Abstract
Studies of brain morphometry may illuminate the effects of pediatric mild traumatic brain injury (TBI; e.g., concussion). However, no published studies have examined cortical thickness in the early injury phases of pediatric mild TBI using an appropriate comparison group. The current study used an automated approach (i.e., FreeSurfer) to determine whether cortical thickness differed in children following a mild TBI or a mild orthopedic injury (OI), and to examine whether post-acute cortical thickness predicted post-acute and chronic post-concussive symptoms (PCS). Children ages 8.00-16.99 years with mild TBI (n = 136) or OI (n = 70) were recruited at emergency department visits to two children's hospitals, during which parents rated children's pre-injury symptoms retrospectively. Children completed a post-acute (3–24 days post-injury) assessment, which included a 3 Tesla MRI, and 3- and 6-month post-injury assessments. Parents and children rated PCS at each assessment. Cortical thickness was estimated using FreeSurfer. Linear mixed effects and multi-variable negative binomial regression models were used to test study aims, with false discovery rate (FDR) correction for multiple comparisons. Groups differed significantly on left parietal cortical thickness (TBI > OI) after FDR correction. Cortical thickness also varied by brain subregion and age, but not sex. Groups differed significantly on PCS post-acutely (TBI > OI), but not at 3 or 6 months. Right frontal thickness was positively related to post-acute PCS in both groups. Right cingulum thickness predicted chronic PCS in the OI group only. Results highlight the complexity of predicting outcomes of pediatric mild TBI from post-acute neuroimaging biomarkers.
Keywords: cortical thickness, mild TBI, orthopedic injury, pediatric TBI, structural MRI
Introduction
Pediatric traumatic brain injury (TBI) is a major global public health concern that affects millions of children annually.1 Roughly 85–90% of all TBI sustained by children are classified as being mild in severity.1,2 The lack of a clinical biomarker for detecting mild TBI complicates accurate clinical diagnosis and prognostication.3 Advanced neuroimaging techniques such as structural magnetic resonance imaging (MRI) have yielded promising results that may address this gap.3 However, few studies have examined brain morphology using quantitative MRI following pediatric mild TBI, limiting our understanding of whether and how mild TBI disrupts the structure of the developing brain.
Existing studies of alterations of brain morphology following pediatric mild TBI have focused on cortical thickness. In typical development, cortical gray matter thinning occurs in a predictable pattern at variable rates across brain regions as children develop into adolescence.4,5 However, the few available studies of cortical thickness following pediatric mild TBI have provided mixed results.6–9 Cortical gray matter abnormalities have been reported in small samples of children with mild TBI at least 3-4 months post-injury relative to uninjured healthy controls.6,7 More recently, however, no differences in cortical thickness were observed 6 months post-injury in a large cohort of children with mild TBI compared with children with orthopedic injury (OI).8 The discrepancies between these studies could reflect differences between comparison groups. Recent evidence suggests that the brain structure of children with OI may differ from that of healthy children who are unexposed to traumatic injury.9 Therefore, differences in brain structure between children with mild TBI and healthy children could reflect pre-morbid differences between children who are at risk for childhood injury versus healthy children.9
Even less is known about the post-acute effects of pediatric mild TBI on cortical thickness. The only known study of the early injury phase (i.e., ∼21 days post-injury) in mild TBI included a small sample and found no differences in cortical thickness relative to typically developing children.6 Even when visible abnormalities are present on computed tomography (CT) or MRI after mild TBI, quantitative estimates of brain macrostructure (e.g., cortical thickness and gray matter volume) may not vary.10,11 This suggests that morphological differences would likely be difficult to detect during early phases of injury.8,12 However, further study of the early effects of pediatric mild TBI on brain morphology is warranted, especially using a more appropriate comparison group, such as children with mild OI.9
The relationship of brain morphology to clinical outcomes after mild TBI also deserves further study. Previous research using quantitative MRI has found positive correlations between cortical thickness at chronic stages of mild TBI and post-concussive symptoms (PCS) in children.8 However, research is needed that examines the relation between cortical thickness and PCS severity in early phases of mild TBI.
Also unclear is whether expected trajectories of cortical development, as reflected in age-related thinning across childhood and early adulthood, are altered in children with pediatric mild TBI.4,5 Age-related cortical thinning was not observed in the presence of a positive self-reported history of sports-related concussion (i.e., a form of mild TBI)13 in a cohort of adolescent and young adult males,14 suggesting that early injuries may disrupt the developmental trajectory of cortical gray matter thinning during adolescence and young adulthood. However, altered developmental trajectories have not been found in younger samples of children with mild TBI,7,8 and no published study has examined age-related changes in cortical thickness in post-acute pediatric mild TBI.
The current study sought to address these gaps by examining post-acute (defined here as the first few weeks post-injury) cortical thickness in children with mild TBI (n = 136) or mild OI (n = 70). Given previous inconsistencies in the literature, we tested the null hypothesis that the two injury groups would not demonstrate differences in post-acute cortical thickness. However, we did expect similar associations of age with cortical thickness for both groups, with reduced thickness at older ages.8 We also examined relations between post-acute cortical thickness and PCS at the 3 and 6 months post-injury assessments. Research on children in the chronic stages of mild TBI provided a rationale to examine potential group differences in associations of cortical thickness with PCS.
Methods
Study design and procedure
Data were drawn from a larger study of pediatric mild TBI that involved a prospective, concurrent cohort design with longitudinal follow-up. Children with mild TBI or OI between the ages of 8.00 and 16.99 years at the time of injury were recruited during emergency department (ED) visits within 24 h post-injury at Nationwide Children's Hospital (NCH) in Columbus, OH and at Rainbow Babies and Children's Hospital (RBCH) in Cleveland, OH. Recruitment occurred over a period of 48 months to accrue the desired sample size.
Information regarding the child's injury and acute clinical presentation was obtained at the ED visit, during which parents also provided retrospective ratings of their child's pre-injury symptoms. Acute symptoms were assessed using the Standardized Assessment of Concussion (SAC).15 Parents provided the address of the family residence, and the 2010 Census tract median family income was obtained to help derive estimates of socioeconomic status. Enrolled children returned for three further assessments: post-acute and 3 and 6 months post-injury. At the post-acute visit, which occurred from 3-24 days post-injury, children completed an MRI as well as ratings of current PCS. Parents also rated current PCS. Ratings of current PCS also were obtained from children and parents at 3 and 6 months post-injury using the Health and Behavior Inventory (HBI).16
As summarized in Ware and colleagues,17 a total of 315 children (mild TBI = 195, OI = 120) were recruited in the ED for the study at large. All but one of the children who returned for the post-acute MRI completed the MRI. Of the participants with usable post-acute MRI data, 152 (mild TBI = 98, OI = 54) also completed the assessment at 3 months post-injury and 135 (mild TBI = 93, OI = 42) completed the assessment at 6 months post-injury. A comparison of demographic characteristics between the participants who did and did not return for the post-acute and chronic (3- and 6-month) assessments is available elsewhere.17
Participants and recruitment
Participants for the current study included children with mild TBI or mild OI who completed an MRI and relevant measures at the post-acute assessment (i.e., within 2 weeks of the injury). A comparison group of children with mild OI not involving the head was included to distinguish the effects of mild TBI from injury in general, account for the stressful experience of suffering a traumatic injury, and control for pre-morbid functioning and demographics.9,18–21
Diagnoses were made by treating physicians during the ED visit. Regardless of physician diagnosis, all children with mild TBI in the current study met the study criteria.22 Specifically, they had to have experienced a blunt head trauma that resulted in at least one of the following: 1) an observed loss of consciousness (LOC) for less than 30 min; 2) Glasgow Coma Scale (GCS) score of 13 or greater (GCS was 13 or 14 in cases with no LOC or other signs or symptoms)23; or 3) at least two acute signs of symptoms of concussion as noted on standard case reports form (e.g., post-traumatic amnesia, focal neurological deficits, skull fracture, vomiting, headache, dizziness, or other mental status changes).
Children with OI were included if they experienced an upper or lower extremity fracture with an Abbreviated Injury Scale (AIS) score of 4 or less.24 Children with OI were excluded from the study if they showed any evidence of head trauma or any symptoms of concussion. Children with mild TBI were eligible if they had a co-occurring OI.
Both groups were subject to the following exclusion criteria: 1) any other severe injury as defined by an AIS score greater than 4; 2) any upper extremity injury likely to interfere with neuropsychological testing (e.g., dominant or nondominant hand, arm or shoulder injury; 3) hypoxia, hypotension, or shock during or following the injury; 4) alcohol or drug ingestion involved with the injury; 5) history of previous TBI requiring hospitalization; 6) pre-morbid neurological disorder or intellectual disability; 7) injury resulting from abuse or assault; 8) history of severe psychiatric disorder requiring hospitalization; or 9) any contraindication to MRI. Children who were administered analgesic medication, including narcotics, were not excluded from either group. Additionally, children with a history of learning or attention problems were not excluded because they are at increased risk for sustaining traumatic injuries.25
Demographic information
Demographic information was collected during the post-acute assessment. A socioeconomic status (SES) composite index was computed by averaging sample z-scores for years of maternal education, median family income for census tract, and the Duncan Socioeconomic Index,26 a measure of occupational prestige. The two-subtest version of the Wechsler Abbreviated Scale of Intelligence-Second Edition, which includes the Matrix Reasoning and Vocabulary subtests, was used to estimate children's Full Scale IQ.27
Post-concussive symptoms
Parent ratings of pre-morbid symptoms were collected during the ED visit. Post-injury PCS were rated by both the parent and child at each follow-up assessment, using the HBI.16 The HBI has good internal consistency and inter-rater agreement, and has been adopted as a core measure in the Common Data Elements for Pediatric TBI.28 It yields separate scores for cognitive and somatic symptom scales. Both parent and child post-injury ratings at each assessment were included in analyses because these can provide different but complementary information about outcomes in pediatric mild TBI.29
Head injury history
History of previous head injury was assessed though retrospective parent report. Overall, 24 children with mild TBI and 12 children with OI had a previous head injury. Groups did not differ in number of previous head injuries reported. Most children had only one prior head injury (mild TBI n = 17; OI n = 10), although one child in the TBI group had history of four previous injuries. Of the children with positive head injury histories, 19 children in the mild TBI group and 12 children in the OI group had sought medical treatment. Severity of injuries (including treated injuries) ranged from a minor bump on the head or stitches to a facial region to diagnosed concussion.
MRI
Each participant completed a 3 Tesla MRI scan as part of the post-acute assessment. Participants were not sedated during MRI acquisition. MRI scans were completed from 3-24 days post-injury (Mean [M] = 10.85, Median = 11.00, Standard Deviation [SD] = 3.28), with most scans (96%) completed between 3-16 days post-injury. Time between injury and MRI scan did not differ by group [F(1, 197) < 1.00, p = 0.634] or site [F(1, 197) < 1.00, p = 0.892], and there was no group by site interaction [F(1, 197) = 1.47, p = 0.227]. The MRI sequences were based on protocols recommended by the National Institute of Neurological Disorders and Stroke Common Data Elements group30 and included: T1- and T2-weighted, axial T2-weighted fluid attenuated inversion recovery (FLAIR), axial susceptibility weighted, and axial diffusion-weighted sequences.31 Coding of the MRIs for incidental and trauma-related findings is reported elsewhere.32 Briefly, most children had normal MRI (i.e., 51%). Approximately 25–35% of the children with mild TBI (n = 37) and OI (n = 25) had positive findings, including intracranial abnormalities of a structural (most commonly ventricular asymmetry), cystic, or vascular nature, and extracranial abnormalities (most commonly mucosal thickening and polyps). Importantly, the rate of positive findings did not differ significantly between the two injury groups and were not related to outcomes.
MRI at each site was accomplished in about 45–60 min. Periodic reliability checks were conducted by having randomly selected protocols from each site reviewed. Quality checks also were initially conducted through regular phantom scans.
MRI data were obtained at NCH using a Siemens Trio 3T scanner with a 32-channel head coil. High-resolution T1-weighted images were acquired using a three-dimensional (3D) magnetization-prepared rapid gradient echo pulse sequence with the following sequence parameters: 192 contiguous sagittal slices with TR (repetition time) = 2200 msec; TE (echo time) = 4.37 msec; in-plane resolution = 0.90 mm2; slice thickness = 0.90 mm; flip angle = 78° field of view (FOV) = 230 mm2; and an acquisition matrix of 256 mm2.
MRI data were obtained at RBCH using a Philips 3.0T Achieva scanner with an eight-channel head coil. High-resolution T1-weighted images were acquired using a 3D magnetization-prepared rapid gradient echo (T1 3D TFE) pulse sequence with the following sequence parameters: 170 contiguous sagittal slices with TR/TE = 8.9/4.1 msec; in-plane resolution = 0.90 mm2; slice thickness = 0.90 mm; flip angle = 88°; FOV = 240 mm2; and an acquisition matrix = 256 mm2.
Quality assurance and pre-processing
All T1- and T2-weighted images were visually inspected for artifact by an expert blind to group membership. Data were excluded from 11 participants who completed the post-acute MRI because of poor anatomical (i.e., T1-weighted) data quality (i.e., severe motion or scanner-related artifact) or incomplete data acquisition (e.g., failure or refusal to complete the MRI).
Briefly, preprocessing and brain extraction procedures were completed on a remote Linux computing cluster. T1- and T2-weighted DICOM data were converted into NIfTI format using the dcm2niix tool in MRIcron (publicly available software; https://github.com/rordenlab/dcm2niix). During conversion to NIfTI format, T1-weighted images were automatically reoriented to canonical space and auto-cropped. Using the acpcdetect tool of the Automatic Registration Toolbox (freely available at www.nitrc.org/frs/?group_id=90), the T1-weighted images were put into standard alignment. Using the Convert3D Medical Image Processing Tool (freely available at www.itksnap.org/pmwiki/pmwiki.php?n=Downloads.C3D),33 T1-weighted images were resampled with an isotropic voxel resolution of 1 mm3. Intensity correction was completed on all T1-weighted data using the Advanced Normalization Tools (ANTs) version 2.1.0 N4 Bias Field Correction.34 The T2-weighted images were subsequently registered to the T1-weighted images for each participant using ANTs registration (antsRegistrationSyNQuick).
Estimates of cortical thickness
FreeSurfer v6.0.0 was used to automatically parcellate cortical and segment subcortical brain regions from the co-registered T1- and T2-weighted anatomical images (freely available at http://surfer.nmr.mgh.harvard.edu).35 Cortical parcellations were identified and labeled within surface-based processing stream according to the Desikan-Kiliany atlas definitions of gyri and sulci for each hemisphere.36 The current analyses examined the cortical thickness of frontal, cingulum, temporal, parietal, and occipital cortical subregions for each hemisphere. The FreeSurfer labels included in each cortical region are listed in Table 1.
Table 1.
List of FreeSurfer Cortical Regions Included in Each Lobe
| Frontal cortex |
|---|
| Caudal middle frontal |
| Lateral orbitofrontal |
| Medial orbitofrontal |
| Pars opercularis |
| Pars orbitalis |
| Pars triangularis |
| Precentral |
| Rostral middle frontal |
| Superior frontal |
| Frontal pole |
| Cingulate Cortex |
|---|
| Caudal anterior cingulate |
| Isthmus cingulate |
| Posterior cingulate |
| Rostral anterior cingulate |
| Temporal cortex |
|---|
| Banks superior temporal sulcus |
| Entorhinal |
| Fusiform |
| Inferior temporal |
| Middle temporal |
| Parahippocampal |
| Temporal pole |
| Transverse temporal |
| Insula |
| Parietal cortex |
|---|
| Inferior parietal |
| Paracentral |
| Postcentral |
| Superior parietal |
| Supramarginal |
| Precuneus |
| Occipital cortex |
|---|
| Lateral occipital |
| Lingual |
| Cuneus |
Statistical analysis
Demographic data were analyzed using analysis of variance (ANOVA) for continuous variables (e.g., age, SES) and chi-square techniques for categorical variables (e.g., sex).
For Aim 1, linear mixed effects models were computed using the R lmerTest package37,38 to investigate the relations between group, brain region, age, sex, and their interactions (fixed effects) as predictors of cortical thickness of the frontal, cingulate, temporal, parietal, and occipital cortices in each hemisphere separately, with site (i.e., NCH, RBCH) and participant entered as random effects. Full factorial models were initially computed to test for interaction effects among group, region, age, and sex, as demonstrated by the following model formula:
Thickness ∼ Group * Region * Age * Sex + (1|Site) + (1|Subject)
The full factorial models did not account for a significant increase in model fit compared with main effects models; and the model for left temporal thickness failed to converge at tolerance = 0.003. Therefore, non-significant interaction effects (adjusted for multiple comparisons) were trimmed from final models, resulting in final (main effects) models as demonstrated by the following model formula:
Thickness ∼ Group + Region + Age + Sex + (1|Site) + (1|Subject)
For Aim 2, multiple multivariable negative binomial regression analyses were used to examine relations among cortical thickness and post-acute and chronic (3 and 6 months post-injury) somatic and cognitive PCS ratings (separate analyses for each follow-up assessment). Group, average thickness of each cortical region (i.e., mean thickness of frontal, cingulate, temporal, parietal, occipital cortical subregions) for each hemisphere separately, and their interactions were included as predictors to test whether associations between average cortical thickness and somatic or cognitive PCS differed in children with mild TBI versus OI; age, sex, and pre-morbid PCS were included as covariates. Parent and child PCS ratings were used as outcome measures.
The false discovery rate (FDR) was used to correct for multiple comparisons for all analyses.39 Continuous predictors in Aim 1 and 2 models were mean centered. Mixed models are generalizations of multiple regression models. A power analysis, conducted using G*Power v3.1,40 indicated that, in a regression analysis, the current sample size (n = 206) was sufficiently powered (1-β = .80) to detect a change in R2 of about 0.038 for a single predictor at α = 0.05, assuming 10 total predictors.
Results
Demographic characteristics
Sample characteristics and demographic data are presented in Table 2. Across sites, the mild TBI and OI groups did not differ significantly in age, sex, or Full Scale IQ. The groups also did not differ on parent's ratings of pre-injury somatic or cognitive symptoms. However, SES was significantly higher in the mild TBI group compared with the OI group, and race significantly differed between the groups, with a significantly higher proportion of white and Asian children in the mild TBI group compared with the OI group (Table 2). No site differences were observed in sex, age, or Full Scale IQ, either across groups or within groups (uncorrected p values ≥0.183).
Table 2.
Sample Demographics and Injury Characteristics
| |
Group |
|
|||
|---|---|---|---|---|---|
| Variable |
OI |
Mild TBI |
|
||
| Mean/n | SD/% | Mean/n | SD/% | p value | |
| Age (years) | 12.36 | 2.41 | 12.51 | 2.65 | 0.676 |
| Full Scale IQ | 97.94 | 14.44 | 98.47 | 14.59 | 0.806 |
| Socioeconomic status (z-score) | −0.22 | 0.88 | 0.13 | 1.02 | 0.011 |
| Race (White/Asian) | 27 | 38.57 | 74 | 54.41 | 0.031 |
| Sex (Male) | 46 | 65.71 | 91 | 66.91 | 0.987 |
| Emergency department PCS* | 24.50 | 2.79 | 23.04 | 3.88 | 0.002 |
| Pre-morbid somatic PCS-Parent | 2.77 | 4.23 | 3.63 | 4.00 | 0.162 |
| Pre-morbid cognitive PCS-Parent | 9.58 | 7.81 | 10.29 | 7.66 | 0.539 |
| Post-acute somatic PCS-Parent | 3.19 | 4.17 | 7.57 | 5.47 | < 0.001 |
| Post-acute cognitive PCS-Parent | 9.04 | 8.33 | 12.58 | 8.98 | 0.006 |
| 3-month somatic PCS-Parent | 2.72 | 3.71 | 3.43 | 3.98 | 0.276 |
| 3-month cognitive PCS-Parent | 9.04 | 8.24 | 10.03 | 8.27 | 0.479 |
| 6-month somatic PCS-Parent | 1.95 | 2.46 | 2.83 | 3.8 | 0.112 |
| 6-month cognitive PCS-Parent | 8.71 | 8.61 | 9.15 | 8.38 | 0.784 |
| Post-acute somatic PCS-Child | 4.81 | 5.04 | 9.29 | 5.89 | < 0.001 |
| Post-acute cognitive PCS-Child | 9.84 | 8.2 | 14.03 | 8.35 | < 0.001 |
| 3-month somatic PCS-Child | 4.43 | 4.41 | 4.81 | 4.87 | 0.628 |
| 3-month cognitive PCS-Child | 8.77 | 7.27 | 9.34 | 8.08 | 0.662 |
| 6-month somatic PCS-Child | 3.48 | 4.01 | 4.49 | 4.67 | 0.198 |
| 6-month cognitive PCS-Child | 8.52 | 7.65 | 8.39 | 7.97 | 0.925 |
| Mechanism of Injury | 0.001 | ||||
| Fall | 30 | 42.86 | 46 | 33.82 | |
| Motor vehicle related | 2 | 2.86 | 6 | 4.41 | |
| Struck by an object | 16 | 22.86 | 35 | 25.74 | |
| Struck by a person | 7 | 10.00 | 35 | 25.74 | |
| Bicycle related | 5 | 7.12 | 12 | 8.82 | |
| Glasgow Coma Scale | - | - | 14.84 | 0.45 | - |
Uncorrected p-value < 0.05
Based on the Standardized Assessment of Concussion.
OI, orthopedic injury; TBI, traumatic brain injury; SD, standard deviation; PCS, post-concussive symptoms.
Of the total sample of children with mild TBI, 38 completed a CT scan during the acute ED visit. Positive findings (subdural hematoma n = 2, frontal parenchymal contusion n = 1, subdural bleed n = 1, skull fracture n = 3) were found in four children.
Group differences in cortical thickness
Results from the linear mixed effects predicting cortical thickness are presented in Table 3 (fixed effects) and Supplementary Table S1 (random effects), and are also summarized in Figure 1.
Table 3.
Fixed Linear Mixed Effects Results for Aim 1
| |
Cortical region |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |
Frontal |
Cingulum |
Temporal |
Parietal |
Occipital |
||||||||||
| |
Statistic* |
||||||||||||||
| F | p | B | F | p | B | F | p | B | F | p | B | F | p | B | |
| Left hemisphere | |||||||||||||||
| Group | 1.89 | 0.171 | 0.03 | 1.419 | 0.235 | 0.03 | 1.41 | 0.237 | 0.03 | 8.12 | 0.005 | 0.05 | 2.42 | 0.121 | 0.02 |
| Subregion | 232.12 | < 0.001 | - | 86.787 | < 0.001 | - | 419.08 | < 0.001 | - | 732.86 | < 0.001 | - | 237.97 | < 0.001 | - |
| Age | 33.19 | < 0.001 | −0.02 | 38.364 | < 0.001 | −0.03 | 5.59 | 0.019 | −0.01 | 49.71 | < 0.001 | −0.02 | 33.29 | < 0.001 | −0.02 |
| Sex | 0.03 | 0.856 | <0.01 | 0.079 | 0.779 | −0.01 | <0.01 | 0.984 | <0.01 | 0.08 | 0.776 | <0.01 | 1.23 | 0.269 | 0.02 |
| Right hemisphere | |||||||||||||||
| Group | 5.02 | 0.026 | 0.05 | 0.358 | 0.555 | 0.01 | 3.57 | .060 | 0.04 | 4.69 | 0.032 | 0.04 | 0.33 | 0.565 | 0.01 |
| Subregion | 175.47 | < 0.001 | - | 72.89 | < 0.001 | - | 336.61 | < 0.001 | - | 861.62 | < 0.001 | - | 375.14 | < 0.001 | - |
| Age | 42.25 | < 0.001 | −0.03 | 24.51 | < 0.001 | −0.02 | 4.51 | 0.035 | −0.01 | 44.70 | < 0.001 | −0.02 | 37.91 | < 0.001 | −0.02 |
| Sex | 3.11 | 0.080 | −0.04 | 0.90 | 0.343 | −0.02 | 0.02 | 0.879 | <0.01 | 0.07 | 0.787 | <0.01 | 0.01 | 0.913 | <0.01 |
Italic = unadjusted p value <0.05.
Bolded = false discovery rate adjusted p value <0.05.
Parameter estimates for cortical subregions varied.
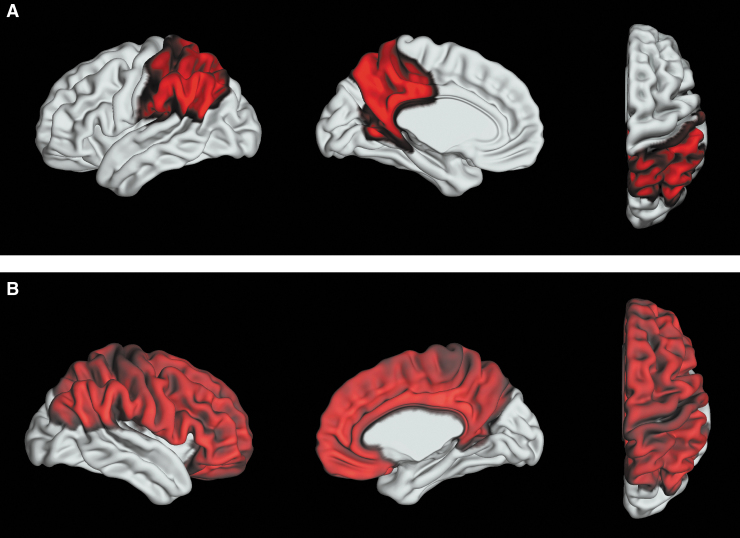
FIG. 1.
Summary of results for Aim 1: the mild TBI group showed (A) robustly (adjusted p value <0.05) increased left parietal cortical thickness and (B) less robustly (unadjusted p value <0.05) increased cortical thickness in the right parietal and frontal cortices as compared to the OI group.
The mild TBI and OI groups differed significantly in the thickness of the left parietal cortex after FDR correction, with greater left parietal cortical thickness (see top of Fig. 1) in the mild TBI group relative to the OI group (mild TBI: M = 2.43, SD = 0.13; OI: M = 2.38, SD = 0.14). Follow-up univariate multivariable linear regression revealed that the mild TBI group had greater cortical thickness relative to the OI group in all of the left parietal subregions (listed in Table 1) except for the left paracentral cortical region. Nominally significant group differences in the same direction (i.e., mild TBI > OI) were found in right frontal (mild TBI: M = 2.71, SD = 0.18; OI: M = 2.67, SD = 0.18) and parietal (mild TBI: M = 2.46, SD = 0.13; OI: M = 2.42, SD = 0.13) cortical thickness, but did not survive FDR correction (bottom of Fig. 1).
As expected, thickness differed significantly between cortical subregions. Consistent with the expected reductions in thickness with maturation, age at injury was significantly negatively associated with post-acute cortical thickness after FDR correction in bilateral frontal, cingulum, parietal, and occipital cortices, but not after FDR correction in bilateral temporal cortices. Sex was not significantly associated with post-acute cortical thickness. The random effects, site and participant, generally contributed significant variability to cortical thickness after FDR correction (Supplementary Table S1). Results were similar after controlling for race and SES, which differed between groups. Finally, a history of previous head injury was unrelated to cortical thickness in any of the regions in the children with mild TBI or OI (all uncorrected p values ≥0.10).
Post-acute and chronic PCS
The mild TBI and OI groups significantly differed on post-acute somatic and cognitive PCS ratings (mild TBI > OI), but not on 3- or 6-month post-injury PCS ratings after FDR correction (see Table 2 for descriptive data).
Associations of cortical thickness and PCS
Parent ratings
The multivariable negative binomial regression analyses predicting parent PCS ratings are summarized in Supplementary Tables S2 (somatic) and S3 (cognitive). Across groups, right frontal thickness was positively associated with parent's post-acute somatic PCS ratings after FDR correction. Positive associations across groups that were nominally significant but did not survive FDR correction included bilateral frontal thickness with parent's post-acute cognitive PCS ratings. Several group by thickness interactions were nominally significant, but did not survive FDR correction, including the group by left temporal thickness interaction for parent's 3-month somatic PCS ratings and the group by right cingulum thickness interaction for parent's post-acute cognitive PCS ratings.
The covariate age was significantly positively associated with parent's post-acute somatic PCS ratings after FDR correction. Sex was not significantly associated with parent's PCS ratings. Pre-morbid symptoms were significantly positively associated with parent's post-acute and chronic PCS ratings.
Child ratings
Analyses predicting children's PCS ratings are summarized in Supplementary Tables S4 (somatic) and S5 (cognitive). Right cingulum cortical thickness was positively associated with children's post-acute somatic and 6-month cognitive PCS ratings after FDR correction. Positive associations across groups that were nominally significant but did not survive FDR correction included bilateral frontal thickness with children's post-acute somatic PCS ratings, and right cingulum thickness with children's 3- and 6-month somatic PCS ratings.
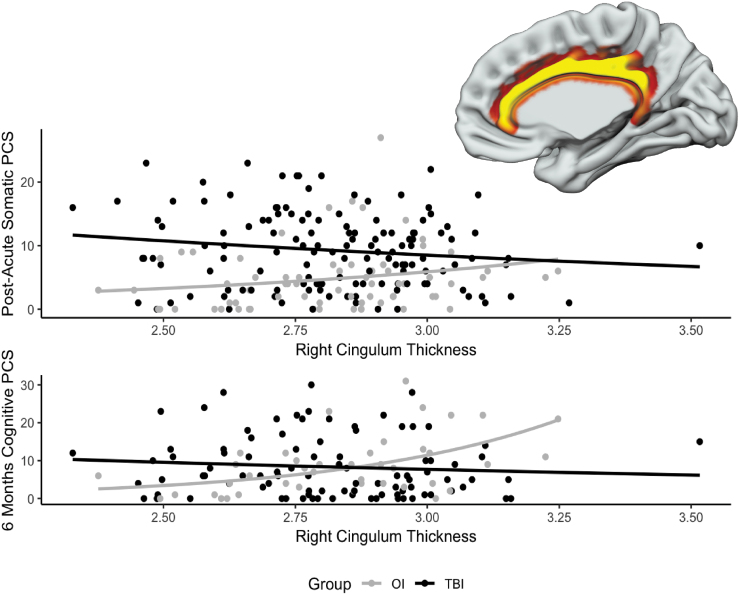
The group by right cingulum thickness interaction was a significant predictor of children's post-acute somatic PCS and 6-month cognitive PCS ratings after FDR correction. Follow-up analyses, shown in Figure 2, revealed that the association of right cingulum thickness with post-acute somatic PCS ratings was non-significant but negative in the mild TBI group (B = -0.45, p = 0.157) and non-significant but positive in the OI group (B = 1.19, p = 0.104). Similarly, the association of right cingulum thickness with children's 6-month cognitive PCS ratings was non-significant but negative in the mild TBI group (B = -0.69, p = 0.259), but significant and positive in the OI group (B = 2.63, p < 0.001). Several other group by thickness interactions were nominally significant, but did not survive FDR correction, including group by right frontal thickness for children's 6-month cognitive PCS, group by right cingulum thickness for children's 6-month somatic PCS ratings, and left and right occipital thickness for children's 6-month somatic PCS ratings.
FIG. 2.
Graphs illustrating the significant group by average thickness of the right cingulum cortex (shown in the inset) interaction on children's ratings of post-acute somatic (top) and 6-month cognitive (bottom) post-concussive symptoms.
The covariates age and sex were generally not associated with children's PCS ratings. Pre-morbid symptoms were significantly positively associated with children's chronic cognitive PCS ratings after FDR correction.
Discussion
The current study used data from a multisite study of demographically similar children with mild TBI or mild OI to conduct a novel investigation of post-acute (defined as the first few weeks post-injury) cortical thickness in relation to PCS. Overall, the results provide evidence of group differences in post-acute left parietal cortical thickness and, less robustly (i.e., did not survive FDR correction), right frontal and parietal cortical thickness, with increased thickness observed in the children with mild TBI versus OI. These findings were surprising for several reasons. Most children exhibit no visible trauma-related abnormalities on MRI following mild TBI, and that is true for this sample as well. Instead, mild TBI is more often characterized by subtle and diffuse alterations at the microstructural level.41 The absence of macrostructural changes in pediatric mild TBI several months post-injury suggests that morphological differences would be difficult to detect in early phases of injury.8,10 However, increased frontal and parietal cortical thickness and brain volume in post-acute mild TBI also have been found in adults with mild TBI and could reflect morphological alterations induced by biomechanical force.42,43 Pre-clinical evidence of increased cortical thickness in similar brain regions on the day following trauma suggests that edema could possibility account for the current findings.44
Whether MRI signal intensity is affected by alterations in any of these microbiological structures, and how such alterations would influence the definition of gray and white matter boundaries during automated parcellation, is unclear.45 Cortical structure and development is highly complex, and several microstructural features, including dendrites, vasculature, and intracortical myelin, could be affected by pediatric mild TBI.45 Pain may mediate many PCS in mild TBI, especially early post-injury, and can be associated with increased cortical thickness as well.46,47 However, more research in neurobiological outcomes of pediatric pain is needed. Another possible explanation for the current findings is that group differences in cortical thickness existed prior to injury. However, the children with mild TBI and OI in the current sample show few differences in other respects in pre-morbid status. Specifically, Full Scale IQ and pre-injury somatic and cognitive symptoms were similar in the two injury groups. Further investigation using a longitudinal design could help to clarify the nature of the observed macrostructural changes across recovery following mild TBI.
The current results are inconsistent with the only published study of post-acute cortical thickness in pediatric mild TBI, which found no alterations in children with mild TBI within 21 days post-injury compared with healthy controls.6 This discrepancy is most likely attributable to differences between the comparison groups in the two studies (i.e., children with OI vs. typically developing children). The use of children with OI as a comparison group has several advantages. An OI group is more clinically relevant than a typically developing group. Clinicians need to determine if a child's injuries warrant further assessment for TBI, and typically developing children do not serve the practical need for distinguishing between patients who sustained a TBI versus other injuries. More specific to neuroimaging studies is recent evidence that youth and young adults with mild TBI and OI are more similar on MRI than either group is to healthy controls.9 Previous studies also have found children with TBI to have elevated rates of behavior problems relative to uninjured children, but not to children with other injuries; thus, an OI group provides a means of controlling for behaviors that predispose to injury.48 Although these findings have not been extended to younger children or to brain morphology, they may help account for the discrepancies between the findings of the current study and those reported previously.6 Discrepancies might also reflect the different FreeSurfer versions used in Mayer and colleagues compared with the current study.6,49
Expected differences in thickness between cortical subregions and age-related cortical thinning were observed in both injury groups. Monotonic cortical thinning with increased age is typical of children as early as preschool and continues into young adulthood.45,50–53 The current results specifically found cortical thinning of 0.015- 0.03 mm per year of age across both groups, consistent with cross-sectional studies of typically developing children.50 In contrast, no sex differences were observed in cortical thickness. Earlier studies reported different, regionally-specific cortical thickness trajectories across development in girls compared with boys.54 However, several more recent studies of typical development have failed to find sex differences.45,51,52,55
Finally, although somatic and cognitive PCS were more severe in children with mild TBI compared with OI within the first 2 post-injury weeks (i.e., post-acute), they did not differ between the groups at 3 or 6 months post-injury. Post-acute somatic and 6-month cognitive PCS were positively associated with right frontal and cingulum cortical thickness in both groups. Less robust (i.e., did not survive FDR correction) associations also were observed in both groups among thickness of the bilateral frontal cortices and post-acute PCS and thickness of the right cingulum and chronic (3 months) PCS. However, right cingulum cortex thickness was only significantly associated with chronic (6 months) cognitive PCS in the children with OI after FDR correction; right frontal and cingulum and bilateral occipital cortical thickness also were significantly related to 6-month PCS ratings in children with OI, but not after FDR correction. In agreement with a recent study that found significant relations between chronic (i.e., at least 6 months post-injury) cortical thickness and PCS in children with mild TBI and OI,56 significant relations were observed in the current study between post-acute cortical thickness and post-acute and chronic PCS ratings. However, none of the relations differentially predicted symptom recovery in the mild TBI group. Instead, greater post-acute cortical thickness was associated with greater chronic symptoms either in both groups or only in the children with OI. The results may suggest that children whose cortical thickness is developmentally less mature (i.e., thicker) are likely to demonstrate more post-injury symptoms regardless of the nature of their injury.
Although the current study provided a much-needed examination of post-acute cortical thickness in children with mild TBI versus OI, several limitations warrant acknowledgement. Significant site differences suggest that some variability in cortical thickness could reflect a possible methodological artifact. Different collection platforms can introduce systematic differences that distort structural MRI information and confound results from subsequent, automated processing pipelines (e.g., FreeSurfer).57 Variability in quantitative anatomical data can be introduced in multi-site studies through the use of scanners of different vendors and scanning platforms.58 Even data from single-site studies of a single MRI scanner can be influenced by changes in hardware or software upgrades that can occur over the course of data collection. MRI data quality and subsequent analyses also can be influenced by factors pertaining to scanner environment and noise, including static magnetic field inhomogeneity, imaging gradient nonlinearity, and differences in subject positioning.59–62
Although image quality was controlled in the current study, motion artifact is of considerable concern in MRI studies,63 particularly those involving children with neurodevelopmental problems.64–66 Common motion artifacts can include blurring and ringing.63 Significant head motion during MRI acquisition can influence results from automated brain segmentation pipelines, including FreeSurfer,63,67 and can also yield data that is not usable. Impulsivity and hyperactivity, both of which are associated with increased risk for TBI in children,25 have been linked with more severe motion artifacts in youth with attention-deficit/hyperactivity disorder.66 Age is also related to extent of motion artifact in children, with younger children generally showing greater motion artifact than older children.67 A similar effect of age on motion parameters during diffusion MRI acquisition also has been shown in pediatric mild TBI.31 The current study only examined cortical thickness at a single time. Injury-related changes in cortical thickness might be more readily detected at more remote times post-injury. Further research that included longitudinal neuroimaging would be beneficial. Finally, we did not collect information about contact sport involvement, which can result in increased risk of exposure to repetitive blows (i.e., subclinical) to the head in children.68 Such effects may have had an impact on the children in both of the injury groups.
The current results suggest that cortical thickness may be altered by mild TBI in the first few weeks following injury and that post-acute cortical thickness can predict early and chronic PCS in children with mild TBI and OI, although not differentially. Prospective, longitudinal studies are needed to elucidate whether intra-individual changes in brain morphology occur across time after mild TBI and are related to the rate of recovery.
Supplementary Material
Funding Information
National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development, Predicting Outcomes in Children with Mild Traumatic Brain Injury, 1R01HD076885.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1. Ruff R.M., Iverson G.L., Barth J.T., Bush S.S., and Broshek D.K.; NAN Policy and Planning Committee. (2009). Recommendations for diagnosing a mild traumatic brain injury: a National Academy of Neuropsychology Education Paper. Arch. Clin. Neuropsychol. 24, 3–10 [DOI] [PubMed] [Google Scholar]
- 2. Gilchrist J., Thomas K., Xu L., McGuire L.C., and Coronado V.G. (2011). Nonfatal traumatic brain injuries related to sports and recreation activities among persons aged ≤19 years—United States, 2001–2009. MMWR 60, 1337–1342 [PubMed] [Google Scholar]
- 3. Mayer A.R., Kaushal M., Dodd A.B., Hanlon F.M., Shaff N.A., Mannix R., Master C.L., Leddy J.J., Stephenson D., Wertz C.J., Suelzer E.M., Arbogast K.B., and Meier T.B. (2018). Advanced biomarkers of pediatric mild traumatic brain injury: progress and perils. Neurosci. Biobehav. Rev. 94, 149–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sowell E.R. (2004). Longitudinal mapping of cortical thickness and brain growth in normal children. J. Neurosci. 24, 8223–8231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giedd J.N., Raznahan A., Alexander-Bloch A., Schmitt E., Gogtay N., and Rapoport J.L. (2015). Child psychiatry branch of the National Institute of Mental Health Longitudinal Structural Magnetic Resonance Imaging Study of Human Brain Development. Neuropsychopharmacology 40, 43–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mayer A.R., Hanlon F.M., and Ling J.M. (2015). Gray matter abnormalities in pediatric mild traumatic brain injury. J. Neurotrauma 32, 723–730 [DOI] [PubMed] [Google Scholar]
- 7. Urban K.J., Riggs L., Wells G.D., Keightley M., Chen J.-K., Ptito A., Fait P., Taha T., and Sinopoli K.J. (2017). Cortical thickness changes and their relationship to dual-task performance following mild traumatic brain injury in youth. J. Neurotrauma 34, 816–823 [DOI] [PubMed] [Google Scholar]
- 8. Bigler E.D., Finuf C., Abildskov T.J., Goodrich-Hunsaker N.J., Petrie J.A., Wood D.M., Hesselink J.R., Wilde E.A., and Max J.E. (2018). Cortical thickness in pediatric mild traumatic brain injury including sports-related concussion. Intl. J. Psychophysiol. 132, 99–104 [DOI] [PubMed] [Google Scholar]
- 9. Wilde E.A., Ware A.L., Li X., Wu T.C., McCauley S.R., Barnes A., Newsome M., Biekman B., Hunter J.V., Chu Z., and Levin H. (2018). Orthopedic injured versus uninjured comparison groups for neuroimaging research in mild traumatic brain injury. J. Neurotrauma 36, 239–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bigler E.D., Jantz P.B., Farrer T.J., Abildskov T.J., Dennis M., Gerhardt C.A., Rubin K.H., Stancin T., Taylor H.G., Vannatta K., and Yeates K.O. (2015). Day of injury CT and late MRI findings: cognitive outcome in a pediatric sample with complicated mild traumatic brain injury. Brain Inj. 29, 1062–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Königs M., Pouwels P.J., Ernest van Heurn L., Bakx R., Jeroen Vermeulen R., Carel Goslings J., Poll-The B.T., van der Wees M., Catsman-Berrevoets C.E., and Oosterlaan J. (2018). Relevance of neuroimaging for neurocognitive and behavioral outcome after pediatric traumatic brain injury. Brain Imaging Behav. 12, 29–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bigler E.D. (2015). Neuropathology of mild traumatic brain injury: correlation to neurocognitive and neurobehavioral findings., in: Brain Neurotrauma: Molecular, Neuropsychological, and Rehabilitation Aspects., Kobeissy, F.H. (ed). CRC Press/Taylor & Francis: Boca Raton, FL. [PubMed] [Google Scholar]
- 13. McCrory P., Feddermann-Demont N., Dvořák J., Cassidy J.D., McIntosh A., Vos P.E., Echemendia R.J., Meeuwisse W., and Tarnutzer A.A. (2017). What is the definition of sports-related concussion: a systematic review. Br, J. Sports Med. 51, 877–887 [DOI] [PubMed] [Google Scholar]
- 14. Albaugh M.D., Orr C., Nickerson J.P., Zweber C., Slauterbeck J.R., Hipko S., Gonyea J., Andrews T., Brackenbury J.C., Watts R., and Hudziak J.J. (2015). Postconcussion symptoms are associated with cerebral cortical thickness in healthy collegiate and preparatory school ice hockey players. J. Pediatr. 166, 394-400.e1. [DOI] [PubMed] [Google Scholar]
- 15. McCrea M., Kelly J.P., Randolph C., Kluge J., Bartolic E., Finn G., and Baxter B. (1998). Standardized assessment of concussion (SAC): on-site mental status evaluation of the athlete. J. Head Trauma Rehabil. 13, 27–35 [DOI] [PubMed] [Google Scholar]
- 16. Ayr L.K., Yeates K.O., Taylor H.G., and Browne M. (2009). Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. J. Int. Neuropsychol. Soc. 15, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ware A.L., Shukla A., Goodrich-Hunsaker N.J., Lebel C., Wilde E.A., Abildskov T.J., Bigler E.D., Cohen D.M., Mihalov L.K., Bacevice A., Bangert B.A., Taylor H.G., and Yeates K.O. (2019). Post-acute white matter microstructure predicts post-acute and chronic post-concussive symptom severity following mild traumatic brain injury in children. NeuroImage Clin. 25, 102106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mathias J.L., Dennington V., Bowden S.C., and Bigler E.D. (2013). Community versus orthopaedic controls in traumatic brain injury research: how comparable are they? Brain Inj. 27, 887–895 [DOI] [PubMed] [Google Scholar]
- 19. Loder R.T., Warschausky S., Schwartz E.M., Hensinger R.N., and Greenfield M.L. (1995). The psychosocial characteristics of children with fractures. J. Pediatr. Orthop. 15, 41–46 [DOI] [PubMed] [Google Scholar]
- 20. Ozer K., Gillani S., Williams A., and Hak D.J. (2010). Psychiatric risk factors in pediatric hand fractures. J. Pediatr. Orthop. 30, 324–327 [DOI] [PubMed] [Google Scholar]
- 21. Uslu M., Uslu R., Eksioglu F., and Ozen N.E. (2007). Children with fractures show higher levels of impulsive-hyperactive behavior. Clin. Orthop. Relat. Res. 460, 192–195 [DOI] [PubMed] [Google Scholar]
- 22. Carroll L., Cassidy J.D., Holm L., Kraus J., and Coronado V. (2004). Methodological issues and research recommendations for mild traumatic brain injury: the WHO collaborating centre task force on mild traumatic brain injury. J. Rehabil. Med. 36, 113–125 [DOI] [PubMed] [Google Scholar]
- 23. Teasdale G. and Jennett B. (1974). Assessment of coma and impaired consciousness. a practical scale. Lancet 2, 81–84 [DOI] [PubMed] [Google Scholar]
- 24. Greenspan L., McLellan B.A., and Greig H. (1985). Abbreviated Injury Scale and Injury Severity Score: a scoring chart. J. Trauma 25, 60–64 [DOI] [PubMed] [Google Scholar]
- 25. Lee L.-C., Harrington R.A., Chang J.J., and Connors S.L. (2008). Increased risk of injury in children with developmental disabilities. Res. Dev. Disabil. 29, 247–255 [DOI] [PubMed] [Google Scholar]
- 26. Stevens G. and Cho J.H. (1985). Socioeconomic indexes and the new 1980 census occupational classification scheme. Soc. Sci. Res. 14, 142–168 [Google Scholar]
- 27. Wechsler D. (2011). Wechsler Abbreviated Scale of Intelligence–2nd Edition (WASI-II), manual. Pearson: San Antonio, TX [Google Scholar]
- 28. Adelson P.D., Pineda J., Bell M.J., Abend N.S., Berger R.P., Giza C.C., Hotz G., and Wainwright M.S. (2012). Common Data Elements for pediatric traumatic brain injury: recommendations from the Working Group on Demographics and Clinical Assessment. J. Neurotrauma 29, 639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hajek C.A., Yeates K.O., Taylor H.G., Bangert B., Dietrich A., Nuss K.E., Rusin J., and Wright M. (2010). Agreement between parents and children on ratings of post-concussive symptoms following mild traumatic brain injury. Child Neuropsychol. 17, 17–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Duhaime A.-C., Holshouser B., Hunter J.V., and Tong K. (2012). Common Data Elements for neuroimaging of traumatic brain injury: pediatric considerations. J. Neurotrauma 29, 629–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Goodrich-Hunsaker N.J., Abildskov T.J., Black G., Bigler E.D., Cohen D.M., Mihalov L.K., Bangert B.A., Taylor H.G., and Yeates K.O. (2018). Age- and sex-related effects in children with mild traumatic brain injury on diffusion magnetic resonance imaging properties: a comparison of voxelwise and tractography methods. J. Neurosci. Res. 96, 626–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mayer A.R., Cohen D.M., Wertz C.J., Dodd A.B., Shoemaker J., Pluto C., Zumberge N.A., Park G., Bangert B.A., Lin C., Minich N.M., Bacevice A.M., Bigler E.D., Campbell R.A., Hanlon F.M., Meier T.B., Oglesbee S.J., Phillips J.P., Pottenger A., Shaff N.A., Taylor H.G., Yeo R.A., Arbogast K.B., Leddy J.J., Master C.L., Mannix R., Zemek R.L., and Yeates K.O. (2019). Radiologic common data elements rates in pediatric mild traumatic brain injury. Neurology 94, e241–e253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yushkevich P.A., Piven J., Hazlett H.C., Smith R.G., Ho S., Gee J.C., and Gerig G. (2006). User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage 31, 1116–1128 [DOI] [PubMed] [Google Scholar]
- 34. Avants B.B., Tustison N., and Song G. (2008). Advanced normalization tools (ANTS). OR Insight 1–35 [Google Scholar]
- 35. Fischl B. (2012). FreeSurfer. NeuroImage 62, 774–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Destrieux C., Fischl B., Dale A., and Halgren E. (2010). Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage 53, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuznetsova A., Brockhoff P.B., and Christensen R.H.B. (2017). lmerTest Package: Tests in linear mixed effects models. J. Stat. Software 82 [Google Scholar]
- 38. Bates D., Mächler M., Bolker B., and Walker S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Software 67 [Google Scholar]
- 39. Benjamini Y. and Hochberg Y. (1995). Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 57, 289–300 [Google Scholar]
- 40. Faul F., Erdfelder E., Buchner A., and Lang A.-G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 41, 1149–1160 [DOI] [PubMed] [Google Scholar]
- 41. Giza C.C. and Hovda D.A. (2014). The new neurometabolic cascade of concussion. Neurosurgery 75, S24–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X., Xie H., Cotton A.S., Tamburrino M.B., Brickman K.R., Lewis T.J., McLean S.A., and Liberzon I. (2015). Early cortical thickness change after mild traumatic brain injury following motor vehicle collision. J. Neurotrauma 32, 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Blatter D.D., Bigler E.D., Gale S.D., Johnson S.C., Anderson C.V., Burnett B.M., Ryser D., Macnamara S.E., and Bailey B.J. (1997). MR-based brain and cerebrospinal fluid measurement after traumatic brain injury: correlation with neuropsychological outcome. A.J.N.R. Am. J. Neuroradiol. 18, 1–10 [PMC free article] [PubMed] [Google Scholar]
- 44. Lewén A., Fredriksson A., Li G.L., Olsson Y., and Hillered L. (1999). Behavioural and morphological outcome of mild cortical contusion trauma of the rat brain: influence of NMDA-receptor blockade. Acta Neurochir. 141, 193–202 [DOI] [PubMed] [Google Scholar]
- 45. Walhovd K.B., Fjell A.M., Giedd J., Dale A.M., and Brown T.T. (2016). Through thick and thin: a need to reconcile contradictory results on trajectories in human cortical development. Cereb. Cortex bhv301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Newsome M.R., Wilde E.A., Bigler E.D., Liu Q., Mayer A.R., Taylor B.A., Steinberg J.L., Tate D.F., Abildskov T.J., Scheibel R.S., Walker W.C., and Levin H.S. (2018). Functional brain connectivity and cortical thickness in relation to chronic pain in post-911 veterans and service members with mTBI. Brain Inj. 32, 1235–1243 [DOI] [PubMed] [Google Scholar]
- 47. Hubbard C.S., Becerra L., Heinz N., Ludwick A., Rasooly T., Wu R., Johnson A., Schechter N.L., Borsook D., and Nurko S. (2016). Abdominal pain, the adolescent and altered brain structure and function. PLOS One 11, e0156545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goldstrohm S., and Arffa S. (2005). Preschool children with mild to moderate traumatic brain injury: an exploration of immediate and post-acute morbidity. Arch. Clin. Neuropsychol. 20, 675–695 [DOI] [PubMed] [Google Scholar]
- 49. Gronenschild E.H.B.M., Habets P., Jacobs H.I.L., Mengelers R., Rozendaal N., van Os J., and Marcelis M. (2012). The effects of FreeSurfer Version, Workstation Type, and Macintosh Operating System Version on anatomical volume and cortical thickness measurements. PLoS One 7, e38234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ducharme S., Albaugh M.D., Nguyen T.V., Hudziak J.J., Mateos-Pérez J.M., Labbe A., Evans A.C., and Karama S.; Brain Development Cooperative Group. (2016). Trajectories of cortical thickness maturation in normal brain development—the importance of quality control procedures. Neuroimage 125, 267–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou D., Lebel C., Treit S., Evans A., and Beaulieu C. (2015). Accelerated longitudinal cortical thinning in adolescence. NeuroImage 104, 138–145 [DOI] [PubMed] [Google Scholar]
- 52. Jernigan T.L., Brown T.T., Bartsch H., and Dale A.M. (2016). Toward an integrative science of the developing human mind and brain: focus on the developing cortex. Dev. Cogn. Neurosci. 18, 2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Brown T.T., Kuperman J.M., Chung Y., Erhart M., McCabe C., Hagler D.J., Venkatraman V.K., Akshoomoff N., Amaral D.G., Bloss C.S., Casey B.J., Chang L., Ernst T.M., Frazier J.A., Gruen J.R., Kaufmann W.E., Kenet T., Kennedy D.N., Murray S.S., Sowell E.R., Jernigan T.L., and Dale A.M. (2012). Neuroanatomical assessment of biological maturity. Curr. Biol. 22, 1693–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sowell E.R., Peterson B.S., Kan E., Woods R.P., Yoshii J., Bansal R., Xu D., Zhu H., Thompson P.M., and Toga A.W. (2007). Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb. Cortex 17, 1550–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Raznahan A., Lerch J.P., Lee N., Greenstein D., Wallace G.L., Stockman M., Clasen L., Shaw P.W., and Giedd J.N. (2011). Patterns of coordinated anatomical change in human cortical development: a longitudinal neuroimaging study of maturational coupling. Neuron 72, 873–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Bigler E.D., Finuf C., Abildskov T.J., Goodrich-Hunsaker N.J., Petrie J.A., Wood D.-M., Hesselink J.R., Wilde E.A., and Max J.E. (2018). Cortical thickness in pediatric mild traumatic brain injury including sports-related concussion. Intl. J. Psychophysiol. 132, 99–104 [DOI] [PubMed] [Google Scholar]
- 57. Backhausen L.L., Herting M.M., Buse J., Roessner V., Smolka M.N., and Vetter N.C. (2016). Quality control of structural MRI images applied using FreeSurfer—a hands-on workflow to rate motion artifacts. Front. Neurosci. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wilde E.A., Provenzale J.M., Taylor B.A., Boss M., Zuccolotto A., Hachey R., Pathak S., Tate D.F., Abildskov T.J., and Schneider W. (2018). Assessment of quantitative magnetic resonance imaging metrics in the brain through the use of a novel phantom. Brain Inj. 32, 1266–1276 [DOI] [PubMed] [Google Scholar]
- 59. Focke N.K., Helms G., Kaspar S., Diederich C., Tóth V., Dechent P., Mohr A., and Paulus W. (2011). Multi-site voxel-based morphometry—not quite there yet. Neuroimage 56, 1164–1170 [DOI] [PubMed] [Google Scholar]
- 60. Jovicich J., Czanner S., Greve D., Haley E., van der Kouwe A., Gollub R., Kennedy D., Schmitt F., Brown G., Macfall J., Fischl B., and Dale A. (2006). Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage 30, 436–443 [DOI] [PubMed] [Google Scholar]
- 61. Littmann A., Guehring J., Buechel C., and Stiehl H.S. (2006). Acquisition-related morphological variability in structural MRI. Acad. Radiol. 13, 1055–1061 [DOI] [PubMed] [Google Scholar]
- 62. Vovk U., Pernus F., and Likar B. (2007). A review of methods for correction of intensity inhomogeneity in MRI. IEEE Trans. Med. Imaging 26, 405–421 [DOI] [PubMed] [Google Scholar]
- 63. Reuter M., Tisdall M.D., Qureshi A., Buckner R.L., van der Kouwe A.J.W., and Fischl B. (2015). Head motion during MRI acquisition reduces gray matter volume and thickness estimates. NeuroImage 107, 107–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Afacan O., Erem B., Roby D.P., Roth N., Roth A., Prabhu S.P., and Warfield S.K. (2016). Evaluation of motion and its effect on brain magnetic resonance image quality in children. Pediatr. Radiol. 46, 1728–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brown T.T., Kuperman J.M., Erhart M., White N.S., Roddey J.C., Shankaranarayanan A., Han E.T., Rettmann D., and Dale A.M. (2010). Prospective motion correction of high-resolution magnetic resonance imaging data in children. NeuroImage 53, 139–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rauch S.L. (2005). Neuroimaging and attention-deficit/hyperactivity disorder in the 21st century: what to consider and how to proceed. Biol. Psychiatry 57, 1261–1262 [DOI] [PubMed] [Google Scholar]
- 67. Blumenthal J.D., Zijdenbos A., Molloy E., and Giedd J.N. (2002). Motion artifact in magnetic resonance imaging: implications for automated analysis. NeuroImage 16, 89–92 [DOI] [PubMed] [Google Scholar]
- 68. Slobounov S.M., Walter A., Breiter H.C., Zhu D.C., Bai X., Bream T., Seidenberg P., Mao X., Johnson B., and Talavage T.M. (2017). 0. NeuroImage Clin. 14, 708–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.