Abstract
Introduction: Encrustation of implanted urinary tract devices is associated with significant morbidity. Pellethane® is a polyether-based compound noted for its strength, porosity, and resistance to solvents. We assessed Pellethane thermoplastic polyurethane (TPU) with and without surface coatings 2-hydroxyethyl methacrylate (HEMA) and tetraethylene glycol dimethyl ether (TETRA) for the potential to resist encrustation in an artificial urine environment.
Materials and Methods: Samples of Pellethane TPU, HEMA Pellethane TPU, TETRA Pellethane TPU, and hydrogel-coated ureteral stent (Cook®) were suspended in a batch-flow model with an artificial urine solution (AUS). Every 48 hours for 90 days, 40% of the solution was replaced with fresh AUS. All samples were stored in a 37°C incubator. Subsequently, the samples were thoroughly dried for 48 hours before weighing. Scanning electron microscopy was used to assess the degree of encrustation. Nu-Attom Inductively Coupled Plasma Mass Spectrometry (ICP-MS) was used to determine the precise compositions of the encrustation specifically with regard to calcium, magnesium, and phosphate.
Results: At the conclusion of the 90-day trial, the samples were analyzed, and the average mass changes were as follows: stent 63.78%, uncoated Pellethane TPU 11.50%, HEMA-coated Pellethane TPU 2.90%, and TETRA-coated Pellethane TPU 0.60%. Pellethane TPU products, and specifically those coated with HEMA and TETRA, exhibited less average mass increase and a lesser propensity to form encrustation than the traditional urinary tract stent. The mass increases noted on coated Pellethane devices were primarily ionic, whereas that of the stent was not.
Conclusion: Pellethane, particularly with an HEMA-based preventative coating, may serve as a favorable alternative to traditional urinary stent material, providing its improved resistance to encrustation.
Keywords: urine, encrustation, urinary tract devices, Pellethane, stent
Introduction
The formation of encrustation on the surface of implanted urinary tract devices remains a persistent impediment to urological care; encrustation appears to be associated with increased morbidity, causing significant patient pain and discomfort throughout the course of urological treatment.1 Previous studies have indicated that removing an excessively retained and encrusted stent can be up to seven times costlier than that of an appropriately timed removal of a stent, thereby placing a needless burden on the patient and the health care system.2 Encrustation may vary drastically depending on urine composition, the presence of bacterial infection, and the composition of the device itself; the most common forms of encrustation deposits are magnesium ammonium phosphate and calcium based3 To address this recurrent problem, there remains a demand for novel, alternative device materials with the capability to reduce the potential for encrustation formation while effectively performing their intended purpose.
Thermoplastic polyurethane (TPU) has been successfully used in a myriad of medical applications, including artificial hearts and digestive system prostheses.4 Pellethane® TPU (Lubrizol Thermedics) is an aromatic polyether-based TPU noted for its high strength, flexibility, and resistance to highly caustic solvents.4 Due to its ideal mechanical and functional properties, including durability, fabricability, flexibility, porosity, and capacity to maintain applied surface coatings, TPU might prove to be a feasible alternative to current urinary tract device materials.
In addition to a more sanguine stent material, interest has also arisen with regard to the potential advantageous properties of encrustation, limiting surface coatings that may be applied to urinary tract materials to prolong the potential lifespan of these devices. Surface coatings ranging from antibacterial agents to natural glucosaminoglycans have previously been shown to reduce biofilm formation and encrustations with varying degrees of success.5 However, to date, no study has assessed the potential encrustation properties of either uncoated Pellethane TPU or Pellethane TPU with an encrustation-resistant surface coating.
This study investigated uncoated TPU along with TPU coated with either a hydrophilic polymer (hydrogel) (i.e., poly 2-hydroxyethyl methacrylate) (HEMA) or tetraethylene glycol dimethyl ether (TETRA), a precursor molecule that may form a poly (ethylene glycol)-like hydrophilic linear polymer. HEMA is noted for its chemical stability and ability to resist cell adhesion. Due to these properties, HEMA is often incorporated into the design of devices such as contact lenses to reduce and or prevent the potential negative impact of biofilm formation on the surface of the eye.6,7 Similarly, TETRA has been noted to resist protein adsorption and platelet adhesion, particularly under flow conditions that closely mimic the conditions a device would experience when implanted into the urinary tract.8–10
The primary objective of this study was to assess the mineral encrustation formation on Pellethane TPU with and without biofilm-resistant surface treatments in an environment resembling that of human urine with comparison to a commonly deployed hydrogel-coated ureteral stent (Cook Medical, Inc.).
Materials and Methods
In this study, samples of Pellethane TPU, HEMA-Pellethane, and TETRA-Pellethane were obtained and coated. Samples were then extracted in ethanol to prevent the inadvertent removal of low-molecular-weight components (i.e., encrustations) and to prevent the inadvertent removal of any of the respective coatings, which, in both cases, would falsely lower the final weight of the study materials at the end of the experimentation period.
HEMA and TETRA coatings were applied by using radiofrequency (RF) plasma deposition. Such coatings, applied in a low-pressure vapor atmosphere energized with an oscillation RF field, are ultrathin (10–100 nm), delamination-resistant and, under appropriate conditions, are capable of maintaining a chemical structure closely resembling the vapor that is flowed through the RF plasma reactor. The deposition apparatus and deposition conditions have been described in a number of previous publications.11–15 Specific reaction conditions used for HEMA are argon etching (to clean the starting surface) (250 mT/20 watts/5 minutes), CH4 plasma pre-coating (to inhibit delamination of the HEMA layer [140 mT/80 watts/5 minutes]), and HEMA (250 mT/100 watts/1 minute, then 6 watts/25 minutes). Specific reaction condition used for TETRA is argon etching (to clean the starting surface) (350 mT/20 watts/5 minutes, tetraglyme [350 mT/80 watts/1 minute, then 10 watts/20 minutes]).
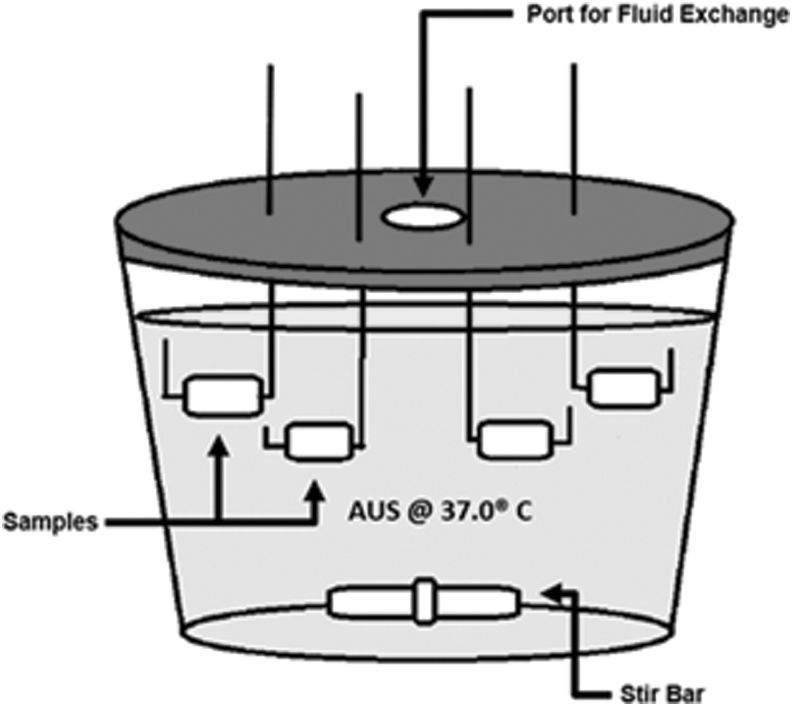
Previous urological studies have indicated that batch-flow models with static agitation encrust more readily compared with continuous flow models.16 An artificial urine solution (AUS) was chosen for use in this experiment to alleviate any uncertainty in real patient urine variability due to dietary fluctuations and lack of specific patient to patient reproducibility17,18 (Table 1). Solutions A, B, and C were each prepared separately and combined as needed in the reaction vessel to prevent the precipitation of brushite14,16,17 (Fig. 1).
Table 1.
Artificial Urine Solution Compositions
| Quantity added | ||
|---|---|---|
| Solution A | Potassium dihydrogen orthophosphate | 7.62 g |
| Magnesium chloride hexahydrate | 3.64 g | |
| Urea | 16.00 g | |
| Deionized water | 1000 mL | |
| Solution B | Calcium chloride hexahydrate | 5.32 g |
| Chicken ovalbumin | 20.00 g | |
| Deionized water | 1000 mL | |
| Solution C | Urease | 0.50 g |
| Deionized water | 400 mL | |
FIG. 1.
Reaction vessel for batch-flow encrustation model. Vessel consists of a glass container with a plastic lid. Lid is modified to include one central port to allow 40% solution replacement every 48 hours. Samples are suspended in AUS through perforations in the lid. The reaction vessel was kept in an incubator at 37.0°C ± 0.1°C, and solution was agitated with a magnetic stir bar at 250 RPM. AUS = artificial urine solution.
The Pellethane TPU samples were then compared with a radio opaque hydrogel coated ureteral stent (Cook Medical, Inc.), which is used clinically at our institution. All the urinary device materials were cut into similarly massed pieces and labeled respectively. The glass reaction vessel (Fig. 1) was placed in a 37°C incubator and agitated with a magnetic Teflon stir bar for 24 hours a day; agitation was interrupted only to allow for the exchange of AUS materials. All urinary device materials were suspended in the AUS from the lid of the reaction vessel. Every 48 hours for 90 days, 40% of the solution was replaced with fresh AUS. At the conclusion of the 90-day trial, samples were dried for 48 hours in a sterile fume hood before weighing. Encrustation was quantified via weight change and percent change in mass according to Gleeson et al.19
Scanning electron microscopy
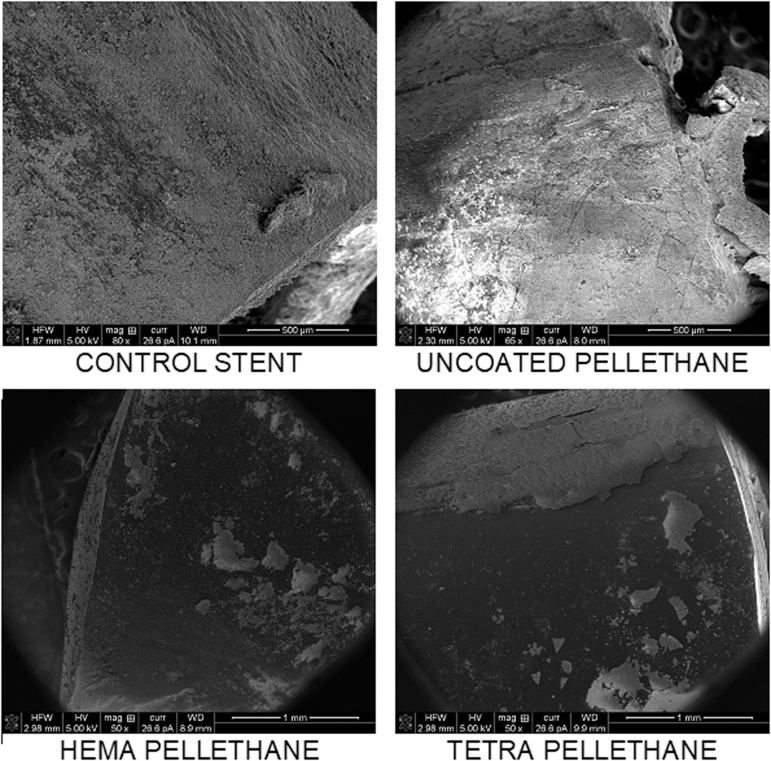
After thoroughly drying, samples were examined by using a FEI Magellan 400 XHR scanning electron microscopy (SEM). Images were qualitatively assessed for the degree of encrustation formed on several areas of the outer surfaces (end with exposed lumen vs middle, continuous sections of the device) of each respective urinary device. This method precluded analysis of the lumen of each sample.
Mass spectrometry analysis
All sample containers for the inductively coupled plasma (ICP) mass spectrometer (MS) experiments were pre-washed with nitrous acid (Fisher Scientific), for ultra-trace elemental analysis, before usage. Dried specimens of stent, and Pellethane TPU (coated and uncoated) were submerged in 2% HNO3 (v/v) followed by ultrasonication for 1.5 hours by using a Bransonic® ultrasonic cleaner to dissolve encrusted minerals. Each sonicated sample was further diluted by 100-fold using 2% HNO3 before mass spectrometry analysis. The presence of magnesium (Mg), calcium (Ca), and phosphorus (P) was measured by using an Attom High-Resolution Inductively Coupled Plasma Mass Spectrometry (ICP-MS) (Nu instruments, UK) at University of California, Irvine. Isotopes of 26Mg and 43Ca were measured at low resolution (R = 300) with 72Ge as an internal standard. 31P isotopes were measured at medium resolution (R = 2500). Standard solution of Mg, Ca, and P for standard curves were obtained from Inorganic Ventures (United States), using the following prepared standard solutions of known concentrations of the elements: Mg (100, 50, 25, 12.5, 6.25 ppb), Ca (1000, 500, 250, 125, 62.5 ppb), and P (20, 10, 5, 2.5, 1.25 ppm). Mg and Ca standard curves were prepared with a trace amount of 72Ge as an internal standard.
Results
At the conclusion of the 90-day trial, all sets of samples were analyzed, and the average mass changes were as follows: stent 63.78%, uncoated Pellethane TPU 11.50%, HEMA-coated Pellethane TPU 2.90%, and TETRA-coated Pellethane TPU 0.60%. Overall, Pellethane TPU products exhibited less mass increase and reduced propensity to form encrustation than the traditional urinary tract stent (Table 2 and Fig. 2). The mass increases on Pellethane devices were primarily ionic, whereas that of the stent was not, suggesting that the stent encrustation may be composed of alternative materials present in the AUS such as proteins, urea, and bacteria.
Table 2.
Average Mass Changes of All Urinary Tract Device Samples After a 90-Day Trial
| Material | Average initial mass (mg) | Average final mass (mg) | Average percent change in mass |
|---|---|---|---|
| Stent | 138 | 231 | 63.78% |
| Uncoated Pellethane® | 134 | 149 | 11.50% |
| HEMA-Coated Pellethane | 140 | 144.5 | 2.90% |
| TETRA-Coated Pellethane | 142 | 143 | 0.60% |
HEMA = 2-hydroxyethyl methacrylate; TETRA = tetraethylene glycol dimethyl ether.
FIG. 2.
Scanning electron microscopy images of all tested urinary tract device materials in the same respective positions. Regions that appear white and or gray represent heavily encrusted surfaces, whereas dark surfaces are devoid of encrustation.
ICP-MS was used to detect the presence of magnesium, calcium, and phosphorus encrusted on each of the materials (Table 3). The average mass of magnesium detected was as follows: stent 0.067 mg, uncoated Pellethane TPU 0.022 mg, HEMA-coated Pellethane TPU 0.006 mg, and TETRA-coated Pellethane TPU 0.027 mg. The average mass of calcium detected was as follows: stent 0.312 mg, uncoated Pellethane TPU 0.164 mg, HEMA-coated Pellethane TPU 0.052 mg, and TETRA-coated Pellethane TPU 0.183 mg. The average mass of phosphorus detected was as follows: stent 1.149 mg, uncoated Pellethane TPU 0.348 mg, HEMA-coated Pellethane TPU 0.360 mg, and TETRA-coated Pellethane TPU 0.530 mg (Table 4).
Table 3.
Presence of Magnesium, Calcium, and Phosphorous Content Detected by Inductively Coupled Plasma Mass Spectrometry
| Material | Magnesium* |
Calcium* |
Phosphorus** |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ppb | SD | %RSD | ppb | SD | %RSD | ppb | SD | %RSD | ||
| Stent | Set 1 | 125.57 | 3.70 | 2.95 | 564.09 | 10.39 | 1.84 | 417.70 | 2.62 | 0.63 |
| Set 2 | 41.33 | 1.31 | 3.18 | 215.70 | 4.63 | 2.15 | 545.37 | 3.24 | 0.59 | |
| Pellethane | Set 1 | 36.85 | 0.94 | 2.56 | 242.06 | 5.59 | 2.31 | 193.91 | 1.59 | 0.82 |
| Set 2 | 17.38 | 0.60 | 3.44 | 168.42 | 8.02 | 4.76 | 8.70 | 1.32 | 15.23 | |
| HEMA-Coated Pellethane | Set 1 | 7.79 | 0.37 | 4.74 | 87.68 | 4.74 | 5.41 | 84.33 | 1.30 | 1.54 |
| Set 2 | 7.55 | 0.55 | 7.32 | 43.08 | 2.95 | 6.84 | 292.55 | 1.95 | 0.67 | |
| TETRA-Coated Pellethane | Set 1 | 33.14 | 1.81 | 5.45 | 185.38 | 4.01 | 2.16 | 360.69 | 2.28 | 0.63 |
| Set 2 | 33.53 | 1.04 | 3.11 | 271.80 | 5.26 | 1.94 | 367.47 | 17.68 | 4.81 | |
Isotopes measured in low resolution (R = 300): 26Mg, 43Ca, 72Ge (internal standard)
Isotopes measured in medium resolution (R = 2500): 31P.
%RSD = % relative standard deviation; SD = standard deviation.
Table 4.
Average Amount of Mg, Ca, and P Detected by Inductively Coupled Plasma Mass Spectrometry Encrusted on Different Materials After the 90-Day Trial
| Material | Magnesium | Calcium | Phosphorus |
|---|---|---|---|
| Uncoated Pellethane | 0.067 mg | 0.312 mg | 1.149 mg |
| HEMA-Coated Pellethane | 0.022 mg | 0.164 mg | 0.348 mg |
| TETRA-Coated Pellethane | 0.006 mg | 0.052 mg | 0.360 mg |
| Stent | 0.027 mg | 0.183 mg | 0.530 mg |
Among the three ions being measured, magnesium ions were observed to be lower in abundance compared with calcium ions, whereas phosphorus was observed with the greatest abundance. The Pellethane materials were all observed to have lower magnesium, calcium, and phosphorus content as compared with the stent. Among the three Pellethane materials tested, HEMA-coated Pellethane TPU had the least amount of encrustation as suggested by the low abundance of magnesium, calcium, and phosphorus detected.
Discussion
Encrustation of implanted urinary tract devices, particularly stents, remains a persistent obstacle to urological care. This encrustation varies drastically from patient to patient, possibly due to genetic factors, diet, urine volume, and urine composition. Regardless of these, encrustations may hinder efficient stent removal and may contribute to stent-related patient discomfort and urinary tract infection.2
Although encrustation deposit material is often complex in its composition, the most commonly occurring components encountered are magnesium ammonium phosphate and calcium phosphate.3 To analyze these components in the encrustation formed on all the samples of urinary tract devices tested in this study, ICP-MS was used. Across all materials, phosphorous was observed in the greatest abundance throughout the encrustations tested. These results are expected since encrustation is partly attributed to the formation and eventual accumulation of magnesium ammonium phosphate and calcium phosphate crystals.
Overall, Pellethane specimens exhibited less average mass increases when compared with the non-Pellethane stent material. Notably, the majority of the mass increases on Pellethane specimens were ionic whereas the increases seen on the hydrogel stent could not be explained by ion accumulation with respect to calcium, phosphorous, or magnesium. Further study is warranted to investigate the potential capability of Pellethane products to impede encrustation specifically, as our findings suggest that this may also be likely. In addition, future studies are needed to examine the interaction between different bacteria and the various formulations of Pellethane with regard to biofilm formation. Our findings may potentially be of clinical significance, especially in individuals who require a long-term implanted urinary device (e.g., to overcome an ureteral stricture). Also, despite the commonly intended short-term usage of these devices, complications arise due to forgotten stents that become massively encrusted.2,20 Lastly, it would be of interest to analyze these newer coatings with regard to the bacterial communities that might colonize on them.
As aforementioned, surface treatment of urinary tract devices, particularly with well-known coatings such as HEMA and TETRA, has been proposed as approaches to extend the longevity of Pellethane products over traditional stents with regard to damage caused by encrustation and additional resistance to mechanical change over time. Among all Pellethane materials tested, HEMA-coated Pellethane TPU had the least amount of encrustation, as suggested by the low abundance of magnesium, calcium, and phosphorus detected. Although our findings indicated that TPU coated in HEMA and TETRA accumulated less average mass increase when compared with traditional urinary tract device materials, these coatings did not significantly impact encrustation levels (p = 0.23, Table 5).
Table 5.
Statistical Analysis of Primary Experiment
| Variables | Stent (S) (n = 2) | Uncoated Pellethane (UP) (n = 2) | HEMA-coated Pellethane (HP) (n = 2) | TETRA-coated Pellethane (TP) (n = 2) | p* | Post hoc analysis** |
|---|---|---|---|---|---|---|
| Mean initial mass (mg) | 141 | 134 | 140 | 142 | 0.16 | N/A |
| Mean final mass (mg) | 231 | 149 | 145 | 143 | <0.001 | S>UP,HP,TP (p < 0.001) |
| Mean Δ mass (mg) | 90 | 15 | 5 | 1 | <0.001 | S>UP,HP,TP (p < 0.001) |
| Encrustation characteristics | ||||||
| Encrustation of PO43−(mg) | 0.39 | 0.08 | 0.15 | 0.17 | 0.43 | N/A |
| Encrustation of Mg2+ (mg) | 0.07 | 0.02 | 0.01 | 0.03 | 0.25 | N/A |
| Encrustation of Ca2+ (mg) | 0.31 | 0.16 | 0.18 | 0.05 | 0.14 | N/A |
Unpaired data >2 groups required the use of one-way ANOVA for analysis; p < 0.05 determined as statistically significant.
Tukey Honest Significant Differences method used for post hoc analysis.
Future areas of study include, but are not limited to, the design of surface-treated Pellethane ureteral stents for pilot testing in animal models, as well as the investigation of the specific bacterial profile of Pellethane and various other common surface coatings that may be feasible in a urological context.
Conclusion
HEMA-based surface coatings coupled with Pellethane products, HEMA, may provide advantageous anti-encrustation properties when compared with traditional urinary device materials.
Abbreviations Used
- %RSD
% relative standard deviation
- AUS
artificial urine solution
- HEMA
2-hydroxyethyl methacrylate
- HEMA-P
HEMA-coated Pellethane TPU
- ICP-MS
Nu-Attom Inductively Coupled Plasma Mass Spectrometry
- RF
radiofrequency
- SD
standard deviation
- SEM
scanning electron microscopy
- TETRA
tetraethylene glycol dimethyl ether
- TETRA-P
TETRA-coated Pellethane TPU
- TPU
thermoplastic polyurethane
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1 TR001414. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1. Lange D, Bidnur S, Hoag N, Chew BH. Ureteral stent-associated complications-where we are and where we are going. Nat Rev Urol 2015;12:17–25 [DOI] [PubMed] [Google Scholar]
- 2. Sancaktutar AA, Söylemez H, Bozkurt Y, Penbegül N, Atar M. Treatment of forgotten ureteral stents: How much does it really cost? A cost-effectiveness study in 27 patients. Urol Res 2012;40:317–325 [DOI] [PubMed] [Google Scholar]
- 3. Stickler DJ. Clinical complications of urinary catheters caused by crystalline biofilms: Something needs to be done. J Intern Med 2014;276:120–129 [DOI] [PubMed] [Google Scholar]
- 4. Marois Y, Guidoin R.. Biocompatibility of Polyurethanes. In: Madame Curie Bioscience Database [Internet]. Austin, TX: Landes Bioscience, 2000–2013. Available from: https://www.ncbi.nlm.nih.gov/books/NBK6422 [Google Scholar]
- 5. Wang L, Zhang S, Keatch R, Corner G, Nabi G, Murdoch S, Davidson F, Zhao Q. In-vitro antibacterial and anti-encrustation performance of silver-polytetrafluoroethylene nanocomposite coated urinary catheters. J Hosp Infect 103:55–63. doi: 10.1016/j.jhin.2019.02.012 [DOI] [PubMed] [Google Scholar]
- 6. Hu XH, Zhang GJ, Tan HP, Li D, Chen XY, Zhang YS. Synthesis and surface modification of chitosan containing hydrogel for ophthalmic drug delivery, Mater Technol 2014;29:144–151 [Google Scholar]
- 7. Montheard JP, Chatzoloulos M, Chappard D. 2-hydroxyethyl methacrylate (HEMA): Chemical properties and applications in biomedical fields. J Macromol Sci C 1992;32:1–34 [Google Scholar]
- 8. Alinejad M, Henry C, Nikafshar S, et al. Lignin-based polyurethanes: Opportunities for bio-based foams, elastomers, coatings and adhesives. Polymers (Basel) 2019;11:DOI: 10.3390/polym11071202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang M, Horbett TA. Tetraglyme coatings reduce fibrinogen and von Willebrand factor adsorption and platelet adhesion under both static and flow conditions. J Biomed Mater Res A 2009;89A:791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Uwais ZA, Hussein MA, Samad MA, Al-Aqeeli N. Surface modification of metallic biomaterials for better tribological properties: A review. Arabian J Sci Eng 2017;42:4493–4512 [Google Scholar]
- 11. López GP, Ratner BD, Tidwell CD, Haycox CL, Rapoza RJ, Horbett TA. Glow discharge plasma deposition of tetraethylene glycol dimethyl ether for fouling-resistant biomaterial surfaces. J Biomed Mater Res 1992;26:415–439 [DOI] [PubMed] [Google Scholar]
- 12. López GP, Ratner BD, Tidwell CD, Rapoza RJ, Horbett TA. Plasma deposition of ultrathin films of poly (2-hydroxyethyl methacrylate): Surface analysis and protein adsorption measurements. Macromolecules 1993;26:3247–3253 [Google Scholar]
- 13. Aninwene GE, Stout D, Yang Z, Webster TJ. Nano-BaSO4: A novel antimicrobial additive tellethane. Int J Nanomed 2013;8:1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shen M, Martinson L, Wagner MS, Castner DG, Ratner BD, Horbett TA. PEO-like plasma polymerized tetraglyme surface interactions with leukocytes and proteins: In vitro and in vivo studies. J Biomater Sci Polym Ed 2002;13:367–390 [DOI] [PubMed] [Google Scholar]
- 15. Ratner BD, Chilkoti A, López GP. Chapter 7: Plasma deposition and treatment for biomaterial applications. In: D'Agostino R. Plasma Deposition, Treatment, and Etching of Polymers. Academic Press, San Diego, CA, 1990, pp. 463–516 [Google Scholar]
- 16. Jones DS, Djokic J, Gorman SP. Characterization and optimization of experimental variables within a reproducible bladder encrustation model and in vitro evaluation of the efficacy of urease inhibitors for the prevention of medical device-related encrustation. J Biomed Mater Res B 2006;76B:1–7 [DOI] [PubMed] [Google Scholar]
- 17. Shaheen T, Edirisinghe T, Gabriel M, Bourdoumis A, Buchholz N, Knight M. In vitro encrustation of a semi-permanent polymer-covered nitinol ureter stent: An artificial urine model. Urolithiasis 2014;42:203–207 [DOI] [PubMed] [Google Scholar]
- 18. Gilmore BF, Jones DS, Gorman SP, Ceri H. Models for the assessment of biofilm and encrustation formation on urological materials. Biomater Tissue Eng Urol 2009;59–81. DOI: 10.1533/9781845696375.1.59 [DOI]
- 19. Gleeson MJ, Glueck JA, Feldman L, Griffith DP, Noon GP. Comparative in vitro encrustation studies of biomaterials in human urine. ASAIO Trans 1989;35:495. [DOI] [PubMed] [Google Scholar]
- 20. Sali GM, Joshi HB. Ureteric stents: Overview of current clinical applications and economic implications. Int J Urol 2019. [Epub ahead of print]; DOI: 10.1111/iju.14119 [DOI] [PubMed]