Abstract
We report here the development of oncolytic adenoviruses (Ads) that have reduced toxicity, enhanced tumor tropism, produce strong antitumor response, and can overcome resistance to immune checkpoint inhibitor therapy in breast cancer. We have shown that LyP-1 receptor (p32) is highly expressed on the surface of breast cancer cells and tumors from cancer patients, and that increased stromal expression of transforming growth factor β-1 (TGFβ-1) is associated with triple-negative breast cancer. Therefore, we constructed oncolytic Ads, AdLyp.sT and mHAdLyp.sT, in which the p32-binding LyP-1 peptide was genetically inserted into the adenoviral fiber protein. Both AdLyp.sT and mHAdLyp.sT express sTGFβRIIFc, a TGFβ decoy that can inhibit TGFβ pathways. mHAdLyp.sT is an Ad5/48 chimeric hexon virus in which hypervariable regions (HVRs 1–7) of Ad5 are replaced with the corresponding Ad48 HVRs. AdLyp.sT and mHAdLyp.sT exhibited better binding, replication, and produced higher sTGFβRIIFc protein levels in breast cancer cell lines compared with Ad.sT or mHAd.sT control viruses without LyP-1 peptide modification. Systemic delivery of mHAdLyp.sT in mice resulted in reduced hepatic/systemic toxicity compared with Ad.sT and AdLyp.sT. Intravenous delivery of AdLyp.sT and mHAdLyp.sT elicited a strong antitumor response in a human MDA-MB-231 bone metastasis model in mice, as indicated by bioluminescence imaging, radiographic tumor burden, serum TRACP 5b and calcium, and body weight analyses. Furthermore, intratumoral delivery of AdLyp.sT in 4T1 model in immunocompetent mice inhibited tumor growth and metastases, and augmented anti-PD-1 and anti-CTLA-4 therapy. Based on these studies, we believe that AdLyp.sT and mHAdLyp.sT can be developed as potential targeted immunotherapy agents for the treatment of breast cancer.
Keywords: metastatic breast cancer, oncolytic adenovirus, transforming growth factor β, LyP-1, liver-detargeted, systemic therapy, immunotherapy
Introduction
Despite the latest breakthroughs in medical research, metastatic breast cancer remains an incurable disease and the frequent cause of cancer-related deaths worldwide.1,2 In advanced breast cancers, the rapid acquisition of therapy resistance seems to limit the effectiveness of newly developed targeted therapy and immunotherapy. Therefore, it is important to develop novel therapies that can target tumor cells and signaling molecules specific for metastasis, and circumvent various resistance mechanisms.3,4
Recently, there has been a growing interest in understanding the role of transforming growth factor β (TGFβ) signaling in promoting tumor growth and metastases and inducing therapy resistance. Under physiological conditions TGFβ is a potent growth inhibitor. However, aberrant TGFβ expression has been reported in advanced malignancies, including breast cancer.5,6 It has been shown that aberrant TGFβ signaling can alter the tumor microenvironment (TME), promote angiogenesis and epithelial–mesenchymal transition (EMT) while suppressing antitumor immunity, and contribute significantly to metastasis and tumor immune escape.7,8 Thus, several inhibitors of TGFβ pathway are currently being developed and tested in animal studies and clinical trials for many cancer types.9–14
Our laboratory has previously developed an oncolytic adenovirus Ad.sT, expressing soluble TGFβ receptor II fused with human IgG Fc fragment (sTGFβRIIFc).15,16 sTGFβRIIFc acts as the decoy protein preventing TGFβ binding to tumor cells.15,16 In immunodeficient mouse models, systemic delivery of Ad.sT inhibited skeletal metastases of human breast and prostate cancer cells.16,17 In the breast and renal immunocompetent mouse models, direct inoculation of a similar sTGFβRIIFc expressing oncolytic virus rAd.sT into subcutaneous tumors inhibited protumorigenic signals and produced immune activation.18 rAd.sT can also overcome resistance to immune checkpoint inhibitors (ICIs) and improve the antitumor effects of anti-PD-1 and anti-CTLA-4 antibody therapy.18
To treat metastatic cancers, the preferred route to deliver adenoviral vectors would be via systemic administration. Two key limitations in the use of Ad5-based adenoviruses (Ads) for systemic administration are the limited tumor tropism and liver/systemic toxicities. To enhance tumor tropism, in this study, a 9-amino acid-long tumor homing-cell penetrating LyP-1 peptide was inserted into the HI loop of Ad5 adenoviral fiber to generate AdLyp.sT. This novel AdLyp.sT vector will target LyP-1 receptor, which is expressed on the breast cancer cell surface, tumor macrophages, and tumor lymphatics, but not readily detectable on normal tissues.19–21
To bypass the hepatic uptake and produce minimum hepatic and systemic toxicity, we have previously engineered a liver detargeted oncolytic adenovirus (mHAd.sT) expressing sTGFβRIIFc that has Ad5/48 chimeric hexon. Because Ad48 hexon hypervariable regions (HVRs) 1–7 have reduced capacity to bind with FactorX, the virus expressing Ad48 hexon is expected to have reduced liver uptake.22–27 In this study, we generated mHAdLyp.sT, an oncolytic adenovirus that has both LyP-1 peptide to enhance tropism to breast cancers and Ad5/48 chimeric hexon to reduce liver/systemic toxicities.
The goals of this study were to examine the following: (i) the prevalence of LyP-1 receptor expression in breast cancer cells and in the tumors from breast cancer patients, and if aberrant TGFβ expression is associated with advanced breast cancer; (ii) whether modification of oncolytic viruses with LyP-1 peptide1 will enhance virus binding and replication, and sTGFβRIIFc production in breast cancer cells; (iii) if upon systemic delivery, mHAdLyp.sT will have reduced hepatic uptake and produce minimum hepatic and systemic toxicity; (iv) if systemic administration of AdLyp.sT and mHAdLyp.sT in human breast tumor models in immunodeficient mouse will have improved inhibition of the skeletal metastases and the tumor-induced bone destruction; and (v) if in immunocompetent model, intratumoral inoculation of AdLyp.sT will produce antitumor growth and antimetastasis effects, and augment anti-PD-1/anti-CTLA-4 antibody therapy.
We report here that breast cancer cells and human breast tumors ubiquitously express LyP-1 receptors and high levels of stromal TGFβ-1 proteins in triple-negative breast cancer (TNBC) patients. Inclusion of LyP-1 peptide in AdLyp.sT and mHAdLyp.sT improves the virus binding and replication, and transgene expression. Systemic administration of mHAdLyp.sT in mice showed reduced hepatic/systemic cytotoxicity, while both AdLyp.sT and mHAdLyp.sT produced effective antitumor and antibone metastasis activities in a human TNBC mouse model. Finally, our studies indicated that direct inoculation of AdLyp.sT into murine syngeneic tumors inhibited tumor growth and lung metastasis, and enhanced efficacy of anti-PD-1/anti-CTLA-4 therapy in a mouse TNBC model. Overall, our studies suggest that AdLyp.sT and mHAdLyp.sT can be potentially developed for metastatic breast cancer therapy and enhancing ICI therapy.
Materials and Methods
Case selection and tissue slide preparation
Fifty-two paired (tumor and matched normal tissue samples adjacent to tumors) formalin-fixed, paraffin-embedded (FFPE), breast tissue blocks with initial diagnosis from 26 breast cancer patients were derived by expert pathologists. These surgical specimens were collected from 2004 to 2011 in the NorthShore University HealthSystem (Illinois). The FFPE blocks were cut into 5-μm-thick sections and the sectioned tissue slides were prepared according to an established and verified standard protocol. Relevant clinical and pathological information of these patients was retrieved from the NorthShore Epic electronic health records (EHRs) by authorized personnel. To better understand the relationship between molecular targets of interest (LyP-1 receptor and TGFβ) with the human epidermal growth factor receptor 2 (HER2) and hormone receptor statuses of patients, we used the sample pool consisting of similar numbers of molecular subtypes. The protocol was approved by the NorthShore Institutional Review Board, and written informed consent was previously obtained from each patient for breast cancer research through the NorthShore Biospecimen Repository.
Cell lines and adenoviruses
Human mammary tumor cell lines, MCF-7 (ATCC, Manassas, VA), MDA-MB-231 (ATCC), and MDA-MB-231-luc2,16 and the mouse mammary tumor cell line, 4T1 (ATCC), were maintained as described earlier.28 All media components were purchased from Thermo Fisher Scientific (Waltham, MA).
To create oncolytic adenoviruses, viral or target genes were modified and cloned in a shuttle vector and subjected to homologous recombination with adenoviral genomic DNA derived from adenoviral mutant dl01/07 using published methods.15 In particular, the LyP-1 receptor binding sequence encoding nine amino acids (LyP-1 peptide: CGNKRTRGC) was introduced into the HI loop of fiber to create p309-FibHI-Lyp-1. AdLyp.sT was obtained by homologous recombination between p309-FibHI-Lyp-1 and pTG-sTGFβRIIFc. The latter contains the sTGFβRIIFc gene to inhibit TGFβ signaling.
To create mHAdLyp.sT, p309 FibHI-Lyp-1-TGFβRIIFc was first generated to have both sequences of LyP-1 peptide and the TGFβ inhibitory ligand: sTGFβRIIFc. It was subjected to homologous recombination with a previously constructed Ad5/48 chimeric hexon containing vector, pTG hex5/48,26 to generate mHAdLyp.sT. The other key components—mutant E1A (01/07), ADP (adenoviral death protein), and ITR (inverted terminal repeats), are sequentially positioned as described previously.15,26 All adenoviral vectors were amplified in HEK293 cells (ATCC) and purified as described earlier.
Immunofluorescence, Western blot, and immunohistochemistry staining
For immunofluorescence staining, cells were seeded and, when reaching ∼50% confluences on Fisherbrand™ cover glasses in six-well plates, were subjected to staining. Cells were fixed with 3.7% paraformaldehyde and permeabilized with 0.5% Triton X-100 in phosphate-buffered saline (PBS). One percent goat serum was used for blocking. Rabbit anti-GC1q R (p23, the receptor for LyP-1) antibody from Abcam (Cat#: ab101267, 1:200 dilution; Cambridge, United Kingdom) and goat anti-rabbit IgG H&L (Alexa Fluor® 488; Abcam) were used for detection. Cells were mounted with ProLong® Gold Antifade Reagent with DAPI (Invitrogen, Carlsbad, CA) and viewed using a Leica microscope equipped with epifluorescence illumination and appropriate fluorescence filters for DAPI and Alexa Fluor 488. Fluorescence images in each wavelength were collected using a CoolSNAP CCD camera and were relayed to the IPLab Spectrum image analysis software. Images were subjected to identical grayscale normalization and contrast enhancement and were then subjected to pseudocolor overlay to produce the color images.
Cells grown in 100 mm dishes were collected for Western blot using a protocol described previously,27 using rabbit anti-GC1q R (p23) antibody (ab101267; Abcam) or anti-actin antibody (A2066; Sigma-Aldrich, St. Louis, MO) as the primary antibody.
For immunocytochemistry analysis, paraffin sections were deparaffinized and rehydrated by a standard protocol.29,30 Proteolytic-induced epitope retrieval followed by saponin/hydrogen peroxide solution was utilized to promote epitope availability and block endogenous peroxidase activity. The tris phosphate buffer (TBS) blocking solution contained 5% goat serum, 5% BSA, 0.1% gelatin, 0.5% Triton X-100, and 0.05% Tween 20. The primary antibodies: rabbit anti-GC1q R (p23) antibody (ab101267, 1:200 dilution; Abcam) and mouse anti-TGFβ-1 antibody [TB21] (ab27969, 1:200 dilution; Abcam) in 2% BSA TBS were used for detection, followed by TBS containing 0.1% Tween 20 TBST washes and incubation with corresponding biotinylated secondary antibodies from the VECTASTAIN® Elite® ABC HRP Kit (Burlingame, CA). Vector DAB Peroxidase (HRP) Substrate Kit and hematoxylin Gill III (Newcomer, Middleton, WI) were utilized for chromogenic reaction and counterstaining. The slides were dehydrated, mounted, and observed by a Nikon Eclipse TE200 Inverted Microscope. The Nikon DS-Fi1 camera and NIS-Elements BR 3.10 software were used for documenting micrographs. Higher magnification was used when necessary for identification.
Adenoviral binding, replication, and sTGFβRIIFc expression in MDA-MB-231 and 4T1 cells
In vitro virus binding and replication assays were performed according to our established protocols as described earlier.17 For virus binding assay, 1 × 105 cells per well were seeded in 24-well plates. The next day, cells were washed two times with ice-cold PBS and exposed to 2.5 × 104 viral particles (VPs) per cell corresponding viruses in ice-cold serum-free cell culture media for 1 h at 4°C. At the end of 1 h, the plates were switched to 37°C for 5 min to allow viruses to be internalized. Cells were then immediately washed twice with room-temperature PBS, followed by the incubation with complete cell culture media at 37°C for 24 h.
Cell fixing and sTGFβRIIFc staining were performed by the procedures previously described.27,28 Goat anti-human IgG, Fcγ fragment-specific antibody from Jackson ImmunoResearch Laboratories (Cat#: 109005098; West Grove, PA) was used to detect sTGFβRIIFc. Positive cells were counted with 100 × magnification in the 2.54 mm2 field area in our digital image system for microscopes previously described.28 Regular viral infection and protein expression assays were also performed according to our established protocol as described earlier.17,27 In short, cell culture media were collected, and sTGFβRIIFc levels in the media were determined by enzyme-linked immunosorbent assay (ELISA) using previously described methods.15,17,27
Animal studies
All animal experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the NorthShore University HealthSystem.
Toxicity studies and innate immune responses
Six-week-old female NU/NU nude mice (Charles River Laboratories, Wilmington, MA) were injected with 1 × 1011 VPs per mouse of corresponding adenoviruses via tail vein. Each group contained four mice. Mice were euthanized 3 days after the injection. The livers were photographed. The liver samples were collected and processed for hematoxylin and eosin (H&E) staining, immunohistochemistry (IHC) staining by the anti-human IgG, Fcγ fragment-specific antibody (Cat#: 109005098; Jackson ImmunoResearch Laboratories), and viral genome copy measurement by quantitative PCR by the methods described in our previous publications.26 Mice spleens were also processed for H&E staining and IHC staining of sTGFβRIIFc.
Mouse blood samples were collected (0.5 mL) and serum samples were used to determine the sTGFβRIIFc levels by ELISA as described earlier.16 The commercially available kits were utilized to determine serum levels of alanine transaminase (ALT) (EALT-100; BioAssay Systems, Hayward, CA), aspartate transaminase (AST) (EASTR-100; BioAssay Systems), and lactate dehydrogenase (LDH) (ab197000; Abcam, Cambridge, MA). Serum tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β levels were determined by ELISA as previously described.27
Bone metastasis model and bioluminescence imaging
To establish bone metastasis, MDA-MB-231-luc2 cells (2.0 × 105/mouse) were injected into the left heart ventricle of 4-week-old female nude mice on day 0, as described earlier.17,31 On day 7, mice were subjected to bioluminescence imaging (BLI) in dorsal and ventral positions using Xenogen IVIS and the laboratory's standard protocol previously described.16 Signal intensities were quantified as the total flux (photons/seconds) within regions of interests (ROIs) in both left and right hind limbs using Living Image software 3.0 (Caliper Life Sciences, Hopkinton, MA) as described.16,17,31 We obtained 70 mice that had photon flux of ROIs within the range of 1.0 × 105–1.0 × 106 photons per second. Mice were divided into seven groups, with statistically indistinguishable BLI signals among each group (an average of about 2.8 × 105–6.2 × 105 photons/second per group).
For treatment groups, various viral vectors were administered via tail vein on days 8 and 12 as described in the Results section. In précis, we included four groups that received a low therapeutic dose (LTD) of Ad.sT, AdLyp.sT, mHAd.sT, or mHAdLyp.sT (a total of 5 × 1010 VPs/mouse), and two groups treated with a high therapeutic dose (HTD) of mHAd.sT or mHAdLyp.sT (a total of 2.0 × 1011 VPs/mouse), individually (n = 10). The control group of mice (n = 10) was administered with the buffer alone. BLI was conducted weekly for the duration of the study. All mice were euthanized according to the standard protocol after blood was collected via cardiac puncture on day 23.
X-ray radiography analysis, body weight measurements, and quantifications of calcium and TRACP 5b in serum
Mice were subjected to X-ray radiography in the prone position using Faxitron (Faxitron X-Ray Corporation, Wheeling, IL) on day 15 and 22. Radiographic tumor burden from bone lesions was quantified in the femur and tibia of both hind limbs using ImageJ software (NIH, Bethesda, MD) as described earlier.17,31 All mice, together with control mice that did not receive MDA-MB-231-luc2 cells (normal, n = 10) or any treatments (buffer, n = 10), were also subjected to body weight measurement once a week. Quantification of calcium and TRACP 5b in mouse serum was performed using our published methods.17,31
4T1 xenograft model and therapeutic analysis with/without ICIs
To establish the mouse mammary tumor syngeneic model, we injected 2 × 106 4T1 cells per mouse subcutaneously (day 0) into the dorsal right flank of 30 female BALB/c mice (4–6 weeks old). Mice were monitored every day. On day 6, tumor dimensions were measured (in mm) using a caliper. Tumor volumes were calculated by the following formula: tumor volume = (width2 × length)/2. Then, tumor-bearing mice were divided into six groups, without statistical differences between each group (n = 5). On day 7, 9, and 11 post-tumor cell implantation, tumor-bearing mice were administered 100 μL of PBS buffer, Ad.sT, or AdLyp.sT (5.0 × 1010 VPs per mouse/each injection) intratumorally (10 mice for each virus). On days 8, 10, 13, and 15, anti-PD-1 and anti-CTLA-4 antibodies were administered together intraperitoneally (0.2 mg per mouse per antibody) in the groups indicated in the Results section.
The tumor volumes were monitored on day 10, 14, 17, 21, 24, and 27. The body weights were measured on day 6, 14, 17, 21, 24, 27, 31, and day 35. Mice were euthanized on day 35. The whole lungs were excised and photographed. Lung surface tumor lesions were counted for each group. Parts of the lungs were processed for H&E staining according to our published protocol.18 Pulmonary metastatic burden was quantified by using ImageJ software (NIH) for each lung section.
Statistical analyses
All data were analyzed using GraphPad Prism software version 5 (GraphPad software, San Diego, CA). For prognostic and clinicopathological significance analysis, the chi-squared test and Fisher's exact test were used to assess for independence among variables. The chi-squared (χ2) test applied an approximation assuming multiple samples, while the Fisher's exact test ran an exact procedure for two variables. Data are presented in the tables. For all other statistical analyses, data are plotted as the mean ± standard error of the mean. Longitudinal data were analyzed using a two-way repeated-measure ANOVA followed by Bonferroni post hoc pairwise tests for the data obtained over the time course. For multiple group analyses, one-way ANOVA followed by Bonferroni post hoc tests adjusting for multiplicity was performed. Student's t-tests were performed to compare two sets of data. A Fisher's exact test was used for the bone metastasis incidence data. Differences were considered significant at two-sided p < 0.05.
Results
LyP-1 receptor (p32) expression on the surface of TNBC cell lines and on tumor samples of breast cancer patients
We have examined the prevalence of two relevant molecular biomarkers, LyP-1 receptor (p32) and TGFβ-1, in breast cancer cell lines and in the human breast tumors. Immunofluorescence staining showed that the two human breast cancer cell lines, MDA-MB-231 and MCF-7, exhibit strong LyP-1 receptor staining on cell surface and internal membrane networks (Fig. 1A). LyP-1 receptors were localized more on plasma membrane and endoplasmic reticulum-like membrane networks in MDA-MB-231 cells (Fig. 1A, upper panel). In MCF-7 cells, LyP-1 receptors were enriched in Golgi-like juxtanuclear compartments (Fig. 1A, middle panel). 4T1, a highly tumorigenic and invasive mouse mammary tumor cell line, showed a staining pattern similar to that of MDA-MB-231 (Fig. 1A, lower panel). To be noted, both human MDA-MB-231 and mouse 4T1 are TNBC cell lines and are more aggressive than MCF-7, an estrogen/progesterone receptor-positive and HER2-negative cell line.
Figure 1.
LyP-1 receptor (p32) and/or TGFβ-1 expression in breast cancer cell lines and patients. (A) LyP-1 receptor (p32) expression in human and mouse breast cancer cells by immunofluorescence staining. In MDA-MB-231 and 4T1 cells, LyP-1 receptors were localized more on plasma membrane (white arrows) and endoplasmic reticulum-like membrane networks (white triangular arrowheads). In MCF-7 cells, they were more enriched in Golgi-like juxtanuclear compartments (white arrows). Scale bar = 25 μm. (B) LyP-1 receptor expression in breast cancer cell lines by Western blot. Note that mouse LyP-1 receptors in 4T1 cells have a slightly different banding pattern from those in human breast cancer cells. All experiments were repeated three times. (C) Representative images of LyP-1 receptor and TGFβ-1 expression in breast cancer patient samples. Yellow arrows point to positive p32 IHC staining in tumor cells; red open arrows point to TGFβ-1 staining in tumor stroma. Magnification is indicated by scale bars. IHC, immunohistochemistry; TGFβ-1, transforming growth factor β-1.
The expression of LyP-1 receptors in these three breast cancer cell lines was further verified by Western blot analysis (Fig. 1B). LyP-1 receptors in mouse 4T1 cells exhibit a different banding pattern from those seen in human breast cancer cells, suggesting that human and murine Lyp-1 receptors, although they can react with our LyP-1 receptor antibody, show species specificity. We further analyzed LyP-1 receptor expression in human tumors derived from breast cancer patients. The FFPE samples obtained from 26 patients before they received any therapy were subjected to IHC staining of LyP-1 receptors. Tumor cells in all these samples showed medium (Fig. 1C, lower left panel) to strong (Fig. 1C, upper left panel) staining for LyP-1 receptor. The statistical analysis indicated that LyP-1 receptor expression levels are not associated with any clinical and/or pathological parameters in these patients.
Tumor stromal expression of TGFβ-1 is associated with TNBC and is a poor prognostic marker of overall survival in breast cancer patients
Because there is increased evidence in the literature suggesting that aberrant transforming growth factor β (TGFβ) signaling is closely associated with tumor growth and metastases in advanced cancers, including breast cancer, we analyzed for TGFβ-1 expression in these patient samples. Distinct TGFβ-1 expression patterns were observed among them. Some samples contained increased TGFβ-1 expressing cells in tumor stroma (Fig. 1C, upper right panel), while others had fewer TGFβ-1 expressing cells (Fig. 1C, lower right panel). Relevant clinical and pathological information of these patients was retrieved from the NorthShore Epic EHRs by authorized pathologists. Our sample pool consists of five patients who are negative for HER2&ER&PR (TNBC), five patients who are HER2 positive only, seven HER2 (−)/ER&PR (+) patients, and nine patients with all positive markers [HER&ER&PR (+)].
Our statistical analysis showed that a strong TGFβ-1 expression in tumor stroma was significantly associated with these molecular statuses (χ2 = 10.85, p = 0.0126; Table 1). Furthermore, Fisher's exact test suggested that the difference in TGFβ-1 expression between TNBC and ER/PR/HER2 (+) tumors was most significant (p = 0.005). The tumor stromal expression of TGFβ-1 was also significantly associated with overall survival (OS) (p = 0.0084, Fisher; Table 1). Two other major clinical and pathological parameters significantly associated with progression-free survival and overall survival (OS) in this group were molecular subtypes and metastasis status (Supplementary Table S1).
Table 1.
Associations between transforming growth factor β-1 expressions in tumor-associated stromal cells by immunohistochemistry and clinical and pathological parameters
| Clinicopathological Parameters | n | TGFβ-1 in TASC |
p | |
|---|---|---|---|---|
| High, n = 9 | Low to None, n = 17 | |||
| Molecular subtype | ||||
| TNBC | 5 | 4 | 1 | χ2 = 10.85, p = 0.0126; TNBC vs. ER/PR/HER2 (+): p = 0.005 (Fisher) |
| HER2 (+)/ER&PR (−) | 5 | 3 | 2 | |
| HER2 (−)/ER&PR (+) | 7 | 2 | 5 | |
| HER&ER&PR (+) | 9 | 0 | 9 | |
| Histological grade | ||||
| 1 | 1 | 0 | 1 | χ2 = 0.7876, p = 0.6745 (chi-square) |
| 2 | 7 | 2 | 5 | |
| 3 | 18 | 7 | 11 | |
| Age | ||||
| <50 | 7 | 2 | 5 | p = 1.000 (Fisher) |
| ≥50 | 19 | 7 | 12 | |
| Tumor size, cm | ||||
| ≤2 | 14 | 4 | 10 | p = 0.6828 (Fisher) |
| >2 | 12 | 5 | 7 | |
| Lymph node status | ||||
| Negative | 13 | 4 | 9 | χ2 = 1.393, p = 0.7071 (chi-square) |
| 1–3 | 4 | 2 | 2 | |
| 4–9 | 5 | 1 | 4 | |
| 10 or more | 4 | 2 | 2 | |
| Metastasis | ||||
| None | 22 | 6 | 16 | p = 0.1039 (Fisher) |
| Yes | 4 | 3 | 1 | |
| TNM stage | ||||
| I | 10 | 3 | 7 | χ2 = 0.3107, p = 0.8561 (chi-square) |
| II | 7 | 3 | 4 | |
| III | 9 | 3 | 6 | |
| Progression-free survival, years | ||||
| <5 | 7 | 4 | 3 | p = 0.1881 (Fisher) |
| ≥5 | 19 | 5 | 14 | |
| Overall survival, years | ||||
| <5 | 4 | 4 | 0 | p = 0.0084 (Fisher) |
| ≥5 | 22 | 5 | 17 | |
HER2, human epidermal growth factor receptor 2; TGFβ-1, transforming growth factor β-1; TNBC, triple-negative breast cancer.
Construction of AdLyp.sT and mHAdLyp.sT, viral replication, virus binding, and vector-mediated sTGFβRIIFc protein expression in TNBC cell lines
Our laboratory has previously developed a platform of oncolytic adenoviruses expressing sTGFβRIIFc. Ad.sT is the original cytomegalovirus (CMV) promoter-regulated Ad5-based virus expressing sTGFβRIIFc (named as Ad.sTβRFc in some of our previous publications).15,16 To reduce hepatic/systemic toxicity associated with systemic delivery of Ad.sT, we have previously created mHAd.sT, a liver detargeted virus, by replacing Ad5 HVRs of Ad.sT with Ad48 HVRs (named as mHAd.sTβRFc in the previous publication.27
To enhance tumor tropism, we have now created AdLyp.sT and mHAdLyp.sT by introducing LyP-1 peptide, a 9-amino acid-long tumor homing-cell peptide, into the HI loop of the Ad.sT and mHAd.sT fiber, respectively. Thus, these viruses can bind to the LyP-1 receptor expressed on the breast cancer cells. In addition to tumor cells, AdLyp.sT and mHAdLyp.sT should also target LyP-1 receptor expressing tumor macrophages and lymphatics, but not the normal tissues. Simultaneously, they can express sTGFβRIIFc and therefore inhibit aberrant TGFβ signaling associated with many cancers (Fig. 2A).
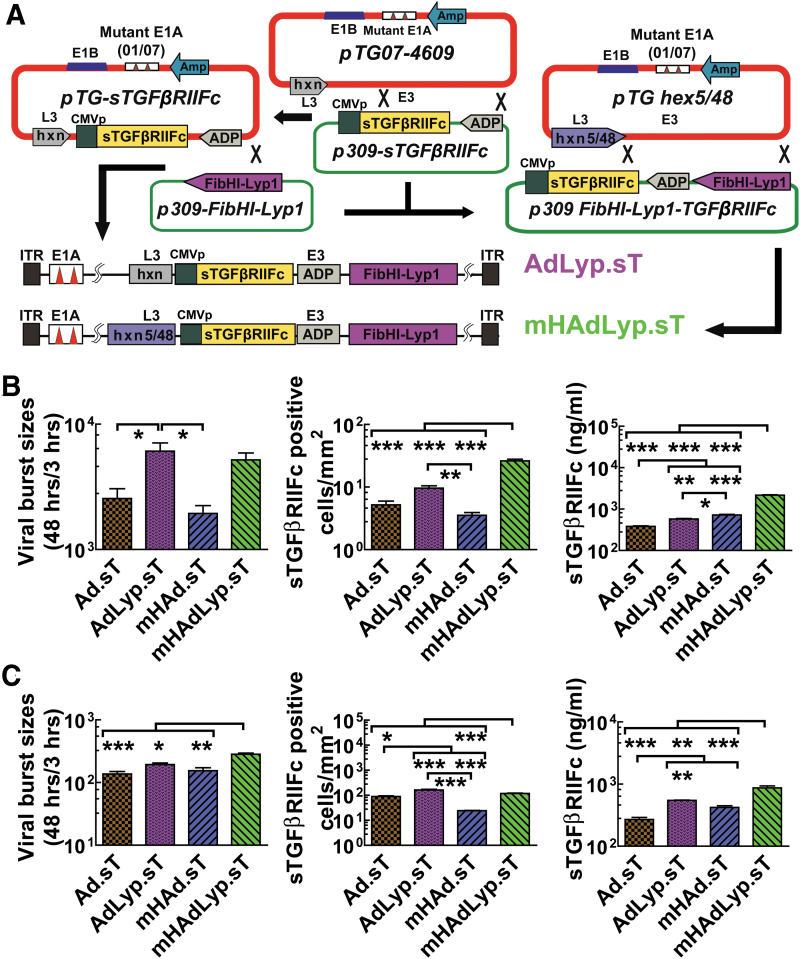
Figure 2.
Construction of LyP-1-modified adenoviruses expressing sTGFβRIIFc (AdLyp.sT and mHAdLyp.sT) and their replication, binding, and sTGFβRIIFc expression in MDA-MB-231 and 4T1 cells. (A) Schematic diagram of adenoviral constructs of AdLyp.sT and mHAdLyp.sT. LyP-1 peptide: CGNKRTRGC was introduced into the HI loop of fiber to create p309-FibHI-Lyp-1. The TGFβ inhibitory ligand: sTGFβRIIFc gene was introduced by pTG-sTGFβRIIFc or shuttle vectors. Please note that sTGFβRIIFc expression is under the control of the cytomegalovirus promoter shown as (CMVp). Ad5/48 chimeric hexon containing vector: pTG hex5/48 was used to generate mHAdLyp.sT. All viruses have two small deletions, 01/07 (amino acids 4–25, and amino acids 111–123) in the E1A region, and deletion in E3 but contain adenoviral death protein (labeled as ADP in the diagram). The maps are not drawn to scale. (B) The replication, binding, and sTGFβRIIFc expression of adenoviruses in human MDA-MB-231 cells. Shown are corresponding results of AdLyp.sT and mHAdLyp.sT and their controls: Ad.sT and mHAd.sT. Viral replication was indicated by the ratio of hexon proteins between 48 and 3 h after the infection (left panel). Viral binding was determined by numbers of cells expressing sTGFβRIIFc 24 h after the incubation with viruses for 1 h (middle panel). sTGFβRIIFc productions in cell culture media 48 h after the infection were quantified by ELISA (right panel). (C) The replication (left panel), binding (middle panel), and sTGFβRIIFc expression (right panel) of adenoviruses in mouse 4T1 cells. One-way ANOVA followed by Bonferroni's multiple comparison test was used to calculate p-value for each set of the experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. All experiments were repeated three times. Ad, adenovirus; ELISA, enzyme-linked immunosorbent assay; Hxn, hexon; ITR, inverted terminal repeats.
AdLyp.sT and mHAdLyp.sT were examined for their replication potential and sTGFβRIIFc expression in breast cancer cells. We used human MDA-MB-231 and mouse 4T1 cells in this study because they are two very well-studied TNBC cell lines and we have documented their cell surface LyP-1 receptor expression. Two previously published adenoviruses, Ad.sT and mHAd.sT, were used as controls. To demonstrate their replication potentials, the viral burst sizes were calculated as the ratio of viral hexon protein production at 48 h relative to the levels seen at 3 h, as previously described.27 In human MDA-MB-231 cells, the viral titers of AdLyp.sT and mHAdLyp.sT were significantly more than double that of Ad.sT and mHAd.sT, respectively (Fig. 2B, left panel, p < 0.05). This result suggested that the LyP-1 peptide facilitates viral spread and production in the breast tumor cells. Moreover, mHAdLyp.sT exhibited a higher viral replication potential in mouse 4T1 cells (Fig. 2C, left panel, one-way ANOVA test showed that the viral titer of mHAdLyp.sT was significantly higher than that of all other viruses).
Next, the viral binding and entry into the tumor cells were quantified by the viral binding assay. In both cell lines, mHAdLyp.sT produced a significantly higher numbers of sTGFβRIIFc-positive cells than Ad.sT or mHAd.sT (Fig. 2B, C, middle panel). AdLyp.sT resulted in a significant increase of binding affinity when compared with Ad.sT and mHAd.sT, especially in mouse 4T1 cells. Similar results were observed when we quantified secreted sTGFβRIIFc protein in cell culture media by ELISA (Fig. 2B, C, right panel). Taken together, these results indicated that AdLyp.sT and mHAdLyp.sT have the increased ability to bind, replicate, and produce sTGFβRIIFc protein in breast cancer cells in vitro.
Systemic administration of mHAdLyp.sT in nude mice resulted in reduced uptake in the liver and spleen, hepatotoxicity and systemic toxicity, and attenuated innate immune response
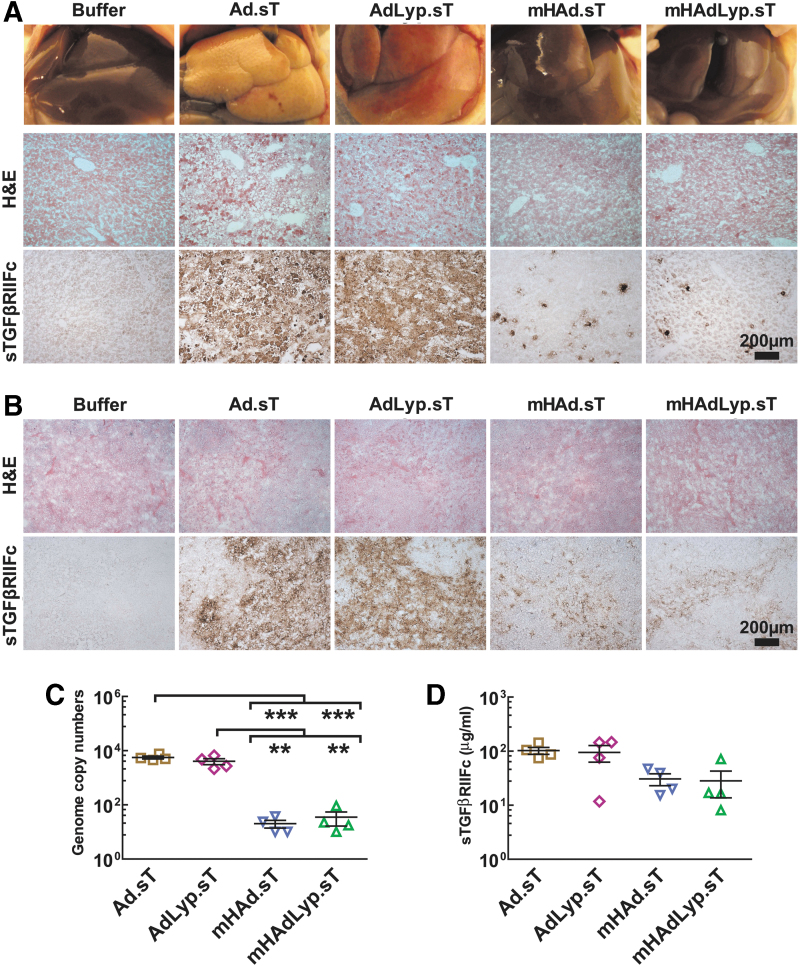
To compare the toxicity profiles of AdLyp.sT and mHAdLyp.sT, we administered these and Ad.sT and mHAd.sT viruses (1 × 1011 VPs/mouse) into different groups of nude mice via tail vein. Mice were carefully monitored for 72 h, and then, blood samples and liver/spleen tissues were harvested. Livers from Ad5-based Ad.sT- and AdLyp.sT-treated mice had an abnormal pale/yellow appearance, while livers from Ad5/48 chimeric hexon-based mHAd.sT, mHAdLyp.sT, and the buffer-treated groups had a normal bright red appearance (Fig. 3A, upper panel). H&E stain showed liver necrosis in these pale/yellow tissues (Fig. 3A, middle panel).
Figure 3.
Systemic administration of mHAd.sT and mHAdLyp.sT in nude mice exhibited reduced liver and spleen uptake. (A) Shown are gross liver morphology (upper panel), H&E staining (middle panel), and IHC staining of sTGFβRIIFc expression (lower panel) in the liver 3 days after intravenous injection of viruses. Scale bar = 200 μm. (B) H&E staining (upper panel) and IHC staining of sTGFβRIIFc expression (lower panel) in the spleen 3 days after intravenous injection of viruses are shown. Scale bar = 200 μm. (C) Viral DNA copy numbers in the liver (n = 4) were measured and shown. (D) sTGFβRIIFc levels in mouse serum (n = 4) were measured and shown. p-Value comparisons by one-way ANOVA are shown for (C) (**p < 0.01, ***p < 0.001). H&E, hematoxylin and eosin.
We also detected more sTGFβRIIFc-positive cells in Ad.sT- or AdLyp.sT-treated groups by IHC staining (brown stained cells in Fig. 3A, lower panel). While no distinct gross pathological and morphological changes of spleen were observed among these groups (data not shown and Fig. 3B, upper panel), the splenic uptake of adenoviruses, indicated by sTGFβRIIFc expression, was much more obvious in Ad.sT- and AdLyp.sT-treated groups (Fig. 3B, lower panel).
We further quantified adenoviral uptake in the liver by measuring viral genomic DNA. Viral DNA copy numbers in the mHAd.sT- and mHAdLyp.sT-treated groups were both significantly lower than those in the Ad.sT (p < 0.001) and AdLyp.sT (p < 0.01) group (Fig. 3C). Interestingly, no significant difference in the sTGFβRIIFc levels in the mouse serum was observed among these groups (Fig. 3D), suggesting that they all had similar levels of the sTGFβRIIFc protein in their circulation. Thus, compared with Ad.sT and AdLyp.sT, both mHAd.sT and mHAdLyp.sT exhibited reduced uptake in the liver and spleen.
We next examined if the systemic administration of mHAd.sT and mHAdLyp.sT elicited reduced hepatic and systemic toxicity and weaker proinflammatory cytokine responses. To investigate the hepatotoxicity, we analyzed for ALT and AST levels in mouse serum. The serum ALT level in mHAd.sT- or mHAdLyp.sT-treated mice was significantly lower than that in the corresponding Ad.sT or AdLyp.sT group (Fig. 4A). Since ALT is found primarily in the liver and any elevation of the ALT is a direct indication of viral-induced liver injury, our findings indicated significantly reduced hepatotoxicity of mHAd.sT or mHAdLyp.sT. The average serum AST levels in both Ad.sT and AdLyp.sT groups were also increased compared with the buffer, mHAd.sT-, or mHAdLyp.sT-treated mice. However, these AST levels were not significantly different (Fig. 4B). It is probably because (i) unlike ALT, AST is found in a variety of tissues, including the liver, brain, pancreas, heart, kidneys, lungs, and skeletal muscles; thus, although AST levels are indicative of tissue injury, they are not specific to the liver per se; (ii) compared with ALT, AST is short-lived and has half-life of less than 16 h, and (iii) it is also likely that those adenoviruses could potentially be taken up by other tissues, which can also produce AST. Taken together, these results indicate that systemic administration of mHAd.sT or mHAdLyp.sT could specifically reduce hepatic damage.
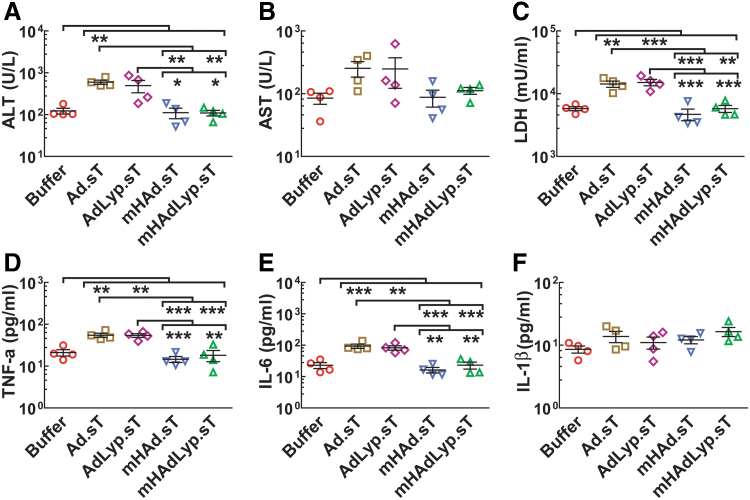
Figure 4.
Systemic administration of mHAd.sT and mHAdLyp.sT in nude mice produced reduced hepatic and systemic toxicity, and reduced proinflammatory cytokine responses. (A) Serum ALT levels, (B) serum AST levels, (C) serum LDH levels, (D) serum TNF-α levels, (E) serum IL-6 levels, and (F) serum IL-1β levels n = 4 in each group. One-way ANOVA followed by Bonferroni's multiple comparison test was used to calculate p-value for each set of the experiments. *p < 0.05, **p < 0.01, and ***p < 0.001. ALT, alanine transaminase; AST, aspartate transaminase; IL, interleukin; LDH, lactate dehydrogenase; TNF-α, tumor necrosis factor-α.
Next, we examined the serum LDH, TNF-α, IL-6, and IL-1β levels in 72-h samples. There was a significant increase of LDH levels in the Ad.sT- and AdLyp.sT-treated groups, suggesting that AdLyp.sT is similar to Ad.sT in causing cell death and tissue damages. However, the corresponding modified Ad5/48 hexon viruses, mHAd.sT and mHAdLyp.sT, produced much reduced cytotoxicity. In fact, LDH levels in mHAd.sT- and mHAdLyp.sT-treated groups were similar to the buffer group 72 h after the virus injections (Fig. 4C).
Similar results were obtained regarding the serum levels of TNF-α and IL-6, two proinflammatory cytokines that are closely associated with innate inflammation, suggesting attenuated inflammatory responses induced by mHAd.sT and mHAdLyp.sT (Fig. 4D, E). We did not detect the significant changes of another proinflammatory cytokine, IL-1β, among these groups (including the buffer group) (Fig. 4F). This is consistent with the previous findings that following systemic administration of Ad5, serum IL-1β levels reach peak within half an hour, in the period preceding the rise in TNF-α and IL-6 in an innate inflammation response.32
Systemic administration of LyP-1-modified AdLyp.sT and mHAdLyp.sT viruses inhibits bone metastases in a human MDA-MB-231 experimental metastasis model
Next, we investigated if following the systemic delivery of Ad.sT, AdLyp.sT, mHAd.sT, or mHAdLyp.sT, breast cancer metastasis can be inhibited in a mouse experimental metastasis model. MDA-MB-231-luc2 cells were inoculated in the left heart ventricle of female nude mice to produce skeletal metastases. Viral vectors were administered intravenously on day 8 and 12. Four animal groups received an LTD (two doses, 2.5 × 1010 VPs/mouse each, a total of 5 × 1010 VPs/mouse) of each virus. Given the superior safety profile of Ad5/48 chimeric hexon viruses shown in Figs. 3 and 4, two groups were treated with HTD (two doses, 1.0 × 1011 VPs/mouse each, a total of 2.0 × 1011 VPs/mouse) of mHAd.sT or mHAdLyp.sT.
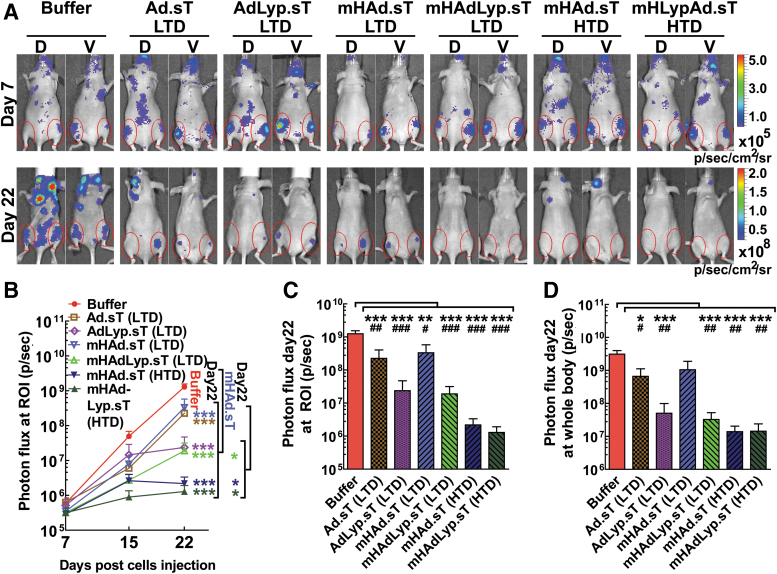
In our laboratory, previously we had conduced dose dependence toxicity studies for Ad.sT and mHAd.sT in immune-deficient mice. Those studies had guided us to use the LTD and HTD amounts used in this study. To minimize the number of mice in our current experiment, we therefore decided to use only LTD and HTD as they do provide us with a range and guide as to the toxicity, safety, and therapeutic profile of our viruses. Similar to the results shown earlier (Figs. 3 and 4), Ad.sT and AdLyp.sT resulted in significant hepatic and systemic toxicity; therefore, the HTD of Ad.sT and AdLyp.sT was not administered. Mice were subjected to BLI on days 7, 15, and 22 to monitor tumor growth over time. Figure 5A shows BLI of a representative mouse from each group at day 7 and 22. The red circles indicate the ROIs that are identified as skeletal metastases in hind limbs, as described in our previous publications.16,27
Figure 5.
Systemic administration of low dose of AdLyp.sT and high dose of mHAd.sT or mHAdLyp.sT enhanced inhibitory activity against MDA-MB-231 bone metastasis as measured by the BLI of the hind limbs and whole body. (A) Representative whole-body dorsal and ventral BLI images on day 7 and 22 in buffer and each treatment group are shown. BLI signals in the hind limbs are pointed out with red circles (ROI), indicating bone metastases. (B) Bone metastases at ROI were quantified in buffer and each treatment group, and BLI photon flux progressions are shown. (C) Bone metastases at ROI on day 22 by BLI photon flux in buffer and each treatment group are shown. (D) Whole-body BLI photon fluxes on day 22 in buffer and each treatment group are shown. n = 10 in each group. Data are plotted as mean ± SEM. Two-way ANOVA (over time course) or one-way ANOVA followed by Bonferroni's tests was used to calculate p-value for (B–D); *p < 0.05, **p < 0.01, and ***p < 0.001. The Student's t-tests between buffer and each treatment group were also performed for (C, D); #p < 0.05, ##p < 0.01, and ###p < 0.001. BLI, bioluminescence imaging; ROI, regions of interest; SEM, standard error of the mean.
The BLI signal intensity of combined dorsal and ventral ROIs in each mouse was quantified. In the buffer-treated group, there was a time-dependent increase in BLI signals (Fig. 5B). All five treatment groups exhibited significant reductions of signal intensities in these ROIs on day 22 when compared with the buffer group by two-way ANOVA followed by Bonferroni post hoc tests adjusting for multiplicity (p < 0.001, n = 10). Moreover, three treatment groups (the LTD of mHAdLyp.sT, the HTD of mHAd.sT, and the HTD of mHAdLyp.sT) exhibited significant reductions compared with the LTD of mHAd.sT on day 22 by two-way ANOVA (p < 0.05). We further analyzed BLI photon flux on ROIs by one-way ANOVA and Student's t-test on day 22 samples (Fig. 5C). Four treatment groups (the LTD of AdLyp.sT, the LTD of mHAdLyp.sT, the HTD of mHAd.sT, and the HTD of mHAdLyp.sT) exhibited similar statistical significance against the buffer-treated group (p < 0.001 in both one-way ANOVA and t-test, Fig. 5C). This result suggested that the low doses of Lyp-1-modified viruses (AdLyp.sT and mHAdLyp.sT) and the high doses of liver detargeted viruses (mHAd.sT and mHAdLyp.sT) were all more effective in inhibiting the skeletal tumor growth compared with the low doses of unmodified virus (Ad.sT) and liver detargeted virus (mHAd.sT).
Since MDA-MB-231-luc2 cells can also metastasize to the liver, lungs, and brain, we analyzed whole-body BLI photon flux on day 22. Again, four treatment groups (the LTD of AdLyp.sT, the LTD of mHAdLyp.sT, the HTD of mHAd.sT, and the HTD of mHAdLyp.sT) exhibited similar statistical significance against the buffer-treated group (p < 0.001 in one-way ANOVA and p < 0.01 in t-test, Fig. 5D). These results further indicated that Lyp-1-modified viruses have enhanced inhibitory effect against breast cancer metastases in the mouse experimental model.
To further examine the effect of Lyp-1-modified viruses to inhibit bone metastases, we also preformed radiographic analyses on day 15 and 22. Figure 6A shows representative X-ray images on day 22 from each group. Osteolytic lesions on the skeletal tumors are marked by yellow arrows. Lesion sizes in the hind limb bones for each mouse were quantified by using ImageJ software. Compared with the buffer group, a significant inhibition of lesion size progression was observed for each of the treatment groups on day 22 (p < 0.001 in two-way ANOVA followed by Bonferroni post hoc tests adjusting for multiplicity, n = 10, Fig. 6B). On day 15, only the HTD of mHAdLyp.sT exhibited significant inhibition (p < 0.05). Also, both the HTD groups of mHAdLyp.sT and mHAd.sT were more effective in inhibiting lesion size progression on day 22 than the LTD of mHAd.sT (p < 0.01). These results suggested that the HTD of mHAdLyp.sT is most effective in inhibiting bone metastases, followed by the HTD of mHAd.sT, and the LTD of mHAd.sT is least effective in this analysis.
Figure 6.
Effects of adenoviral vectors on MD-MB-231 tumor burden: radiographic, blood, and body weight analysis. (A) Representative radiographs of mouse hind limbs on days 22 in the buffer and each treatment group are shown. Yellow arrows indicate the sites of osteolytic lesions. (B) Lesion sizes in the hind limb bones for each mouse during the duration of the study were calculated using ImageJ software. Results shown are the average lesion area in the hind limbs in buffer group and each of the treatment groups. (C) Lesion sizes in the hind limb bones on day 22 were calculated and are shown. (D) Bone metastasis-free incidences (mice without X-ray-positive lesions) on day 22 are shown. (E) Mice body weights during the duration of the study are shown. (F) Serum TRACP 5b concentration on day 22 in units per liter. (G) Serum samples collected on day 22 were used to measure calcium levels. n = 10 in each group. Data are plotted as the mean ± SEM. Two-way ANOVA (over time course) or one-way ANOVA followed by Bonferroni's tests was used to calculate p-value for (B, C, E, F, G); a Fisher's exact test was used for the bone metastasis incidence data; *p < 0.05, **p < 0.01, and ***p < 0.001. For (C, F, G), p-value comparisons between two groups by Student's t-tests are also shown; #p < 0.05, ##p < 0.01, and ###p < 0.001.
When we used one-way ANOVA and paired t-test to further analyze day 22 data (Fig. 6C), we found that only the LTD of mHAd.sT showed a significant difference compared with the buffer group in t-test (p < 0.05), while all the other treatment groups showed a much higher significant difference (p < 0.001). Also, only the HTD of mHAdLyp.sT produced an antitumor response that was significantly better than the LTD of mHAd.sT on day 22 (p < 0.05). Indeed, in the group treated with the HTD of mHAdLyp.sT, 8 out of 10 (80%) mice were free of osteolytic lesions in hind limbs (p < 0.001, compared with the buffer group, Fig. 6D). This inhibitory effect is better than that observed in the HTD of mHAd.sT or any of the other two LTD of LyP-1-modified viruses (p < 0.05, compared with the buffer group). The LTD of Ad.sT or mHAd.sT did not exhibit a significant difference from the buffer groups in terms of the number of skeletal tumor-free mice. Thus, among all the treatment groups, the HTD of mHAdLyp.sT is the most effective modality to combat breast cancer bone metastases in this model.
We also monitored the animal body weights once a week, as an indicator of cancer cachexia. On day 22, the average body weight of the buffer group mice dropped 22% from that on day 15; the average body weight was 17.68 g on day 22 versus 22.46 g on day 15 (Fig. 6E). It was significantly lower than that of all treatment groups and normal group (naive mice without tumors) (the HTD of mHAdLyp.sT and mHAd.sT, and normal: p < 0.001; all other treatment groups: p < 0.01; Fig. 6E). Of note, there was no significant difference in the day 22 body weight between the normal group and the HTD of mHAdLyp.sT or mHAd.sT. These results suggested that HTD treatments with the two liver detargeted viruses performed the best in preventing cancer cachexia.
To further investigate the effect of the HTD of mHAdLyp.sT to inhibit tumor-induced osteolytic bone destruction, we measured two biomarkers in mouse serum. Serum tartrate-resistant acid phosphatase 5b (TRACP 5b) is considered to be a good indicator of the osteoclastic bone resorption and turnover. Two treatment groups, the LTD of AdLyp.sT and the HTD of mHAdLyp.sT, as well as the normal group, have significantly lower serum TRACP 5b levels compared with that of the buffer group (p < 0.05 in one-way ANOVA; p < 0.001 in t-test; Fig. 6F).
Since osteolytic bone destruction results in hypercalcemia, the serum calcium levels were also measured. When compared with the buffer group by t-test, the HTD of mHAdLyp.sT and mHAd.sT both lowered calcium levels (p < 0.001; Fig. 6G) and was statistically similar to the age-matched normal mice (p < 0.001). By one-way ANOVA, the HTD of mHAdLyp.sT had the most significant reduction (p < 0.05) among all the treatment groups. When compared with either the normal group or the least potent treatment group (the LTD of mHAd.sT), the HTD of mHAdLyp.sT also performed best among all treatment groups (Fig. 6G). Taken together, our data support the conclusion that the HTD of mHAdLyp.sT is the most effective treatment in suppressing bone metastasis in this experimental mouse model.
Intratumoral delivery of AdLyp.sT improves the antitumor effects of Ad.sT and anti-PD-1 and anti-CTLA-4 immunotherapy in a highly aggressive 4T1 syngeneic immunocompetent mouse model
We have recently shown that in the 4T1 syngeneic mouse model, intratumoral inoculation of an oncolytic adenovirus expressing sTGFβRIIFc (rAd.sT) can significantly improve the inhibition of tumor growth and metastases by anti-PD-1 and anti-CTLA-4 antibodies. In rAd.sT, the viral replication is regulated by the human telomerase reverse transcriptase promoter (TERTp). In the oncolytic adenoviruses investigated in this study, Ad.sT and AdLyp.sT, a more robust and general promoter, CMV, drives the sTGFβRIIFc gene expression. Thus, in this study, we investigated the effects of intratumoral delivery of Ad.sT and AdLyp.sT in inhibiting tumor growth and metastases alone and in combination with ICIs. It is known that 4T1 tumors are resistant to anti-PD-1 and anti-CTLA-4 therapy,33–36 and our unpublished data (not shown here) have also confirmed that observation.
Moreover, in a preliminary study using intratumoral delivery of Ad.sT, our combination therapy with a single ICI agent, anti-PD-1 or anti-CTLA-4, did not produce significant improvement in the antitumor response (data not shown). Unfortunately, we did not conduct immunotherapy using a combination of AdLyp.sT and single ICI. Although anti-PD-1 and anti-CTLA-4 produce significant inhibition of tumor growth and metastases, we do not observe complete eradication of the tumors or metastases by this combination (Fig. 7). Therefore, we were very interested to further improve anti-PD-1 and anti-CTLA-4 in TNBC models such as the 4T1 tumor model.
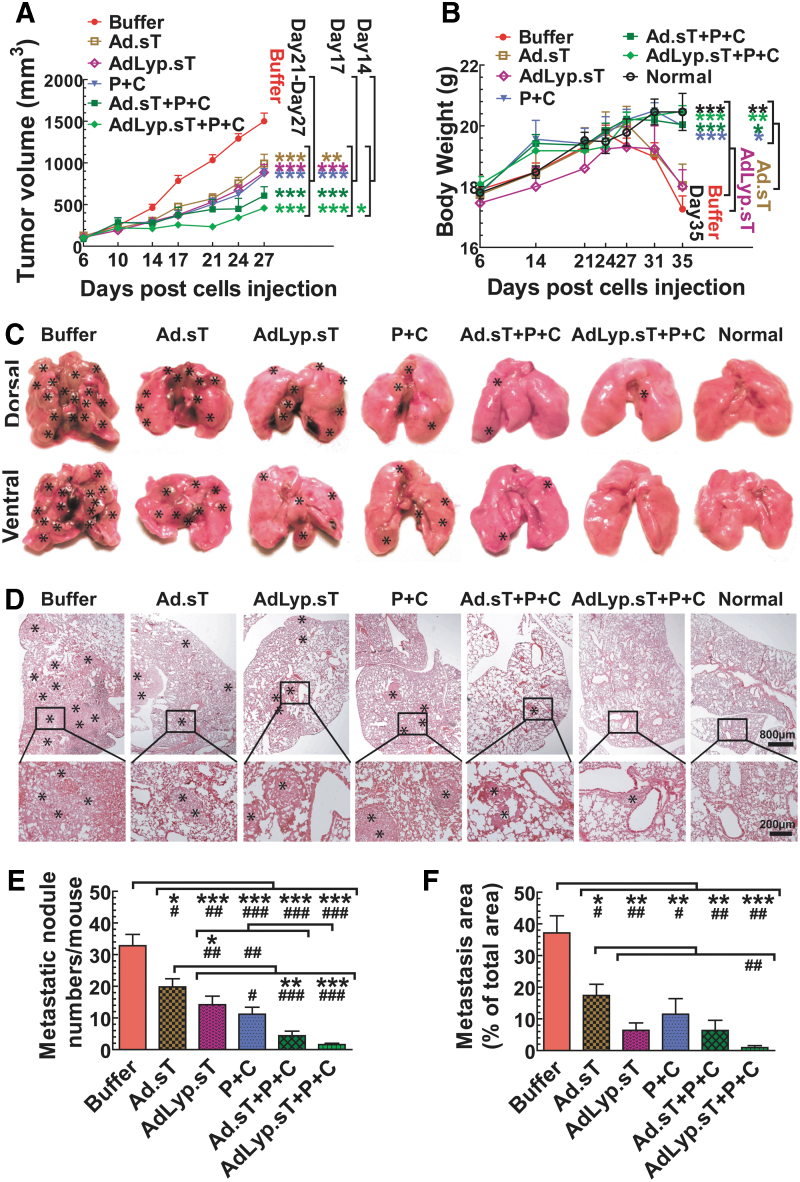
Figure 7.
Intratumoral delivery of Ad.sT and AdLyp.sT enhanced the immune checkpoint inhibitor therapy on 4T1 tumor growth and spontaneous metastasis in the syngeneic mouse model. (A) Progression of primary tumor sizes measured by using calipers in the buffer and each treatment group is shown. (B) Mice body weights during the duration of the study are shown. (C) Representative images of the gross appearances of lungs at the end of the study are shown. *Indicates the site of lung macrometastases. (D) Representative images of H&E-stained lung sections of the buffer and each treatment group are shown. *Indicates the site of lung micrometastases and macrometastases. Magnification is indicated by scale bars. (E) Visible metastatic foci on the lung surface were counted by gross examination and shown. (F) Pulmonary metastases were also determined by microscopic inspection, and the percentage of metastasis area was quantified using ImageJ software. n = 5 for each group. Data are plotted as the mean ± SEM. Two-way ANOVA (over time course) or one-way ANOVA followed by Bonferroni's tests was used to calculate p-value for (A, B, E, F). *p < 0.05, **p < 0.01, and ***p < 0.001. For (E, F), p-value comparisons between two groups by Student's t-tests are also shown; #p < 0.05, ##p < 0.01, and ###p < 0.001.
4T1 tumors were established in BALB/c mice, and treated with Ad.sT or AdLyp.sT and anti-PD-1/anti-CTLA-4, either alone or in combinations, as described in the Materials and Methods section. Primary tumor growth was followed over time till day 27. In this model, from day 27 onward, severe ulcerations are produced in some of the primary tumors, and therefore, tumor volume readings beyond day 27 are less reliable. There was significant inhibition of tumor growth on day 21, 24, and 27 in mice treated with either Ad.sT or AdLyp.sT and/or anti-PD-1/anti-CTLA-4, compared with the buffer group (p < 0.001) (Fig. 7A). However, only the triple therapy combination of AdLyp.sT and anti-PD-1/anti-CTLA-4 produced significant inhibition on day 14 (p < 0.05). These results suggested that the combination of AdLyp.sT and anti-PD-1/anti-CTLA-4 was the most effective approach. On day 17, Ad.sT alone was effective (p < 0.01 compared with the buffer), however, all other treatment groups produced better inhibition of tumor growth (p < 0.001).
All mice were euthanized on day 35, by which time some of the mice had reached the level 2 of the body condition score. The body weights of Ad.sT- or AdLyp.sT-treated mice were reduced significantly by day 35 when compared with the combinations of anti-PD-1/anti-CTLA-4, or anti-PD-1/anti-CTLA-4 alone, or normal mice (p < 0.01 or p < 0.05, Fig. 7B). Since loss of body weight is an indicator of cancer cachexia and poor survival during the late stage of 4T1 tumor progression, we concluded that the combination of AdLyp.sT and anti-PD-1/anti-CTLA-4 was also most effective in preventing cancer cachexia and poor survival (p < 0.01, compared with Ad.sT or AdLyp.sT alone on day 35).
Next, we examined the effects of our therapy modalities on the spontaneous lung metastasis, as lung is the predominant metastatic site in this 4T1 mouse model. At the end of this study, mice were euthanized and all visible superficial lung metastatic foci from each mouse were photographed and counted by gross examination (Fig. 7C, E). There was significant inhibition of the numbers of macrometastases on the lung surface in all the treatment groups compared with the buffer group (p < 0.001, p < 0.01, or p < 0.05 by either one-way ANOVA or t-test; Fig. 7E). However, further analysis of data showed that only the combination of AdLyp.sT and anti-PD-1/anti-CTLA-4 has p < 0.001 in both one-way ANOVA and t-test when compared with the buffer group or Ad.sT alone, the weakest treatment group. Thus, the combination of AdLyp.sT and anti-PD-1/anti-CTLA-4 was also the most effective in inhibiting lung macrometastases.
We further conducted histological examination of lung metastases (Fig. 7D). The total lung micrometastatic burden in each group was determined as described in the Materials and Methods section (Fig. 7F). Again, while all the treatment groups significantly inhibited the lung micrometastatic burden compared with the buffer group (p < 0.001, p < 0.01, or p < 0.05 by either one-way ANOVA or t-test; Fig. 7F), the combination of AdLyp.sT and anti-PD-1/anti-CTLA-4 was the most effective (p < 0.001 by one-way ANOVA). When compared with Ad.sT alone by t-test, the combination of AdLyp.sT and anti-PD-1/anti-CTLA-4 also produced significant inhibition (p < 0.01, Fig. 7F). These results indicated that in the 4T1 tumor model, intratumoral inoculation of AdLyp.sT can significantly improve anti-PD-1+anti-CTLA-4-mediated inhibition of tumor metastasis.
Discussion
We are reporting here the construction of two novel oncolytic adenoviruses—AdLyp.sT and mHAdLyp.sT. These oncolytic adenoviruses express sTGFβRIIFc, a soluble TGFβ decoy that can bind with TGFβ-1 and thus can inhibit aberrant TGFβ signaling associated with breast cancer. Both these viruses express LyP-1, a tumor homing peptide inserted in the HI loop of the fiber, and can specifically target breast cancer cells.
A unique feature of mHAdLyp.sT is that HVRs 1–7 of Ad5 hexon were replaced by the corresponding Ad48 HVRs. Because of this genetic modification, systemic administration of mHAdLyp.sT bypasses the liver uptake and produces minimum hepatic/systemic toxicity. Our studies described here have shown that both AdLyp.sT and mHAdLyp.sT elicited strong antitumor and antimetastasis responses in the human breast cancer immunodeficient mouse model. These antitumor responses were in general superior to Ad.sT and mHAd.sT, the corresponding unmodified viruses that do not express the LyP-1 peptide. In addition, because mHAdLyp.sT can be administered systemically with the HTD, the best inhibitory effect was observed with this modality. In this study, we have also shown that intratumoral delivery of AdLyp.sT was effective in inhibiting the tumor growth and metastasis, and enhanced ICI therapy in an immunocompetent mouse tumor model.
TGFβ as a target for breast cancer therapy
In the recent years, there have been significant advances in understanding the key regulators of the TME and immune system. TGFβ is one of the key factors that have a direct influence on tumor evasion of immune response and in promoting tumor growth and metastasis. The outcome of the TGFβ signaling pathway is dependent on the cell and tissue context. In invasive tumors, TGFβ singling plays the oncogenic role and promotes tumor progression by several mechanisms. These include activating EMT and stimulating angiogenesis.8,37 TGFβ can also recruit myeloid-derived suppressor cells, Tregs, and M1 macrophages, known to induce immunosuppression.7 In addition, TGFβ can destroy the production and functions of immune cells such as NK cells, dendritic cells, and CD8+ CTLs that are critical in producing antitumor responses.8,38 Thus, over the years, several TGFβ inhibitors, including receptor kinase inhibitors, antiligand antisense oligonucleotides, and monoclonal antibodies, have been explored to treat advanced tumors both in the preclinical setting and in the clinical trials in cancer patients. However, till to date, none of the anti-TGFβ inhibitors has been approved by the FDA for clinical usage.
To block the TGFβ pathway, we have previously constructed oncolytic adenoviruses that express sTGFβRIIFc. We have reported that intratumoral administration of one of these viruses, rAd.sT, can inhibit TGFβ-regulated genes such as CTGF, CXCR4, IL-11, PTHrP, N-cadherin, vimentin, VEGFA, and VEGFR in 4T1 tumors established in the syngeneic mouse.18 These TGFβ targets are well-documented inducers/markers of tumor growth, EMT, angiogenesis, and metastases. Moreover, rAd.sT produced a favorable shift to the production and recruitment of antitumor immunomodulatory factors and immune cells in the mouse tumors, peripheral blood, and spleen, suggesting a systemic immune activation directed toward 4T1 tumors.18 Thus, our approach of targeting aberrant TGFβ signaling is quite effective in disrupting the protumorigenic TME and overcoming immune tolerance.
The results presented in this study further document that our sTGFβRIIFc expressing oncolytic viruses elicit strong inhibitory effects in a human breast cancer metastasis model in immune deficient mice. Although we did not conduct bioactivity results of vector-induced sTGFβRIIFc expression in this study, previous studies from our laboratory as well as from others have documented the biological effects of sTGFβRIIFc.18,28,39,40 Furthermore, our oncolytic viruses are also effective in inhibiting mouse mammary tumor growth and its spontaneous metastasis to the lungs in a syngeneic mouse tumor model.
Viral replication: an added advantage of using oncolytic adenoviruses for targeting TGFβ pathways
Although several approaches to target TGFβ are being examined in many laboratories, we believe that one advantage of using oncolytic adenoviruses expressing sTGFβRIIFc is that in addition to inhibiting TGFβ pathways, viral replication will directly cause tumor cell death. Whether the viruses are delivered intratumorally or via systemic route (as described in our studies here), viral uptake into the tumors will produce viral replication within the tumors, cause tumor cell death, release tumor antigens, and activate dendritic cells and tumor-specific cytotoxic immune cell responses.41,42 Thus, our oncolytic viruses AdLyp.sT and mHAdLyp.sT can produce antitumor responses by targeting multiple steps—including targeting tumor cells, TME, and activating the immune system, both locally and systemically.
One point regarding the differences in the response to oncolytic adenoviruses by mouse and human cancer cells is worth discussing here. Because our oncolytic viruses do not exhibit significant replication in mouse cells (including 4T1 cells), while the effect on adenovirus binding and hence transgene expression can be enhanced by LyP-1 modification, no significant improvements in the viral replication were observed by LyP-1-modified viruses in 4T1 cells. However, oncolytic viruses do replicate in human cancer cells, and LyP-1-modified viruses bind better with human breast cancer cells; therefore, LyP-1-modified viruses produce both enhanced viral replication and transgene expression in human cancer cells.
Addressing key barriers in the systemic delivery of oncolytic adenoviruses
In this study, we have attempted to address the key barriers in the use of oncolytic adenoviruses—massive systemic toxicity induced by the systemic therapy of oncolytic adenoviruses, the prevalence of preexisting adenovirus neutralizing Abs, and the lack of their tumor specificity.16,26,43,44 Regarding systemic toxicity, it is known that the commonly used Ad5-based viruses produce toxicity mainly from viral sequestration and destruction by the liver Kupffer cells. To address this issue, we have created the hexon chimeric Ad5/Ad48 virus (mHAdLyp.sT). Since Ad48 hexon HVRs 1–7 have much reduced factor X binding, the Ad5/Ad48 chimeric hexon virus will have attenuated hepatic uptake.22–27 In fact, we have shown here that mHAdLyp.sT indeed has the diminished capacity of liver sequestration and produces reduced hepatotoxicity and decreased systemic toxicity.
Although we have documented that in immune-deficient mice, the liver detargeted viruses have reduced uptake in the liver as measured by viral DNA copy numbers (Fig. 3C), in future, we will also conduct these distribution studies in multiple tissues in more relevant immunocompetent mouse models. Also, because functionally significant Ad5-specific neutralizing antibodies are directed primarily against the HVRs of Ad5 hexon in both human and mouse, the hexon chimeric Ad5/Ad48 virus can circumvent pre-existing antivector immunity.24,25,45,46
To address the issue of tumor tropism, our new virus mHAdLyp.sT described in this study has LyP-1 peptide-enhanced breast tumor tropism. As shown, mHAdLyp.sT exhibits strong antitumor effects in the experimental bone metastasis model. Moreover, because of the improved safety profile, we could also administer a higher therapeutic dose of mHAdLyp.sT further improving its efficacy in targeting metastases. LyP-1-modified mHAdLyp.sT virus will target the LyP-1 receptor, which is expressed on the cancer cells, tumor macrophages, and tumor lymphatics, but not on the normal tissues.19,20,47 Thus, in the potential future clinical trials in breast cancer, the systemic administration of LyP-1-modified Ad5/48 chimeric hexon-based mHAdLyp.sT is expected to have attenuated hepatic uptake causing reduced hepatic/systemic toxicity, but will have enhanced tropism for the breast tumors.
mHAdLyp.sT has two levels of tumor selective targeting/replication. First, the virus will target the tumor cells and the tumor lymphatics via LyP-1, a tumor-specific peptide enabling it to selectively target the breast tumors. Second, the viral backbone used to construct the oncolytic mHAdLyp.sT vector selectively replicates in the tumor cells, producing tumor lyses. Upon the arrival of mHAdLyp.sT in the tumors, large amounts of sTGFβRIIFc, the TGFβ decoy will be produced in the tumors. sTGFβRIIFc will be also secreted into the blood stream. sTGFβRIIFc will bind with TGFβ to inhibit aberrant TGFβ signaling, relieving TGFβ-induced immune suppression. Thus, mHAdLyp.sT will target tumor cells as well as remodel tumor stroma potentially enhancing T cell trafficking into the tumors. It should be also noted that in LyP-1-modified viruses, the LyP-1 peptide is generically linked with the H1 lops of fiber; we do not believe that the LyP-1 peptide can be dissociated from the fiber when it is being formed in the cytoplasm, as large amounts of LyP-1 viruses can be grown in the cell culture.
Another point worth noting is that following systemic delivery of oncolytic viruses, the level of transgene expression can be also possibly impacted by additional factors, including vector-induced cytotoxicity in the infected tissues. For example, while Ad5-based viruses have increased uptake in the liver and spleen, due to vector-induced toxicity, these vectors tend to destroy these tissues, thus potentially reducing sTGFβRIIFc production in the blood. Conversely, this will be true for liver detargeted viruses—because they have reduced uptake in the liver, could be more stable in those resident tissues, and continue to produce sTGFβRIIFc in the blood. Therefore, following adenoviral administrations, the amount of sTGFβRIIFc in the blood is somewhat difficult to interpret. Despite that, as shown in Fig. 3D, Ad5-based viruses do indeed produce about five times more protein in the blood (about 100 μg/mL), compared with Ad5/48-based viruses, which produce about 20 μg/mL in the blood. It is true that liver detargeted viruses have reduced uptake in the liver, which appear dramatically different than the Ad5-based viruses (Fig. 3A). However, it should be noted that liver detargeted viruses also produce reduced levels of sTGFβRIIFc in the blood (about fivefold), although it does not appear as dramatic (perhaps due to log scale used in Fig. 3D).
Targeting TGFβ to overcome resistance to anticheckpoint inhibitor therapy
In recent years, ICIs, including anti-PD-1, anti-PD-L1, and anti-CTLA-4, have shown promise as antitumor agents, and are approved for the treatment of several malignancies.48–51 Clinical trials in breast cancer patients have shown that about 20% of TNBC responded favorably to anti-PD-1 antibodies.52–55 However, many TNBC patients are resistant to anti-PD-1/PD-L1 and anti-CTLA-4 treatments.52–55 Therefore, there is a significant interest in exploring novel therapeutic approaches to improve anti-PD-1 and anti-CTLA-4 therapies for TNBC. Aberrant TGFβ signaling is one of the key factors in producing resistance to ICI therapy.56
In this study, IHC analysis of the patient tumor samples showed that elevated stromal TGFβ-1 was associated with more aggressive TNBC phenotype and poor OS, indicating resistance to the current therapies. As discussed above, we believe that aberrant TGFβ signaling can produce a TME that is resistant to ICI therapy. So far, the only approved ICI therapy for TNBC is the combination of anti-PD-L1 antibody (atezolizumab) and nab-paclitaxel (Abraxane) for PD-L1-positive, unresectable, locally advanced, or metastatic TNBC patients.57 However, TNBC patients with low levels of PD-L1 or even some patients with high PD-L1 are resistant to this combination therapy.57 In that regard, our results that AdLyp.sT can augment anti-PD-1 and anti-CTLA-4 responses in a poorly immunogenic and ICI-resistant 4T1 tumor model33–36 are quite important.
In this initial study using our novel oncolytic viruses, we have focused primarily on describing their safety, toxicity, and therapeutic profile. In our next series of experiments, we will conduct additional studies to document intratumoral gene expression and to define the key factors in more relevant immunocompetent models as shown previously for the unmodified oncolytic virus rAd.sT.18 We will also examine the effect of intravenous delivery of our oncolytic adenoviruses in improving the ICI therapy in immunocompetent mouse models. In the future, we also plan to elucidate the underlying mechanisms how AdLyp.sT and ICIs work collectively to convert immunologically cold tumors to into hot ones, thus activating the immune system to destroy cancer cells.
AdLyp.sT and mHAdLyp.sT have the potential of targeting several other malignancies
Although in this study we have focused on targeting TNBC, it is worth noting that many other cancers could also benefit from our LyP-1-modified oncolytic virus approach targeting TGFβ signaling. LyP-1 receptor has been shown to be overexpressed in many cancers, including breast, prostate, melanoma, glioblastomas, pancreas, and in general is considered an excellent target for cancer therapy.19–21,47 Likewise, aberrant TGFβ signaling has also been shown to promote tumor growth and metastases of many cancers, including prostate, kidney, and gastrointestinal cancers.58,59 Therefore, we believe that our targeted oncolytic viral approach described here has the potential of an excellent alternative to these ongoing approaches9–14,60,61 to target multiple cancers and to improve ICI therapies.
In conclusion, based on our studies described here, we believe that both AdLyp.sT and mHAdLyp.sT can be further developed as promising treatment strategies to control metastatic breast cancer as well as to augment immunotherapy.
Supplementary Material
ACKNOWLEDGMENTS
We are thankful to the Kovler Family Foundation, Mr. and Mrs. Richard Hulina, Mr. Jimmie Alford and Ms Maree Bullock, Maxine and James Farrell, Carol Gollob Foundation, and an anonymous donor for their generous gifts. We are thankful to Drs. Bruce Brockstein, Michael Caplan, Janardan Khandekar, Charles Brendler, and Ted Mazzone for their continuous support.
AUTHOR DISCLOSURE
No competing financial interests exist for any of the authors.
FUNDING INFORMATION
The research was funded, in part, by grants from the Department of Defense Breast Cancer Research Program Award DAMAD17-03-0703, the National Cancer Institute grant R01CA127380, Cancer Gene Therapy funds from NorthShore, and the NorthShore Auxiliary Breast Cancer Research award to Prem Seth.
Supplementary Material
References
- 1. Cancer Facts and Statistics. American Cancer Society. www.cancer.org/research/cancerfactsstatistics/index (last accessed June05, 2020)
- 2. Kimbung S, Loman N, Hedenfalk I. Clinical and molecular complexity of breast cancer metastases. Semin Cancer Biol 2015;35:85–95 [DOI] [PubMed] [Google Scholar]
- 3. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019;321:288–300 [DOI] [PubMed] [Google Scholar]
- 4. Diana A, Franzese E, Centonze S, et al. Triple-negative breast cancers: systematic review of the literature on molecular and clinical features with a focus on treatment with innovative drugs. Curr Oncol Rep 2018;20:76. [DOI] [PubMed] [Google Scholar]
- 5. de Kruijf EM, Dekker TJA, Hawinkels LJAC, et al. The prognostic role of TGF-β signaling pathway in breast cancer patients. Ann Oncol 2012;24:384–390 [DOI] [PubMed] [Google Scholar]
- 6. Ivanovic V, Todorovic-Rakovic N, Demajo M, et al. Elevated plasma levels of transforming growth factor-beta 1 (TGF-beta 1) in patients with advanced breast cancer: association with disease progression. Eur J Cancer 2003;39:454–461 [DOI] [PubMed] [Google Scholar]
- 7. Batlle E, Massague J. Transforming growth factor-beta signaling in immunity and cancer. Immunity 2019;50:924–940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ahmadi A, Najafi M, Farhood B, et al. Transforming growth factor-beta signaling: tumorigenesis and targeting for cancer therapy. J Cell Physiol 2019;234:12173–12187 [DOI] [PubMed] [Google Scholar]
- 9. Courau T, Nehar-Belaid D, Florez L, et al. TGF-beta and VEGF cooperatively control the immunotolerant tumor environment and the efficacy of cancer immunotherapies. JCI Insight 2016;1:e85974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ravi R, Noonan KA, Pham V, et al. Bifunctional immune checkpoint-targeted antibody-ligand traps that simultaneously disable TGFbeta enhance the efficacy of cancer immunotherapy. Nat Commun 2018;9:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Loffek S. Transforming of the tumor microenvironment: implications for tgf-beta inhibition in the context of immune-checkpoint therapy. J Oncol 2018;2018:9732939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mariathasan S, Turley SJ, Nickles D, et al. TGFbeta attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature 2018;554:544–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmgaard RB, Schaer DA, Li Y, et al. Targeting the TGFbeta pathway with galunisertib, a TGFbetaRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. J Immunother Cancer 2018;6:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jiao S, Subudhi SK, Aparicio A, et al. Differences in tumor microenvironment dictate T helper lineage polarization and response to immune checkpoint therapy. Cell 2019;179:1177–1190.e1113. [DOI] [PubMed] [Google Scholar]
- 15. Seth P, Wang ZG, Pister A, et al. Development of oncolytic adenovirus armed with a fusion of soluble transforming growth factor-beta receptor II and human immunoglobulin Fc for breast cancer therapy. Hum Gene Ther 2006;17:1152–1160 [DOI] [PubMed] [Google Scholar]
- 16. Hu Z, Gerseny H, Zhang Z, et al. Oncolytic adenovirus expressing soluble TGFbeta receptor II-Fc-mediated inhibition of established bone metastases: a safe and effective systemic therapeutic approach for breast cancer. Mol Ther 2011;9:1609–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu Z, Gupta J, Zhang Z, et al. Systemic delivery of oncolytic adenoviruses targeting transforming growth factor-beta inhibits established bone metastasis in a prostate cancer mouse model. Hum Gene Ther 2012;23:871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang Y, Xu W, Peng D, et al. An oncolytic adenovirus targeting transforming growth factor beta inhibits protumorigenic signals and produces immune activation: a novel approach to enhance anti-PD-1 and anti-CTLA-4 therapy. Hum Gene Ther 2019;30:1117–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Laakkonen P, Porkka K, Hoffman JA, et al. A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat Med 2002;8:751–755 [DOI] [PubMed] [Google Scholar]
- 20. Fogal V, Zhang L, Krajewski S, et al. Mitochondrial/cell-surface protein p32/gC1qR as a molecular target in tumor cells and tumor stroma. Cancer Res 2008;68:7210–7218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Song N, Zhao L, Zhu M, et al. Recent progress in LyP-1-based strategies for targeted imaging and therapy. Drug Deliv 2019;26:363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Waddington SN, McVey JH, Bhella D, et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 2008;132:397–409 [DOI] [PubMed] [Google Scholar]
- 23. Kalyuzhniy O, Di Paolo NC, Silvestry M, et al. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc Natl Acad Sci U S A 2008;105:5483–5488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Roberts DM, Nanda A, Havenga MJ, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature 2006;441:239–243 [DOI] [PubMed] [Google Scholar]
- 25. Bradley RR, Maxfield LF, Lynch DM, et al. Adenovirus serotype 5-specific neutralizing antibodies target multiple hexon hypervariable regions. J Virol 2012;86:1267–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Z, Krimmel J, Hu Z, et al. Systemic Delivery of a novel liver-detargeted oncolytic adenovirus causes reduced liver toxicity but maintains the antitumor response in a breast cancer bone metastasis model. Hum Gene Ther 2011;22:1137–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Xu W, Zhang Z, Yang Y, et al. Ad5/48 hexon oncolytic virus expressing sTGFbetaRIIFc produces reduced hepatic and systemic toxicities and inhibits prostate cancer bone metastases. Mol Ther 2014;22:1504–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang Z, Hu Z, Gupta J, et al. Intravenous administration of adenoviruses targeting transforming growth factor beta signaling inhibits established bone metastases in 4T1 mouse mammary tumor model in an immunocompetent syngeneic host. Cancer Gene Ther 2012;19:630–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saha D, Rabkin SD. Immunohistochemistry for tumor-infiltrating immune cells after oncolytic virotherapy. Methods Mol Biol 2020;2058:179–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bottai G, Raschioni C, Losurdo A, et al. An immune stratification reveals a subset of PD-1/LAG-3 double-positive triple-negative breast cancers. Breast Cancer Res 2016;18:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xu W, Neill T, Yang Y, et al. The systemic delivery of an oncolytic adenovirus expressing decorin inhibits bone metastasis in a mouse model of human prostate cancer. Gene Ther 2015;22:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Shayakhmetov DM, Li Z-Y, Ni S, et al. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J Immunol 2005;174:7310–7319 [DOI] [PubMed] [Google Scholar]
- 33. Rodriguez-Ruiz ME, Rodriguez I, Garasa S, et al. Abscopal effects of radiotherapy are enhanced by combined immunostimulatory mAbs and are dependent on CD8 T cells and crosspriming. Cancer Res 2016;76:5994–6005 [DOI] [PubMed] [Google Scholar]
- 34. Duan X, Chan C, Guo N, et al. Photodynamic therapy mediated by nontoxic core-shell nanoparticles synergizes with immune checkpoint blockade to elicit antitumor immunity and antimetastatic effect on breast cancer. J Am Chem Soc 2016;138:16686–16695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kim K, Skora AD, Li Z, et al. Eradication of metastatic mouse cancers resistant to immune checkpoint blockade by suppression of myeloid-derived cells. Proc Natl Acad Sci U S A 2014;111:11774–11779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pulaski BA, Ostrand-Rosenberg S. Reduction of established spontaneous mammary carcinoma metastases following immunotherapy with major histocompatibility complex class II and B7.1 cell-based tumor vaccines. Cancer Res 1998;58:1486–1493 [PubMed] [Google Scholar]
- 37. Valcourt U, Carthy J, Okita Y, et al. Analysis of epithelial-mesenchymal transition induced by transforming growth factor beta. Methods Mol Biol 2016;1344:147–181 [DOI] [PubMed] [Google Scholar]
- 38. Tauriello DVF, Palomo-Ponce S, Stork D, et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018;554:538–543 [DOI] [PubMed] [Google Scholar]
- 39. Yang YA, Dukhanina O, Tang B, et al. Lifetime exposure to a soluble TGF-beta antagonist protects mice against metastasis without adverse side effects. J Clin Invest 2002;109:1607–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gupta J, Robbins J, Jilling T, et al. TGFbeta-dependent induction of interleukin-11 and interleukin-8 involves SMAD and p38 MAPK pathways in breast tumor models with varied bone metastases potential. Cancer Biol Ther 2011;11:311–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Marelli G, Howells A, Lemoine NR, et al. Oncolytic viral therapy and the immune system: a double-edged sword against cancer. Front Immunol 2018;9:866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Vloten JP, Workenhe ST, Wootton SK, et al. Critical interactions between immunogenic cancer cell death, oncolytic viruses, and the immune system define the rational design of combination immunotherapies. J Immunol 2018;200:450–458 [DOI] [PubMed] [Google Scholar]
- 43. Reid T, Warren R, Kirn D. Intravascular adenoviral agents in cancer patients: lessons from clinical trials. Cancer Gene Ther 2002;9:979–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamamoto Y, Nagasato M, Yoshida T, et al. Recent advances in genetic modification of adenovirus vectors for cancer treatment. Cancer Sci 2017;108:831–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sumida SM, Truitt DM, Lemckert AA, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol 2005;174:7179–7185 [DOI] [PubMed] [Google Scholar]
- 46. Bruder JT, Semenova E, Chen P, et al. Modification of Ad5 hexon hypervariable regions circumvents pre-existing Ad5 neutralizing antibodies and induces protective immune responses. PLoS One 2012;7:e33920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Teesalu T, Sugahara KN, Ruoslahti E. Tumor-penetrating peptides. Front Oncol 2013;3:216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res 2019;25:4592–4602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. George S, Rini BI, Hammers HJ. Emerging role of combination immunotherapy in the first-line treatment of advanced renal cell carcinoma: a review. JAMA Oncol 2019;5:411–421 [DOI] [PubMed] [Google Scholar]
- 50. Khair DO, Bax HJ, Mele S, et al. Combining immune checkpoint inhibitors: established and emerging targets and strategies to improve outcomes in melanoma. Front Immunol 2019;10:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Le DT, Durham JN, Smith KN, et al. Mismatch-repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. O'Donnell JS, Long GV, Scolyer RA, et al. Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev 2017;52:71–81 [DOI] [PubMed] [Google Scholar]
- 53. Pusztai L, Karn T, Safonov A, et al. New strategies in breast cancer: immunotherapy. Clin Cancer Res 2016;22:2105–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vonderheide RH, Domchek SM, Clark AS. Immunotherapy for breast cancer: what are we missing? Clin Cancer Res 2017;23:2640–2646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Saha P, Nanda R. Concepts and targets in triple-negative breast cancer: recent results and clinical implications. Ther Adv Med Oncol 2016;8:351–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miao Y, Yang H, Levorse J, et al. Adaptive immune resistance emerges from tumor-initiating stem cells. Cell 2019;177:1172–1186.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schmid P, Adams S, Rugo HS, et al. Atezolizumab and Nab-Paclitaxel in advanced triple-negative breast cancer. N Engl J Med 2018;379:2108–2121 [DOI] [PubMed] [Google Scholar]
- 58. Korkut A, Zaidi S, Kanchi RS, et al. A pan-cancer analysis reveals high-frequency genetic alterations in mediators of signaling by the TGF-beta superfamily. Cell Syst 2018;7:422–437 e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Massague J. TGFbeta in cancer. Cell 2008;134:215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu J, Liao S, Diop-Frimpong B, et al. TGF-beta blockade improves the distribution and efficacy of therapeutics in breast carcinoma by normalizing the tumor stroma. Proc Natl Acad Sci U S A 2012;109:16618–16623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Muraoka RS, Dumont N, Ritter CA, et al. Blockade of TGF-beta inhibits mammary tumor cell viability, migration, and metastases. J Clin Invest 2002;109:1551–1559 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.