Abstract
Monoclonal antibodies (mAbs) are a crucial asset for human health and modern medicine, however, the repeated administration of mAbs can be highly immunogenic. Drug immunogenicity manifests in the generation of anti-drug antibodies (ADAs), and some mAbs show immunogenicity in up to 70% of patients. ADAs can alter a drug’s pharmacokinetic and pharmacodynamic properties, reducing drug efficacy. In more severe cases, ADAs can neutralize the drug’s therapeutic effects or cause severe adverse events to the patient. While some contributing factors to ADA formation are known, the molecular mechanisms of how therapeutic mAbs elicit ADAs are not completely clear. Accurate ADA detection is necessary to provide clinicians with sufficient information for patient monitoring and clinical intervention. However, ADA assays present unique challenges because both the analyte and antigen are antibodies, so most assays are cumbersome, costly, time consuming, and lack standardization. This review will discuss aspects related to ADA formation following mAb drug administration. First, we will provide an overview of the prevalence of ADA formation and the available diagnostic tools for their detection. Next, we will review studies that support possible molecular mechanisms causing the formation of ADA. Finally, we will summarize recent approaches used to decrease the propensity of mAbs to induce ADAs.
Keywords: monoclonal antibodies, anti-drug antibodies, immune response, immunogenicity, neutralizing antibodies
Introduction
In the last three decades, the pharmaceutical industry experienced a massive shift toward the use of protein drugs, often referred to as “biologics.” Biologics offer higher specificity and better characterized mechanisms of action compared to small molecule drugs, and their use has revolutionized the treatment of a wide range of diseases and disorders. In general, monoclonal antibodies (mAbs) are the most widely used class of biologics (1).
Monoclonal antibodies account for a growing number of blockbuster drugs with their US sales reaching over $24 billion (2), and will maintain a dominant position in the pharmaceutical market that exceeds $125 billion by the end of 2020 (3).
To date, over 73 mAbs have been approved by the United States Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Hundreds more mAbs are in different stages of clinical developmental. mAbs are used for various clinical indications including cancer, chronic autoimmune and inflammatory diseases, allergies, infections, transplantations, and cardiovascular diseases (4).
The mechanism of action (MOA) of mAbs can vary across different use cases. For example, the anti-CD20 rituximab induces cell death by binding to surface receptors, resulting in a signaling cascade that leads to apoptosis (5). Other mAbs, including the anti-HER-2 trastuzumab, block receptor-ligand interactions to achieve a desired effect, either by blocking the receptor domain to inhibit an activation signal by removing a soluble ligand entirely from circulation (6). mAbs can also induce fragment crystallizable (Fc)-dependent effector functions such as antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), which are important for the anti-CD20 drug obinutuzumab that is used for the treatment of lymphoproliferative disorders (7). Other mAbs target specific proteins involved in pathogenesis of disease, such as anti-TNFα mAbs infliximab and adalimumab that are used to treat inflammatory bowel disease (IBD) and rheumatoid arthritis (RA) (8). Other mAbs in this category are omalizumab, an anti-IgE mAb that is used to treat patients with allergic asthma (9), palivizumab which targets an epitope in the A antigenic site of the F protein of the respiratory syncytial virus (RSV) (10), and bezlotoxumab which binds and neutralizes Clostridium difficile toxin B (11). Some mAbs, such as cetuximab and panitumumab (12), target the epidermal growth factor receptor (EGFR) which is overexpressed in a number of cancers. In recent years, checkpoint inhibitor mAbs were also developed to manipulate anti-tumor T-cell responses, like the anti-PD-1 nivolumab that is used to treat melanoma and non-small cell lung cancer (13).
The tremendous progress in mAb discovery began in 1975, when Köhler and Milstein reported in vitro screening and production of murine mAbs from hybridomas (14). In the late 1980s, murine mAbs were in rapid clinical development, but had significant drawbacks as they were often induced allergic reactions and the formation of human anti-mouse antibodies (HAMA). Examples include T101 used to treat chronic lymphocytic leukemia (CLL) and cutaneous T cell lymphoma (CTCL), and 9.2.27 to treat melanoma (15). Additionally, murine mAbs exhibited a relatively short half-life in humans, possibly due to low affinity toward the human neonatal Fc receptor (FcRn) (16), and were relatively poor recruiters of effector functions, crucial for some mAb efficacy (17).
To overcome the immunogenicity and reduced effector function of murine mAbs, chimeric antibodies (mouse–human) were next developed by fusing the antigen-specific variable domain of a murine mAb with the constant domains of a human mAb. This resulted in chimeric mAbs of approximately 65% human origin by amino acid content (18). Human gene sequences were mostly taken from the κ light chain and the IgG1 heavy chain, as IgG1 has the highest efficiency in activating complement and cytotoxic effector cells, and the κ light chain is more common in human serum antibodies (19, 20). The development of chimeric mAbs indeed reduced immunogenicity and increased efficacy. For example, metastatic colorectal carcinoma patients who received the chimeric mAb 17-1A did not show any toxic or allergic reactions, and the chimeric antibody was significantly less immunogenic than its parental murine antibody (21).
Chimeric mAbs exhibited an extended half-life and reduced immunogenicity, but they still presented a considerably high propensity for ADA induction (22). Aiming to further reduce mAb immunogenicity, humanized mAbs were developed by grafting the murine complementarity determining regions (CDR) onto framework regions (FR) of the human mAb heavy and light chain variable domains (VH and VL, respectively), for mAbs that are approximately 95% human (23). mAb humanization often significantly reduces immunogenicity and ADA formation (24).
Technological advances of phage display technology (25, 26) based on human single chain Fv (scFv) libraries (27) next enabled the discovery of antibodies comprised entirely of human genes. These human mAbs were additionally aided by the more recent development of transgenic mouse strains expressing human antibody variable domains (28–30).
While both humanized and fully human mAbs reduce immunogenic potential and show properties similar to human endogenous IgGs, they fail to completely eliminate mAb immunogenicity and ADA formation (31). Table 1 summarizes mAbs that are currently approved in the US and EU, along with their reported immunogenicity rates.
TABLE 1.
Approved mAb and their reported ADA rates.
| International non-proprietary name | Brand name | Target | Format | Indication first approved or reviewed | First EU/US approval year | %ADA | %ntADA | References |
| Adalimumab | Humira | TNFa | Human IgG1 | Rheumatoid arthritis | 2003/2002 | 28% | Not reported | (139, 140, 141) |
| Alemtuzumab | Lemtrada; MabCampath, Campath-1H | CD52 | Humanized IgG1 | Multiple sclerosis; chronic myeloid leukemia# | 2013; 2001#/2014;2001# | 67.1–75.4 | Not reported | (102, 103) |
| Alirocumab | Praluent | PCSK9 | Human IgG1 | High cholesterol | 2015/2015 | 5.1% | 1.30% | (142) |
| Atezolizumab | Tecentriq | PD-L1 | Humanized IgG1 | Bladder cancer | 2017/2016 | 30–48% | Not reported | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761034s010lbl.pdf |
| Avelumab | Bavencio | PD-L1 | Human IgG1 | Merkel cell carcinoma | 2017/2017 | 4.10% | Not reported | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761069s002lbl.pdf |
| Basiliximab | Simulect | IL-2R | Chimeric IgG1 | Prevention of kidney transplant rejection | 1998/1998 | 1.17% | Not reported | https://www.accessdata.fda.gov/drugsatfda_docs/label/2003/basnov010203LB.htm |
| Belimumab | Benlysta | BLyS | Human IgG1 | Systemic lupus erythematosus | 2011/2011 | 0–4.8% | Not reported | (74) |
| Benralizumab | Fasenra | IL-5R α | Humanized IgG1 | Asthma | 2018/2017 | 15.62% | Not reported | (143) |
| Bevacizumab | Avastin | VEGF | Humanized IgG1 | Colorectal cancer | 2005/2004 | 0% | 0% | (144) |
| Bezlotoxumab | Zinplava | Clostridium difficile enterotoxin B | Human IgG1 | Prevention of Clostridium difficile infection recurrence | 2017/2016 | 0% | 0% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761046Orig1s000ClinPharmR.pdf |
| Brodalumab | Siliq, LUMICEF | IL-17R | Human IgG2 | Plaque psoriasis | 2017/2017 | 2.70% | 0% | (145) |
| Burosumab | Crysvita | FGF23 | Human IgG1 | X-linked hypophosphatemia | 2018/2018 | 0% | 0% | https://www.ultragenyx.com/file.cfm/29/docs/Crysvita_Full_Prescribing_Information.pdf |
| Canakinumab | Ilaris | IL-1β | Human IgG1 | Muckle-Wells syndrome | 2009/2009 | <1% | 0% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/125319Orig1s085,086,087MedR.pdf |
| Cemiplimab | Libtayo | PD-1 | Human mAb | Cutaneous squamous cell carcinoma | 2019/2018 | 1.30% | Not reported | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761097s000lbl.pdf |
| Cetuximab | Erbitux | EGFR | Chimeric IgG1 | Colorectal cancer | 2004/2004 | 22.36% | Not reported | (90) |
| Crizanlizumab | Adakveo | CD62 (aka P-selectin) | Humanized IgG2 | Sickle cell disease | In review/2019 | 0–1.6% | 0% | https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/761128s000lbl.pdf |
| Daratumumab | Darzalex | CD38 | Human IgG1 | Multiple myeloma | 2016/2015 | 0.70% | Not reported | https://www.ema.europa.eu/en/documents/variation-report/darzalex-h-c-4077-ii-0002-epar-assessment-report-variation_en.pdf |
| Denosumab | Prolia | RANK-L | Human IgG2 | Bone Loss | 2010/2010 | 0% | 0% | (146) |
| Dinutuximab | Unituxin | GD2 | Chimeric IgG1 | Neuroblastoma | 2015/2015 | 28% | Not reported | (147) |
| Durvalumab | IMFINZI | PD-L1 | Human IgG1 | Bladder cancer | 2018/2017 | 2.90% | Not reported | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761069s002lbl.pdf |
| Eculizumab | Soliris | C5 | Humanized IgG2/4 | Paroxysmal nocturnal hemoglobinuria | 2007/2007 | 0% | 0% | (148) |
| Elotuzumab | Empliciti | SLAMF7 | Humanized IgG1 | Multiple myeloma | 2016/2015 | 33.30% | Not reported | (149) |
| Emapalumab, emapalumab-lzsg | Gamifant | IFNg | Human IgG1 | Primary hemophagocytic lymphohistiocytosis | In review/2018 | 5% | 1.60% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761107Orig1s000MultidisciplineR.pdf |
| Erenumab | Aimovig | CGRP receptor | Human IgG2 | Migraine prevention | 2018/2018 | 8.90% | 0% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761077Orig1s000SumR.pdf |
| Evolocumab | Repatha | PCSK9 | Human IgG2 | High cholesterol | 2015/2015 | 0.16% | 0% | (150) |
| Evolocumab | Dupixent | IL-4R α | Human IgG4 | Atopic dermatitis | 2017/2017 | 2–6% | 4–9% | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761055s007lbl.pdf |
| Fremanezumab | Ajovy | CGRP | Humanized IgG2 | Migraine prevention | 2019/2018 | 0.4–1.6% | 0.06–0.9% | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761089s000lbl.pdf |
| Galcanezumab | Emgality | CGRP | Humanized IgG4 | Migraine prevention | 2018/2018 | 12.50% | Most ADA were ntADA | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761063Orig1s000ClinPharmR.pdf |
| Golimumab | Simponi | TNFa | Human IgG1 | Rheumatoid and psoriatic arthritis, ankylosing spondylitis | 2009/2009 | 31.70% | Not reported | (151) |
| Guselkumab | TREMFYA | IL-23 p19 | Human IgG1 | Plaque psoriasis | 2017/2017 | 5.50% | 0.40% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761061Orig1s000MultidisciplineR.pdf |
| Ibalizumab, ibalizumab-uiyk | Trogarzo | CD4 | Humanized IgG4 | HIV infection | 2019/2018 | 0.83% | 0.83% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761065Orig1s000ClinPharmR.pdf |
| Infliximab | Remicade | TNF | Chimeric IgG1 | Crohn’s disease | 1999/1998 | 66.70% | Not reported | (139; 152) |
| Ipilimumab | Yervoy | CTLA-4 | Human IgG1 | Metastatic melanoma | 2011/2011 | 26%, 1.1–5.4% | Not reported, 0% | (153), United States Product Information 2018 |
| Ixekizumab | Taltz | IL-17a | Humanized IgG4 | Psoriasis | 2016/2016 | 9% | Not reported | (154) |
| Lanadelumab | Takhzyro | Plasma kallikrein | Human IgG1 | Hereditary angioedema attacks | 2018/2018 | 12% | Not reported | https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/761090s000lbl.pdf |
| Mepolizumab | Nucala | IL-5 | Humanized IgG1 | Severe eosinophilic asthma | 2015/2015 | 3% | <1% | (155) |
| Mogamulizumab | Poteligeo | CCR4 | Humanized IgG1 | Mycosis fungoides or Sézary syndrome | 2018/2018 | 3.90% | 0% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761051Orig1s000MultidisciplineR.pdf |
| Natalizumab | Tysabri | a4 integrin | Humanized IgG4 | Multiple sclerosis | 2006/2004 | 8–9% | Not reported | (156) |
| Necitumumab | Portrazza | EGFR | Human IgG1 | Non-small cell lung cancer | 2015/2015 | 4.10% | 1.40% | https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/125547s000lbl.pdf |
| Nivolumab | Opdivo | PD1 | Human IgG4 | Melanoma, non-small cell lung cancer | 2015/2014 | 12.7%, 4.1–37.8% | 0.8%, 0–4.6% | (157) https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125554s070lbl.pdf |
| Obiltoxaximab | Anthim | B. anthracis PA | Chimeric IgG1 | Prevention of inhalational anthrax | In review/2016 | 0% | 0% | (158) |
| Obinutuzumab | Gazyva, Gazyvaro | CD20 | Humanized IgG1 | Chronic lymphocytic leukemia | 2014/2013 | 7% | Not reported | https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125486s017s018lbl.pdf |
| Ocrelizumab | OCREVUS | CD20 | Humanized IgG1 | Multiple sclerosis | 2018/2017 | 0.9%, 0.2–0.5% | 0.15%, 0–0.2% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761053Orig1s000ClinPharmR.pdf, (159) |
| Ofatumumab | Arzerra | CD20 | Human IgG1 | Chronic lymphocytic leukemia | 2010/2009 | <1% | Not reported | https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/125326s062lbl.pdf |
| Olaratumab | Lartruvo | PDGFRα | Human IgG1 | Soft tissue sarcoma | 2016/2016 | 3.50% | 3.50% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761038Orig1s000MultiDisciplineR.pdf |
| Omalizumab | Xolair | IgE | Humanized IgG1 | Asthma | 2005/2003 | 0% | 0% | (160) |
| Palivizumab | Synagis | RSV | Humanized IgG1 | Prevention of respiratory syncytial virus infection | 1999/1998 | 1.80% | 0% | (161) |
| Panitumumab | Vectibix | EGFR | Human IgG2 | Colorectal cancer | 2007/2006 | 4.60% | 1.60% | https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/125147s080lbl.pdf |
| Pembrolizumab | Keytruda | PD1 | Humanized IgG4 | Melanoma | 2015/2014 | 1.80% | 0.50% | (162) |
| Pertuzumab | Perjeta | HER2 | humanized IgG1 | Breast Cancer | 2013/2012 | 0.60% | Not reported | (163) |
| Ramucirumab | Cyramza | VEGFR2 | Human IgG1 | Gastric cancer | 2014/2014 | 3.80% | 0.18% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2014/125477Orig1s000MedR.pdf |
| Ravulizumab (ALXN1210) | Ultomiris | C5 | Humanized IgG2/4 | Paroxysmal nocturnal hemoglobinuria | 2019/2018 | >0.5% | 0% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761108Orig1s000MultidisciplineR.pdf |
| Raxibacumab | (Pending) | B. anthracis PA | Human IgG1 | Anthrax infection | NA/2012 | 0% | 0% | https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/125349s000lbl.pdf |
| Reslizumab | Cinqaero, Cinqair | IL-5 | Humanized IgG4 | Asthma | 2016/2016 | 4.8–5.4%, 5% | Not reported, 0% | (164; 165) |
| Risankizumab | Skyrizi | IL-23 p19 | Humanized IgG1 | Plaque psoriasis | 2019/2019 | 24% | 14% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761105Orig1s000MultidisciplineR.pdf |
| Rituximab | MabThera, Rituxan | CD20 | Chimeric IgG1 | Non-Hodgkin lymphoma | 1998/1997 | 26–37%, 12.5% | Not reported | (73; 144) |
| Romosozumab | Evenity | Sclerostin | Humanized IgG2 | Osteoporosis in postmenopausal women at increased risk of fracture | NA/2019 | 18.10% | 4.60% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761062Orig1s000MultidisciplineR.pdf |
| Sarilumab | Kevzara | IL-6R | Human IgG1 | Rheumatoid arthritis | 2017/2017 | 14–19.3% | 1.8–3.3% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2017/761037Orig1s000ChemR.pdf |
| Secukinumab | Cosentyx | IL-17a | Human IgG1 | Psoriasis | 2015/2015 | 0.41% | 0.20% | (166) |
| Siltuximab | Sylvant | IL-6 | Chimeric IgG1 | Castleman disease | 2014/2014 | 0.20% | 0% | (167) |
| Tildrakizumab | Ilumya | IL-23 p19 | Humanized IgG1 | Plaque psoriasis | 2018/2018 | 6.8–8.8%, 4.1–8.2% | 2.7–3.34%, 0.6–3.2% | https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/761067Orig1s000MultdisciplineR.pdf, (168) |
| Tocilizumab | RoActemra, Actemra | IL-6R | Humanized IgG1 | Rheumatoid arthritis | 2009/2010 | 5 | Not reported | (169) |
| Trastuzumab | Herceptin | HER2 | Humanized IgG1 | Breast cancer | 2000/1998 | 16.30% | Not reported | (144) |
| Ustekinumab | Stelara | IL-12/23 | Human IgG1 | Psoriasis | 2009/2009 | 6.50% | Not reported | (170) |
| Vedolizumab | Entyvio | α4β7 integrin | humanized IgG1 | Ulcerative colitis, Crohn’s disease | 2014/2014 | 17% | Not reported | (171) |
In the past decade, next-generation sequencing (NGS) technologies enabled a rapid increase in the capacity to sequence human and animal genomes (32). Like many other areas of modern biology, NGS is now frequently used in basic and applied immunology. NGS is often applied for sequencing the VH and VL antibody domains (33–36), as well as T-cell receptors (37, 38) and antibody derivative [e.g., scFv, F(ab)] libraries screened using display systems (39–41). NGS analysis of B cells can elucidate the features of antibody immune responses at a molecular level, and has been further exploited for advanced mAb discovery and engineering (42–44).
In addition to NGS of bulk populations, single-cell sequencing comprises an important group of technologies for antibody discovery, as single cell data is necessary to reveal the native VH and VL pairing. Previous studies were able to obtain VH and VL chain pairing from isolated plasmablasts (PB) in immunized mice (34, 45, 46) and antigen-specific PB from tetanus-vaccinated human patients (33).
A recently introduced technology combines proteomic analyses of antibodies in blood or secretions with NGS analysis of antibody-encoding B cells. Proteomics thus provides invaluable information about the molecular, monoclonal properties of human serum antibodies in health and disease (46–48). All of the above recently developed technologies have expedited mAb discovery and revolutionized our understanding about the nature of the immune responses, including in the formation of ADAs following immunization and administration of mAbs.
Monoclonal antibodies immunogenicity is mainly manifested in ADA generation (49). The formation of ADAs alters a drug’s bioavailability and pharmacokinetic and pharmacodynamic properties, and most often reduces drug efficacy (50, 51). ADAs have a significant impact on mAb drug safety, as they can lead to serious adverse immune reactions in the clinic (52). Patients with ADAs can be stratified by their effect on the clinical treatment course. Patients are designated as having primary loss of response (LOR) when the administrated mAb fails to show any efficacy within several weeks following treatment initiation, or secondary LOR when patients show significant side effects or the drug loses effectiveness over time despite an initial therapeutic response (53–55).
For multiple decades, many studies focused on possible mechanisms that govern ADA formation, development of improved assays for ADA detection, and advancement of tools for immunogenicity and prediction of ADA formation. This review provides an overview on these topics, underlining the challenges and potential solutions for this important research field. While this review focuses on ADA as an important outcome of mAb immunogenicity, there are other immunogenicity outcomes such as allergic reactions, cytopenia, and anaphylaxis that are widely reviewed elsewhere (56).
The Molecular Mechanisms That Lead to ADA Formation
Anti-drug antibodies can be generated by a T-cell dependent or independent B cell activation pathway. In the T-cell dependent pathway, mAbs act as antigens and are internalized by antigen presenting cells (APCs), processed, and presented to T cells via the cognate interaction between the MHC class II molecules and T-cell receptor. Depending on the cytokine milieu during this interaction, several different immune responses can occur (57). In the T-cell dependent pathway, ADAs are generated when a T helper cell (Th) differentiates into a Th1 or Th2 phenotype and, following their cognate interactions with B cells, induces the proliferation of plasma cells (PC) that secrete ADAs. Previous studies showed that a Th2 response mostly induce ADA production of the IgG4 isotype, in comparison to the Th1 response, that in the case of anti-factor VIII elicits the generation of IgG1 and IgG2 ADA (58, 59).
For example, infliximab-specific Th2 cells can be detected in circulation after infliximab infusion, and these cells were correlated with the presence of infliximab-specific ADA (60). Interestingly, this cellular response was observed mostly in patients with hypersensitivity reactions, rather than in the LOR group. In another study, T cell epitopes of infliximab and rituximab were identified by isolating antibody-specific T cells after repeated rounds of antibody-loaded dendritic cells (DCs) in co-culture (61). These T cells were specific to peptides derived from VH and VL and encompassed CDRs and FRs, reflecting the immunogenicity of the chimeric part of these antibodies. Importantly, these peptides were also eluted from antibody-loaded DCs, highlighting the importance of MHC Class II antigen presentation in the ADA formation process.
In contrast, for the T cell independent pathway mAbs with multiple epitopes can crosslink B cell receptors (BCRs) and stimulate B cells to differentiate into PC to produce ADAs (62–66). It was previously demonstrated that impurities and aggregates of the mAbs may increase the number of adjacent epitopes on the mAb, potentially steering the immune response toward a T-cell independent pathway by B cell crosslinking (67–70).
Drug and Patient Characteristics Contributing to ADA Formation
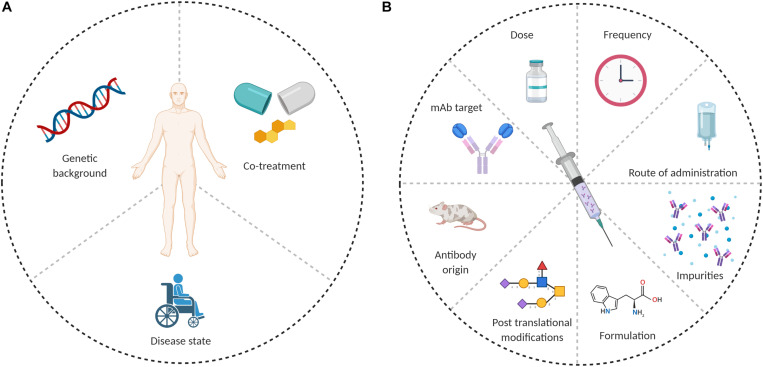
Anti-drug antibodies formation depends on the interplay between several factors, which can be patient-related or drug-related. Possible causes for ADA formation are summarized in Figure 1.
FIGURE 1.
Possible causes of ADA formation. (A) Patient related and (B) drug related.
Patient-Related Factors
The study of why and how ADAs are generated is complicated by the fact that some patients develop ADAs and some, with the same clinical indication and receiving the same therapeutic mAb, do not. The extent of immunogenicity thus differs among patients receiving the same mAb, which could be related to the immune pathways underlying the pathogenesis of the disease (71). For example, RA patients have a higher likelihood of developing ADAs toward a mAb drug than spondyloarthritis patients (57). When examining a specific disease or immune target, different mAbs may have a varying effect on the induction of ADAs. RA patients develop higher ADA levels when treated with two different mAbs (72). In multiple sclerosis (MS) patients, treatment with rituximab (chimeric anti-CD20 mAb) generated an unwanted immune response in up to 37% of patients (73). On the contrary, belimumab (a fully human anti B-cell activating factor (BAFF) mAb), which is used to treat systemic lupus erythematosus (SLE) patients, showed low rates induction ADA (74). Of note, in autoimmune diseases the hyperactivation of both the innate and adaptive immune responses may further complicate the study of mAb immunogenicity (57, 75). On the other hand, when administering mAbs to cancer patients, ADA formation often depends on the stage of the cancer. ADA levels tend to be higher in early stages of the disease than in later stages (76).
Much of the variability in the propensity of administrated mAb to induce ADA formation may result from different immune contexts; Principally, disease status and HLA alleles, which could promote or inhibit an ADA response. The idea that ADA formation is often derived from a T-dependent response has recently led to studies focusing on how ADA formation correlates with HLA polymorphism in the population. Although limited by sample size, Benucci et al. showed that patients with the HLA-DRβ-11, HLA-DQ-03, and HLA-DQ-05 alleles were at a higher risk to develop ADA responses after treatment with an anti-TNF mAb (5 different mAbs were included in this study) (77). Another report revealed that a G1m1 allotype in the IgG1 created a protease cleavage site in the CH3 domain of the antibody Fc and enabled presentation of a CH315–29 peptide epitope (78). The CH315–29 peptide epitope was tolerated in patients with a G1m1 allotype. However, donors homozygous for nG1m1 did not natively display the G1m1 MHC-II peptide and developed T cell CD4+ responses against antibody therapeutics containing the G1m1 allotype sequence; these ADA were also correlated with HLA-DRB1∗07 allele. Some therapeutic mAbs (including trastuzumab) do not harbor this allotype, which could partially explain differences in immunogenicity across different mAb drugs (78, 79). This allotype difference could impact future development of antibody products, since ∼40% of the Caucasian population is homozygous for nG1m1, and thus may be at a greater risk for ADA generation (80). In two recent studies, ADA formation against infliximab and adalimumab was correlated with the HLADQA1∗05A > G genotype in IBD patients (81, 82). One detailed recent study examined the immune response to natalizumab, a humanized monoclonal IgG4 antibody to α4 integrins that is used to treat patients with MS, and that induces ADA formation in ∼6% of the patients. The immune response was found to be polyclonal and targeted different epitopes of the natalizumab idiotype, with a single immunodominant T cell epitope spanning the FR2-CDR2 region of the VL (83). Generation of a T cell-dependent ADA response is also a multifactorial process, depending not only on the existence of a potential MHC-II peptide epitope in the mAb, but also on the ability of that epitope to be processed, presented and recognized by T cells. The influence of HLA allotypes on the probability of ADA responses should be considered during the design of immunogenicity studies and clinical trials for mAb development. Conclusions from studies that rely on smaller cohorts might not have general applicability for ADA predictions if the study population has substantially different MHC-II gene backgrounds from a larger treatment population.
Drug-Related Factors
The molecular mechanisms that lead to induction of ADAs were initially related to the murine origin of the first mAbs, which were recognized as “non-self” by the human immune system. Unfortunately, even the use of complete human antibody genes has not completely eliminated immunogenicity and the associated induction of ADA (84). Fully human mAbs contain new epitopes in the CDRs that can steer the immune response through an idiotype/anti-idiotype interaction (85, 86). As discussed above, mAb-derived peptides presented by MHC-II are necessary for T cell-dependent ADA formation. Efforts to remove T cell epitopes during mAb engineering are used consistently, but the high genetic variability of human populations greatly complicates efforts to remove all MHC-II-binding peptides from human mAbs (87, 88).
Changes in Fc glycosylation may also affect ADA induction. The removal of N-linked glycosylation of the Fc was shown to reduce immunogenicity (89). Fully human mAbs lacking Fc functions were also shown to be immunogenic and have direct effects on the ability to recruit macrophages and activate complement. For example, galactose-α-1,3-galactose, which is a foreign glycan not found in humans, is present on the antigen-binding (Fab) portion of the cetuximab VH (a chimeric mAb used in cancer therapy targeting the EGF receptor). This glycan was shown to induce ADA formation of the IgE isotype, and was responsible for anaphylactic reactions in patients (90, 91). On the other hand, immunogenicity is sometimes linked to impurities in the formulation process, and not necessarily due to glycosylation differences. A review of the differences between 18 biosimilars and mAbs originators concluded that the differences between them are mainly in glycosylation patterns, and do not impact immunogenicity (92).
Other drug related factors that play a role in mAb immunogenicity are “danger signals” that are released by tissues undergoing stress, damage or abnormal death. The danger model was first suggested in 1994, were it was first postulated that the immune system responds to substances that cause damage, rather than to those that are simply foreign (93, 94). In the case of therapeutic antibodies, process related impurities (such as aggregates and residual DNA or proteins from the mAb expression system) can influence immunogenicity (95).
The mAb target may also have high importance for the MOA of ADA formation. We recently found that repeated administration of infliximab (a TNFα antagonist) results in a vaccine-like response, where ADA formation is governed by the extrafollicular T cell-independent immune response (96). The administration of infliximab blocks TNFα and shifts the immune response toward the marginal zone (MZ) instead of the germinal center (GC), as observed in TNFα knockout mice (97). Another possible explanation is that a strong T cell-independent immune response in the MZ may be induced by a drug/ADA/TNFα immunocomplex (IC). As a trimer, TNFα may form “super complexes” upon engagement with TNFα antagonistic antibodies (98–100).
Another example of mAb target importance is alemtuzumab, a mAb specific to the CD52 lymphocyte cell surface glycoprotein. Alemtuzumab is used to treat MS (101) and induces ADAs in about 85% of patients, of which around 92% develop neutralizing ADAs (102). Alemtuzumab’s high frequency of ADA induction may be related to CD52 expression patterns. Alemtuzumab targets APCs, which include DCs, monocytes, and memory B cells, based on their CD52 expression. When monocytes repopulate, they encounter the circulating mAb that rapidly presents antigen to the antigen-specific lymphocytes (103, 104). Memory B cells often exhibit homeostatic expansion following treatment with alemtuzumab (105), which could complement ADA generation.
mAb dosage and schedule are other possible factors influencing ADA formation rates. Increased numbers of injections and higher mAb doses are associated with higher ADA risk, although some cases of chronic treatment and higher doses have lower immunogenicity (92, 106). For example, rituximab, a chimeric mAb anti-CD20, targets surface antigens on pre-B cells and B cells before their differentiation into PCs. As rituximab selectively depletes CD20 positive B cells, it does not affect mature PCs and does not have a propensity to elicit ADAs (107).
Assays for Immunogenicity Assessment and Tools for Immunogenicity Reduction
Pre-clinical Setting
Due to the growing importance of mAb immunogenicity, there has been a growing need for tools to assess immunogenicity and reduce the propensity of mAbs to induce ADAs. Great efforts in tools such as in silico prediction algorithms and cell based experimental assays are facilitating immunogenicity assessment, especially during the initial development phases of the mAb (108).
In silico CD4+ T cell epitope prediction models are often used to identify potentially immunogenic MHC-II peptide epitopes. These algorithms are based on the affinity of mAb-derived peptides to MHC-II (109–111).
With recent advances in proteomics and sequencing, several MHC-II peptide epitope databases have been constructed that provide a library of MHC-II binding data to enable immunogenicity prediction (112). Most algorithms that predict the immunogenic sequences recognized by T cells are later confirmed by assessing peptide binding to MHC molecules (88, 113). For example, a strong correlation was found between in silico evaluation of T cell epitopes from a recombinant Fc fusion protein, and the immunogenicity rate when administered to patients in a clinical trial (114). While such predictive algorithms are common used, they capture only a fraction of the system’s complexity. Most CD4 + T cell epitope prediction algorithms are based on binding affinity and stability to MHC-II molecules (88, 110), but fail to consider other essential factors in the recognition of T cell epitopes. Among these factors are protease cleavage sites (115), T cell precursor frequency (116), and peptide and T cell competition (117).
Experimental tools are also used to make pre-clinical predictions about mAb immunogenicity risk. These include HLA binding assays, DC related assays, T cell stimulation assays, peripheral blood mononuclear cell (PBMC) stimulation assays, and various animal models (115). HLA binding and DC antigen presentation assays can evaluate potential T cell epitopes derived from the mAb, while T cell and PBMC stimulation assays examine whether a mAb can activate immune cells in vitro and ex vivo in terms of cell proliferation and cytokine release. For example, T cell epitopes in the variable regions of infliximab and rituximab were able to stimulate peripheral blood mononuclear cells (PBMCs) to secrete a variety of cytokines (61). In another study, the immunogenicity of secukinumab, an anti- interleukin-17A mAb used to treat plaque psoriasis, was assessed by examining T-cell proliferation (118).
Each of these experimental tools has limitations in assessing and predicting immunogenicity. While considered reliable and straightforward, most of the experimental assays are labor intensive and are impractical to implement with a large number of mAb candidates. These assays are often performed with cells derived from a naïve population, where the frequency of antigen-specific cells is relatively low and precludes a clear positive result due to low signal-to-noise ratios (88).
Other advancements are being made in the development of mAbs to which patients will be more tolerant. A previous study identified a set of naturally occurring human regulatory T cell epitopes (“Tregitopes”), present in the Fc and Fab domains of IgG, that induce tolerance when co-administered with other proteins (119). When incubated with PBMCs in vitro, Tregitopes activated CD4+ T cells and increased expression of regulatory cytokines, chemokines, and CD25/Foxp3. When were administered in vivo with protein antigens, Tregitopes inhibited T cell proliferation, reduced effector cytokine expression, and induced antigen-specific adaptive tolerance. Co-administration of Tregitopes along with mAbs may be a useful tool for tolerization of mAbs.
Clinical Settings
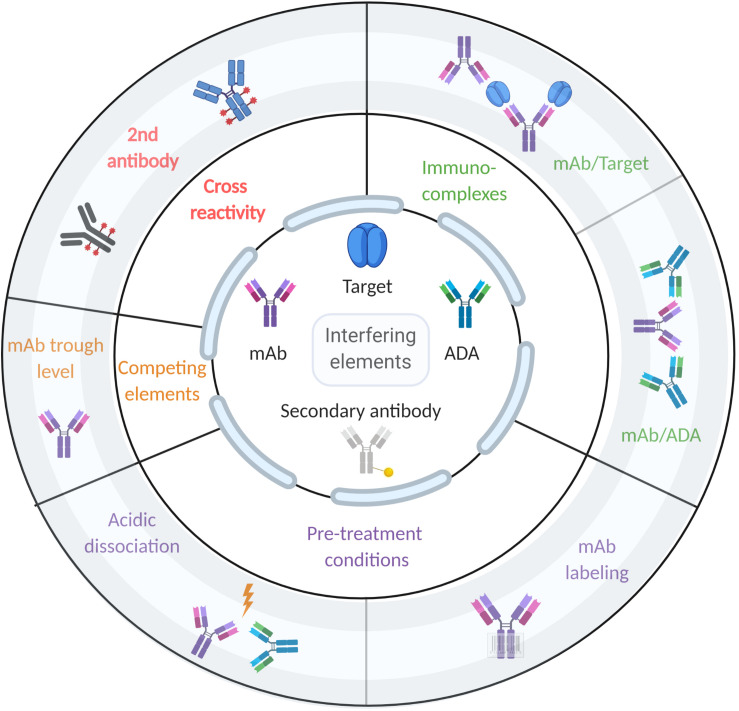
Early and accurate ADA detection is extremely important for patients treated with biologics, especially for mAbs (120). ADA detection is required to provide the clinicians with sufficient information to monitor treatment and determine optimal intervention strategies (121). Detection of ADA against therapeutic mAbs is highly challenging since both the drug and the analyte are antibodies. Moreover, immunoassays are prone to biases due to the presence of the drug and immune-complexes in patients’ serum. Historically, studies of the response following mAb administration and ADA prevalence have been inconsistent, partly due to the various assay formats used to monitor immunogenicity in clinical trials (122). Each available format has its limitations that can reduce the assay’s utility in clinical and research settings, and also complicate interpretation of the data. Some assays have poor dynamic range and may generate false-negative results because of interfering interactions with the active drug, or false-positive results due to other antibodies like rheumatoid factor (123). Figure 2 shows the competing factors which affect accurate measurement of ADAs.
FIGURE 2.
Factors that affect ADA detection in immunoassays. The center of the figure designates the components that could interfere with ADA detection (i.e., mAb, target, ADA, and secondary antibody). The middle circle designates the type of interference, while the outer circle provides examples of such interferences.
An ELISA-based bridging assay is one of the most commonly used assays for ADA screening, where the mAb drug is used to first capture ADA present in the patient sera, and the latter are detected by adding additional labeled mAb as a secondary probe. Bridging ELISA assays are used for ADA detection of a large variety of mAbs, and some include an acidic step to dissociate ADA from the mAb. The excess mAb is then captured or removed, and free ADA can be detected. These assays often have significantly higher background and suffer from low sensitivity due to the disassociation of antibodies. Bridging assays can also result in false-negatives, as they are more likely to “miss” low affinity IgM ADAs present in early stages of the immune response (124). Most ELISA-based bridging assays are also sensitive to the mAbs’ trough levels (levels of circulating mAb at sampling time). ADA and mAbs tend to form high molecular weight immune-complexes, making ADA detection more challenging (125). To overcome this challenge, several drug-tolerant assays have been developed to measure ADA levels in the presence of high mAb concentrations (126). Most of these assays also use an acidic treatment step. Several other techniques have been reported to evaluate serum ADA levels. These assays include radio-immunoassays (127), Biotin-drug Extraction with Acid Dissociation (BEAD) (128), Precipitation and Acid dissociation (PANDA) (129), Affinity Capture Elution ELISA (ACE) (130), and Homogenous Mobility Shift Assay (HMSA) (131); these assays have been reviewed in detail elsewhere (126). While these assays presumably detect all serum ADA, they primarily provide qualitative measures to assist healthcare providers deciding on appropriate patient interventions, and many (if not all) studies underestimate actual ADA levels. These assays also lack standardization that could enable comparisons of ADA levels across health centers. The great diversity in these assays poses tremendous difficulty in studying ADA levels between different mAbs, across studies of the same mAb, and across different assays.
In a clinical context, it important both to assess ADA levels in patient serum, and also to assess the presence of neutralizing antibodies that interfere with biological and clinical activity of the mAb. The neutralizing effect of ADAs can be assayed by testing whether ADAs in serum inhibit binding of the mAb to its target (132). Several cell-based assays were developed to detect ntADA in patients’ serum. One of these assays is a functional ADA cell-based assay that was developed to quantify the activity of TNFα antagonists. This assay assesses both drug activity and ntADA levels (133), but correlations between the clinical outcome and assay results were not thoroughly tested. Another assay developed for ntADA detection is the reporter gene assay, which is based on excretion of IL8 by HT29 cells due to TNFα stimulation (77). When the assay was applied to sera samples with low-level ADA, it detected ntADA even prior to clinical LOR to the mAb, which allows the prediction of clinical LOR with high probability.
While these assays are accurate and sensitive, they require an active cell line, which complicates assay implementation. We recently reported on a newly developed quantitative bio-immunoassay for quantifying ADA specific to TNFα antagonists. The bio-immunoassay was further modified to easily assess the neutralization capacity of ADA using an in vitro assay (96). This assay can be readily used in a clinical setting that performs routine ADA measurements.
Other clinical approaches to reduce immunogenicity include active interference of the T cell responses to mAbs, thereby inducing individual tolerance of the immune system (“tolerization”).
For example, administration of methotrexate (MTX) with infliximab reduced ADA formation in RA patients (134). MTX also reversed high ADA levels in infantile Pompe disease patients treated with rituximab, when administered alongside bortezomib, a proteasome activity inhibitor that leads to cell death (135). Azathioprine is also an immunosuppressive drug that can be given in combination with infliximab or adalimumab to improve treatment and reduce immunogenicity and ADA formation (136–138). However, such non-specific immunosuppressive approaches have potentially harmful side effects that must be balanced with the patient’s overall treatment plan.
Concluding Remarks
Monoclonal antibodies have the potential to treat a wide range of diseases and disorders, but they can be highly immunogenic and induce undesirable ADA responses. ADAs can reduce mAb drug efficacy by altering its bioavailability and/or accelerating clearance from circulation. While the molecular mechanisms of ADA generation are not fully understood, it is dependent on both patient and drug characteristics. While early ADAs were related to the murine origin of the first mAb therapeutics, ADAs also occur against fully human mAbs. Indeed, complete humanization cannot completely abrogate mAb immunogenicity and ADA formation. The questions of why and how ADA are generated also depend on variability of the reported immunogenicity rates, which emphasizes the need for standardized clinical assays for ADA detection. Understanding the mechanisms of ADA generation and the major factors that influence immunogenicity of mAbs will help us design safer mAbs with lower drug rejection rates. Recent and ongoing efforts to study mAb immunogenicity at the molecular level is augmenting our understanding of these mechanisms that lead to ADA formation, which may help provide new guidelines to improve the safety and efficacy of mAb therapeutics.
Author Contributions
AV-M, MG-G, BD, and YW wrote the sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Colette Worcester for assistance with manuscript preparation.
Footnotes
Funding. Funding for this work was provided by the United States-Israel Binational Science Foundation #2017359, and by US NIH grants R21AI143407, R21AI144408, and DP5OD023118.
References
- 1.Chan JCN, Chan ATC. Biologics and biosimilars: what, why and how? ESMO Open. (2017) 2:e000180 10.1136/esmoopen-2017-000180 [DOI] [Google Scholar]
- 2.Aggarwal RS. What’s fueling the biotech engine-2012 to 2013. Nat Biotechnol. (2014) 32:32–9. 10.1038/nbt.2794 [DOI] [PubMed] [Google Scholar]
- 3.Ecker DM, Jones SD, Levine HL. The therapeutic monoclonal antibody market. MAbs. (2015) 7:9–14. 10.4161/19420862.2015.989042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grilo AL, Mantalaris A. The increasingly human and profitable monoclonal antibody market. Trends Biotechnol. (2019) 37:9–16. 10.1016/j.tibtech.2018.05.014 [DOI] [PubMed] [Google Scholar]
- 5.Alas S, Bonavida B. Rituximab inactivates signal transducer and activation of transcription 3 (STAT3) activity in B-non-hodgkin’s lymphoma through inhibition of the interleukin 10 autocrine / paracrine loop and results in down-regulation of Bcl-2 and sensitization to cytot. Cancer Res. (2001) 61:5137–44. [PubMed] [Google Scholar]
- 6.Nahta R, Esteva FJ. Herceptin: mechanisms of action and resistance. Cancer Lett. (2006) 232:123–38. 10.1016/j.canlet.2005.01.041 [DOI] [PubMed] [Google Scholar]
- 7.Said R, Tsimberidou AM. Obinutuzumab for the treatment of chronic lymphocytic leukemia and other B-cell lymphoproliferative disorders. Expert Opin Biol Ther. (2017) 17:1463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim H, Lee SH, Lee HT, Lee JU, Son JY, Shin W, et al. Structural biology of the TNFalpha antagonists used in the treatment of rheumatoid arthritis. Int J Mol Sci. (2018) 19:768. 10.3390/ijms19030768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulman ES. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am J Respir Crit Care Med. (2001) 164:S6–11. [DOI] [PubMed] [Google Scholar]
- 10.Rogovik AL, Carleton B, Solimano A, Goldman RD. Palivizumab for the prevention of respiratory syncytial virus infection. Can Fam Physician. (2010) 56:769–72. [PMC free article] [PubMed] [Google Scholar]
- 11.Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, et al. Bezlotoxumab for prevention of recurrent clostridium difficile infection. N Engl J Med. (2017) 376:305–17. [DOI] [PubMed] [Google Scholar]
- 12.García-Foncillas J, Sunakawa Y, Aderka D, Wainberg Z, Ronga P, Witzler P, et al. Distinguishing features of cetuximab and panitumumab in colorectal cancer and other solid tumors. Front Oncol. (2019) 9:849. 10.3389/fonc.2019.00849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rajan A, Kim C, Heery CR, Guha U, Gulley JL. Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: role in advanced cancers. Hum Vaccin Immunother. (2016) 12:2219–31. 10.1080/21645515.2016.1175694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. (1975) 256:495–7. 10.1038/256495a0 [DOI] [PubMed] [Google Scholar]
- 15.Schroff RW, Foon KA, Beatty SM, Oldham RK, Morgan AC., Jr. Human anti-murine immunoglobulin responses in patients receiving monoclonal antibody therapy. Cancer Res. (1985) 45:879–85. [PubMed] [Google Scholar]
- 16.Pyzik M, Rath T, Lencer WI, Baker K, Blumberg RS. FcRn: the architect behind the immune and nonimmune functions of IgG and albumin. J Immunol. (2015) 194:4595–603. 10.4049/jimmunol.1403014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. (2001) 13:1551–9. 10.1093/intimm/13.12.1551 [DOI] [PubMed] [Google Scholar]
- 18.Morrison SL, Johnson MJ, Herzenberg LA, Oi VT. Chimeric human antibody molecules: mouse antigen-binding domains with human constant region domains. Proc Natl Acad Sci USA. (1984) 81:6851–5. 10.1073/pnas.81.21.6851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLennan IC, Howard A, Gotch FM, Quie PG. Effector activating determinants on IgG. I. The distribution and factors influencing the display of complement, neutrophil and cytotoxic B-cell determinants on human IgG sub-classes. Immunology. (1973) 25:459–69. [PMC free article] [PubMed] [Google Scholar]
- 20.Reff ME, Carner K, Chambers KS, Chinn PC, Leonard JE, Raab R, et al. Depletion of B cells in vivo by a chimeric mouse human monoclonal antibody to CD20. Blood. (1994) 83:435–45. 10.1182/blood.v83.2.435.bloodjournal832435 [DOI] [PubMed] [Google Scholar]
- 21.LoBuglio AF, Wheeler RH, Trang J, Haynes A, Rogers K, Harvey EB, et al. Mouse/human chimeric monoclonal antibody in man: kinetics and immune response. Proc Natl Acad Sci USA. (1989) 86:4220–4. 10.1073/pnas.86.11.4220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Presta LG. Engineering of therapeutic antibodies to minimize immunogenicity and optimize function. Adv Drug Deliv Rev. (2006) 58:640–56. 10.1016/j.addr.2006.01.026 [DOI] [PubMed] [Google Scholar]
- 23.Jones PT, Dear PH, Foote J, Neuberger MS, Winter G. Replacing the complementarity-determining regions in a human antibody with those from a mouse. Nature. (1986) 321:522–5. 10.1038/321522a0 [DOI] [PubMed] [Google Scholar]
- 24.Hwang WY, Foote J. Immunogenicity of engineered antibodies. Methods. (2005) 36:3–10. 10.1016/j.ymeth.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 25.Winter G, Griffiths AD, Hawkins RE, Hoogenboom HR. Making antibodies by phage display technology. Annu Rev Immunol. (1994) 12:433–55. 10.1146/annurev.iy.12.040194.002245 [DOI] [PubMed] [Google Scholar]
- 26.Vaughan TJ, Williams AJ, Pritchard K, Osbourn JK, Pope AR, Earnshaw JC, et al. Human antibodies with sub-nanomolar affinities isolated from a large non-immunized phage display library. Nat Biotechnol. (1996) 14:309–14. 10.1038/nbt0396-309 [DOI] [PubMed] [Google Scholar]
- 27.Azriel-Rosenfeld R, Valensi M, Benhar I. A human synthetic combinatorial library of arrayable single-chain antibodies based on shuffling in vivo formed CDRs into general framework regions. J Mol Biol. (2004) 335:177–92. 10.1016/j.jmb.2003.10.053 [DOI] [PubMed] [Google Scholar]
- 28.Green LL, Hardy MC, Maynard-Currie CE, Tsuda H, Louie DM, Mendez MJ, et al. Antigen-specific human monoclonal antibodies from mice engineered with human Ig heavy and light chain YACs. Nat Genet. (1994) 7:13–21. 10.1038/ng0594-13 [DOI] [PubMed] [Google Scholar]
- 29.Cohenuram M, Saif MW. Panitumumab the first fully human monoclonal antibody: from the bench to the clinic. Anticancer Drugs. (2007) 18:7–15. 10.1097/cad.0b013e32800feecb [DOI] [PubMed] [Google Scholar]
- 30.Rau R. Adalimumab (a fully human anti-tumour necrosis factor alpha monoclonal antibody) in the treatment of active rheumatoid arthritis: the initial results of five trials. Ann Rheum Dis. (2002) 61(Suppl. 2):ii70–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baker MP, Reynolds HM, Lumicisi B, Bryson CJ. Immunogenicity of protein therapeutics: the key causes, consequences and challenges. Self Nonself. (2010) 1:314–22. 10.4161/self.1.4.13904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Luciani F, Bull RA, Lloyd AR. Next generation deep sequencing and vaccine design: today and tomorrow. Trends Biotechnol. (2012) 30:443–52. 10.1016/j.tibtech.2012.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeKosky BJ, Ippolito GC, Deschner RP, Lavinder JJ, Wine Y, Rawlings BM, et al. High-throughput sequencing of the paired human immunoglobulin heavy and light chain repertoire. Nat Biotechnol. (2013) 31:166–9. 10.1038/nbt.2492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.DeKosky BJ, Kojima T, Rodin A, Charab W, Ippolito GC, Ellington AD, et al. In-depth determination and analysis of the human paired heavy- and light-chain antibody repertoire. Nat Med. (2015) 21:86–91. 10.1038/nm.3743 [DOI] [PubMed] [Google Scholar]
- 35.Doria-Rose NA, Schramm CA, Gorman J, Moore PL, Bhiman JN, DeKosky BJ, et al. Developmental pathway for potent V1V2-directed HIV-neutralizing antibodies. Nature. (2014) 509:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu X, Zhou T, Zhu J, Zhang B, Georgiev I, Wang C, et al. Focused evolution of HIV-1 neutralizing antibodies revealed by structures and deep sequencing. Science. (2011) 333:1593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robins HS, Campregher PV, Srivastava SK, Wacher A, Turtle CJ, Kahsai O, et al. Comprehensive assessment of T-cell receptor beta-chain diversity in alphabeta T cells. Blood. (2009) 114:4099–107. 10.1182/blood-2009-04-217604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosati E, Dowds CM, Liaskou E, Henriksen EKK, Karlsen TH, Franke A. Overview of methodologies for T-cell receptor repertoire analysis. BMC Biotechnol. (2017) 17:61. 10.1186/s12896-017-0379-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glanville J, Zhai W, Berka J, Telman D, Huerta G, Mehta GR, et al. Precise determination of the diversity of a combinatorial antibody library gives insight into the human immunoglobulin repertoire. Proc Natl Acad Sci USA. (2009) 106:20216–21. 10.1073/pnas.0909775106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vaisman-Mentesh A, Wine Y. Monitoring phage biopanning by next-generation sequencing. Methods Mol Biol. (2018) 1701:463–73. 10.1007/978-1-4939-7447-4_26 [DOI] [PubMed] [Google Scholar]
- 41.Wang B, DeKosky BJ, Timm MR, Lee J, Normandin E, Misasi J, et al. Functional interrogation and mining of natively paired human VH:VL antibody repertoires. Nat Biotechnol. (2018) 36:152–5. 10.1038/nbt.4052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Georgiou G, Ippolito GC, Beausang J, Busse CE, Wardemann H, Quake SR. The promise and challenge of high-throughput sequencing of the antibody repertoire. Nat Biotechnol. (2014) 32:158–68. 10.1038/nbt.2782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddy ST, Ge X, Miklos AE, Hughes RA, Kang SH, Hoi KH, et al. Monoclonal antibodies isolated without screening by analyzing the variable-gene repertoire of plasma cells. Nat Biotechnol. (2010) 28:965–9. 10.1038/nbt.1673 [DOI] [PubMed] [Google Scholar]
- 44.Saggy I, Wine Y, Shefet-Carasso L, Nahary L, Georgiou G, Benhar I. Antibody isolation from immunized animals: comparison of phage display and antibody discovery via V gene repertoire mining. Protein Eng Des Sel. (2012) 25:539–49. 10.1093/protein/gzs060 [DOI] [PubMed] [Google Scholar]
- 45.Lee J, Boutz DR, Chromikova V, Joyce MG, Vollmers C, Leung K, et al. Molecular-level analysis of the serum antibody repertoire in young adults before and after seasonal influenza vaccination. Nat Med. (2016) 22:1456–64. 10.1038/nm.4224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lavinder JJ, Wine Y, Giesecke C, Ippolito GC, Horton AP, Lungu OI, et al. Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc Natl Acad Sci USA. (2014) 111:2259–64. 10.1073/pnas.1317793111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Boutz DR, Horton AP, Wine Y, Lavinder JJ, Georgiou G, Marcotte EM. Proteomic identification of monoclonal antibodies from serum. Anal Chem. (2014) 86:4758–66. 10.1021/ac4037679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wine Y, Boutz DR, Lavinder JJ, Miklos AE, Hughes RA, Hoi KH, et al. Molecular deconvolution of the monoclonal antibodies that comprise the polyclonal serum response. Proc Natl Acad Sci USA. (2013) 110:2993–8. 10.1073/pnas.1213737110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nat Rev Rheumatol. (2013) 9:164–72. 10.1038/nrrheum.2013.4 [DOI] [PubMed] [Google Scholar]
- 50.Atzeni F, Talotta R, Salaffi F, Cassinotti A, Varisco V, Battellino M, et al. Immunogenicity and autoimmunity during anti-TNF therapy. Autoimmun Rev. (2013) 12:703–8. 10.1016/j.autrev.2012.10.021 [DOI] [PubMed] [Google Scholar]
- 51.De Groot AS, Scott DW. Immunogenicity of protein therapeutics. Trends Immunol. (2007) 28:482–90. 10.1016/j.it.2007.07.011 [DOI] [PubMed] [Google Scholar]
- 52.Hansel TT, Kropshofer H, Singer T, Mitchell JA, George AJ. The safety and side effects of monoclonal antibodies. Nat Rev Drug Discov. (2010) 9:325–38. 10.1038/nrd3003 [DOI] [PubMed] [Google Scholar]
- 53.de Vries MK, Wolbink GJ, Stapel SO, de Groot ER, Dijkmans BA, Aarden LA, et al. Inefficacy of infliximab in ankylosing spondylitis is correlated with antibody formation. Ann Rheum Dis. (2007) 66:133–4. 10.1136/ard.2006.057745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yanai H, Hanauer SB. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. (2011) 106:685–98. 10.1038/ajg.2011.103 [DOI] [PubMed] [Google Scholar]
- 55.Ben-Horin S, Chowers Y. Review article: loss of response to anti-TNF treatments in Crohn’s disease. Aliment Pharmacol Ther. (2011) 33:987–95. 10.1111/j.1365-2036.2011.04612.x [DOI] [PubMed] [Google Scholar]
- 56.Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology. (2013) 2:e26333. 10.4161/onci.26333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Talotta R, Rucci F, Canti G, Scaglione F. Pros and cons of the immunogenicity of monoclonal antibodies in cancer treatment: a lesson from autoimmune diseases. Immunotherapy. (2019) 11:241–54. 10.2217/imt-2018-0081 [DOI] [PubMed] [Google Scholar]
- 58.Reding MT, Lei S, Lei H, Green D, Gill J, Conti-Fine BM. Distribution of Th1- and Th2-induced anti-factor VIII IgG subclasses in congenital and acquired hemophilia patients. Thromb Haemost. (2002) 88:568–75. 10.1055/s-0037-1613257 [DOI] [PubMed] [Google Scholar]
- 59.Reding MT. Immunological aspects of inhibitor development. Haemophilia. (2006) 12(Suppl. 6):30–5; discussion 35–6. [DOI] [PubMed] [Google Scholar]
- 60.Vultaggio A, Petroni G, Pratesi S, Nencini F, Cammelli D, Milla M, et al. Circulating T cells to infliximab are detectable mainly in treated patients developing anti-drug antibodies and hypersensitivity reactions. Clin Exp Immunol. (2016) 186:364–72. 10.1111/cei.12858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamze M, Meunier S, Karle A, Gdoura A, Goudet A, Szely N, et al. Characterization of CD4 T cell epitopes of infliximab and rituximab identified from healthy donors. Front Immunol. (2017) 8:500. 10.3389/fimmu.2017.00500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B-cell activation by T-cell-independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev. (2000) 176:154–70. 10.1034/j.1600-065x.2000.00607.x [DOI] [PubMed] [Google Scholar]
- 63.Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. (2004) 21:379–90. 10.1016/j.immuni.2004.06.019 [DOI] [PubMed] [Google Scholar]
- 64.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. (2006) 203:305–10. 10.1084/jem.20052036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.El Shikh ME, El Sayed RM, Szakal AK, Tew JG. T-independent antibody responses to T-dependent antigens: a novel follicular dendritic cell-dependent activity. J Immunol. (2009) 182:3482–91. 10.4049/jimmunol.0802317 [DOI] [PubMed] [Google Scholar]
- 66.Taillardet M, Haffar G, Mondière P, Asensio MJ, Gheit H, Burdin N, et al. The thymus-independent immunity conferred by a pneumococcal polysaccharide is mediated by long-lived plasma cells. Blood. (2009) 114:4432–40. 10.1182/blood-2009-01-200014 [DOI] [PubMed] [Google Scholar]
- 67.Yin L, Chen X, Vicini P, Rup B, Hickling TP. Therapeutic outcomes, assessments, risk factors and mitigation efforts of immunogenicity of therapeutic protein products. Cell Immunol. (2015) 295:118–26. 10.1016/j.cellimm.2015.03.002 [DOI] [PubMed] [Google Scholar]
- 68.Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. (2009) 9:15–27. 10.1038/nri2454 [DOI] [PubMed] [Google Scholar]
- 69.Kumar S, Singh SK, Wang X, Rup B, Gill D. Coupling of aggregation and immunogenicity in biotherapeutics: T- and B-cell immune epitopes may contain aggregation-prone regions. Pharm Res. (2011) 28:949–61. 10.1007/s11095-011-0414-9 [DOI] [PubMed] [Google Scholar]
- 70.Fehr T, Bachmann MF, Bucher E, Kalinke U, Di Padova FE, Lang AB, et al. Role of repetitive antigen patterns for induction of antibodies against antibodies. J Exp Med. (1997) 185:1785–92. 10.1084/jem.185.10.1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ben-Horin S, Heap GA, Ahmad T, Kim H, Kwon T, Chowers Y. The immunogenicity of biosimilar infliximab: can we extrapolate the data across indications? Expert Rev Gastroenterol Hepatol. (2015) 9(Suppl. 1):27–34. 10.1586/17474124.2015.1091307 [DOI] [PubMed] [Google Scholar]
- 72.Spinelli FR, Valesini G. Immunogenicity of anti-tumour necrosis factor drugs in rheumatic diseases. Clin Exp Rheumatol. (2013) 31:954–63. [PubMed] [Google Scholar]
- 73.Dunn N, Juto A, Ryner M, Manouchehrinia A, Piccoli L, Fink K, et al. Rituximab in multiple sclerosis: frequency and clinical relevance of anti-drug antibodies. Mult Scler. (2018) 24:1224–33. 10.1177/1352458517720044 [DOI] [PubMed] [Google Scholar]
- 74.Blair HA, Duggan ST. Belimumab: a review in systemic lupus erythematosus. Drugs. (2018) 78:355–66. 10.1007/s40265-018-0872-z [DOI] [PubMed] [Google Scholar]
- 75.Tovey MG, Lallemand C. Immunogenicity and other problems associated with the use of biopharmaceuticals. Ther Adv Drug Saf. (2011) 2:113–28. 10.1177/2042098611406318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Brummelen EM, Ros W, Wolbink G, Beijnen JH, Schellens JH. Antidrug antibody formation in oncology: clinical relevance and challenges. Oncologist. (2016) 21:1260–8. 10.1634/theoncologist.2016-0061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benucci M, Damiani A, Li Gobbi F, Bandinelli F, Infantino M, Grossi V, et al. Correlation between HLA haplotypes and the development of antidrug antibodies in a cohort of patients with rheumatic diseases. Biologics. (2018) 12:37–41. 10.2147/btt.s145941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stickler MM, Reddy A, Xiong JM, Hinton PR, DuBridge R, Harding FA. The human G1m1 allotype associates with CD4+ T-cell responsiveness to a highly conserved IgG1 constant region peptide and confers an asparaginyl endopeptidase cleavage site. Genes Immun. (2011) 12:213–21. 10.1038/gene.2010.68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci USA. (1992) 89:4285–9. 10.1073/pnas.89.10.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Johnson MJ, de Lange G, Cavalli-Sforza LL. Ig gamma restriction fragment length polymorphisms indicate an ancient separation of Caucasian haplotypes. Am J Hum Genet. (1986) 38:617–40. [PMC free article] [PubMed] [Google Scholar]
- 81.Sazonovs A, Kennedy NA, Moutsianas L, Heap GA, Rice DL, Reppell M, et al. HLA-DQA1∗05 carriage associated with development of anti-drug antibodies to infliximab and adalimumab in patients with Crohn’s disease. Gastroenterology. (2020) 158:189–99. [DOI] [PubMed] [Google Scholar]
- 82.Wilson A, Peel C, Wang Q, Pananos AD, Kim RB. HLADQA1∗05 genotype predicts anti-drug antibody formation and loss of response during infliximab therapy for inflammatory bowel disease. Aliment Pharmacol Ther. (2020) 51:356–63. 10.1111/apt.15563 [DOI] [PubMed] [Google Scholar]
- 83.Cassotta A, Mikol V, Bertrand T, Pouzieux S, Le Parc J, Ferrari P, et al. A single T cell epitope drives the neutralizing anti-drug antibody response to natalizumab in multiple sclerosis patients. Nat Med. (2019) 25:1402–7. 10.1038/s41591-019-0568-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pecoraro V, De Santis E, Melegari A, Trenti T. The impact of immunogenicity of TNFalpha inhibitors in autoimmune inflammatory disease. A systematic review and meta-analysis. Autoimmun Rev. (2017) 16:564–75. 10.1016/j.autrev.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 85.Harding FA, Stickler MM, Razo J, DuBridge RB. The immunogenicity of humanized and fully human antibodies: residual immunogenicity resides in the CDR regions. MAbs. (2010) 2:256–65. 10.4161/mabs.2.3.11641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jerne NK. The generative grammar of the immune system. EMBO J. (1985) 4:847–52. 10.1002/j.1460-2075.1985.tb03709.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Griswold KE, Bailey-Kellogg C. Design and engineering of deimmunized biotherapeutics. Curr Opin Struct Biol. (2016) 39:79–88. 10.1016/j.sbi.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jawa V, Cousens LP, Awwad M, Wakshull E, Kropshofer H, De Groot AS. T-cell dependent immunogenicity of protein therapeutics: preclinical assessment and mitigation. Clin Immunol. (2013) 149:534–55. 10.1016/j.clim.2013.09.006 [DOI] [PubMed] [Google Scholar]
- 89.Zhou Q, Qiu H. The mechanistic impact of N-glycosylation on stability, pharmacokinetics, and immunogenicity of therapeutic proteins. J Pharm Sci. (2019) 108:1366–77. 10.1016/j.xphs.2018.11.029 [DOI] [PubMed] [Google Scholar]
- 90.Chung CH, Mirakhur B, Chan E, Le QT, Berlin J, Morse M, et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-alpha-1,3-galactose. N Engl J Med. (2008) 358:1109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berg EA, Platts-Mills TA, Commins SP. Drug allergens and food–the cetuximab and galactose-alpha-1,3-galactose story. Ann Allergy Asthma Immunol. (2014) 112:97–101. 10.1016/j.anai.2013.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Doevendans E, Schellekens H. Immunogenicity of innovative and biosimilar monoclonal antibodies. Antibodies. (2019) 8:21. 10.3390/antib8010021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gallucci S, Matzinger P. Danger signals: SOS to the immune system. Curr Opin Immunol. (2001) 13:114–9. 10.1016/s0952-7915(00)00191-6 [DOI] [PubMed] [Google Scholar]
- 94.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. (1994) 12:991–1045. 10.1146/annurev.iy.12.040194.005015 [DOI] [PubMed] [Google Scholar]
- 95.Niazi S. Biosimilarity: The FDA Perspective. Boca Raton, FL: CRC Press. (2018). 10.1146/annurev.iy.12.040194.005015 [DOI] [Google Scholar]
- 96.Vaisman-Mentesh A, Rosenstein S, Yavzori M, Dror Y, Fudim E, Ungar B, et al. Molecular landscape of anti-drug antibodies reveals the mechanism of the immune response following treatment with TNFα antagonists. Front Immunol. (2019) 10:2921. 10.3389/fimmu.2019.02921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. (1996) 184:1397–411. 10.1084/jem.184.4.1397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Schie KA, Kruithof S, Ooijevaar-de Heer P, Derksen NIL, van de Bovenkamp FS, Saris A, et al. Restricted immune activation and internalisation of anti-idiotype complexes between drug and antidrug antibodies. Ann Rheum Dis. (2018) 77:1471–9. 10.1136/annrheumdis-2018-213299 [DOI] [PubMed] [Google Scholar]
- 99.Arnoult C, Brachet G, Cadena Castaneda D, Azzopardi N, Passot C, Desvignes C, et al. Crucial role for immune complexes but not FcRn in immunization against Anti-TNF-alpha antibodies after a single injection in mice. J Immunol. (2017) 199:418–24. 10.4049/jimmunol.1601246 [DOI] [PubMed] [Google Scholar]
- 100.Bar-Yoseph H, Pressman S, Blatt A, Vainberg SG, Maimon N, Starosvetsky E, et al. Infliximab-tumor necrosis factor complexes elicit formation of anti-drug antibodies. Gastroenterology. (2019) 157:1338–51.e8. [DOI] [PubMed] [Google Scholar]
- 101.Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet. (2012) 380:1819–28. [DOI] [PubMed] [Google Scholar]
- 102.Baker D, Herrod SS, Alvarez-Gonzalez C, Giovannoni G, Schmierer K. Interpreting lymphocyte reconstitution data from the pivotal phase 3 trials of alemtuzumab. JAMA Neurol. (2017) 74:961–9. 10.1001/jamaneurol.2017.0676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Li Z, Richards S, Surks HK, Jacobs A, Panzara MA. Clinical pharmacology of alemtuzumab, an anti-CD52 immunomodulator, in multiple sclerosis. Clin Exp Immunol. (2018) 194:295–314. 10.1111/cei.13208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Thomas K, Eisele J, Rodriguez-Leal FA, Hainke U, Ziemssen T. Acute effects of alemtuzumab infusion in patients with active relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm. (2016) 3:e228. 10.1212/nxi.0000000000000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cox AL, Thompson SA, Jones JL, Robertson VH, Hale G, Waldmann H, et al. Lymphocyte homeostasis following therapeutic lymphocyte depletion in multiple sclerosis. Eur J Immunol. (2005) 35:3332–42. 10.1002/eji.200535075 [DOI] [PubMed] [Google Scholar]
- 106.Herskovitz J, Ryman J, Thway T, Lee S, Zhou L, Chirmule N, et al. Immune suppression during preclinical drug development mitigates immunogenicity-mediated impact on therapeutic exposure. AAPS J. (2017) 19:447–55. 10.1208/s12248-016-0026-8 [DOI] [PubMed] [Google Scholar]
- 107.Cohen SB, Emery P, Greenwald MW, Dougados M, Furie RA, Genovese MC, et al. Rituximab for rheumatoid arthritis refractory to anti-tumor necrosis factor therapy: Results of a multicenter, randomized, double-blind, placebo-controlled, phase III trial evaluating primary efficacy and safety at twenty-four weeks. Arthritis Rheum. (2006) 54:2793–806. 10.1002/art.22025 [DOI] [PubMed] [Google Scholar]
- 108.Gokemeijer J, Jawa V, Mitra-Kaushik S. How close are we to profiling immunogenicity risk using in silico algorithms and in vitro methods? An industry perspective. AAPS J. (2017) 19:1587–92. 10.1208/s12248-017-0143-z [DOI] [PubMed] [Google Scholar]
- 109.Messitt TJ, Terry F, Moise L, Martin W, De Groot AS. A comparison of two methods for T cell epitope mapping: “cell free” in vitro versus immunoinformatics. Immunome Res. (2011) 7:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yin L, Calvo-Calle JM, Dominguez-Amorocho O, Stern LJ. HLA-Dm constrains epitope selection in the human CD4 T cell response to vaccinia virus by favoring the presentation of peptides with longer HLA-DM-mediated half-lives. J Immunol. (2012) 189:3983–94. 10.4049/jimmunol.1200626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. (2018) 13:612–32. [DOI] [PubMed] [Google Scholar]
- 112.Schlessinger A, Ofran Y, Yachdav G, Rost B. Epitome: database of structure-inferred antigenic epitopes. Nucleic Acids Res. (2006) 34:D777–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fleri W, Paul S, Dhanda SK, Mahajan S, Xu X, Peters B, et al. The immune epitope database and analysis resource in epitope discovery and synthetic vaccine design. Front Immunol. (2017) 8:278. 10.3389/fimmu.2017.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Koren E, De Groot AS, Jawa V, Beck KD, Boone T, Rivera D, et al. Clinical validation of the “in silico” prediction of immunogenicity of a human recombinant therapeutic protein. Clin Immunol. (2007) 124:26–32. 10.1016/j.clim.2007.03.544 [DOI] [PubMed] [Google Scholar]
- 115.Manoury B, Mazzeo D, Fugger L, Viner N, Ponsford M, Streeter H, et al. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat Immunol. (2002) 3:169–74. 10.1038/ni754 [DOI] [PubMed] [Google Scholar]
- 116.Harrington CJ, Paez A, Hunkapiller T, Mannikko V, Brabb T, Ahearn M, et al. Differential tolerance is induced in T cells recognizing distinct epitopes of myelin basic protein. Immunity. (1998) 8:571–80. 10.1016/s1074-7613(00)80562-2 [DOI] [PubMed] [Google Scholar]
- 117.Kedl RM, Kappler JW, Marrack P. Epitope dominance, competition and T cell affinity maturation. Curr Opin Immunol. (2003) 15:120–7. 10.1016/s0952-7915(02)00009-2 [DOI] [PubMed] [Google Scholar]
- 118.Karle A, Spindeldreher S, Kolbinger F. Secukinumab, a novel anti-IL-17A antibody, shows low immunogenicity potential in human in vitro assays comparable to other marketed biotherapeutics with low clinical immunogenicity. MAbs. (2016) 8:536–50. 10.1080/19420862.2015.1136761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.De Groot AS, Moise L, McMurry JA, Wambre E, Van Overtvelt L, Moingeon P, et al. Activation of natural regulatory T cells by IgG Fc-derived peptide “Tregitopes”. Blood. (2008) 112:3303–11. 10.1182/blood-2008-02-138073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Krieckaert C, Rispens T, Wolbink G. Immunogenicity of biological therapeutics: from assay to patient. Curr Opin Rheumatol. (2012) 24:306–11. 10.1097/bor.0b013e3283521c4e [DOI] [PubMed] [Google Scholar]
- 121.Hart MH, de Vrieze H, Wouters D, Wolbink GJ, Killestein J, de Groot ER, et al. Differential effect of drug interference in immunogenicity assays. J Immunol Methods. (2011) 372:196–203. 10.1016/j.jim.2011.07.019 [DOI] [PubMed] [Google Scholar]
- 122.Vincent FB, Morand EF, Murphy K, Mackay F, Mariette X, Marcelli C. Antidrug antibodies (ADAb) to tumour necrosis factor (TNF)-specific neutralising agents in chronic inflammatory diseases: a real issue, a clinical perspective. Ann Rheum Dis. (2013) 72:165–78. 10.1136/annrheumdis-2012-202545 [DOI] [PubMed] [Google Scholar]
- 123.Tatarewicz S, Miller JM, Swanson SJ, Moxness MS. Rheumatoid factor interference in immunogenicity assays for human monoclonal antibody therapeutics. J Immunol Methods. (2010) 357:10–6. 10.1016/j.jim.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 124.Collet-Brose J, Couble PJ, Deehan MR, Nelson RJ, Ferlin WG, Lory S. Evaluation of multiple immunoassay technology platforms to select the anti-drug antibody assay exhibiting the most appropriate drug and target tolerance. J Immunol Res. (2016) 2016:5069678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang YM, Fang L, Zhou L, Wang J, Ahn HY. A survey of applications of biological products for drug interference of immunogenicity assays. Pharm Res. (2012) 29:3384–92. 10.1007/s11095-012-0833-2 [DOI] [PubMed] [Google Scholar]
- 126.Bloem K, Hernandez-Breijo B, Martinez-Feito A, Rispens T. Immunogenicity of therapeutic antibodies: monitoring antidrug antibodies in a clinical context. Ther Drug Monit. (2017) 39:327–32. 10.1097/ftd.0000000000000404 [DOI] [PubMed] [Google Scholar]
- 127.Svenson M, Geborek P, Saxne T, Bendtzen K. Monitoring patients treated with anti-TNF-α biopharmaceuticals: assessing serum infliximab and anti-infliximab antibodies. Rheumatology. (2007) 46:1828–34. 10.1093/rheumatology/kem261 [DOI] [PubMed] [Google Scholar]
- 128.Lofgren JA, Wala I, Koren E, Swanson SJ, Jing S. Detection of neutralizing anti-therapeutic protein antibodies in serum or plasma samples containing high levels of the therapeutic protein. J Immunol Methods. (2006) 20:101–8. 10.1016/j.jim.2005.10.007 [DOI] [PubMed] [Google Scholar]
- 129.Zoghbi J, Xu Y, Grabert R, Theobald V, Richards S. A breakthrough novel method to resolve the drug and target interference problem in immunogenicity assays. J Immunol Methods. (2015) 426:62–9. 10.1016/j.jim.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 130.Schmidt E, Hennig K, Mengede C, Zillikens D, Kromminga A. Immunogenicity of rituximab in patients with severe pemphigus. Clin Immunol. (2009) 132:334–41. 10.1016/j.clim.2009.05.007 [DOI] [PubMed] [Google Scholar]
- 131.Hernandez-Breijo B, Chaparro M, Cano-Martinez D, Guerra I, Iborra M, Cabriada JL, et al. Standardization of the homogeneous mobility shift assay protocol for evaluation of anti-infliximab antibodies. Application of the method to Crohn’s disease patients treated with infliximab. Biochem Pharmacol. (2016) 122:33–41. 10.1016/j.bcp.2016.09.019 [DOI] [PubMed] [Google Scholar]
- 132.Gupta S, Indelicato SR, Jethwa V, Kawabata T, Kelley M, Mire-Sluis AR, et al. Recommendations for the design, optimization, and qualification of cell-based assays used for the detection of neutralizing antibody responses elicited to biological therapeutics. J Immunol Methods. (2007) 321:1–18. 10.1016/j.jim.2006.12.004 [DOI] [PubMed] [Google Scholar]
- 133.Lallemand C, Kavrochorianou N, Steenholdt C, Bendtzen K, Ainsworth MA, Meritet JF, et al. Reporter gene assay for the quantification of the activity and neutralizing antibody response to TNFα antagonists. J Immunol Methods. (2011) 373:229–39. 10.1016/j.jim.2011.08.022 [DOI] [PubMed] [Google Scholar]
- 134.Maini RN, Breedveld FC, Kalden JR, Smolen JS, Davis D, Macfarlane JD, et al. Therapeutic efficacy of multiple intravenous infusions of anti-tumor necrosis factor alpha monoclonal antibody combined with low-dose weekly methotrexate in rheumatoid arthritis. Arthritis Rheum. (1998) 41:1552–63. [DOI] [PubMed] [Google Scholar]
- 135.Banugaria SG, Prater SN, McGann JK, Feldman JD, Tannenbaum JA, Bailey C, et al. Bortezomib in the rapid reduction of high sustained antibody titers in disorders treated with therapeutic protein: lessons learned from Pompe disease. Genet Med. (2013) 15:123–31. 10.1038/gim.2012.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ruffolo C, Scarpa M, Bassi N. Infliximab, azathioprine, or combination therapy for Crohn’s disease. N Engl J Med. (2010) 363:1086–7; author reply 1087–8. [DOI] [PubMed] [Google Scholar]
- 137.Anderson PJ. Tumor necrosis factor inhibitors : clinical implications of their different immunogenicity profiles. Semin Arthritis Rheum. (2005) 34:19–22. 10.1016/j.semarthrit.2005.01.005 [DOI] [PubMed] [Google Scholar]
- 138.Garces S, Demengeot J, Benito-Garcia E. The immunogenicity of anti-TNF therapy in immune-mediated inflammatory diseases: a systematic review of the literature with a meta-analysis. Ann Rheum Dis. (2013) 72:1947–55. 10.1136/annrheumdis-2012-202220 [DOI] [PubMed] [Google Scholar]
- 139.Quistrebert J, Hassler S, Bachelet D, Mbogning C, Musters A, Tak PP, et al. Incidence and risk factors for adalimumab and infliximab anti-drug antibodies in rheumatoid arthritis: a European retrospective multicohort analysis. Semin Arthritis Rheum (2019) 48:967–75. 10.1016/j.semarthrit.2018.10.006 [DOI] [PubMed] [Google Scholar]
- 140.Paul S, Moreau AC, Del Tedesco E, Rinaudo M, Phelip JM, Genin C, et al. Pharmacokinetics of adalimumab in inflammatory bowel diseases: a systematic review and meta-analysis. Inflamm Bowel Dis. (2014) 20:1288–95. 10.1097/mib.0000000000000037 [DOI] [PubMed] [Google Scholar]
- 141.Bartelds GM, Krieckaert CL, Nurmohamed MT, van Schouwenburg PA, Lems WF, Twisk JW, et al. Development of antidrug antibodies against adalimumab and association with disease activity and treatment failure during long-term follow-up. JAMA. (2011) 305:1460–8. 10.1001/jama.2011.406 [DOI] [PubMed] [Google Scholar]
- 142.Roth EM, Goldberg AC, Catapano AL, Torri A, Yancopoulos GD, Stahl N, et al. Antidrug antibodies in patients treated with alirocumab. N Engl J Med. (2017) 376:1589–90. 10.1056/nejmc1616623 [DOI] [PubMed] [Google Scholar]
- 143.Yan L, Wang B, Chia YL, Roskos LK. Population pharmacokinetic modeling of benralizumab in adult and adolescent patients with asthma. Clin Pharmacokinet. (2019) 58:943–58. 10.1007/s40262-019-00738-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Saffari F, Jafarzadeh A, Kalantari Khandani B, Saffari F, Soleimanyamoli S, Mohammadi M. Immunogenicity of rituximab, trastuzumab, and bevacizumab monoclonal antibodies in patients with malignant diseases. Int J Cancer Manag. (2018) 11:e64983. [Google Scholar]
- 145.Bagel J, Lebwohl M, Israel RJ, Jacobson A. Immunogenicity and skin clearance recapture in clinical studies of brodalumab. J Am Acad Dermatol. (2020) 82:344–51. 10.1016/j.jaad.2019.05.094 [DOI] [PubMed] [Google Scholar]
- 146.Chen Q, Hu C, Liu Y, Song R, Zhu W, Zhao H, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of single-dose denosumab in healthy Chinese volunteers: a randomized, single-blind, placebo-controlled study. PLoS One. (2018) 13:e0197984. 10.1371/journal.pone.0197984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ozkaynak MF, Gilman AL, London WB, Naranjo A, Diccianni MB, Tenney SC, et al. Trial of chimeric antibody 14.18 with GM-CSF, IL-2, and isotretinoin in high-risk neuroblastoma patients following myeloablative therapy: children’s oncology group study ANBL0931. Front Immunol. (2018) 9:1355. 10.3389/fimmu.2018.01355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hillmen P, Muus P, Szer J, Hill A, Hochsmann B, Kulasekararaj A, et al. Assessment of human antihuman antibodies to eculizumab after long-term treatment in patients with paroxysmal nocturnal hemoglobinuria. Am J Hematol. (2016) 91:E16–7. [DOI] [PubMed] [Google Scholar]
- 149.Passey C, Mora J, Dodge R, Gibiansky L, Sheng J, Roy A, et al. An integrated assessment of the effects of immunogenicity on the pharmacokinetics, safety, and efficacy of elotuzumab. AAPS J. (2017) 19:557–67. 10.1208/s12248-016-0033-9 [DOI] [PubMed] [Google Scholar]
- 150.Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, Ruzza A, et al. Long-term efficacy and safety of evolocumab in patients with hypercholesterolemia. J Am Coll Cardiol. (2019) 74:2132–46. 10.1016/j.jacc.2019.08.1024 [DOI] [PubMed] [Google Scholar]
- 151.Leu JH, Adedokun OJ, Gargano C, Hsia EC, Xu Z, Shankar G. Immunogenicity of golimumab and its clinical relevance in patients with rheumatoid arthritis, psoriatic arthritis and ankylosing spondylitis. Rheumatology. (2019) 58:441–6. 10.1093/rheumatology/key309 [DOI] [PubMed] [Google Scholar]
- 152.Ben-Horin S, Yavzori M, Katz L, Kopylov U, Picard O, Fudim E, et al. The immunogenic part of infliximab is the F(ab’)2, but measuring antibodies to the intact infliximab molecule is more clinically useful. Gut. (2010) 60:41–8. 10.1136/gut.2009.201533 [DOI] [PubMed] [Google Scholar]
- 153.Kverneland AH, Enevold C, Donia M, Bastholt L, Svane IM, Nielsen CH. Development of anti-drug antibodies is associated with shortened survival in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology. (2018) 7:e1424674. 10.1080/2162402x.2018.1424674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gordon KB, Blauvelt A, Papp KA, Langley RG, Luger T, Ohtsuki M, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. (2016) 375:345–56. [DOI] [PubMed] [Google Scholar]
- 155.Ortega H, Lemiere C, Llanos JP, Forshag M, Price R, Albers F, et al. Outcomes following mepolizumab treatment discontinuation: real-world experience from an open-label trial. Allergy Asthma Clin Immunol. (2019) 15:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, et al. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. (2005) 353:1912–25. [DOI] [PubMed] [Google Scholar]
- 157.Agrawal S, Statkevich P, Bajaj G, Feng Y, Saeger S, Desai DD, et al. Evaluation of immunogenicity of nivolumab monotherapy and its clinical relevance in patients with metastatic solid tumors. J Clin Pharmacol. (2017) 57:394–400. 10.1002/jcph.818 [DOI] [PubMed] [Google Scholar]
- 158.Nagy CF, Leach TS, King A, Guttendorf R. Safety, pharmacokinetics, and immunogenicity of obiltoxaximab after intramuscular administration to healthy humans. Clin Pharmacol Drug Dev. (2018) 7:652–60. 10.1002/cpdd.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Song A, Hendricks R, Chung S, Wang Q, Chin P, Garren H. Immunogenicity with repeated dosing of ocrelizumab in patients with multiple sclerosis (P2.087). Neurology (2016) 86:P2.087. 10.1002/cpdd.410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Somerville L, Bardelas J, Viegas A, D’Andrea P, Blogg M, Peachey G. Immunogenicity and safety of omalizumab in pre-filled syringes in patients with allergic (IgE-mediated) asthma. Curr Med Res Opin. (2014) 30:59–66. 10.1185/03007995.2013.844115 [DOI] [PubMed] [Google Scholar]
- 161.Null D, Jr., Pollara B, Dennehy PH, Steichen J, Sanchez PJ, Givner LB, et al. Safety and immunogenicity of palivizumab (Synagis) administered for two seasons. Pediatr Infect Dis J. (2005) 24:1021–3. 10.1097/01.inf.0000183938.33484.bd [DOI] [PubMed] [Google Scholar]
- 162.van Vugt MJH, Stone JA, De Greef R, Snyder ES, Lipka L, Turner DC, et al. Immunogenicity of pembrolizumab in patients with advanced tumors. J Immunother Cancer. (2019) 7:212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kirschbrown WP, Wang B, Nijem I, Ohtsu A, Hoff PM, Shah MA, et al. Pharmacokinetic and exposure-response analysis of pertuzumab in patients with HER2-positive metastatic gastric or gastroesophageal junction cancer. Cancer Chemother Pharmacol. (2019) 84:539–50. 10.1007/s00280-019-03871-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zou L, Pukac L, Shalit Y, Hickey L, Garin M, Liu P. Immunogenicity assessment of intravenous administration of reslizumab in patients with asthma in phase 3 clinical studies. Eur Respir J. (2018) 52:A1032. [Google Scholar]
- 165.Maspero J. Reslizumab in the treatment of inadequately controlled asthma in adults and adolescents with elevated blood eosinophils: clinical trial evidence and future prospects. Ther Adv Respir Dis. (2017) 11:311–25. 10.1177/1753465817717134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mrowietz U, Leonardi CL, Girolomoni G, Toth D, Morita A, Balki SA, et al. Secukinumab retreatment-as-needed versus fixed-interval maintenance regimen for moderate to severe plaque psoriasis: a randomized, double-blind, noninferiority trial (SCULPTURE). J Am Acad Dermatol. (2015) 73:27–36.e1. 10.1016/j.jaad.2015.04.011 [DOI] [PubMed] [Google Scholar]
- 167.Deisseroth A, Ko CW, Nie L, Zirkelbach JF, Zhao L, Bullock J, et al. FDA approval: siltuximab for the treatment of patients with multicentric Castleman disease. Clin Cancer Res. (2015) 21:950–4. 10.1158/1078-0432.ccr-14-1678 [DOI] [PubMed] [Google Scholar]
- 168.Kimball AB, Kerbusch T, van Aarle F, Kulkarni P, Li Q, Blauvelt A, et al. Assessment of the effects of immunogenicity on the pharmacokinetics, efficacy and safety of tildrakizumab. Br J Dermatol. (2020) 182:180–189. 10.1158/1078-0432.ccr-14-1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Burmester GR, Choy E, Kivitz A, Ogata A, Bao M, Nomura A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis. (2017) 76:1078–85. 10.1136/annrheumdis-2016-210297 [DOI] [PubMed] [Google Scholar]
- 170.Chiu H-Y, Chu TW, Cheng Y-P, Tsai T-F. The association between clinical response to ustekinumab and immunogenicity to ustekinumab and prior adalimumab. PLoS One. (2015) 10:e0142930. 10.1371/journal.pone.0142930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ungar B, Kopylov U, Yavzori M, Fudim E, Picard O, Lahat A, et al. Association of vedolizumab level, anti-drug antibodies, and alpha4beta7 occupancy with response in patients with inflammatory bowel DIseases. Clin Gastroenterol Hepatol. (2018) 16:697–705.e7. 10.1016/j.cgh.2017.11.050 [DOI] [PubMed] [Google Scholar]