234: 239–251. 10.1016/j.pscychresns.2015.08.016 [DOI] [PubMed] [Google Scholar]
- Sanchez-Puelles C, Calleja-Felipe M, Ouro A, Bougamra G, Arroyo A, Diez I, Erramuzpe A, Cortes J, Martinez-Hernandez J, Lujan R, et al. 2019. PTEN activity defines an axis for plasticity at cortico-amygdala synapses and influences social behavior. Cereb Cortex 10.1093/cercor/bhz103 [DOI] [PubMed] [Google Scholar]
- Sandin S, Lichtenstein P, Kuja-Halkola R, Hultman C, Larsson H, Reichenberg A. 2017. The heritability of autism spectrum disorder. JAMA 318: 1182–1184. 10.1001/jama.2017.12141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenck C, Bundlie S, Patterson A, Mahowald M. 1987. Rapid eye movement sleep behavior disorder: a treatable parasomnia affecting older adults. JAMA 257: 1786–1789. 10.1001/jama.1987.03390130104038 [DOI] [PubMed] [Google Scholar]
- Séjourné J, Llaneza D, Kuti OJ, Page DT. 2015. Social behavioral deficits coincide with the onset of seizure susceptibility in mice lacking serotonin receptor 2c. PLoS ONE 10: e0136494 10.1371/journal.pone.0136494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman JL, Yang M, Lord C, Crawley JN. 2010. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci 11: 490–502. 10.1038/nrn2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skokauskas N, Gallagher L. 2010. Psychosis, affective disorders and anxiety in autistic spectrum disorder: prevalence and nosological considerations. Psychopathology 43: 8–16. 10.1159/000255958 [DOI] [PubMed] [Google Scholar]
- Smith GD, White J, Lugo JN. 2016. Superimposing status epilepticus on neuron subset-specific PTEN haploinsufficient and wild type mice results in long-term changes in behavior. Sci Rep 6: 36559 10.1038/srep36559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperow M, Berry RB, Bayazitov IT, Zhu G, Baker SJ, Zakharenko SS. 2012. Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration. J Physiol 590: 777–792. 10.1113/jphysiol.2011.220236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stessman HA, Xiong B, Coe BP, Wang T, Hoekzema K, Fenckova M, Kvarnung M, Gerdts J, Trinh S, Cosemans N, et al. 2017. Targeted sequencing identifies 91 neurodevelopmental-disorder risk genes with autism and developmental-disability biases. Nat Genet 49: 515–526. 10.1038/ng.3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stores G. 2008. Rapid eye movement sleep behaviour disorder in children and adolescents. Dev Med Child Neurol 50: 728–732. 10.1111/j.1469-8749.2008.03071.x [DOI] [PubMed] [Google Scholar]
- Thomas A, Burant A, Bui N, Graham D, Yuva-Paylor LA, Paylor R. 2009. Marble burying reflects a repetitive and perseverative behavior more than novelty-induced anxiety. Psychopharmacology 204: 361–373. 10.1007/s00213-009-1466-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilot AK, Gaugler MK, Yu Q, Romigh T, Yu W, Miller RH, Frazier TW II, Eng C. 2014. Germline disruption of Pten localization causes enhanced sex-dependent social motivation and increased glial production. Hum Mol Genet 23: 3212–3227. 10.1093/hmg/ddu031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga EA, Pastore M, Prior T, Herman GE, McBride KL. 2009. The prevalence of PTEN mutations in a clinical pediatric cohort with autism spectrum disorders, developmental delay, and macrocephaly. Genet Med 11: 111–117. 10.1097/GIM.0b013e31818fd762 [DOI] [PubMed] [Google Scholar]
- Vogt D, Cho KKA, Lee AT, Sohal VS, Rubenstein JLR. 2015. The parvalbumin/somatostatin ratio is increased in Pten mutant mice and by human PTEN ASD alleles. Cell Rep 11: 944–956. 10.1016/j.celrep.2015.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vriend JL, Corkum PV, Moon EC, Smith IM. 2011. Behavioral interventions for sleep problems in children with autism spectrum disorders: current findings and future directions. J Pediatr Psychol 36: 1017–1029. 10.1093/jpepsy/jsr044 [DOI] [PubMed] [Google Scholar]
- Wang P, Mei F, Hu J, Zhu M, Qi H, Chen X, Li R, McNutt MA, Yin Y. 2017. PTENα modulates CaMKII signaling and controls contextual fear memory and spatial learning. Cell Rep 19: 2627–2641. 10.1016/j.celrep.2017.05.088 [DOI] [PubMed] [Google Scholar]
- Weigelt S, Koldewyn K, Kanwisher N. 2012. Face identity recognition in autism spectrum disorders: a review of behavioral studies. Neurosci Biobehav Rev 36: 1060–1084. 10.1016/j.neubiorev.2011.12.008 [DOI] [PubMed] [Google Scholar]
- Yeung KS, Tso WWY, Ip JJK, Mak CCY, Leung GKC, Tsang MHY, Ying D, Pei SLC, Lee SL, Yang W, et al. 2017. Identification of mutations in the PI3K-AKT-mTOR signalling pathway in patients with macrocephaly and developmental delay and/or autism. Mol Autism 8: 66 10.1186/s13229-017-0182-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Blundell J, Ogawa S, Kwon CH, Zhang W, Sinton C, Powell CM, Parada LF. 2009. Pharmacological inhibition of mTORC1 suppresses anatomical, cellular, and behavioral abnormalities in neural-specific Pten knock-out mice. J Neurosci 29: 1773–1783. 10.1523/jneurosci.5685-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]